Abstract
Cytosolic Ca2+ is a versatile second messenger that can regulate multiple cellular processes simultaneously. This is accomplished in part through Ca2+ waves and other spatial patterns of Ca2+ signals. To investigate the mechanism responsible for the formation of Ca2+ waves, we examined the role of inositol 1,4,5-trisphosphate receptor (InsP3R) isoforms in Ca2+ wave formation. Ca2+ signals were examined in hepatocytes, which express the type I and II InsP3R in a polarized fashion, and in AR4-2J cells, a nonpolarized cell line that expresses type I and II InsP3R in a ratio similar to what is found in hepatocytes but homogeneously throughout the cell. Expression of type I or II InsP3R was selectively suppressed by isoform-specific DNA antisense in an adenoviral delivery system, which was delivered to AR4-2J cells in culture and to hepatocytes in vivo. Loss of either isoform inhibited Ca2+ signals to a similar extent in AR4-2J cells. In contrast, loss of the basolateral type I InsP3R decreased the sensitivity of hepatocytes to vasopressin but had little effect on the initiation or spread of Ca2+ waves across hepatocytes. Loss of the apical type II isoform caused an even greater decrease in the sensitivity of hepatocytes to vasopressin and resulted in Ca2+ waves that were much slower and delayed in onset. These findings provide evidence that the apical concentration of type II InsP3Rs is essential for the formation of Ca2+ waves in hepatocytes. The subcellular distribution of InsP3R isoforms may critically determine the repertoire of spatial patterns of Ca2+ signals.
Cytosolic Ca2+ exerts wide ranging effects as a second messenger (1). In hepatocytes Ca2+ regulates such diverse functions as bile secretion, glucose release, cell metabolism, gene transcription, and apoptosis (2). Spatial patterns of Ca2+ signals, such as Ca2+ waves, help encode the information that is responsible for such regulation (1, 2). Ca2+ waves have specifically been implicated in the regulation of such processes as fluid and electrolyte secretion, exocytosis, cell-cell communication, and morphogenesis (3–7). In hepatocytes, Ca2+ wave formation is due entirely to the inositol 1,4,5-trisphosphate (InsP3)2 receptor (InsP3R), because that is the only intracellular Ca2+ release channel in this cell type (8) and because Ca2+ waves in hepatocytes do not rely on influx of extracellular Ca2+ (9). However, it is not entirely understood how InsP3Rs regulate the formation and spread of Ca2+ waves.
There are three isoforms of the InsP3R, each with distinct biophysical properties (10–12). There is also variability in the expression and subcellular distribution of each isoform among different cell and tissue types (8, 13–16). Hepatocytes express only the type I and the type II isoforms of the InsP3R (8). In addition, the type I InsP3R is distributed relatively uniformly throughout the hepatocyte, whereas the type II isoform is concentrated in the apical region (8). It has been hypothesized that the expression and subcellular distribution of each isoform determines the spatial patterns of Ca2+ signals in cells. Because the type II InsP3R is the isoform with the highest affinity for InsP3 (17), it has been suggested that Ca2+ waves begin in the apical region of hepatocytes because the type II isoform is concentrated there (8). The purpose of this study was to determine whether the subcellular distribution of InsP3R isoforms is important for organizing the pattern of Ca2+ waves.
EXPERIMENTAL PROCEDURES
Materials
Acetylcholine (ACh), arginine-vasopressin, bovine serum albumin, and penicillin-streptomycin were obtained from Sigma. Dulbecco’s modified Eagle’s medium and Liebowitz 15 (L-15) medium were from Invitrogen. Fluo-4/acetoxymethyl ester, TO-PRO-3, and rhodamine-conjugated phalloidin were from Molecular Probes (Eugene, OR). All other reagents were of the highest quality commercially available.
Cloning of Rat cDNA Sequences for Type I, II, and III Isoforms of the InsP3R
The cDNA for the type I and type II isoforms of the InsP3R was synthesized by reverse transcription-PCR using template RNA isolated from the brain and the liver of rats, respectively. RNA from RIN cells was used as a template for the cDNA of the type III isoform. The RNA was isolated by the RNAqueous™ kit (Ambion Inc., Austin, TX). The sequences for each forward primer contained an XbaI site, and the sequence for each reverse primer contained a HindIII site. Sequences were designed to be ~200 bp in length in order to maximize their stability upon production by adenovirus. Vector NTI software (Invitrogen) was used to identify regions of each isoform that had minimal (16–19%) homology with the other two isoforms (Fig. 1). This approach was taken so that each of the three antisense sequences would have little likelihood of inhibiting expression of InsP3R isoforms in a nonspecific fashion. The resulting primers for each of the three isoforms were as follows: (a) type I isoform 5′(5′-aagcttt-ACATCTGCAGGGCCTGTAACAA-3′, position 4699–4720) and type I isoform 3′ (5′-ctagaGCAGTGGTAAACTCGGAACACG-3′, position 4873–4895), which generates a 196-bp cDNA fragment; (b) type II isoform 5′ (5′-agctttTTCTCAGCCCTCCTTTGGGTAG-3′, position 6787–6809) and type II isoform 3′ (5′ - tctagaTTCCCACAAAACTCACCAGGA-3′, position 6964–6986), which generates a 199-bp cDNA fragment; (c) type III isoform 5′ (5′-aagctttTGTGGGGTAGCATCTCCTTCAA - 3′, position 6739–6761) and type III isoform 3′ (5′-tctagaGAAGGACCAGTGCCACAATGAG-3′, position 6920–6942), which generates a 203-bp cDNA fragment. The obtained cDNA sequence for each isoform was then subcloned into the dual promoter (pCR II) vector (Invitrogen) for amplification, and then the identities were confirmed by direct sequencing.
FIGURE 1. Sequence alignments for InsP3 receptor regions used for antisense constructs.
A, sense sequence used for antisense for the type I InsP3R is shown in alignment with the same base pair positions for the type II and the type III isoforms of the InsP3R. Asterisk indicates nucleotides that are found in identical positions in all three sequences. The sequences for the type I, II, and III InsP3Rs are from rat brain, liver, and endocrine pancreas, respectively. B, sense sequence used for the type II antisense construct is shown in alignment with the corresponding base pair positions of the type I and the type III isoforms of the InsP3R. C, alignment of the sense sequence for the type III antisense construct with the corresponding sequences from the type I and type II isoforms of the InsP3R. In each case, there is minimal homology with the sequence used for the antisense construct.
Antisense Oligonucleotides and Adenovirus Construction
Each cDNA was cloned into an adenovirus vector in an antisense orientation as described previously (18). Briefly, the cDNA sequence for each InsP3R isoform was directionally subcloned into the adenoviral shuttle vector pCMVPLPASR, between the HindIII and XbaI polycloning sites in an antisense orientation, and each of the three antisense sequences was confirmed by sequencing. This construct was co-transfected with the plasmid pJM17 into 293 cells. Homologous recombination resulted in adenoviral particles expressing the antisense construct (19). The resultant adenoviruses were tested by PCR to ensure that each expressed the correct sequence. Individual viral plaques were isolated and amplified, and then the recombinant adenoviruses were purified and concentrated using CsCl step gradients followed by dialysis against 10% glycerol in phosphate-buffered saline. Viral stocks consisting of 8 × 1010 plaque-forming units (pfu)/ml for adenoviral antisense for the type I InsP3R, 7 × 1010 pfu/ml for the type II receptor, and 5 × 1010 pfu/cell for the type III receptor were produced after four rounds of amplification for each construct. Viruses were then stored in aliquots at −80 °C in buffer with 10% glycerol. DsRed and enhanced green fluorescent protein (EGFP) (Clontech) were used to form adenoviral DsRed and EGFP constructs, respectively. These were amplified and purified as described above and then used to monitor the efficacy of infection.
Infection Conditions
Both cell lines and animals were used for adenoviral studies. AR4-2J pancreatoma and RIN insulinoma cell lines (ATCC, Manassas, VA) were cultured in high glucose Dulbecco’s modified Eagle’s medium with 10% calf serum and antibiotics. Cells were seeded onto culture dishes at a density of 8 × 105 cells/dish. After an initial incubation period of 24 h, the cells were infected with adenovirus at a multiplicity of infection (m.o.i.) of 40 followed by a further 24–48 h of incubation. Male Sprague-Dawley rats (200–225 g; Charles River Breeding Laboratories) were used for all animal studies. For adenoviral studies, animals were anesthetized with 4% pentobarbital (0.4 ml intraperitoneally) and then adenoviral constructs (5 × 109 pfu/ml) were injected via the portal vein, which was exposed by laparotomy. After repair of the surgical wound, animals were allowed to recover and then were sacrificed 48 h later. At the time of sacrifice, livers were used for immunofluorescence or else used to isolate hepatocyte couplets and triplets as described below. All animal experiments were performed under the guidelines of the Yale University IACUC.
Immunoblotting
Immunoblots were used to test the efficacy of adenoviral antisense constructs and were performed as described previously (8, 13). Briefly, cells were lysed at 4 °C with lysis buffer; the lysate underwent centrifugation, and the protein concentration of the supernatant was determined spectrophotometrically. Twenty five µg of total cellular protein was separated by SDS-PAGE using a 7.5% polyacrylamide gel. Membranes were blocked with nonfat milk and then incubated at room temperature with InsP3R isoform-specific antibodies (8, 13). An affinity-purified polyclonal antibody directed against the C terminus of the type I isoform of the InsP3R (11) was used at a dilution of 1:1000, an affinity-purified polyclonal antibody against the C terminus of the type II isoform (20) was used at 1:100, and a monoclonal antibody against the N terminus of the type III isoform (11) was used at a dilution of 1:500. Membranes were washed and incubated with peroxidase-conjugated secondary antibodies, and then protein-antibody conjugates were detected by enhanced chemiluminescence (Amersham Biosciences).
Immunofluorescence
Confocal immunofluorescence to detect the subcellular distribution of InsP3R isoforms was performed as described previously (8, 13). Briefly, frozen rat liver sections were fixed in 4% formaldehyde, followed by tissue permeabilization in 0.5% Triton X-100. After blocking steps, the liver sections were incubated with primary antibody against specific InsP3R isoforms and then rinsed with phosphate-buffered saline and 1% bovine serum albumin. The specimens were then incubated with Alexa 488 secondary antibody (Molecular Probes) and co-labeled with rhodamine-conjugated phalloidin (Molecular Probes) to facilitate the recognition of the apical and the basolateral pole of hepatocytes (8, 9). For AR4-2J cells, the isolated cells were seeded onto glass coverslips and incubated for 48 h at 37 °C, then fixed with 4% formaldehyde, and permeabilized with 0.5% Triton X-100. Primary and secondary antibodies were the same as used for liver immunofluorescence, and TO-PRO-3 (Molecular Probes) was used to label the nucleus of the cultured cells. An MRC-1024 confocal microscope (Bio-Rad) was used for all imaging studies. Images were obtained by excitation at 488 nm and observation at 505–550 nm to detect Alexa 488. Tissue specimens were excited at 543 nm and observed at >585 nm to detect rhodamine phalloidin, whereas cells in culture were excited at 647 nm and observed at >680 nm to detect TO-PRO-3.
Isolation of Hepatocytes
Isolated rat hepatocyte couplets and triplets were used for single cell imaging, because these cells maintain structural and functional polarity in short term culture (8, 9, 21). Cells were isolated in the Cell Isolation Core of the Yale Liver Center, as described previously (8, 9, 21). Briefly, rat livers were perfused with Hanks’ A and then Hanks’ B medium containing 0.05% collagenase (Roche Applied Science) and 0.8 units of trypsin inhibitor (Sigma) per unit of tryptic activity. Livers were minced and passed through serial nylon mesh filters, and the resultant cells were washed. Isolated hepatocytes were resuspended in L-15 medium with 50 units of penicillin and 50 mg of streptomycin. The cells were then seeded onto glass coverslips and incubated at 37 °C for 2–4 h before used.
Measurement of Cytosolic Ca2+
Cytosolic Ca2+ was monitored in individual cells and subcellular regions by time lapse confocal microscopy as described previously (8, 11, 13, 22). Briefly, AR4-2J cells or isolated rat hepatocytes were incubated with Fluo-4/acetoxymethyl ester (6 µm) for 30 min at 37 °C. Coverslips seeded with the cells were transferred to a custom-built perfusion chamber on the stage of an MRC-1024 confocal microscope (Bio-Rad), and the cells were then perfused with HEPES-buffered solution. Fluo-4 was excited at 488 nm and observed at 505–550 nm. In most experiments a ×63 objective was used to observe the cells, but a ×20 objective was used in a limited series of studies to monitor population responses to agonists. Increases in Ca2+ were expressed as percent increase in Fluo-4 fluorescence intensity.
Statistics
All results are expressed as mean ± S.D. Student’s t test was used for comparisons between groups, whereas repeated measures ANOVA was used for comparisons among larger groups. A p value less than 0.05 was used to indicate a statistically significant difference. GraphPad Prism software (San Diego, CA) was used for all statistical tests.
RESULTS
Adenoviral Antisense Can Selectively Decrease Expression of Specific InsP3R Isoforms
Antisense sequences were designed for regions of each InsP3R isoform with little homology to the other two isoforms (Fig. 1), and an adenoviral delivery system was used. AR4-2J cells were used as a tool to examine the efficacy of the adenoviral antisense constructs because these cells, like hepatocytes, are epithelia that almost exclusively express the types I and II InsP3R (20). In addition, hepatocytes and AR4-2J cells express these two isoforms in similar proportions (20), and AR4-2J cells can be induced to differentiate into a hepatocyte phenotype (23). Confocal immunofluorescence confirmed that AR4-2J cells express the type I and II isoforms of the InsP3R (Fig. 2). Both isoforms were distributed uniformly throughout the cytosol (Fig. 2, A and D). The cells were co-labeled with the nuclear stain TO-PRO-3 (Fig. 2, B and E), which suggested the presence of the type I isoform of the InsP3R in the nucleus as well (Fig. 2C), although the nuclear labeling may have been nonspecific. There was minimal nuclear expression of the type II isoform (Fig. 2F). Thus, the cytosolic distribution of these two receptor isoforms was similar in AR4-2J cells. The efficacy of the isoform-specific adenoviral antisense constructs was determined in several steps. First, the efficiency of adenoviral infection was optimized by infecting cells with an adenovirus-DsRed construct. This allowed visual confirmation of successful infection. The efficiency of infection of AR4-2J cells was 100% using an m.o.i. of 40 (not shown), so this m.o.i. was used for infection with antisense constructs as well. Immunoblotting was then performed to analyze the effects of each adenoviral antisense construct. Treatment of AR4-2J cells with the adenoviral antisense construct for the type I InsP3R decreased the expression of the type I InsP3R (Fig. 3A) but not the type II InsP3R (Fig. 3B). Similarly, treatment of these cells with the construct for the type II InsP3R decreased the expression of the type II InsP3R (Fig. 3C), without affecting the expression of the type I InsP3R (Fig. 3D). In each case, expression of the respective isoform decreased markedly by 24 h post-infection and remained low after 48 h. Cells infected with an m.o.i. of less than 40 exhibited less pronounced decreases in the expression of the corresponding InsP3R isoform (not shown). Expression of type I and type II InsP3R was not inhibited by infection with adenovirus construct for DsRed (Fig. 3, A and C), demonstrating that the inhibition was not a nonspecific effect of adenoviral infection. Finally, the efficacy of the adenoviral antisense construct for the type III InsP3R was examined. RIN cells were used for these experiments because this cell type nearly exclusively expresses the type III isoform (11, 20). The expression of this isoform in RIN cells was decreased only slightly 24 h after infection but decreased dramatically 48 h after infection (Fig. 3E). The basis for the longer time interval needed to decrease expression of this isoform is unclear. The type III InsP3R is degraded as quickly as the other two InsP3R isoforms following stimulation of phosphoinositide hydrolysis (20). However, InsP3R turnover is much slower in nonstimulated cells (24), so it is possible that the type III isoform has a longer half-life than the other two isoforms. These results demonstrate that each of the three adenoviral antisense constructs effectively inhibits expression of the corresponding InsP3R isoform.
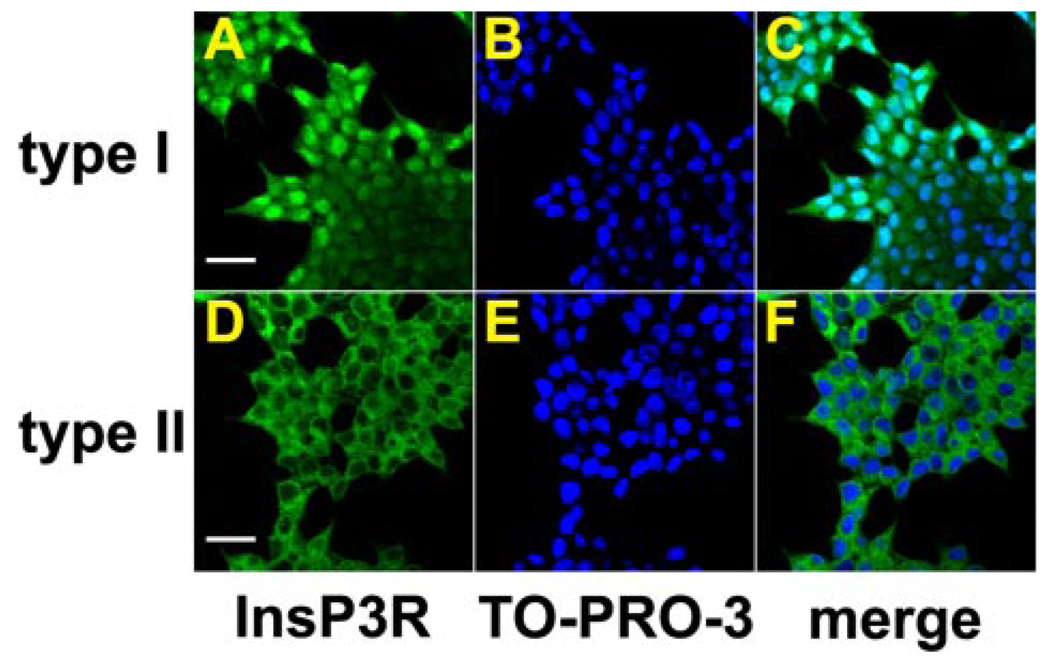
FIGURE 2. The type I and type II isoforms of the InsP3R are distributed homogeneously in the cytosol of AR4-2J cells.
Images were obtained by confocal immunofluorescence. Cells were double-labeled with antibodies specific for either the type I or the type II isoform of the InsP3R (green) and with TO-PRO-3 to stain the cell nucleus. A, labeling of type I InsP3R (scale bar, 20 µm). B, nuclear staining with TO-PRO-3. C, merged image shows that this isoform of the receptor is distributed throughout the cytosol and is found within the nucleus as well. D, labeling of the type II isoform of the InsP3R (scale bar, 20 µm). E, nuclear staining with TO-PRO-3. F, merged image shows that this isoform also is distributed throughout the cytosol.
FIGURE 3. Efficacy and specificity of adenoviral antisense constructs for each InsP3R isoform.
A, effect of type I antisense (AS) on expression of the type I InsP3R in AR4-2J cells. The adenoviral construct markedly decreases expression of the type I isoform both 24 and 48 h after infection (left and right lanes, respectively), relative to uninfected control cells (ctrl). In contrast, expression of this isoform was not decreased 24 or 48 h after infection with the adenoviral DsRed construct (left and right lanes, respectively). B, expression of the type II isoform is not decreased 24 or 48 h after infection with the antisense construct for the type I InsP3R (left and right lanes, respectively), relative to uninfected control cells. C, effect of type II antisense on expression of the type II InsP3R in AR4-2J cells. The adenoviral construct markedly decreases expression of the type II isoform both 24 and 48 h after infection (left and right lanes, respectively), relative to uninfected control cells. In contrast, expression of this isoform was not decreased 24 or 48 h after infection with the adenoviral DsRed construct. D, expression of the type I isoform is not decreased 24 or 48 h after infection with the antisense construct for the type II InsP3R (left and right lanes, respectively), relative to uninfected control cells. E, effect of type III antisense on expression of the type III InsP3R in RIN cells. The adenoviral construct markedly decreases expression of the type III isoform 48 h (right) but not 24 h (left) after infection, relative to uninfected control cells. Each immunoblot was performed using 25 µg of protein/lane, and cells were infected with each adenovirus at a concentration of 5 ± 10 10 pfu/ml (m.o.i. = 40).
Type I and II InsP3Rs Both Contribute to Ca2+ Signals in AR4-2J Cells
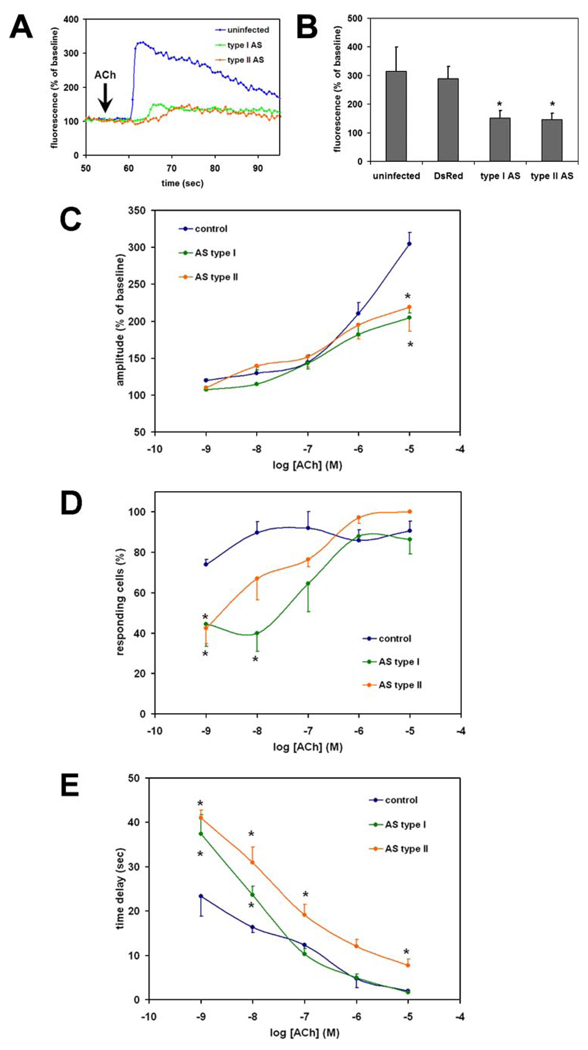
To investigate the relative contribution of the type I and II InsP3R isoforms to Ca2+ signaling in AR4-2J cells, the cells were infected with adenoviral constructs as described above. Cells were then stimulated with ACh (10 µm) to induce an InsP3-mediated increase in cytosolic Ca2+ (Fig. 4A). The amplitude of the ACh-induced Ca2+ signal was reduced by 75% in cells treated with adenoviral antisense for either the type I or type II InsP3R (p < 10−10 relative to uninfected controls, n = 20–22 cells in each group) (Fig. 4, A and B). Infection with the adenovirus DsRed construct did not affect the amplitude of the ACh-induced Ca2+ signal (p = 0.12), suggesting that the inhibition was not a nonspecific effect of adenoviral infection (n = 20 in both the noninfected and DsRed group) (Fig. 4B). The amplitude of the ACh-induced Ca2+ signal was not reduced significantly in cells treated with adenoviral antisense for either the type I or II InsP3R if cells were stimulated with lower concentrations of ACh (Fig. 4C). To characterize the effects of InsP3R isoforms on Ca2+ signals more completely, the relationship between ACh concentration and percent of responding cells (Fig. 4D) and time delay before a Ca2+ signal begins (Fig. 4E) were examined. The percent of cells responding to ACh was lower than controls in cells treated with adenoviral antisense for either the type I or II InsP3R, but only at minimal concentrations of ACh (Fig. 4D). Similarly, the time delay before the onset of ACh-induced Ca2+ signals was lower in controls than in cells treated with adenoviral antisense for either the type I or II InsP3R. The time delay was prolonged in cells expressing either type of antisense and stimulated with lower ACh concentrations, but the time delay persisted at higher ACh concentrations only in those cells expressing antisense for the type II InsP3R (Fig. 4E). These findings demonstrate that the loss of InsP3Rs induced by the adenoviral constructs have functional consequences for Ca2+ signaling, and furthermore suggest that the type I and the type II isoforms of the InsP3R contribute to Ca2+ signaling to a similar extent in AR4-2J cells.
FIGURE 4. Type I and II InsP3R contribute similarly to Ca2+ signaling in AR4-2J cells.
Cells were loaded with Fluo-4 and stimulated with ACh (10 µm), and the resulting cytosolic Ca2+ signal was monitored by confocal microscopy. A, tracings from representative single cells that were not transfected (blue) or transfected with antisense for the type I (red) or type II (green) InsP3R. B, summary of results. The amplitude of the ACh-induced Ca2+ signal was not decreased relative to uninfected controls in cells infected with adenoviral DsRed, but it was decreased significantly and to a similar extent in cells infected with either adenoviral antisense (*, p < 10−10 for both antisense groups; n = 20–22 cells for each of the four groups). Values shown are mean ± S.D. C, amplitude of the ACh-induced Ca2+ signal for a range of ACh concentrations. Cells lacking either the type I or type II InsP3R have a decreased response to ACh relative to nontransfected controls, but only for high ACh concentrations (*, p < 0.05). D, percentage of cells in which ACh increases cytosolic Ca2+ in AR4-2J cells. Cells lacking either the type I or type II InsP3R have decreased sensitivity to low concentrations of ACh relative to nontransfected controls (*, p < 0.05). E, time lag between exposure to ACh and onset of Ca2+ signal (measured only in those cells in which a Ca2+ increase occurs). A significant delay is detected upon stimulation with a range of concentrations of ACh in AR4-2J cells lacking either type I or type II InsP3Rs (*, p < 0.05). C–E, an average of 130 cells was examined in each experimental group at each concentration of ACh. Values are mean ± S.E.
Type I or Type II InsP3R Expression Can Be Selectively Inhibited in Hepatocytes in Vivo
Adenoviral DsRed and EGFP constructs were used to determine the efficiency of the adenoviral delivery system in vivo. For these studies, constructs were injected into the portal vein, and the rats were sacrificed 48 h later to analyze liver sections for expression of DsRed or EGFP in hepatocytes. Portal injection of 600 µl containing a viral titer of 5 × 109 pfu/ml was found to be optimal for infection, because confocal fluorescence demonstrated that this resulted in expression of DsRed or EGFP in nearly 100% of hepatocytes (not shown). Injection of greater amounts of virus often caused toxicity, whereas injection of lower amounts resulted in expression in less than 90% of hepatocytes. These findings demonstrate that portal injection of adenoviral constructs can be used for highly efficient delivery into hepatocytes. Adenoviral antisense constructs were injected as described above, and then rat hepatocytes were isolated 48 h later for immunoblotting to analyze the effects of each construct on InsP3R expression (Fig. 5). The adenoviral antisense for the type I isoform markedly reduced the expression of that isoform in hepatocytes, whereas adenoviral antisense for the type II isoform did not reduce expression of the type I InsP3R (Fig. 5A). Similarly, the adenoviral antisense for the type II isoform markedly reduced expression of that isoform in hepatocytes, whereas antisense for the type I isoform did not affect expression of the type II isoform (Fig. 5B). It is recognized that the type I and II InsP3Rs are distributed in characteristic patterns in individual hepatocytes (8); the type I isoform is distributed uniformly throughout the cytosol (Fig. 6), whereas the type II isoform is concentrated in the pericanalicular region (Fig. 7). Therefore, rat liver sections also were analyzed for the expression of both isoforms 48 h after injection of the adenoviral constructs. The adenoviral antisense for the type I isoform markedly reduced the expression of that isoform throughout the liver, but it did not affect expression of the type II isoform (Fig. 6). Similarly, the adenoviral antisense for the type II isoform markedly reduced expression of that isoform in hepatocytes, but did not affect expression of the type I isoform (Fig. 7). The adenoviral antisense for the type III InsP3R was used as a negative control because hepatocytes do not express this isoform (8, 20). Infection with this construct did not inhibit expression of either the type I or the type II InsP3R (Fig. 8). These findings demonstrate that portal injection of these adenoviral antisense constructs can be used to selectively inhibit expression of the corresponding InsP3R isoforms in hepatocytes.
FIGURE 5. Adenoviral antisense constructs selectively reduce expression of InsP3R isoforms in hepatocytes in vivo.
The adenoviral constructs were injected in the portal vein as described under “Experimental Procedures,” and after 48 h the liver was excised; hepatocytes were isolated, and Western blotting was performed. A, portal injection of adenovirus for type I InsP3R antisense (ASI) reduces expression of type I InsP3R in hepatocytes relative to control (CT), whereas injection of adenovirus for type II InsP3R antisense (ASII) does not affect type I InsP3R expression. B, portal injection of adenovirus for type II InsP3R antisense reduces expression of type II InsP3R in hepatocytes, whereas injection of adenovirus for type I InsP3R antisense does not. In each case, densitometry measurements were normalized by densitometry values obtained for the loading control, actin. Results are representative of those observed in at least three separate experiments. IB, immunoblot; cntl, control.
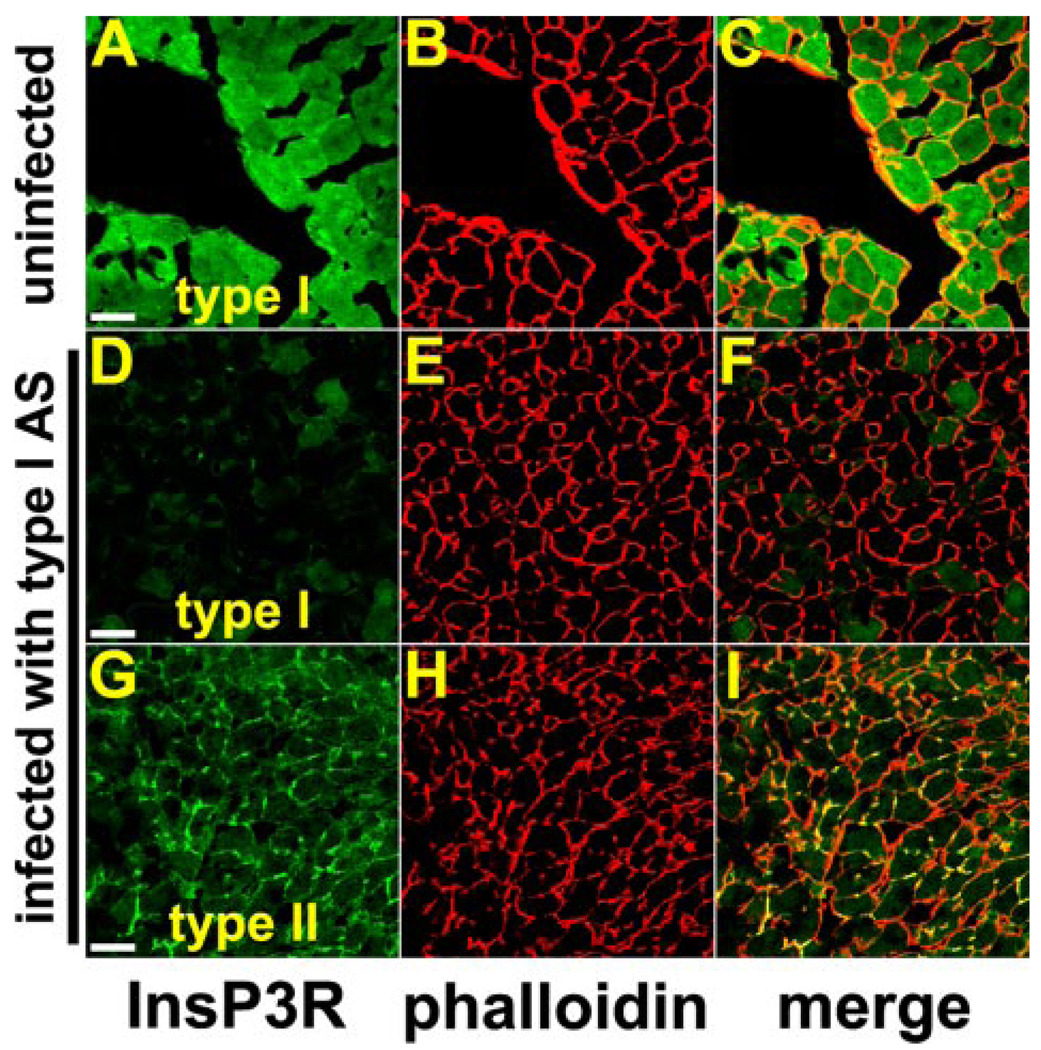
FIGURE 6. Adenoviral antisense for the type I InsP3R specifically inhibits expression of the type I isoform in hepatocytes in vivo.
The adenoviral construct was injected in the portal vein as described under “Experimental Procedures,” and after 48 h the liver was excised, sectioned, stained with isoform-specific InsP3R antibodies (green), counterstained with the actin stain rhodamine phalloidin (red) to outline individual hepatocytes, and examined by confocal microscopy. A–C, distribution of type I InsP3R in control (noninfected) rat liver. The type I InsP3R is distributed throughout the cytosol of hepatocytes under normal conditions, as has been described previously (8). Scale bar, 20 µm. D–F, labeling for the type I InsP3R is nearly absent from hepatocytes 48 h after portal injection of adenoviral antisense. G–I, distribution of type II InsP3R in hepatocytes 48 h after injection of the adenoviral construct. The type II InsP3R is concentrated in the canalicular (apical) region, identified by the yellow (double) labeling in the merged image in I. This is the same as the distribution of this isoform in hepatocytes under normal conditions (Fig. 7, A–C) (8). Therefore, the distribution of the type II isoform is not affected by the adenoviral antisense construct for the type I InsP3R.
FIGURE 7. Adenoviral antisense for the type II InsP3R specifically inhibits expression of the type II isoform in hepatocytes in vivo.
The adenoviral construct was injected in the portal vein as described under “Experimental Procedures,” and after 48 h the liver was excised, sectioned, stained with isoform-specific InsP3R antibodies (green), counterstained with the actin stain rhodamine phalloidin (red) to outline individual hepatocytes, and examined by confocal microscopy. A–C, distribution of type II InsP3R in control (noninfected) rat liver. The type II InsP3R is concentrated in the canalicular region of hepatocytes under normal conditions, as has been described previously (8). Scale bar, 20 µm. D–F, labeling for the type II InsP3R is nearly absent from hepatocytes 48 h after portal injection of adenoviral antisense. G–I, distribution of type I InsP3R in hepatocytes 48 h after injection of the adenoviral construct. The type I InsP3R is distributed throughout the cytosol of hepatocytes, similar to what is observed under normal conditions (Fig. 6, A–C). Therefore, the distribution of the type I isoform is not affected by the adenoviral antisense construct for the type II InsP3R.
FIGURE 8. Adenoviral antisense for the type III InsP3R does not inhibit expression of the type I or type II isoform in hepatocytes in vivo.
The adenoviral construct was injected in the portal vein as described under “Experimental Procedures,” and after 48 h the liver was excised, sectioned, stained with isoform-specific InsP3R antibodies (green), counterstained with the actin stain rhodamine phalloidin (red) to outline individual hepatocytes, and examined by confocal microscopy. A–C, distribution of type I InsP3R in hepatocytes 48 h after injection of the adenoviral construct. The type I InsP3R is distributed throughout the cytosol of hepatocytes, similar to what is observed under normal conditions (Fig. 6, A–C). D–F, distribution of type II InsP3R in hepatocytes 48 h after injection of the adenoviral construct. The type II InsP3R is concentrated in the canalicular region, identified by the yellow (double) labeling in the merged image in F. This is the same as the distribution of this isoform in hepatocytes under normal conditions (Fig. 7, A–C). Therefore, neither the distribution of the type I nor the type II isoform is affected by the adenoviral antisense construct for the type III InsP3R. Scale bars, 20 µm.
Type I and II InsP3R Have Distinct Effects on Ca2+ Signaling in Hepatocytes
To determine the effects of type I and II InsP3R on Ca2+ signaling, hepatocytes were isolated from rats treated with each of the isoform-specific antisense constructs and from untreated rats. The isolated hepatocytes were stimulated with vasopressin (0.1–100 nm) to induce an InsP3-mediated increase in cytosolic Ca2+ (25), and the resulting Ca2+ signals were analyzed. Significant differences were detected among the concentration-response curves (p < 0.005 by repeated measures ANOVA), and this reflected that hepatocytes from noninfected control animals responded differently from hepatocytes lacking the type I (p < 0.05) or type II (p < 0.01) but not the type III InsP3R (p > 0.05; Dunnett’s post-test for multiple comparisons). Specifically, an increase in Ca2+ was seen in response to stimulation with vasopressin in most (84–100%) hepatocytes isolated from uninfected animals or from animals treated with antisense for type III InsP3R, regardless of the concentration of vasopressin used (Fig. 9A). Hepatocytes lacking the type I InsP3R were minimally responsive to lower (0.1–1 nm) concentrations of vasopressin, but they responded with the same frequency as negative controls upon stimulation with maximal (10–100 nm) vasopressin concentrations (Fig. 9A). Like hepatocytes lacking the type I InsP3R, hepatocytes lacking the type II InsP3R were minimally responsive to stimulation with low concentrations of vasopressin. Unlike hepatocytes lacking the type I isoform, however, hepatocytes lacking the type II isoform responded less frequently than negative controls (~70% versus 100%) even after stimulation with maximal concentrations of vasopressin (Fig. 9A). It is unlikely that the decreased sensitivity of hepatocytes lacking the type I or II InsP3R reflected nonspecific effects of adenovirus or antisense, because cells treated with antisense for the type III isoform responded the same as noninfected controls. There is also a concentration-dependent delay between stimulation with vasopressin and the initiation of Ca2+ signaling in hepatocytes (26), so the effect of InsP3R isoforms on this delay was examined as well (Fig. 9B). The delay in uninfected hepatocytes decreased progressively from 156 to 29 s as the vasopressin concentration was increased from 0.1 to 100 nm, and these values were not significantly different in hepatocytes treated with antisense for type III InsP3R. However, the delay time was significantly prolonged in hepatocytes lacking the type I InsP3R that were treated with 1–10 nm vasopressin as well as in hepatocytes lacking the type II InsP3R that were treated with 1–100 nm vasopressin (p < 0.05). Although the delay in hepatocytes stimulated with 0.1 nm vasopressin was not significant from what was observed in controls, this may in part reflect the low percentages of adenovirus-treated hepatocytes that responded to this minimal concentration of vasopressin (16% of cells lacking the type I isoform and 6% of cells lacking type II). Together these findings demonstrate that Ca2+ signaling is impaired in hepatocytes lacking either the type I or type II InsP3R but that the impairment is greater in cells lacking the type II isoform.
FIGURE 9. InsP3R isoforms affect vasopressin-induced Ca2+ signals in hepatocytes in a concentration-dependent fashion.
Hepatocytes were isolated 48 h after portal injection of adenoviral antisense constructs, and then vasopressin-induced Ca2+ signals were examined by confocal microscopy. A, percentage of cells in which vasopressin increases cytosolic Ca2+ in hepatocytes. Cells lacking the type I InsP3R have decreased sensitivity to low concentrations of vasopressin, whereas cells lacking the type II isoform have decreased sensitivity to vasopressin at all concentrations (*, p < 0.005 by repeated measures ANOVA). B, time lag between exposure to vasopressin and onset of Ca2+ signal (measured only in those cells in which a Ca2+ increase occurs). A significant delay is detected upon stimulation with a range of concentrations of vasopressin in hepatocytes lacking either type I or type II InsP3Rs (*, p < 0.05). A minimum of 30–40 cells was examined in each experimental group at each concentration of vasopressin. Values are mean ± S.D.
Type I and II InsP3R Have Distinct Effects on Ca2+ Waves in Hepatocytes
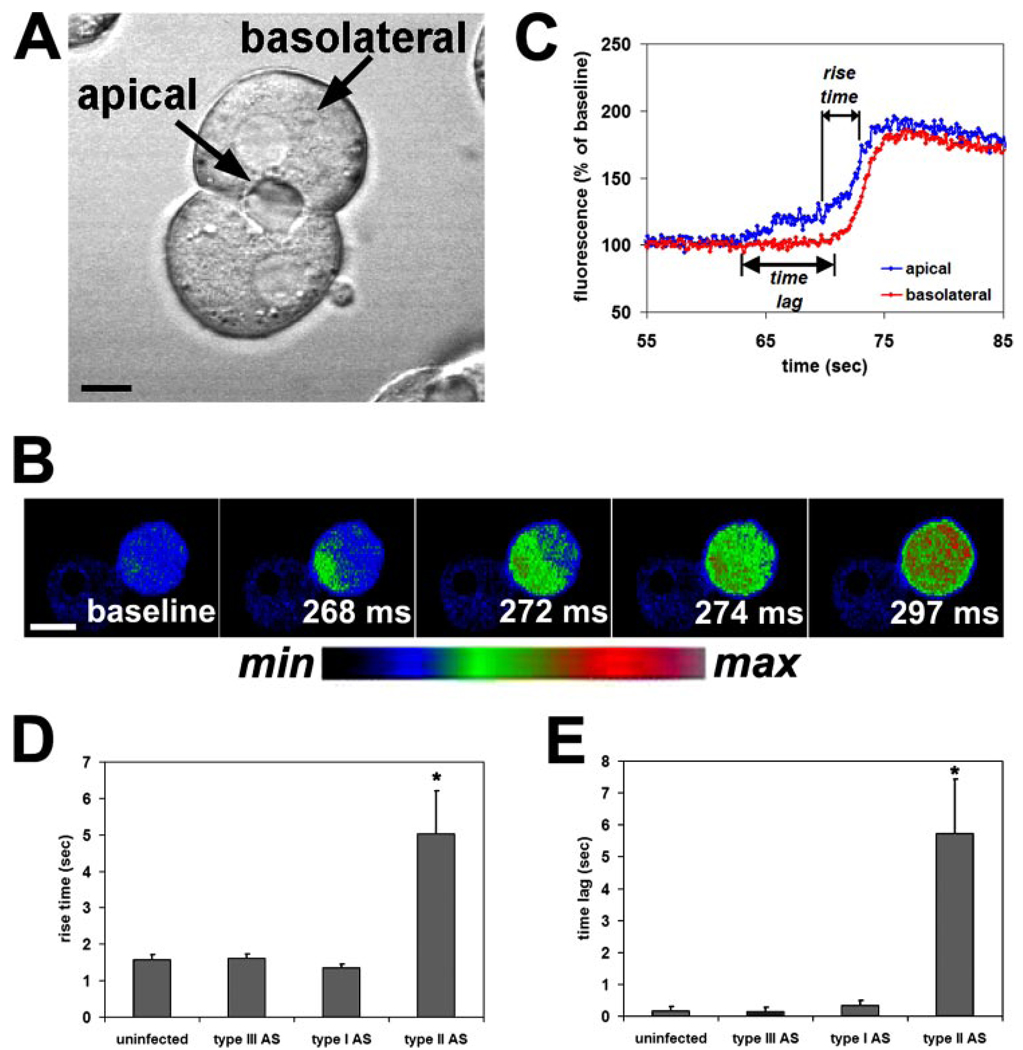
Ca2+ waves occur in a polarized fashion in hepatocytes (8, 9) and in other epithelia (4, 13, 22). Cell polarity is preserved in isolated rat hepatocyte couplets (Fig. 10A) (8, 21, 27), so they are a particularly good cell system in which to examine epithelial Ca2+ wave formation. Vasopressin (10 nm) induced polarized, apical-to-basal Ca2+ waves in hepatocytes (Fig. 10B), similar to what has been described previously (8, 9). To quantify the effects of InsP3R isoforms on Ca2+ waves, the time for the apical component of the Ca2+ signal to increase from 20 to 80% of its maximum value (rise time) and the time for Ca2+ wave to travel from the apical to the basolateral region (time lag) were measured (Fig. 10C). The rise time was 1.58 ± 0.13 s (mean ± S.E., n = 18) in hepatocytes from untreated animals, and this value was not significantly different in hepatocytes treated with antisense for type I or III InsP3R (n = 18 and 19, respectively). However, the rise time was increased over 3-fold to 5.04 ± 1.16 s (n = 16) in hepatocytes lacking the type II isoform (p < 0.005; Fig. 10D). Similarly, the time lag required for Ca2+ waves to cross the cell was 167 ± 121 ms (n = 18) in hepatocytes from untreated animals, and this value was not significantly different in hepatocytes treated with antisense for type I or III InsP3R (n = 18 and 19, respectively). However, the time lag was increased over 30-fold to 5.73 ± 1.71 s (n = 16) in hepatocytes lacking the type II isoform (p < 0.005; Fig. 10E). Thus, loss of the type I InsP3R has little effect on the kinetics of Ca2+ waves in hepatocytes, whereas loss of the type II isoform severely retards both the rate of onset of Ca2+ waves and the speed at which they cross the cell.
FIGURE 10. Loss of the type II InsP3R selectively impairs Ca2+ waves in hepatocytes.
Hepatocyte couples and triplets were stimulated with vasopressin (10 nm), and Ca2+ wave kinetics were analyzed. A, transmission image of an isolated rat hepatocyte couplet illustrates the apical and basolateral region of the cells. Scale bar, 10 µm. B, serial confocal images of a couplet loaded with the Ca2+ dye Fluo-4 and stimulated with vasopressin (10 nm) illustrates a Ca2+ wave spreading from the apical to the basolateral pole of one of the cells. Images are pseudocolored according to the color scale below. Scale bar, 20 µm. C, graphical representation of the apical and basolateral components of a Ca2+ wave in a single hepatocyte. The tracing illustrates measurement of the rise time (time required for the apical Ca2+ signal to increase from 20 to 80% of its peak) and the time lag (time required for the signal to move from the apical to the basolateral region). D, rise time is significantly delayed in cells from animals treated with the adenoviral antisense (AS) for the type II InsP3R (*, p < 0.005) but not in cells from animals treated with the type I or III antisense. E, time lag also is significantly delayed in cells from animals treated with the adenoviral antisense (AS) for the type II InsP3R (*, p < 0.005) but not in cells from animals treated with the type I or III antisense. Values are mean ± S.E.
DISCUSSION
Here we examined the role of InsP3R isoforms in the formation of Ca2+ waves. Ca2+ waves can be the result of Ca2+ release from InsP3Rs (28, 29) or ryanodine receptors (30), or they can result from the coordinated release of Ca2+ from both types of receptors (9). In hepatocytes, Ca2+ waves are formed entirely by InsP3Rs because this cell type does not express the ryanodine receptor (8). Ca2+ waves can be formed entirely by a single InsP3R isoform, as occurs in Xenopus oocytes (28, 29, 31), which express only a single type of InsP3R that is similar to the mammalian type I isoform (32). Ca2+ waves also occur in cells that express multiple, spatially segregated InsP3R isoforms. For example, Ca2+ waves in the nonpigmented epithelium of the ocular ciliary bilayer are the result of sequential Ca2+ release from apical type III InsP3Rs, followed by basolateral, type I InsP3Rs (14, 22). Similarly, Ca2+ waves in hepatocytes are the result of sequential Ca2+ release from apical type II InsP3Rs, followed by basolateral, type I InsP3Rs (8). Despite these observations about the pattern of Ca2+ waves in such cells, there has not been direct evidence that Ca2+ waves result from the coordinated release of Ca2+ from multiple, spatially segregated InsP3R isoforms. This study demonstrates directly the essential role of type II InsP3Rs in the formation of Ca2+ waves in hepatocytes, because selective loss of this isoform severely delayed both the initiation and spread of Ca2+ waves. Although the ability to generate a Ca2+ signal in hepatocytes also depended on expression of type I InsP3Rs, the morphology of Ca2+ waves appeared normal in those cells lacking the type I isoform in which signals could be initiated. There are several reasons why the type II isoform may play a more critical role in triggering Ca2+ waves in hepatocytes. First, single channel studies suggest that the open probability of the type I InsP3R is decreased by high concentrations of Ca2+ (10, 33), whereas the type II isoform is not (12), so the type II InsP3R may be better suited to act as a trigger for Ca2+ signals. Cofactors may modify the single channel behavior of InsP3Rs in intact cells (34), which may detract from this explanation. Second, the type II InsP3R is the isoform with the highest affinity for InsP3 (17), so it may preferentially initiate Ca2+ signals on that basis. Third, because the type II isoform is concentrated in a single subcellular region in hepatocytes, this may enhance local feedback activation of the receptor by Ca2+ (35, 36). Loss of type II InsP3Rs slowed not only initiation but also spread of Ca2+ waves. Because Ca2+ is a co-agonist for the InsP3R (10), one possible explanation is that a decreased apical Ca2+ signal results in less effective activation of other nearby InsP3Rs and that this diminished effect propagates across the hepatocyte. Alternatively, type II InsP3Rs are present not only apically but also in the basolateral region, albeit in lower amounts than in the apical region (8). Because this isoform has a higher affinity than the type I isoform for InsP3 (17), it is possible that loss of type II InsP3Rs or of type I–II heterodimers (37) from the basolateral region may inhibit the spread of Ca2+ waves. Although loss of the type II InsP3R had a distinct effect on Ca2+ signaling in hepatocytes, loss of either the type I or type II isoform had similar effects on Ca2+ signaling in AR4-2J cells. This was unexpected, because the two isoforms are expressed in similar ratios in hepatocytes and AR4-2J cells. Because the two isoforms are not spatially segregated in AR4-2J cells, one possible explanation is that the two isoforms form heterotetramers in those cells (37). If the heterotetramers behave like type II InsP3Rs, then loss of either isoform might similarly impair Ca2+ signaling. The findings in hepatocytes and AR4-2J cells together demonstrate that Ca2+ waves are determined not only by the InsP3R isoforms that are expressed, but by their subcellular distribution as well.
To our knowledge, this is the first study of the effect of InsP3R isoforms on the spatial pattern of Ca2+ signals. Several previous studies have examined the effects of InsP3R isoforms on the temporal pattern of Ca2+ signals. In one study, three separate DT40 lymphocyte cell lines were generated, each expressing only one of the three isoforms of the InsP3R (38). That study suggested that the type II InsP3R supports sustained and long term Ca2+ oscillations, whereas the type I isoform can support repetitive Ca2+ signals that are only transient and erratic, and the type III isoform generally supports only a single transient increase in Ca2+ rather than repetitive Ca2+ signals (38). Studies in vascular myocytes expressing the type I with or without the type II InsP3R found that Ca2+ oscillations occurred only in myocytes expressing both isoforms and that oscillations were abolished by type II-specific antisense (39). Most recently, an approach using small interfering RNA compared the effects of types I and III InsP3Rs on Ca2+ signaling. Loss of type I InsP3R from HeLa cells impaired Ca2+ signaling and eliminated Ca2+ oscillations, whereas loss of the type III isoform enhanced Ca2+ oscillations (40). Moreover, knockdown of the type III isoform in COS-7 cells converted transient Ca2+ signals into oscillatory ones, even though this isoform accounts for 93% of InsP3Rs in that cell type (40). Because Ca2+ oscillations and other temporal patterns of Ca2+ signals encode critical signaling information (1, 41, 42), these previous studies together suggest that the pattern of InsP3R isoform expression establishes the repertoire of temporal signals available to the cell. This study establishes the complementary concept in the spatial domain that the expression and subcellular distribution of InsP3R isoforms form the repertoire of spatial signaling patterns that are available.
What is the physiological role of Ca2+ waves in hepatocytes? Ca2+ waves are thought to direct morphogenesis in developing embryos (3), but their role in polarized epithelia is instead likely to relate to secretion. For example, the apical- to-basal pattern of Ca2+ waves in pancreatic acinar cells results in sequential activation of apical and then basal ion channels that together direct apical fluid and electrolyte secretion (4, 5). Primitive hepatocytes from the little skate Raja erinacea lack apical type II InsP3Rs and form Ca2+ signals randomly throughout the cell rather than as a polarized Ca2+ wave (43). Perhaps as a result, secretion is slow and inefficient in skate liver (44). The initial, sub-plasmalemmal component of Ca2+ signals also directs exocytosis, both in the apical region of polarized epithelia (5) and in the presynaptic region of neurons (45). This requires micromolar range Ca2+ concentrations and is likely mediated by local activation of synaptotagmin (46). Finally, Ca2+ waves travel from cell to cell in isolated hepatocyte couplets and triplets (47–49) as well as in the intact liver (6, 7). Formation of intercellular Ca2+ waves may be because of the apical localization of type II InsP3Rs, because this is also the region in which gap junctions are localized in hepatocytes, and because InsP3-mediated Ca2+ signaling coordinates cell-to-cell Ca2+ waves via gap junctions in the liver (47). Intercellular Ca2+ waves are important for the regulation of both bile secretion and glucose release in the liver (50). Loss of InsP3Rs results in impairments in both Ca2+ signaling and secretion in bile duct epithelial cells (51), but it is not known whether these effects are because of loss of specific InsP3R isoforms. Therefore, it is likely that future work will investigate directly the link between particular InsP3R isoforms, formation of subcellular Ca2+ signals, and regulation of specific physiological effects such as secretion.
Acknowledgment
We thank Reed P. Hickey for technical assistance.
Footnotes
This work was supported by a postdoctoral research fellowship award from the American Liver Foundation, a grant-in-aid from the American Heart Association, and National Institutes of Health Grants DK45710, DK57751, DK34989, DK61747, DK07356, and TW01451.
The abbreviations used are: InsP3, inositol 1,4,5-trisphosphate; InsP3R, inositol 1,4,5-trisphosphate receptor; ACh, acetylcholine; pfu, plaque-forming units; EGFP, enhanced green fluorescent protein; m.o.i., multiplicity of infection; ANOVA, analysis of variance.
REFERENCES
- 1.Berridge MJ, Lipp P, Bootman MD. Nat. Rev. Mol. Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 2.Leite MF, Nathanson MH. In: The Liver: Biology and Pathobiology. Arias IM, editor. Philadelphia: Lippincott, Williams, and Wilkins; 2001. pp. 537–554. [Google Scholar]
- 3.Gilland E, Miller AL, Karplus E, Baker R, Webb SE. Proc. Natl. Acad. Sci. U. S. A. 1999;96:157–161. doi: 10.1073/pnas.96.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kasai H, Augustine GJ. Nature. 1990;348:735–738. doi: 10.1038/348735a0. [DOI] [PubMed] [Google Scholar]
- 5.Ito K, Miyashita Y, Kasai H. EMBO J. 1997;16:242–251. doi: 10.1093/emboj/16.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nathanson MH, Burgstahler AD, Mennone A, Fallon MB, Gonzalez CB, Saez JC. Am. J. Physiol. 1995;269:G167–G171. doi: 10.1152/ajpgi.1995.269.1.G167. [DOI] [PubMed] [Google Scholar]
- 7.Robb-Gaspers LD, Thomas AP. J. Biol. Chem. 1995;270:8102–8107. doi: 10.1074/jbc.270.14.8102. [DOI] [PubMed] [Google Scholar]
- 8.Hirata K, Pusl T, O’Neill AF, Dranoff JA, Nathanson MH. Gastroenterology. 2002;122:1088–1100. doi: 10.1053/gast.2002.32363. [DOI] [PubMed] [Google Scholar]
- 9.Nathanson MH, Burgstahler AD, Fallon MB. Am. J. Physiol. 1994;267:G338–G349. doi: 10.1152/ajpgi.1994.267.3.G338. [DOI] [PubMed] [Google Scholar]
- 10.Bezprozvanny I, Watras J, Ehrlich BE. Nature. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- 11.Hagar RE, Burgstahler AD, Nathanson MH, Ehrlich BE. Nature. 1998;396:81–84. doi: 10.1038/23954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramos-Franco J, Fill M, Mignery GA. Biophys. J. 1998;75:834–839. doi: 10.1016/S0006-3495(98)77572-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirata K, Dufour J-F, O’Neill AF, Bode H-P, Cession D, St-Pierre MV, LaRusso NF, Leite MF, Nathanson MH. Hepatology. 2002;36:284–296. doi: 10.1053/jhep.2002.34432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirata K, Nathanson MH, Burgstahler AD, Okazaki K, Mattei E, Sears ML. Investig. Ophthalmol. Vis. Sci. 1999;40:2046–2053. [PubMed] [Google Scholar]
- 15.Sugiyama T, Yamamoto-Hino M, Wasano K, Mikoshiba K, Hasegawa M. J. Histochem. Cytochem. 1996;44:1237–1242. doi: 10.1177/44.11.8918898. [DOI] [PubMed] [Google Scholar]
- 16.Yule DI, Ernst SA, Ohnishi H, Wojcikiewicz RJH. J. Biol. Chem. 1997;272:9093–9098. doi: 10.1074/jbc.272.14.9093. [DOI] [PubMed] [Google Scholar]
- 17.Newton CL, Mignery GA, Südhof TC. J. Biol. Chem. 1994;269:28613–28619. [PubMed] [Google Scholar]
- 18.Lu CY, Giordano FJ, Rogers KC, Rothman A. J. Mol. Cell. Cardiol. 1996;28:1703–1713. doi: 10.1006/jmcc.1996.0160. [DOI] [PubMed] [Google Scholar]
- 19.McGrory WJ, Bautista DS, Graham FL. Virology. 1988;163:614–617. doi: 10.1016/0042-6822(88)90302-9. [DOI] [PubMed] [Google Scholar]
- 20.Wojcikiewicz RJH. J. Biol. Chem. 1995;270:11678–11683. doi: 10.1074/jbc.270.19.11678. [DOI] [PubMed] [Google Scholar]
- 21.Graf J, Gautam A, Boyer JL. Proc. Natl. Acad. Sci. U. S. A. 1984;81:6516–6520. doi: 10.1073/pnas.81.20.6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirata K, Nathanson MH, Sears ML. Proc. Natl. Acad. Sci. U. S. A. 1998;95:8381–8386. doi: 10.1073/pnas.95.14.8381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen CN, Slack JMW, Tosh D. Nat. Cell Biol. 2000;2:879–887. doi: 10.1038/35046522. [DOI] [PubMed] [Google Scholar]
- 24.Joseph SK. J. Biol. Chem. 1994;269:5673–5679. [PubMed] [Google Scholar]
- 25.Nathanson MH, Moyer MS, Burgstahler AD, O’Carroll A-M, Brownstein MJ, Lolait SJ. J. Biol. Chem. 1992;267:23282–23289. [PubMed] [Google Scholar]
- 26.Rooney TA, Sass EJ, Thomas AP. J. Biol. Chem. 1989;264:17131–17141. [PubMed] [Google Scholar]
- 27.Gautam A, Ng O-C, Boyer JL. Hepatology. 1987;7:216–223. doi: 10.1002/hep.1840070203. [DOI] [PubMed] [Google Scholar]
- 28.Marchant J, Callamaras N, Parker I. EMBO J. 1999;18:5285–5299. doi: 10.1093/emboj/18.19.5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parker I, Ivorra I. Science. 1990;250:977–979. doi: 10.1126/science.2237441. [DOI] [PubMed] [Google Scholar]
- 30.Cheng H, Lederer WJ, Cannell MB. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- 31.Lechleiter J, Girard S, Peralta E, Clapham D. Science. 1991;252:123–126. doi: 10.1126/science.2011747. [DOI] [PubMed] [Google Scholar]
- 32.Parys JB, Bezprozvanny I. Cell Calcium. 1995;18:353–363. doi: 10.1016/0143-4160(95)90051-9. [DOI] [PubMed] [Google Scholar]
- 33.Finch EA, Turner TJ, Goldin SM. Science. 1991;252:443–446. doi: 10.1126/science.2017683. [DOI] [PubMed] [Google Scholar]
- 34.Michikawa T, Hirota J, Kawano S, Hiraoka M, Yamada M, Furuichi T, Mikoshiba K. Neuron. 1999;23:799–808. doi: 10.1016/s0896-6273(01)80037-4. [DOI] [PubMed] [Google Scholar]
- 35.Yao Y, Choi J, Parker I. J. Physiol. (Lond.) 1995;482:533–553. doi: 10.1113/jphysiol.1995.sp020538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swillens S, Dupont G, Combettes L, Champeil P. Proc. Natl. Acad. Sci. U. S. A. 1999;96:13750–13755. doi: 10.1073/pnas.96.24.13750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Onoue H, Tanaka H, Tanaka K, Doira N, Ito Y. Biochem. Biophys. Res. Commun. 2000;267:928–933. doi: 10.1006/bbrc.1999.2065. [DOI] [PubMed] [Google Scholar]
- 38.Miyakawa T, Maeda A, Yamazawa T, Hirose K, Kurosaki T, Iino M. EMBO J. 1999;18:1303–1308. doi: 10.1093/emboj/18.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morel JL, Fritz N, Lavie JL, Mironneau J. Arterioscler. Thromb. Vasc. Biol. 2003;23:1567–1575. doi: 10.1161/01.ATV.0000089013.82552.5D. [DOI] [PubMed] [Google Scholar]
- 40.Hattori M, Suzuki AZ, Higo T, Miyauchi H, Michikawa T, Nakamura T, Inoue T, Mikoshiba K. J. Biol. Chem. 2004;279:11967–11975. doi: 10.1074/jbc.M311456200. [DOI] [PubMed] [Google Scholar]
- 41.Dolmetsch RE, Xu K, Lewis RS. Nature. 1998;392:933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- 42.Li W, Llopis J, Whitney M, Zlokarnik G, Tsien RY. Nature. 1998;392:936–941. doi: 10.1038/31965. [DOI] [PubMed] [Google Scholar]
- 43.Nathanson MH, O’Neill AF, Burgstahler AD. J. Exp. Biol. 1999;202:3049–3056. doi: 10.1242/jeb.202.22.3049. [DOI] [PubMed] [Google Scholar]
- 44.Nathanson MH, Mariwalla K. Am. J. Physiol. 1996;270:R561–R570. doi: 10.1152/ajpregu.1996.270.3.R561. [DOI] [PubMed] [Google Scholar]
- 45.Llinas R, Sugimori M, Silver RB. Science. 1992;256:677–679. doi: 10.1126/science.1350109. [DOI] [PubMed] [Google Scholar]
- 46.Fernandez-Chacon R, Konigstorfer A, Gerber S, Garcia J, Matos M, Stevens C, Brose N, Rizo J, Rosenmund C, Sudhof TC. Nature. 2001;410:41–49. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- 47.Nathanson MH, Burgstahler AD. Mol. Biol. Cell. 1992;3:113–121. doi: 10.1091/mbc.3.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tordjmann T, Berthon B, Claret M, Combettes L. EMBO J. 1997;16:5398–5407. doi: 10.1093/emboj/16.17.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tordjmann T, Berthon B, Jacquemin E, Clair C, Stelly N, Guillon G, Claret M, Combettes L. EMBO J. 1998;17:4695–4703. doi: 10.1093/emboj/17.16.4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nathanson MH, Rios-Velez L, Burgstahler AD, Mennone A. Gastroenterology. 1999;116:1176–1183. doi: 10.1016/s0016-5085(99)70021-1. [DOI] [PubMed] [Google Scholar]
- 51.Shibao K, Hirata K, Robert ME, Nathanson MH. Gastroenterology. 2003;125:1175–1187. doi: 10.1016/s0016-5085(03)01201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]