Abstract
The highly infectious bacterium Francisella tularensis is a facultative intracellular pathogen and the causative agent of tularemia. TolC, which is an outer membrane protein involved in drug efflux and type I protein secretion, is required for the virulence of the F. tularensis live vaccine strain (LVS) in mice. Here, we show that an LVS ΔtolC mutant colonizes livers, spleens, and lungs of mice infected intradermally or intranasally, but it is present at lower numbers in these organs than in those infected with the parental LVS. For both routes of infection, colonization by the ΔtolC mutant is most severely affected in the lungs, suggesting that TolC function is particularly important in this organ. The ΔtolC mutant is hypercytotoxic to murine and human macrophages compared to the wild-type LVS, and it elicits the increased secretion of proinflammatory chemokines from human macrophages and endothelial cells. Taken together, these data suggest that TolC function is required for F. tularensis to inhibit host cell death and dampen host immune responses. We propose that, in the absence of TolC, F. tularensis induces excessive host cell death, causing the bacterium to lose its intracellular replicative niche. This results in lower bacterial numbers, which then are cleared by the increased innate immune response of the host.
Francisella tularensis is the etiological agent of tularemia. F. tularensis is classified as a category A agent of bioterrorism by the U.S. Centers for Disease Control and Prevention (http://emergency.cdc.gov/agent/agentlist-category.asp) due to its low infectious dose, ease of aerosol dissemination, and capacity to cause high morbidity and mortality (19). There are two clinically relevant subspecies of F. tularensis: subsp. tularensis, which is extremely pathogenic in humans, and subsp. holarctica, which causes a less severe clinical presentation (48). The most severe form of the disease is pneumonic tularemia caused by the inhalation of aerosolized F. tularensis subsp. tularensis (19). The F. tularensis subsp. holarctica-derived live vaccine strain (LVS) was used for many years as the vaccination against tularemia. However, the basis for its attenuation is unknown, and it is no longer in use as a vaccine (46). The LVS is highly virulent in mice, where it causes a disease closely resembling human tularemia (30). These features make the LVS an important model for the study of tularemia. An additional Francisella species, F. novicida, causes disease only in immunocompromised individuals. F. novicida, like the LVS, is highly virulent in mice and widely used as a model of tularemia (20).
F. tularensis is a Gram-negative, facultative intracellular pathogen (50). Although factors important for the virulence of F. tularensis are beginning to be identified, the molecular mechanisms behind the extreme pathogenicity of this organism still are largely unknown. In vivo, F. tularensis is a stealth pathogen, evading host cell defenses and dampening host proinflammatory responses. F. tularensis produces an unusual lipopolysaccharide that has low toxicity and does not activate host cells in a TLR4-dependent manner (4, 22). A critical aspect of the pathogenesis of F. tularensis is its ability to escape the phagosome and replicate within the cytosol of a variety of host cells, including both murine and human macrophages and dendritic cells (2, 3, 16, 25, 49). Although F. tularensis does have an extracellular phase (24), it is thought that cytosolic replication allows the bacteria to grow to large numbers while avoiding detection by the host immune system.
Host cells respond to F. tularensis invasion by inducing cell death pathways, including apoptosis and pyroptosis (32, 38). In the intrinsic apoptotic pathway, cytochrome c is released from mitochondria into the cytosol, leading to caspase-9 activation and ultimately to the activation of effector caspases such as caspase-3 and -7 (10). In pyroptosis, caspase-1 is activated through the inflammasome complex, resulting in the release of proinflammatory cytokines such as interleukin-1ß (IL-1ß) (6, 32). Lai and coworkers demonstrated that the infection of murine J774 macrophage-like cells with the LVS activated the intrinsic apoptotic pathway as early as 12 h postinfection. Activated caspase-3, but not caspase-1, was detected in the infected cells (38). In contrast, Mariathasan et al. found that the infection of preactivated murine peritoneal macrophages by either the LVS or strain U112 (F. novicida) triggered pyroptosis and the release of IL-1ß (42). In both studies, the induction of cell death was dependent upon the bacteria escaping the phagosome and initiating cytosolic replication. Weiss and colleagues isolated mutants of strain U112 that were attenuated in vivo and caused increased cell death in tissue culture compared to that caused by wild-type U112 (53). This suggests that although host cells initiate death pathways in response to F. tularensis infection, the bacteria has the ability to actively reduce cell death, and this is important for virulence.
In addition to triggering death pathways, host cells respond to invading bacteria by mounting a proinflammatory response to alert neighboring cells of the impending bacterial threat (17). However, F. tularensis has been shown to actively suppress these innate host responses. Telepnev and coworkers showed that the LVS disrupted toll-like receptor signaling and blocked the secretion of the proinflammatory cytokines tumor necrosis factor alpha (TNF-α) and IL-1ß by murine and human macrophages (51, 52). Similarly, Bosio and colleagues showed that the LVS inhibited the innate immune response of murine pulmonary dendritic cells to bacterial ligands, and the infection of mice with the fully virulent Schu4 strain (F. tularensis subsp. tularensis) caused an overall state of immunosuppression in the lungs (8, 9).
The genome analysis of F. tularensis identified only a few potential virulence factors, suggesting that the bacterium uses novel factors to achieve its high level of pathogenicity (40). Unique to F. tularensis is a 33.9-kb region of DNA termed the Francisella pathogenicity island (FPI) (29, 40, 45). The FPI encodes genes that are essential for intracellular survival and virulence, including iglABCD and pdpABCD (45). F. tularensis lacks type III and IV secretion systems, which is surprising considering its intracellular nature. These secretion systems commonly are used by intracellular pathogens to deliver effector proteins inside host cells to manipulate host cell responses (14, 26). F. tularensis does contain genes encoding a type IV pilus biogenesis system that also functions in the secretion of soluble proteins by a type II-like mechanism and that are important for virulence (12, 31, 54). Finally, F. tularensis appears to contain a functioning type I secretion system that is critical for pathogenesis (28).
Type I secretion systems function in the secretion of a variety of toxins and other virulence factors directly from the cytoplasm to the extracellular milieu in a single energized step (33, 37). The type I system consists of three separate components: an outer membrane channel-forming protein, a periplasmic adaptor or membrane fusion protein, and an inner membrane pump that typically belongs to the ATP-binding cassette family. The TolC protein of Escherichia coli, which functions in hemolysin secretion, is the prototypical outer membrane channel component (37). In addition to protein secretion, TolC functions in the efflux of small noxious molecules, conferring multidrug resistance (37). F. tularensis contains three TolC paralogs, TolC, FtlC, and SilC, with TolC and FtlC exhibiting significant homology to the E. coli TolC protein (28, 35). In a previous study we created tolC and ftlC deletion mutants in the F. tularensis LVS (28). We found that both TolC and FtlC participate in multidrug resistance in F. tularensis, but only the ΔtolC mutant was attenuated for virulence in mice by the intradermal route. Thus, tolC is a critical virulence factor of F. tularensis and likely functions in type I secretion in addition to multidrug efflux.
Here, we delineate the molecular mechanisms behind the attenuation of the LVS ΔtolC mutant in mice infected by both the intradermal and intranasal routes. In vivo organ burden assays revealed that the ΔtolC strain is decreased for the bacterial colonization of liver, spleen, and most prominently, lungs. In vitro experiments revealed that the ΔtolC mutant is hypercytotoxic to murine macrophages, causing increased apoptosis via a mechanism involving caspase-3 but not caspase-1. In addition, the LVS ΔtolC mutant was hypercytotoxic toward human macrophages and elicited the significantly increased secretion of the proinflammatory chemokines CXCL8 (also known as IL-8) and CCL2 (also known as monocyte chemoattractant protein [MCP-1]). Taken together, these data demonstrate a critical role for TolC, likely via a TolC-secreted toxin(s), in the successful intracellular lifestyle of F. tularensis, its ability to evade host innate immune responses, and its overall virulence.
MATERIALS AND METHODS
Bacteria.
The F. tularensis LVS ΔtolC mutant strain DTH1 and plasmid-complemented strain DTH1/pGPTA (tolC+) were described previously (28). Unless otherwise noted, the parental LVS and mutant strains were grown on Mueller-Hinton II chocolate agar plates (MHC; BD Biosciences) or in Mueller-Hinton broth (MHB; BD Biosciences) as described previously (12).
Mouse infection experiments and organ burden assays.
For intranasal infections of mice, stocks were prepared by growing bacteria as a lawn on six-cysteine heart agar (CHaB; Difco) plates at 37°C, 5% CO2. Bacteria then were scraped from the plates, washed with phosphate-buffered saline (PBS), and resuspended in 15 ml of MHB supplemented with 10% sucrose. Serial dilutions then were made and aliquots frozen at −80°C. Bacterial counts for each dilution were determined by CFU assay.
For lethal intranasal infections, groups of five C3H/HeN mice (6 to 8 weeks old; Jackson Laboratories) were inoculated intranasally with 20 μl of a thawed bacterial aliquot that was chosen to provide a dose of approximately 105 CFU. The actual infectious doses were determined by retrospective CFU counts. The animals were observed twice daily and monitored for survival for 18 days.
For the intradermal and intranasal organ burden assays, groups of 15 C3H/HeN mice either were intradermally or intranasally inoculated with a sublethal dose of the LVS or DTH1, and for both experiments an additional mouse was infected with PBS to serve as a control. For the intradermal infections, the bacteria were grown in MHB overnight to an optical density at 600 nm (OD600) of 0.2 and diluted in PBS to inoculate approximately 105 microorganisms per mouse in a 100-μl injection. For the intranasal infections, stocks prepared and administered as described above were chosen to inoculate 5 × 103 CFU per mouse. Actual infectious doses were determined for all infections by CFU counts. Five mice from each group were sacrificed on days 2, 4, and 7 postinfection (p.i.) for the intradermal infections and on days 3, 5, and 9 p.i. for the intranasal infections, and the necropsy of the lung, liver, and spleen was performed. The organs were homogenized in 1 ml PBS using Stomacher bags (Seward), and then neat homogenate and serial dilutions were plated to determine the CFU.
For histopathology, organs were fixed in 10% neutral-buffered formalin, embedded in paraffin, sectioned at 1 to 4 μm, stained with hematoxylin and eosin, dehydrated in graded alcohols, cleared with Histoclear (National Diagnostics), and mounted on glass slides. The sectioning and staining procedures were performed by McClain Laboratories (Smithtown, NY). The tissue sections were examined by light microscopy.
All animal research protocols were approved by the Institutional Animal Care and Use Committee of Stony Brook University.
Preparation of cells for tissue culture and bacterial infections.
Murine bone marrow-derived macrophages (muBMDM) were obtained as described previously (23) from the femurs of female wild-type C3H/HeN or C57BL/6 mice (Jackson Laboratories) or from caspase-1−/− C57BL/6 mice (41). The muBMDM were seeded in 24-well plates (Corning) at concentrations of 1.5 × 105 cells per well and used for experiments the following day. For each experiment, a single colony of a freshly streaked strain was picked and grown in MHB to late-log phase (16 to 18 h). One-ml aliquots of the bacterial cultures were centrifuged, resuspended in bone marrow medium (BMM) (23), and added at a multiplicity of infection (MOI) of 50 to the muBMDM, and the plates were centrifuged (5 min, 800 × g) to facilitate contact between the macrophages and bacteria. Bacterial concentrations initially were estimated by the OD600 of the suspension culture, and actual numbers of viable bacteria were determined by CFU counts. After 2 h of coculture at 37°C, 5% CO2, the infected muBMDM were washed three times with PBS and incubated with 5 μg/ml gentamicin for 1 h to kill any remaining extracellular bacteria. The cells then were washed three times with PBS, 1 ml of fresh BMM without antibiotics was added back, and the plates were incubated until the designated time points.
Human MDM (huMDM) were isolated as described previously (23) from healthy human donors and plated at 2 × 105 cells/well in 24-well plates (Corning). The cells were cultured for 5 days in the presence of 10 ng/ml macrophage colony stimulating factor (Sigma) and infected the following day. For cytotoxicity (lactate dehydrogenase [LDH]) experiments, the infection of huMDM with F. tularensis strains was carried out as described above for the muBMDM. For chemokine detection experiments, bacterial inocula were prepared as described above, but the huMDM were infected at an MOI of 25.
Human umbilical vein endothelial cells (HUVEC) were isolated by collagenase perfusion as previously described (34) and seeded in a 48-well plate (BD Biosciences) at a density of 2.3 × 105 cells/well. Bacterial inocula were prepared as described above for the muBMDM infections and added to the HUVEC at an MOI of 75 without centrifugation, and cultures were incubated for 24 h.
LDH assay.
Macrophage infection assays were conducted as described above. At designated time points, conditioned media were collected and analyzed for LDH release using the CytoTox 96 NonRadioactive Cytotoxicity Assay (Promega) according to the manufacturer's protocols. Background LDH release was measured in medium conditioned by uninfected cells, while total LDH release (equal to 100%) was measured from uninfected cells that were lysed by freezing and thawing. The percentage of LDH release was calculated by subtracting the background LDH release value from the LDH release value of the samples, and this number then was divided by the total LDH release value and multiplied by 100.
TUNEL staining.
Macrophages infected as described above were analyzed for apoptosis by terminal deoxynucleotidyltransferase-mediated UTP-biotin nick end labeling (TUNEL) staining using the In Situ Cell Death Detection kit, TMR red (Roche), according to the manufacturer's protocols. The cells were visualized by fluorescence microscopy using a Zeiss Axiovert S100 microscope. Images were captured using a Spot camera (Diagnostic Instruments) and processed with Adobe Photoshop. To calculate the percentage of TUNEL-positive cells, the number of TUNEL-positive cells was divided by the total number of cells in 10 different fields.
Caspase-3 detection.
Macrophages were isolated as described above and seeded in a 96-well, white-walled plate (Corning) at a concentration of 2 × 104 cells/well. Three wells each were used for the LVS, DTH1, and DTH1/pGPTA infections as well as for an uninfected negative control. Macrophage infections were conducted as described above. At 17 h p.i., activated caspase-3/-7 was detected using the Caspase-Glo 3/7 assay (Promega) according to the manufacturer's instructions. Caspase-3/-7 activation was measured using a FLUOstar Optima luminometer (BMG Labtech).
To detect mature caspase-3, infected muBMDM were stained with a rabbit polyclonal antibody targeted specifically against cleaved caspase-3 (Trevigen). muBMDM were infected as described above. At 17 and 24 h p.i., the cells were washed with sterile PBS, fixed with paraformaldehyde for 1 h at 25°C, permeabilized with 0.1% Triton X-100 for 10 min, washed with PBS, and incubated overnight at 4°C with the anti-cleaved caspase-3 antibody diluted 1:500 in PBS plus 1% bovine serum albumin (BSA). The cells were washed three times with PBS and stained with a tetramethyl rhodamine isothiocyanate (TRITC)-conjugated, anti-rabbit IgG secondary antibody (Invitrogen) diluted 1:1,000 in PBS plus 1% BSA. The caspase-3-positive cells were quantified using fluorescence microscopy as described for TUNEL staining.
Detection of CXCL8, CCL2, and IL-1β secretion.
muBMDM, huMDM, and HUVEC infections were conducted as described above. At 24 h p.i., the conditioned media from the various wells were clarified by centrifugation (12,000 × g, 10 min) and stored at −80°C until assayed. Enzyme-linked immunosorbent assay (ELISA) kits (Antigenix) were used according to the manufacturer's instructions to detect CXCL8 (IL-8), IL-1β, and CCL2 (MCP-1) release from the human cells. Quantikine ELISA kits (R&D Systems) were used to detect IL-1β release from the murine macrophages.
Statistical analysis.
The CFU/g results obtained from the organ burden assays were compared using the Mann-Whitney test for nonparametric data with one-tailed probability (P) values. The mouse survival curves were compared using the log-rank test. All other results were analyzed for significance using data obtained from three independent experiments with multiple replicates unless otherwise stated. P values were calculated by one-way analysis of variance and Tukey's multiple-comparison posttest. Statistical calculations were performed using Prism 4.0 (GraphPad Software). P < 0.05 was considered significant.
RESULTS
The LVS ΔtolC exhibits decreased colonization within the lung, liver, and spleen of mice infected by the intradermal route.
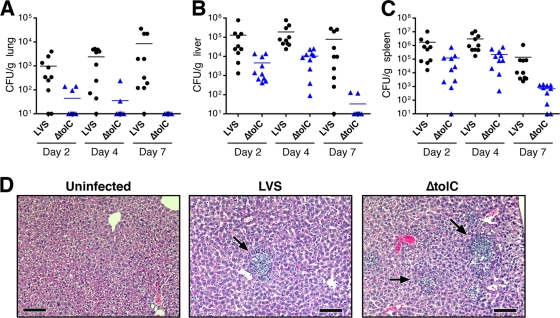
We previously found that TolC is required for the virulence of the LVS in the mouse model of tularemia by the intradermal route (28). To understand the basis for the attenuation of the ΔtolC mutant, we analyzed the dissemination to and colonization of the livers, spleens, and lungs of mice infected with sublethal doses of the mutant or parental strains. Mice inoculated intradermally with 105 CFU were sacrificed on days 2, 4, and 7 postinfection (p.i.), and organs were removed for bacterial enumeration and histology. As shown in Fig. 1, both the LVS and LVS ΔtolC were detected in all three organs by day 2 p.i., indicating the dissemination of the mutant strain. For both strains, bacterial loads were highest in the spleen and peaked at day 4, after which bacterial colonization decreased (Fig. 1C). Notably, the ΔtolC strain exhibited the decreased colonization of liver, spleen, and lung compared to that of mice infected with the LVS (Fig. 1). In both the liver and spleen, CFU for the ΔtolC mutant were consistently 1 to 2 logs lower than those obtained with the parental LVS. The largest differences in colonization were seen on day 7 p.i., indicating that the tolC mutant was being cleared more rapidly from the mice than the wild-type LVS. The CFU of the ΔtolC mutant in the liver and spleen increased from days 2 to 4 p.i., similarly to the wild-type strain (Fig. 1B and C). Therefore, the mutant is capable of replicating within these tissues. In agreement with these observations, the analysis of liver sections from day 4 p.i. showed that the ΔtolC mutant formed characteristic granuloma-like lesions similarly to the wild-type LVS (Fig. 1D) (47). Spleens from infected mice also exhibited comparable pathology for both the LVS and ΔtolC mutant (data not shown).
FIG. 1.
LVS ΔtolC is defective for colonizing the organs of mice inoculated by the intradermal route. C3H/HeN mice were infected intradermally with a sublethal dose (105 CFU) of the LVS or ΔtolC mutant. Organs were harvested on days 2, 4, and 7 p.i., and CFU/g of organ weight were determined for the lung (A), liver (B), and spleen (C). The limit of detection per organ was 10 CFU. Two independent experiments with 5 mice per time point per bacterial strain were performed, and these data were combined. The bars indicate mean CFU values. Colonization by the ΔtolC mutant was significantly decreased compared to that of the wild-type LVS at each time point in each organ (P < 0.01). (D) Hematoxylin- and eosin-stained sections obtained from infected livers harvested at day 4 p.i. show that the wild-type LVS and ΔtolC mutant cause similar pathology. Arrows indicate granuloma-like lesions. Scale bars = 100 μm.
The in vivo growth defect of the ΔtolC mutant was particularly prominent in the lungs. CFU were obtained from only 3 of 10 mice on day 2 p.i., and whereas CFU for the wild-type LVS increased from days 2 to 7, CFU for the mutant decreased during the course of the experiment to undetectable levels (Fig. 1A). Thus, in contrast to the colonization of liver and spleen by the ΔtolC strain, the mutant showed no evidence for replication within the lung. The analysis of lung sections taken on day 4 p.i. did not reveal much pathology for either the wild-type or ΔtolC LVS compared to that of uninfected controls (data not shown). This was not surprising given the low overall bacterial numbers in the lung (Fig. 1A) and the fact that F. tularensis infections cause only limited lung pathology (18).
The LVS ΔtolC is attenuated for the infection of mice by the intranasal route.
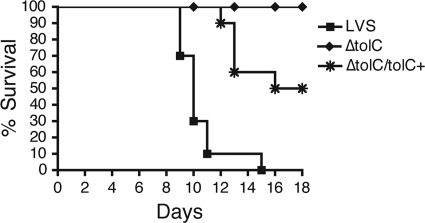
The analysis of intradermally infected mice suggested that the defect of the LVS ΔtolC mutant was most severe in the lung. To assess directly the role of TolC in lung colonization, we first analyzed the virulence of the mutant and wild-type LVS in mice via the intranasal route of infection. Mice were inoculated with a lethal dose of 105 CFU of the LVS or ΔtolC mutant and monitored for survival for 18 days. All mice infected with the LVS died as a result of the infection by day 15 (Fig. 2). In contrast, the ΔtolC mutant was highly attenuated, with no mice succumbing to infection during the course of the experiment. The complementation of the LVS ΔtolC mutant with a tolC expression plasmid restored virulence, with 50% of mice dying by day 18 (Fig. 2). These results demonstrate that TolC is critical for the virulence of F. tularensis by the intranasal route.
FIG. 2.
TolC is required for virulence of the LVS in mice by the intranasal route. C3H/HeN mice were infected intranasally with a lethal dose (1 × 105 CFU) of the LVS, ΔtolC, or complemented strain (ΔtolC tolC+). The animals were monitored for survival for 18 days. Two independent experiments with 5 mice per bacterial strain were performed, and the data were combined. The ΔtolC mutant was significantly attenuated compared to the parental LVS (P < 0.0001), and complementation significantly restored virulence to the ΔtolC mutant (P < 0.01).
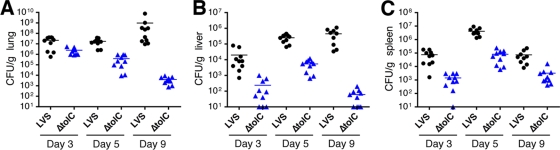
We next conducted an organ burden analysis. Mice inoculated intranasally with sublethal doses of 5 × 103 CFU were sacrificed on days 3, 5, and 9 p.i., and the lungs, livers, and spleens were harvested for bacterial enumeration and histology. As shown in Fig. 3, CFU for the ΔtolC mutant were consistently one or more logs lower than those for the parental LVS. Bacterial loads were highest in the lung, as expected for intranasal inoculation, and both the LVS and ΔtolC mutant disseminated from the lung to the liver and spleen. CFU for both strains peaked in the liver and spleen on day 5 p.i. and then stayed relatively constant or decreased from days 5 to 9 (Fig. 3B and C). Liver and spleen CFU for the tolC mutant increased from days 3 to 5 p.i., indicating the ability of the mutant to replicate in these organs, but to lower numbers than those of the wild-type LVS. By day 9 p.i., numbers for the ΔtolC mutant dropped significantly, particularly in the liver, indicating the greater clearance of the mutant compared to that of the parental LVS. As seen with the intradermal infections, the phenotype of the ΔtolC mutant was strongest in the lung. In this organ, the mutant did not increase in CFU from days 3 to 5 p.i. but instead decreased steadily throughout the duration of the experiment (Fig. 3A). This is in contrast to the wild-type LVS, which increased in CFU from days 3 to 9. The ΔtolC mutant apparently was able to replicate in the lung early after inoculation, since the CFU/g lung tissue obtained at day 3 p.i. were higher than those obtained from the input dose alone (mouse lung weights ranged from approximately 0.10 to 0.15 g). In addition, the histological analysis of lung sections taken from mice on day 5 p.i. revealed no obvious differences in pathology between lungs infected with the wild-type LVS and those infected with the ΔtolC mutant (data not shown). Collectively, these results indicate that by both the intradermal and intranasal routes the LVS ΔtolC mutant was defective for replication within the lungs by 4 to 5 days p.i.
FIG. 3.
LVS ΔtolC is defective for colonizing the organs of mice inoculated by the intranasal route. C3H/HeN mice were intranasally infected with a sublethal dose (5 × 103 CFU) of the LVS or ΔtolC mutant. Organs were harvested on days 3, 5, and 9 p.i., and CFU/g of organ weight were determined for the lung (A), liver (B), and spleen (C). The limit of detection per organ was 10 CFU. Two independent experiments with 5 mice per time point per bacterial strain were performed, and the data were combined. The bars indicate mean CFU values. Colonization by the ΔtolC mutant was significantly decreased compared to that of the wild-type LVS at each time point in each organ (P < 0.05 for lung day 3 p.i.; P < 0.0001 for all other comparisons).
The LVS ΔtolC is hypercytotoxic to host macrophages.
Given that TolC typically functions in the export of bacterial toxins such as hemolysin, we reasoned that the attenuation of the LVS ΔtolC mutant in the mouse model of tularemia could be explained by the loss of the secretion of a F. tularensis toxin or virulence factor. To compare the toxicity of the ΔtolC mutant to that of wild-type LVS toward host cells, we infected murine bone marrow-derived macrophages (muBMDM) cultured from C3H/HeN mice and measured lactate dehydrogenase release (LDH) as a marker for cell death. Cells were infected at a multiplicity of infection (MOI) of 50, and LDH release was quantified at 7, 9, 17, and 24 h p.i. Surprisingly, rather than being less cytotoxic, the ΔtolC mutant at each time point caused significantly more LDH release than the wild-type LVS (Fig. 4 and data not shown). The complementation of the ΔtolC mutant with a tolC expression plasmid reduced the toxicity of the mutant back to wild-type levels (Fig. 4). The largest fold increase in LDH release caused by the mutant versus the wild-type LVS (∼3-fold) was observed consistently at 17 and 24 h p.i.; these time points were chosen for subsequent experiments.
FIG. 4.
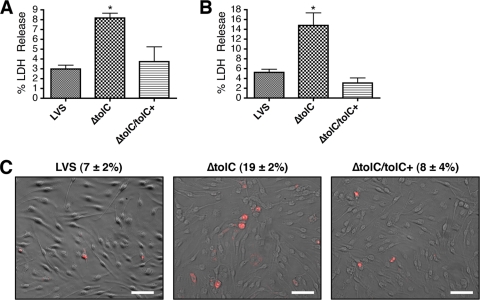
LVS ΔtolC is hypercytotoxic to murine macrophages. muBMDM cultured from C3H/HeN mice were infected with the LVS, ΔtolC mutant, or complemented strain (ΔtolC tolC+) at an MOI of 50. Cytotoxicity was quantified by measuring LDH release at 17 (A) or 24 h p.i. (B). Bars represent means ± standard errors of the means (SEM) of three independent experiments. The ΔtolC mutant caused significantly increased LDH release compared to that of the wild-type LVS (P < 0.05). (C) muBMDM infected as described for panel B were assayed for apoptosis at 17 h p.i. by TUNEL staining. The images show overlays of TUNEL-positive cells (red) and corresponding phase-contrast images. The percentages of TUNEL-positive cells ± SEM were calculated from 10 separate fields and represent the averages from three independent experiments. The ΔtolC mutant caused significantly increased TUNEL staining compared to that of the wild-type LVS (P < 0.05). Scale bars = 50 μm.
To determine if the hypercytotoxicity of the ΔtolC mutant was a result of increased programmed cell death, we infected muBMDM as described above and performed terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assays. TUNEL-positive cells were quantified by fluorescence microscopy. As shown in Fig. 4C, muBMDM infected with the ΔtolC mutant exhibited an approximately 3-fold increase in the number of TUNEL-positive cells compared to those of macrophages infected with the wild-type LVS or the complemented strain. The increase in TUNEL-positive cells induced by the LVS ΔtolC mirrored the increased cell death measured by LDH release. Thus, the ΔtolC mutant is defective in suppressing host cell death responses.
The hypercytotoxicity of the LVS ΔtolC is caspase-1 independent and correlates with increased caspase-3 activation.
To determine if host caspase-1 is required for the LVS-induced cell death, we compared LDH released from caspase-1-deficient and wild-type muBMDM infected with the LVS or ΔtolC strain. The caspase-1-deficient macrophages were cultured from caspase-1−/− C57BL/6 mice (41). The LVS ΔtolC mutant caused the same trend of increased cell death at 24 h p.i. in the wild-type C57BL/6 muBMDM (Fig. 5A) as that observed for the C3H/HeN macrophages (Fig. 4). In addition, the ΔtolC mutant was hypercytotoxic toward muBMDM from the caspase-1−/− C57BL/6 mice (Fig. 5A). This demonstrates that caspase-1 is not required for the hypercytotoxicity of the LVS ΔtolC mutant. Caspase-1 activation leads to the maturation and release of IL-1ß. We assayed conditioned media from C3H/HeN muBMDM infected with either the wild-type or ΔtolC LVS for the presence of IL-1ß. In agreement with a lack of caspase-1 involvement, neither strain caused IL-1β release above levels obtained from uninfected control cells (data not shown).
FIG. 5.
Hypercytotoxicity of the LVS ΔtolC is independent of caspase-1 and involves caspase-3. (A) muBMDM isolated from wild-type or caspase-1-deficient C57BL/6 mice were infected with the LVS or ΔtolC mutant at an MOI of 50. Cytotoxicity was quantified by measuring LDH release at 24 h p.i. The ΔtolC mutant caused significantly increased LDH release compared to that of the wild-type LVS for the caspase-1-deficient muBMDM (P < 0.05). Bars represent means ± SEM from three replicate samples. A representative experiment is shown. The experiment was repeated with similar results. (B) muBMDM isolated from C3H/HeN mice were uninfected or were infected with the LVS, ΔtolC mutant, or complemented strain (ΔtolC tolC+) at an MOI of 50. Activated caspase-3/-7 was measured at 17 h p.i. using a luminescence-based assay. Infection with the ΔtolC mutant caused a significant increase in caspase-3/-7 activity compared to that of the wild-type LVS (P < 0.05). Bars represent means ± SEM from three replicate samples. A representative experiment is shown. The experiment was repeated twice more with similar results. (C) muBMDM from C3H/HeN mice were infected as described for panel B and probed with an antibody against mature caspase-3 followed by a TRITC-conjugated secondary antibody. The images show overlays of caspase-3-positive cells (red) and corresponding phase-contrast images. The percentages of caspase-3-positive cells ± SEM were calculated from 10 separate fields and represent the averages from three independent experiments. The ΔtolC mutant caused significantly increased staining compared to that of the wild-type LVS (P < 0.05). Bars = 50 μm.
To examine the role of caspase-3 in the hypercytoxicity of the LVS ΔtolC mutant, we assayed infected C3H/HeN muBMDM using a luminescence-based method that measures caspase-3/-7 activity. Macrophages infected with the ΔtolC mutant exhibited significantly higher caspase-3/-7 activity at 17 h p.i. compared to those of cells infected with the wild-type LVS or complemented strain (Fig. 5B). As an additional assay for caspase-3 activation, we infected muBMDM with the LVS, ΔtolC, or complemented strain and detected the mature form of caspase-3 at 24 h p.i. by immunofluorescence microscopy. As shown in Fig. 5C, muBMDM infected with the ΔtolC mutant displayed ∼3-fold more mature caspase-3-positive cells than macrophages infected with the LVS. Collectively, the increased caspase-3 activation triggered by the LVS ΔtolC correlates well with the hypercytotoxicity of the mutant toward muBMDM, suggesting that the increased apoptosis is mediated by a caspase-3-dependent pathway.
The LVS ΔtolC is hypercytotoxic toward human macrophages and elicits the increased secretion of proinflammatory chemokines.
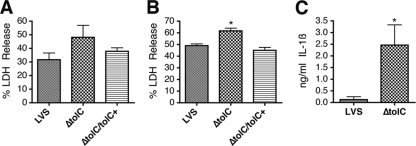
To determine if the F. tularensis ΔtolC mutant was hypercytotoxic to human monocyte-derived macrophages (huMDM), we infected huMDMs with the LVS or ΔtolC mutant and quantified cell death by measuring LDH release at 17 and 24 h p.i. As shown in Fig. 6, huMDM infected with the ΔtolC mutant exhibited increased LDH release compared to that of cells infected with the wild-type LVS or complemented strain. Therefore, the suppression of host cell death pathways in both human and murine macrophages appears to be dependent upon TolC. The level of LDH released by the huMDM upon infection with both wild-type and mutant strains was higher than that found for muBMDM. This likely is due to the higher infectivity of F. tularensis for huMDM (7). To assess caspase-1 involvement in the death of infected human macrophages, we measured the secretion of IL-1ß. At 24 h p.i. there was a significant increase in IL-1β release from huMDM infected by the ΔtolC mutant compared to levels for cells infected by the wild-type strain (Fig. 6C), implying that caspase-1 is activated in human macrophages in response to infection by the ΔtolC mutant. The complementation of the ΔtolC mutant reduced IL-1β levels back to those obtained with the wild-type strain (data not shown).
FIG. 6.
LVS ΔtolC is hypercytotoxic to human macrophages and elicits increased secretion of IL-1β. huMDM were infected with the LVS, ΔtolC mutant, or complemented strain (ΔtolC tolC+) at an MOI of 50. Cytotoxicity was quantified by measuring LDH release at 17 (A) or 24 h p.i. (B). Bars represent means ± SEM from three independent experiments. The ΔtolC mutant caused significantly increased LDH release compared to that of the wild-type LVS at 24 h p.i. (P < 0.05). (C) huMDM were infected with the LVS or ΔtolC mutant at an MOI of 25. IL-1β release was quantified by ELISA of conditioned media at 24 h p.i. Bars represent means ± SEM from three independent experiments. The ΔtolC mutant caused a significant increase in IL-1ß secretion compared to the wild-type LVS (P < 0.05).
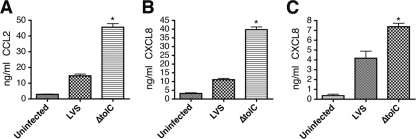
F. tularensis has been shown to play an active role in dampening innate immune responses (8, 22, 51). To determine if TolC, in addition to suppressing proapoptotic pathways, participates in the manipulation of host immune responses, we examined the secretion of the proinflammatory chemokines CXCL8 and CCL2 by infected huMDMs and human umbilical vein endothelial cells (HUVEC). Either huMDM or HUVEC were incubated with the LVS or ΔtolC mutant, and at 24 h p.i. the conditioned medium was removed and analyzed for the presence of CXCL8 or CCL2. huMDM infected with the ΔtolC mutant produced significantly larger amounts of CCL2 and CXCL8 than cells infected with the wild-type LVS (Fig. 7A and B). The complementation of the ΔtolC mutant reduced CCL2 and CXCL8 secretion back to levels obtained with the wild-type strain (data not shown). In addition, although we did not detect a significant increase in the amount of CCL2 secreted by HUVEC (data not shown), the infection of HUVEC with the LVS ΔtolC caused the significantly higher secretion of CXCL8 compared to that of cells infected with the wild-type LVS (Fig. 7C). These results suggest that a TolC-related function is required for F. tularensis to suppress proinflammatory immune responses in human host cells.
FIG. 7.
LVS ΔtolC elicits increased secretion of proinflammatory chemokines from human cells. huMDM were uninfected or infected with the LVS or ΔtolC mutant at an MOI of 25. The secretion of CCL2 (A) or CXCL8 (B) was quantified by the ELISA of conditioned medium at 24 h p.i. (C) HUVEC were incubated with medium alone or with the LVS or ΔtolC mutant at an MOI of 75. The secretion of CXCL8 was quantified by the ELISA of conditioned medium at 24 h p.i. The ΔtolC mutant caused the significantly increased secretion of the proinflammatory chemokines compared to that of the wild-type LVS (P < 0.05). Bars represent means ± SEM from three replicate samples. Each graph shows a representative experiment. Each experiment was repeated twice more with similar results.
DISCUSSION
In this study, we demonstrated that TolC is critical for the virulence of F. tularensis LVS in mice by the intranasal route of infection, as we found previously for infections by the intradermal route (28). Therefore, TolC is a general virulence factor of F. tularensis and represents a potential therapeutic target. To understand the role of TolC during pathogenesis, we analyzed bacterial colonization within the livers, spleens, and lungs of both intradermally and intranasally infected mice. The ΔtolC mutant spread to all organs following inoculation at each site, demonstrating that TolC is not required for F. tularensis to spread systemically. Although the ΔtolC mutant spread to all organs, CFU counts for the mutant were consistently lower by one or more logs compared to those of the parental LVS. The lower organ burden levels are likely a key part of the attenuation of the ΔtolC mutant, since this may allow the host to gain an upper hand against the invading bacteria.
Despite the organ colonization defect, CFU for the ΔtolC mutant roughly paralleled the wild-type LVS in the liver and spleen for mice infected by both the intradermal and intranasal routes. Importantly, both the mutant and wild-type strains increased in numbers between the first and second time points, indicating that the mutant retains the ability to replicate in these organs. In agreement with these observations, the ΔtolC mutant caused pathology characteristic of F. tularensis infections in both the liver and spleen. This suggests that TolC is critical for a step in pathogenesis other than intracellular replication.
The greatest difference in organ colonization between the wild-type and LVS ΔtolC strain was observed in the lungs. For both the intradermal and intranasal routes of infection, the number of CFU for the wild-type LVS increased in the lung from the first through the last time points. In contrast, the number of CFU for the ΔtolC mutant decreased over the three time points, with the mutant completely cleared from the lungs in the intradermal infections. These results suggest that TolC function is particularly important for the colonization of F. tularensis within the lung. Moreover, given that the tolC mutant is highly attenuated for virulence in mice, this finding further suggests that the successful colonization of the lungs is critical for a lethal infection.
The organ colonization data we obtained correlate well with in vitro tissue culture studies showing that the LVS ΔtolC replicates within muBMDM but to lower numbers than those of the wild-type LVS (28). Similarly, we found that the ΔtolC mutant retained the ability to replicate in both the murine hepatocyte cell line FL83B and the human lung epithelial cell line A549 (G. J. Platz and D. G. Thanassi; unpublished data). The ΔtolC mutant exhibited growth kinetics similar to those of the wild-type LVS in FL83B cells, but the replication of the mutant was consistently one log lower in the A549 cells. The ability of the ΔtolC mutant to grow intracellularly makes this strain a plausible vaccine candidate, as it should provoke a strong cellular immune response. Indeed, preliminary vaccination studies showed that the LVS ΔtolC provided full protection against subsequent lethal challenge with the parental LVS (S. Chakraborty, P. Mena, and D. G. Thanassi; unpublished data).
In vitro studies showed that the ΔtolC mutant is hypercytotoxic to host macrophages. This could explain the growth defect of the mutant in tissue culture cells and mouse organs; the bacteria may kill its host cell too quickly and lose its intracellular replicative niche. Compared to infection with the parental LVS, muBMDM infected with the ΔtolC mutant exhibited approximately 3-fold higher rates of cell death. This increased cell death was correlated with caspase-3 activation and was independent of caspase-1. In agreement with a lack of caspase-1 activation, we did not detect the increased release of IL-1ß by the muBMDM. Our results match those of Lai et al., who reported that the infection of J774 murine macrophage-like cells with the LVS triggered apoptosis by the intrinsic pathway, with the activation of caspase-3 but not caspase-1 (38). In contrast, Mariathasan et al. reported that the infection of preactivated murine peritoneal macrophages by either the LVS or strain U112 triggered cell death via the inflammsome complex and caspase-1 activation (32, 42). The different cell death pathways detected by these studies may reflect the different infection conditions used; our infection conditions most closely resemble those used by Lai et al. (38).
We found that the LVS ΔtolC mutant was hypercytotoxic toward huMDM, demonstrating conserved functions of F. tularensis TolC in the human and murine hosts. In contrast to what was seen in murine macrophages, we observed the increased secretion of IL-1ß from huMDM infected with the ΔtolC mutant, indicating the activation of caspase-1. We do not yet know if this increased IL-1ß release indicates that caspase-1 plays a direct role in the hypercytotoxicity of the ΔtolC mutant. Experiments to address this issue using specific inhibitors of caspase-1 or caspase-3 were unsuccessful due to the toxicity of the inhibitors toward the human macrophages (D. C. Bublitz, M. B. Furie, and D. G. Thanassi, unpublished data). Nevertheless, our results support the idea that F. tularensis induces different cell death pathways depending on the specific infection conditions and host cell type and suggest that the TolC-related inhibitory function is active against different cell death pathways. Similarly to our LVS ΔtolC mutant, two transposon mutants of strain U112 isolated by Weiss and coworkers displayed hypercytotoxicity toward murine macrophages (53). One of these mutations was in a gene of unknown function, and the other was in a putative transcriptional regulator (53). Although these U112 mutants were shown to modulate inflammasome/caspase-1-mediated cell death, it is possible that the same or similar effectors are connected to the phenotypes we observed for the LVS ΔtolC mutant. A difference between our mouse and cell culture infection results is that we obtained the full complementation of the cytotoxicity phenotype of the ΔtolC mutant in the macrophage infection assays (Fig. 4 and 6) but only partial complementation for the virulence of the mutant in the lethal infection assay (Fig. 2). This difference may reflect stricter requirements for proper tolC expression in the animal infections compared to those for the cell culture experiments. In the complementing plasmid, tolC is expressed from a constitutive promoter and at a higher copy number than that of the native gene on the F. tularensis chromosome.
The inhibition of host cell death is increasingly recognized as a strategy used by intracellular pathogens to prolong the time they have to replicate (21). Bacteria employ a variety of mechanisms to inhibit apoptosis, including the activation of host cell survival pathways, the prevention of cytochrome c release from mitochondria, and the inhibition of caspases (21). Many intracellular pathogens, including Shigella and Legionella, use secretion systems to deliver effector proteins into host cells to suppress apoptosis (1, 15, 36). Others, such as Neisseria and Wolbachia, employ surface proteins to inhibit apoptosis (5, 43). TolC is located in the bacterial outer membrane and could directly interact with host proteins to mediate the suppression of apoptosis upon infection by F. tularensis. However, we favor the hypothesis that TolC is required for the secretion of a toxin via the type I secretion pathway. In support of TolC functioning in type I secretion in F. tularensis, we found that a tolC transposon insertion mutant of strain U112 (27) was defective for hemolysin secretion (G. J. Platz and D. G. Thanassi, unpublished data). Hemolysin is a prototypical type I secretion substrate, but the LVS and most other strains of F. tularensis lack hemolytic activity (39). Work is under way to identify the putative TolC-secreted toxin or toxins in the LVS and fully virulent strains of F. tularensis.
In addition to causing increased cell death, the infection of huMDM by the LVS ΔtolC caused a significant increase in the secretion of the proinflammatory chemokines CXCL8 and CCL2 compared to infection by the wild-type LVS. CXCL8 secretion also was significantly increased in HUVEC exposed to the ΔtolC mutant. CXCL8 functions to attract and activate neutrophils (44), whereas CCL2 recruits monocytes, memory T cells, and dendritic cells to sites of tissue injury and infection (11). During pathogenesis, the increased secretion of these proinflammatory chemokines would bring additional immune cells to the site of infection and contribute to the clearance of the pathogen. These data suggest that the attenuation in the virulence of the ΔtolC mutant is due not only to hypercytotoxicity and premature loss of its intracellular niche but also to the increased activation of the host innate immune system. This increased proinflammatory response could explain the more rapid clearance of the ΔtolC mutant observed in the mouse organ burden assays.
F. tularensis is known to suppress or interfere with innate immune responses by macrophages, dendritic cells, and endothelial cells (8, 9, 51, 52, and D. C. Bublitz and M. B. Furie, unpublished data). Furthermore, a recent study found that a component secreted by F. tularensis acted to suppress proinflammatory responses of uninfected bystander cells (13). Based on our findings, we propose that the ability of F. tularensis to actively suppress proinflammatory host responses is at least partially dependent upon TolC, likely via a TolC-secreted factor or factors. Note that HUVEC are not professional phagocytes and do not readily take up F. tularensis (22). This implies that the TolC-secreted factor(s) is able to dampen host immune responses from extracellular as well as intracellular locations.
In conclusion, our data show that tolC is required for the virulence of F. tularensis in mice by both the intradermal and intranasal routes of infection, and that tolC is required for the maximal colonization of mouse organs. In addition, our data show that TolC functions to suppress both the proapoptotic and proinflammatory innate responses of host cells. We propose that these functions are carried out by a F. tularensis toxin or toxins that require TolC for secretion by the type I pathway. Taking our in vivo and cell culture infection data together, we propose the following model for TolC function in pathogenesis. Effectors secreted via TolC interfere with death pathways of infected host cells, providing the bacteria extended time to replicate in the protected intracellular niche. The ΔtolC mutant kills its host cells too quickly and therefore replicates to lower numbers. Effectors secreted via TolC also function to dampen proinflammatory responses of host cells. Thus, the ΔtolC mutant not only replicates to lower numbers compared to the wild-type strain, but the bacteria also are cleared more rapidly due to the increased activation of the host innate immune system. The host-suppressive activities related to TolC function likely are critical to the extreme virulence of F. tularensis.
Acknowledgments
We thank James Bliska (Stony Brook University) for providing the caspase-1−/− mice and for the use of the fluorescence microscope, Indralatha Jayatilaka (Stony Brook University) for preparing the huMDM, Gloria Monsalve (Stony Brook University) for assistance with mouse experiments, Neta Dean (Stony Brook University) for the use of the luminometer, Jason Huntley (University of Texas Southwestern Medical Center) for assistance with the intranasal infection protocol, and Adrianus Van Der Velden (Stony Brook University) for the critical reading of the manuscript.
This work was supported by National Institutes of Health grant AI055621 and Northeast Biodefense Center grant U54-AI057158-Lipkin.
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 22 December 2009.
REFERENCES
- 1.Abu-Zant, A., S. Jones, R. Asare, J. Suttles, C. Price, J. Graham, and Y. A. Kwaik. 2007. Anti-apoptotic signalling by the Dot/Icm secretion system of L. pneumophila. Cell Microbiol. 9:246-264. [DOI] [PubMed] [Google Scholar]
- 2.Anthony, L. D., R. D. Burke, and F. E. Nano. 1991. Growth of Francisella spp. in rodent macrophages. Infect. Immun. 59:3291-3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bar-Haim, E., O. Gat, G. Markel, H. Cohen, A. Shafferman, and B. Velan. 2008. Interrelationship between dendritic cell trafficking and Francisella tularensis dissemination following airway infection. PLoS Pathog. 4:e1000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker, J. H., J. Weiss, M. A. Apicella, and W. M. Nauseef. 2006. Basis for the failure of Francisella tularensis lipopolysaccharide to prime human polymorphonuclear leukocytes. Infect. Immun. 74:3277-3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bazzocchi, C., S. Comazzi, R. Santoni, C. Bandi, C. Genchi, and M. Mortarino. 2007. Wolbachia surface protein (WSP) inhibits apoptosis in human neutrophils. Parasite Immunol. 29:73-79. [DOI] [PubMed] [Google Scholar]
- 6.Bergsbaken, T., S. L. Fink, and B. T. Cookson. 2009. Pyroptosis: host cell death and inflammation. Nat. Rev. Microbiol. 7:99-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolger, C. E., C. A. Forestal, J. K. Italo, J. L. Benach, and M. B. Furie. 2005. The live vaccine strain of Francisella tularensis replicates in human and murine macrophages but induces only the human cells to secrete proinflammatory cytokines. J. Leukoc. Biol. 77:893-897. [DOI] [PubMed] [Google Scholar]
- 8.Bosio, C. M., H. Bielefeldt-Ohmann, and J. T. Belisle. 2007. Active suppression of the pulmonary immune response by Francisella tularensis Schu4. J. Immunol. 178:4538-4547. [DOI] [PubMed] [Google Scholar]
- 9.Bosio, C. M., and S. W. Dow. 2005. Francisella tularensis induces aberrant activation of pulmonary dendritic cells. J. Immunol. 175:6792-6801. [DOI] [PubMed] [Google Scholar]
- 10.Budihardjo, I., H. Oliver, M. Lutter, X. Luo, and X. Wang. 1999. Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol. 15:269-290. [DOI] [PubMed] [Google Scholar]
- 11.Carr, M. W., S. J. Roth, E. Luther, S. S. Rose, and T. A. Springer. 1994. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc. Natl. Acad. Sci. USA 91:3652-3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chakraborty, S., M. Monfett, T. M. Maier, J. L. Benach, D. W. Frank, and D. G. Thanassi. 2008. Type IV pili in Francisella tularensis: roles of pilF and pilT in fiber assembly, host cell adherence, and virulence. Infect. Immun. 76:2852-2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chase, J. C., J. Celli, and C. M. Bosio. 2009. Direct and indirect impairment of human dendritic cell function by virulent Francisella tularensis Schu S4. Infect. Immun. 77:180-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christie, P. J., K. Atmakuri, V. Krishnamoorthy, S. Jakubowski, and E. Cascales. 2005. Biogenesis, architecture, and function of bacterial type IV secretion systems. Annu. Rev. Microbiol. 59:451-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark, C. S., and A. T. Maurelli. 2007. Shigella flexneri inhibits staurosporine-induced apoptosis in epithelial cells. Infect. Immun. 75:2531-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clemens, D. L., B. Y. Lee, and M. A. Horwitz. 2004. Virulent and avirulent strains of Francisella tularensis prevent acidification and maturation of their phagosomes and escape into the cytoplasm in human macrophages. Infect. Immun. 72:3204-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole, L. E., K. L. Elkins, S. M. Michalek, N. Qureshi, L. J. Eaton, P. Rallabhandi, N. Cuesta, and S. N. Vogel. 2006. Immunologic consequences of Francisella tularensis live vaccine strain infection: role of the innate immune response in infection and immunity. J. Immunol. 176:6888-6899. [DOI] [PubMed] [Google Scholar]
- 18.Conlan, J. W., W. Chen, H. Shen, A. Webb, and R. KuoLee. 2003. Experimental tularemia in mice challenged by aerosol or intradermally with virulent strains of Francisella tularensis: bacteriologic and histopathologic studies. Microb. Pathog. 34:239-248. [DOI] [PubMed] [Google Scholar]
- 19.Dennis, D. T., T. V. Inglesby, D. A. Henderson, J. G. Bartlett, M. S. Ascher, E. Eitzen, A. D. Fine, A. M. Friedlander, J. Hauer, M. Layton, S. R. Lillibridge, J. E. McDade, M. T. Osterholm, T. O'Toole, G. Parker, T. M. Perl, P. K. Russell, and K. Tonat. 2001. Tularemia as a biological weapon: medical and public health management. JAMA 285:2763-2773. [DOI] [PubMed] [Google Scholar]
- 20.Ellis, J., P. C. Oyston, M. Green, and R. W. Titball. 2002. Tularemia. Clin. Microbiol. Rev. 15:631-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faherty, C. S., and A. T. Maurelli. 2008. Staying alive: bacterial inhibition of apoptosis during infection. Trends Microbiol. 16:173-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forestal, C. A., J. L. Benach, C. Carbonara, J. K. Italo, T. J. Lisinski, and M. B. Furie. 2003. Francisella tularensis selectively induces proinflammatory changes in endothelial cells. J. Immunol. 171:2563-2570. [DOI] [PubMed] [Google Scholar]
- 23.Forestal, C. A., H. Gil, M. Monfett, C. E. Noah, G. J. Platz, D. G. Thanassi, J. L. Benach, and M. B. Furie. 2008. A conserved and immunodominant lipoprotein of Francisella tularensis is proinflammatory but not essential for virulence. Microb. Pathog. 44:512-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forestal, C. A., M. Malik, S. V. Catlett, A. G. Savitt, J. L. Benach, T. J. Sellati, and M. B. Furie. 2007. Francisella tularensis has a significant extracellular phase in infected mice. J. Infect. Dis. 196:134-137. [DOI] [PubMed] [Google Scholar]
- 25.Fortier, A. H., S. J. Green, T. Polsinelli, T. R. Jones, R. M. Crawford, D. A. Leiby, K. L. Elkins, M. S. Meltzer, and C. A. Nacy. 1994. Life and death of an intracellular pathogen: Francisella tularensis and the macrophage. Immunol. Ser. 60:349-361. [PubMed] [Google Scholar]
- 26.Galan, J. E., and H. Wolf-Watz. 2006. Protein delivery into eukaryotic cells by type III secretion machines. Nature 444:567-573. [DOI] [PubMed] [Google Scholar]
- 27.Gallagher, L. A., E. Ramage, M. A. Jacobs, R. Kaul, M. Brittnacher, and C. Manoil. 2007. A comprehensive transposon mutant library of Francisella novicida, a bioweapon surrogate. Proc. Natl. Acad. Sci. USA 104:1009-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gil, H., G. J. Platz, C. A. Forestal, M. Monfett, C. S. Bakshi, T. J. Sellati, M. B. Furie, J. L. Benach, and D. G. Thanassi. 2006. Deletion of TolC orthologs in Francisella tularensis identifies roles in multidrug resistance and virulence. Proc. Natl. Acad. Sci. USA 103:12897-12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gray, C. G., S. C. Cowley, K. K. Cheung, and F. E. Nano. 2002. The identification of five genetic loci of Francisella novicida associated with intracellular growth. FEMS Microbiol. Lett. 215:53-56. [DOI] [PubMed] [Google Scholar]
- 30.Green, M., G. Choules, D. Rogers, and R. W. Titball. 2005. Efficacy of the live attenuated Francisella tularensis vaccine (LVS) in a murine model of disease. Vaccine 23:2680-2686. [DOI] [PubMed] [Google Scholar]
- 31.Hager, A. J., D. L. Bolton, M. R. Pelletier, M. J. Brittnacher, L. A. Gallagher, R. Kaul, S. J. Skerrett, S. I. Miller, and T. Guina. 2006. Type IV pili-mediated secretion modulates Francisella virulence. Mol. Microbiol. 62:227-237. [DOI] [PubMed] [Google Scholar]
- 32.Henry, T., A. Brotcke, D. S. Weiss, L. J. Thompson, and D. M. Monack. 2007. Type I interferon signaling is required for activation of the inflammasome during Francisella infection. J. Exp. Med. 204:987-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holland, I. B., L. Schmitt, and J. Young. 2005. Type 1 protein secretion in bacteria, the ABC-transporter dependent pathway (review). Mol. Membr. Biol. 22:29-39. [DOI] [PubMed] [Google Scholar]
- 34.Huang, A. J., M. B. Furie, S. C. Nicholson, J. Fischbarg, L. S. Liebovitch, and S. C. Silverstein. 1988. Effects of human neutrophil chemotaxis across human endothelial cell monolayers on the permeability of these monolayers to ions and macromolecules. J. Cell. Physiol. 135:355-366. [DOI] [PubMed] [Google Scholar]
- 35.Huntley, J. F., P. G. Conley, K. E. Hagman, and M. V. Norgard. 2007. Characterization of Francisella tularensis outer membrane proteins. J. Bacteriol. 189:561-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knodler, L. A., B. B. Finlay, and O. Steele-Mortimer. 2005. The Salmonella effector protein SopB protects epithelial cells from apoptosis by sustained activation of Akt. J. Biol. Chem. 280:9058-9064. [DOI] [PubMed] [Google Scholar]
- 37.Koronakis, V., J. Eswaran, and C. Hughes. 2004. Structure and function of TolC: the bacterial exit duct for proteins and drugs. Annu. Rev. Biochem. 73:467-489. [DOI] [PubMed] [Google Scholar]
- 38.Lai, X. H., and A. Sjostedt. 2003. Delineation of the molecular mechanisms of Francisella tularensis-induced apoptosis in murine macrophages. Infect. Immun. 71:4642-4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lai, X. H., S. Y. Wang, H. Edebro, and A. Sjostedt. 2003. Francisella strains express hemolysins of distinct characteristics. FEMS Microbiol. Lett. 224:91-95. [DOI] [PubMed] [Google Scholar]
- 40.Larsson, P., P. C. Oyston, P. Chain, M. C. Chu, M. Duffield, H. H. Fuxelius, E. Garcia, G. Halltorp, D. Johansson, K. E. Isherwood, P. D. Karp, E. Larsson, Y. Liu, S. Michell, J. Prior, R. Prior, S. Malfatti, A. Sjostedt, K. Svensson, N. Thompson, L. Vergez, J. K. Wagg, B. W. Wren, L. E. Lindler, S. G. Andersson, M. Forsman, and R. W. Titball. 2005. The complete genome sequence of Francisella tularensis, the causative agent of tularemia. Nat. Genet. 37:153-159. [DOI] [PubMed] [Google Scholar]
- 41.Lilo, S., Y. Zheng, and J. B. Bliska. 2008. Caspase-1 activation in macrophages infected with Yersinia pestis KIM requires the type III secretion system effector YopJ. Infect. Immun. 76:3911-3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mariathasan, S., D. S. Weiss, V. M. Dixit, and D. M. Monack. 2005. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J. Exp. Med. 202:1043-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Massari, P., Y. Ho, and L. M. Wetzler. 2000. Neisseria meningitidis porin PorB interacts with mitochondria and protects cells from apoptosis. Proc. Natl. Acad. Sci. USA 97:9070-9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Modi, W. S., M. Dean, H. N. Seuanez, N. Mukaida, K. Matsushima, and S. J. O'Brien. 1990. Monocyte-derived neutrophil chemotactic factor (MDNCF/IL-8) resides in a gene cluster along with several other members of the platelet factor 4 gene superfamily. Hum. Genet. 84:185-187. [DOI] [PubMed] [Google Scholar]
- 45.Nano, F. E., N. Zhang, S. C. Cowley, K. E. Klose, K. K. Cheung, M. J. Roberts, J. S. Ludu, G. W. Letendre, A. I. Meierovics, G. Stephens, and K. L. Elkins. 2004. A Francisella tularensis pathogenicity island required for intramacrophage growth. J. Bacteriol. 186:6430-6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oyston, P. C., A. Sjostedt, and R. W. Titball. 2004. Tularaemia: bioterrorism defence renews interest in Francisella tularensis. Nat. Rev. Microbiol. 2:967-978. [DOI] [PubMed] [Google Scholar]
- 47.Rasmussen, J. W., J. Cello, H. Gil, C. A. Forestal, M. B. Furie, D. G. Thanassi, and J. L. Benach. 2006. Mac-1+ cells are the predominant subset in the early hepatic lesions of mice infected with Francisella tularensis. Infect. Immun. 74:6590-6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Svensson, K., P. Larsson, D. Johansson, M. Bystrom, M. Forsman, and A. Johansson. 2005. Evolution of subspecies of Francisella tularensis. J. Bacteriol. 187:3903-3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tärnvik, A. 1989. Nature of protective immunity to Francisella tularensis. Rev. Infect. Dis. 11:440-451. [PubMed] [Google Scholar]
- 50.Tarnvik, A., and L. Berglund. 2003. Tularaemia. Eur. Respir. J. 21:361-373. [DOI] [PubMed] [Google Scholar]
- 51.Telepnev, M., I. Golovliov, T. Grundstrom, A. Tarnvik, and A. Sjostedt. 2003. Francisella tularensis inhibits Toll-like receptor-mediated activation of intracellular signalling and secretion of TNF-alpha and IL-1 from murine macrophages. Cell Microbiol. 5:41-51. [DOI] [PubMed] [Google Scholar]
- 52.Telepnev, M., I. Golovliov, and A. Sjostedt. 2005. Francisella tularensis LVS initially activates but subsequently down-regulates intracellular signaling and cytokine secretion in mouse monocytic and human peripheral blood mononuclear cells. Microb. Pathog. 38:239-247. [DOI] [PubMed] [Google Scholar]
- 53.Weiss, D. S., A. Brotcke, T. Henry, J. J. Margolis, K. Chan, and D. M. Monack. 2007. In vivo negative selection screen identifies genes required for Francisella virulence. Proc. Natl. Acad. Sci. USA 104:6037-6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zogaj, X., S. Chakraborty, J. Liu, D. G. Thanassi, and K. E. Klose. 2008. Characterization of the Francisella tularensis subsp. novicida type IV pilus. Microbiology 154:2139-2150. [DOI] [PubMed] [Google Scholar]