Abstract
Purpose
To identify those metallothionein and α-crystallin/small heat-shock genes induced by toxic metals in human lens cells and to evaluate the levels of these metals between young and aged human lenses.
Methods
Human SRA01/04 and primary human lens epithelial cells were cultured and exposed to Cd2+, Cu2+, and Zn2+. The levels of lens metallothioneins (Ig, If, Ih, Ie, and IIa) and α-crystallin/small heat-shock (αA-crystallin, αB-crystallin, and HSP27) genes were analyzed by semiquantitative and quantitative competitive RT-PCR. The content of aluminum, cadmium, calcium, chromium, copper, iron, lead, magnesium, manganese, nickel, potassium, sodium, and zinc in young (mean, 32.8 years), middle-aged (mean, 52.3 years), and old (mean, 70.5 years) human lenses was analyzed by inductively coupled plasma-emission spectroscopy.
Results
Lens metallothioneins (Ig, If, Ih, Ie, and IIa) and α-crystallin/small heat-shock genes (αA-crystallin, αB-crystallin, and HSP27) were differentially induced by specific metals in SRA01/04 human lens epithelial cells. Cd2+ and Zn2+, but not Cu2+, induced the metallothioneins, whereas Cd2+ and Cu2+, but not Zn2+, induced αB-crystallin and HSP27. αA-crystallin was induced by Cu2+ only. Similar responses of the metallothionein IIa gene were detected in identically treated primary human lens epithelial cells. Cd2+ and Zn2+ induced metallothionein IIa to five times higher levels than metallothionein Ig. Of 13 different metals, only iron was altered, exhibiting an 81% decrease in old versus young lenses.
Conclusions
Induction of metallothioneins and α-crystallin/small heat shock proteins by different metals indicates the presence of metal-specific lens regulatory pathways that are likely to be involved in protection against metal-associated stresses.
Toxic metals and the genes that they induce are associated with cell death, oxidative stress, and lens cataract. Human exposures to toxic metals such as iron, copper, cadmium, lead, aluminum, and others, arise from widespread sources, including cigarette smoke, air pollution, leaching of landfills, industrial waste, emissions from fossil fuels, fertilizers, and corrosion of plumbing.1,2 Cadmium has a biological half-life in humans of up to 30 years,3 and large amounts of Cd2+ have been detected in the lenses of chronic smokers4 who also exhibit early cataract formation.5 Increased Cd2+ levels have been reported in cataract versus clear human lenses.4 Fe2+ and Cu2+ participate in Fenton-type reactions associated with oxidative stress and cataract.6 Hyperferritinemia7 and defects in Cu2+ transport, including Wilson disease and Menkes syndrome,8 result in specific types of human cataract.
Biological systems have evolved numerous gene pathways to regulate and detoxify heavy metals. One major group of proteins that are believed to regulate and protect against metals is the metallothioneins (MTs). There are 16 known isoforms of MTs in humans, grouped into four classes: I, II, III, and IV. MTs are 6- to 7-kDa polypeptides9 that bind a wide spectrum of metals and are rapidly induced by metals and other agents in numerous tissues.9 In addition to metals, they are induced by steroids in rat fibroblasts10 and primary fibroblasts in human skin,11 carcinogens in mice,12 chemicals that induce oxidative stress in rodent cells,13 and UV-induced DNA damage.14
We have shown that the human lens expresses MT classes I and II including MT isoforms Ia, Ig, If, Ih, Ie, and IIa.15 Only one isoform, MTIIa, is specific for the lens epithelium, whereas the MTI isoforms are expressed at lower levels in both the lens epithelium and lens fibers.15 In addition, MTIIa exhibits increased expression in age-related cataract compared with clear human lenses,16 suggesting a possible role for MTIIa in lens protection.
Multiple studies have demonstrated a direct role for MTs in protecting multiple cell types against a wide range of insults that are associated with metal exposure, oxidative stress, and cataract. Overexpression of MT in a human trophoblastic cell line has been shown to protect against cadmium-induced apoptosis.17 MT I- and II-null mice are more sensitive than wild-type mice to metal exposure and oxidative stress18–22; however, no one has examined the lenses of these animals. Overexpression of MTIa in a human retinal pigment epithelial cell line provides direct protection against Cd2+ exposure, heme- and iron-induced oxidation, and UV light-induced apoptosis.23
In addition to the MTs, the α-crystallin/small heat-shock genes have been shown to be induced by metals in nonlens systems. Like MTs, αB-crystallin and HSP27 have been shown to be induced by Cd2+ in astrocytes.24 In addition to metals, the small heat-shock proteins (sHSPs) are induced by a wide variety of agents, including increasing hypertonicity in retinal pigment epithelial cells25 and canine lens epithelial cells26; vasopressin in human vascular smooth muscle cells27; TGF-β in human trabecular meshwork cells28 and rat lenses29; heat shock in human and monkey trabecular meshwork30 and various rat tissues including central nervous tissue, liver, lung, spleen, adrenal glands, and hypophysis31 and astrocytoma cells32; hydrogen peroxide treatment in human and monkey trabecular meshwork cells30; and glucocorticoids in fibroblasts.33 To date, no one has examined the levels of α-crystallin/sHSPs induced by metals in lens cells.
MTs’ exact functions in lens cells remain unknown, but numerous studies have demonstrated a direct role for α-crystallin/sHSPs in lens protection. Overexpression of αA- and αB-crystallin has been shown to protect lens epithelial cells against stress-induced apoptosis.34–36 αA-crystallin–null mice exhibit lens opacities at an early age36,37 and the growth rate of lens epithelial cells isolated from these animals is reduced by 50%.36
Based on the association between toxic metals and lens cataract and the detection of increased expression of MTIIa in age-related cataract compared with clear lenses,16 we sought to define further the magnitude and specificity of lens MT15 and α-crystallin/sHSP inductions in response to three commonly studied metals: Cd2+, Cu2+, and Zn2+. To survey the metal content of aging human lenses, we also determined the levels of 13 different metals between young, middle-aged, and old lenses.
Establishing the metal-induced expression patterns of these genes in human lens epithelial (HLE) cells is important, because it is essential to examine their responses in cultured lens cells before proceeding to functional and in vivo studies. Because the lens epithelium is a transcriptionally active region of the lens and is essential for the growth, differentiation, and homeostasis of the entire lens,38,39 and, because approximately 90% of the lens MTs are confined to this lens region,15 the lens epithelium would be expected to be particularly responsive to toxic metals. In addition, significant levels of α-crystallin/sHSPs that may also respond to metals24 are localized to this part of the lens. Because lens epithelial cells occupy the most anterior portion of the lens and are readily exposed to environmental insults, and because these cells contain most of the enzymes and transport systems in the lens,40–42 this region of the lens would be expected to be particularly prone to direct and/or indirect damage associated with toxic metals.
Our results provide evidence that the human lens epithelium responds to specific metals through the differential induction of five MT isoforms and three α-crystallin/sHSPs, including αA-crystallin which, to our knowledge, has not been shown to be induced by metals and/or stress. Consistent with the detection of increased MTIIa expression in age-related cataract compared with clear human lenses,16 MTIIa is the primary MT isoform induced in HLE cells. Different MTs and α-crystallin/sHSPs are induced by different metals, which suggests specific roles for these genes in lens metal regulation and/or protection. With the exception of iron levels, which dramatically decrease with age, the levels of 12 different metals in healthy human lenses remain constant with age, indicating that toxic metals do not accumulate in clear human lenses.
Materials and Methods
HLE Culture
HLE cells (SRA01/04)43 were grown and cultured in Dulbecco’s modified Eagle’s medium supplemented with 15% fetal bovine serum, gentamicin (50 units/mL; Invitrogen, Gaithersburg, MD) and PSN (penicillin-streptomycin-neomycin) antibiotic mix (50 U/mL; Invitrogen), at 36.5°C in the presence of 5% CO2. The methods for establishing primary cultures of human lens epithelial cells have been described.44 Briefly, pieces of capsule and epithelium obtained from infants who underwent surgery for retinopathy of prematurity were washed once with Ca2+- and Mg2+-free phosphate-buffered saline and collected with a microsuction pipette. Small fragments of capsule (1–2 mm2) with epithelial cells attached were placed in a 60-mm culture dish in Dulbecco’s modified Eagle’s medium supplemented with 20% fetal calf serum (Falcon; BD Biosciences, Oxnard, CA) as explants, until a confluent monolayer was formed in approximately 2 weeks.
Cells from explant cultures were dissociated with trypsin-EDTA solution (Invitrogen) and collected by centrifugation. They were subcultured in the initial medium at 36.5°C in a humidified atmosphere with 5% CO2. The procedure was repeated for additional subcultures.
Metal Treatments
Cells were exposed to the indicated concentrations of CdCl2, CuCl2, and ZnCl2 dissolved in water, as previously described45 and optimal induction conditions were determined. At indicated times, cells were washed with PBS, and total RNA was isolated with extraction reagent (Trizol; Invitrogen), as specified by the manufacturer. Cell viability in response to metal treatment was assessed by trypan blue exclusion.46 Cell viability is expressed as the standard deviation for three separate cell populations treated identically.
Semiquantitative RT-PCR
Gene-specific primers were designed using the BLAST program and GenBank database (http://www.nlm.nih.ncbi.gov/, National Center for Biotechnology Information, Bethesda, MD). Primer sequences, Gen-Bank accession numbers, annealing temperatures, and product lengths for all gene-specific primers used in this study are indicated in Table 1. RT-PCR was performed with 100 ng of RNA, with a commercial RT-PCR system used according to the manufacturer’s protocol (OneStep; Invitrogen). Products were separated by gel electrophoresis on 1.5% agarose gels and visualized by ethidium bromide staining and sequenced to ensure specificity. Product formation for indicated genes was linear over 20 to 30 PCR cycles.
Table 1.
Primers Used for RT-PCR
| Gene | Primer Sequence | Annealing Temperature | Product Length | Accession No. |
|---|---|---|---|---|
| MTIIa | AAGTCCCAGCGAACCCGCGT | 52 | 237 | J00271 |
| MTIIa | CAGCAGCTGCACTTGTCCGACGC | 52 | 237 | J00271 |
| MTIe | GCTCCAGCATCCCCTTTGCT | 57 | 211 | M10942 |
| MTIe | CACATCAGGCACAGCAGCTG | 57 | 211 | M10942 |
| MTIf | GCTTCTCTCTTGGAAAGTCC | 55 | 226 | M10943 |
| MTIf | GGCATCAGTCGCAGCCGCTG | 55 | 226 | M10943 |
| MTIg | GCCTCTTCCCTTCTCGCTTG | 55 | 217 | J03910 |
| MTIg | GACATCAGGCGCAGCAGCTG | 55 | 217 | J03910 |
| MTIh | GAACTCCAGTCTCACCTCGG | 55 | 213 | X64834 |
| MTIh | GACATCAGGCACAGCAGCTG | 55 | 213 | X64834 |
| αA-crystallin | CCACCTCGGCTCCCTCGTCCTAAG | 64 | 492 | NM_000394 |
| αA-crystallin | CCATGTCCCCAAGAGCGGCACTAC | 64 | 492 | NM_000394 |
| αB-crystallin | AGCCGCCTCTTTGACCAGTTCTTC | 60 | 452 | NM_001885 |
| αB-crystallin | GCGGTGACAGCAGGCTTCTCTTC | 60 | 452 | NM_001885 |
| HSP27 | CGCGCTCAGCCGGCAACTCAG | 64 | 419 | XM_055937 |
| HSP27 | AGGGGTGGGCATCCGGGCTAAGG | 64 | 419 | XM_055937 |
| GAPDH | CCACCCATGGCAAATTCCATGGCA | 52 | 600 | XM_006959 |
| GAPDH | TCTAGACGGCAGGTCAGGTCCACC | 52 | 600 | XM_006959 |
Quantitative Competitive RT-PCR
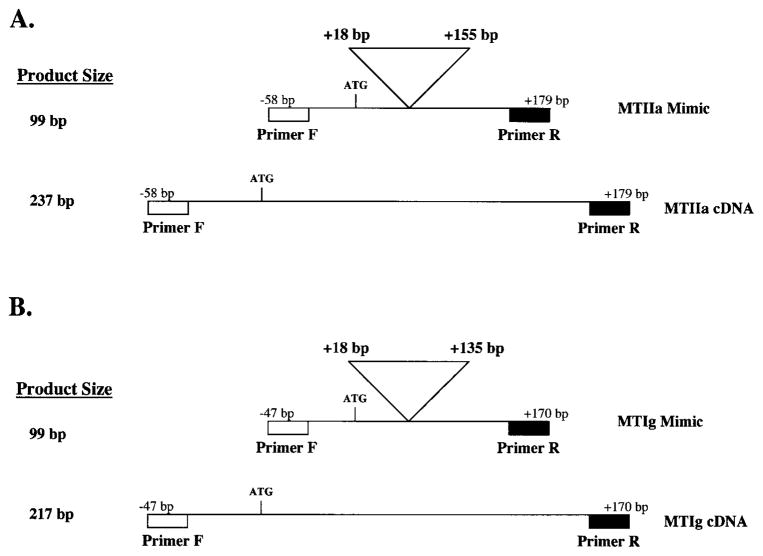
For PCR, mimic cDNAs (Fig. 1)—identical in sequence with the MTIIa and MTIg cDNA sequences, with the exception of a 138-bp internal deletion (base pairs +18 to +155 from the start of translation) for the MTIIa mimic cDNA and a 118-bp internal deletion (base pairs +18 to +135 from the start of translation) for the MTIg mimic cDNA—were synthesized and used to compete with endogenous MT cDNAs. Indicated transcripts were reverse transcribed and amplified in the presence or absence of the mimic cDNAs, as recommended by the manufacturer (One Step; Invitrogen), and the same primers and conditions described for semiquantitative RT-PCR. The MT mimic cDNAs contain the same primer binding sites and sequences as do the endogenous MT mRNAs except for the indicated deletions. The amount of MTIIa or MTIg mimic cDNA needed to compete equally with a fixed amount of total RNA is proportional to the amount of transcript present. Increasing amounts (0.1–500 pg) of mimic DNA template competed with a constant amount of RNA (300 ng) in the presence or absence of 1.0 μCi of α32P-CTP (250 μCi/mmol; Amersham Biosciences, Piscataway, NJ). MTIIa products were excised from the gel and incorporated radioactivity monitored by scintillation counting. The resultant counts were corrected for background and the number of cytosine residues present in each PCR product. The amount of MTIIa transcript per nanogram total RNA was determined by calculating the amount of mimic DNA (in counts per minute; cpm) required to compete equally with the endogenous transcript present in 300 ng total RNA.
Figure 1.
Schematic representation of the MTIIa mimic cDNA (A). A 138-bp internal sequence (+18 to +155 bp from the start of translation) was deleted from the MTIIa cDNA to create the 99-bp MTIIa mimic cDNA. Shown for comparison is the full-length 237-bp MTIIa cDNA. Schematic representation of the MTIg mimic cDNA (B). A 118-bp internal sequence (+18 to +135 base pairs, from the start of translation) was deleted from the MTIg cDNA to create the 99-bp MTIg mimic cDNA. Shown for comparison is the full-length 217-bp MTIg cDNA. Indicated are the primer binding sites.
Metal Analysis of Human Lenses
Forty-five clear, decapsulated human lenses ranging from 21 to 72 years of age were dried in a 60°C incubator overnight. Dry weights for individual lenses were determined. Triplicate groups of five—young lenses, average age 25.8, 34.2, and 38.4 years; middle-aged lenses, average age 51.6, 53.0, and 54.8 years; and old lenses, average age 69.2, 70.6, and 71.8 years—were predigested overnight in 5 mL concentrated HNO3, after which 3 mL of 30% H2O2 was added. Digests were heated to 120°C.47 After they were cooled, samples were brought to a final volume of 50 mL with deionized water and then filtered (0.2 μm). Samples were assayed for elemental metal concentrations by inductively coupled plasma emission spectroscopy (ICP) by the Analytical Laboratory at the National Research Center for Coal and Energy at West Virginia University. Elemental concentrations for each sample were corrected for differences in dry weights. Differences between age group means were explored by analysis of variance and the Tukey-Kramer honest significant difference (HSD) procedure (JMP; SAS Institute, Cary, NC).
Results
Quantification of MTIIa Levels Compared with MTIg Levels Induced by Cd2+, Cu2+, and Zn2+ in HLE Cells
To determine the relative levels of a class II MT in comparison with a class I MT induced by toxic metals in HLE cells, we examined the induction levels of MTIIa and MTIg on exposure to Cd2+, Cu2+, and Zn2+ by quantitative competitive RT-PCR. Cd2+, Cu2+, and Zn2+ were evaluated as inducers in these experiments, because these are the metals that have been used in most studies on MT and α-crystallin/sHSP induction in non-lens systems.9,24 MTIIa was chosen, because this MT isoform exhibits increased expression in age-related cataract compared with clear human lenses,16 and MTIg was chosen as a representative class I MT.
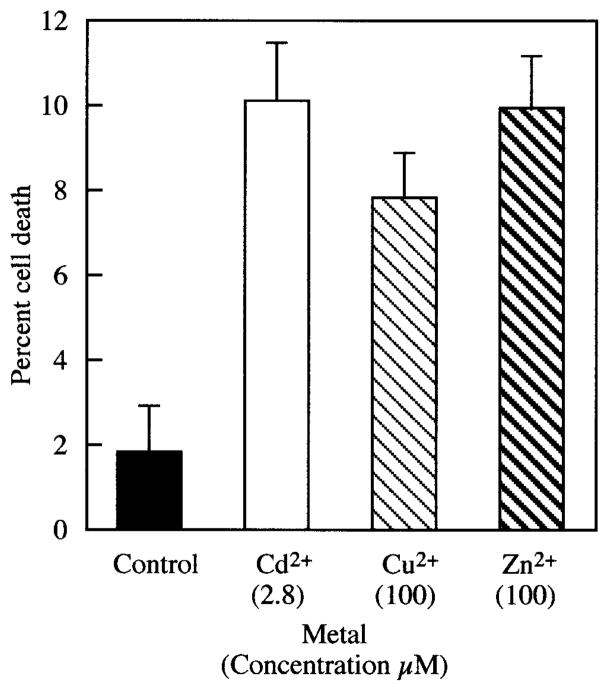
In preliminary studies, maximum MT induction occurred after 8-hour treatments with 2.8 μM Cd2+, 100 μM Cu2+, and 100 μM Zn2+ (data not shown). Trypan blue exclusion detected no more than 10% cell death under these conditions (Fig. 2). Longer exposure times or higher metal concentrations resulted in decreased cell viability and consequent loss of gene expression (data not shown).
Figure 2.
Cell toxicity resulting from Cd2+, Cu2+, and Zn2+ treatment of HLE cells at the metal concentrations shown below the metals (in micromolar). HLE cells were treated with indicated metals for 8 hours, and cell death was examined by trypan blue exclusion. Data are the mean ± SD of results in three separate experiments.
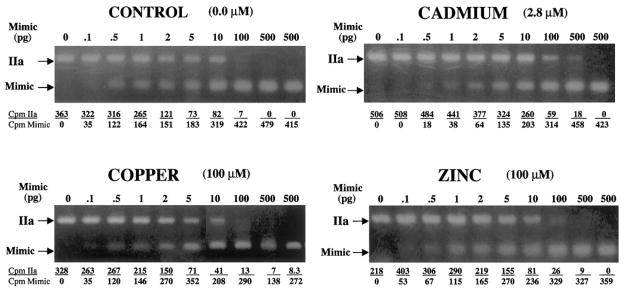
One to 2 pg MTIIa mimic DNA equally competed with the amount of MTIIa transcript present in 300 ng of untreated control RNA (Fig. 3). The level of MTIIa transcript present in untreated control cells is therefore between 0.003 and 0.006 pg/ng total RNA. By comparison, the amount of mimic DNA required to compete equally with the amount of MTIIa transcript present in 300 ng of RNA from Cd2+-treated cells was 10 to 20 pg (Fig. 3). The level of induced MTIIa transcript in Cd2+-treated cells is therefore between 0.033 and 0.067 pg./ng total RNA. The amount of mimic DNA required to compete equally with the amount of MTIIa transcript present in 300 ng of RNA from Zn2+-treated cells was between 2 and 4 pg (Fig. 3). The level of induced MTIIa transcript in Zn2+-treated cells is therefore between 0.007 and 0.013 pg/ng total RNA. Treatment with Cu2+ showed little or no difference in the levels of MTIIa transcript (0.003–0.006 pg/ng total RNA) when compared with untreated control cells (0.003–0.006 pg MTIIa per ng total RNA; Fig. 3). These data demonstrate that MTIIa is induced in HLE cells by Cd2+ (10–20-fold), and Zn2+(2–4-fold), whereas Cu2+ treatment did not induce the MTIIa transcript.
Figure 3.
Ethidium bromide–stained gels showing quantitative competitive RT-PCR analysis of MTIIa induced after 8 hours of Cd2+, Cu2+, or Zn2+ treatment. RNA (300 ng) was amplified in the presence of increasing amounts (0–500 pg) of competing MTIIa mimic DNA. Indicated are the metal concentrations, the 237-bp MTIIa cDNA, the 99-bp MTIIa mimic DNA PCR products, and the calculated radioactivity incorporated in each PCR product.
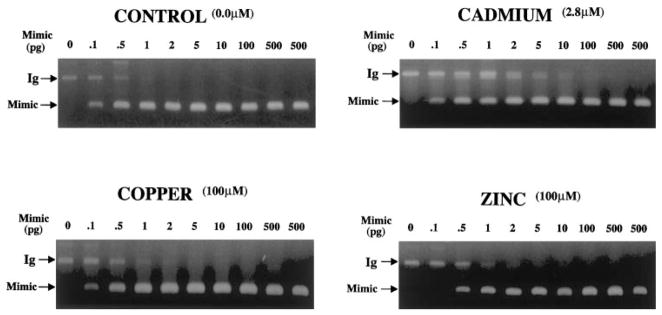
In contrast to MTIIa, 0.1 pg MTIg mimic DNA competed equally with the amount of MTIg transcript present in 300 ng of untreated control RNA. The level of MTIg transcript present in untreated control cells was therefore 0.0003 pg/ng total RNA. The amount of mimic DNA required to compete equally with the amount of MTIg transcript present in 300 ng of RNA from Cd2+-treated cells was approximately 1 pg (Fig. 4). The level of MTIg transcript in Cd2+-treated cells was therefore approximately 0.003 pg/ng total RNA. The amount of mimic DNA required to compete equally with the amount of MTIg transcript present in 300 ng of RNA from Zn2+-treated cells was between 0.1 and 0.5 pg (Fig. 4). The level of MTIg transcript in Zn2+-treated cells was therefore between 0.0004 and 0.0018 pg/ng total RNA. Consistent with the previous experiments, treatment with Cu2+ showed little or no difference in the levels of MTIg transcript (0.0003 pg/ng total RNA) when compared with untreated control cells (0.0003 pg MTIg per ng total RNA; Fig. 4).
Figure 4.
Ethidium bromide–stained gels showing quantitative competitive RT-PCR analysis of MTIg induced after 8 hours of Cd2+, Cu2+, or Zn2+ treatment, in conditions identical with those described in Figure 3. Indicated are the metal concentrations, the 217-bp MTIg cDNA, and the 99-bp MTIg mimic PCR products.
Collectively, these data demonstrate that although the patterns of induction of MTIIa and MTIg in response to metals are similar, MTIIa is induced at five times higher levels than MTIg in response to these metals in HLE cells. These genes exhibit specific inductions for different metals, in that Cd2+ and Zn2+ activated them but Cu2+ did not.
Induction of MTIIa in Primary HLE Cells
To provide confidence that inductions detected for MTIIa are not restricted to the transformed SRA01/04 cells, the induction levels of MTIIa were also examined under identical conditions in untransformed primary HLE cells by semiquantitative RT-PCR (Fig. 5). In these cells, MTIIa was induced to high levels by Cd2+ and low levels by Zn2+ when compared with untreated control cells (Fig. 5). Although slight induction was detected with Cu2+ treatment (Fig. 5), these data demonstrate that similar induction patterns for MTIIa occur in untransformed primary HLE cells and are likely to be paralleled in vivo.
Figure 5.
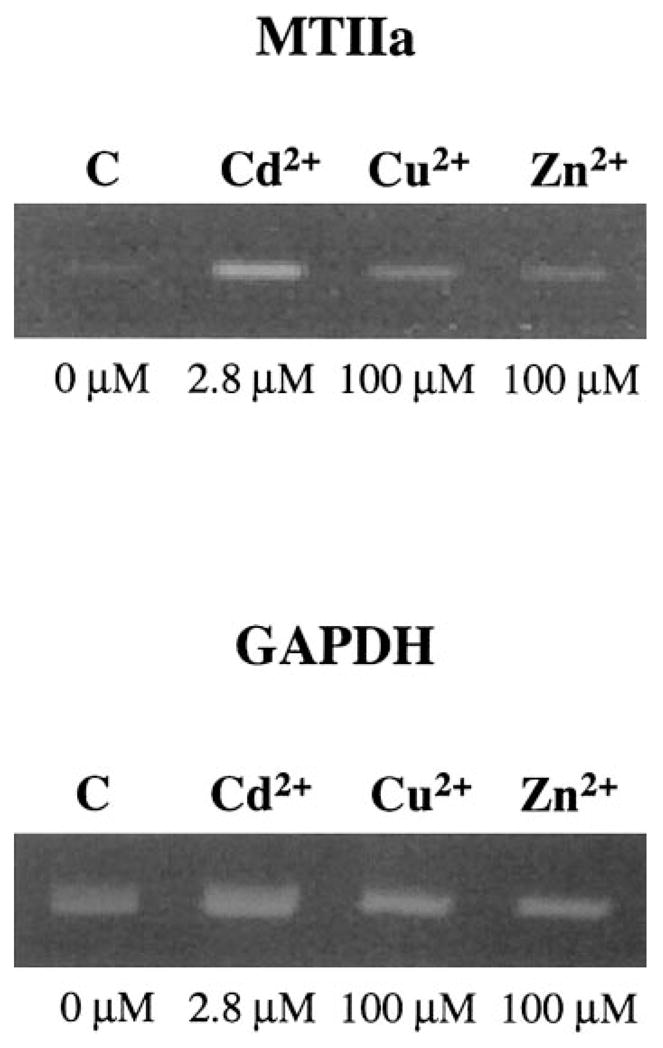
Ethidium bromide–stained gel showing the levels of MTIIa detected by RT-PCR in 50 ng RNA isolated from primary HLE cells induced by Cd2+, Cu2+, and Zn2+ for 8 hours at the indicated concentrations for a total of 28 PCR cycles. Shown as the control are the corresponding GAPDH levels.
Identification of the Spectrum of Metallothionein and Small Heat-Shock Genes Induced by Cd2+, Cu2+, and Zn2+ in HLE Cells
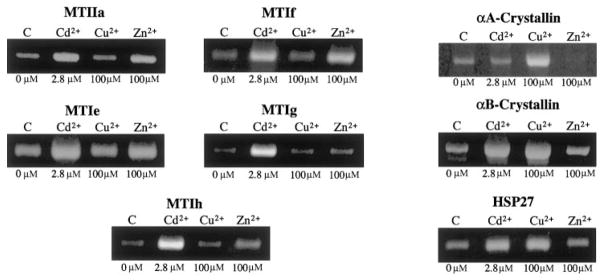
The spectrum and metal specificity for five of the previously identified lens MT isoforms15 and three lens α-crystallin/sHSPs induced by Cd2+, Cu2+, and Zn2+ in SRA01/04 HLE cells were evaluated by semiquantitative RT-PCR. As in the previous studies (Figs. 3, 4), MTs IIa and Ig were induced by Cd2+ and Zn2+, but not by Cu2+ (Fig. 6). MTs Ie, If, and Ih were also induced by Cd2+ and Zn2+, but not by Cu2+ (Fig. 6). αB-crystallin and HSP27 were induced by Cd2+ and Cu2+, but not by Zn2+, and αA-crystallin was induced only by Cu2+ (Fig. 6).
Figure 6.
Ethidium bromide–stained gels showing the levels of metallothionein (left) and small heat-shock (right) genes detected by RT-PCR in 100 ng of RNA isolated from HLE cells induced by Cd2+, Cu2+, and Zn2+ for 8 hours at the indicated concentrations.
Analysis of 13 Different Metals in Decapsulated Human Lenses
To establish whether metal levels change in the aging human lens, the levels of 13 different metals were evaluated by ICP. Elemental profiles in aging lenses are presented in Table 2. Sodium and potassium concentrations were highest, followed by magnesium and calcium. Of the other metals, zinc was found in the highest concentration, followed by iron and copper. Levels of aluminum, manganese, nickel, cadmium, chromium, and lead were below the levels of detection (Table 2). Of the elements analyzed, only iron exhibited an age-dependent pattern. Iron concentration was highest in young lenses, exhibited increasing variability in middle-aged lenses, and decreased by 81% in the old lenses.
Table 2.
Concentration of Elements in Human Lenses from Individuals of Different Ages
| Age Group (mean) |
|||
|---|---|---|---|
| Element | Young (32.8 y) | Middle (52.3 y) | Old (70.5 y) |
| Sodium | 8267 ± 931 | 6632 ± 798 | 8326 ± 859 |
| Potassium | 4479 ± 468 | 5478 ± 1007 | 5574 ± 326 |
| Magnesium | 176.5 ± 3.0 | 180.2 ± 20.2 | 148.2 ± 15.3 |
| Calcium | 119.7 ± 28.1 | 78.0 ± 21.6 | 136.0 ± 37.7 |
| Zinc | 32.15 ± 9.39 | 25.82 ± 3.45 | 25.79 ± 3.96 |
| Iron | 12.42 ± 0.21a | 10.67 ± 2.07a | 2.22 ± 1.21b |
| Copper | 1.094 ± 0.168 | 0.677 ± 0.029 | 0.949 ± 0.304 |
| Cadmium | ND* | ND | ND |
Data are concentrations (μg/g) ± SE (n = 3). Superscript letters denote significant differences by the Tukey-Kramer HSD comparison. Nine groups of five lenses each were digested and assessed for metal concentration by ICP and the results expressed in micrograms of metal per gram of dry lens. ND, not detectable.
Below the level of detection (<0.191 μg/L).
Discussion
In the present study, five MTs shown to be expressed in the human lens,15 including isoforms Ie, If, Ig, Ih, and IIa, and three lens α-crystallin/sHSPs, including αA-crystallin, αB-crystallin, and HSP27, were differentially induced by specific metals in HLE cells. These inductions are likely to be present in vivo, because similar inductions of MTIIa were observed in primary cultures of HLE cells. To our knowledge, this is the first demonstration of αA-crystallin induction by metals or other stresses and provides evidence that αA-crystallin could be a stress-responsive gene that protects lens cells against metal-associated damage.
Activation of these genes is metal specific in HLE cells, in that Cd2+ and Zn2+, but not Cu2+, induced the MT genes (Ie, If, Ig, Ih, and IIa), whereas Cd2+ and Cu2+, but not Zn2+, induced two of the three α-crystallin/sHSP genes (αB-crystallin and HSP27). αA-crystallin induction was observed only with exposure to Cu2+. The differential induction of these genes by specific metals indicates that the encoded proteins are likely to have different roles in lens regulation of, and/or protection against, specific metals.
MTIIa was induced at five times higher levels than MTIg, indicating that MTIIa is the primary MT responding to metals in lens cells. This is consistent with its reported increased expression in age-related cataract compared with clear lenses16 and its lens epithelium specificity.15
The present data address the induction of these genes at concentrations of metals that resulted in no more than 10% cell death over a relatively short incubation time. We could not examine higher levels of these metals or longer exposure times, because significant cell lethality and consequent loss of gene expression were observed with higher metal concentrations or with longer exposure times (data not shown).
Differential induction of these genes by specific metals suggests that the lens may use metal-specific transcriptional mechanisms to regulate specific genes. These responses are probably mediated by previously identified metal-responsive transcription factors. One of these, which is known to regulate the expression of mouse MTs I and II in nonlens cells by binding to metal responsive regulatory elements (MREs) in the promoters of these genes, is the MRE-binding transcription factor (MTF)-1.48 MTF-1 has been shown to activate the expression of MTs I and II through specific heavy metals including Cd2+ and Cu2+.49 MTF-1–null mice lose their ability to express MTs I and II.49 Like the MTs, the promoters for the α-crystallin/sHSPs, including αB-crystallin and HSP27, are known to contain binding sites for multiple stress-related transcription factors, including a near-perfect MRE that is located in the promoter of the rat αB-crystallin gene.24 They also contain other stress-associated regulatory elements, including heat shock responsive elements (HSEs) and AP1-like consensus sequences.50–52
Several studies have suggested that numerous metals are associated with cataract,4–8 and increased Cd2+ levels have been demonstrated in cataractous versus clear human lenses.4 Although intact cataractous lenses are not readily available and no conclusions regarding the presence of metals in human cataracts can be drawn from the present results, no differences were detected in the levels of 12 metals among young, middle-aged, and old healthy lenses. In contrast to the data reported for human cataract,4 cadmium was not even detectable in the clear lenses analyzed in the present report, suggesting that increased cadmium levels are specific to cataractous lenses. Possibly MT-metal complexes are secreted by the normal lens and retained by cataracts. An 81% decrease in iron levels was detected between young and middle-aged versus old human lenses. Although altered iron regulation is associated with cataract,6 the significance of this result is open to speculation. We do not think that decreased iron results from sample contamination, because no differences were detected in the levels of 12 other metals examined, three separate groups of five lenses possessed similar iron levels, and contamination is very unlikely to be reflected in the decreased level of iron detected in a single group. Although the lens capsule was excluded from the lens metal contents reported, we are certain that our results would not be affected by inclusion of the lens capsule, because its weight is insignificant compared with that of the remainder of the lens. Relative to the sensitivity of presently available techniques, the extremely large number of human lens epithelia and capsules that would be required to assay the metal content of this tissue (approximately 0.3 g) makes this examination unfeasible. Inclusion of water weight could also affect our measurements.
Future studies are needed to determine the exact function of induced MTs and α-crystallin/sHSPs in HLE cells. It is likely that MTs are capable of protecting these cells against damage induced by toxic metals and possibly other insults associated with cataract, because metallothioneins have been shown to protect numerous tissues, including retinal pigment epithelial cells,23 against toxic metals, oxidative stress, and insults by UV light. MTs are likely to protect lens cells through direct metal binding and scavenging of free radicals, as they have been demonstrated to do in nonlens systems. Indeed, it is estimated that MTs are 50 times more efficient as free radical scavengers than is reduced glutathione, on a molar basis.53 The present data also provide evidence that α-crystallin/sHSPs may have a role in lens metal protection. α-Crystallin/sHSPs protect against protein aggregation, and it is possible that they are induced in response to metals to prevent protein aggregation or other damage resulting from exposure to metal. Regardless of their exact functions, the inductions of these genes in human lens cells indicate that they are likely to play significant roles in lens metal regulation and/or protection. Future studies will examine their ability to provide direct protection to lens cells against metals and other cataract-associated insults.
Acknowledgments
Supported by National Eye Institute Grants EY13022 (MK) and EY00484 and EY07003 (VNR).
The authors thank Frank Giblin of the Oakland University Eye Research Institute and J. Fielding Hejtmancik and Ignacio R. Rodriguez of the National Eye Institute for invaluable advice and discussion throughout the course of the work and the Lions Eye Bank of Oregon and the West Virginia Eye Bank for providing the lenses used in the study.
Footnotes
Commercial relationships policy: N.
This report was in partial fulfillment of the West Virginia University, Department of Biology, Organismal Biology PhD requirements for JRH.
References
- 1.Ruffett I, Ayres J, McBride D. Possible chemical pollution. Practitioner. 1992;236:13–16. [PubMed] [Google Scholar]
- 2.Artic Monitoring and Assessment Program (AMAP) AMAP Report of Issues of Concern. Oslo, Norway: AMAP; 2000. Heavy metals. available at http://www.amap.no/assess/soaer7.htm/ [Google Scholar]
- 3.Grubb BR, DuVal GE, Morris JS, Bentley PJ. Accumulation of cadmium by the eye with special reference to the lens. Toxicol Appl Pharmacol. 1985;77:444–450. doi: 10.1016/0041-008x(85)90184-x. [DOI] [PubMed] [Google Scholar]
- 4.Ramakrishnan S, Sulochana KN, Selvaraj T, Abdul Rahim A, Lakshmi M, Arunagiri K. Smoking of beedies and cataract: cadmium and vitamin C in the lens and blood. Br J Ophthalmol. 1995;79:202–206. doi: 10.1136/bjo.79.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clayton RM, Cuthbert J, Seth J, Phillips CI, Bartholmew RS, Reid JM. Epidemiological and other studies in the assessment of factors contributing to cataractogenesis. Ciba Found Symp. 1984;106:25–47. doi: 10.1002/9780470720875.ch3. [DOI] [PubMed] [Google Scholar]
- 6.Brown NP, Bron AJ. Lens Disorders: a Clinical Manual of Cataract Diagnosis. Butterworth-Heinemann; Oxford, UK: 1996. [Google Scholar]
- 7.Girelli D, Corrocher R, Bisceglia L, et al. Molecular basis for the recently described hereditary hyperferritinemia-cataract syndrome: a mutation in the iron-responsive element of ferritin L-subunit gene (the “Verona mutation”) Blood. 1995;86:4050–4053. [PubMed] [Google Scholar]
- 8.Cuthbert JA. Wilson’s disease: update of a systemic disorder with protean manifestations. Gastroenterol Clin North Am. 1998;27:655–664. doi: 10.1016/s0889-8553(05)70025-x. [DOI] [PubMed] [Google Scholar]
- 9.Kagi JHR, Schaffer A. Biochemistry of metallothionein. Biochemistry. 1988;27:8509–8515. doi: 10.1021/bi00423a001. [DOI] [PubMed] [Google Scholar]
- 10.Karin M, Haslinger A, Holtgreve RI, Krauter P, Westphal HM, Beato M. Characterization of DNA sequences through which cadmium and glucocorticoid hormones induce human metallothionein-IIa gene. Nature. 1984;308:513–519. doi: 10.1038/308513a0. [DOI] [PubMed] [Google Scholar]
- 11.Angel P, Poting A, Mallick U, Rahmsdorf HJ, Schorpp M, Herrlich P. Induction of metallothionein and other mRNA species by carcinogens and tumor promoters in primary human skin fibroblasts. Mol Cell Biol. 1986;6:1760–1766. doi: 10.1128/mcb.6.5.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bauman JW, Liu J, Liu YP, Klaassen CD. Increase in metallothionein produced by chemicals that induce oxidative stress. Toxicol Appl Pharmacol. 1991;110:347–354. doi: 10.1016/s0041-008x(05)80017-1. [DOI] [PubMed] [Google Scholar]
- 13.Fornace AJ, Jr, Schalch H, Alamo I., Jr Coordinate induction of metallothionein I and II in rodents cells by UV-irradiation. Mol Cell Biol. 1988;8:4716–4720. doi: 10.1128/mcb.8.11.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oguro T, Yoshida T. Effect of ultraviolet A on ornithine decarboxylase and metallothionein gene expression in mouse skin. Photodermatol Photoimmunol Photomed. 2001;17:71–78. doi: 10.1034/j.1600-0781.2001.017002071.x. [DOI] [PubMed] [Google Scholar]
- 15.Oppermann B, Zhang W, Magabo K, Kantorow M. Identification and spatial analysis of metallothioneins expressed by the adult human lens. Invest Ophthalmol Vis Sci. 2001;42:188–193. [PMC free article] [PubMed] [Google Scholar]
- 16.Kantorow M, Kays T, Horwitz J, Huang Q, Sun J, Piatigorsky J, Carper D. Differential display detects altered gene expression between cataractous and normal human lenses. Invest Ophthalmol Vis Sci. 1998;39:2344–2354. [PubMed] [Google Scholar]
- 17.McAleer MF, Tuan RS. Metallothionein overexpression in human trophoblastic cells protects against cadmium-induced apoptosis. In Vitro Mol Toxicol. 2001;14:25–42. doi: 10.1089/109793301316882522. [DOI] [PubMed] [Google Scholar]
- 18.Park JD, Liu Y, Klaassen CD. Protective effect of metallothionein against the toxicity of cadmium and other metals (1) Toxicology. 2001;163:93–100. doi: 10.1016/s0300-483x(01)00375-4. [DOI] [PubMed] [Google Scholar]
- 19.Michalska AE, Choo KH. Targeting and germ-line transmission of a null mutation at the metallothionein I and II loci in mouse. Proc Natl Acad Sci USA. 1993;90:8088–8092. doi: 10.1073/pnas.90.17.8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masters BA, Kelly EJ, Quaife CJ, Brinster RL, Palmiter RD. Targeted disruption of metallothionein I and II genes increases sensitivity to cadmium. Proc Natl Acad Sci USA. 1994;91:584–588. doi: 10.1073/pnas.91.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Liu Y, Michalska AE, Choo KH, Klaassen CD. Metallothionein plays less of a protective role in cadmium-metallothionein-induced nephrotoxicity than in cadmium chloride-induced hepatotoxicity. J Pharmacol Exp Ther. 1996;276:1216–1223. [PubMed] [Google Scholar]
- 22.Kelly EJ, Quaife CJ, Froelick GJ, Palmiter RD. Metallothionein I and II protect against zinc deficiency and zinc toxicity in mice. J Nutr. 1996;126:1782–1790. doi: 10.1093/jn/126.7.1782. [DOI] [PubMed] [Google Scholar]
- 23.Lu H, Hunt DM, Ganti R, et al. Metallothionein protects retinal pigment epithelial cells against apoptosis and oxidative stress. Exp Eye Res. 2002;74:83–92. doi: 10.1006/exer.2001.1101. [DOI] [PubMed] [Google Scholar]
- 24.Head MW, Hurwitz L, Goldman JE. Transcriptional regulation of αB-crystallin in astrocytes: analysis of HSF and AP1 activation by different types of physiological stress. J Cell Sci. 1996;109:1029–1039. doi: 10.1242/jcs.109.5.1029. [DOI] [PubMed] [Google Scholar]
- 25.Lin LR, Carper D, Yokoyama T, Reddy VN. The effect of hypertonicity on aldose reductase, alpha B-crystallin, and organic osmolytes in the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1993;34:2352–2359. [PubMed] [Google Scholar]
- 26.Dasgupta S, Hohman TC, Carper D. Hypertonic stress induces alpha B-crystallin expression. Exp Eye Res. 1992;54:461–470. doi: 10.1016/0014-4835(92)90058-z. [DOI] [PubMed] [Google Scholar]
- 27.Kaida T, Kozawa O, Ito T, et al. Vasopressin stimulates the induction of heat shock protein 27 and alphaB-crystallin via protein kinase C activation in vascular smooth muscle cells. Exp Cell Res. 1999;246:327–337. doi: 10.1006/excr.1998.4277. [DOI] [PubMed] [Google Scholar]
- 28.Welge-Lussen U, May CA, Eichhorn M, Bloemendal H, Lütjen-Drecoll E. αB-crystallin in the trabecular meshwork is inducible by transforming growth factor-beta. Invest Ophthalmol Vis Sci. 1999;40:2235–2241. [PubMed] [Google Scholar]
- 29.Sun JK, Iwata T, Zigler JS, Jr, Carper DA. Differential gene expression in male and female rat lenses undergoing cataract induction by transforming growth factor-beta (TGF-beta) Exp Eye Res. 2000;70:169–181. doi: 10.1006/exer.1999.0771. [DOI] [PubMed] [Google Scholar]
- 30.Tamm ER, Russell P, Johnson DH, Piatigorsky J. Human and monkey trabecular meshwork accumulate alpha B-crystallin in response to heat shock and oxidative stress. Invest Ophthalmol Vis Sci. 1996;37:2402–2413. [PubMed] [Google Scholar]
- 31.Inaguma Y, Hasegawa K, Goto S, Ito H, Kato K. Induction of the synthesis of hsp27 and alpha B crystallin in tissues of heat-stressed rats and its suppression by ethanol or an alpha 1-adrenergic antagonist. J Biochem. 1995;117:1238–1243. doi: 10.1093/oxfordjournals.jbchem.a124850. [DOI] [PubMed] [Google Scholar]
- 32.Inaguma Y, Shinohara H, Goto S, Kato K. Translocation and induction of alpha B crystallin by heat shock in rat glioma (GA-1) cells. Biochem Biophys Res Commun. 1992;182:844–850. doi: 10.1016/0006-291x(92)91809-5. [DOI] [PubMed] [Google Scholar]
- 33.Scheier B, Foletti A, Stark G, Aoyama A, Dobbeling U, Rusconi S, Klemenz R. Glucocorticoids regulate the expression of the stress protein alpha B-crystallin. Mol Cell Endocrinol. 1996;123:187–198. doi: 10.1016/s0303-7207(96)03922-6. [DOI] [PubMed] [Google Scholar]
- 34.Andley UP, Patel HC, Xi JH. The R116C mutation in alpha A-crystallin diminishes its protective ability against stress-induced lens epithelial cell apoptosis. J Biol Chem. 2002;277:10178–10186. doi: 10.1074/jbc.M109211200. [DOI] [PubMed] [Google Scholar]
- 35.Andley UP, Song Z, Wawrousek EF, Fleming TP, Bassnett S. Differential protective activity of alpha A- and alpha B-crystallin in lens epithelial cells. J Biol Chem. 2000;275:36823–36831. doi: 10.1074/jbc.M004233200. [DOI] [PubMed] [Google Scholar]
- 36.Andley UP, Song Z, Wawrousek EF, Bassnett S. The molecular chaperone alpha A-crystallin enhances lens epithelial cell growth and resistance to UVA stress. J Biol Chem. 1998;273:31252–31261. doi: 10.1074/jbc.273.47.31252. [DOI] [PubMed] [Google Scholar]
- 37.Brady JP, Garland D, Duglas-Tabor Y, Robison WG, Jr, Groome A, Wawrousek EF. Targeted disruption of the mouse αA-crystallin gene induces cataract and cytoplasmic inclusion bodies containing the small heat shock protein αB-crystallin. Proc Natl Acad Sci USA. 1997;94:884–889. doi: 10.1073/pnas.94.3.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piatigorsky J. Lens differentiation in vertebrates: a review of cellular and molecular features. Differentiation. 1981;19:134–135. doi: 10.1111/j.1432-0436.1981.tb01141.x. [DOI] [PubMed] [Google Scholar]
- 39.Bloemendal H. Molecular Biology of the Eye Lens. New York: John Wiley; 1981. [Google Scholar]
- 40.Reddy VN. Metabolism of glutathione in the lens. Exp Eye Res. 1971;11:310–328. doi: 10.1016/s0014-4835(71)80043-x. [DOI] [PubMed] [Google Scholar]
- 41.Spector A. Aging of the lens and cataract formation. In: Sekuler R, Kline D, Dismukes K, editors. Aging and Human Visual Function. New York: Alan Liss; 1982. pp. 27–43. [Google Scholar]
- 42.Reddan JR. Control of cell division in the ocular lens, retina and vitreous humour. In: McDevitt DS, editor. Cell Biology of the Eye. New York: Academic Press; 1982. pp. 299–375. [Google Scholar]
- 43.Ibaraki N, Chen SC, Lin LR, Okamoto H, Pipas JM, Reddy VN. Human lens epithelial cell line. Exp Eye Res. 1998;67:577–585. doi: 10.1006/exer.1998.0551. [DOI] [PubMed] [Google Scholar]
- 44.Arita T, Lin LR, Reddy VN. Differentiation of human lens epithelial cells in tissue culture. Exp Eye Res. 1988;47:905–910. doi: 10.1016/0014-4835(88)90072-3. [DOI] [PubMed] [Google Scholar]
- 45.Foster R, Jahroudi N, Varshney U, Gedamu L. Structure and expression of the human metallothionein IG gene: differential promoter activity of two linked metallothionein I genes in response to heavy metals. J Biol Chem. 1988;263:11528–11535. [PubMed] [Google Scholar]
- 46.Ausubel I, Frederick M. Current Protocols in Molecular Biology. Vol. 2. John Wiley and Sons, Inc; 1998. [Google Scholar]
- 47.Jones JB, Jr, Case VW. Sampling, handling and analyzing plant tissue samples. In: Westerman RL, editor. Soil Testing and Plant Analysis. 3. Madison, WI: Soil Science Society of America; 1999. pp. 389–426. [Google Scholar]
- 48.Gunes C, Heuchel R, Georgiev O, et al. Embryonic lethality and liver degeneration in mice lacking the metal-responsive transcriptional activator MTF-1. EMBO J. 1998;17:2846–2854. doi: 10.1093/emboj/17.10.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heuchel R, Radtke F, Georgiev O, Stark G, Aguet M, Schaffner W. The transcription factor MTF-1 is essential for basal and heavy metal-induced metallothionein gene expression. EMBO J. 1994;13:2870–2875. doi: 10.1002/j.1460-2075.1994.tb06581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iwaki A, Iwaki T, Goldman JE, Liem RKH. Multiple mRNAs of rat brain α-crystallin B chain result from alternative transcriptional initiation. J Biol Chem. 1990;265:22197–22203. [PubMed] [Google Scholar]
- 51.Frederikse PH, Piatigorsky J. Novel and heat inducible binding of HSF to α-crystallin regulatory sequences [ARVO Abstract] Invest Ophthalmol Vis Sci. 1994;35:2073. Abstract nr 3792. [Google Scholar]
- 52.Srinivasan AN, Bhat SP. Complete structure and expression of the rat αB-crystallin gene. DNA Cell Biol. 1994;13:651–661. doi: 10.1089/dna.1994.13.651. [DOI] [PubMed] [Google Scholar]
- 53.Miura T, Muraoka S, Ogiso T. Antioxidant activity of metallothionein compared with reduced glutathione. Life Sci. 1997;60:301–309. doi: 10.1016/s0024-3205(97)00156-2. [DOI] [PubMed] [Google Scholar]