Abstract
A Salmonella enterica serovar Hadar strain resistant to tigecycline (MIC, 16 μg/ml) was isolated. Molecular characterization revealed the presence of a plasmid-borne tet(A) variant associated with Tn1721 mediating a rise of the MIC for tigecycline when transferred to Escherichia coli. Additionally, a truncating mutation in ramR was detected. Transformation with wild-type ramR but not with the mutated ramR lowered the MIC for tigecycline. Characterization of this Salmonella isolate implicates ramR in resistance to tigecycline.
Tigecycline is a novel broad-spectrum antibiotic belonging to the class of glycylcyclines and is chemically derived from the tetracycline minocycline (17). Tigecycline is notable for its antibacterial activity against an extraordinarily broad range of bacteria, with only few naturally resistant exceptions, namely, Proteus spp., Morganella morganii, Providencia spp., and Pseudomonas aeruginosa. In particular, the in vitro and in vivo activities of tigecycline against multidrug-resistant pathogens like methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus (VRE), extended-spectrum beta-lactamase (ESBL)-expressing Enterobacteriaceae, and carbapenem-resistant strains make this drug a promising agent for difficult-to-treat infections (14, 18, 27). Resistance in isolates of usually susceptible pathogens has so far only rarely been described and has been limited to isolates of Klebsiella pneumoniae (3, 23), Enterobacter cloacae (12), Acinetobacter baumannii (21), Escherichia coli (13), Enterococcus faecalis (31), and Staphylococcus aureus (15). Although the exact mechanisms of resistance could not be definitely determined, a common finding in these sporadic isolates as well as in the naturally resistant species is overexpression of different efflux pumps: AcrAB RND-type efflux pumps in Enterobacteriaceae, certain other RND-type efflux pumps in Pseudomonas aeruginosa (MexXY) and Acinetobacter species (AdeABC and AdeIJK), and MATE family efflux pumps in Staphylococcus aureus (7, 8, 12, 13, 15, 24-26, 30). Although genes conferring resistance to tetracyclines do not seem to have an effect on susceptibility to tigecycline (9, 11), mutants of Tet(A) and Tet(B) with altered substrate specificities have been isolated that demonstrated low-level resistance against an early glycylcycline (10) and tigecycline (29). So far, tigecycline resistance due to Tet(A) or Tet(B) variants has not been described in clinical isolates.
MICs for tigecycline were determined in this study by broth microdilution (5, 6) using a commercially available tigecycline panel (MERLIN Diagnostika GmbH, Bornheim-Hersel, Germany) with freshly prepared (<12-h-old) Mueller-Hinton II broth (BBL, BD Bioscience, Sparks, MD). All MICs were interpreted according to EUCAST clinical breakpoints as susceptible, intermediate, or resistant.
We isolated a Salmonella enterica serovar Hadar strain (VA5649) resistant to tigecycline with a MIC of 16 μg/ml. The patient had no known history of tigecycline exposure. Molecular analysis for the presence of known tet genes (2) revealed the presence of tet(A). Sequencing of the full open reading frame of the tet(A) gene revealed a previously described (29) double frameshift mutation compared to the RP1-linked tet(A) gene (gene id number gi:42508), leading to the substitution of amino acids 201, 202, and 203 (serine, phenylalanine, and valine to alanine, serine, and phenylalanine, respectively) in the interdomain loop. This variant was found to elevate the MIC for the glycylcycline GAR-936, now termed tigecycline (29). In order to analyze whether the tet(A) gene resides on a plasmid, we extracted plasmid DNA from S. enterica VA5649 and transformed competent tetracycline-susceptible E. coli (Top Ten; Invitrogen) with the DNA preparation. Tetracycline-resistant clones could be isolated that carried the same tet(A) gene as the Salmonella isolate, confirming that the tet(A) gene is localized on a plasmid. This transformed E. coli clone [E. coli DH10B with Tn1721-tet(A) plasmid] also exhibited an elevated MIC for tigecycline (MIC, 0.25 μg/ml), compared to the untransformed E. coli (strain DH10B) with a MIC of 0.065 μg/ml (Table 1). In order to exclude effects of other genes on the natural plasmid, the tet(A)/tetR(A) unit [54 nucleotides upstream of the tet(A) stop codon and 57 nucleotides downstream of the tetR(A) stop codon; primers AGGATCCTAGCTTGCCGGAAGTCGCCTTGA and AAAGCTTATGTTGTCTACATGGCTCTGC; reference sequence gi:48194] was amplified from the plasmid and cloned into the pSKII vector. E. coli carrying the pSKII-tet(A)/tetR construct {E. coli DH10B with pSKII[Tn1721-tet(A)]} showed the same MIC increase for tigecycline (0.25 μg/ml) as E. coli carrying the natural plasmid, compared to a strain carrying an empty pSKII vector (E. coli DH10B with pSKII; MIC, 0.065 μg/ml) (Table 1). Although E. coli DH10B with pSKII[Tn1721-tet(A)] remained in the susceptible range, the elevation of the MIC suggested that the tet(A) variant contributes to tigecycline resistance in the Salmonella isolate. Furthermore, we confirmed the observation that this variant also confers resistance against minocycline (Table 1), a property commonly attributed only to Tet(B) among the tetracycline efflux pumps (4). Sequencing of the upstream and downstream regions of the plasmid starting from the tet(A) locus showed that it is situated in a highly conserved Tn1721 element, which has 3,163 nucleotides deleted at the 5′ end, compared with the complete Tn1721 element (gi:48194). No mutations in the tetR(A) regulator or the intergenic region between tet(A) and tetR(A), which harbors regulatory sequences, were found on comparison with the Tn1721 reference sequence (gi:48194). Subsequent database searches revealed that Tn1721-associated tet(A) commonly harbors the above-described amino acid exchanges in the interdomain loop, which may suggest that bacteria carrying the Tn1721 element may generally be less susceptible to tigecycline.
TABLE 1.
MICs and relative ramA and acrB expression levels of strains used in this study
| Isolate | MIC (μg/ml) (susceptibility)a |
Relative expression ofd: |
||||
|---|---|---|---|---|---|---|
| Tigecyclineb | Ciprofloxacinc | Chloramphenicolc | Minocyclinec | ramA | acrB | |
| S. enterica serovar Hadar VA5649 | 16 (R) | 1.0 (I) | 8.0 (I) | 32 (R) | 1 | 1 |
| E. coli DH10B | 0.065 (S) | 0.002 (S) | NDe | 0.75 (S) | ND | ND |
| E. coli DH10B + Tn1721-tet(A) plasmid | 0.25 (S) | 0.002 (S) | ND | 8 (R) | ND | ND |
| E. coli DH10B + pSKII | 0.065 (S) | ND | ND | 0.75 (S) | ND | ND |
| E. coli DH10B + pSKII[Tn1721-tet(A)] | 0.25 (S) | ND | ND | 8.0 (R) | ND | ND |
| S. enterica TY2313-WT | 0.25 (S) | ND | 2.0 (S) | ND | 0.005 | 0.225 |
| S. enterica serovar Hadar VA5649(ramR-2313-WT) | 2 (I) | 0.25 (S) | 2.0 (S) | ND | 0.044 | 0.171 |
| S. enterica serovar Hadar VA5649(ramR-5649) | 16 (R) | 1.0 (I) | 8.0 (I) | ND | 1.932 | 1.569 |
| S. enterica serovar Hadar VA5649(pACYC177) | 16 (R) | 1.0 (I) | 8.0 (I) | ND | 1.189 | 1 |
S, susceptible; I, intermediate; R, resistant (according to EUCAST clinical breakpoints [www.eucast.org]).
Tested by broth microdilution.
Tested by Etest.
Measured by quantitative RT-PCR and normalized to expression levels of VA5649 (expression of 1).
ND, not determined.
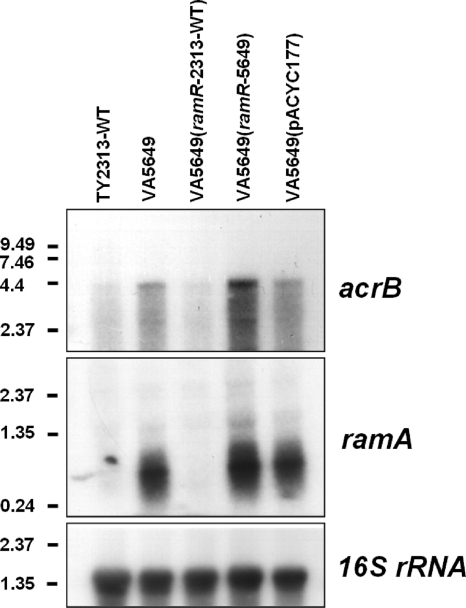
The high MIC for tigecycline in S. enterica VA5649 suggested additional resistance mechanisms. Resistance to tigecycline in Enterobacter cloacae and Klebsiella pneumoniae has been linked to overexpression of ramA, a positive regulator of the AcrAB efflux system (12, 26). The AcrAB system is an efflux pump that confers low-level resistance to a wide range of antibiotics (19, 20). Recently, mutations in ramR, a putative negative regulator of ramA, were shown to result in overexpression of ramA and resistance to ciprofloxacin in Salmonella (1, 16, 22). We reasoned that ramR may be involved in resistance to tigecycline in Salmonella and sequenced the coding region of ramR and the intergenic region between ramA and ramR, as previously described (1). Sequencing revealed, besides two silent nucleotide exchanges (207T>C and 330G>A), deletion of cytosine 515, resulting in a frameshift after amino acid 171 with a divergent C terminus and truncation of 22 amino acids. ramR was cloned from an S. enterica isolate (TY2313) with a wild-type MIC for tigecycline (MIC, 0.25 μg/ml) and from the resistant strain S. enterica VA5649 and inserted into the pACYC177 low-copy-number vector. Transformation of S. enterica VA5649 with ramR-2313 [Salmonella enterica serovar Hadar VA5649(ramR-2313-WT)] lowered the MIC for tigecycline from 16 μg/ml to 2 μg/ml (Table 1). In contrast, transformation of VA5649 with either the mutated ramR [Salmonella enterica serovar Hadar VA5649(ramR-5649)] or the empty pACYC177 vector [Salmonella enterica serovar Hadar VA5649(pACYC177)] did not lower the tigecycline MIC (Table 1). Furthermore, MICs for two other known substrates of AcrAB, ciprofloxacin and chloramphenicol (determined by Etest; AB Biodisk, Solna, Sweden), were affected in a similar manner. S. enterica serovar Hadar VA5649, S. enterica serovar Hadar VA5649(ramR-5649), and S. enterica serovar Hadar VA5649(pACYC177) exhibited MICs of 1.0 μg/ml for ciprofloxacin and 8.0 μg/ml for chloramphenicol, which were both interpreted as intermediate according to EUCAST clinical breakpoints, yet the introduction of wild-type ramR in S. enterica serovar Hadar VA5649(ramR-2313) lowered the MIC for ciprofloxacin to 0.25 μg/ml and the MIC for chloramphenicol to 2.0 μg/ml, which were both interpreted as susceptible. As RamR had been suggested to be a negative regulator of ramA, we analyzed expression of ramA in the different strains by Northern blotting (Fig. 1) (hybridization probes for ramA were generated with primers ATGACCATTTCCGCTCAGGTTA and TCAATGCGTACGACCATG and for acrB and 16S rRNA we used the reverse transcription-PCR [RT-PCR] primers described in reference 16) and by quantitative RT-PCR as described previously (16) (Table 1). While ramA expression was virtually absent in the wild-type strain S. enterica TY2313, ramA was massively overexpressed in the tigecycline-resistant strain S. enterica serovar Hadar VA5649. Introduction of wild-type ramR into VA5649 [S. enterica serovar Hadar VA5649(ramR-2313-WT)] effectively repressed ramA. In contrast, transformation with the mutated allele in S. enterica serovar Hadar VA5649(ramR-5649) or the empty vector in S. enterica serovar Hadar VA5649(pACYC177) did not repress ramA expression. Furthermore, upregulation of ramA in strains carrying only the mutated ramR gene, S. enterica serovar Hadar VA5649, S. enterica serovar Hadar VA5649(ramR-5649), and S. enterica serovar Hadar VA5649(pACYC177), was paralleled by upregulated acrB expression, while acrB expression was low in the wild-type strain, S. enterica TY2313, and the complemented strain S. enterica serovar Hadar VA5649(ramR-2313-WT). These findings support the concept of RamR being a repressor of ramA and of RamA being an activator of the AcrAB system.
FIG. 1.
RamR is a repressor of ramA. Expression levels of ramA and acrB were analyzed in different strains by Northern blotting. Total RNA was extracted from mid-log-phase cultures of the indicated strains. Three micrograms of total RNA was loaded onto each lane. The filter was hybridized consecutively in the following order with [32P]dCTP-labeled probes of ramA, acrB, and 16S rRNA. Expected sizes of bands: ramA, ∼350 bp; acrB, ∼4,000 bp; 16S rRNA, ∼1,500 bp. Values on the left margin are band sizes in kbp.
In conclusion, we report here a clinical Salmonella isolate highly resistant to tigecycline and have characterized the underlying molecular mechanisms. Our results imply that the combination of the two low-level resistance mechanisms, Tn1721-tet(A) and inactivation of ramR, results in complete resistance to tigecycline. The prevalence of Tn1721-tet(A) in clinical isolates is currently unknown but may be low, and its clinical significance might be limited. Recent studies did not find full resistance to tigecycline in tet(A)-carrying E. coli isolates, yet the presence of the Tn1721-associated tet(A) was not specifically investigated (28). None of the tetracycline-resistant Salmonella strains from our collection (n = 17) carried this gene (data not shown). It will be important to systematically address this issue for a broader range of bacteria in future studies. Mutations in ramR have so far only been described in Salmonella resistant to ciprofloxacin. We have shown for the first time that mutations in ramR also mediate resistance to tigecycline, presumably by upregulation of ramA. Thus, a preceding therapy with ciprofloxacin (and presumably other antibiotics) may affect susceptibility to tigecycline, even though the patient has never been treated with this drug.
Footnotes
Published ahead of print on 22 December 2009.
REFERENCES
- 1.Abouzeed, Y. M., S. Baucheron, and A. Cloeckaert. 2008. ramR mutations involved in efflux-mediated multidrug resistance in Salmonella enterica serovar Typhimurium. Antimicrob. Agents Chemother. 52:2428-2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aminov, R. I., J. C. Chee-Sanford, N. Garrigues, B. Teferedegne, I. J. Krapac, B. A. White, and R. I. Mackie. 2002. Development, validation, and application of PCR primers for detection of tetracycline efflux genes of gram-negative bacteria. Appl. Environ. Microbiol. 68:1786-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bratu, S., D. Landman, A. George, J. Salvani, and J. Quale. 2009. Correlation of the expression of acrB and the regulatory genes marA, soxS and ramA with antimicrobial resistance in clinical isolates of Klebsiella pneumoniae endemic to New York City. J. Antimicrob. Chemother. 64:278-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chopra, I., and M. Roberts. 2001. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 65:232-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clinical Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 7th ed. M7-A7. Clinical Laboratory Standards Institute, Wayne, PA.
- 6.Clinical Laboratory Standards Institute. 2008. Performance standards for antimicrobial susceptibility testing; eighteenth informational supplement. M100-S18. Clinical Laboratory Standards Institute, Wayne, PA.
- 7.Damier-Piolle, L., S. Magnet, S. Bremont, T. Lambert, and P. Courvalin. 2008. AdeIJK, a resistance-nodulation-cell division pump effluxing multiple antibiotics in Acinetobacter baumannii. Antimicrob. Agents Chemother. 52:557-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dean, C. R., M. A. Visalli, S. J. Projan, P. E. Sum, and P. A. Bradford. 2003. Efflux-mediated resistance to tigecycline (GAR-936) in Pseudomonas aeruginosa PAO1. Antimicrob. Agents Chemother. 47:972-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fluit, A. C., A. Florijn, J. Verhoef, and D. Milatovic. 2005. Presence of tetracycline resistance determinants and susceptibility to tigecycline and minocycline. Antimicrob. Agents Chemother. 49:1636-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guay, G. G., M. Tuckman, and D. M. Rothstein. 1994. Mutations in the tetA(B) gene that cause a change in substrate specificity of the tetracycline efflux pump. Antimicrob. Agents Chemother. 38:857-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirata, T., A. Saito, K. Nishino, N. Tamura, and A. Yamaguchi. 2004. Effects of efflux transporter genes on susceptibility of Escherichia coli to tigecycline (GAR-936). Antimicrob. Agents Chemother. 48:2179-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keeney, D., A. Ruzin, and P. A. Bradford. 2007. RamA, a transcriptional regulator, and AcrAB, an RND-type efflux pump, are associated with decreased susceptibility to tigecycline in Enterobacter cloacae. Microb. Drug Resist. 13:1-6. [DOI] [PubMed] [Google Scholar]
- 13.Keeney, D., A. Ruzin, F. McAleese, E. Murphy, and P. A. Bradford. 2008. MarA-mediated overexpression of the AcrAB efflux pump results in decreased susceptibility to tigecycline in Escherichia coli. J. Antimicrob. Chemother. 61:46-53. [DOI] [PubMed] [Google Scholar]
- 14.Kelesidis, T., D. E. Karageorgopoulos, I. Kelesidis, and M. E. Falagas. 2008. Tigecycline for the treatment of multidrug-resistant Enterobacteriaceae: a systematic review of the evidence from microbiological and clinical studies. J. Antimicrob. Chemother. 62:895-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McAleese, F., P. Petersen, A. Ruzin, P. M. Dunman, E. Murphy, S. J. Projan, and P. A. Bradford. 2005. A novel MATE family efflux pump contributes to the reduced susceptibility of laboratory-derived Staphylococcus aureus mutants to tigecycline. Antimicrob. Agents Chemother. 49:1865-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Regan, E., T. Quinn, J. M. Pages, M. McCusker, L. Piddock, and S. Fanning. 2009. Multiple regulatory pathways associated with high-level ciprofloxacin and multidrug resistance in Salmonella enterica serovar Enteritidis: involvement of ramA and other global regulators. Antimicrob. Agents Chemother. 53:1080-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petersen, P. J., N. V. Jacobus, W. J. Weiss, P. E. Sum, and R. T. Testa. 1999. In vitro and in vivo antibacterial activities of a novel glycylcycline, the 9-t-butylglycylamido derivative of minocycline (GAR-936). Antimicrob. Agents Chemother. 43:738-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peterson, L. R. 2008. A review of tigecycline: the first glycylcycline. Int. J. Antimicrob. Agents 32(Suppl. 4):S215-S222. [DOI] [PubMed] [Google Scholar]
- 19.Piddock, L. J. 2006. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin. Microbiol. Rev. 19:382-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poole, K. 2005. Efflux-mediated antimicrobial resistance. J. Antimicrob. Chemother. 56:20-51. [DOI] [PubMed] [Google Scholar]
- 21.Reid, G. E., S. A. Grim, C. A. Aldeza, W. M. Janda, and N. M. Clark. 2007. Rapid development of Acinetobacter baumannii resistance to tigecycline. Pharmacotherapy 27:1198-1201. [DOI] [PubMed] [Google Scholar]
- 22.Ricci, V., and L. J. Piddock. 2009. Ciprofloxacin selects for multidrug resistance in Salmonella enterica serovar Typhimurium mediated by at least two different pathways. J. Antimicrob. Chemother. 63:909-916. [DOI] [PubMed] [Google Scholar]
- 23.Ruzin, A., F. W. Immermann, and P. A. Bradford. 2008. Real-time PCR and statistical analyses of acrAB and ramA expression in clinical isolates of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 52:3430-3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruzin, A., D. Keeney, and P. A. Bradford. 2005. AcrAB efflux pump plays a role in decreased susceptibility to tigecycline in Morganella morganii. Antimicrob. Agents Chemother. 49:791-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruzin, A., D. Keeney, and P. A. Bradford. 2007. AdeABC multidrug efflux pump is associated with decreased susceptibility to tigecycline in Acinetobacter calcoaceticus-Acinetobacter baumannii complex. J. Antimicrob. Chemother. 59:1001-1004. [DOI] [PubMed] [Google Scholar]
- 26.Ruzin, A., M. A. Visalli, D. Keeney, and P. A. Bradford. 2005. Influence of transcriptional activator RamA on expression of multidrug efflux pump AcrAB and tigecycline susceptibility in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 49:1017-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stein, G. E., and W. A. Craig. 2006. Tigecycline: a critical analysis. Clin. Infect. Dis. 43:518-524. [DOI] [PubMed] [Google Scholar]
- 28.Tuckman, M., P. J. Petersen, A. Y. Howe, M. Orlowski, S. Mullen, K. Chan, P. A. Bradford, and C. H. Jones. 2007. Occurrence of tetracycline resistance genes among Escherichia coli isolates from the phase 3 clinical trials for tigecycline. Antimicrob. Agents Chemother. 51:3205-3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tuckman, M., P. J. Petersen, and S. J. Projan. 2000. Mutations in the interdomain loop region of the tetA(A) tetracycline resistance gene increase efflux of minocycline and glycylcyclines. Microb. Drug Resist. 6:277-282. [DOI] [PubMed] [Google Scholar]
- 30.Visalli, M. A., E. Murphy, S. J. Projan, and P. A. Bradford. 2003. AcrAB multidrug efflux pump is associated with reduced levels of susceptibility to tigecycline (GAR-936) in Proteus mirabilis. Antimicrob. Agents Chemother. 47:665-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Werner, G., S. Gfrorer, C. Fleige, W. Witte, and I. Klare. 2008. Tigecycline-resistant Enterococcus faecalis strain isolated from a German intensive care unit patient. J. Antimicrob. Chemother. 61:1182-1183. [DOI] [PubMed] [Google Scholar]