Abstract
Background & Aims
Polarity is critical for hepatocyte function. Ca2+ waves are polarized in hepatocytes because the inositol 1,4,5-trisphosphate receptor (InsP3R) is concentrated in the pericanalicular region, but the basis for this localization is unknown. We examined whether pericanalicular localization of the InsP3R and its action to trigger Ca2+ waves depends on lipid rafts.
Methods
Experiments were performed using isolated rat hepatocyte couplets and pancreatic acini, plus SkHep1 cells as nonpolarized controls. The cholesterol depleting agent methyl-beta-cyclodextrin (mβCD) was used to disrupt lipid rafts. InsP3R isoforms were examined by immunoblot and immunofluorescence. Ca2+ waves were examined by confocal microscopy.
Results
Type II InsP3Rs initially were localized to only some endoplasmic reticulum fractions in hepatocytes, but redistributed into all fractions in mβCD-treated cells. This InsP3R isoform was concentrated in the pericanalicular region, but redistributed throughout the cell after mβCD treatment. Vasopressin-induced Ca2+ signals began as apical-to-basal Ca2+ waves, and mβCD slowed the wave speed and prolonged the rise time. MβCD had a similar effect on Ca2+ waves in acinar cells but did not affect Ca2+ signals in SkHep1 cells, suggesting that cholesterol depletion has similar effects among polarized epithelia, but this is not a nonspecific effect of mβCD.
Conclusions
Lipid rafts are responsible for the pericanalicular accumulation of InsP3R in hepatocytes, and for the polarized Ca2+ waves that result. Signaling microdomains exist not only in the plasma membrane, but also in the nearby endoplasmic reticulum, which in turn, helps establish and maintain structural and functional polarity.
Cytosolic Ca2+ regulates a wide variety of functions in hepatocytes, including glucose release, gene transcription, apoptosis, and bile secretion.1–3 Ca2+ is able to regulate such a range of cell functions because of the diverse temporal and spatial patterns of Ca2+ signals that can be encoded. For example, temporal patterns of Ca2+ signals such as Ca2+ oscillations encode information in the oscillation frequency, and frequency modulation can differentially regulate expression of transcription factors and cytokines.4,5 Similarly, spatial patterns encode information in the form of Ca2+ waves, which can regulate fluid and electrolyte secretion and exocytosis.6,7 Ca2+ signaling in hepatocytes is mediated by inositol 1,4,5-trisphosphate (InsP3),8 so Ca2+ signaling patterns depend on the expression and subcellular distribution of isoforms of the InsP3 receptor (InsP3R). The type II InsP3R is the predominant isoform in hepatocytes,9 and that isoform is expressed most heavily in the pericanalicular region.8 However, it is not known why the type II InsP3R accumulates there. Hepatocytes also express the type I isoform of the InsP3R, and in neurons, this isoform is drawn to the region of the plasma membrane because it associates with the cytoskeletal linker protein 4.1N.10,11 Protein 4.1N belongs to the band 4.1 family of proteins that also includes 4.1R and 4.1G, both of which are cytoskeletal linker proteins expressed widely, including in pancreas and liver.12 Protein 4.1N is the only 1 of these that is thought to bind to the type I InsP3R, but 4.1N is not expressed in hepatocytes13 or in liver cell lines.14 No binding partners for the type II InsP3R have been identified, but the canalicular membrane of hepatocytes contains lipid rafts,15 and in other cells lipid rafts can establish signaling microdomains.16 Therefore, we examined whether lipid rafts are responsible for the pericanalicular accumulation of the type II InsP3R in hepatocytes, and whether this in turn is responsible for shaping Ca2+ waves in these cells.
Materials and Methods
Animals and Materials
Male Sprague-Dawley rats obtained from Charles River Laboratories (Wilmington, MA) and weighing 100–250 g were used for all experiments. Animals were housed in temperature- and light-controlled rooms and maintained on a standard diet. Dulbecco’s modified Eagle’s medium and other tissue culture reagents were from Invitrogen (Basel, Switzerland). ER-Tracker, fluo-4/AM, mag-fluo-4/AM, and rhodamine-conjugated phalloidin were from Molecular Probes (Eugene, OR). Monoclonal antibodies against calreticulin and caveolin-1 were from BD Biosciences (San Jose, CA), antibodies for Na+/K+ ATPase were from Santa Cruz (Santa Cruz, CA), and for MRP2 were from Abcam (Cambridge, MA). Antibodies specific for InsP3R isoforms I and II were used for immunoblotting and immunofluorescence. Type I InsP3R antibodies from affinity-purified specific rabbit polyclonal antiserum directed against the 19 C-terminal residues of the mouse type I InsP3R8 and monoclonal antibodies directed against sarcoplasmic/endoplasmic reticulum (ER) Ca2+-ATPase (SERCA) type 2b were commercially obtained from Affinity Bioreagents (Golden, CO). Type II InsP3R antibodies from affinity-purified specific rabbit polyclonal antiserum directed against the 18 C-terminal residues of the rat type II InsP3R9 were kindly provided by Richard Wojcikiewicz (State University of New York, Syracuse, NY). Methyl-beta-cyclodextrin (mβCD), filipin, cholesterol, acetylcholine (ACh), and arginine8-vasopressin were obtained from Sigma-Aldrich (St. Louis, MO). All other chemicals were of the highest quality commercially available.
Isolation of Hepatocytes and Pancreatic Acinar Cells
Isolated rat hepatocyte couplets were prepared in the Cell Isolation Core of the Yale Liver Center as described previously.8 This cell preparation was used because hepatocyte couplets retain structural and functional polarity in short-term culture.17–19 Briefly, rat livers were perfused with Hanks’ A and then Hanks’ B medium containing 0.05% collagenase (Boehringer Mannheim Biochemicals, Indianapolis, IN) and 0.8 U trypsin inhibitor (Sigma) per unit tryptic activity. Livers were then excised, minced, and passed through serial nylon mesh filters, and the resultant cells were washed. These cells were suspended at a concentration of 1.5 × 105 cells/mL in L-15 medium (GIBCO, Grand Island, NY) containing 50 U penicillin and 50 mg streptomycin/mL, and were plated onto glass coverslips. Cells were incubated at 37°C and used 2–4 hours after plating. Cell viability was measured 2 hours after plating by trypan blue exclusion and exceeded 90%.
Pancreatic acini were isolated as described previously.20 Briefly, fasted rats were anesthetized and killed, and then the pancreas was surgically removed and placed in a Ca2+-Mg2+-free buffer (pH 7.4). The tissue was finely diced and then transferred to a siliconized flask containing 1 mL digestion buffer. The tissue was shaken, serially filtered through nylon mesh, and then rinsed. Acini were maintained at room temperature and used within 3 hours of isolation. Hepatocytes or acinar cells were cultured with 5 mmol/L mβCD for 30 minutes at 37°C to disrupt cholesterol-bearing lipid rafts.21 Control dishes were treated with culture medium alone. In selected experiments, cells cultured with mβCD were then placed in culture with mβCD preloaded with cholesterol (242 μg cholesterol/mL) for 90 minutes. This protocol allows depleted cholesterol to be restored to cells,22,23 and was performed to determine whether the effects of mβCD were reversible.
Cell Fractionation
OptiPREP gradient (Accurate Chem. & Sci. Corp., Westbury, NY) analysis was performed using a modification of previously published protocols.21 Rat hepatocytes in short-term (2–4-hour) culture were homogenized by 20 passages through a 27–30-gauge needle in lysis buffer (250 mmol/L sucrose, 10 mmol/L Tris-HCl, pH 7.4; 1% Triton-X 100 was added in studies to detect caveolin) containing protease inhibitor on ice, and then centrifuged at 500×g for 10 minutes to remove cell debris. Supernatants were placed at the top of the tube, and then run through with a discontinuous 0%–45% Optiprep gradient. After centrifugation at 100,000×g in a Beckman SW41 ultracentrifuge (Fullerton, CA) overnight at 4°C, 10 serial 1-mL fractions were collected from the top of the gradient.
Immunoblot Analysis
Immunoblots of isolated hepatocytes were prepared as described previously.8 Protein concentration was determined,24 and then proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis using a 4%–20% polyacrylamide gel. Gels were subsequently transferred to protein nitrocellulose membranes. The membranes were blocked for 1 hour and incubated at 4°C overnight with the primary antibodies. The antibody against type I InsP3R was used at a dilution of 1:1000, the antibody against type II InsP3R was used at a dilution of 1:100, the antibody against MRP2 was used at a dilution of 1:2000, the antibody against Na+/K+ ATPase was used at a dilution of 1:100, the antibodies against SERCA 2b and caveolin each were used at a dilution of 1:500, and the antibody against calreticulin was used at a dilution of 1:1000. Membranes were washed, incubated for 1 hour with peroxidase-conjugated antirabbit (1:5000) or antimouse (1:4000) secondary IgG antibody, and revealed by enhanced chemiluminescence (Amersham, Arlington Heights, IL).
Confocal Immunofluorescence Microscopy
Confocal immunofluorescence was performed on isolated hepatocytes and on rat liver sections, as described previously.8 Cells were fixed with cold acetone and permeabilized with Triton X-100. After blocking steps, specimens were labeled with primary antibodies, rinsed with phosphate-buffered saline, and incubated with secondary antibodies. Cells also were labeled with rhodamine-conjugated phalloidin to facilitate identification of the canalicular membrane.17,25 For negative control studies, cells were incubated with secondary antibodies, but primary antibodies were omitted. Specimens were examined with a Zeiss LSM 510 Laser Scanning Confocal Microscope equipped with a krypton/argon mixed gas laser (Thornwood, NY). To ensure specificity of staining, images were obtained using confocal machine settings at which no fluorescence was detectable in negative control samples labeled with secondary antibodies alone. Double-labeled specimens were serially excited at 488 nm and observed at 505–550 nm to detect Alexa 488; then they were excited at 543 nm and observed at >585 nm to detect rhodamine. Triple-labeled specimens were serially excited at 488 nm and observed at 505–550 nm to detect Alexa 488, excited at 543 nm, and observed at >585 nm to detect Alexa 555, and excited at 633 nm and observed at >650 nm to detect Alexa 647. All images were collected using a Zeiss LSM 510 Meta Laser Scanning Confocal Microscope. For filipin/caveolin double-labeling studies, isolated rat hepatocyte couplets were incubated with filipin (0.05 mg/mL) for 60 minutes, then fixed and stained with anticaveolin primary antibody and then Alexa 555 secondary antibody as described above. Filipin fluorescence was determined by 2-photon microscopy using an excitation wavelength of 735 nm and an emission wavelength range of 410–490 nm,26 while Alexa 555 labeling of caveolin antibodies was detected simultaneously using confocal microscopy as above.
Ca2+ Measurements
Cytosolic Ca2+ (Cai2+) measurements were performed by time-lapse confocal microscopy.27,28 Hepatocytes were incubated with fluo-4/AM (6 μmol/L) for 30 minutes at 37°C.27 Pancreatic acinar cells were also loaded with fluo-4/AM, but at room temperature.20,29 Coverslips containing the dye-loaded cells were transferred to a chamber on the stage of a Zeiss LSM 510 Confocal Microscope, perfused with a HEPES-buffered solution, and observed. Cai2+ was monitored in these cells by exciting the specimen at 488 nm and detecting emission signals above 515 nm. A krypton-argon laser was used for excitation. Images of apical and basolateral increases in Cai2+ were obtained at a rate of ~5 frames per second. Neither autofluorescence nor other background signals were detectable at the machine settings used. Changes in fluorescence F were normalized by the initial fluorescence (F0) and are expressed as (F/F0) × 100%.27 Hepatocytes and SKHep1 cells were observed during perfusion with vasopressin, which causes an InsP3-mediated increase in Cai2+ in both of these cell types.1,30 Similarly, pancreatic acinar cells were stimulated with ACh, which causes an InsP3-mediated increase in Cai2+ in these cells.20 Apical and basal regions were identified within the cytosol of individual cells, and the Cai2+ signals in those regions were determined from the recorded images.
ER Imaging
The subcellular distribution of the ER in isolated rat hepatocyte couplets was visualized in 2 different ways, using a low-affinity Ca2+ dye to label ER Ca2+ stores and using an ER membrane dye.31 ER Ca2+ stores were labeled by loading cells with mag-fluo-4/AM (6 μmol/L) for 30 minutes at room temperature. Cells were examined using a Bio-Rad MRC 1024 confocal imaging system (Hercules, CA) equipped with a krypton/argon laser. Fluo-4 fluorescence was excited using the 488-nm line of a krypton/argon laser, and emitted fluorescence >515 nm was collected.31 The ER membrane was labeled by loading cells with ER-tracker (100 nmol/L) for 30 minutes at room temperature. Cells were examined by 2-photon microscopy using the BioRad MRC 1024 system plus a mode-locked, fsec-pulsed Spectra-Physics Tsunami Titanium:Sapphire laser with a Millenia X pump laser (Mountain View, CA) for 2-photon excitation. ER-Tracker was excited at 790 nm, and 2-photon fluorescence was observed at 500–540 nm using custom-built external detectors.31
Statistics
Values listed are mean ± SEM. Statistical comparisons were made using Student t test. A value of P < .05 was taken as significant.
Results
The Type II InsP3R Is Localized to the Pericanalicular ER in Hepatocytes
The InsP3R is the principal Ca2+ release channel in hepatocytes, and the predominant InsP3R isoform in hepatocytes is the type II InsP3R, which is concentrated in the canalicular region.8 The InsP3R generally is in the ER membrane,1 although it has been identified in the plasma membrane as well in some cells.32,33 To determine whether the InsP3R in hepatocytes is in the canalicular membrane or the nearby ER, we performed triple-label confocal immunofluorescence studies of rat liver. Sections were stained for type II InsP3R, submembranous actin, and the canalicular membrane transporter MRP2. This revealed that the type II InsP3R colocalizes with actin beneath the canalicular membrane, rather than with MRP2 on the plasma membrane (Figure 1). This intracellular rather than plasma membrane distribution of the type II InsP3R is consistent with the observation that Ca2+ wave speed in hepatocytes is not decreased in Ca2+-free medium.17 These findings also suggest that the ER and thus ER Ca2+ stores would also be found in the canalicular region. To investigate the subcellular distribution of the ER, we labeled isolated rat hepatocyte couplets with the ER membrane dye ER-Tracker, and examined the couplets using 2-photon microscopy (Figure 2A). Second, to determine whether ER Ca2+ stores are in the pericanalicular region, couplets were loaded with mag-fluo-4, which preferentially labels ER Ca2+ stores owing to its low affinity for Ca2+.31 These couplets were examined using confocal microscopy (Figure 2B). These findings demonstrated that the ER and its Ca2+ stores are distributed throughout the hepatocyte, including in the pericanalicular region. This is consistent with earlier electron microscopic findings that the ER extends to the pericanalicular region as well.19 Together, these findings show that the type II InsP3R in hepatocytes is concentrated in the pericanalicular ER, in close association with the canalicular membrane.
Figure 1.
The type II InsP3R is concentrated in the pericanalicular region of hepatocytes. Confocal immunofluorescence of rat liver shows that the type II InsP3 receptor (blue) colocalizes with submembraneous actin (red) rather than with the apical plasma membrane protein MRP2 (green) in rat hepatocytes.
Figure 2.
The endoplasmic reticulum is distributed throughout the cell in isolated hepatocyte couplets. (A) Isolated rat hepatocyte couplet labeled with the ER membrane dye ER-Tracker visualized by 2-photon microscopy shows that the ER is distributed throughout the apical and basolateral region. (B) Confocal image of an isolated rat hepatocyte couplet loaded with the low-affinity calcium dye mag-fluo-4, which selectively labels ER Ca2+stores. This shows that ER Ca2+stores also are distributed throughout the apical and basolateral region.
The InsP3R Redistributes After Cholesterol Depletion
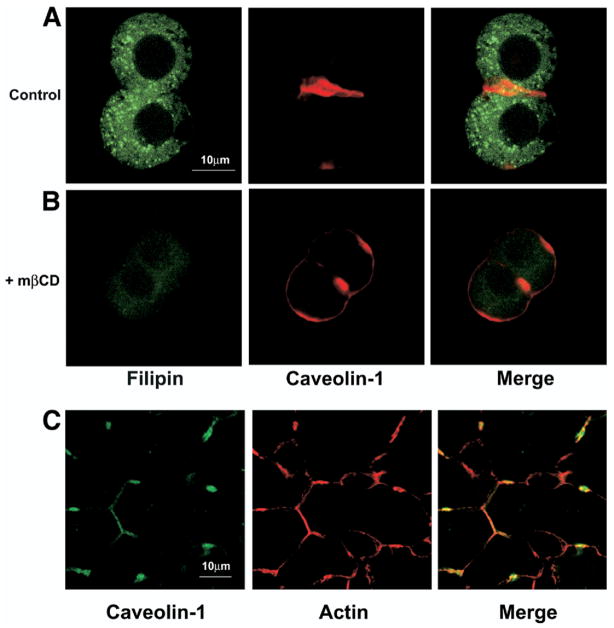
Lipid rafts within membranes have increased cholesterol content, and cholesterol depletion with mβCD disrupts the rafts and releases raft-associated proteins.34 The effects of mβCD on cholesterol depletion and disruption of lipid rafts was examined directly in isolated rat hepatocyte couplets by double labeling the couplets with the fluorescent cholesterol stain filipin plus immunostaining for caveolin (Figure 3). Filipin staining was eliminated and caveolin redistributed from the canalicular membrane to the entire plasma membrane in couplets treated with mβCD. This confirms that mβCD depletes cholesterol and disrupts lipid rafts in isolated rat hepatocyte couplets. To determine whether the type II InsP3R is associated with lipid rafts, we compared the distribution of the receptor in isolated rat hepatocyte couplets before and after treatment with mβCD (5 mmol/L) for 30 minutes. The subcellular distribution of InsP3R isoforms was examined by immunoblot of cell fractions and by confocal immunofluorescence. Immunoblot analysis showed that the type II InsP3R initially was localized to only 3 of 5 ER fractions in hepatocytes (membrane fractions 6–8), but redistributed into all 5 ER fractions (fractions 6–10) in cells treated with mβCD (Figure 4A). This is in contrast to the ER marker calreticulin, which was consistently in all 5 ER fractions. The type I isoform of the InsP3R, which accounts for only a minor fraction of InsP3Rs in hepatocytes9 but is distributed throughout the basolateral region,8 was in cell membrane fractions 7–10 both before and after cholesterol depletion. Separate blots were performed with different ER and plasma membrane markers, as additional controls. This analysis similarly showed that the type II InsP3R initially was localized to only 3 of 5 ER fractions in hepatocytes (membrane fractions 6–8), but redistributed into all 5 ER fractions (fractions 6–10) in cells treated with mβCD (Figure 4B). The ER marker SERCA 2b was consistently in all 5 ER fractions, similar to what was observed with calreticulin and consistent with the observation that SERCA 2b staining is detected throughout the cytoplasm in immunofluorescence of liver sections.35 Caveolin, which is known to partition into lipid rafts in the plasma membrane, initially localized to only 2 of 3 plasma membrane fractions but redistributed into all 3 fractions in cells treated with mβCD. Isolated rat hepatocyte couplets also were examined by confocal immunofluorescence to identify the subcellular regions in which the type II InsP3R redistributes. The type II InsP3R was expressed primarily in a pericanalicular distribution under control conditions, but moved into the basolateral region as well after treatment with mβCD (Figure 5). Line scanning was used to quantify subcellular fluorescence labeling (Figure 6A), and then apical/basolateral fluorescence ratios were calculated so that measurements would be independent of individual fluorescence intensity values, to account for variations in labeling among cells. Under control conditions, the ratio of apical to basolateral fluorescence was 38.7 ± 6.3. After treatment with mβCD, the apical to basolateral fluorescence ratio decreased to 2.9 ± 0.4 (P < .0001, n = 20 cells in 10 couplets; Figure 6B). Similar results were obtained when apical and basolateral fluorescence was measured within 2-dimensional regions of interest rather than along 1-dimensional line scans (not shown). In contrast to the redistribution of InsP3R labeling that was observed, the subcellular pattern of actin staining was not affected by treatment with mβCD (Figure 6A); the apical/basolateral fluorescence ratio was 17.8 ± 5.4 under control conditions, while the ratio in the presence of mβCD was 16.3 ± 3.3 (P = .80, n = 20). In separate experiments, cholesterol was added back to couplets after depletion by mβCD to determine whether the redistribution of type II InsP3R was reversible. The ratio of apical to basolateral fluorescence increased only slightly but significantly (to 8.6 ± 2.4; P < .05, n = 10) in these couplets, relative to what was observed in mβCD-depleted couplets. The observation that the effects of mβCD were not completely reversible in this cell system likely reflects the fact that isolated rat hepatocyte couplets are polarized only because they are incompletely separated during cell isolation, and all couplets gradually lose polarity during the several hours after isolation.19 Therefore, there is not likely to be a driving force to reestablish aspects of polarity once its loss is hastened by treatment with mβCD. Together, these findings provide evidence that the pericanalicular localization of the type II InsP3R in hepatocytes depends upon the presence of lipid rafts.
Figure 3.
mβCD depeletes cholesterol and redistributes caveolin in isolated rat hepatocyte couplets. Isolated rat hepatocyte couplets were double-labeled with the cholesterol dye filipin (green) and immunofluorescent staining of caveolin (red). Filipin was visualized by 2-photon microscopy while caveolin was visualized simultaneously by confocal microscopy. (A) Under control conditions filipin staining is present in a punctuate pattern throughout each hepatocyte, while caveolin is localized to the plasma membrane and is most concentrated along the canalicular membrane. (B) After treatment with mβCD there is little residual filipin staining, and caveolin staining redistributes along the entire plasma membrane. Resultsarerepresentativeof what was observed in 5 couplets under each condition. (C) Rat liver section double-labeled for caveolin-1 (green) and the actin stain phalloidin (red) examined by confocal immunofluorescence. Phalloidin staining is specific for filamentous actin, which outlines the plasma membrane in hepatocytes. The staining is most intense along the canalicular membrane, where actin is most concentrated. Areas of colocalization (yellow) in the merged image confirm that caveolin is most concentrated along the canalicular membrane of hepatocytes under normal conditions.
Figure 4.
The type II InsP3R is in a cholesterol-dependent fraction of the ER. Membranes were collected from isolated rat hepatocytes, and then separated by density gradient into 10 fractions. (A) Distribution of types I and II InsP3R, along with calreticulin, MRP2, and the Na+/K+-ATPase. The type II InsP3R is in fractions 6–8 under control conditions but spreads into fractions 9–10 after cells have been treated with mβCD (5 mmol/L) for 30 minutes. The distribution of the type I InsP3R (fractions 7–10) is not affected by treatment with mβCD. Calreticulin is used to identify ER fractions, while MRP2 and Na+/K+-ATPase are markers for the apical and basolateral plasma membrane, respectively. (B) Distribution of type II InsP3R, along with SERCA 2b and caveolin-1. The type II InsP3R is in fractions 6–8 under control conditions but spreads into fractions 9–10 after cells have been treated with mβCD (5 mmol/L) for 30 minutes. SERCA 2b is used to identify ER fractions, while caveolin is a marker for plasma membrane lipid rafts. Note that caveolin is in (plasma membrane) fractions 3–4 under control conditions but spreads into fraction 5 after treatment with mβCD.
Figure 5.
The type II InsP3R redistributes to the basolateral region after cholesterol depletion. Confocal fluorescence image of an isolated rat hepatocyte couplet labeled with antibody against type II InsP3R receptor (green), plus Alexa 647 phalloidin (red) to label actin, which is most concentrated along the apical membrane. The type II InsP3R is concentrated in the apical region of hepatocytes under control conditions. After treatment with mβCD, the InsP3R is more diffusely distributed throughout the couplet. The InsP3R receptor was labeled with the same antibody used for immunoblots. No InsP3R labeling was detected in cells stained with secondary antibody alone (not shown).
Figure 6.
Quantification of the redistribution of the type II InsP3R. (A) Line scan analysis was performed to measure fluorescence intensity along the (red) line crossing each couplet (top panels). Fluorescence intensity from InsP3R staining (green tracing) and actin staining (red tracing) along the scan line is graphically depicted in the bottom panels. (B) Apical/basolateral fluorescence intensity ratio reveals that basolateral fluorescence labeling of type II InsP3R increases significantly relative to apical fluorescence after treatment with mβCD (*P < .0001; n = 15 couplets). Fluorescence ratios were used so that measurements would be independent of individual fluorescence intensity values, to account for variations in labeling among cells.
Propagation of Ca2+ Waves Is Impaired by Cholesterol Depletion
Ca2+ waves occur in an apical-to-basal fashion in hepatocytes8,17 and other polarized epithelia,25,29,36 and this is thought to be the functional correlate of the apical localization of the InsP3R.37 Therefore, we compared Ca2+ signaling in isolated rat hepatocyte couplets before and after treatment with mβCD. Couplets were stimulated with vasopressin (10 nmol/L), which induces polarized, apical-to-basal Ca2+ waves in hepatocytes.8 To quantify the effects of cholesterol depletion on Ca2+ signaling, the Ca2+ wave speed and the rise time required for the Ca2+ signal to increase from 20% to 80% of its peak were determined (Figure 7A–B). Under control conditions, Ca2+ waves spread from the apical to the basolateral region in 280 ± 3 milliseconds (n = 30 cells in 15 couplets). However, this time interval slowed to 950 ± 6 milliseconds (P < 10−8) in hepatocytes treated with mβCD. This corresponded to a Ca2+ wave speed of 54 ± 6 μm/second and a rise time of 0.9 ± 0.04 seconds under control conditions, and a wave speed of 15 ± 1 mmol/L/seconds (P < 10−6; Figure 7C) and a rise time of 1.8 ± 0.2 seconds (P < .01; Figure 7D) in couplets pretreated with mβCD. These findings demonstrate that redistribution of InsP3R from the apical to the basolateral region is associated with slower initiation and spread of Ca2+ waves in hepatocytes.
Figure 7.
Cholesterol depletion slows Ca2+ waves in hepatocytes. (A) Serial confocal images of an isolated rat hepatocyte couplet loaded with the Ca2+ dye fluo-4 and then stimulated with vasopressin (10 nmol/L). Fluorescence was monitored in the apical (A) and basolateral (B) regions. Region of interest is outlined in yellow; serial images demonstrate that a Ca2+ wave begins in the apical region of the cell (white arrow in middle panel). This and subsequent Ca2+ images are pseudocolored according to the scale shown at bottom. (B) Graphical representation of the fluorescence increase detected in the apical and basolateral region. Note the time lag between the apical increase in Ca2+ and the basolateral increase; the Ca2+ wave speed is calculated by dividing the distance between the apical and basolateral reference points by the time lag. The rise time is the time required for the Ca2+ signal to increase from 20% to 80% of its maximum value. (C) The Ca2+ wave speed is slowed in couplets treated with mβCD (*P < 10−6, n = 15 couplets in each group). (D) The rise time is prolonged in hepatocytes treated with mβCD (*P < .01, n = 15).
To understand the generality of these observations, Ca2+ signaling was examined in pancreatic acinar cells before and after treatment with mβCD. Like hepatocytes, pancreatic acinar cells are polarized epithelia in which InsP3Rs are concentrated near the apical membrane37 and in which hormone-induced Ca2+ signals begin as polarized Ca2+ waves.7,29 However, acinar cells express all 3 InsP3Rs,38,39 and the predominant isoform is the type III InsP3R,9,37 which is not expressed in hepatocytes.8 Under control conditions, ACh (1 μmol/L) induced a rapid apical-to-basal Ca2+ wave that did not diminish in magnitude as it crossed the cell (Figure 8A–B), as has been reported previously.20,29 Ca2+ wave speed in these cells was 42.5 ± 3.5 μm/second (n = 23; Figure 8C) and the rise time was 1.0 ± 0.1 seconds (Figure 8D). In cells treated with mβCD, Ca2+ wave speed was slowed by over 50%, to 18.4 ± 1.2 μm/second (n = 15, P < .001; Figure 8C), while rise time was increased to 1.7 ± 0.2 seconds (P < .05; Figure 8D). Both the delayed wave speed and the prolonged rise time were reversed by cholesterol repletion (Figure 8C–D). These findings demonstrate that polarized Ca2+ waves depend upon lipid rafts in pancreatic acinar cells, as they do in hepatocytes, and suggest that this is a general feature of polarized epithelia.
Figure 8.
Cholesterol depletion slows Ca2+ waves in pancreatic acinar cells. (A) Transmission image and serial confocal images of a pancreatic acinus loaded with fluo-4 and then stimulated with ACh (1 μmol/L). Fluorescence was monitored in the apical (A) and basolateral (B) region of individual acinar cells. Region of interest in a particular cell is outlined in red; serial images demonstrate that a Ca2+ wave begins in the apical region of the cell. (B) Graphical representation of the fluorescence increase detected in the apical and basolateral region of a typical acinar cell. As in hepatocytes, the apical increase in Ca2+ precedes the basolateral increase, reflecting an apical-to-basal Ca2+ wave. (C) Pretreatment with mβCD significantly slows Ca2+ wave speed (*P < .001, n = 15). This effect reverses in acinar cells that have been reloaded with cholesterol (n = 8). (D) The rise time of Ca2+ signals increases in cells treated with mβCD (*P < .05, n = 15). This effect reverses in acinar cells that have been reloaded with cholesterol (n = 8).
To determine whether mβCD has nonspecific effects on InsP3-mediated Ca2+ signaling, SKHep1 cells were examined before and after treatment with mβCD. SKHep1 cells were used because they are a liver-derived epithelial cell line that is not polarized. Furthermore, like hepatocytes,8 SKHep1 cells lack ryanodine receptors,30 so that Ca2+ signaling depends entirely on InsP3Rs in these cells. The InsP3R was diffusely distributed throughout the cytosol in SkHep1 cells, and this pattern was unchanged after treatment with mβCD (Figure 9A). Vasopressin-induced Ca2+ signaling also was monitored in SKHep1 cells before and after treatment with mβCD (Figure 9B). The rise time for Ca2+ signals in untreated cells was 4.5 ± 0.2 seconds, and was 4.7 ± 0.2 seconds in treated cells (n = 15 cells in each group, P = .52; Figure 9C). This finding demonstrates that treatment with mβCD does not induce nonspecific effects on Ca2+ signaling pathways.
Figure 9.
Cholesterol depletion does not affect Ca2+ signals in SKHep1 cells. (A) Confocal immunofluorescence shows that the InsP3R does not redistribute in SKHep1 cells after treatment with mβCD. SKHep1 cells were used as a control of mβCD treatment because they are a nonpolarized liver cell line. Cells were triple labeled to reveal actin (red) and the type II (green) and type III (blue) InsP3R; these are the 2 InsP3R isoforms expressed in this cell line. (B) Serial confocal images of an SKHep1 cell loaded with fluo-4 and then stimulated with vasopressin (100 nmol/L). (C) The rise time of vasopressin-induced Ca2+ signals is not prolonged by treatment with mβCD (P = .52, n = 15).
Discussion
The current work provides evidence that the pericanalicular localization of InsP3Rs in hepatocytes depends upon the presence of detergent-resistant membranes, or lipid rafts. Although the existence of lipid rafts remains controversial,40 treatment with mβCD is a well-accepted approach to disrupt putative rafts, and is based on cholesterol depletion.21,40 This was the only method used to disrupt rafts here because the other method commonly used, treatment with fumonisin B1 to remove sphingolipids,21 requires pretreatment of cells for 72 hours, which is longer than hepatocytes can remain polarized in isolated cell culture.19 Disruption of the rafts through cholesterol depletion allowed InsP3Rs to redistribute into the basolateral region, with concomitant slowing of the onset and speed of Ca2+ waves. These findings may be of broader significance, because cholesterol depletion had a similar effect on Ca2+ waves in pancreatic acinar cells. Although the InsP3R is most concentrated in the apical region in both hepatocytes and pancreatic acinar cells, the receptor is almost exclusively the type II isoform in hepatocytes,8 but includes all 3 isoforms in pancreatic acinar cells.38,39 Moreover, periapical accumulation of the InsP3R appears to be a general feature of polarized epithelial cells, and has been reported in cholangiocytes,25 salivary acinar, and duct cells,39 and the nonpigmented ciliary epithelium of the eye.36 These observations suggest that lipid rafts may be necessary to localize InsP3Rs to the apical region in most or all polarized epithelia. It is unclear from the current work whether the pericanalicular, type II InsP3Rs in hepatocytes are contained in rafts within a specialized region of ER, or if the InsP3Rs instead are linked to proteins within rafts in the canalicular membrane. Lipid rafts within the ER have been described previously, and can contain proteins such as caveolin-3 and the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase,41 as well as the cellular prion protein,21 which is the precursor protein responsible for transmissible spongiform encephalopathies. However, the efficacy of mβCD in reducing ER cholesterol is not well established. Moreoever, several proteins have been identified that link the InsP3R to plasma membrane proteins. For example, protein 4.1N links the type I InsP3R to syndecan-2.11 However, protein 4.1N is not expressed in liver,14 and does not bind to the type II or III InsP3R,11 which are the periapical isoforms in hepatocytes,8 and cholangiocytes,25 respectively. Members of the Homer family of proteins also bind to the InsP3R and link it to metabotropic glutamate receptors42,43 and transient receptor potential channels.44 Homer proteins are heavily expressed in brain but only weakly expressed in liver,43 and it is not known which hepatic cell types express Homer. In addition, Homer binds to the type I InsP3R,42 but there is no evidence that it binds to either the type II or type III InsP3R. On the other hand, transient receptor potential channels in platelets coimmunoprecipitate with the type II InsP3R.45 Therefore, there is precedence for linker proteins drawing InsP3Rs to the region of the plasma membrane, but specific linker proteins for the type II InsP3R in hepatocytes and their binding partners within the canalicular membrane have not yet been identified.
The pericanalicular accumulation of InsP3Rs in polarized epithelia has been called the “trigger zone,” because it defines the subcellular region that triggers the formation of Ca2+ waves.37,46 There are 3 isoforms of the InsP3R, and no 1 isoform is required to trigger Ca2+ waves. For example, the trigger zone is comprised of type II InsP3Rs in hepatocytes,8 type III InsP3Rs in cholangiocytes,25 and all 3 isoforms in pancreatic acinar cells.38,39 InsP3 is generated by phospholipase C near plasma membrane hormone receptors, which are often on the basolateral membrane, so it is perhaps counterintuitive that Ca2+ signals instead would begin at the apical membrane. Certain biophysical properties of the type II47 and III48 InsP3Rs may explain why these receptors initiate Ca2+ waves at the trigger zone, but an alternative explanation is that clustering of InsP3Rs, which occurs in the apical region, lowers the threshold for Ca2+ release.49,50 Indeed, the rise time is prolonged and the Ca2+ wave speed is reduced in hepatocytes with decreased expression of the type II InsP3R,51 just as these parameters of Ca2+ signaling are reduced merely by redistribution of the receptor. Once initiated in the apical region, Ca2+ waves may propagate into and across the basolateral region through 1 of several mechanisms. Pancreatic acinar cells express the ryanodine receptor (RyR) Ca2+ release channel in the basolateral region,52 and the RyR is activated by Ca2+ itself, which results in Ca2+-induced Ca2+ release. Therefore, Ca2+ waves in pancreatic acinar cells are initiated by InsP3 in the trigger zone, but then propagate across the cell by RyR-mediated Ca2+-induced Ca2+ release.20,53 Other epithelia, including cholangiocytes, do not express the RyR.25 However, this cell type expresses low levels of InsP3Rs in the basolateral region.25 Because Ca2+ can coactivate the InsP3R,54 Ca2+ waves in cholangiocytes likely propagate by a modified form of Ca2+-induced Ca2+ release in which Ca2+ released from the apical trigger zone sensitizes basolateral InsP3Rs and thus allows Ca2+ waves to form on that basis. The mechanism of Ca2+ wave propagation in hepatocytes is less clear. Pharmacologic evidence using RyR inhibitors suggests that the spread of Ca2+ waves across hepatocytes depends upon serial activation of apical InsP3Rs, and then basolateral RyRs,17 similar to what occurs in pancreatic acinar cells.20 However, RT-PCR studies suggest that hepatocytes lack RyRs.8 Recently, though, a truncated but functional form of the RyR was detected in hepatocytes.55 The subcellular distribution of this novel RyR variant and its role in the formation of Ca2+ waves has yet to be determined.
Ca2+ signals regulate a number of important processes in the liver, and the spatial location of the signal within the cell determines its specificity. For example, Ca2+ signals within the nucleus have different effects on gene transcription than signals within the cytosol,2 Ca2+ signals generated near mitochondria are more effective in inducing apoptosis,3 and pericanalicular signals are thought to play a role in directing canalicular motility.56 The spatial information contained within Ca2+ waves is thought to play an essential role in epithelial secretion. Studies in pancreatic acinar cells have shown that the apical-to-basal progression of Ca2+ waves serially activates ion channels on the apical, and then basolateral membrane.6,7 The result of this coordinated series of events is to direct fluid and electrolyte secretion into the lumen.6,7 The apical accumulation of InsP3Rs observed in most polarized epithelia also permits the local Ca2+ concentration in the apical region to reach as high as 5–10 μmol/L.32 This Ca2+ concentration would be toxic elsewhere in the cell but is required to activate synaptotagmins, and thus is necessary for targeting and fusion of vesicles to the apical plasma membrane.57 Therefore, apical localization of InsP3Rs is important not only for fluid and electrolyte secretion, but for regulated insertion of transporters into the plasma membrane and for exocytosis as well. What would happen to secretion without this subcellular organization? A clue to this may be found in the little skate, Leucoraja erinacea. Hepatocytes in this primitive elasmobranch express a single isoform of the InsP3R, which is distributed uniformly throughout the cytosol.58 As a result, Ca2+ signals begin at random locations in these cells and no coordinated Ca2+ waves are observed.58 Moreover, the rate of bile secretion is nearly 2 orders of magnitude slower in skate liver than in mammalian liver.58 Another clue to the importance of apical InsP3Rs may be found in studies of cholangiocytes from cholestatic patients and in animal models of cholestasis. After bile duct ligation, rat cholangiocytes lose apical InsP3Rs, and this is associated with selective loss of Ca2+-mediated ductular bicarbonate secretion.59 Similarly, expression of apical InsP3Rs is decreased or absent in cholangiocytes from patients with primary biliary cirrhosis, sclerosing cholangitis, common bile duct obstruction, and biliary atresia, suggesting that this may represent a final common pathway for the development of cholestatic disorders.59 Further work will be needed to identify the factors that localize apical InsP3Rs to lipid microdomains in hepatocytes, and to demonstrate the importance of this level of subcellular organization for bile secretion in health and disease.
Acknowledgments
The authors thank Wayne Grant, Sohail Husain, Michele Rodrigues, Kathy Harry, and Emma Kruglov for technical assistance.
Supported by NIH Grants DK45710, DK61747, and DK34989.
Abbreviations used in this paper
- ACh
acetylcholine
- Cai2+
Cytosolic Ca2+
- ER
endoplasmic reticulum
- F0
initial fluorescence
- InsP3R
inositol 1,4,5-trisphosphate receptor
- MβCD
methyl-beta-cyclodextrin
- RyR
ryanodine receptor
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Leite MF, Nathanson MH. Calcium signaling in the liver. In: Arias IM, Boyer JL, Chisari FV, Fausto N, Schachter D, Shafritz DA, editors. The liver biology and pathobiology. 4. Philadelphia, PA: Lippincott Willliams & Wilkins; 2001. pp. 537–554. [Google Scholar]
- 2.Pusl T, Wu JJ, Zimmerman TL, Zhang L, Ehrlich BE, Berchtold MW, Hoek JB, Karpen SJ, Nathanson MH, Bennett AM. Epidermal growth factor-mediated activation of the ETS domain transcription factor Elk-1 requires nuclear calcium. J Biol Chem. 2002;277:27517–27527. doi: 10.1074/jbc.M203002200. [DOI] [PubMed] [Google Scholar]
- 3.Mendes CC, Gomes DA, Thompson M, Souto NC, Goes TS, Goes AM, Rodrigues MA, Gomez MV, Nathanson MH, Leite MF. The type III inositol 1,4,5-trisphosphate receptor preferentially transmits apoptotic Ca2+ signals into mitochondria. J Biol Chem. 2005;280:40892–40900. doi: 10.1074/jbc.M506623200. [DOI] [PubMed] [Google Scholar]
- 4.Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392:933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- 5.Li W, Llopis J, Whitney M, Zlokarnik G, Tsien RY. Cell-permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression. Nature. 1998;392:936–941. doi: 10.1038/31965. [DOI] [PubMed] [Google Scholar]
- 6.Ito K, Miyashita Y, Kasai H. Micromolar and submicromolar Ca2+ spikes regulating distinct cellular functions in pancreatic acinar cells. EMBO J. 1997;16:242–251. doi: 10.1093/emboj/16.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kasai H, Augustine GJ. Cytosolic Ca2+ gradients triggering unidirectional fluid secretion from exocrine pancreas. Nature. 1990;348:735–738. doi: 10.1038/348735a0. [DOI] [PubMed] [Google Scholar]
- 8.Hirata K, Pusl T, O’Neill AF, Dranoff JA, Nathanson MH. The type II inositol 1,4,5-trisphosphate receptor can trigger Ca2+ waves in rat hepatocytes. Gastroenterology. 2002;122:1088–1100. doi: 10.1053/gast.2002.32363. [DOI] [PubMed] [Google Scholar]
- 9.Wojcikiewicz RJ. Type I, II, and III inositol 1,4,5-trisphosphate receptors are unequally susceptible to down-regulation and are expressed in markedly different proportions in different cell types. J Biol Chem. 1995;270:11678–11683. doi: 10.1074/jbc.270.19.11678. [DOI] [PubMed] [Google Scholar]
- 10.Zhang S, Mizutani A, Hisatsune C, Higo T, Bannai H, Nakayama T, Hattori M, Mikoshiba K. Protein 4.1N is required for translocation of inositol 1,4,5-trisphosphate receptor type 1 to the basolateral membrane domain in polarized Madin-Darby canine kidney cells. J Biol Chem. 2003;278:4048–4056. doi: 10.1074/jbc.M209960200. [DOI] [PubMed] [Google Scholar]
- 11.Maximov A, Tang TS, Bezprozvanny I. Association of the type 1 inositol (1,4,5)-trisphosphate receptor with 4.1N protein in neurons. Mol Cell Neurosci. 2003;22:271–283. doi: 10.1016/s1044-7431(02)00027-1. [DOI] [PubMed] [Google Scholar]
- 12.Parra M, Gascard P, Walensky LD, Snyder SH, Mohandas N, Conboy JG. Cloning and characterization of 4.1G (EPB41L2), a new member of the skeletal protein 4.1 (EPB41) gene family. Genomics. 1998;49:298–306. doi: 10.1006/geno.1998.5265. [DOI] [PubMed] [Google Scholar]
- 13.Walensky LD, Blackshaw S, Liao D, Watkins CC, Weier HU, Parra M, Huganir RL, Conboy JG, Mohandas N, Snyder SH. A novel neuron-enriched homolog of the erythrocyte membrane cytoskeletal protein 4.1. J Neurosci. 1999;19:6457–6467. doi: 10.1523/JNEUROSCI.19-15-06457.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sehgal S, Guerra MT, Kruglov EA, Wang J, Nathanson MH. Protein 4.1N does not interact with the inositol 1,4,5-trisphosphate receptor in an epithelial cell line. Cell Calcium. 2005;38:469–480. doi: 10.1016/j.ceca.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 15.Mazzone A, Tietz P, Jefferson J, Pagano R, LaRusso NF. Isolation and characterization of lipid microdomains from apical and basolateral plasma membranes of rat hepatocytes. Hepatology. 2006;43:287–296. doi: 10.1002/hep.21039. [DOI] [PubMed] [Google Scholar]
- 16.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 17.Nathanson MH, Burgstahler AD, Fallon MB. Multistep mechanism of polarized Ca2+ wave patterns in hepatocytes. Am J Physiol. 1994;267:G338–G349. doi: 10.1152/ajpgi.1994.267.3.G338. [DOI] [PubMed] [Google Scholar]
- 18.Graf J, Gautam A, Boyer JL. Isolated rat hepatocyte couplets: a primary secretory unit for electrophysiologic studies of bile secretory function. Proc Natl Acad Sci U S A. 1984;81:6516–6520. doi: 10.1073/pnas.81.20.6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gautam A, Ng OC, Boyer JL. Isolated rat hepatocyte couplets in short-term culture: structural characteristics and plasma membrane reorganization. Hepatology. 1987;7:216–223. doi: 10.1002/hep.1840070203. [DOI] [PubMed] [Google Scholar]
- 20.Leite MF, Burgstahler AD, Nathanson MH. Ca2+ waves require sequential activation of inositol trisphosphate receptors and ryanodine receptors in pancreatic acini. Gastroenterology. 2002;122:415–427. doi: 10.1053/gast.2002.30982. [DOI] [PubMed] [Google Scholar]
- 21.Sarnataro D, Campana V, Paladino S, Stornaiuolo M, Nitsch L, Zurzolo C. PrP(C) association with lipid rafts in the early secretory pathway stabilizes its cellular conformation. Mol Biol Cell. 2004;15:4031–4042. doi: 10.1091/mbc.E03-05-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nyasae LK, Hubbard AL, Tuma PL. Transcytotic efflux from early endosomes is dependent on cholesterol and glycosphingolipids in polarized hepatic cells. Mol Biol Cell. 2003;14:2689–2705. doi: 10.1091/mbc.E02-12-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christian AE, Haynes MP, Phillips MC, Rothblat GH. Use of cyclodextrins for manipulating cellular cholesterol content. J Lipid Res. 1997;38:2264–2272. [PubMed] [Google Scholar]
- 24.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.Hirata K, Dufour JF, Shibao K, Knickelbein R, O’Neill AF, Bode HP, Cassio D, St Pierre MV, Larusso NF, Leite MF, Nathanson MH. Regulation of Ca(2+) signaling in rat bile duct epithelia by inositol 1,4,5-trisphosphate receptor isoforms. Hepatology. 2002;36:284–296. doi: 10.1053/jhep.2002.34432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leventhal AR, Chen W, Tall AR, Tabas I. Acid sphingomyelinase-deficient macrophages have defective cholesterol trafficking and efflux. J Biol Chem. 2001;276:44976–44983. doi: 10.1074/jbc.M106455200. [DOI] [PubMed] [Google Scholar]
- 27.Schlosser SF, Burgstahler AD, Nathanson MH. Isolated rat hepatocytes can signal to other hepatocytes and bile duct cells by release of nucleotides. Proc Natl Acad Sci U S A. 1996;93:9948–9953. doi: 10.1073/pnas.93.18.9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nathanson MH, Burgstahler AD. Coordination of hormone-induced calcium signals in isolated rat hepatocyte couplets: demonstration with confocal microscopy. Mol Biol Cell. 1992;3:113–121. doi: 10.1091/mbc.3.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nathanson MH, Padfield PJ, O’Sullivan AJ, Burgstahler AD, Jamieson JD. Mechanism of Ca2+ wave propagation in pancreatic acinar cells. J Biol Chem. 1992;267:18118–18121. [PubMed] [Google Scholar]
- 30.Leite MF, Hirata K, Pusl T, Burgstahler AD, Okazaki K, Ortega JM, Goes AM, Prado MA, Spray DC, Nathanson MH. Molecular basis for pacemaker cells in epithelia. J Biol Chem. 2002;277:16313–16323. doi: 10.1074/jbc.M109207200. [DOI] [PubMed] [Google Scholar]
- 31.Echevarria W, Leite MF, Guerra MT, Zipfel WR, Nathanson MH. Regulation of calcium signals in the nucleus by a nucleoplasmic reticulum. Nat Cell Biol. 2003;5:440–446. doi: 10.1038/ncb980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fadool DA, Ache BW. Plasma membrane inositol 1,4,5-trisphosphate-activated channels mediate signal transduction in lobster olfactory receptor neurons. Neuron. 1992;9:907–918. doi: 10.1016/0896-6273(92)90243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan AA, Steiner JP, Klein MG, Schneider MF, Snyder SH. IP3 receptor: localization to plasma membrane of T cells and cocapping with the T cell receptor. Science. 1992;257:815–818. doi: 10.1126/science.1323146. [DOI] [PubMed] [Google Scholar]
- 34.Keller P, Simons K. Cholesterol is required for surface transport of influenza virus hemagglutinin. J Cell Biol. 1998;140:1357–1367. doi: 10.1083/jcb.140.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lievremont JP, Hill AM, Tran D, Coquil JF, Stelly N, Mauger JP. Intracellular calcium stores and inositol 1,4,5-trisphosphate receptor in rat liver cells. Biochem J. 1996;314(Pt 1):189–197. doi: 10.1042/bj3140189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirata K, Nathanson MH, Burgstahler AD, Okazaki K, Mattei E, Sears ML. Relationship between inositol 1,4,5-trisphosphate receptor isoforms and subcellular Ca2+ signaling patterns in non-pigmented ciliary epithelia. Invest Ophthalmol Vis Sci. 1999;40:2046–2053. [PubMed] [Google Scholar]
- 37.Nathanson MH, Fallon MB, Padfield PJ, Maranto AR. Localization of the type 3 inositol 1,4,5-trisphosphate receptor in the Ca2+ wave trigger zone of pancreatic acinar cells. J Biol Chem. 1994;269:4693–4696. [PubMed] [Google Scholar]
- 38.Yule DI, Ernst SA, Ohnishi H, Wojcikiewicz RJ. Evidence that zymogen granules are not a physiologically relevant calcium pool. Defining the distribution of inositol 1,4,5-trisphosphate receptors in pancreatic acinar cells. J Biol Chem. 1997;272:9093–9098. doi: 10.1074/jbc.272.14.9093. [DOI] [PubMed] [Google Scholar]
- 39.Lee MG, Xu X, Zeng W, Diaz J, Wojcikiewicz RJ, Kuo TH, Wuytack F, Racymaekers L, Muallem S. Polarized expression of Ca2+ channels in pancreatic and salivary gland cells. Correlation with initiation and propagation of [Ca2+]i waves. J Biol Chem. 1997;272:15765–15770. doi: 10.1074/jbc.272.25.15765. [DOI] [PubMed] [Google Scholar]
- 40.Munro S. Lipid rafts: elusive or illusive? Cell. 2003;115:377–388. doi: 10.1016/s0092-8674(03)00882-1. [DOI] [PubMed] [Google Scholar]
- 41.Li C, Duan W, Yang F, Zhang X. Caveolin-3-anchored microdomains at the rabbit sarcoplasmic reticulum membranes. Biochem Biophys Res Commun. 2006;344:1135–1140. doi: 10.1016/j.bbrc.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 42.Tu JC, Xiao B, Yuan JP, Lanahan AA, Leoffert K, Li M, Linden DJ, Worley PF. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron. 1998;21:717–726. doi: 10.1016/s0896-6273(00)80589-9. [DOI] [PubMed] [Google Scholar]
- 43.Xiao B, Tu JC, Petralia RS, Yuan JP, Doan A, Breder CD, Ruggiero A, Lanahan AA, Wenthold RJ, Worley PF. Homer regulates the association of group 1 metabotropic glutamate receptors with multivalent complexes of homer-related, synaptic proteins. Neuron. 1998;21:707–716. doi: 10.1016/s0896-6273(00)80588-7. [DOI] [PubMed] [Google Scholar]
- 44.Yuan JP, Kiselyov K, Shin DM, Chen J, Shcheynikov N, Kang SH, Dehoff MH, Schwarz MK, Seeburg PH, Muallem S, Worley PF. Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell. 2003;114:777–789. doi: 10.1016/s0092-8674(03)00716-5. [DOI] [PubMed] [Google Scholar]
- 45.Rosado JA, Sage SO. Coupling between inositol 1,4,5-trisphosphate receptors and human transient receptor potential channel 1 when intracellular Ca2+ stores are depleted. Biochem J. 2000;350(Pt 3):631–635. [PMC free article] [PubMed] [Google Scholar]
- 46.Kasai H, Li YX, Miyashita Y. Subcellular distribution of Ca2+ release channels underlying Ca2+ waves and oscillations in exocrine pancreas. Cell. 1993;74:669–677. doi: 10.1016/0092-8674(93)90514-q. [DOI] [PubMed] [Google Scholar]
- 47.Ramos-Franco J, Fill M, Mignery GA. Isoform-specific function of single inositol 1,4,5-trisphosphate receptor channels. Biophys J. 1998;75:834–839. doi: 10.1016/S0006-3495(98)77572-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hagar RE, Burgstahler AD, Nathanson MH, Ehrlich BE. Type III InsP3 receptor channel stays open in the presence of increased calcium. Nature. 1998;396:81–84. doi: 10.1038/23954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yao Y, Choi J, Parker I. Quantal puffs of intracellular Ca2+ evoked by inositol trisphosphate in Xenopus oocytes. J Physiol. 1995;482(Pt 3):533–553. doi: 10.1113/jphysiol.1995.sp020538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swillens S, Dupont G, Combettes L, Champeil P. From calcium blips to calcium puffs: theoretical analysis of the requirements for interchannel communication. Proc Natl Acad Sci U S A. 1999;96:13750–13755. doi: 10.1073/pnas.96.24.13750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hernandez E, Leite MF, Guerra MT, Kruglov EA, Bruna-Romero O, Rodrigues MA, Gomes DA, Giordano FJ, Dranoff JA, Nathanson MH. The spatial distribution of inositol 1,4,5-trisphosphate receptor isoforms shapes Ca2+ waves. J Biol Chem. doi: 10.1074/jbc.M700746200. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leite MF, Dranoff JA, Gao L, Nathanson MH. Expression and subcellular localization of the ryanodine receptor in rat pancreatic acinar cells. Biochem J. 1999;337(Pt 2):305–309. [PMC free article] [PubMed] [Google Scholar]
- 53.Husain SZ, Prasad P, Grant WM, Kolodecik TR, Nathanson MH, Gorelick FS. The ryanodine receptor mediates early zymogen activation in pancreatitis. Proc Natl Acad Sci U S A. 2005;102:14386–14391. doi: 10.1073/pnas.0503215102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- 55.Pierobon N, Renard-Rooney DC, Gaspers LD, Thomas AP. Ryanodine receptors in liver. J Biol Chem. 2006;281:34086–34095. doi: 10.1074/jbc.M607788200. [DOI] [PubMed] [Google Scholar]
- 56.Nathanson MH, Burgstahler AD, Mennone A, Fallon MB, Gonzalez CB, Saez JC. Ca2+ waves are organized among hepatocytes in the intact organ. Am J Physiol. 1995;269:G167–G171. doi: 10.1152/ajpgi.1995.269.1.G167. [DOI] [PubMed] [Google Scholar]
- 57.Fernandez-Chacon R, Konigstorfer A, Gerber SH, Garcia J, Matos MF, Stevens CF, Brose N, Rizo J, Rosenmund C, Sudhof TC. Synaptotagmin I functions as a calcium regulator of release probability. Nature. 2001;410:41–49. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- 58.Nathanson MH, O’Neill AF, Burgstahler AD. Primitive organization of cytosolic Ca(2+) signals in hepatocytes from the little skate Raja erinacea. J Exp Biol. 1999;202:3049–3056. doi: 10.1242/jeb.202.22.3049. [DOI] [PubMed] [Google Scholar]
- 59.Shibao K, Hirata K, Robert ME, Nathanson MH. Loss of inositol 1,4,5-trisphosphate receptors from bile duct epithelia is a common event in cholestasis. Gastroenterology. 2003;125:1175–1187. doi: 10.1016/s0016-5085(03)01201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]