Abstract
The pathogenesis of human immunodeficiency virus (HIV) associated encephalopathy is attributed to infiltration of the central nervous system (CNS) by HIV-1 infected mononuclear cells that transmigrate across the blood brain barrier (BBB). The endothelial tight junctions (TJ) of the blood brain barrier (BBB) play a critical role in controlling cellular traffic into the CNS. Neuropathogenesis of HIV-1 is exacerbated by drugs of abuse such as methamphetamine (Meth) which are capable of dysregulating BBB function. HIV-1 viral proteins like gp120 are both neurotoxic and cytotoxic and have been implicated in the development of HIV-1 dementia (HAD). We hypothesize that gp120 in synergy with Meth can alter BBB permeability via the modulation of tight junction expression. We investigated the effect of Meth and/or gp120 on the basal expression of TJ proteins ZO-1, JAM-2, Occludin, Claudin-3 and Claudin-5, using in vitro cultures of the primary brain microvascular endothelial cells (BMVEC). Further, the functional effects of TJ modulation were assessed using an in vitro BBB model, that allowed measurement of BBB permeability using TEER measurements and transendothelial migration of immunocompetent cells. Our results show that both Meth and gp120 individually and in combination, modulated TJ expression, and these effects involved Rho-A activation. Further, both Meth and gp120 alone and in combination significantly decreased transendothelial resistance across the in vitro BBB and the enhanced transendothelial migration of immunocompetent cells across the BBB. An understanding of the mechanisms of BBB breakdown that lead to neurotoxicity is crucial to the development of therapeutic modalities for Meth abusing HAD patients.
Keywords: Methamphetamine; Neuropathogenesis; HIV-1, HIV-1 associated dementia; Blood brain barrier; Tight junction; Drug abuse
1. Introduction
The most prominent neurological complication associated with human immunodeficiency virus (HIV-1) infection, excluding opportunistic infections, is a syndrome of combined cognitive and motor dysfunction referred to as either HIV encephalopathy, AIDS dementia complex (ADC) or HIV-1 associated dementia (HAD) (Gendelman et al., 1997; Krebs et al., 2000; Nath, 2002;Kolson, 2002; Sleasman and Goodenow, 2003; Trujillo et al., 2005). Neurological dysfunction is the initial complication of AIDS in ~20% of patients and >50% of all AIDS patients experience some form of neurological abnormality during their disease (Ozdener, 2005;Trujillo et al., 2005). Although the clinical and pathological complications of HIV encephalopathy have been well characterized, the pathogenesis of this progressive central nervous system (CNS) disorder remains poorly defined. The pathogenesis of HIV encephalopathy may be partly attributed to infiltration of the CNS by HIV-1 infected mononuclear cells since the transmigration ofHIV-1 infected monocytes and lymphocytes across the blood brain barrier (BBB) into the CNS is a pre-requisite for the development of HIV encephalopathy (Boven et al., 2005; Eugenin et al., 2006; Gendelman et al., 1997; Nottet et al., 1996; Persidsky et al., 1999; Krebs et al., 2000; Schmidtmayerova et al., 1996; Worthylake and Burridge, 2001; Wu et al., 2000; Nottet, 1999; Lipton, 1991).
Many HIV-related neurological impairments may be worsened by the use of psych ostimulants such as methamphetamine (Meth) which are capable of influencing brain function, probably through modifying the BBB function (Hawkins and Davis, 2005; Bradley et al., 1998; Bradbury, 1993). Meth acts as a co-factor in HIV-1 infection resulting in exacerbated neurodegenerative changes (Theodore et al., 2007). Recent studies by Theodore et al. (2006a,b,c) and Conant et al. (2004) have demonstrated thatMethandHIV-1 viral protein Tat interact resulting in the release of pro-inflammatory cytokines, chemokines and matrix metalloproteinases, further causing enhanced neurotoxicity. HIV-1 viral proteins induce elevation of TNF-α and IL1-β which may predispose the dopaminergic neurons to subsequent damage by Meth (Theodore et al., 2006a,b,c; Flora et al., 2003).
The endothelial tight junctions (TJ) of the BBB play a critical role in controlling cellular traffic into the CNS (Andras et al., 2005, 2003; Aurrand-Lions et al., 2001, 2002; Balda et al., 1996; Balda and Matter, 1998; Luscinskas et al., 2002; Ma et al., 2000; Schneeberger and Lynch, 2004;Won, 1997).Themajormolecular components of the TJ include the transmembranous and structural proteins, occludin, junctional adhesion molecule (JAM) and claudins and the submembranous peripheral Zona occludin (ZO) proteins (Aurrand-Lions et al., 2001, 2002; Furuse et al., 1998, 1999; Bazzoni et al., 2000; Bradley et al., 1998). ZO proteins are essential for targeting TJ structures, and they are linked to the actin cytoskeleton and related signal transducing mechanisms, critical for TJ function. The apical filamentous actin (F-actin) cytoskeleton organization is influenced by the Rho GTPase family, which also controls TJ function. (Bazzoni et al., 2000; Luscinskas et al., 2002; Mehta and Malik, 2006; Tsukita et al., 1997). JAM proteins are not connected to the ancillary proteins of the cytoplasm, but they affect passage of cells when endothelial or mononuclear cells are activated (Andras et al., 2005, 2003; Aurrand-Lions et al., 2001, 2002). TJ and ZOare highly sensitive to the microenvironment and respond to inflammatory cytokines in vitro which results in an alteration in the subcellular localization and dissociation of the occludin/ZO complex, associated with an impaired BBB.
The HIV-1 envelope glycoprotein gp120 is both neurotoxic and cytotoxic and has been implicated in the development of HIV-1 dementia (Li et al., 2005; Nath et al., 2000). It is also known to cause oxidative stress and is associated with disruption of the BBB (Price et al., 2005).Gp120 can damage the endothelial cells of the BBB, thereby compromising its integrity leading to migration of HIV-infected cells into the brain (Iyengar et al., 1999; Toneatto et al., 1999). Gp120 can activate the vascular component of the blood brain barrier (Cioni and Annunziata, 2002). Kanmogne et al. have shown that treatment of cultured human brain endothelial cells with gp120 for 24 h resulted in increased permeability of the endothelial monolayer and dysregulation of the ZO-1, ZO-2 and occludin and no effect on the expression of claudin-1 and claudin-5 (Kanmogne et al., 2005, 2007). We hypothesize that gp120 in synergy with Meth can alter BBB permeability via the modulation of tight junction expression, and a thorough understanding of the mechanisms of BBB breakdown that lead to neurotoxicity is crucial to the development of therapeutic modalities for Meth abusing HAD patients. Limited studies have been done to investigate the effects of methamphetamine on tight junction modulation. Further, no studies have investigated the combined effect of gp120 and methamphetamine on JAM-2, ZO-1, Occludin and Claudin-3, -5 expression, all of which are key tight junction proteins that play a role in modulation of BBB permeability. In this study, to investigate the effect of Meth and/or gp120 on the basal expression of TJ proteins, we used in vitro cultures of primary brain microvascular endothelial cells (BMVEC). The functional effects of TJ modulation by Meth and/or gp120 will be studied using an in vitro BBB model, that allows measurement of BBB permeability using TEER measurements and transendothelial migration of peripheral blood mononuclear cells (PBMC).
2. Results
2.1. Methamphetamine and/or gp120 treatment increases transendothelial electrical resistance (TEER) across the in vitro BBB
Transendothelial electrical resistance across the in vitro BBB was measured using an ohm meter Millicell-ERS system (Millipore, Bedford, MA Cat #MERS 000 01). The BBB was treated with Meth (10, 25 and 50 nM) for 24, 48 and 72 h followed by replacement of fresh media prior to recording of TEER. TEER measurements of the blank inserts with media alone were subtracted from the TEER readings obtained from inserts with confluent endothelial and astrocyte monolayers. The TEER values represent the tightness or integrity of the in vitro BBB. No significant decrease in TEER measurements was observed at baseline for the untreated control or treated samples. Our results suggest that treatment with Meth resulted in a significant loss of BBB integrity at 24, 48 and 72 h post treatment (Fig. 1). Results shown in Fig. 1 indicate that Meth treatment significantly decreased the TEER across the BBB in a dose dependent manner and that the maximal decrease in TEER was observed at the highest Meth concentration of 50nM and at a time period of 48 h post Meth treatment.
Fig. 1.
The kinetics and dose response effect of Meth on the TEER across the in vitro BBB. Transendothelial electrical resistance across the in vitro BBB was measured using a Millicell-ERS system (Millipore, Bedford, MA Cat # MERS 000 01). The BBB was treated with Meth (10, 25 and 50 nM) for 24, 48 and 72 h followed by replacement of fresh media prior to recording of TEER. TEER measurements (Ω/cm2) are expressed as mean±SD of 3 separate experiments done in triplicate. Statistical significance was determined using ANOVA based on comparison with the untreated control for each time point. Our results indicate that Meth treatment significantly decreased the TEER across the BBB in a dose dependent manner and that the maximal decrease in TEER was observed at a Meth concentration of 50 nM and at 48 h post treatment.
To observe the effects of gp120 alone and a combination of gp120 and Meth, the BBB was treated with gp120 alone (50ng/ml) and Meth (10 nM and 50 nM) for 48 h. Our results (Fig. 2) show that treatment of BBB with Meth in combination with gp120 resulted in a significant decrease in TEER suggesting that Meth acts as a co-factor in the neuropathogenesis of HIV-1 by altering BBB integrity in conjunction with the HIV-1 viral protein gp120.
Fig. 2.
Effect of Meth and/or gp120 on the TEER across the in vitro BBB. Transendothelial electrical resistance across the in vitro BBB was measured using a Millicell-ERS system (Millipore, Bedford, MA Cat # MERS 000 01). The BBB was treated with Meth (10 nM and 50 nM) and/or gp120 (50 ng/ml) for 48 h followed by replacement of fresh media prior to recording of TEER. TEER measurements (Ω/cm2) are expressed as mean±SD of 3 separate experiments done in triplicate. Statistical significance was determined using ANOVA. The asterisk (*) denotes statistically significant differences between the treated samples with the untreated control; the symbol (§)denotes statistically significant differences between the Meth treated samples and the combination samples treated with both concentrations of Meth and gp120 respectively; and the symbol (†) denotes statistically significant differences between the gp120 treated samples and the combination samples treated with both concentrations of Meth and gp120 respectively. Our results show that treatment of BBB with Meth and gp120 alone and in combination resulted in a significant decrease in TEER.
2.2. Effect of methamphetamine and gp120 on tight junction gene expression
BMVEC(1×106 cells/ml)were treated in vitro with Meth(10nM)± gp120 (50 ng) for 48 h. The 48 h time point was chosen based on dose and time kinetics experiments. RNA is isolated, reverse transcribed and real time quantitative PCR used to quantitate gene expression using specific primers for ZO-1, JAM-2, Claudin-3, Claudin-5 and Occludin (Table 1). Relative expression of mRNA species was calculated using the comparative CT method and expressed as Transcript accumulation Index (TAI) (Bustin, 2002). All data are controlled for quantity of RNA input by performing measurements on an endogenous reference gene, β-actin and the 18S RNA as internal controls. Additionally, results on RNA from treated samples are normalized to the results obtained on RNA from the control untreated sample. Fig. 3b shows an amplification plot of a representative quantitative PCR run. Our results (Fig. 3a) show that at a lower concentration (10 nM) of Meth a decrease in Claudin-3 (18% decrease, TAI=0.82±0.26, p=NS), and Claudin-5 (13%decrease, TAI=0.86± 0.26; p=NS) gene expression was observed but no statistically significant differences were observed when compared to the untreated control (TAI=1.0). Further at the same concentration of Meth, ZO-1(TAI=1.03±0.31; p=NS), JAM-2 (TAI=1.042±0.046; p=NS), and Occludin (TAI=1.037±0.09; p=NS), there showed no change in gene expression as compared to the untreated control (TAI=1.0). Treatment with Gp120 (50 ng/ml) alone significantly decreased ZO-1 (54% decrease, TAI=0.46±0.06; p=0.006), JAM-2 (27% decrease, TAI=0.73±0.03; p<0.05), and Claudin-3 (23% decrease, TAI=0.77±0.15; p<0.05) gene expression and no significant change was observed in Claudin-5 (TAI=0.94±0.038; p=NS); and Occludin (TAI=1.19±0.23; p=NS) gene expression. However,when BMVEC were treated with a combination of Meth (10nm) and gp120 (50ng/ml) a significant decrease was observed in ZO-1 (68% decrease, TAI=0.32±0.07; p<0.00001), JAM-2 (32% decrease, TAI=0.69±0.05; p<0.00001), Claudin-3 (43% decrease, TAI=0.57±0.12; p <0.006), and Claudin-5 (46% decrease, TAI=0.54±0.04; p=0.005), gene expression while a significant increase in Occludin (51% increase, TAI=1.51±0.18 p=0.012) gene expression was observed, when compared to the untreated control (TAI=1.0).
Table 1.
Sequence and size of primers used in quantitative real time PCR
| Primer | PCR product size (bp) |
Primer sequence |
|---|---|---|
| Β-Actin | 548 | 5′-TGACGGGGTCACCACACTGTGCCCATCTA |
| 3′-AGTCATAGTCCGCCTAGAAGCATTTGCGGT | ||
| ZO-1 | 461 | 5′-GCCAGCAACTTTCAGACCACCAAA |
| 3′-TGGCCACCACAGTATGACCATCTT | ||
| JAM-2 | 294 | 5′-AGGGCGACTTCAGACACAAGTCAT |
| 3′-TAGGAACCCAACTCAGTGAACGCA | ||
| Occludin | 355 | 5′-ATGACTTCAGGCAGCCTCGTTACA |
| 3′-TCAGCAGCAGCCATGTACTCTTCA | ||
| Claudin-3 | 163 | 3′-TCAGCAGCAGCCATGTACTCTTCA |
| 3′-GGTGGCCGTGTACTTCTTCT | ||
| Claudin-5 | 706 | 5′-GCACATGCAGTGCAAAGTGTACGA |
| 3′-TTCATTCCGTCTGTTAAGGGCAGG | ||
| Rho A | 239 | 5′-AGAGGTGTATGTGCCCACAGTGTT |
| 3′-GCACGTTCCCACAGAAATGCTTGA |
Fig. 3.
a: Meth modulates the gene expression of TJ proteins in BMVEC. BMVEC (1×106 cells/ml) were treated in vitro with Meth (10 nM) ± gp120 (50 ng) for 48 h. RNA was extracted, reverse transcribed, cDNA amplified and the ZO-1, JAM-2, Claudin-3, Claudin-5 and Occludin gene expressions were determined by real time quantitative PCR. Relative expression of mRNA species was calculated using the comparative CT method. Data are the mean±SD of 3 separate experiments done in triplicate. Statistical significance was determined using ANOVA based on comparison with the untreated control. Our results show that the most significant differences in TJ expression were observed when the BMVEC were treated with a combination of Meth and gp120. b: An amplification plot from a representative real time quantitative PCR run. The line across the amplification plot is called the threshold, which determines the CT value and is selected where all data are undergoing exponential amplification. The threshold is a numerical value assigned automatically by the software for each run above the calculated baseline.
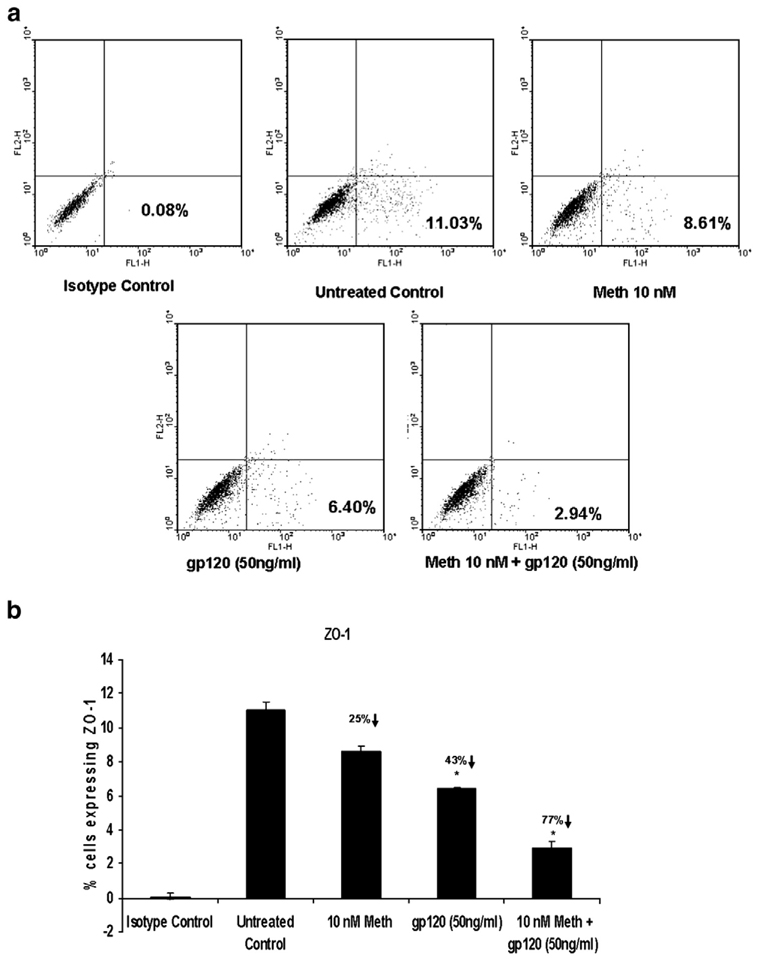
2.3. Effect of methamphetamine and/or gp120 on tight junction expression by FACS analysis
In addition to gene expression studies, we also quantitated the ZO-1 and JAM-2 expression by FACS analysis. BMVEC (1×106 cells/ml) were treated in vitro with Meth (10 nM) ± gp120 (50 ng) for 48 h. The results (Fig. 4a and b and Fig.5a and b) are expressed as the percentage of BMVEC cells expressing ZO-1 and JAM-2. Meth (10 nM) treatment decreased ZO-1 expression by 25% (8.81±0.28%; p=0.058), and increased JAM-2 expression by 16% (9.35±0.64%; p=0.078). Gp120 (50 ng/ml) alone significantly decreased ZO-1 expression by 43% (6.75±0.49%; p=0.02), and decreased JAM-2 expression by 36% (5.16±0.76%; p=0.029) while a combination of Meth (10 nM)+Gp120 (50 ng/ml) further significantly decreased ZO-1 expression by 77% (2.72±0.31%; p=0.014), and decreased JAM-2 expression by 63% (3.02± 0.16%; p=0.016) (Fig. 4a and b and Fig.5a and b).
Fig. 4.
Meth and/or gp120 modulates the ZO-1 expression in BMVEC as measured by FACS analysis. BMVEC (1×106 cells/ml) were treated in vitro with Meth (10 nM) ± gp120 (50 ng) for 48 h. The number of ZO-1 expressing BMVEC cells was quantitated using FACS analysis. Stained cells were subjected to light scatter analysis and a fixed population of cells was gated after quadrant markers were set, based on the isotype control and represented as FL-1 (FITC-labeled) on the X axis. Cells positive for ZO-1 were expressed as a percentage of the total cells gated. a: Dot plot presentation of data from a representative experiment. b: Graphical representation of data expressed as the mean±SD of 3 separate experiments done in triplicate. Statistical significance was determined using ANOVA based on comparison with the untreated control. Our results show that Meth and gp120 alone and in combination significantly decreased ZO-1 expression.
Fig. 5.
Meth and/or gp120 modulates the JAM-2 expression in BMVEC as measured by FACS analysis. BMVEC (1×106 cells/ml) were treated in vitro with Meth (10 nM) ± gp120 (50 ng) for 48 h. The number of JAM-2 expressing BMVEC cells was quantitated using FACS analysis. Stained cells were subjected to light scatter analysis and a fixed population of cells was gated after quadrant markers were set, based on the isotype control and represented as FL-2 (PE-labeled) on the Y axis. Cells positive for JAM-1 were expressed as a percentage of the total cells gated. a: Dot plot presentation of data from a representative experiment. b: Graphical representation of data expressed as the mean ± SD of 3 separate experiments done in triplicate. Statistical significance was determined using ANOVA based on comparison with the untreated control. Our results show that Meth significantly increased JAM-2 expression, while gp120 alone and in combination with Meth significantly decreased JAM-2 expression.
2.4. Effect of methamphetamine and gp120 on transmigration of peripheral blood mononuclear cells (PBMC) across the BBB
In addition to measuring the effect of Meth and gp120 on transendothelial resistance across the BBB, yet another functional assay that assesses BBB integrity by quantitating the transendothelial migration of PBMC across the BBB was done. Our results (Fig. 6a) showed that treatment of the BBB with Meth significantly increased percentage transmigration of PBMC by 30.6% (p=0.04) and 47% (p=0.02) at 10 and 50 nM concentrations respectively as compared to the untreated control (expressed as a 100%). Gp120 (50 ng/ml) alone also significantly enhanced percentage transmigration by 63% (p=0.011) as compared to the untreated control. Further, a combination of Meth at 10 nM or 50 nM plus gp120 (50 ng/ml) increased percentage transmigration to 71% (p=0.010) and 88% (p=0.001) respectively. To evaluate the contribution of the combined effect of Meth and gp120 on BBB integrity, statistical comparisons were made between Meth (10 nM or 50 nM) or gp120 (50 ng/ml) treatments alone and the combination treatment with Meth (10 nM or 50 nM) and gp120 (50 ng/ml) respectively. A significant increase of 31% (p=0.009) and 23% (p=0.008) in transmigration of PBMC across the BBB was observed with Meth (10 nM or 50 nM)+gp120 (50 ng/ml) as compared to treatment with Meth (10 nM) or Meth (50 nM) alone respectively. Further, only a small but not statistically significant increase in transmigration of PBMC across the BBB was observed with Meth (10 nM)+gp120 (50 ng/ ml) (6%; p=NS) and Meth (50 nM)+gp120 (50 ng/ml) (15%; p=0.08 [NS]) as compared to treatment with gp120 (50 ng/ml) alone respectively. These results indicate that Meth acts as a co-factor with HIV-1 viral protein gp120 in the breakdown of the BBB.
Fig. 6.
a: Effect of Meth and/or gp120 on transmigration of PBMC across the in vitro BBB. The in vitro BBB was treated with Meth (10 and 50 nM) alone and/or gp120 (50 ng/ml) for 24 h prior to the transmigration assay. PBMC (5×105 cells) are added per well into the upper chamber of the insert and the chambers are then incubated for 3 h at 37 °C, in 5% CO2 during which cells migrate to the lower chamber in response to a RANTES chemotaxic gradient. The percentage of cells that transmigrated across the BBB with respect to the untreated control was calculated. Data are the mean ± SD of 3 separate experiments done in triplicate. Statistical significance was determined using ANOVA based on the following comparisons. The asterisk (*) denotes statistically significant differences between the treated samples and the untreated control; the symbol (§) denotes statistically significant differences between the Meth treated samples and the combination samples treated with both concentrations of Meth and gp120 respectively; and the symbol (†) denotes statistically significant differences between the gp120 treated samples and the combination samples treated with both concentrations of Meth and gp120 respectively. Our results show that treatment of BBB with Meth and gp120 alone and in combination resulted in a significant increase in transmigration across the in vitro BBB. b: Transmigration of HIV-1 infected and non-infected PBMC across the in vitro BBB treated with Meth. The in vitro BBB was treated with Meth (10 and 50 nM) alone for 24 h prior to the transmigration assay. HIV-1 infected/non-infected PBMC (5×105 cells) are added per well into the upper chamber of the insert and the chambers are then incubated for 3 h at 37 °C, in 5% CO2 during which cells migrate to the lower chamber in response to a RANTES chemotaxic gradient. The percentage of cells that transmigrated across the BBB with respect to the untreated control was calculated. Data are the mean ± SD of 3 separate experiments done in triplicate. Statistical significance was determined using ANOVA. Comparisons were made between the transmigration of HIV-1 infected PBMC as compared to the transmigration of HIV-1 negative normal PBMC at the comparative concentration of Meth treatment (10 nM and 50 nM) respectively and between the respective untreated controls. Our results support the role of Meth in the exacerbation of the neuropathogenesis of HIV-1.
Since, we did not obtain statistically significant increases in transmigration in the Meth + gp120 combination experiments when compared to gp120 alone as outlined above, we did additional transmigration experiments using HIV-1 infected PBMC to investigate the additive effects of Meth and HIV-1. BBB were treated with Meth at 10 and 50 nM concentrations for 24 h and the comparative effects of HIV-1 infected and uninfected PBMC on transmigration across the BBB were observed. We observed a 37% increase (p<0.004) in transmigration of HIV-1 infected PBMC as compared to the uninfected control. Meth treatment further increased the transmigration of both the HIV-1 infected and uninfected PBMC. A significant increase in the transmigration of HIV-1 infected as compared to the transmigration of uninfected PBMC was observed with both Meth (10 nM) (43% increase; p=0.031) or Meth (50 nM) (46% increase; p=0.004) treatments of the BBB respectively (Fig. 6b). These results indicate that Meth abuse in HIV-1 infected subjects may contribute to the neuropathogenesis of HAD via the breakdown of the BBB and exacerbating the potential comorbid interactions between drug abuse and HIV-1 on the CNS.
2.5. Effect of Meth on Rho-A expression
The signaling molecule Rho-A is of particular significance with regard to TJ function and regulation. (Hirase et al., 2001; Nusrat et al., 1995; Persidsky et al., 2006). BMVEC (1×106 cells/ml) were treated in vitro with Meth (10 nM) ± gp120 (50 ng/ml) for 48 h and gene expression of Rho-A was quantitated by real time PCR. Our results (Fig. 7a) show that the Rho-A gene expression increased 41% (TAI=1.41±0.21; p=0.04), 157% (TAI=2.571± 0.07; p<0.0001), and 290% (TAI=3.9±0.20; p<0.0001), on treatment with Meth (10 nM) alone, gp120 (50 ng/ml) alone and a combination Meth (10 nM)+gp120 (50 ng/ml) respectively as compared to the untreated control (TAI=1.00). Additionally, using western blot analysis both total and phospho-protein expressions of Rho-A were quantitated using specific total and phospho-Rho-A antibodies. Measurement of p-Rho-A expression is done within 30 min of exposure to Meth±gp120, while total Rho-A protein was measured 48 h post treatment with Meth±gp120. Our results (Fig. 7b) show that treatment of BMVEC significantly increased total Rho-A protein expression (expressed as % increase with respect to the untreated control) by 44% (p<0.05), 72% (p<0.01) and 69% (p<0.01) and p-Rho-A protein expression by 95.7% (p<0.001), 46.7% (p<0.05) and 104.5% (p<0.001) with Meth (10 nM), gp120 (50 ng/ml) and a combination Meth (10 nM)+p120 (50 ng/ml) respectively as compared to the untreated control (100%). These data suggest that both Meth and gp120 alone and in combination, significantly increased both gene and total and phospho-protein expression of Rho-A.
Fig. 7.
a: Meth modulates the gene expression of Rho-A in BMVEC. BMVEC (1×106 cells/ml) were treated in vitro with Meth (10 nM) ± gp120 (50 ng) for 48 h. RNA was extracted, reverse transcribed, cDNA amplified and the Rho-A gene expression was determined by real time quantitative PCR. Relative expression of mRNA species was calculated using the comparative CT method. Data are the mean ± SD of 3 separate experiments done in triplicate. Statistical significance was determined using ANOVA based on comparison with the untreated control. b: Meth and/or gp120 modulates Rho-A and phospho-Rho-A protein expression using western blot analysis in BMVEC. BMVEC (3×106 cells/ml) were cultured with or without Meth and/or gp120 or 48 h, cells were lysed, protein extracted and followed by separation on a 4–20% Tris glycine gradient gel and protein expression quantitated using specific total and phospho-Rho-A antibodies. A) Data from a representative western blot experiment showing (i) β-actin protein expression (42 kDa band) showing no change in β-actin protein expression with Meth and/or gp120 treatment. (ii) Rho-A protein expression (~22 kDa band) showing a significant increase in Rho-A expression with Meth and/or gp120 treatment, (ii) p-Rho-A protein expression (~26 kDa band) showing a significant increase in Rho-A expression with Meth and/or gp120 treatment. B) Graphical representation of the densitometric analysis of the Rho-A protein band showing the relative intensity (expressed as pixels units) of the band of this target protein as compared to the expression of β-actin. Data shown are mean ± SD of results from 2 separate experiments done in triplicate. C) Graphical representation of the densitometric analysis of the phospho-Rho-A protein band showing the relative intensity (expressed as pixels units) of the band of this target protein as compared to the expression of β-actin. Data shown are mean ± SD of results from 2 separate experiments done in triplicate. Statistical significance was determined using ANOVA based on comparison with the untreated control. Our results show that Meth and gp120 alone and in combination, significantly modulated the expression of both the total and phospho-forms of the Rho-A protein.
We also evaluated the effect of Meth (10 nM) and or gp120 (50 ng/ml) on expression of p-Rho-A by FACS analysis and Immunofluorescent microscopy. Our FACS analysis results are expressed as the percentage of BMVEC cells expressing p-Rho-A, and show the time dependent increase in phospho-Rho-A activation at 10, 20 and 30 min after exposure to Meth (10 nM)+Gp120 (50 ng/ml) (Fig. 8a). Treatment with Meth (10 nM) and gp120 (50 ng/ml) alone increased the phospho-Rho-A expression to 9.86% (p=0.012) and 12.27% (p=0.003) respectively, while a combination of Meth (10 nM)+gp120 (50 ng/ml) further increased phospho-Rho-A expression to 15.43% (p=0.007), 17.98% (p=0.0001) and 22.41% (p=0.006) at 10, 20 and 30 min post treatment as compared to the untreated control (1.11%). Our immunofluorescent results also indicate that Meth treatment alone and in conjunction with gp120 upregulated p-Rho-A expression by BMVEC (Fig. 8b).
Fig. 8.
Effect of Meth and/or gp120 on phospho-Rho-A protein expression in BMVEC. BMVEC were treated in vitro with Meth (10 nM) ± gp120 (50 ng) for 10, 20 and 30 min and p-Rho-A expression quantitated using FACS analysis (a) and immunofluorescent staining (b). a: Histogram (grey) representation of a typical experiment showing the increase phenotypic expression of p-Rho-A with Meth and/or gp120 treatment. The overlayed black lined histogram represents the untreated control. b: Representative fluorescence micrograph images of confluent BMVEC monolayers after treatment with Meth and/or gp120. All fluorescent images taken using the Leica confocal microscope (TCS SP2 AOBS, Leica Microsystems Heidelberg GmbH), 63× objective, oil immersion. a) Transparence background image; b) β-actin control (uniform bright red fluorescence staining imaged by using laser 633 nm (red), Cy-5 fluorescence image was red c) phospho-Rho-A staining in the untreated control sample; d) BMVEC cells treated with Meth (10 nM) alone; e) BMVEC cells treated with gp120 (50 ng/ml) alone; d) BMVEC cells treated with Meth (10 nM) + gp120 (50 ng/ml); Micrographs c–e imaged by using laser 488 nm (blue), and the FITC fluorescence was green. All images were taken 30 min after treatment with Meth and/or gp120. Our results indicate that Meth treatment alone and in conjunction with gp120 upregulated p-Rho-A expression by BMVEC.
3. Discussion
The BBB represents a complex cellular system consisting of brain microvascular endothelial cells (BMVEC), pericytes, perivascular microglia, astrocytes, and basal lamina (Bradbury, 1993). BMVEC form a unique, tightly interconnected, cellular monolayer. Special characteristics of BMVEC include the presence of tight junctions. TJ in the BBB are composed of an intricate combination of transmembrane and cytoplasmic proteins linked to an actin based cytoskeleton that allows the TJ to form a tight seal while still remaining capable of rapid modulation and regulation. TJ formation and disruption is a process that involves a complex interaction between different TJ proteins and can be modulated by HIV-1 viral proteins (Andras et al., 2005; Balda and Matter, 1998; Toborek et al., 2005).
Methamphetamine abuse by patients infected with HIV-1 results in exacerbated neurodegenerative changes suggesting that methamphetamine acts as a co-factor in HIV-1 neuropathogenesis (Theodore et al., 2007; 2006a,b,c; Mahajan et al., 2006; Conant et al., 2004; Nath et al., 2001). HIV-1 viral proteins such as gp120 can cause toxicity to dopaminergic neurons, and this toxicity is synergistic with methamphetamine which also acts on the dopaminergic system (Volkow et al., 2001a,b; Xie et al., 2002). The neurotransmitter dopamine is involved in the regulation of several endothelial cell functions, and the fact that both methamphetamine and gp120 act on the dopiminergic system leads us to speculate that they may either alone or synergistically regulate the TJ modulation in BMVEC thereby compromising BBB integrity and exacerbating the HIV-1 neuropathogenesis process.
The major molecular components of the TJ's include the transmembranous and structural proteins, occludin, JAM and claudins and the submembranous peripheral ZO proteins (Aurrand-Lions et al., 2001, 2002; Furuse et al., 1998, 1999). ZO proteins are essential for targeting TJ structures, and they are linked to the actin cytoskeleton and related signal transducing mechanisms, critical for TJ function (Bazzoni et al., 2000; Luscinskas et al., 2002; Mehta and Malik, 2006; Tsukita et al., 1997). JAM proteins are not connected to the ancillary proteins of the cytoplasm, but they affect passage of cells when endothelial or mononuclear cells are activated (Aurrand-Lions et al., 2002; 2001; Martin-Padura et al., 1998). TJ and ZO are highly sensitive to the microenvironment and respond to inflammatory cytokines in vitro which results in an alteration in the subcellular localization and dissociation of the occludin/ZO complex, associated with an impaired BBB (Bradley et al., 1998; Collins et al., 2006; Tsukita et al., 1997).We evaluated the TJ proteins JAM-2, ZO-1, Claudin-3, Claudin-5 and Occludin which we believe are most relevant to the pathophysiological conditions that alter BBB integrity in HIV-1 infection.
Our results suggest that both Meth and/or gp120 can alter TJ expression in the brain endothelium. These alterations in TJ expression result in breakdown of BBB integrity as reflected by enhanced transendothelial migration and a significant decrease in transendothelial electrical resistance across the BBB. We observed that gp120 treatment resulted in a significant decrease in ZO-1, JAM-2 and Claudin-3 gene expression while there was a small decrease in Claudin-5 and Occludin gene expression, no statistically significant change was observed. Kanmogne et al. have reported that gp120 treatment of BMVEC resulted in a decrease in ZO-1, ZO-2 and Occludin expression and no change in Claudin-5 expression (Kanmogne et al., 2002, 2005, 2007; Wong et al., 2004). Meth treatment showed a small decrease in ZO-1, Claudin-3, and Claudin-5 gene expression but no statistically significant differences were observed when compared to the untreated control. Further, Meth treatment did not result in any change in JAM-2 and Occludin gene expression as compared to the untreated control. However, when BMVEC were treated with a combination of Meth and gp120 further decrease in ZO-1, JAM-2, Claudin-3, and Claudin-5 gene expression and an increase in Occludin gene expression were observed when compared to untreated control or the Meth or gp120 alone treated cultures. Our results suggest that both Meth and/or gp120 alone and synergistically can alter TJ expression in the brain endothelium. There are variable reports in literature on TJ regulation and its role in BBB permeability. Regulation of paracellular permeability by JAM-2 was observed by Aurrand-Lions et al. (2002). In the context of HIV-1 and drug abuse both gp120 and Meth potentially decrease TJ expression, however it may well be that TJ disruption, may involve redistribution but not degradation of certain TJ proteins. Further both gp120 and Meth may elicit an inflammatory response that can result in the increased expression of Occludin that we observe in our study. Inflammation has been shown to result in an increase in ZO-1 expression, which can be attributed to increase in ZO-1 transcription (Asanuma et al., 2004; Wong et al., 2004). Claudin expression however, has been shown to remain unchanged in spite of an inflammation stimulus, suggesting that it may play a major role in maintaining TJ integrity (Huber et al., 2001a). Claudin-3 and Claudin-5 have been reported to play a key role in TJ integrity at the BBB (Furuse et al., 1999, 1998; Huber et al., 2001b). The alterations in TJ gene expression that we observed in our study were similar to those reported in literature and we believe that these alterations in TJ expression may result in breakdown of BBB integrity as reflected by enhanced transendothelial migration and a significant decrease in TEER across the BBB. The ability of Meth and gp120 alone and in combination to alter functional changes like transendothelial migration of immunocompetent cells and TEER validates the role of Meth in the exacerbation of the neuropathogenesis of HIV-1. It is important to note that although Meth and gp120 modulate the expression of several TJ proteins, they did not have a significant effect on the actin cytoskeleton as reflected by similar levels of actin staining in untreated controls and in the Meth and/or gp120 treated samples in our immunofluorescent studies. Both our western blot studies as well as our real time quantitative PCR studies showed no significant differences in the β-actin protein and gene expression in the gp120 and/or Meth treated and control BMVEC cultures.
Signaling molecules that directly control actin cytoskeleton organization are of particular significance with regard to TJ function regulation. Several studies have implicated Rho-A in TJ functions (Persidsky et al., 2006; Hirase et al., 2001; Furuse et al., 1999, 1998; Nusrat et al., 1995). We therefore investigated the effect of Meth and/or gp120 on Rho-A expression in BMVEC. TJ structure and function are dynamic and subject to Rho-A regulation, even after the junctional complex has been fully assembled and tightly sealed (Hirase et al., 2001). Several processes such as membrane transport, organization of TJ, chemotaxis, cytokinesis, and cell polarity are also regulated by Rho proteins and require cytoskeletal changes. Thus signaling pathways activated by RhoGTPases coordinate junction assembly, stability and function (Lang et al., 1996). Rho/RhoK activation in BMVECs could be an underlying cause of BBB impairment during HAD and that loss of TJ integrity was associated with Rho activation (Persidsky et al., 2006; Adamson et al., 1999; Wojciak-Stothard et al., 1999). Man et al. have reported that the interaction between chemokine MIP-1-α and its ligand CCR5 promotes T cells transendothelial migration via Rho kinase (Man et al., 2007). Further studies by Adamson et al. suggest that the cell adhesion molecule ICAM-1 mediates activation of Rho proteins facilitating rearrangement of the endothelial actin cytoskeleton and resulting in T lymphocyte migration through the tight blood brain barrier of the CNS (Adamson et al., 1999).
Our results showed that both gp120 and/or Meth treatment resulted in a significant increase in Rho-A gene and protein expression. These results suggest that TJ modulation by gp120 and/or Meth involves the activation of Rho kinase (Rho-A).
Under various pathological conditions, tissues are incessantly exposed to reactive oxygen species produced by infiltrating inflammatory cells. Oxidative stress has been shown to play an important role in the toxic effects of Meth (Cubells et al., 1994; Lee et al., 2004). Meth treatment can also lead to augmentation of excitatory neurotransmission which may result in enhanced oxidative stress. Meth produces selective degeneration of dopamine (DA) neuron terminals and Meth induced neurotoxicity involves dopamine-dependent intracellular oxidative stress suggesting that dopamine-dependent events play a key role in MA neurotoxicity. Meth induced oxidative stress can also activate myosin light chain kinase (MLCK), resulting in the enhanced phosphorylation of Rho in BMVEC which is associated with loss of TJ integrity (Persidsky et al., 2006; Haorah et al., 2005; Adamson et al., 1999). Thus we speculate that Meth treatment of the BMVEC cells resulted in increased oxidative stress and release of reactive oxygen species which alter BBB integrity, causing cytoskeleton rearrangements and redistribution and disappearance of TJ proteins. Signaling pathways such as Rho-A are activated as a consequence of these events and specific inhibitors of the Rho-A activation could prevent reactive oxygen species (ROS)-induced transmigration of PBMC across an in vitro model of the BBB.
Thus, gp120 independently can modulate TJ expression thereby compromising BBB integrity, however methamphetamine too alone and in synergy with gp120 could modulate dopaminergic function, and may also make the dopaminergic system more vulnerable to HIV-1. Although, we did not measure the release of cytokines in response to gp120 and or Meth, in the media in our BBB model system, cytokines like TNF-α and chemokines like MCP-1 have been implicated in the Meth and HIV-1 viral protein tat induced neurotoxicity, possibly by exacerbating BBB dysfunction (Theodore et al., 2007). Understanding the mechanisms that lead to the Meth induced selective neurotoxicity of dopaminergic neurons in the context of HIV-1 neuropathogenesis would help the development of therapeutic modalities for methamphetamine abusing HIV-1 patients.
4. Experimental procedures
4.1. Cell culture
Primary cultures of both human BMVEC (Cat# ACBRI666) and Normal Human astrocytes (NHA) (Cat# ACBRI666) were obtained from Applied Cell Biology Research Institute (ACBRI) Kirkland, WA. Characterization of BMVEC demonstrated that >95% cells were positive for cytoplasmic VWF/Factor VIII. BMVEC were cultured in CS-C complete serum-free medium (ABCRI, Cat SF-4Z0-500) with attachment factors (ABCRI, Cat # 4Z0-210) and Passage Reagent Group™ (ABCRI, Cat # 4Z0-800). BMVEC are obtained at passage 2 for each experiment and are used for all experimental paradigms between 2 and 8 passages within the 6 to 27 cumulative population doublings. Kinetics (24, 48, 72 h) and dose response studies of Meth (10, 25 and 50 nM) and/or combination experiments with gp120 (50 ng/ml) were done to determine the effect of Meth and/or gp120 on TJ expression. The Meth concentrations (10, 25 and 50 nM) used in our study are well within the physiological range and have been used by other investigators in prior studies (NIDA info Facts, 2005; Anonymous, 2005; Boddiger, 2005; Urbina and Jones, 2004; Chang et al., 2005). HIV-1 gp120 [recombinant] (Mtropic/R5 strain, Bal), was commercially obtained from Chiron Corp., Emeryville, CA. Appropriate dilutions of stock solutions for use in culture were made in culture media. Gp120 concentration used in culture was 50 ng/ml, which is comparable to levels found in vivo during active infection with HIV-1 (Baritaki et al., 2005; Singh et al., 2004). Denatured (heat inactivated) gp120 is used as control.
4.2. In vitro BBB model
We validated the in vitro BBB model by Persidsky et al. in our laboratory and used this model to examine BBB properties like permeability and transendothelial migration of monocytes (Persidsky and Gendelman, 1997; Persidsky et al., 1997). The BBB model used consists of 2-compartment wells in a 12 well culture plate with the upper compartment separated from the lower by a 3 µm PET insert (surface area=0.3 cm2) on which the BMVEC cells are grown to confluency on the upper side while a confluent layer of human astrocytes is grown on the underside. Formation of a BBB takes aminimum of 5 days. Formation of an intact BBB was measured by determining transendothelial electrical resistance (TEER) using Millicell-ERS microelectrodes (Millipore, Bedford, MA) and inulin permeability. Both measures confirm formation of a functionally intact in vitro BBB by day 5 of culture (Mahajan et al., 2004).
4.3. HIV-1 infection of PBMC
PBMC were isolated by the standard density gradient technique using the Ficoll–Hypaque method (Böyum, 1974). Briefly, heparinized blood was diluted 1:1 with Hank's buffer and layered on 15 ml Ficoll-Paque® Plus (Amersham-Pharmacia, Piscataway, NJ Cat # 17-1440-03), in a 50 ml culture tube. Samples were centrifuged for 20 min at 700 ×g and 20 °C without applying a brake. The PBMC interface was carefully removed by pipetting and was washed twice with Hank's buffer by stepwise centrifugation for 15 min at 300 ×g and for 10 min at 90 ×g for platelet removal. PBMC were resuspended in 2 ml of complete RPMI media and total number of cells counted using a hemocytometer. PBMC (3×106 cells/ml) were infected with HIV-1IIIB (NIH AIDS Research and Reference Reagent Program) at a concentration of 10 3.0TCID50/ml cells equivalent to 10 ng viral isolate/ml of culture media for 3 h, and then washed with Hank's buffered saline and cells reconstituted in RPMI media fortified with 10% FBS and incubated at 37 °C/5%CO2 for 7 days. Levels of p24 in the culture supernatants were measured using a commercially available p24 ELISA kit (Zeptometrix, Buffalo, NY) 7 days post infection. These infected PBMC were then washed and reconstituted in fresh culture medium and used in the transmigration assay.
4.4. Transmigration assay
All transmigration experiments are conducted using well established protocols on day 6 of BBB culture after membrane integrity has been established by TEER measurement and permeability to inulin (Ricardo-Dukelow et al., 2007; Banks et al., 1997; Persidsky and Gendelman, 1997; Persidsky et al., 1997). Transmigration assays were done in the presence and absence of a chemotaxic gradient. The chemoattractant used was recombinant RANTES (10 ng/ml) added to the lower chamber just prior to the start of the transmigration assay. The artificial BBB was treated with Meth (10 and 50 nM) alone and/or gp120 (50 ng/ml) for 24 h prior to the transmigration assay. PBMC (5×105 cells) are added per well into the upper chamber of the insert, the chambers are then incubated for 3 h at 37 °C, in 5% CO2 after which the contents of the lower chamber are collected after scraping the bottom well. Appropriate controls, which include insert only, insert with astrocytes only, inserts with endothelial cells only to compare with co-cultures as well as co-cultures with and without the addition of chemokine RANTES as chemoattractant were used. Percent transmigration was calculated with respect to the initial total number of cells added to the upper chamber by counting the cells using a hemocytometer. Cell viability was assessed by trypan blue staining. The viability of the transmigrated cells for all the experiments in the study was >90%.
4.5. RNA extraction
Cytoplasmic RNA was extracted by an acid guanidinium-thiocyanate-phenol-chloroform method as described (Chomczynski and Saachi, 1987). Cultured cells were centrifuged and resuspended in a 4 M solution of guanidinium thiocynate. Cells were lysed by repeated pipetting and then phenol-chloroform extracted in the presence of sodium acetate. After centrifugation, RNA was precipitated from the aqueous layer by adding an equal volume of isopropanol and the mixture was kept at −20 °C for 1 h and then centrifuged to sediment the RNA. The RNA pellet was washed with 75% ethanol to remove any traces of guanidinium and DNAse (1 U/µg RNA) treated to remove any DNA contamination. The final pellet was dried and resuspended in diethyl pyrocarbonate (DEPC) water and the amount of RNA is quantitated using a Nano-Drop ND-1000 spectrophotometer (Nano-Drop™ Wilmington, DE) and isolated RNA is stored at −80 °C until used.
4.6. Real time quantitative PCR (Q-PCR)
Q-PCR was used to quantitate the effect of methamphetamine and/or gp120 on TJ expression in BMVEC cultures. Approximately 1×106 BMVEC cells were treated with Meth (50nM) alone and/or with gp120 (50 ng/ml) for 48 h and cells were harvested, and RNA extracted as described above. The RNA is then reverse transcribed to cDNA using the reverse transcriptase kit from Promega (Promega Inc, Madison, WI; Cat # A3500). Relative abundance of each mRNA species is quantitated using real time quantitative PCR using specific primers (Table 1) using the Brilliant® SYBR® green Q-PCR master mix from Stratagene (Stratagene Inc, La Jolla, CA; Cat # 600548-51). To provide precise quantification of initial target in each PCR reaction, the amplification plot is examined at a point during the early log phase of product accumulation. This is accomplished by assigning a fluorescence threshold above background and determining the time point at which each sample's amplification plot reaches the threshold (defined as the threshold cycle number or CT) (Fig. 3b). The threshold is a numerical value assigned automatically by the software for each run above the calculated baseline. The CT value assigned to a particular sample thus reflects the point during the amplification reaction at which sufficient number of amplicons has accumulated above the baseline. After a suitable threshold is selected, the data is analyzed and CT values for each sample determined. Differences in threshold cycle number (CT) are used to quantify the relative amount of PCR target contained within each tube. Relative expression of mRNA species is calculated using the comparative CT method. All data are controlled for quantity of RNA input by performing measurements on an endogenous reference gene, β-actin. In addition, results on RNA from treated samples are normalized to results obtained on RNA from the control untreated sample. Briefly, the analysis is performed as follows: for each sample, a difference in CT values (ΔCT) was calculated for each mRNA by taking the mean CT of duplicate tubes and subtracting the mean CT of the duplicate tubes for the reference RNA (β-actin) measured on an aliquot from the same RT reaction. The ΔCT for the treated sample was then subtracted from the ΔCT for the untreated control sample to generate a DDCT. The mean of these DDCT measurements was then used to calculate expression of the test gene relative to the reference gene and normalized to the untreated control as follows: Relative Expression/Transcript Accumulation Index=2−ΔΔCT. This calculation assumes that all PCR reactions are working with ~100% efficiency (Bustin, 2002; Radonić et al., 2004).
4.7. FACS analysis
Flow cytometric analysis was used to study the effect of methamphetamine and/or gp120 on TJ expression in BMVEC cultures. Approximately 1×106 BMVEC cells were treated with Meth (10 nM) alone and/or with gp120 (50 ng/ml) for 48 h and cells were harvested, trypsinized, washed and suspended in staining buffer. FACS conditions were optimized by adjusting the settings for photomultiplier tube voltage and compensation using appropriate staining controls and quadrant markers were set using a specified isotype control. (Shapiro, 1994). Primary antibodies (mAbs) against ZO-1 (Cat #2304; Santacruz Biotech, Santacruz, CA) and JAM-2 (Cat # GTX70418; Gene-Tex, Inc, San Antonio, TX) were used. Matched isotype controls (IgG2A and IgG1 respectively) were obtained from R&D Systems, Inc, Minneapolis, MN. FITC and PE-conjugated secondary antibodies specific for both anti ZO-1 and anti-JAM-2 primary antibodies were used to determine percentage of cells expression these antigens. After staining procedures, cells were then washed in PBS, and resuspended in staining buffer prior to flow cytometry using a FACS Calibur instrument (BD Biosciences, San Jose, CA).
4.8. Immunofluorescent staining of endothelial cells
BMVEC cells were grown to 70% confluence on a glass cover slip, and were treated with Meth (10 nM) ± gp120 (50 ng/ml) for 10, 20, 30 and 60 min followed by fixation for 10 min at 37 °C in 2% formaldehyde followed by permeabilization with ice-cold 90% methanol. Cells were then washed in 1× phosphate buffer saline (PBS) and stained with specific primary antibodies against p-Rho-A and β-actin. Standard immunofluorescent staining procedures outlined in the Immunocytochemistry: laboratory manuals, Cold Spring Harbor Press (New York, 1988) and Acadenmic Press (1989) were followed (Harlow and Lane, 1993; Bullock and Petrusz, 1989). Fluoro-linked secondary antibodies (FITC-labeled anti-Rabbit for p-Rho A; Cat # 554020, BD Pharmingen, CA and Cy-5 labelled for β-actin Cat # 81-1616, Zymed Inc, San Fransisco, CA) against the specific primary antibodies were used for detection by immunofluorescent microscopy. Appropriate internal controls are used during all immunostaining procedures. Imaging was done using a Leica Confocal Laser Scanning Microscope (TCS SP2 AOBS, leica Microsystems Heidelberg GmbH) with an oil immersion objective lens 63×. HeNe 633 nm laser was applied to excite Cy5, Argon ion laser was applied to excite FITC.
4.9. Western blot analysis
Approximately 1×106 BMVEC cells were treated with Meth (10 nM) alone and/or with gp120 (50 ng/ml) for 72 h and cells were washed and lysed using the M-PER® protein extraction reagent (Cat # 78503, Pierce, Inc, Rockford, IL). Immunodetection using standard western blotting techniques adapted from current protocols in Immunology were used (Current Protocols in Immunology, Copyright©, 2007 by John Wiley and Sons, Inc, Hoboken, NJ). 30 µg total protein extracted from cells was loaded per lane and separated by a 4–20% SDS-Tris glycine PAGE. Gels are blotted onto nitrocellulose membranes and membranes are probed with Rho-A (Cat #sc418, Santacruz Biotech) and p-Rho-A (Cat #sc32954-R, Santacruz Biotech, Santacruz CA) antibodies against the total and phosphorylated forms of the Rho protein.
4.10. Statistical analysis
All results were expressed as mean±SD, and a value of p<0.05 was considered significant. Statistically significant effects of Meth and/or gp120 on TEER measurement, percentage transmigration of PBMC and Rho-A, JAM-2, ZO-1, Claudin-3, Claudin-5 and Occludin expression will be assessed using a one way ANOVA. To determine the significance between the groups a Bonferroni post hoc test was done. The statistical software package GraphPad Prism software (GraphPad Prism Software, Inc. San Diego, CA) was used.
Acknowledgments
This work was supported in part by grants from the Margaret Duffy and Robert Cameron Troup Memorial Fund of Kaleida Health and the Kaleida Health Foundation.
Abbreviations
- HIV-1
human immunodeficiency virus-1
- ADC
AIDS dementia complex
- BBB
Blood Brain Barrier
- BMVEC
brain microvascular endothelial cells
- CNS
Central nervous system
- DEPC
diethyl pyrocarbonate
- FITC
Fluorescein
- HAD
HIV-1 associated dementia
- IL1-β
Interleukin-1 beta
- JAM-2
Junctional adhesion molecule-2
- Meth
Methamphetamine
- MLCK
myosin light chain kinase
- NHA
Normal Human astrocytes
- PBS
Phosphate buffer saline
- PE
Phycoerythrin
- ROS
reactive oxygen species
- TAI
Transcript Accumulation Index
- TEER
transendothelial electrical resistance
- TJ
Tight junctions
- TNF-α
tumor necrosis factor-alpha
- ZO-1
Zona Occludins
REFERENCES
- Adamson P, Etienn S, Couraud PO, Calder V, Greenwood J. Lymphocyte migration through brain endothelial cell monolayers involves signaling through endothelial ICAM-1 via a rho-dependent pathway. J. Immunol. 1999;1(5):2964–2973. 162. [PubMed] [Google Scholar]
- Andras IE, Pu H, Tian J, Deli MA, Nath A, Hennig B, Toborek M. HIV-1 Tat protein alters tight junction protein expression and distribution in cultured brain endothelial cells. J. Neurosci Res. 2003;74:255. doi: 10.1002/jnr.10762. [DOI] [PubMed] [Google Scholar]
- Andras IE, Pu H, Tian J, Deli MA, Nath A, Hennig B, Toborek M. Signaling mechanisms of HIV-1 Tat-induced alterations of claudin-5 expression in brain endothelial cells. J. Cereb. Blood Flow Metab. 2005;25(9):1159–1170. doi: 10.1038/sj.jcbfm.9600115. [DOI] [PubMed] [Google Scholar]
- Asanuma M, Miyazaki I, Higashi Y, Tsuji T, Ogawa N. Specific gene expression and possible involvement of inflammation in methamphetamine-induced neurotoxicity. Ann. N. Y. Acad. Sci. 2004;1025:69–75. doi: 10.1196/annals.1316.009. [DOI] [PubMed] [Google Scholar]
- Anonymous. HIV and drugs. Meth use develops stronger link to HIV risk. AIDS Policy Law. 2005;20(13):5. [PubMed] [Google Scholar]
- Aurrand-Lions M, Duncan L, Ballestrem C, Imhof BA. JAM-2, a Novel immunoglobulin superfamily molecule, expressed by endothelial and lymphatic cell. J. Biol. Chem. 2001;276(4):2733–2741. doi: 10.1074/jbc.M005458200. [DOI] [PubMed] [Google Scholar]
- Aurrand-Lions M, Johnson-Leger C, Lamagna C, Ozaki H, Kita T, Imhof BA. Junctional adhesion molecules and interendothelial junctions. Cells Tissues Organs. 2002;172(3):152–160. doi: 10.1159/000066967. [DOI] [PubMed] [Google Scholar]
- Balda M, Matter K. Tight junctions. J. Cell Sci. 1998;111(5):541–547. doi: 10.1242/jcs.111.5.541. [DOI] [PubMed] [Google Scholar]
- Balda MS, Whitney JA, Flores C, Gonzalez S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J. Cell Biol. 1996;134(4):1031–1049. doi: 10.1083/jcb.134.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Akerstrom V. HIV-1 protein gp120 crosses the blood-brain barrier: role of adsorptive endocytosis. Life Sci. 1997;61:PL119–PL125. doi: 10.1016/s0024-3205(97)00597-3. [DOI] [PubMed] [Google Scholar]
- Baritaki S, Dittmar MT, Spandidos DA, Krambovitis E. In vitro inhibition of R5 HIV-1 infectivity by X4 V3-derived synthetic peptides. Int. J. Mol. Med. 2005;16(2):333–336. [PubMed] [Google Scholar]
- Bazzoni G, Martinez-Estrada OM, Orsenigo F, Cordenonsi M, Citi S, Dejana E. Interaction of junctional adhesion molecule with the tight junction components ZO-1, cingulin, and occludin. J. Biol. Chem. 2000;275(27):20520–20526. doi: 10.1074/jbc.M905251199. [DOI] [PubMed] [Google Scholar]
- Boddiger D. Metamphetamine use linked to rising HIV transmission. Lancet. 2005;365(9466):1217–1218. doi: 10.1016/S0140-6736(05)74794-2. [DOI] [PubMed] [Google Scholar]
- Boven LA, Middel J, Breij EC, Schotte D, Verhoef J, Soderland C, Nottet HS. Interactions between HIV-infected monocyte-derived macrophages and human brain microvascular endothelial cells result in increased expression of CC chemokines. J. Neurovirol. 2005;6:382. doi: 10.3109/13550280009018302. [DOI] [PubMed] [Google Scholar]
- Böyum A. Separation of blood leukocytes, granulocytes and lymphocytes. Tissue Antigens. 1974;4:269. [PubMed] [Google Scholar]
- Bradbury MW. The blood-brain barrier. Exp. Physiol. 1993;78(4):453–472. doi: 10.1113/expphysiol.1993.sp003698. [DOI] [PubMed] [Google Scholar]
- Bradley M, Denker, Nigam SK. Molecular structure and assembly of the tight junction. Am. J. Physiol. Renal. Physiol. 1998;274:F1–F9. doi: 10.1152/ajprenal.1998.274.1.F1. [DOI] [PubMed] [Google Scholar]
- Bullock GR, Petrusz P, editors. Techniques in Immunocytochemistry Volumes 1, 2, 3 and 4. Volume 1. Academic Press; 1989. p. 186. [Google Scholar]
- Bustin SA. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. J. Mol. Endocrinol. 2002;29(1):23–39. doi: 10.1677/jme.0.0290023. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Saachi N. Single step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Conant K, St Hillaire C, Anderson C, Galey D, Wang J, Nath A. Human immunodeficiency virus type 1 Tat and methamphetamine affect the release and activation of matrix-degrading proteinases. J. Neurovirol. 2004;10(1):21–28. doi: 10.1080/13550280490261699. [DOI] [PubMed] [Google Scholar]
- Cioni C, Annunziata P. Circulating gp120 alters the blood-brain barrier permeability in HIV-1 gp120 transgenic mice. Neurosci. Lett. 2002;330(3):299–301. doi: 10.1016/s0304-3940(02)00814-5. [DOI] [PubMed] [Google Scholar]
- Collins NT, Cummins PM, Colgan OC, Ferguson G, Birney YA, Murphy RP, Meade G, Cahill PA. Cyclic strain-mediated regulation of vascular endothelial occludin and ZO-1: influence on intercellular tight junction assembly and function. Arterioscler Thromb. Vasc. Biol. 2006;26(1):62–68. doi: 10.1161/01.ATV.0000194097.92824.b3. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Speck O, Grob CS. Additive effects of HIV and chronic methamphetamine use on brain metabolite abnormalities. Am. J. Psychiatry. 2005;162(2):361–369. doi: 10.1176/appi.ajp.162.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubells JF, Rayport S, Rajendran G, Sulzer D. Methamphetamine neurotoxicity involves vacuolation of endocytic organelles and dopamine-dependent intracellular oxidative stress. J. Neurosci. 1994;Vol 14:2260–2271. doi: 10.1523/JNEUROSCI.14-04-02260.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Current Protocols in Immunology, Copyright ©, 2007. Hoboken, NJ: John Wiley and Sons, Inc; [Google Scholar]
- Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J. Neurosci. 2006;26(4):1098–1106. doi: 10.1523/JNEUROSCI.3863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora G, Lee YW, Nath A, Hennig B, Maragos W, Toborek M. Methamphetamine potentiates HIV-1 Tat protein-mediated activation of redox-sensitive pathways in discrete regions of the brain. Exp. Neurol. 2003;179(1):60–70. doi: 10.1006/exnr.2002.8048. [DOI] [PubMed] [Google Scholar]
- Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J. Cell Biol. 1998;141(7):1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Sasaki H, Tsukita S. Manner of interaction of heterogeneous claudin species within and between tight junction strands. J. Cell Biol. 1999;147:891. doi: 10.1083/jcb.147.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman HE, Persidsky Y, Ghorpade A, Limoges J, Stins M, Fiala M, Morrisett R. The neuropathogenesis of the AIDS dementia complex. AIDS. 1997;11 Suppl A:S35. [PubMed] [Google Scholar]
- Haorah J, Heilman D, Knipe B, Chrastil J, Leibhart J, Ghorpade A, Miller DW, Persidsky Y. Ethanol-induced activation of myosin light chain kinase leads to dysfunction of tight junctions and blood-brain barrier compromise. Alcohol Clin. Exp. Res. 2005;29(6):999–1009. doi: 10.1097/01.alc.0000166944.79914.0a. [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies: a laboratory manual, Cold Spring Harbor Press (New York, 1988) In: Beesley JE, editor. Immunocytochemistry: a Practical Approach. IRL Press; 1993. pp. 215–216. [Google Scholar]
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2005;57(2):173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Hirase TS, Kawashima EY, Wong M, Ueyama T, Rikitake Y, Tsukita S, Yokoyama M, Staddon JM. Regulation of tight junction permeability and Occludin phosphorylation by RhoA-p160ROCK-dependent and -independent mechanism. J. Biol. Chem. 2001;276(13):10423–10431. doi: 10.1074/jbc.M007136200. [DOI] [PubMed] [Google Scholar]
- Huber JD, Witt KA, Hom S, Egleton RD, Mark KS, Davis TP. Inflammatory pain alters blood-brain barrier permeability and tight junctional protein expression. Am. J. Physiol. Heart Circ. Physiol. 2001a;280(3):H1241–H1248. doi: 10.1152/ajpheart.2001.280.3.H1241. [DOI] [PubMed] [Google Scholar]
- Huber JD, Egleton RD, Davis TP. Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neurosci. 2001b;24:719. doi: 10.1016/s0166-2236(00)02004-x. [DOI] [PubMed] [Google Scholar]
- Iyengar S, Schwartz DH, Hildreth JE. T cell-tropic HIV gp120 mediates CD4 and CD8 cell chemotaxis through CXCR4 independent of CD4: implications for HIV pathogenesis. J. Immunol. 1999;162(10):6263–6267. [PubMed] [Google Scholar]
- Kanmogne GD, Kennedy RC, Grammas P. HIV-1 gp120 proteins and gp160 peptides are toxic to brain endothelial cells and neurons: possible pathway for HIV entry into the brain and HIV-associated dementia. J. Neuropathol. Exp. Neurol. 2002;61(11):992–1000. doi: 10.1093/jnen/61.11.992. [DOI] [PubMed] [Google Scholar]
- Kanmogne GD, Primeaux C, Grammas P. HIV-1 gp120 proteins alter tight junction protein expression and brain endothelial cell permeability: implications for the pathogenesis of HIV-associated dementia. J. Neuropathol. Exp. Neurol. 2005;64(6):498–505. doi: 10.1093/jnen/64.6.498. [DOI] [PubMed] [Google Scholar]
- Kanmogne GD, Schall K, Leibhart J, Knipe B, Gendelman HE, Persidsky Y. HIV-1 gp120 compromises blood-brain barrier integrity and enhances monocyte migration across blood-brain barrier: implication for viral neuropathogenesis. J. Cereb. Blood Flow Metab. 2007;27(1):123–134. doi: 10.1038/sj.jcbfm.9600330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolson DL. Neuropathogenesis of central nervous system HIV-1 infection. Clin. Lab. Med. 2002;22:703. doi: 10.1016/s0272-2712(02)00009-4. [DOI] [PubMed] [Google Scholar]
- Krebs FC, Ross H, McAllister J, Wigdahl B. HIV-1-associated central nervous system dysfunction. Adv. Pharmacol. 2000;49:315. doi: 10.1016/s1054-3589(00)49031-9. [DOI] [PubMed] [Google Scholar]
- Lang P, Gesbert F, Delespine-Carmagnat M, Stancou R, Pouchelet M, Bertoglio J. Protein kinase A phosphorylation of RhoA mediates the morphological and functional effects of cyclic AMP in cytotoxic lymphocytes. EMBO J. 1996;15(3):510–519. [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Namkoong K, Kim DH, Kim KJ, Cheong YH, Kim SS, Lee WB, Kim KY. Hydrogen peroxide-induced alterations of tight junction proteins in bovine brain microvascular endothelial cells. Microvasc. Res. 2004;68(3):231–238. doi: 10.1016/j.mvr.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Li W, Galey D, Mattson MP, Nath A. Molecular and cellular mechanisms of neuronal cell death in HIV dementia. Neurotox Res. 2005;8(12):119–134. doi: 10.1007/BF03033824. [DOI] [PubMed] [Google Scholar]
- Lipton SA. HIV-related neurotoxicity. Brain Pathol. 1991;1:193. doi: 10.1111/j.1750-3639.1991.tb00659.x. [DOI] [PubMed] [Google Scholar]
- Luscinskas FW, Ma S, Nusrat A, Parkos CA, Shaw SK. The role of endothelial cell lateral junctions during leukocyte trafficking. Immunol. Rev. 2002;186:57. doi: 10.1034/j.1600-065x.2002.18606.x. [DOI] [PubMed] [Google Scholar]
- Ma TY, Tran D, Hoa N, Nguyen D, Merryfield M, Tarnawski A. Mechanism of extracellular calcium regulation of intestinal epithelial tight junction permeability: role of cytoskeletal involvement. Microsc. Res. Tech. 2000;51:156. doi: 10.1002/1097-0029(20001015)51:2<156::AID-JEMT7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Mahajan S, Schwartz SA, Sykes D, Chawda R, Aalinkeel R, Nair MPN. Effect of HIV Peptides on Trans-endothelial Migration of Dendritic Cells Across the Blood Brain Barrier. 12th International Congress of Immunology and 4th Annual Conference of FOCIS; July, 2004; Montreal, Canada. Medimond Press; 2004. pp. 153–159. [Google Scholar]
- Mahajan SD, Zihua Hu, Reynolds JL, Aalinkeel R, Schwartz SA, Nair MPN. Methamphetamine modulates gene expression patterns in monocyte derived mature dendritic cells: implications for HIV-1 pathogenesis. Mol. Diagn. Ther. 2006;10(4):257–269. doi: 10.1007/BF03256465. 2006. [DOI] [PubMed] [Google Scholar]
- Man SM, Ma YR, Shang DS, Zhao WD, Li B, Guo DW, Fang WG, Zhu L, Chen YH. Peripheral T cells overexpress MIP-1alpha to enhance its transendothelial migration in Alzheimer's disease. Neurobiol. Aging. 2007;28(4):485–496. doi: 10.1016/j.neurobiolaging.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Martin-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A, Simmons D, Dejana E. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J. Cell Biol. 1998;142:117. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol. Rev. 2006;86(1):279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- National Institute on Drug Abuse (NIDA) INFO FACTS. 2005 May
- Nath A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J. Infect. Dis. 2002;186 Suppl 2:S193. doi: 10.1086/344528. [DOI] [PubMed] [Google Scholar]
- Nath A, Anderson C, Jones M, Maragos W, Booze R, Mactutus C, Bell J, Hauser KF, Mattson M. Neurotoxicity and dysfunction of dopaminergic systems associated with AIDS dementia. J. Psychopharmacol. 2000;14(3):222–227. doi: 10.1177/026988110001400305. [DOI] [PubMed] [Google Scholar]
- Nath A, Maragos WF, Avison MJ, Schmitt FA, Berger JR. Acceleration of HIV dementia with methamphetamine and cocaine. J. Neurovirol. 2001;7(1):66–71. doi: 10.1080/135502801300069737. [DOI] [PubMed] [Google Scholar]
- Nottet HS. Interactions between macrophages and brain microvascular endothelial cells: role in pathogenesis of HIV-1 infection and blood-brain barrier function. J. Neurovirol. 1999;5:659. doi: 10.3109/13550289909021294. [DOI] [PubMed] [Google Scholar]
- Nottet HS, Persidsky Y, Sasseville VG, Nukuna AN, Bock P, Zhai QH, Sharer LR, McComb RD, Swindells S, Soderland C, Gendelman HE. Mechanisms for the transendothelial migration of HIV-1-infected monocytes into brain. J. Immunol. 1996;156:1284. [PubMed] [Google Scholar]
- Nusrat A, Giry M, Turner JR, Colgan SP, Parkos CA, Carnes D, Lemichez E, Boquet P, Madara JL. Rho protein regulates tight junctions and perijunctional actin organization in polarized epithelia. Proc. Natl. Acad. Sci. U. S. A. 1995;92(23):10629–10633. doi: 10.1073/pnas.92.23.10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdener H. Molecular mechanisms of HIV-1 associated neurodegeneration. J. Biosci. 2005;30(3):391–405. doi: 10.1007/BF02703676. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Gendelman HE. Development of laboratory and animal model systems for HIV-1 encephalitis and its associated dementia. J. Leukoc. Biol. 1997;62(1):100–106. doi: 10.1002/jlb.62.1.100. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Stins M, Way D, Witte MH, Weinand M, Kim KS, Bock P, Gendelman HE, Fiala M. A model for monocyte migration through the blood-brain barrier during HIV-1 encephalitis. J. Immunol. 1997;158(7):3499–3510. [PubMed] [Google Scholar]
- Persidsky Y, Ghorpade A, Rasmussen J, Limoges J, Liu XJ, Stins M, Fiala M, Way D, Kim KS, Witte MH, Weinand M, Carhart L, Gendelman HE. Microglial and astrocyte chemokines regulate monocyte migration through the blood-brain barrier in human immunodeficiency virus-1 encephalitis. Am J Pathol. 1999;155:1599. doi: 10.1016/S0002-9440(10)65476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persidsky Y, Heilman D, Haorah J, Zelivyanskaya M, Persidsky R, Weber GA, Shimokawa H, Kaibuchi K, Ikezu T. Rho-mediated regulation of tight junctions during monocyte migration across blood-brain barrier in HIV-1 encephalitis (HIVE) Blood. 2006;107(12):4770–4780. doi: 10.1182/blood-2005-11-4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TO, Ercal N, Nakaoke R, Banks WA. HIV-1 viral proteins gp120 and Tat induce oxidative stress in brain endothelial cells. Brain Res. 2005;1045(1–2):57–63. doi: 10.1016/j.brainres.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Radonić A, Thulke S, Mackay IM, Landt O, Siegert W, Nitsche A. Guideline to reference gene selection for quantitative real-time PCR. Biochem. Biophys. Res. Commun. 2004;313(4):856–862. doi: 10.1016/j.bbrc.2003.11.177. [DOI] [PubMed] [Google Scholar]
- Ricardo-Dukelow M, Kadiu I, Rozek W, Schlautman J, Persidsky Y, Ciborowski P, Kanmogne GD, Gendelman HE. HIV-1 infected monocyte-derived macrophages affect the human brain microvascular endothelial cell proteome: new insights into blood-brain barrier dysfunction for HIV-1-associated dementia. J.Neuroimmunol. 2007 Apr.185(1–2):37–46. doi: 10.1016/j.jneuroim.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidtmayerova H, Nottet HS, Nuovo G, Raabe T, Flanagan CR, Dubrovsky L, Gendelman HE, Cerami A, Bukrinsky M, Sherry B. Human immunodeficiency virus type 1 infection alters chemokine beta peptide expression in human monocytes: implications for recruitment of leukocytes into brain and lymph nodes. Proc. Natl. Acad. Sci. U. S. A. 1996;93(2):700–704. doi: 10.1073/pnas.93.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am. J. Physiol. Cell Physiol. 2004;286(6):C1213–C1228. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- Shapiro H. Practical Flow Cytometry. 3rd Edition. New York: Wiley-Liss; 1994. [Google Scholar]
- Singh IN, Goody RJ, Dean C, Ahmad NM, Lutz SE, Knapp PE, Nath A, Hauser KF. Apoptotic death of striatal neurons induced by human immunodeficiency virus-1 Tat and gp120: differential involvement of caspase-3 and endonuclease G. J. Neurovirol. 2004;10(3):141–151. doi: 10.1080/13550280490441103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleasman JW, Goodenow MM. HIV-1 infection. J. Allergy Clin. Immunol. 2003;111 2 Suppl:S582–S592. doi: 10.1067/mai.2003.91. [DOI] [PubMed] [Google Scholar]
- Theodore S, Cass WA, Maragos WF. Involvement of cytokines in human immunodeficiency virus-1 protein Tat and methamphetamine interactions in the striatum. Exp. Neurol. 2006a;199(2):490–498. doi: 10.1016/j.expneurol.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Theodore S, Cass WA, Maragos WF. Methamphetamine and human immunodeficiency virus protein Tat synergize to destroy dopaminergic terminals in the rat striatum. Neuroscience. 2006b;137(3):925–935. doi: 10.1016/j.neuroscience.2005.10.056. [DOI] [PubMed] [Google Scholar]
- Theodore S, Stolberg S, Cass WA, Maragos WF. Human immunodeficiency virus-1 protein tat and methamphetamine interactions. Ann. N. Y. Acad. Sci. 2006c;1074:178–190. doi: 10.1196/annals.1369.018. [DOI] [PubMed] [Google Scholar]
- Theodore S, Cass WA, Nath A, Maragos WF. Progress in understanding basal ganglia dysfunction as a common target for methamphetamine abuse and HIV-1 neurodegeneration. Curr HIV Res. 2007;5(3):301–313. doi: 10.2174/157016207780636515. Review. [DOI] [PubMed] [Google Scholar]
- Toborek M, Lee YW, Flora G, Pu H, Andras IE, Wylegala E, Hennig B, Nath A. Mechanisms of the blood-brain barrier disruption in HIV-1 infection. Cell Mol.Neurobiol. 2005;25(1):181–199. doi: 10.1007/s10571-004-1383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toneatto S, Finco O, van der Putten H, Abrignani S, Annunziata P. Evidence of blood-brain barrier alteration and activation in HIV-1 gp120 transgenic mice. AIDS. 1999;13(17):2343–2348. doi: 10.1097/00002030-199912030-00005. [DOI] [PubMed] [Google Scholar]
- Trujillo JR, Jaramillo-Rangel G, Ortega-Martinez M, Penalva de Oliveira AC, Vidal JE, Bryant J, Gallo RC. International NeuroAIDS: prospects of HIV-1 associated neurological complications. Cell Res. 2005;15(11–12):962–969. doi: 10.1038/sj.cr.7290374. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Furuse M, Itoh M. Molecular architecture of tight junctions: occludin and ZO-1. Soc. Gen. Physiol. 1997;Ser 52:69–76. [PubMed] [Google Scholar]
- Urbina A, Jones K. Crystal methamphetamine, its analogues, and HIV infection: medical and psychiatric aspects of a new epidemic. Clin. Infect. Dis. 2004;38:890–894. doi: 10.1086/381975. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am. J. Psychiatry. 2001a;158(12):2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am. J. Psychiatry. 2001b;158(3):377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Wojciak-Stothard B, Williams L, Ridley AJ. Monocyte adhesion and spreading on human endothelial cells is dependent on Rho-regulated receptor clustering. J.Cell Biol. 1999;145:1293. doi: 10.1083/jcb.145.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong D, Dorovini-Zis K, Vincent SR. Cytokines, nitric oxide, and cGMP modulate the permeability of an in vitro model of the human blood-brain barrier. Exp.Neurol. 2004;190(2):446–455. doi: 10.1016/j.expneurol.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Won V. Phosphorylation of occludin correlates with occludin localization and function at the tight junction. Am.J. Physiol.Cell Physiol. 1997;273:C1859–C1867. doi: 10.1152/ajpcell.1997.273.6.C1859. [DOI] [PubMed] [Google Scholar]
- Worthylake RA, Burridge K. Leukocyte transendothelial migration: orchestrating the underlying molecular machinery. Curr. Opin. Cell Biol. 2001;13:569. doi: 10.1016/s0955-0674(00)00253-2. [DOI] [PubMed] [Google Scholar]
- Wu DT, Woodman SE, Weiss JM, McManu CM, D'Aversa TG, Hesselgesser J, Major EO, Nath A, Berman JW. Mechanisms of leukocyte trafficking into the CNS. J. Neurovirol. 2000;1(6 Suppl):S82. [PubMed] [Google Scholar]
- Xie T, Tong L, Barrett T, Yuan J, Hatzidimitriou G, McCann UD, Becker KG, Donovan DM, Ricaurte GA. Changes in gene expression linked to methamphetamine-induced dopaminergic neurotoxicity. J. Neurosci. 2002;22:274–283. doi: 10.1523/JNEUROSCI.22-01-00274.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]