Abstract
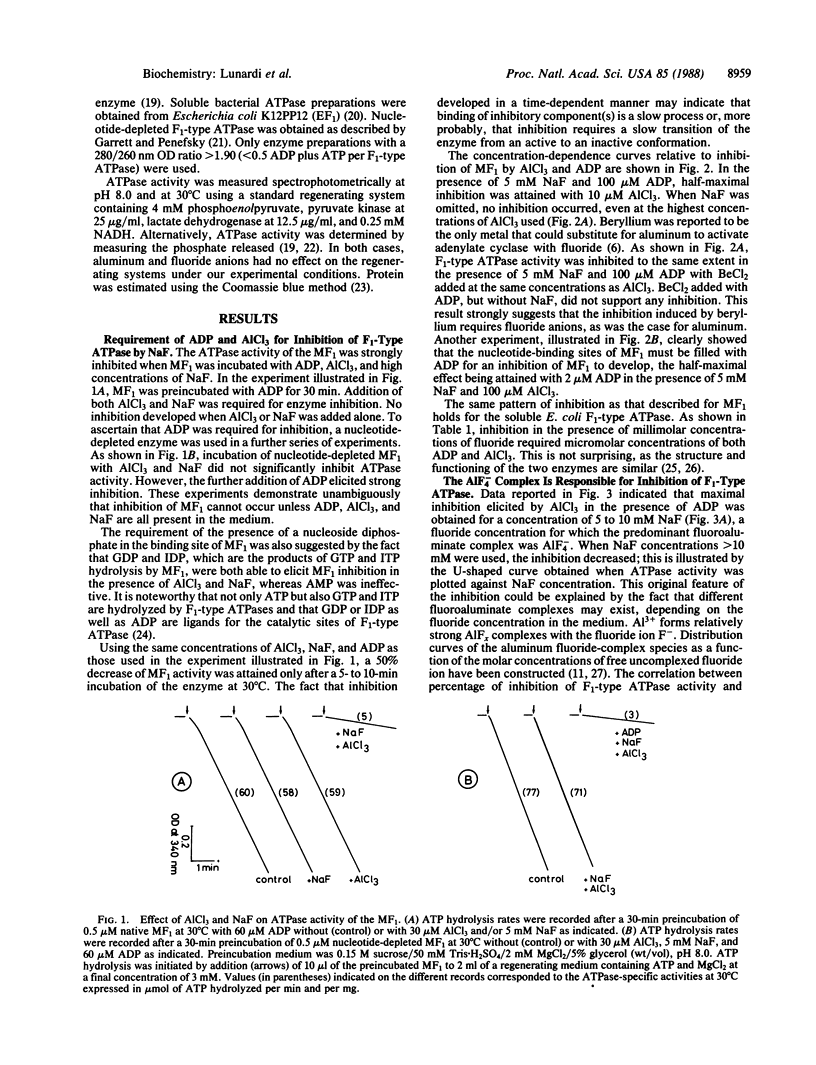
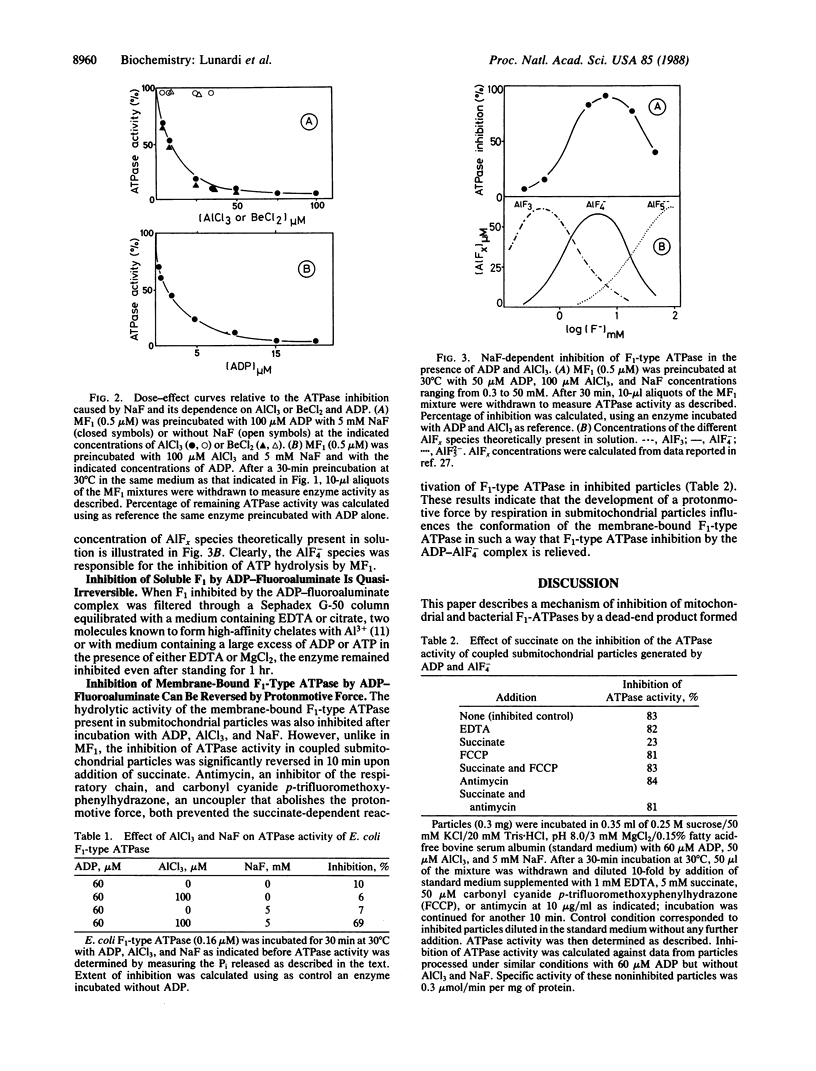
Inhibition of the mitochondrial and bacterial F1-type ATPases [of ATP phosphohydrolase (H+-transporting), EC 3.6.1.34] by fluoride was found to depend on the presence of aluminum and ADP at the catalytic site(s) of F1-type ATPase. AIF-4 was demonstrated to be the active fluoroaluminate species. The identical pattern of inhibition of F1-type ATPase activity obtained in the presence of ADP and NaF with beryllium, a metal that forms fluoride complexes strictly tetracoordinated, suggests that aluminum acts through a tetrahedral complex. Inhibition of isolated F1-type ATPase by AIF-4 in the presence of ADP cannot be reversed by ADP, ATP, or chelators of aluminum. However, the inhibition of the ATPase activity of the F1 sector in submitochondrial particles caused by AIF-4 and ADP was reversed upon addition of an oxidizable substrate. Uncouplers prevented the reversal of inhibition, suggesting that the protonmotive force generated by respiration was responsible for the relief of inhibition. Because of structural similarities between AIF4- and , AIF4- is postulated to mimic the phosphate group of ATP and form an abortive complex with ADP at the active site(s) of F1-type ATPase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bigay J., Deterre P., Pfister C., Chabre M. Fluoride complexes of aluminium or beryllium act on G-proteins as reversibly bound analogues of the gamma phosphate of GTP. EMBO J. 1987 Oct;6(10):2907–2913. doi: 10.1002/j.1460-2075.1987.tb02594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigay J., Deterre P., Pfister C., Chabre M. Fluoroaluminates activate transducin-GDP by mimicking the gamma-phosphate of GTP in its binding site. FEBS Lett. 1985 Oct 28;191(2):181–185. doi: 10.1016/0014-5793(85)80004-1. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carlier M. F., Didry D., Melki R., Chabre M., Pantaloni D. Stabilization of microtubules by inorganic phosphate and its structural analogues, the fluoride complexes of aluminum and beryllium. Biochemistry. 1988 May 17;27(10):3555–3559. doi: 10.1021/bi00410a005. [DOI] [PubMed] [Google Scholar]
- Chernyak B. C., Kozlov I. A. Adenylylimidodiphosphate release from the active site of submitochondrial particles ATPase. FEBS Lett. 1979 Aug 15;104(2):215–219. doi: 10.1016/0014-5793(79)80817-0. [DOI] [PubMed] [Google Scholar]
- Garrett N. E., Penefsky H. S. Interaction of adenine nucleotides with multiple binding sites on beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1975 Sep 10;250(17):6640–6647. [PubMed] [Google Scholar]
- Harris D. A. The interactions of coupling ATPases with nucleotides. Biochim Biophys Acta. 1978 Mar 10;463(3-4):245–273. doi: 10.1016/0304-4173(78)90002-2. [DOI] [PubMed] [Google Scholar]
- Harris D. A., von Tscharner V., Radda G. K. The ATPase inhibitor protein in oxidative phosphorylation. The rate-limiting factor to phosphorylation in submitochondrial particles. Biochim Biophys Acta. 1979 Oct 10;548(1):72–84. doi: 10.1016/0005-2728(79)90188-9. [DOI] [PubMed] [Google Scholar]
- Howlett A. C., Sternweis P. C., Macik B. A., Van Arsdale P. M., Gilman A. G. Reconstitution of catecholamine-sensitive adenylate cyclase. Association of a regulatory component of the enzyme with membranes containing the catalytic protein and beta-adrenergic receptors. J Biol Chem. 1979 Apr 10;254(7):2287–2295. [PubMed] [Google Scholar]
- Issartel J. P., Favre-Bulle O., Lunardi J., Vignais P. V. Is pyrophosphate an analog of adenosine diphosphate for beef heart mitochondrial F1-ATPase. J Biol Chem. 1987 Oct 5;262(28):13538–13544. [PubMed] [Google Scholar]
- Issartel J. P., Lunardi J., Vignais P. V. Characterization of exchangeable and nonexchangeable bound adenine nucleotides in F1-ATPase from Escherichia coli. J Biol Chem. 1986 Jan 15;261(2):895–901. [PubMed] [Google Scholar]
- Knowles A. F., Penefsky H. S. The subunit structure of beef heart mitochondrial adenosine triphosphatase. Physical and chemical properties of isolated subunits. J Biol Chem. 1972 Oct 25;247(20):6624–6630. [PubMed] [Google Scholar]
- Lange A. J., Arion W. J., Burchell A., Burchell B. Aluminum ions are required for stabilization and inhibition of hepatic microsomal glucose-6-phosphatase by sodium fluoride. J Biol Chem. 1986 Jan 5;261(1):101–107. [PubMed] [Google Scholar]
- Macdonald T. L., Humphreys W. G., Martin R. B. Promotion of tubulin assembly by aluminum ion in vitro. Science. 1987 Apr 10;236(4798):183–186. doi: 10.1126/science.3105058. [DOI] [PubMed] [Google Scholar]
- Martin R. B. The chemistry of aluminum as related to biology and medicine. Clin Chem. 1986 Oct;32(10):1797–1806. [PubMed] [Google Scholar]
- Penefsky H. S. Differential effects of adenylyl imidodiphosphate on adenosine triphosphate synthesis and the partial reactions of oxidative phosphorylation. J Biol Chem. 1974 Jun 10;249(11):3579–3585. [PubMed] [Google Scholar]
- Penefsky H. S. Reversible binding of Pi by beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1977 May 10;252(9):2891–2899. [PubMed] [Google Scholar]
- Robinson J. D., Davis R. L., Steinberg M. Fluoride and beryllium interact with the (Na + K)-dependent ATPase as analogs of phosphate. J Bioenerg Biomembr. 1986 Dec;18(6):521–531. doi: 10.1007/BF00743148. [DOI] [PubMed] [Google Scholar]
- Roux B., Fellous G., Godinot C. Circular dichroism and nucleotide and phosphate-induced conformational changes of mitochondrial adenosinetriphosphatase. Biochemistry. 1984 Jan 31;23(3):534–537. doi: 10.1021/bi00298a021. [DOI] [PubMed] [Google Scholar]
- Schuster S. M., Ebel R. E., Lardy H. A. Kinetic studies on rat liver and beef heart mitochondrial ATPase. Evidence for nucleotide binding at separate regulatory and catalytic sites. J Biol Chem. 1975 Oct 10;250(19):7848–7853. [PubMed] [Google Scholar]
- Senior A. E. ATP synthesis by oxidative phosphorylation. Physiol Rev. 1988 Jan;68(1):177–231. doi: 10.1152/physrev.1988.68.1.177. [DOI] [PubMed] [Google Scholar]
- Sitaramayya A., Virmaux N., Mandel P. On the mechanism of light activation of retinal rod outer segments cyclic GMP phosphodiesterase (light activation-influence of bleached rhodopsin and KF-deinhibition). Exp Eye Res. 1977 Aug;25(2):163–169. doi: 10.1016/0014-4835(77)90128-2. [DOI] [PubMed] [Google Scholar]
- Solheim L. P., Fromm H. J. pH kinetic studies of bovine brain hexokinase. Biochemistry. 1980 Dec 23;19(26):6074–6080. doi: 10.1021/bi00567a020. [DOI] [PubMed] [Google Scholar]
- Sternweis P. C., Gilman A. G. Aluminum: a requirement for activation of the regulatory component of adenylate cyclase by fluoride. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4888–4891. doi: 10.1073/pnas.79.16.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton S. V., Bender G. R., Marquis R. E. Fluoride inhibition of proton-translocating ATPases of oral bacteria. Infect Immun. 1987 Nov;55(11):2597–2603. doi: 10.1128/iai.55.11.2597-2603.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignais P. V., Satre M. Recent developments on structural and functional aspects of the F1 sector of H+-linked ATPases. Mol Cell Biochem. 1984;60(1):33–71. doi: 10.1007/BF00226299. [DOI] [PubMed] [Google Scholar]
- Womack F. C., Colowick S. P. Proton-dependent inhibition of yeast and brain hexokinases by aluminum in ATP preparations. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5080–5084. doi: 10.1073/pnas.76.10.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Stadt R. J., de Boer B. L., van Dam K. The interaction between the mitochondrial ATPase (F 1 ) and the ATPase inhibitor. Biochim Biophys Acta. 1973 Feb 22;292(2):338–349. doi: 10.1016/0005-2728(73)90040-6. [DOI] [PubMed] [Google Scholar]


