Summary
Stem cells within the bone marrow (BM) exist in a quiescent state or are instructed to differentiate and mobilize to circulation following specific signals. Matrix metalloproteinase-9 (MMP-9), induced in BM cells, releases soluble Kit-ligand (sKitL), permitting the transfer of endothelial and hematopoietic stem cells (HSCs) from the quiescent to proliferative niche. BM ablation induces SDF-1, which upregulates MMP-9 expression, and causes shedding of sKitL and recruitment of c-Kit+ stem/progenitors. In MMP-9−/− mice, release of sKitL and HSC motility are impaired, resulting in failure of hematopoietic recovery and increased mortality, while exogenous sKitL restores hematopoiesis and survival after BM ablation. Release of sKitL by MMP-9 enables BM repopulating cells to translocate to a permissive vascular niche favoring differentiation and reconstitution of the stem/progenitor cell pool.
Introduction
Stem cells are localized in a microenvironment known as the stem cell “niche,” where they are maintained in an undifferentiated and quiescent state. These niches are critical for regulating the self-renewal and cell fate decisions, yet molecular mechanisms governing survival and maintenance of quiescent stem cells in these specialized environments and why and how these cells are recruited to exit these niches are not well studied. In Drosophila, germ cells lost by normal or induced differentiation are efficiently replaced within their niches (Xie and Spradling, 2000). Stromal cells provide extrinsic signals that maintain the stem cell niche and regulate the repopulation of stem cells. Mice with steel (Sl/Sld) mutation produce deficiency in membrane Kit-ligand (KitL, stem cell factor) in the tissue microenvironment, impairing the proliferation and migration of spermatogonial stem cells (Ohta et al., 2000). In mammals, neurogenesis occurs within an angiogenic niche, which may provide an interface where the microenvironment of stromal cells and circulating factors influence plasticity in the adult central nervous system (Palmer et al., 2000).
BM is a major reservoir for adult organ-specific stem cells, including hematopoietic stem cells (HSCs; Reya et al., 2001), endothelial progenitors (Lyden et al., 2001), neuronal and muscle stem cells (Krause et al., 2001; Blau et al., 2001). Under steady-state conditions, most stem cells are in contact with BM stromal cells, including osteoblasts, and are maintained in G0 phase of cell cycle (Cheng et al., 2000), while a small fraction is in S or G2/M phase of the cell cycle. The equilibrium between these two compartments is dictated by the bioavailability of stem cell-active cytokines, which are bound to the extracellular matrix or tethered to the membrane of stromal cells. Stress, such as BM ablation by cytotoxic agents, switches on sequences of events where HSCs are recruited from their niches to reconstitute hematopoiesis. Treatment with cell cycle cytotoxic agents, including 5-fluorouracil (5-FU), depletes cycling hematopoietic cells. Within 3 days after myelosuppressive stress, the sequential release of chemokines and cytokines that promote stem cell migration into a permissive microenvironment support proliferation, differentiation, and mobilization to the circulation. By day 10 after 5-FU treatment, myeloid, erythroid, lymphoid, and megakaryocytic cell lineages repopulate the BM. In parallel, the mobilized stem cells can be incorporated into specific organs or, in the case of HSCs, expand in extramedullary sites and recirculate back to the BM to replenish the stem and progenitor pool.
HSCs, and other stem cells including cardiac (Orlic et al., 2001), endothelial (Peichev et al., 2000), and epithelial cells express c-Kit, the receptor for KitL, suggesting that a common signaling cascade may govern their proliferation and recruitment. Mutations in KitL or c-Kit produce defects in germ cell and melanocyte development, impairment of hematopoiesis, and increased sensitivity to radiation and chemotherapy (Huang et al., 1992). These observations suggest that KitL plays a key role for maintaining and reconstituting the stem cell pool in adult mice.
Local secretion of proteases may alter the stem cell-stromal cell interaction. The proteolytic cleavage of vascular cell adhesion molecule-1, expressed by BM stromal cells, is triggered by the degranulation of neutrophils in the BM after granulocyte colony stimulating factor (G-CSF) administration and may be an essential step contributing to the mobilization of hematopoietic progenitors (Levesque et al., 2001). Matrix metalloproteinases (MMPs) promote the release of extracellular matrix-bound or cell-surface-bound cytokines (Vu and Werb, 2000), such as vascular endothelial growth factor (VEGF), which then can regulate angiogenesis (Bergers et al., 2000) or osteoclast recruitment (Engsig et al., 2000). Accordingly, we asked whether MMPs contribute to the release of stem cell-active cytokines following stress that shifts stem cells and progenitors from a quiescent to a proliferative niche essential to reconstitute the stem cell pool and hematopoietic recovery.
Results
BM Suppression Induces MMP-9 Expression in BM Cells
While BM suppression with 5-FU results in apoptosis of actively cycling HSCs and progenitor cells, it does not affect HSCs in G0 of cell cycle. This model is ideal for studying factors that promote recruitment of HSCs during hematopoietic reconstitution. We found an increase in pro-MMP-9 (Figure 1A) and active MMP-9 (Figure 1B), but not tissue inhibitor of metalloproteinases (TIMP-1) in supernatants of BM cells of MMP-9 wild-type animals three days after 5-FU treatment. In BM cells of untreated animals, there was a small amount of MMP-9 (Figure 1B) derived from resident neutrophils (Murphy et al., 1982). In wild-type mice, BM hematopoietic and stromal cells expressed MMP-9 three days after treatment with 5-FU (Figure 1C). These data suggest that both pro-MMP-9 and MMP-9 are upregulated in BM cells after myelosuppression.
Figure 1. MMP-9 Is Induced in BM Cells after BM Ablation.
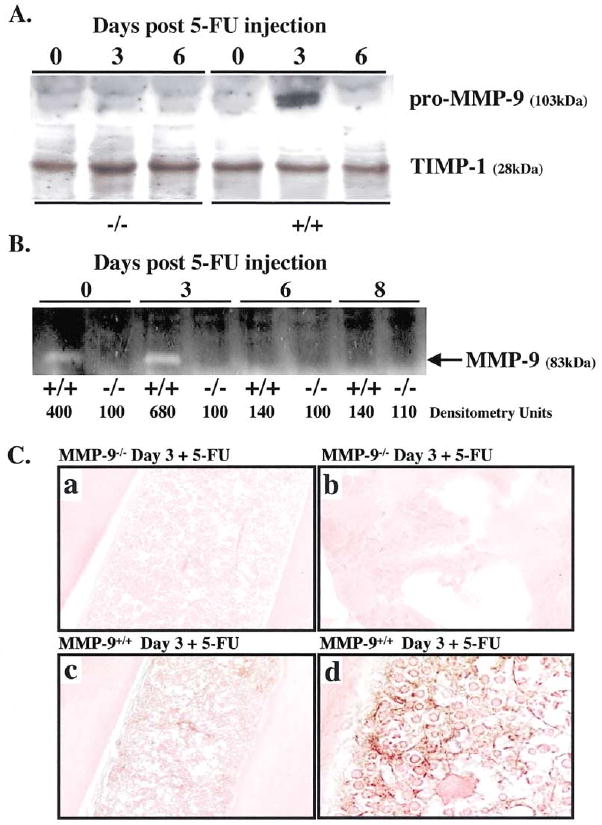
(A and B) MMP-9−/− and MMP-9+/+ mice received a single dose of 5-FU i.v. BM cells obtained at different time points after 5-FU injection were cultured in serum-free medium overnight. BM cell supernatants were assayed for pro-MMP-9 by Western blot (A) and active MMP-9 by gelatin zymography (B). Molecular weight (kDa) are shown (n = 5/group).
(C) Immunohistochemistry of BM sections three days after 5-FU injection for pro-MMP-9, which shows brown staining of stromal and hematopoietic elements in MMP-9+/+ mice (c and d), but not in MMP-9−/− mice (a and b); magnification ×100, (a and c) and ×400 (b and d).
MMP-9−/− Mice Show Delayed Hematopoietic Recovery Following 5-FU Treatment
To understand the role of MMP-9 in promoting HSC recruitment and hematopoietic recovery after BM suppression, we used MMP-9-deficientmice. Under steady-state conditions, adult MMP-9−/− and MMP-9+/+ mice have similar peripheral white blood cell (WBC) counts and both became leukopenic within six days after 5-FU treatment (Figure 2A). However, in 5-FU-treated MMP-9−/− mice, the recovery of WBC (Figure 2A) and platelets (data not shown) was delayed by an additional 8 days. This prolonged delay in hematopoietic recovery resulted in the death of 72% (n = 16, p < 0.001) of 5-FU-treated MMP-9−/− mice, while all of MMP-9+/+ mice survived (Figure 2B). These data suggest that MMP-9 plays a critical role in accelerating hematopoietic reconstitution.
Figure 2. Delayed Hematopoietic Recovery and Increased Mortality in MMP-9−/− Mice after Myelosuppression.
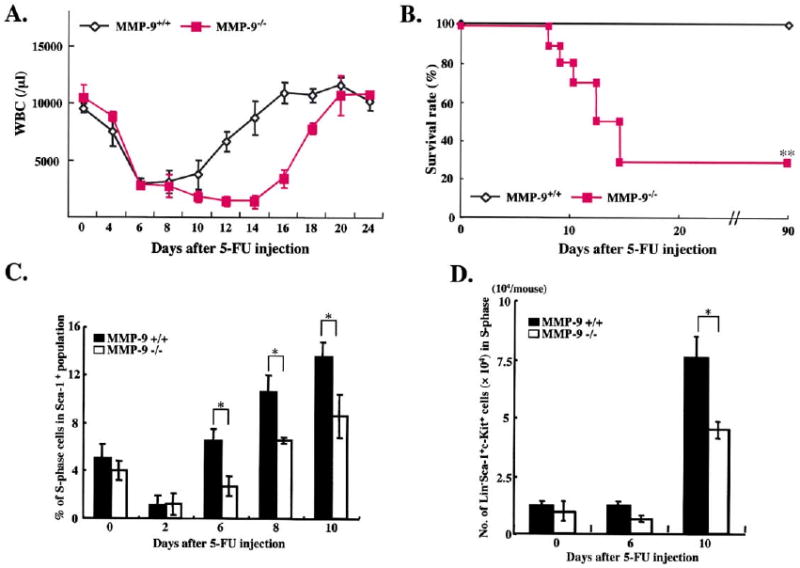
(A and B) MMP-9−/− and MMP-9+/+ mice (n = 16) received a single i.v. dose of 5-FU.
(A) WBC counts were quantified by a Neubauer chamber.
(B) Survival of 5-FU-treated mice was assessed daily (n = 16).
(C and D) Mice treated with 5-FU were sacrificed at different time points. Percentage/total number of Sca-1+ (n = 5) and Lin−Sca-1+c-Kit+ (n = 7) cells, isolated by a combination of magnetic cell isolation (MACS) and flow cytometry (FACS), in S phase was determined for DNA content after propidium iodide staining. The total number of Sca-1+ cells in S phase was higher in MMP-9+/+ as compared to MMP-9−/− mice (2.2 ± 0.05 versus 1.0 ± 0.03 × 105/femur on day 6 and 11.5 ± 0.3 versus 2.1 ± 0.04 × 105/femur on day 10, respectively). All values are given as mean ± SEM. *p < 0.01, **p < 0.001.
Cell cycling, motility, and differentiation of HSCs are accelerated during hematopoietic recovery after BM ablation. We next determined whether the impaired hematopoietic recovery of HSCs in MMP-9−/− mice was due to an alteration in the stem/progenitor cell cycling status. Hematopoietic cells phenotypically marked as Lin−Sca-1+c-Kit+ comprise a large percentage of long-term repopulating HSCs (Cheshier et al., 1999). Under steady-state conditions, the percentage and the total number of Sca-1+ (Figure 2C) and Lin−Sca-1+c-Kit+ cells (Figure 2D) in S phase of cell cycle were not significantly different in MMP-9+/+ and MMP-9−/− mice. However, following 5-FU myeloablation, the number of these cells entering S phase of cell cycle was impaired in MMP-9−/− mice. These data suggest that although during steady-state hematopoiesis there is no difference in the number of cycling repopulating cells, in the recovery phase after myelosuppression, lack of MMP-9 results in a diminished recruitment of cycling cells leading to profound BM hypocellularity.
Restoration of Myeloid and Megakaryocytic Lineages Is Impaired in MMP-9−/− Mice
On day 6 after 5-FU treatment, in MMP-9+/+ mice, BM cellularity increased and hematopoietic cell clusters were seen in close contact to the bone “osteoblastic zone (O),” followed by a shift of cells moving toward the blood vessel-enriched “vascular zone (V)” on day 10 (Figure 3B). However, in the BM of MMP-9−/− animals, there was a paucity of hematopoietic cell clusters in both the osteoblastic and vascular zones, even on day 10 after 5-FU treatment. By day 10, the proportion of differentiated CD11b+Gr-1+ myeloid (Figure 3A) and vWF+ megakaryocytic precursor cells (Figure 3C) in the BM of 5-FU-treated MMP-9−/− mice was dramatically reduced. The impaired recruitment of repopulating stem and progenitor cells into the vascular zone and the lack of differentiation into myeloid and megakaryocytic lineages in MMP-9−/− mice led us to hypothesize that MMP-9 may exert its effects through the release of a stem cell-active cytokine.
Figure 3. Recruitment and Differentiation of Hematopoietic Cells Are Impaired in MMP-9−/− Mice.
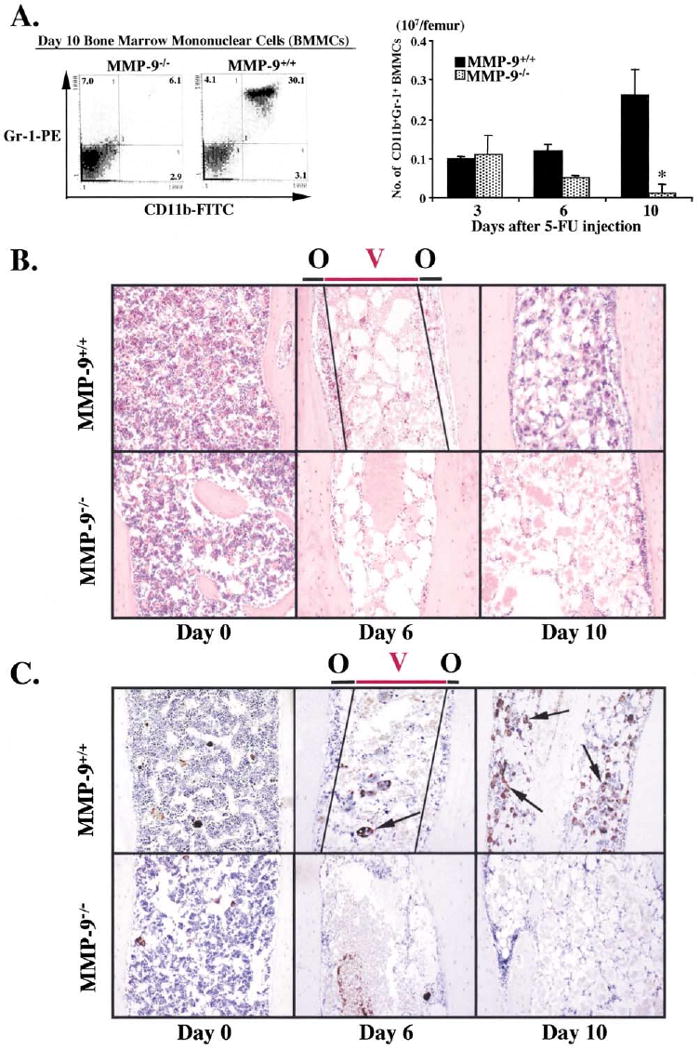
MMP-9−/− and MMP-9+/+ mice were treated with 5-FU and the hematopoietic recovery and the frequency and distribution of myeloid and megakaryocytic precursor cells was evaluated by FACS (A) or immunohistochemistry (B and C).
(A) BM cells obtained from either MMP-9−/− or MMP-9+/+ mice were stained for the myeloid markers CD11b-FITC and Gr-1-PE and analyzed by FACS (left panel). Absolute number of CD11b+/Gr-1+ BM cells per femur was calculated at different time points (right panel, n = 6, *p < 0.01).
(B) H&E staining of femurs from mice after 5-FU treatment. Hematopoietic cell clusters can be detected in close contact to osteoblasts (Osteoblastic zone, O) in the early phase of BM recovery. Over time, abundant clusters of proliferating hematopoietic cells are detected both in the osteoblastic zone and in the vascular-enriched zone (Vascular zone, V) in wild-type animals. In contrast, there is a striking paucity of hematopoietic cell clusters in the osteoblastic and the vascular zone in 5-FU-treated MMP-9−/− mice.
(C) vWF staining (brown) of femurs at different time points following 5-FU treatment. vWF positive megakaryocytes increase during BM recovery in MMP-9+/+ mice, but not in MMP-9−/− mice (arrows; magnification ×100).
Release of Soluble KitL (sKitL) Is Impaired in MMP-9−/− Mice after BM Suppression
Among the known stem cell-active chemokines and cytokines, KitL conveys signals that modulate survival, adhesion, and motility of c-Kit+ HSC and endothelial cells. sKitL is expressed as a membrane (mKitL) form and can be cleaved to a soluble form (sKitL) (Huang et al., 1992). Indeed, plasma levels of sKitL increased 3-fold during the hematopoietic regeneration phase in MMP-9+/+ mice, peaking at 6 days when there was active proliferation in the BM. In contrast, baseline sKitL plasma levels were very low in MMP-9−/− mice and did not increase following BM ablation (Figure 4A). These data suggest that MMP-9 promotes rapid release of sKitL facilitating BM recovery.
Figure 4. MMP-9 Mediated Release of sKitL Enhances Hematopoietic Reconstitution.
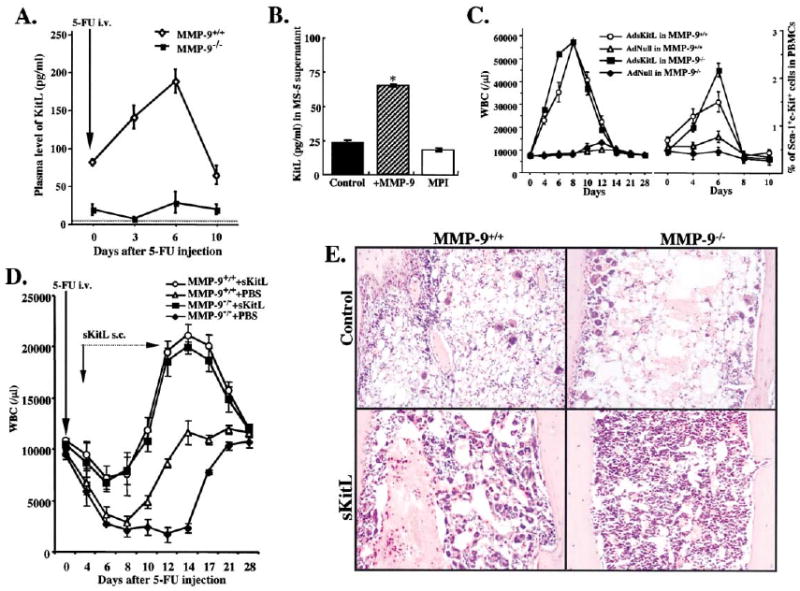
(A) MMP-9−/− and MMP-9+/+ mice were injected i.v. with a single dose of 5-FU and the plasma obtained from peripheral blood (PB) was assayed for sKitL by ELISA (p< 0.05, n = 6/group).
(B) Confluent MS-5 murine stromal cells, which express mKitL, were treated with recombinant active MMP-9 or the MPI CGS 27023A for 24 hr.*p< 0.001.
(C) MMP-9−/− and MMP-9+/+ mice were injected i.v. on day 0 with a single dose of Ad vector encoding for sKitL (AdsKitL) or no transgene (AdNull). PB was taken at indicated days (n = 6/group). Injection of AdsKitL resulted in sKitL plasma levels of 5399 ± 50 and 5126 ± 102 pg/ml on day 5 in MMP-9−/− and MMP-9+/+ mice, respectively. PBMCs were stained for Sca-1 and c-Kit and analyzed by FACS.
(D) MMP-9−/− and MMP-9+/+ mice were injected with recombinant sKitL from day 3–11 after 5-FU therapy (n = 10/group). WBC counts were determined at indicated time points.
(E) H&E staining of BM sections 4 days after 5-FU marrow suppression in MMP-9−/− and MMP-9+/+ mice without (control) and with sKitL Magnification × 200.
Unstimulated BM stromal cells express both mKitL and MMP-9 (Heinrich et al., 1993; Marquez-Curtis et al., 2001). Although MMP-9 was present at low levels in the supernatants of the unstimulated murine BM stromal cell line MS-5, which expresses mKitL, addition of recombinant MMP-9 rapidly promoted the release of sKitL. This shedding was blocked by a synthetic metalloproteinase inhibitor (MPI; Figure 4B). These data suggest that MMP-9 can effectively promote the release of sKitL from stromal cells.
However, MMP-9 expression is upregulated in both hematopoietic and stromal compartments of the BM after myeloablation (Figure 1C). To identify which cellular compartment is more critical in providing bioactive MMP-9 following myelosuppression, we developed chimeric mice by transplanting BM cells from MMP-9−/− mice into lethally irradiated MMP-9+/+ recipients resulting in chimeric mice in which hematopoietic cells are MMP-9 deficient. Transplantation and engraftment of BM from MMP-9+/+ into MMP-9−/− mice resulted in mice with MMP-9-deficient stroma (Supplemental Figure S4a, available at http://www.cell.com/cgi/content/full/109/5/625/DC1). As controls, MMP-9+/+ BM cells were transplanted into lethally irradiated MMP-9+/+ mice and MMP-9−/− BM cells into lethally irradiated MMP-9−/− mice. All mice showed engraftment after 16 days as determined by WBC counts. The majority of MMP-9+/+ recipients transplanted with either MMP-9−/− or MMP-9+/+ BM or MMP-9−/− recipients transplanted with MMP-9+/+ BM survived 5-FU myelosuppression. As expected, high mortality was observed in the MMP-9−/− group transplanted with MMP-9−/− BM cells. These data indicate that MMP-9 expressed either by stromal or hematopoietic cells is sufficient to support hematopoiesis after myelosuppression.
Exogenous sKitL Restores Hematopoietic Recovery and Mobilization in MMP-9−/− Mice
Under steady-state conditions, elevated sKitL levels delivered by adenoviral vector expressing sKitL (AdsKitL) increased WBC in both MMP-9−/− and MMP-9+/+ mice (Figure 4C). Progenitor mobilization determined by either the frequency of mobilized Sca-1+c-Kit+ cells (Figure 4C) or colony-forming unit cells (CFU-Cs) in the peripheral blood (Supplemental Figure S4b at above URL) was enhanced in the MMP-9−/− mice after AdsKitL introduction.
Since MMP-9 augments the release of sKitL, we next asked whether the delayed hematopoietic recovery seen in MMP-9−/− mice could be restored directly by exogenous recombinant sKitL. Treatment of MMP-9−/− mice with recombinant sKitL following 5-FU treatment resulted in rapid recovery of WBC (Figure 4D). In addition, sKitL treatment following 5-FU BM suppression resulted in a rapid increase in BM cellularity and hematopoietic reconstitution in MMP-9−/− mice (Figure 4E) and restored survival of MMP-9−/− mice (decreasing mortality to 17% compared to 72% observed in the PBS group, n = 10 in each group). These data suggest that MMP-9 mediated release of sKitL is essential for promoting stem cell differentiation, accelerating hematopoietic reconstitution following BM ablation.
Chemokines Released upon BM Suppression Promote MMP-9 Expression
But what regulates MMP-9? One clue came from the observation that the chemokine stromal cell-derived factor-1 (SDF-1) increased after myelosuppression (Ponomaryov et al., 2000). Plasma levels of SDF-1 increased following 5-FU treatment, peaking on day 8 (Figure 5A). These data suggest that rapid elevation of chemo/cytokine levels after BM ablation contributes to MMP-9 upregulation, setting the stage for HSC recruitment.
Figure 5. Chemo/Cytokines Induce MMP-9 Expression in BM Hematopoietic Cells.
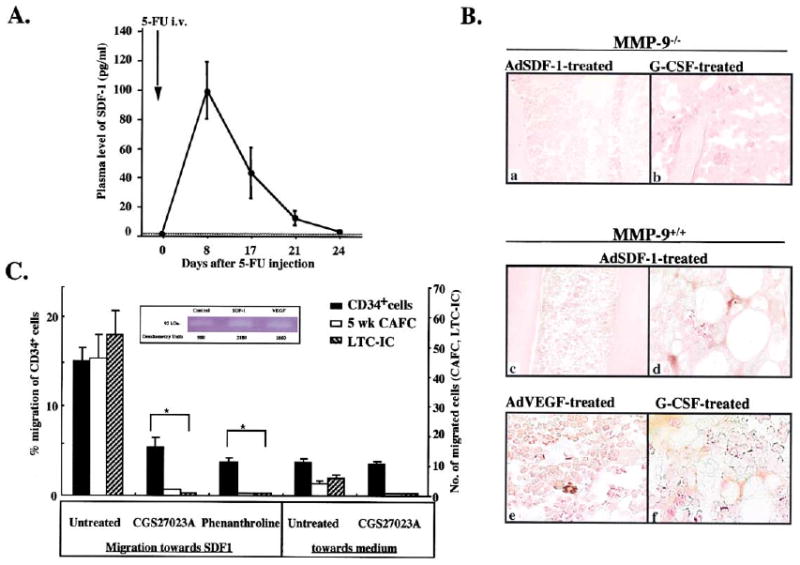
(A) MMP-9+/+ mice were treated with a single dose of 5-FU. At indicated time points, plasma was analyzed for SDF-1 by ELISA (n = 6/time point).
(B) MMP-9−/− and MMP-9+/+ mice received AdSDF-1, AdVEGF, and AdNull vector by a single i.v. injection. BM sections were stained for pro-MMP-9. MMP-9−/− mice treated with G-CSF served as negative control (a and b). BM sections after AdSDF-1 (c and d), AdVEGF (e), and G-CSF (f) in MMP-9+/+ mice. Magnification × 100 (a and c), × 400 (b, d–f).
(C) Human CD34+ cells were plated in Matrigel-coated transwells. MPIs (5-phenyl-1,10-phenanthroline and CGS 27023A) or PBS were added to both chambers. The chemoattractant SDF-1 was added to the lower chamber. Data are shown as a percentage of migrated cells (black bar). Migrated stem cells assayed as absolute number of CAFC at week 5 (open bar) and of LTC-IC (hatched bar; n = 3, *p< 0.05 for the migration of cells treated with/without MPI toward SDF-1).
Insert: Gelatin zymogram of culture supernatants from human CD34+ cells stimulated with/without SDF-1 or VEGF in serum-free medium. Supernatants from CD34+ cell cultures showed gelatinolytic activity for pro-MMP-9 (92 kDa).
To determine whether chemo/cytokines induce MMP-9 expression in vivo, we introduced SDF-1, VEGF, and G-CSF into wild-type mice. BM of the mice treated with SDF-1, VEGF, or G-CSF showed increased immunoreactive MMP-9 on stromal and hematopoietic cells (Figures 5Bc, d, e, and f). These studies raised the possibility that chemo/cytokine-induced MMP-9 activation mediates mobilization of hematopoietic cells.
We found that SDF-1 and VEGF stimulate the release of pro-MMP-9 and induce migration of human CD34+ progenitor and stem cells (5 week cobblestone forming cells [CAFC] and long-term culture initiating cells [LTC-IC]) in a transwell migration assay (Figure 5C). The migration of CD34+ cells was completely blocked by addition of MPIs (CGS 27023A and 5-phenyl-1,10-phenanthroline). Direct incubation of CD34+ cells with MPIs in suspension culture did not alter their proliferation in CAFC or LTC-IC assays (data not shown). These data suggest that chemokines induce the functional expression of MMP-9 on CD34+ cells and their migration.
Chemokine-Induced Mobilization of BM Repopulating Cells Is Impaired in MMP-9-Deficient Mice
Plasma elevation of SDF-1, VEGF, and G-CSF in MMP-9+/+ mice, but not in MMP-9−/− mice mobilized mature WBCs (Figures 6A–6C) and hematopoietic progenitors (CFU-Cs; Figure 6D). The mobilization of hematopoietic cells into the circulation followed the kinetics of plasma elevation of these chemokines (Figures 6A and 6B). We next determined if MMP-9 plays a role in mobilization of cells with repopulating capacity by transplanting peripheral blood mononuclear cells (PBMCs) mobilized by G-CSF as the source of BM repopulating cells into lethally irradiated syngeneic mice. Mice transplanted with G-CSF-mobilized PBMCs harvested from MMP-9+/+ mice 5 days after chemokine treatment showed long-term donor cell engraftment and survival, whereas those transplanted with G-CSF-mobilized PBMCs from MMP-9−/− mice died during the 20 days allowed for engraftment (Figure 6E). These data show that MMP-9 plays a role in mobilization of BM repopulating cells.
Figure 6. Chemo/Cytokine-Induced HSC Mobilization Is Impaired in MMP-9−/− Mice.
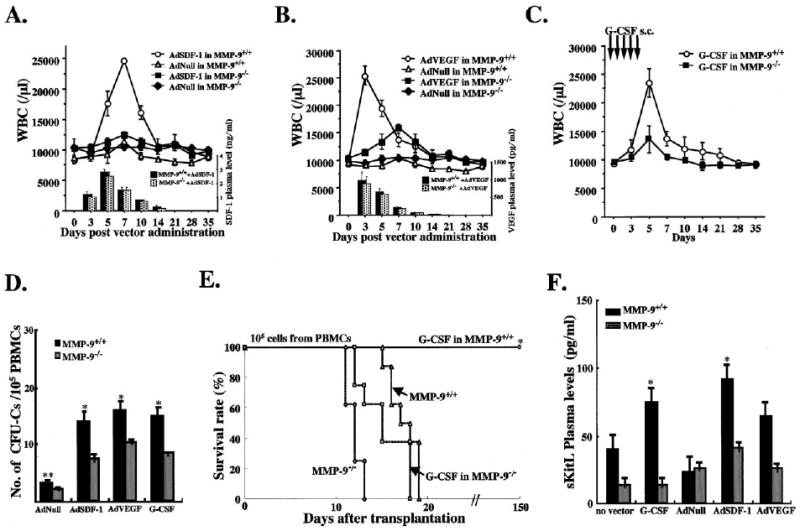
(A–C) MMP-9−/− and MMP-9+/+ mice were injected i.v. with a single dose of AdSDF-1, AdVEGF, and AdNull vector or s.c. with recombinant G-CSF from day 0–5 (n = 10 mice in each group). Elevated chemokine levels for SDF-1 and VEGF were achieved by adenoviral gene delivery of SDF-1 and VEGF ([A] and [B], bar graph insert). WBC counts were determined following AdSDF-1 (A), AdVEGF (B), and G-CSF treatment in MMP-9+/+ mice (C).
(D) Mobilized PBMCs were plated in a colony assay. The number of mobilized progenitor cells (CFU-C) was determined (n = 10, *0.05, **p< 0.01) on day 5 (AdSDF-1), on day 3 (AdVEGF), and on day 5 (G-CSF).
(E) PB of MMP-9−/− and MMP-9+/+ mice treated with or without G-CSF was obtained on day 5. PBMCs were transplanted into lethally irradiated syngeneic animals. Survival of transplanted recipients was monitored (n = 8/group, *p< 0.001).
(F) Plasma of MMP-9+/+ and MMP-9−/− mice 5 days after G-CSF, AdNull, AdSDF-1, and AdVEGF injection and untreated controls was assayed for sKitL (n = 6/group, *p< 0.03).
The necessity of chemokine-induced mobilization on MMP activity was confirmed by administrating a synthetic MPI in vivo, which also decreased WBC counts and blocked chemokine-induced mobilization in MMP-9-competent mice (data not shown). Importantly, mobilization of hematopoietic cells with short-term repopulating potential (CFU-S) was completely blocked in MPI-treated mice (Supplemental Figure S6a available at http://www.cell.com/cgi/content/full/109/5/625/DC1). Lethally irradiated syngeneic mice transplanted with SDF-1-, VEGF-, and G-CSF-mobilized PBMCs showed long-term engraftment in 55%, 40%, and 80% of these mice, respectively (Supplemental Figures S6b, c, and d at above URL). Similar to MMP-9−/− mice, transplantation of PBMCs from MPI-treated mice failed to reconstitute hematopoiesis in lethally irradiated recipients and all recipient mice died within 19 days.
SDF-1 and VEGF Increase sKitL Plasma Levels
If a significant role of MMP-9 activation in the postmyelosuppression process is the release of sKitL, then impaired hematopoietic cell mobilization following SDF-1 and VEGF treatment of MMP-9−/− mice could be due to their inability to produce sKitL. Indeed, we observed increased plasma sKitL levels in MMP-9+/+ mice after treatment with SDF-1, VEGF, and G-CSF as compared to untreated animals (Figure 6F). This chemo/cytokine-induced upregulation of sKitL was impaired in MMP-9−/− mice. These results highlight the importance of MMP-9-induced sKitL release for hematopoietic recovery.
Mobilization of BM-Derived Endothelial Progenitors Is MMP-9 Dependent
c-Kit is expressed on BM-derived progenitors that can give rise to cardiac muscle, skeletal muscle, and endothelial cells. Therefore, we asked if MMP-9 plays a more general role in regulating other tissue-specific stem cells. Elevation of plasma levels of SDF-1 and VEGF in wild-type mice increased the mobilization of circulating endothelial progenitors (CEPs) as assessed by the generation of late outgrowth endothelial cell colony forming units (CFU-EC) and the expression of VEGF-receptor-2 (VEGFR2; Figures 7A and 7B). In contrast, VEGF did not mobilize CFU-EC or VEGFR2+ cells into the circulation of wild-type mice treated with MPI (Figures 7A and 7B). Similarly, VEGF increased the number of CFU-EC in the PB (Figure 7C) and VEGFR2+ cells in the BM of MMP-9+/+, but not in MMP-9−/− mice (Figure 7D). Collectively, these results suggest that MMP-9 activation is required for the recruitment and mobilization of BM-derived CEPs.
Figure 7. Endothelial Cell Progenitor Mobilization Is Blocked in MMP-9−/− Mice.
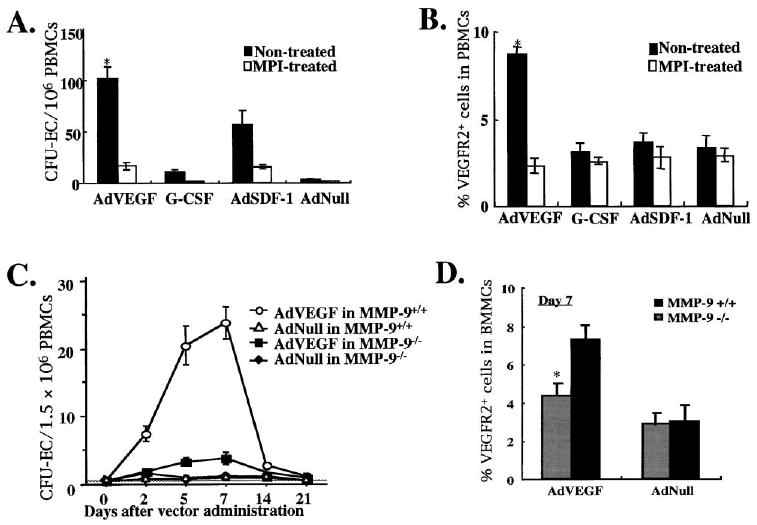
The number of circulating endothelial progenitor cells (CEPs), represented in in vitro cultures as colony forming units of endothelial cells (CFU-EC) were determined in the PB of MMP-9+/+ and MMP-9−/− mice three days after injection with AdVEGF, AdSDF-1, AdNull, and G-CSF in the presence or absence of MPI.
(A and B) CEPs were quantified by the formation of CFU-EC and by VEGFR2+ cells detected by FACS. The increase in circulating CFU-EC in VEGF-treated animals correlated with the number of VEGFR2+ cells (*p < 0.05).
(C and D) MMP-9−/− and MMP-9+/+ mice were injected i.v. with a single dose of AdVEGF and AdNull vector (n = 6/group, *p< 0.05). Mobilized PBMC were assayed for the number of CFU-EC in circulation (C) and the number of VEGFR2+ cells in the BM on day 6 after adenoviral injection (D).
Discussion
Rapid recruitment of HSCs from their quiescent niche is essential to repopulate progenitors of the myeloid and megakaryocytic lineage to avoid life-threatening infection and bleeding after BM suppression. Physiological stress induces rapid recruitment of stem cells from their BM niche with subsequent mobilization to the circulation, where they home to respective organs and either contribute to restore organ function or regenerate the stem and progenitor cell pool. In this study, we demonstrate a key role for MMP-9 to rapidly release the stem cell-active cytokine sKitL, thereby directing stem and progenitor cell recruitment and facilitating hematopoietic reconstitution. This is schematically represented in Figure 8. We show that BM suppression results in a timely upregulation of MMP-9 within the BM microenvironment with the release of sKitL. Increased bioactive sKitL promotes HSC cell cycling and enhances their motility, both of which are essential for HSC and progenitor survival and differentiation. MMP-9 activation also facilitates mobilization of BM repopulating cells into the peripheral circulation, a process that may be essential for reconstitution of the stem cell pool. Collectively, these data introduce a paradigm in stem and progenitor cell biology whereby activation of a metalloproteinase serves as the decisive checkpoint for the rapid reconstitution of the hematopoiesis following life-threatening stressors.
Figure 8. Functional Anatomy and Recruitment of c-Kit+ Stem and Progenitor Cells Is Dictated by MMP-9 Mediated Release of sKitL.
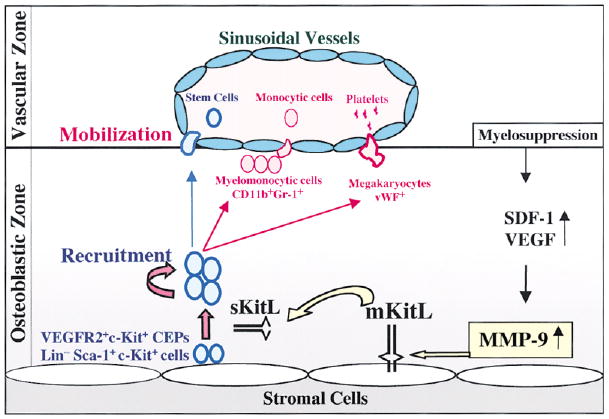
Under steady-state conditions quiescent c-Kit+ HSCs and CEPs reside in a niche in close contact with stromal cells including osteoblasts. Membrane-bound cytokines, such as mKitL not only convey survival signals, but also support the adhesion of stem cells to the stroma. BM ablation or chemokine/cytokine administration induces upregulation of MMP-9 resulting in the release of sKitL sKitL confers signals that enhances mobility of VEGFR2+ endothelial progenitors (CEPs) and Lin−Sca-1+c-Kit+ repopulating cells, translocating them into a vascular-enriched niche favoring differentiation and mobilization to the peripheral circulation.
MMP-9 Alters the Quiescent Stem Cell Niche by Releasing sKitL
The HSCs and BM-derived CEPs reside in a microenvironment where they can readily sense and respond to the stress-induced demands for supporting hematopoiesis and angiogenesis. To meet such a high demand, rapid availability of cytokines is essential for the recruitment of quiescent HSCs and CEPs to a permissive niche where they can proliferate, differentiate, and replenish the exhausted progenitor and precursor pool. We show that this microenvironmental switch depends on the upregulation of MMP-9 by BM cells, resulting in increased bioavailability of sKitL.
MMP-9 cleaves several cytokines and/or their receptors, a process that can either activate or inactivate the cytokines (Vu and Werb, 2000). We hypothesized that active MMP-9 releases membrane-bound KitL (mKitL). A precedent for such a process is the MMP-9-induced VEGF release from the tethered pool within the microenvironment of developing bone (Engsig et al., 2000) or within tumors of pancreatic islets (Bergers et al., 2000). mKitL is a glycoprotein of 248 amino acids that is rapidly cleaved from the cell to release an active soluble protein of 164 amino acids. In contrast, a glycoprotein of 220 amino acids, which lacks the proteolytic cleavage site encoded by differentially spliced exon 6 sequences, remains predominantly membrane associated (Huang et al., 1992). The only enzyme reported to date to cleave mKitL is a mast cell chymase (Longley et al., 1997). The extracellular domain of mKitL has two potential consensus sequences that can behydrolyzed by MMP-9 (Kridel et al., 2001). The rapid increase in plasma sKitL levels in MMP-9+/+ mice and the relative deficiency of sKitL at baseline or after myelosuppression in MMP-9−/− mice strongly suggest that MMP-9 plays a physiological role in releasing sKitL, setting up the stage for hematopoietic reconstitution.
Impairment in MMP-9 Mediated Release of sKitL Results in Delayed Hematopoietic Reconstitution and Increased Mortality after Myeloablation
What is the role of increased sKitL levels in the context of hematopoietic recovery after BM suppression or stem cell mobilization? Rapid hematopoietic recovery following chemotherapy depends on the ability of quiescent stem cells to enter cell cycle (Morrison et al., 1997a, 1997b). KitL stands out as the main upmodulator of stem cell function affecting growth, survival, and/or differentiation of hematopoietic cells and driving hematopoietic reconstitution after BM ablation. Although MMP-9 null mice have cell-associated KitL, the low levels of sKitL, as seen in the MMP-9−/− mice, result in delayed entry of stem cells into the S phase of cell cycle leading to diminished cell motility and differentiation.
The biological significance of sKitL and mKitL in the regulation of postnatal hematopoiesis is not understood. Homozygous steel dickie Sld/Sld mice, which lack mKitL, have defective hematopoiesis under steady-state conditions, suggesting that mKitL may play a role in survival of HSCs. However, since it is difficult to generate transgenic mice completely deficient in sKitL, the physiological significance of sKitL under steady-state conditions remains unknown (Tajima et al., 1998). In accordance with our data, although MMP-9 mediated release of sKitL may not be required for steady-state hematopoiesis, sKitL is essential for the rapid hematopoietic reconstitution during the critical pancytopenic period when there is exhaustion of precursor/progenitor cells.
Despite germ-free conditions where the 5-FU treated mice were kept in our studies, the delay in hematopoietic recovery was translated into the demise of 72% of the 5-FU-treated MMP-9−/− mice, while all of the 5-FU-treated MMP-9+/+ mice survived. We expect that under nonsterile conditions significantly more of the 5-FU treated MMP-9−/− mice would have succumbed to the complications of prolonged neutropenia and thrombocytopenia. These findings underscore the need for rapid shedding of sKitL in addition to constitutively expressed mKitL to restore hematopoiesis.
In humans, rapid BM recovery after repetitive treatment with chemotherapeutic agents is essential to avoid morbidity and mortality secondary to prolonged BM suppression. Indeed, life-threatening infections and bleeding are direct effects of prolonged chemotherapy-related neutropenia and thrombocytopenia. It is conceivable that chronic use of certain chemotherapeutic agents may alter MMP-9 secretion and activation and therefore, induce long-term BM suppression or disrupt trafficking of HSCs contributing to BM failure states. On the other hand, inhibition of MMP-9 may provide a novel mechanism to regulate hematopoiesis in myeloproliferative disorders.
MMP-9 Activation Enhances Stem and Progenitor Cell Recruitment
Upregulation of adhesion molecules and chemokine receptors on HSCs, progenitors, and CEPs facilitate their recruitment within the BM followed by mobilization to the circulation. Our data show that MMP-9 mediated release of sKitL enhances the motogenic potential of stem and progenitor cells, translocating them from their quiescent into a permissive proliferative vascular niche. sKitL is a potent cytokine that not only increases the motility of HSC and progenitors within the BM, but also sets up the stage for hematopoietic cells to be launched to the circulation (Long et al., 1992; Papayannopoulou et al., 1998). Whether sKitL acts as a dominant interfering molecule to reverse adhesion of c-Kit+ HSC and CEPs to mKitL expressed on stromal cells, or whether it has a direct signaling effect increasing cell motility remains to be determined.
After elimination of the cycling stem cell pool with 5-FU treatment, we observed hematopoietic cell clusters in close contact to osteoblasts (osteoblastic zone) on day 3 in both wild-type and MMP-9−/− mice. However, at later time points, hematopoietic clusters in MMP-9−/− mice did not repopulate the inner vascular zone. Based on these results, we postulate that MMP-9 mediated release of sKitL increases the motogenic potential of HSCs, shifting HSCs into a vascular enriched microenvironment where they receive instructions for differentiation and mobilization.
Increased MMP-9 has been detected in the serum of IL-8-treated monkeys and was linked to the mobilization of progenitor cells (Pruijt et al., 1999). These authors hypothesized that the IL-8-induced activation of neutrophils generates high levels of MMP-9. In another report, intravenous injection of recombinant MMP-9 into rabbits produced a rapid, transient neutropenia, followed by a profound neutrophilia and the appearance of immature myeloid cells, including blast cells (Masure et al., 1997). These results can be explained by MMP-9 mediated release of sKitL.
We have previously demonstrated that endothelial-active cytokines, such as VEGF and SDF-1 induce mobilization of BM repopulating cells (Hattori et al., 2001a, 2001b). Here, we demonstrate that MMPs are necessary intermediates downstream of these factors. Cytokine-induced mobilization of hematopoietic progenitors and cells with HSC potential was markedly impaired in MPI-treated or MMP-9−/− mice.
Why are HSCs mobilized after stress? Does it play a critical role in reconstituting the stem cell pool, or is it a coincidental process of moving stem cells out of their quiescent niche? Mobilization of stem and progenitor cells seems to be essential for reconstitution of the stem cell pool in murine myelosuppression models. Indeed, mobilization and localization of BM-derived stem cells to the spleen may facilitate the expansion of the stem/progenitor cell pools. In this regard, MMP-9 activation not only promotes trilineage hematopoietic recovery, but also facilitates mobilization of HSC and CEPs, a process essential for hematopoietic reconstitution and replenishment of stem and progenitor cells.
Endothelial Progenitor Cell Mobilization Is MMP-9 Dependent
BM not only provides a suitable microenvironment for HSCs, but is also a dispensable reservoir for organ-specific stem cells for endothelium, muscle, brain, pancreas, and liver cells (Krause et al., 2001). c-Kit is expressed on HSCs, CEPs (Peichev et al., 2000), and cardiac precursors (Orlic et al., 2001). Selective recruitment of BM-derived stem cells is critical for supporting postnatal organogenesis and tumorigenesis (Coussens et al., 2000; Lyden et al., 2001). We found that mobilization of both HSCs and VEGFR2+ CEPs to the circulation occurs in response to VEGF and is MMP-9 dependent. These data suggest that MMP-9 activation is not only a decisive checkpoint for recruitment of HSCs but also other types of undifferentiated cells, such as endothelial progenitors.
Taken together, these data provide evidence that stem and progenitor cell recruitment from the quiescent niche into a permissive microenvironment following stress depends on MMP-9. This protease acts on different levels by directly releasing sKitL, affects cell mobility and promotes recruitment of stem and progenitor cells, thereby enhancing rapid differentiation. Stem cell-stromal cell interactions are amplified by membrane-anchored cytokines, by transducing proliferation and/or differentiation signals, and by the maintenance of progenitors in a quiescent state under the constraints of limited growth factor concentrations. It is intriguing that the conversion of KitL from a membrane-bound, adhesion/survival-promoting molecule to a soluble survival/motogenic factor by MMP-9 is a critical event in regulating the stem cell niche. Because modulating the bioavailability of local cytokines changes the HSC fate, regulators of enzyme activity leading to proteolytic cleavage are critical elements in the determination of the HSC fate. It will be interesting to determine if MMP-9 is essential for the recruitment of other stem cells that express c-Kit, and if there is an analogous specific protease-dependent regulatory process regulating the stem cell niche for c-Kit negative stem and progenitor cells.
Experimental Procedures
Animal Studies
SCID mice, age and sex-matched (7–8 weeks), weight (>20 g) were purchased from the Jackson Laboratory (Bar Harbor, ME). MMP-9−/− 129Sv mice were generated (Vu et al., 1998) and used after at least eight back crosses to CD1 mice. MMP-9+/+ 129Sv mice were obtained by back crossing MMP-9+/− 129Sv mice with CD1 mice. Mice were maintained in filtered germ-free air Thorensten units.
Administration of 5-FU
MMP-9−/− and MMP-9+/+ mice were injected with 5-FU (250 mg/kg body weight, Pharmacia & Upjohn Company) intravenously (i.v.). At indicated time points, mice were sacrificed. Femurs were flushed and BM cells were used for cell cycle analysis or subjected to magnetic bead isolation (MACS; Miltenyi Biotec). Lineage-negative BM cells were isolated by MACS after labeling the cells with a depletion cocktail of lineage-specific monoclonal antibodies (mAbs), including CD5, CD45R, CD11b, Gr-1, TER119, and 7/4 (Stem Cell Technology, Canada). Lin− cells were MACS isolated with mAb against Sca-1. The separated Lin−Sca-1+ BM cells were >96% positive for Sca-1 (clone E13-161.7; PharMingen, San Diego) and >99% positive for c-Kit (clone 2B8, Bioscience). Less than 4% of the Lin−Sca-1+ cells showed staining for CD11b, CD3, B220, or Gr-1. For cell cycle analysis, whole BM cells of MMP-9−/− and MMP-9+/+ mice were labeled with Sca-1-FITC. Lin−Sca-1+ BM cells were labeled with c-Kit-FITC. Labeled BM cells and Lin−Sca-1+ cells were fixed in ice-cold ethanol for 30 min. After RNase treatment (Sigma, MO), cells were stained with propidium iodide (Molecular-Probes, Oregon). The DNA content of Sca-1+ and Lin−Sca-1+c-Kit+ cells was determined by FACS (Coulter flow cytometer).
Mobilization of BM-Derived Cells by Adenovirus-Delivered Factors
Plasma levels of VEGF, SDF-1, and KitL were elevated by using an adenovirus 5 (Ad)-derived E1a-, E3-deficient (E1a−E3−E4+) vector with an expression cassette in the E1a region containing the transgene cDNA and driven by the cytomegalovirus promoter/enhancer (Hattori et al., 2001b). Mice received the Ad-vector expressing SDF-1 (AdSDF-1) or no transgene (AdNull) at a concentration of 1 × 109 plaque forming units (PFU) and Ad-vector expressing sKitL (AdsKitL) or VEGF165(AdVEGF) at a concentration of 1.5 × 108 PFU. The vector was injected i.v. as a single dose. Recombinant G-CSF (R&D Systems, MN) was administered s.c. daily from day 0–5 at 50 μg/kg body weight. Recombinant murine KitL (PeproTech, NJ) was injected s.c. twice a day at 100 or 200 μg/kg body weight.
CGS 27023A, a potent inhibitor of MMP-9 (IC50 = 8 nM), MMP-2 (IC50 = 11 nM), and MMP-3 (IC50 = 13 nM; Novartis, Basel, Switzerland), was injected at 60 mg/kg body weight s.c. every 3 days starting from day 0.
BM Transplantation
BM cells (3 × 107/mouse) from 8-week-old MMP-9−/− and MMP-9+/+ mice were injected i.v. into lethally irradiated (9.5 Gy from a 137Cs γ-ray source) MMP-9−/− and MMP-9+/+ mice. Sixteen days later, mice were treated with 5-FU as described above. Survival was monitored.
Hematopoietic/Endothelial Progenitor and Stem Cell Assays
Peripheral Blood Analysis
Blood was collected with capillary pipettes by retro-orbital bleeding and WBC counts were determined by using a hemocytometer. PBMCs were isolated from heparinized blood after centrifugation over a discontinuous gradient using Lympholyte-M (Cedarlane, Ontario, Canada).
Progenitor Assay
PBMCs (105) were plated in IMDM containing 1.2% methylcellulose (Fisher Scientific, NJ), 30% FBS, 5 × 10−3 M 2-mercaptoethanol, 2 mM L-glutamine, 0.5 mM hemin (Sigma), murine KitL (20 ng/ml), human erythropoietin (6 U/ml), and murine IL-3 (50 ng/ml). Colonies (>50 cells) were scored after 7 days (37°C, 5% CO2).
CFU-S Assay
Mobilized PBMCs (105/mouse) of chemokine-treated SCID mice were injected i.v. into lethally irradiated syngeneic recipients (9 Gy). Spleens were removed 12 days later and fixed in Bouin's solution. The number of macroscopic spleen colonies was scored.
BM Repopulating Assay
Chemo/cytokine-mobilized PBMCs from SCID mice treated with/without MPI were collected on day 7. PBMCs from G-CSF-treated MMP-9−/− and MMP-9+/+ mice were collected on day 5. Mobilized PBMCs (105) were injected into lethally irradiated syngeneic animals (9.5 Gy). Survival was monitored.
Endothelial Progenitor Assay
Circulating BM-derived CEPs were quantified by colony forming unit-endothelial cell (CFU-EC) as previously described (Hattori et al., 2001a). Mobilized PBMCs were cultured in M199 media (GIBCO-BRL, Gaithersburg, MD) supplemented with 20% FBS (HyClone, UT), ECGF (30 μg/ml), human FGF-basic (Sigma), and heparin. After 5 weeks of culture, adherent cells were scored for colonies (>10 cells). Endothelial colonies were identified by metabolic uptake of DiI-acetylated-LDL (1 μg/ml; PerImmune Inc., Rockville, MD) at 37°C for 5 hr and visualized by fluorescence microscopy. Number of VEGFR2+ cells was quantified by FACS using a Cy2-labeled mAb to VEGFR2 (clone DC101, ImClone, NY; Hattori et al. 2001a).
In Vitro Assays
Murine BM Cultures
Murine BM cells (1 × 106) from MMP-9−/− and MMP-9+/+ BM cells isolated after treatment with/without 5-FU were placed in serum-free medium (X-VIVO-20) overnight. Culture supernatants were analyzed by Western blotting using murine mAb (1 μg/ml) to MMP-9 (clone 7-11C, Oncogene Research-Products); anti-mouse IgG-conjugated to horseradish peroxidase (1:6000 dilution, Santa Cruz Biotechnology, CA) and visualized using ECL chemiluminescence (Amersham Pharmacia-Biotech).
Stromal Cell Line Cultures
Stromal cells (MS-5) were cultured in serum-free medium, and treated with recombinant active MMP-9 (Oncogene, Boston, MA) or an MPI CGS 27023A for 24 hr.
Substrate Zymography
Supernatants from human MACS-isolated CD34+ (5 × 103) cells were collected after overnight incubation in serum-free medium with/without SDF-1 (150 ng/ml, R&D Systems) or VEGF (50 ng/ml, PeproTech). MMP activity was determined by gelatin zymography (Lane et al., 2000).
Immunoassay for Chemokines and Cytokines
Plasma levels of SDF-1, VEGF, and sKitL were measured using commercial available ELISA (R&D Systems).
Immunohistochemistry
BM sections were deparaffinized, rehydrated through an alcohol series, immersed in 0.1% H2O2, and blocked using an Avidin and Biotin blocking kit (Vector, Burlingame, CA). For MMP-9 staining, the M.O.M. immunodetection kit (Vector) was used as recommended. Sections were incubated with MMP-9 mAb (clone 7-11C, Oncogene, MA) for 30 min, washed in PBS, incubated with a biotinylated horse anti-mouse IgG, and incubated for 5 min with A&B reagents (Vector). Von Willebrand Factor (vWF) immmunohistochemistry was performed on deparaffinized sections, treated with 3% H2O2, and incubated with 0.05% pronase E (Sigma). Sections were stained with anti-human vWF/HRP (Dako). Sections were developed with 3,3′-diaminobenzidine substrate and counterstained with eosin and hematoxylin.
In Vitro Transmigration through Reconstituted Basement Membranes
CD34+ cells from PBMC of normal donors were isolated by MACS (Miltenyi Biotec) and showed a purity of >95% by FACS. CD34+ cells were added to 8 μm pore matrigel-coated (BD) transwell inserts (Costar, Cambridge, MA). Recombinant SDF-1 (100 ng/ml) was added to the lower chamber with/without MPIs 5-phenyl-1,10-phenanthroline (1 mM, Sigma), or CGS 27023A (1 nM, Novartis, Switzerland) which were added to both chambers. Transmigrated cells were quantified, placed in a clonogenic assay and in long-term cultures to measure the number of CAFCs and LTC-ICs (Jo et al., 2000).
Statistical Analysis
Results are expressed as mean ± standard deviation. Data were analyzed using the unpaired two-tailed student's t test and the log rank test. P values of < 0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank Harry G. Satterwhite and Rafael Tejada for helpful assistance, Dr. Kei Tashiro (Kyoto University) for helpful comments and Jeanette Wood (Oncology, Novartis) for providing us with the synthetic MPI CGS 27023A. This work was supported by grants from the National Institutes of Health (CA 72006, CA 75072, and AR 46238 to Z.W.) and the Sandler Family Sustaining Foundation (Z.W.), the Doris Duke Charitable Foundation (D.L.), the National Heart, Lung, and Blood Institute (HL-58707, HL-61849, HL-66592, HL-67839 to S.R.), the American Cancer Society (S.R.), Leukemia and Lymphoma Foundation, and the Lupin Foundation (S.R.).
References
- Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau HM, Brazelton TR, Weimann JM. The evolving concept of a stem cell: entity or function? Cell. 2001;105:829–841. doi: 10.1016/s0092-8674(01)00409-3. [DOI] [PubMed] [Google Scholar]
- Cheng T, Rodrigues N, Shen H, Yang Y, Dombkowski D, Sykes M, Scadden DT. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- Cheshier SH, Morrison SJ, Liao X, Weissman IL. In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc Natl Acad Sci USA. 1999;96:3120–3125. doi: 10.1073/pnas.96.6.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engsig MT, Chen QJ, Vu TH, Pedersen AC, Therkidsen B, Lund LR, Henriksen K, Lenhard T, Foged NT, Werb Z, Delaisse JM. Matrix metalloproteinase 9 and vascular endothelial growth factor are essential for osteoclast recruitment into developing long bones. J Cell Biol. 2000;151:879–890. doi: 10.1083/jcb.151.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori K, Dias S, Heissig B, Hackett NR, Lyden D, Tateno M, Hicklin DJ, Zhu Z, Witte L, Crystal RG, et al. Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. J Exp Med. 2001a;193:1005–1014. doi: 10.1084/jem.193.9.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori K, Heissig B, Tashiro K, Honjo T, Tateno M, Shieh JH, Hackett NR, Quitoriano MS, Crystal RG, Rafii S, Moore MA. Plasma elevation of stromal cell-derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood. 2001b;97:3354–3360. doi: 10.1182/blood.v97.11.3354. [DOI] [PubMed] [Google Scholar]
- Heinrich MC, Dooley DC, Freed AC, Band L, Hoatlin ME, Keeble WW, Peters ST, Silvey KV, Ey FS, Kabat D, et al. Constitutive expression of steel factor gene by human stromal cells. Blood. 1993;82:771–783. [PubMed] [Google Scholar]
- Huang EJ, Nocka KH, Buck J, Besmer P. Differential expression and processing of two cell associated forms of the kit-ligand: KL-1 and KL-2. Mol Biol Cell. 1992;3:349–362. doi: 10.1091/mbc.3.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo DY, Rafii S, Hamada T, Moore MA. Chemotaxis of primitive hematopoietic cells in response to stromal cell-derived factor-1. J Clin Invest. 2000;105:101–111. doi: 10.1172/JCI7954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Kridel SJ, Chen E, Kotra LP, Howard EW, Mobashery S, Smith JW. Substrate hydrolysis by matrix metalloproteinase-9. J Biol Chem. 2001;276:20572–20578. doi: 10.1074/jbc.M100900200. [DOI] [PubMed] [Google Scholar]
- Lane WJ, Dias S, Hattori K, Heissig B, Choy M, Rabbany SY, Wood J, Moore MA, Rafii S. Stromal-derived factor 1-induced megakaryocyte migration and platelet production is dependent on matrix metalloproteinases. Blood. 2000;96:4152–4159. [PubMed] [Google Scholar]
- Levesque JP, Takamatsu Y, Nilsson SK, Haylock DN, Simmons PJ. Vascular cell adhesion molecule-1 (CD106) is cleaved by neutrophil proteases in the bone marrow following hematopoietic progenitor cell mobilization by granulocyte colony-stimulating factor. Blood. 2001;98:1289–1297. doi: 10.1182/blood.v98.5.1289. [DOI] [PubMed] [Google Scholar]
- Long MW, Briddell R, Walter AW, Bruno E, Hoffman R. Human hematopoietic stem cell adherence to cytokines and matrix molecules. J Clin Invest. 1992;90:251–255. doi: 10.1172/JCI115844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longley BJ, Tyrrell L, Ma Y, Williams DA, Halaban R, Langley K, Lu HS, Schechter NM. Chymase cleavage of stem cell factor yields a bioactive, soluble product. Proc Natl Acad Sci USA. 1997;94:9017–9021. doi: 10.1073/pnas.94.17.9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- Marquez-Curtis LA, Dobrowsky A, Montano J, Turner AR, Ratajczak J, Ratajczak MZ, Janowska-Wieczorek A. Matrix metalloproteinase and tissue inhibitors of metalloproteinase secretion by haematopoietic and stromal precursors and their production in normal and leukaemic long-term marrow cultures. Br J Haematol. 2001;115:595–604. doi: 10.1046/j.1365-2141.2001.03160.x. [DOI] [PubMed] [Google Scholar]
- Masure S, Paemen L, Van Aelst I, Fiten P, Proost P, Billiau A, Van Damme J, Opdenakker G. Production and characterization of recombinant active mouse gelatinase B from eukaryotic cells and in vivo effects after intravenous administration. Eur J Biochem. 1997;244:21–30. doi: 10.1111/j.1432-1033.1997.00021.x. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Wandycz AM, Hemmati HD, Wright DE, Weissman IL. Identification of a lineage of multipotent hematopoietic progenitors. Development. 1997a;124:1929–1939. doi: 10.1242/dev.124.10.1929. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Wright DE, Weissman IL. Cyclophosphamide/granulocyte colony-stimulating factor induces hematopoietic stem cells to proliferate prior to mobilization. Proc Natl Acad Sci USA. 1997b;94:1908–1913. doi: 10.1073/pnas.94.5.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G, Reynolds JJ, Bretz U, Baggiolini M. Partial purification of collagenase and gelatinase from human polymorphonuclear leucocytes. Analysis of their actions on soluble and insoluble collagens. Biochem J. 1982;203:209–221. doi: 10.1042/bj2030209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta H, Yomogida K, Dohmae K, Nishimune Y. Regulation of proliferation and differentiation in spermatogonial stem cells: the role of c-kit and its ligand SCF. Development. 2000;127:2125–2131. doi: 10.1242/dev.127.10.2125. [DOI] [PubMed] [Google Scholar]
- Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Papayannopoulou T, Priestley GV, Nakamoto B. Anti-VLA4/VCAM-1-induced mobilization requires cooperative signaling through the kit/mkit ligand pathway. Blood. 1998;91:2231–2239. [PubMed] [Google Scholar]
- Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- Ponomaryov T, Peled A, Petit I, Taichman RS, Habler L, Sandbank J, Arenzana-Seisdedos F, Magerus A, Caruz A, Fujii N, et al. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest. 2000;106:1331–1339. doi: 10.1172/JCI10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruijt JF, Fibbe WE, Laterveer L, Pieters RA, Lindley IJ, Paemen L, Masure S, Willemze R, Opdenakker G. Prevention of interleukin-8-induced mobilization of hematopoietic progenitor cells in rhesus monkeys by inhibitory antibodies against the metalloproteinase gelatinase B (MMP-9) Proc Natl Acad Sci USA. 1999;96:10863–10868. doi: 10.1073/pnas.96.19.10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Tajima Y, Moore MA, Soares V, Ono M, Kissel H, Besmer P. Consequences of exclusive expression in vivo of Kit-ligand lacking the major proteolytic cleavage site. Proc Natl Acad Sci USA. 1998;95:11903–11908. doi: 10.1073/pnas.95.20.11903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu TH, Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev. 2000;14:2123–2133. doi: 10.1101/gad.815400. [DOI] [PubMed] [Google Scholar]
- Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–422. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie T, Spradling AC. A niche maintaining germ line stem cells in the Drosophila ovary. Science. 2000;290:328–330. doi: 10.1126/science.290.5490.328. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


