Abstract
By using immunofluorescence microscopy to observe and analyze freshly made HIV-1 virions adsorbed onto cells, we found that they are inherently highly infectious, rather than predominantly defective as previously suggested. Surprisingly, polycations enhance titers 20- to 30-fold by stabilizing adsorption and preventing a previously undescribed process of rapid dissociation, strongly implying that infectivity assays for many viruses are limited not only by inefficient virus diffusion onto cells but also by a postattachment race between entry and dissociation. This kinetic competition underlies inhibitory effects of CCR5 antagonists and explains why adaptive HIV-1 mutations overcome many cell entry limitations by accelerating entry.
It is widely believed that retroviruses, including HIV-1, are predominantly defective, with fewer than 0.1% in plasma or culture media being infectious (4, 23, 24, 30, 45). However, other evidence indicates that diffusion severely limits virion contact with cultured cells and that forcing virions onto cells by spinoculation or magnetic methods greatly increases titers (6, 17, 25, 36, 45). Additionally, polycations, including DEAE-dextran and polybrene, substantially increase titers (5, 12, 13, 20, 25, 47). Reverse transcription is also somewhat inefficient, depending on the virus isolate and cells used. To reconcile these observations and more accurately measure infectivities, we adsorbed HIV-1 onto highly susceptible JC.53 cells (33, 42) at 4°C by spinoculation or brief incubation with concentrated virus, and we analyzed cell-attached virions by immunofluorescence and deconvolution microscopy (29, 39).
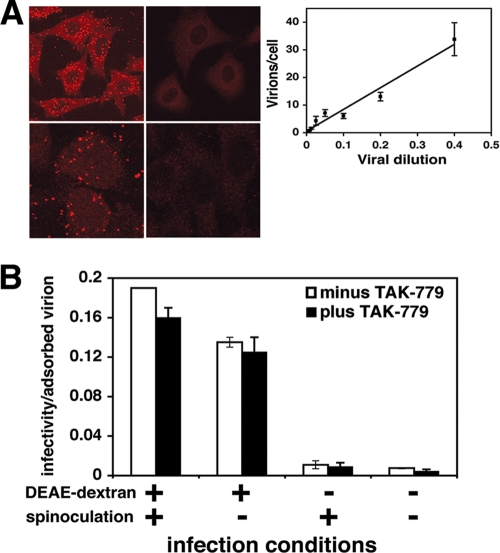
Figure 1A shows fields of adsorbed HIV-1. Cells were fixed for 10 min with 3.7% paraformaldehyde in phosphate-buffered saline (PBS) containing 2% sucrose and then rinsed and incubated for 10 min in 0.5% NP-40 in PBS containing 10% sucrose, and virions were stained using anti-p24 Gag mouse hybridoma 183 (from the NIH AIDS Research and Reference Reagent Program; provided by B. Chesebro). The Z-stack of deconvoluted images was compressed to show all virions. The fluorescent foci had similar intensities, implying that they were single virions. Accordingly, their staining required extraction of lipids with NP-40 (Fig. 1A, lower panels), and the numbers of foci were directly proportional to virus concentrations in the media (Fig. 1A, inset). Similar results were obtained using HIV-1 labeled with Vpr-green fluorescent protein (GFP), and correlative electron microscopy confirmed that these fluorescent foci were single virions (results not shown).
FIG. 1.
Immunofluorescent visualization of HIV-1 virions adsorbed onto HeLa-CD4/CCR5 (clone JC.53) cells and effects of DEAE-dextran and spinoculation on virion infectivity. (A) HIV-1 cell surface visualization. The upper left panel shows a representative large field image of cells containing adsorbed HIV-1NL4-3. The upper right panel shows control cells lacking adsorbed virions. The lower left panel shows a higher-magnification image of two cells containing adsorbed virions. The lower right panel is a replicate culture at the same magnification that was stained without extracting the lipids. The inset shows the effect of virus dilution on the numbers of fluorescent particles counted on the cells. The number of virions/cell was directly proportional to the concentration of HIV-1NL4-3. Fifty to 100 cells were counted per dilution to obtain the average number of adsorbed virions per cell; error bars represent the standard error of the mean (SEM). (B) DEAE-dextran and spinoculation effects on HIV-1 infectivity. Replicate cultures of target JC.53 cells were pretreated with 8 μg/ml DEAE-dextran by incubation at 37°C for 20 min, followed by rinsing in medium lacking DEAE, or were pretreated with medium alone and then spinoculated with HIV-1JRCSF or incubated with virus without centrifugation. One set of cultures was used to determine the number of virions per cell. Other duplicate sets of cultures, one of which was treated with an inhibitory dose of TAK779 20 h after virus incubation, were used to determine virus titers. The small differences in infectivity/adsorbed virion values in cultures subjected to TAK779 were not significant or reproducible (e.g., see Table 1). Differences in infectivity/virion values compared to those in Table 1 reflect the fact that these experiments were performed independently, using different virus preparations and different thawed JC.53 cell aliquots as targets. Averages of the results for two independent experiments are shown, and error bars represent the range. The different infection conditions resulted in the following numbers of virions adsorbed to cells: +DEAE/+spin, 7.9 ± 0.70; +DEAE/−spin, 2.7 ± 0.10; −DEAE/+spin, 5.4 ± 0.30; −DEAE/−spin, 0.81 ± 0.060.
We measured the infectivities of cell-attached HIV-1 following spinoculation (4°C, 30 min, 188 × g) onto cultures pretreated with DEAE-dextran and a subsequent rinsing of the cultures (Table 1) (8). We analyzed molecularly cloned isolates, mostly R5 HIV-1JRCSF, although comparisons were done with R5 HIV-1YU-2, X4 HIV-1HXB3, and HIV-1NL4-3. Virus stocks were produced by transfection of 293T cells with a subsequent expansion in JC.53 cells, followed by filtering virus-containing supernatants and storing aliquots at −80°C. JC.53 cells were inoculated with these viruses, and virion-containing media were harvested 2 days later, 5 h or 24 h after media had been changed. Infectivities of adsorbed virions were surprisingly high, especially those for HIV-1JRCSF, which were ∼0.42 ± 0.09 focus-forming unit (FFU)/virion (n = 4). Virions in 5-h harvests were generally more infectious than were virions that had accumulated in media for 24 h. HIV-1YU-2, HIV-1NL4-3, and HIV-1HXB3 were significantly less infectious, but even in those cases, titers were generally ∼0.05 to 0.08 FFU/virion. Notably, JC.53 cells express high concentrations of CD4 and CCR5 that are optimal for R5 viruses but contain only the endogenous CXCR4 naturally present in HeLa cells (42). Consequently, other cells might be more efficiently infected by X4 viruses. Although we stained the foci of infection 72 h after warming the cultures to 37°C, which conceivably might have allowed secondary infections to occur, this was not a problem, because entry inhibitors added 20 h after warming did not significantly reduce the numbers of infected foci (Table 1 and Fig. 1B).
TABLE 1.
Infectivities of adsorbed HIV-1 virions
| Virus | Condition(s)a | Infection/virionb |
|---|---|---|
| JRCSF (n = 4) | 5 h | 0.42 ± 0.090 |
| 24 h | 0.27 ± 0.034 | |
| NL4-3 (n = 2) | 5 h | 0.071 ± 0.0095 |
| HXB3 (n = 2) | 5 h | 0.083 ± 0.0090 |
| YU2 | Expt 1, 5 h, −TAK | 0.033 |
| Expt 1, 5 h, +TAK | 0.039 | |
| Expt 2, 5 h, −TAK | 0.063 | |
| Expt 2, 5 h, +TAK | 0.083 |
Target cells were spinoculated with virus preparations that had been harvested 5 h or 24 h after medium had been changed on producer cells. For YU2, duplicate cultures were treated 20 h after initiation of infection with (+) or without (−) addition of a completely inhibitory 15 μM TAK779 concentration. Expt, experiment.
Virions were counted after spinoculating concentrated virions onto cells. Appropriately diluted samples were spinoculated onto other cultures for focal infectivity assays as described previously (8). This dilution approach was validated in the inset shown in Fig. 1. Infection/virion values were then obtained by normalizing infections/cell values obtained from virus titers by virions/cell values obtained by counting immunofluorescently stained virus particles adsorbed onto cells.
Because the experiments whose results are shown in Table 1 employed spinoculation onto cultures preincubated with 8 μg/ml DEAE-dextran, we analyzed these treatments. Spinoculation increased adsorption but did not significantly change the infectivity per adsorbed virion (Fig. 1B). In striking contrast, while DEAE-dextran enhanced the numbers of HIV-1JRCSF that were present on the briefly rinsed cultures ∼0.5- to 3-fold, suggesting that it made the virions “stickier,” its major effect was to increase the infectivities of cell-associated virions ∼20- to 30-fold (Fig. 1B).
This suggested that DEAE-dextran moderately increased initial virus attachment or stabilized it during the brief rinsing that preceded further analyses. Additionally, it greatly enhanced the postattachment efficiency of infection. To analyze this, we spinoculated HIV-1JRCSF onto HeLa-CD4/CCR5 (clone JC.53), HeLa-CD4 (clone HI-J), and control HeLa cells in the presence or absence of DEAE-dextran and counted cell-associated virions after briefly rinsing away unadsorbed virions and warming to 37°C. With all three cell clones, the virions remained visible much longer when DEAE-dextran was present (Table 2). Rapid loss of virions from cells lacking DEAE-dextran did not require CD4 or CCR5, suggesting that it was not caused by viral fusion with cell membranes followed by dispersal of virion cores in the cytosol. Moreover, neither the extent of initial attachment nor the rate of virion loss was significantly affected by CD4 or CCR5, consistent with previous results (29, 32, 39). Virion loss was not caused principally by endocytosis and lysosomal degradation, because the lysosomal inhibitor chloroquine at a concentration that blocked degradation of endocytosed gp120 (21) had no effect (results not shown). Moreover, fluorescent foci were lost in an all-or-nothing manner rather than by gradually diminishing in intensity. We conclude that DEAE-dextran does not influence initial HIV-1 attachment onto cells but strongly stabilizes adsorption. Shedding of cell-attached retrovirions was also recently demonstrated by other methods (3, 10, 31, 37).
TABLE 2.
Effect of DEAE-dextran on stability of HIV-1 adsorption
| Time (min) | Average no. of virions per cell ± SEMa |
|||||
|---|---|---|---|---|---|---|
| JC.53 |
HI-J |
HeLa |
||||
| +DEAE-dextranb | −DEAE-dextran | +DEAE-dextran | −DEAE-dextran | +DEAE-dextran | −DEAE-dextran | |
| 0 | 18 ± 1.1 | 16 ± 1.3 | 34 ± 1.6 | 13 ± 0.68 | 20 ± 1.1 | 11 ± 0.66 |
| 15 | 24 ± 1.2 | 7.2 ± 0.43 | N.D. | N.D. | N.D. | N.D. |
| 30 | 19 ± 1.3 | 6.2 ± 0.48 | N.D. | N.D. | N.D. | N.D. |
| 120 | 23 ± 1.3 | 3.2 ± 0.41 | 36 ± 1.7 | 3.9 ± 0.38 | 31 ± 1.7 | 2.0 ± 0.25 |
HIV-1JRCSF virions were adsorbed by spinoculation onto JC.53 cells, or HeLa cells expressing CD4, but not CCR5 (HI-J), or HeLa cells that lack CD4 and CCR5. N.D., not done.
The target cells were treated with (+DEAE-dextran) or without (−DEAE-dextran) 8 μg/ml DEAE-dextran prior to spinoculation. After spinoculation, cells were incubated at 37°C for the indicated times and then fixed and processed for anti-p24 immunofluorescence.
Although virions dissociated rapidly in the absence of DEAE-dextran and were almost entirely gone after ∼30 min at 37°C (Table 2), a small fraction became stably adherent and remained affixed for at least 2 h. This implies that the virions attach onto cells lacking polycations with diverse affinities and that a small proportion eventually becomes stably bound in a manner independent of CD4 or CCR5.
These results substantiate evidence that infectivities of cell-associated HIV-1 depend on kinetic competition between successful entry and process(es) causing viral inactivation (22, 40, 41) and demonstrate that dissociation is the predominant competing process in cultures lacking polycations. Because virion diffusion is severely limiting in culture conditions, dissociated virions would be unlikely to encounter another cell before spontaneously inactivating in the medium (1, 9, 19, 34). Moreover, the large titer enhancements caused by polycations (Fig. 1B) and other adherence factors (2, 11, 15, 44) suggest that nearly all virions enter slowly compared to dissociation (40, 43).
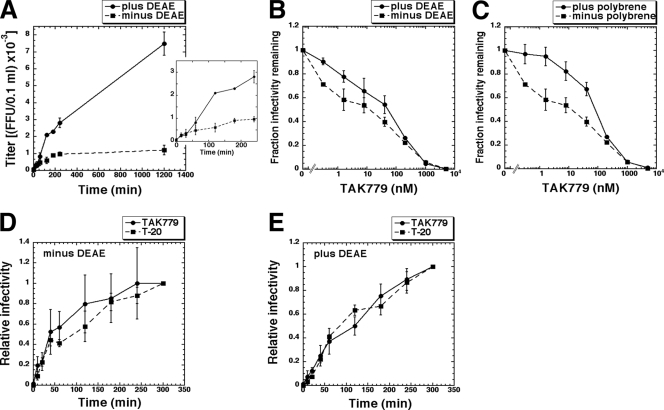
We tested these kinetic interpretations by measuring rates of HIV-1JRCSF entry by using the CCR5 antagonist TAK779 to inactivate virions that had not completed the CCR5-dependent steps of entry (40, 41). There were two kinetic phases, with some virions entering rapidly within the ∼30- to 60-min period required for loosely adherent virions to dissociate and with others entering slowly, taking ∼1 to 20 h (Fig. 2A). In the absence of polycations, approximately half the infections occurred rapidly in competition with dissociation, and the remainder were caused by the small number of stably adsorbed virions that entered leisurely. In contrast, polycations stably affixed all virions and greatly increased the number of virions that were able to enter slowly.
FIG. 2.
Effects of polycations on HIV-1 infection kinetics and on sensitivity to inhibition by the CCR5 antagonist TAK779. (A) HIV-1 infection time course in the absence and presence of DEAE-dextran. Viruses were pelleted by spinoculation at 4°C onto JC.53 cell cultures pretreated with media that lacked or contained 8 μg/ml DEAE-dextran, followed by washing and warming at 37°C. At 0, 15, 30, 60, 120, 180, 240, and 1,200 min, TAK779 was added to yield a completely inhibitory concentration. Foci of infection were stained 72 h after the initial 0-h time point. The inset shows an expanded plot of the same data that includes only the 0- to 240-min time points. The averages of the results for two experiments performed in triplicate are shown. Error bars represent the range. (B and C) Effects of polycations on HIV-1 TAK779 sensitivity. Cells were pretreated with 8 μg/ml DEAE-dextran (B), 8 μg/ml polybrene (C), or medium lacking polycations, and then infected in the absence or presence of serial 5-fold dilutions of TAK779. Virus titers measured at each TAK779 concentration were normalized to titers in the absence of TAK779 (fraction infectivity remaining) and were plotted versus TAK779 concentrations. The averages of the results for three to four experiments performed in quadruplicate are shown. Error bars represent the SEM. (D and E) Kinetics of HIV-1JRCSF escape from TAK779 and T-20. Viruses were pelleted by spinoculation at 4°C onto JC.53 cell cultures pretreated with medium that lacked or contained 8 μg/ml DEAE-dextran, followed by washing and warming at 37°C. At 0, 10, 20, 40, 60, 120, 180, 240, and 300 min, TAK779 or T-20 was added to yield a completely inhibitory concentration. Virus titers were normalized to the final 300-min time point (relative infectivity) and plotted versus the times of inhibitor addition. The averages of the results for three experiments with two to six replicates per experiment are shown. Error bars represent the SEM. At the 300-min time point, virus titers in the absence of DEAE-dextran were approximately 10-fold lower than they were in the presence of DEAE-dextran.
TAK779 lowers the effective CCR5 concentration and thereby slows HIV-1 entry (40). Accordingly, DEAE-dextran and polybrene substantially reduced inhibition of HIV-1JRCSF by TAK779 (Fig. 2B and C). The TAK779 inhibition curves reproducibly had a biphasic shape, especially in the absence of polycations. This is compatible with our evidence that there are two categories of virions that infect JC.53 cells lacking polycations, one that is loosely bound and infects in competition with dissociation and a second that is stably adsorbed and infects more leisurely (Fig. 2A and Table 2). Low concentrations of TAK779 slow entry slightly and preferentially inactivate virions that infect in competition with dissociation, whereas the stably adsorbed virions are less inhibited because they remain on the cells. Consequently, the ± polycation curves are shifted ∼10-fold along the concentration axis at low TAK779 concentrations, whereas they converge at higher concentrations. These interpretations were supported by using diverse inhibitors that slow entry and by using HeLa-CD4/CCR5 cells with a limiting amount of CCR5 that are infected slowly (results not shown).
Presumably, the infectious virions that remain stably attached to cells and sensitive to TAK779 after 60 min (Table 2 and Fig. 2A) might occur in endosomes. Indeed, TAK779 has been reported to penetrate membranes (28). However, we found that blocking endocytosis with dynasore (27) did not alter virion adherence (results not shown). Moreover, the kinetics of HIV-1JRCSF escape from TAK779 and from the membrane-impermeable peptide inhibitors T-20 and C34 that bind irreversibly to a three-stranded coil (3SC) intermediate in the gp41 refolding pathway (14) were not significantly different (Fig. 2D and E). No infections occurred when the inhibitors were present throughout the incubations, implying that viable virions all form 3SCs on cell surfaces. Virions sensitive to TAK779 after ∼60 min were also sensitive to T-20 and C34, confirming their presence on cell surfaces (Fig. 2D and E). Thus, infectious HIV-1 virions form 3SCs and can remain on cell surfaces for many hours before completing entry in a slow stochastic manner. Although these data are compatible with recent evidence that the final stage of HIV-1 entry may occur in endosomes (31), we believe that the presumptive endocytosis rate inferred in that work may have been substantially overestimated due to an absence of polycations and analysis only of virions that completed entry within 90 min.
In contrast to suggestions that polycations enhance titers of many viruses by increasing initial attachments onto cells (12, 13), our evidence suggests that they stabilize adsorption. A corollary is that titers measured for these viruses may be generally limited by dissociation. Furthermore, dissociation is probably a limiting factor in all cultures because adhesion factors, including ICAM-1 incorporated into HIV-1, greatly increase infectivities for cells, including peripheral blood mononuclear cells (PBMCs) that contain corresponding receptors (2, 11, 15, 44, 48), and exogenous factors, including lectins and a semen factor that tightly bind virions onto cells also enhance infectivities (16, 26, 35, 49). Although we conclude that slow diffusion and rapid dissociation severely limit HIV-1 infections in cultures, these kinetic processes are probably less limiting in the context of cell-to-cell transmission and in small intercellular spaces in vivo (7, 18, 38, 46). Considering these issues may improve development of entry inhibitors and vaccines for AIDS.
Acknowledgments
This study was supported by grants CA067358 (D.K.) and RD1AI052051 (T.H.) from the National Institutes of Health.
We thank Bruce Chesebro for donating anti-p24 hybridoma 183 to the NIH AIDS Research and Reference Reagent Program.
Footnotes
Published ahead of print on 30 December 2009.
REFERENCES
- 1.Allison, A. C., and R. C. Valentine. 1960. Virus particle adsorption. III. Adsorption of viruses by cell monolayers and effects of some variables on adsorption. Biochim. Biophys. Acta 40:400-410. [DOI] [PubMed] [Google Scholar]
- 2.Beauséjour, Y., and M. J. Tremblay. 2004. Susceptibility of HIV type 1 to the fusion inhibitor T-20 is reduced on insertion of host intercellular adhesion molecule 1 in the virus membrane. J. Infect. Dis. 190:894-902. [DOI] [PubMed] [Google Scholar]
- 3.Blömer, U., I. Gruh, H. Witschel, A. Haverich, and U. Martin. 2005. Shuttle of lentiviral vectors via transplanted cells in vivo. Gene Ther. 12:67-74. [DOI] [PubMed] [Google Scholar]
- 4.Bourinbaiar, A. S. 1994. The ratio of defective HIV-1 particles to replication-competent infectious virions. Acta Virol. 38:59-61. [PubMed] [Google Scholar]
- 5.Callahan, L. 1994. HIV-1 virion-cell interactions: an electrostatic model of pathogenicity and syncytium formation. AIDS Res. Hum. Retroviruses 10:231-233. [DOI] [PubMed] [Google Scholar]
- 6.Chan, L., D. Nesbeth, T. Mackey, J. Galea-Lauri, J. Gaken, F. Martin, M. Collins, G. Mufti, F. Farzaneh, and D. Darling. 2005. Conjugation of lentivirus to paramagnetic particles via nonviral proteins allows efficient concentration and infection of primary acute myeloid leukemia cells. J. Virol. 79:13190-13194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, P., W. Hubner, M. A. Spinelli, and B. K. Chen. 2007. Predominant mode of human immunodeficiency virus transfer between T cells is mediated by sustained Env-dependent neutralization-resistant virological synapses. J. Virol. 81:12582-12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chesebro, B., and K. Wehrly. 1988. Development of a sensitive quantitative focal assay for human immunodeficiency virus infectivity. J. Virol. 62:3779-3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chuck, A. S., M. F. Clarke, and B. O. Palsson. 1996. Retroviral infection is limited by Brownian motion. Hum. Gene Ther. 7:1527-1534. [DOI] [PubMed] [Google Scholar]
- 10.Cole, C., J. Qiao, T. Kottke, R. M. Diaz, A. Ahmed, L. Sanchez-Perez, G. Brunn, J. Thompson, J. Chester, and R. G. Vile. 2005. Tumor-targeted, systemic delivery of therapeutic viral vectors using hitchhiking on antigen-specific T cells. Nat. Med. 11:1073-1081. [DOI] [PubMed] [Google Scholar]
- 11.Cosma, A., D. Blanc, J. Braun, C. Quillent, C. Barassi, C. Moog, S. Klasen, B. Spire, G. Scarlatti, E. Pesenti, A. G. Siccardi, and A. Beretta. 1999. Enhanced HIV infectivity and changes in GP120 conformation associated with viral incorporation of human leucocyte antigen class I molecules. AIDS 13:2033-2042. [DOI] [PubMed] [Google Scholar]
- 12.Davis, H. E., J. R. Morgan, and M. L. Yarmush. 2002. Polybrene increases retrovirus gene transfer efficiency by enhancing receptor-independent virus adsorption on target cell membranes. Biophys. Chem. 97:159-172. [DOI] [PubMed] [Google Scholar]
- 13.Davis, H. E., M. Rosinski, J. R. Morgan, and M. L. Yarmush. 2004. Charged polymers modulate retrovirus transduction via membrane charge neutralization and virus aggregation. Biophys. J. 86:1234-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckert, D. M., and P. S. Kim. 2001. Mechanisms of viral membrane fusion and its inhibition. Annu. Rev. Biochem. 70:777-810. [DOI] [PubMed] [Google Scholar]
- 15.Fortin, J. F., B. Barbeau, H. Hedman, E. Lundgren, and M. J. Tremblay. 1999. Role of the leukocyte function antigen-1 conformational state in the process of human immunodeficiency virus type 1-mediated syncytium formation and virus infection. Virology 257:228-238. [DOI] [PubMed] [Google Scholar]
- 16.Geijtenbeek, T. B., D. S. Kwon, R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, J. Middel, I. L. Cornelissen, H. S. Nottet, V. N. KewalRamani, D. R. Littman, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587-597. [DOI] [PubMed] [Google Scholar]
- 17.Haim, H., I. Steiner, and A. Panet. 2005. Synchronized infection of cell cultures by magnetically controlled virus. J. Virol. 79:622-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hübner, W., G. P. McNerney, P. Chen, B. M. Dale, R. E. Gordon, F. Y. Chuang, X. D. Li, D. M. Asmuth, T. Huser, and B. K. Chen. 2009. Quantitative 3D video microscopy of HIV transfer across T cell virological synapses. Science 323:1743-1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kabat, D., S. L. Kozak, K. Wehrly, and B. Chesebro. 1994. Differences in CD4 dependence for infectivity of laboratory-adapted and primary patient isolates of human immunodeficiency virus type 1. J. Virol. 68:2570-2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kahn, M. L., S. W. Lee, and D. A. Dichek. 1992. Optimization of retroviral vector-mediated gene transfer into endothelial cells in vitro. Circ. Res. 71:1508-1517. [DOI] [PubMed] [Google Scholar]
- 21.Kozak, S. L., J. M. Heard, and D. Kabat. 2002. Segregation of CD4 and CXCR4 into distinct lipid microdomains in T lymphocytes suggests a mechanism for membrane destabilization by human immunodeficiency virus. J. Virol. 76:1802-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuhmann, S. E., E. J. Platt, S. L. Kozak, and D. Kabat. 2000. Cooperation of multiple CCR5 coreceptors is required for infections by human immunodeficiency virus type 1. J. Virol. 74:7005-7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwon, Y. J., G. Hung, W. F. Anderson, C. A. Peng, and H. Yu. 2003. Determination of infectious retrovirus concentration from colony-forming assay with quantitative analysis. J. Virol. 77:5712-5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Layne, S. P., M. J. Merges, M. Dembo, J. L. Spouge, S. R. Conley, J. P. Moore, J. L. Raina, H. Renz, H. R. Gelderblom, and P. L. Nara. 1992. Factors underlying spontaneous inactivation and susceptibility to neutralization of human immunodeficiency virus. Virology 189:695-714. [DOI] [PubMed] [Google Scholar]
- 25.Le Doux, J. M., N. Landazuri, M. L. Yarmush, and J. R. Morgan. 2001. Complexation of retrovirus with cationic and anionic polymers increases the efficiency of gene transfer. Hum. Gene Ther. 12:1611-1621. [DOI] [PubMed] [Google Scholar]
- 26.Lee, B., G. Leslie, E. Soilleux, U. O'Doherty, S. Baik, E. Levroney, K. Flummerfelt, W. Swiggard, N. Coleman, M. Malim, and R. W. Doms. 2001. cis expression of DC-SIGN allows for more efficient entry of human and simian immunodeficiency viruses via CD4 and a coreceptor. J. Virol. 75:12028-12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macia, E., M. Ehrlich, R. Massol, E. Boucrot, C. Brunner, and T. Kirchhausen. 2006. Dynasore, a cell-permeable inhibitor of dynamin. Dev. Cell 10:839-850. [DOI] [PubMed] [Google Scholar]
- 28.Madani, N., A. M. Hubicki, A. L. Perdigoto, M. Springer, and J. Sodroski. 2007. Inhibition of human immunodeficiency virus envelope glycoprotein-mediated single cell lysis by low-molecular-weight antagonists of viral entry. J. Virol. 81:532-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maréchal, V., F. Clavel, J. M. Heard, and O. Schwartz. 1998. Cytosolic Gag p24 as an index of productive entry of human immunodeficiency virus type 1. J. Virol. 72:2208-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marozsan, A. J., E. Fraundorf, A. Abraha, H. Baird, D. Moore, R. Troyer, I. Nankja, and E. J. Arts. 2004. Relationships between infectious titer, capsid protein levels, and reverse transcriptase activities of diverse human immunodeficiency virus type 1 isolates. J. Virol. 78:11130-11141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyauchi, K., Y. Kim, O. Latinovic, V. Morozov, and G. B. Melikyan. 2009. HIV enters cells via endocytosis and dynamin-dependent fusion with endosomes. Cell 137:433-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mondor, I., S. Ugolini, and Q. J. Sattentau. 1998. Human immunodeficiency virus type 1 attachment to HeLa CD4 cells is CD4 independent and gp120 dependent and requires cell surface heparans. J. Virol. 72:3623-3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montefiori, D., Q. Sattentau, J. Flores, J. Esparza, and J. Mascola. 2007. Antibody-based HIV-1 vaccines: recent developments and future directions. PLoS Med. 4:e348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morgan, J. R., J. M. LeDoux, R. G. Snow, R. G. Tompkins, and M. L. Yarmush. 1995. Retrovirus infection: effect of time and target cell number. J. Virol. 69:6994-7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Münch, J., E. Rucker, L. Standker, K. Adermann, C. Goffinet, M. Schindler, S. Wildum, R. Chinnadurai, D. Rajan, A. Specht, G. Gimenez-Gallego, P. C. Sanchez, D. M. Fowler, A. Koulov, J. W. Kelly, W. Mothes, J. C. Grivel, L. Margolis, O. T. Keppler, W. G. Forssmann, and F. Kirchhoff. 2007. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell 131:1059-1071. [DOI] [PubMed] [Google Scholar]
- 36.O'Doherty, U., W. J. Swiggard, and M. H. Malim. 2000. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J. Virol. 74:10074-10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan, Y. W., J. M. Scarlett, T. T. Luoh, and P. Kurre. 2007. Prolonged adherence of human immunodeficiency virus-derived vector particles to hematopoietic target cells leads to secondary transduction in vitro and in vivo. J. Virol. 81:639-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piguet, V., and Q. Sattentau. 2004. Dangerous liaisons at the virological synapse. J. Clin. Investig. 114:605-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pizzato, M., S. A. Marlow, E. D. Blair, and Y. Takeuchi. 1999. Initial binding of murine leukemia virus particles to cells does not require specific Env-receptor interaction. J. Virol. 73:8599-8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Platt, E. J., J. P. Durnin, and D. Kabat. 2005. Kinetic factors control efficiencies of cell entry, efficacies of entry inhibitors, and mechanisms of adaptation of human immunodeficiency virus. J. Virol. 79:4347-4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Platt, E. J., J. P. Durnin, U. Shinde, and D. Kabat. 2007. An allosteric rheostat in HIV-1 gp120 reduces CCR5 stoichiometry required for membrane fusion and overcomes diverse entry limitations. J. Mol. Biol. 374:64-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Platt, E. J., K. Wehrly, S. E. Kuhmann, B. Chesebro, and D. Kabat. 1998. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J. Virol. 72:2855-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raviv, Y., M. Viard, J. Bess, Jr., and R. Blumenthal. 2002. Quantitative measurement of fusion of HIV-1 and SIV with cultured cells using photosensitized labeling. Virology 293:243-251. [DOI] [PubMed] [Google Scholar]
- 44.Rizzuto, C. D., and J. G. Sodroski. 1997. Contribution of virion ICAM-1 to human immunodeficiency virus infectivity and sensitivity to neutralization. J. Virol. 71:4847-4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rusert, P., M. Fischer, B. Joos, C. Leemann, H. Kuster, M. Flepp, S. Bonhoeffer, H. F. Gunthard, and A. Trkola. 2004. Quantification of infectious HIV-1 plasma viral load using a boosted in vitro infection protocol. Virology 326:113-129. [DOI] [PubMed] [Google Scholar]
- 46.Sowinski, S., C. Jolly, O. Berninghausen, M. A. Purbhoo, A. Chauveau, K. Kohler, S. Oddos, P. Eissmann, F. M. Brodsky, C. Hopkins, B. Onfelt, Q. Sattentau, and D. M. Davis. 2008. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat. Cell Biol. 10:211-219. [DOI] [PubMed] [Google Scholar]
- 47.Thomas, J. A., D. E. Ott, and R. J. Gorelick. 2007. Efficiency of human immunodeficiency virus type 1 postentry infection processes: evidence against disproportionate numbers of defective virions. J. Virol. 81:4367-4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ugolini, S., I. Mondor, and Q. J. Sattentau. 1999. HIV-1 attachment: another look. Trends Microbiol. 7:144-149. [DOI] [PubMed] [Google Scholar]
- 49.Wang, J. H., A. M. Janas, W. J. Olson, V. N. KewalRamani, and L. Wu. 2007. CD4 coexpression regulates DC-SIGN-mediated transmission of human immunodeficiency virus type 1. J. Virol. 81:2497-2507. [DOI] [PMC free article] [PubMed] [Google Scholar]