Abstract
Alpha A-crystallin is a molecular chaperone; it prevents aggregation of denaturing proteins. We have previously demonstrated that upon modification by a metabolic α-dicarbonyl compound, methylglyoxal (MGO), αA-crystallin becomes a better chaperone. Alpha A-crystallin also assists in refolding of denatured proteins. Here, we have investigated the effect of mild modification of αA-crystallin by MGO (with 20-500 μM) on the chaperone function and its ability to refold denatured proteins. Under the conditions used, mildly modified protein contained mostly hydroimidazolone modifications. The modified protein exhibited an increase in chaperone function against thermal aggregation of βL- and γ-crystallins, citrate synthase (CS), malate dehydrogenase (MDH) and lactate dehydrogenase (LDH) and chemical aggregation of insulin. The ability of the protein to assist in refolding of chemically denatured βL- and γ-crystallins, MDH and LDH, and to prevent thermal inactivation of CS were unchanged after mild modification by MGO. Prior binding of catalytically inactive, thermally denatured MDH or the hydrophobic probe, 2-p-toluidonaphthalene-6-sulfonate (TNS) abolished the ability of αA-crystallin to assist in the refolding of denatured MDH. However, MGO-modification of chaperone-null TNS-bound αA-crystallin resulted in partial regain of the chaperone function. Taken together, these results demonstrate that: 1) hydroimidazolone modifications are sufficient to enhance the chaperone function of αA-crystallin but such modifications do not change its ability to assist in refolding of denatured proteins, 2) the sites on the αA-crystallin responsible for the chaperone function and refolding are the same in the native αA-crystallin and 3) additional hydrophobic sites exposed upon MGO modification, which are responsible for the enhanced chaperone function, do not enhance αA-crystallin's ability to refold denatured proteins.
Keywords: AlphaA-crystallin, Chaperone, Protein modification, Methylglyoxal, Hydroimidazolone, Protein refolding
1. Introduction
The mammalian eye lens is a vital organ responsible for the transmission of light for proper vision. Crystallins are the dominant structural proteins within the lens, comprising up to 90 % of total proteins. Crystallins are of three types: α-, β- and γ-crystallin, which interact amongst themselves to maintain transparency [1]. Alpha-crystallin is a major protein in the lens, constituting 40 % of total proteins. It consists of two subunits, αA- and αB-crystallin, which exist in a molar ratio of 3:1 and form polydisperse oligomers of ∼ 40 subunits with an average molecular mass of ∼800 kDa [2]. Alpha-crystallin belongs to the sHsp (small heat shock protein) family, whose structural and functional characteristics are conserved from bacteria to humans [3, 4]. Unlike the classical chaperones, sHsp prevent the aggregation and precipitation of a variety of unrelated proteins [5].
Like other members of the sHsp family, α-crystallin functions as a molecular chaperone. It prevents aggregation of partially denatured proteins [6]. The oligomeric state of the protein appears to be necessary for this function, but this hypothesis is controversial [7, 8]. Support for this role has come from many studies in which client proteins are subjected to denaturing by high temperature or chemicals, in the presence or absence of α-crystallin. It is now apparent that all three domains of α-crystallin, the N-terminus, the crystallin domain and the C-terminus, are important for the chaperone function [5]. Hydrophobic patches within these domains appear to bind to client proteins during the chaperone function. However, short peptides of 10-20 amino acids derived from the native protein could also function as chaperones [9], implying that the whole protein may not be necessary for the chaperone function.
Much support for a role for α-crystallin's chaperone function in the lens has come from genetically modified animals and from studies on human hereditary cataracts. Targeted disruption of αA- or αB-crystallin, or both together, resulted in serious developmental defects or cataract formation in mice [10, 11]. Several point mutations in α-crystallin that have been identified as responsible for human cataracts cause either total or partial loss of chaperone function [12-14]. Furthermore, overexpression of mutant forms of human αA-crystallin that have diminished chaperone function leads to cataract formation in mice [14, 15]. These observations strongly suggest that the chaperone function of α-crystallin is necessary for the lens to remain transparent throughout life.
However, like other proteins, α-crystallin also undergoes post-translational modification in the lens, such as, oxidation, truncation and glycation [16, 17]. Such modifications tend to accumulate in the lens because of its negligible protein turnover. These modifications, in general, have negative consequences on chaperone function. However, one type of modification appears to have a beneficial effect on chaperone function and is by the reaction of a metabolic dicarbonyl compound, methylglyoxal (MGO).
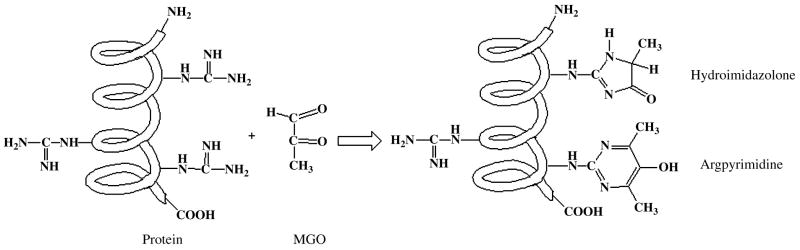
MGO is produced non-enzymatically from sugar fragmentation reactions and decomposition of triose phosphate intermediates during glycolysis, and also during catabolism of threonine and acetone [18]. MGO reacts with arginine and lysine residues on crystallins to produce stable adducts on them. Several such adducts are present in the human lens. They are carboxyethyllysine (CEL) [19], methylglyoxal lysine dimer (MOLD)[20], methylglyoxal-derived imidazoline crosslink (MODIC) [21], hydroimidazolones [22] and argpyrimidine [23]. While MOLD is a lysine-lysine cross linking structure, MODIC is a lysine-arginine crosslinking structure. CEL is a lysine modification and hydroimidazolones and argpyrimidine are arginine modifications (Fig.1). Studies have shown that several of these products accumulate in aging lens proteins [19-22].
Figure 1. MGO-modifications in proteins.
MGO reacts with arginine residues in proteins to form hydroimidazolone and argpyrimidine adducts. Formation of these adducts improves the chaperone function of human αA-crystallin.
We and others have previously shown that MGO-modification of αA-crystallin enhances its chaperone function [24, 25]. We then showed that it was the modification of specific arginine residues to argpyrimidine that led to enhanced chaperone function [25]. Replacement of two of such arginine residues with alanine by site-directed mutagenesis also led to enhanced chaperone function [26]. In addition, conversion of lysine to arginine-like structures followed by MGO modification led to enhanced chaperone function as well [27]. Previous reports showed that mild modification with MGO (500 μM) leads to mostly hydroimidazolone modifications in proteins [28, 29]. Thus, it was possible that the enhancement of chaperone function in MGO-modified αA-crystallin occurred from hydroimidazolne modifications, in addition to argpyrimidine.
Alpha-crystallin performs another potentially significant function: it assists in refolding denatured proteins [30, 31]. In this study we have investigated the relationship between hydroimidazolone modifications and the chaperone function, and assessed the impact of such modifications on the protein refolding ability of MGO-modified αA-crystallin.
2. Materials and Methods
2.1.Chemicals
Dithiothreitol (DTT), guanidine hydrochloride(GdHCl), bovine serum albumin, bovine insulin, malate dehydrogenase (MDH), lactate dehydrogenase (LDH), citrate synthase (CS), oxaloacetic acid, acetyl CoA, ethylene diamine tetraacetic acid disodium salt dihydrate (EDTA), 5,5′-Dithiobis 2-nitro-benzoic acid (DTNB), methylglyoxal (MGO), iodoacetamide (IAA), and chymotrypsin were obtained from Sigma Chemical Co., St. Louis, MO. 2-(p-toluidinyl)naphthalene-6-sulfonate (TNS) was obtained from Molecular Probes (Invitrogen, Carlsbad, CA). Centrifugal filters (100 kDa cutoff) were obtained from Millipore (Bedford, MA). All other chemicals were analytical grade. MGO was purified by low pressure distillation [25].
2.2. Protein purification
Human recombinant αA-crystallin was purified as reported in our previous paper [26]. Human lenses (from a 19 year donor) were homogenized in 2.0 ml of 50 mM sodium phosphate buffer, pH 7.4 containing 100 mM NaCl, 1 mM EDTA and 100 μM PMSF and centrifuged at 10,000 g at 4° C for 30 min. The soluble proteins in the supernatant were fractionated on a Sephacryl S-300 gel filtration column (1.5 × 100 cm) at 4° C. The fractions corresponding to βL- and γ-crystallins were pooled and dialyzed against water and lyophilized.
2.3. Modification of αA-crystallin by MGO
AlphaA-crystallin (5 mg/ml) in 0.1 M phosphate buffer pH 7.4 was incubated with purified MGO (20, 50, 100 and 500 μM) at 37°C in the dark under sterile conditions for 2 days. After 2 days, unbound MGO was removed by dialysis against 50 mM phosphate buffer (pH 7.4) and protein concentration was determined by the Bradford assay (Bio-Rad, Hercules, CA) using BSA as the standard. Since these incubations used much lower concentrations of MGO and shorter incubation period than the previous studies [24, 25, 32], the modified proteins in the present study are henceforth referred to as ‘mildly modified αA-crystallin’. AlphaA-crystallin incubated in the absence of MGO served as the control.
2.4. HPLC Assay for argpyrimidine
Mildly modified αA-crystallin was mixed with 6 N HCl and incubated for 16 h at 110° C. The acid was evaporated in a Savant SpeedVac system and the residue was suspended in 250 μl of water filtered through a 0.45 μm centrifugal filter. Aliquots of all samples were separated by HPLC to measure argpyrimidine. The HPLC conditions were set up according to Wilker et al.[23].
2.5. Identification of hydroimidazolone in mildly modified α-crystallin
AlphaA-crystallin modified with 0, 20, 50, 100, or 500 μM MGO was subjected to SDS-PAGE under denaturing condition on a 12% gel. The Coomassie stained protein band corresponding to αA-crystallin was excised from the gel and digested by trypsin and subjected to mass spectrometry as described previously [27]. Briefly, protein bands are cut from the gel, minced and subjected to in-gel digestion with trypsin. Analysis of proteolytic peptide was performed using a LTQ OrbiTrap XL linear ion trap mass spectrometer (Thermo Fisher Scientific, Walthaw, MA) coupled with an Ultimate 3000 HPLC system (Dionex, Sunnyvale, CA). The spectra were acquired by data-dependent methods, consisting of a full scan and MS/MS on the five most abundant precursor ions at the collision energy of 30%. The obtained data were submitted for a database search using Mascot (Matrix Science, Boston, MA) by setting hydroimidazolone and argpyrimidine modifications on arginine.
2.6. Purification of Nδ-(5-Methyl-4-oxo-5-hydroimidazolone-2-yl)-l-ornithine
(MGH1): MGH1 was isolated from the incubation mixture of MGO (220 μL, 1.4 mmol) and L-arginine (213 mg, 1.2 mmol) in 10 mL phosphate buffer (0.2 M, pH 7.4). The reaction mixture was incubated for 4.5 hr at 40 °C. Subsequently, the pH was adjusted to 4.0 with 1 N HCl. A sample (8 mL) of the solution was adjusted to pH 1.0 with 1 N HCl and subjected to ion exchange chromatography on a Dowex® 50W×4 4-400 column (2.5 cm i.d. × 22 cm, H+-form). Before being conditioned with pyridinium formate (0.2 M, pH 5.0), the column was washed with 1 M NaOH, 1 M HCl, and water. Fractions were collected and analyzed by thin-layer chromatography (TLC). TLC was performed on silica gel 60 F254 plates (Merck) using n-butanol/H2O/HOAc/pyridine 4:2:3:3 as the mobile phase. Visualization of separated material was achieved with ninhydrin. Fractions with material having Rf = 0.48 were pooled and subjected to preparative HPLC as follows.
A Besta HD 2-200 pump (Wilhelmsfeld, Germany) with a Gynkotek fluorescence detector RF-530 and a SERVOGOR 220 pen recorder was used. Chromatographic separations were performed on a stainless steel column (VYDAC 218TP1022, 250 × 25 mm, RP18, 10 μm) using a flow rate of 15 mL/min. The mobile phase used was water (solvent A) and MeOH/water (7:3 v/v; solvent B). To both solvents A and B, 1.2 mL/L heptafluorobutyric acid (HFBA) was added. Samples were injected at 5% B and run isocratically. Fractions with material eluting at tR=62 min having a Rf= 0.48 on TLC were collected, combined, and freeze-dried to yield a colorless amorphic residue (49.22 mg, 11.6 %, MGH1 × 2 HFBA). The chemical structure of the product was confirmed as MGH1 by 1H-, 13C-NMR and high resolution mass spectrometry, which was fully compatible with previously published data [33]. Accurate mass (mean of three measurements ± standard deviation) was determined: m/z 229.1314 ± 0.0007 [M + H]+ (229.1312, calcd for C9H17N4O3).
2.7. Quantification of hydroimidazolone in mildly modified α-crystallin by liquid chromatography and mass spectrometry
MGO-modified proteins (1.2 mg in each case) were enzymatically hydrolyzed as previously described [34] and analyzed by LC/MS. The HPLC apparatus (Jasco, Groβ-Umstadt, Germany) consisted of a pump (PU-2080 Plus) with degasser (LG-2080-02) and quaternary gradient mixer (LG-2080-04), a column oven (Jasco Jetstream II) and an Autosampler (AS-2057 Plus). Chromatographic separations were performed on a stainless steel column packed with RP-18 material (VYDAC CRT, no. 218TP54, 250 × 4.0 mm, RP 18, 5 μm, Hesperia, CA) using a flow rate of 1.0 mL/min. The mobile phase used was water (solvent A) and MeOH/water (7:3 (v/v), solvent B). To both solvents (A and B), 1.2 mL/L heptafluorobutyric acid (HFBA) was added. Analysis was performed at 25 °C column temperature using isocratic elution at 95% A/ 5% B. Mass spectrometric detection was conducted on a API 4000 Q Trap LC/MS/MS system (Applied Biosystems/ MDS Sciex, Concord, ON, Canada) equipped with a turbo ionspray source using electrospray ionisation in positive mode: sprayer capillary voltage 4.0 kV, nebulizing gas flow 50 ml/min, heating gas 60 ml/min at 550°C and curtain gas 40 ml/min. The multiple reaction monitoring (MRM) mode was used, utilizing collision-induced dissociation (CID) of the protonated molecules with compound specific orifice potentials and fragment specific collision energies. MGH1 (m/z 229.2 → 70.1, 114.1, 116.1) was detected at RT=36.5 min. Quantification was performed using the standard addition method using the authentic reference compound of MGH1, which was synthesized as above.
2.8. UV-circular dichroism spectrometry
The conformational changes of αA-crystallin (unmodified and mildly modified) were observed by recording far-UV and near-UV CD spectra at 25°C in a Jasco 810 spectropolarimeter (Jasco, Inc., Japan). The far-UV spectra were recorded between 195-250 nm in a 1 mm path length CD quartz cell, at a scan speed of 20 nm/min. The concentration of protein was 0.2 mg/ml in 10 mM sodium phosphate buffer pH 7.4. The near-UV CD was recorded between 250-350 nm in a 10 mm path length CD quartz cell, at a scan speed of 20 nm/min. The protein concentration was 1 mg/ml in the same phosphate buffer.
2.9. TNS-fluorescence
The surface hydrophobicity of native and MGO-modified αA-crystallin was studied with TNS, a hydrophobic probe. αA-Crystallin (0.1 mg/ml) in 50 mM phosphate buffer (pH 7.4) was incubated with 100 μM TNS for 2 h at 25°C. Fluorescence emission spectra were recorded between 350 and 520 nm using an excitation wavelength of 320 nm. The excitation and emission slit widths were each 5 nm.
2.10. Analysis of chaperone function of mildly modified α-crystallin
The chaperone function of MGO-modified and unmodified αA-crystallin was evaluated using six client proteins: human βL-crystallin, human γ-crystallin, malate dehydrogenase (MDH) lactate dehydrogenase (LDH), CS and insulin. CS and insulin chaperone assays were performed in 96-microwell plates using a micro plate reader (SpectraMax, Model 190; Molecular Devices, Sunnyvale, CA). The total reaction volume was 250 μL. Human βL-crystallin, human γ-crystallin, MDH and LDH chaperone assays were performed in a DU 800 spectrophotometer, using quartz cuvettes with 1 cm pathlengths. The total reaction volume was 500 μl. The following assays were performed to evaluate chaperone function:
βL-crystallin aggregation assay. βL-Crystallin (0.25 mg/ml) was incubated with native or MGO-modified αA-crystallin (0.00625 mg/ml) at 60°C in 50 mM phosphate buffer pH 7.4 and light scattering was monitored at 360 nm in the kinetic mode. βL-Crystallin without αA-crystallin served as control.
γ-crystallin aggregation assay. γ-Crystallin (0.125 mg/ml) was incubated with native or MGO-modified αA-crystallin (0.00625 mg/ml) at 65°C in 50 mM phosphate buffer pH 7.4 and light scattering was monitored at 360 nm in the kinetic mode. γ-Crystallin without αA-crystallin served as control.
CS aggregation assay. Citrate synthase (CS) was dialyzed against 40 mM HEPES buffer, pH 7.4 for 24 hr. CS (0.06 mg/ml) in 40 mM HEPES buffer, pH 7.4) was incubated with native and MGO modified αA-crystallin (0.004 mg/ml) at 43°C and light scattering was monitored at 360 nm [35]. CS without αA-crystallin served as control.
MDH aggregation assay. MDH was dialyzed against 50mM phosphate buffer (pH 7.4) for 24 hr. MDH (0.25 mg/ml) and incubated with native and mildly modified αA-crystallin (0.25 mg/ml) in 50 mM phosphate buffer (pH 7.4) at 50°C and light scattering was monitored at 360 nm. [36] MDH incubated in the absence of αA-crystallin served as the control.
LDH aggregation assay. LDH was dialyzed against 50 mM phosphate buffer (pH 6.7) for 24 hr. LDH (0.25 mg/ml) was incubated with native and mildly modified αA-crystallin (0.0125 mg/ml) in 50 mM phosphate buffer (pH 6.7) at 60°C and light scattering was monitored at 360 nm in the kinetic mode. LDH incubated in the absence of αA-crystallin served as the control.
Insulin aggregation assay. Insulin (0.32 mg/ml) in 50 mM phosphate buffer (pH 7.2) was incubated at 25°C with native and MGO modified αA-crystallin (0.064 mg/ml). Insulin without αA-crystallin served as control. Insulin aggregation was initiated by adding freshly prepared DTT to a final concentration of 20 mM, and light scattering at 400 nm was monitored for 1 hr in the kinetic mode [37].
2.11. Preparation of αA-crystallin-MDH and αA-crystallin-TNS complexes
MDH (1 mg/ml) was incubated with 500 μM of iodoacetamide in 50 mM phosphate buffer (pH 7.4) for 1 hr, in the absence of light, at room temperature. Excess iodoacetamide was removed by dialysis for 24 hr at 4°C against 50 mM phosphate buffer (pH 7.4). MDH activity was measured as described previously [26], and was found to be completely abolished by iodoacetamide treatment. The catalytically inactive MDH (1 mg/ml) was incubated with αA-crystallin (2 mg/ml) at 50°C for 1 hr in 50 mM sodium phosphate buffer, pH 7.4. The complex was centrifuged at 10,000 g for 30 min at 4°C. The clear supernatant was loaded on to Sephacryl S-300 gel filtration column and eluted with 50 mM phosphate buffer (pH 7.4) containing 100 mM NaCl. The αA-crystallin-MDH complex was collected, pooled and concentrated using a 10K Centricon filter (Millipore, Billerica, MA). The concentration of αA-crystallin in the complex was determined by an ELISA using an anti-αA-crystallin monoclonal antibody (see below).
AlphaA-crystallin (5 mg/ml) was incubated with 2.5 mM TNS in 50 mM phosphate buffer (pH 7.4) for 2 hr at room temperature. The complex was extensively dialyzed (48 hrs) against 0.1 M phosphate buffer (pH 7.4) to remove excess TNS. The αA-crystallin-TNS complex (5 mg/ml) was incubated with or without 500 μM MGO for 2 days in 0.1 M phosphate buffer (pH 7.4) under sterile conditions, in the dark. Excess MGO was removed by dialysis against 50 mM phosphate buffer (pH 7.4). The concentration of αA-crystallin in the αA-crystallin-TNS and MGO-modified αA-crystallin-TNS complexes was determined by the Bradford assay, using BSA as the standard.
2.12. ELISA for αA-crystallin
Microplate wells were coated overnight with 0.125, 0.25, 0.5, 1 or 2 μg of human recombinant αA-crystallin per well in 50 mM carbonate buffer (pH 9.6), in triplicate. The wells were then washed three times with PBS-T and incubated with 50 μl of rabbit anti-αA/αB-crystallin polyclonal antibody (1:500) (Assay Designs, Ann Arbor, MI) for 1 hr at 37 °C. Following this step, the wells were washed three times with PBS-T, and incubated for 1 hr at 37 °C with 50 μL of goat anti-rabbit antibody (Sigma Aldrich, St. Louis, MO) diluted in PBS-T (1:5000). After the wells were washed with PBS-T, they were incubated with 100 μl of 3, 3′, 5, 5′-tetramethylbenzidine substrate (Sigma). The enzyme reaction was stopped by the addition of 50 μL of 2N H2SO4, and the absorbance was measured at 450 nm in a Dynex MRX 5000 Microplate Reader. A standard curve for αA-crystallin was generated from the values obtained from recombinant αA-crystallin and from this the concentration of αA-crystallin in the αA-crystallin-MDH complex was determined.
2.13. Thermal inactivation of citrate synthase in the presence of αA-crystallin
Citrate synthase was dialyzed overnight against 40 mM HEPES buffer (pH 7.4). CS (15 μM) was diluted 100-fold in 40 mM HEPES buffer pH 7.4 (final volume 300 μl), in the presence or absence of 30 μM αA-crystallin. The inactivation reaction was started by placing the mixture in a water bath set at 43°C. At regular time intervals, aliquots were withdrawn and stored on ice. The residual CS activity was measured according to the procedure of Zhi et al [38]. Briefly, the reaction mixture consisted of 50 mM Tris-HCl buffer pH 8.0 containing 2 mM EDTA, 100 μM oxaloacetic acid, 100 μM DTNB and 150 μM of acetyl CoA. The activity assay was performed at 25°C and the increase in absorbance at 412 nm was recorded. CS (15 μM) diluted 100-fold into 40 mM HEPES buffer (pH 7.4) and kept on ice served as the control.
2.14. Protein refolding assay
The ability of αA-crystallin to assist in the refolding of denatured proteins was measured, using human βL-crystallin, human γ-crystallin, MDH and LDH as client proteins.
The unfolding and rapid refolding of crystallins was done according to previously published procedure [39]. βL- and γ-crystallins (300 μg each) were denatured in 20mM sodium phosphate buffer (pH 7.4) containing 8M urea in the presence of 60 μg of native or MGO-modified αA-crystallin for 5 hr at room temperature. Rapid refolding was done by diluting 50 μl of incubated samples with 950 μl of 10 mM phosphate buffer (pH 7.4) containing 100 mM NaCl. The mixture was incubated for 1 hr at room temperature and turbidity was measured at 360 nm in a Beckman DU-600 spectrophotometer. The percentage recovery of βL and γ-crystallins was calculated according to previously published procedure [39].
MDH (1 μM) was denatured in 6M GdHCl for 8 hr at 25°C. Refolding was initiated by diluting the denatured MDH 100-fold in a refolding buffer (pH 7.5) containing 50 mM phosphate, 10 mM magnesium acetate, and 5 mM DTT, with or without 30 μM αA-crystallin (native and mildly modified), αA-crystallin-MDH complex, αA-crystallin-TNS complex and MGO-modified αA-crystallin-TNS complex. The enzyme concentration was 10 nM during refolding. The activity of refolded enzyme was assayed by adding 20 μl of refolding mixture at various time intervals to 480 μl of a refolding buffer that contained 0.1 mM NADH and 0.4 mM oxaloacetate. The decrease in absorbance at 340 nm with time was recorded. MDH (1 μM) kept on ice served as the control.
LDH (1 μM) was denatured in 6M GdHCl for 8 hr at 25°C. Refolding was performed as described for the MDH assay, with the exception that 0.4 mM of sodium pyruvate was used as the substrate and the assay was performed at 37°C. The concentration of αA-crystallin (native and MGO-modified) was 30 μM. LDH (1 μM) kept on ice served as control. Similar refolding assays were performed with BSA (30 μM) in the place of αA-crystallin to rule out non-specific protein effects.
3. Results and Discussion
In our previous study, we showed that MGO modification of αA-crystallin enhances its chaperone function [25]. In that study we used high concentrations of MGO (up to 5 mM). Another study that reported improvement in the chaperone function also used similar high concentrations of MGO [24]. The aim of this study was to determine whether the improvement in chaperone occurred at low concentrations of MGO and to identify MGO-modifications responsible for such a change. Another aim was to determine if modification by low concentrations of MGO would adversely affect αA-crystallin's ability to refold denatured proteins, as previously reported with high concentrations of MGO [24].
3.1. Hydroimidazolones are the major modifications of MGO in αA-crystallin
LC/MS-MS spectrometry was used to detect and identify sites where MGO had modified αA-crystallin. As shown in Table 1, at 20 μM MGO out of the 13 arginine residues in αA-crystallin, 4 were already modified to hydroimidazolone (R12, R65, R157 and R163). Hydroimidazolone modification occurred on other arginine residues as the concentration of MGO increased. With 500 μM MGO, we observed hydroimidazolone modification on 11 arginine residues. Argpyrimidine was not detected (by HPLC) in any of the samples (data not shown). The formation of hydroimidazolone at low concentrations of MGO has been observed as the predominant modification in other proteins as well [29, 40]. We previously reported that modification of R21 and R103 to argpyrimidine in αA-crystallin made it a better chaperone [25]. In that study, we used high concentrations of MGO to modify αA-crystallin that resulted in argpyrimidine formation on selected arginine residues. Moreover, in that study we did not investigate hydroimidazolone modifications. In the present study, although R103 was modified to hydroimidazolone, R21 was not. These observations suggest that the chemical nature of MGO modification and the sites of arginine modification depend on MGO concentration; at low concentrations hydroimidazolone is the major modification and at high concentrations it is both hydroimidazolone and argpyrimidine.
TABLE 1. Identification of hydroimidazolone modified sites in MGO-modified αA-crystallin.
| Sequence | Mass (obs.) | Mass (cal.) | Arginine residue modified to hydroimidazolone | Modified sites observed at MGO concentration (μM) | |||
|---|---|---|---|---|---|---|---|
| 20 | 50 | 100 | 500 | ||||
| 12RTLGPFYPSR21 | 1246.6428 | 1246.6458 | R12 | X | X | X | X |
| 50QSLFRTVLDSGISEVR65 | 1859.9748 | 1859.9741 | R54 | X | X | X | |
| 55TVLDSGISEVRSDRDK70 | 1829.9102 | 1829.9119 | R65 | X | X | X | X |
| 66SDRDKFVIFLDVK78 | 1634.8682 | 1634.8668 | R68 | X | X | X | |
| 100HNERQDDHGYISR112 | 1679.7358 | 1679.7400 | R103 | X | X | X | |
| 104QDDHGYISREFHR116 | 1712.7616 | 1712.7655 | R112 | X | X | ||
| 113EFHRR117 | 797.3950 | 797.3932 | R116 | X | |||
| 117RYRLPSNVDQSALSCSLSADGMLTFCGPK145 | 3299.5462 | 3299.5373 | R117 | X | X | ||
| 118YRLPSNVDQSALSCSLSADGMLTFCGPK145 | 3143.4322 | 3143.4362 | R119 | X | |||
| 146IQTGLDATHAERAIPVSR163 | 1988.0402 | 1988.0439 | R157 | X | X | X | X |
| 158AIPVSREEKPTSAPSS173 | 1708.8600 | 1708.8631 | R163 | X | X | X | X |
The MGO concentration in the human lens has been reported to be 1-2 μM [18, 41]. In lens proteins hydroimidazolone is one of the major chemical modifications, concentrations as high as 22 nmoles/mg have been reported [22]. Since αA-crystallin is one of the major proteins of the lens, it is likely that hydroimidazolone formation occurs in this protein and enhances its chaperone function. MGH1 is the major isomer among the three isomers of hydroimidazolone. In the human lens, the reported MGH1 concentrations are 2-14 nmoles/mg protein [22]. We quantified this product in MGO-modified αA-crystallin and found that MGH1 concentration increased with increasing concentrations of MGO (Table. 2). The concentrations with 20, 50 and 100 μM MGO were 1-8 nmoles/mg protein, and were within reported values in the human lens [22]. Thus, we believe that the enhancement in the chaperone function of αA-crystallin we have observed in this study with MGO concentrations up to 100 μM could occur in vivo.
TABLE 2. Quantification of hydroimidazolone (MGH1) in MGO-modified αA-crystallin.
| MGO added (μM) | MGH1 (nmoles)/mg protein |
|---|---|
| 20 | 1.37 |
| 50 | 3.04 |
| 100 | 7.59 |
| 500 | 27.80 |
3.2. Structural changes in mildly modified α-crystallin
Far UV-CD spectra showed no changes in the secondary structure of αA-crystallin after MGO modification (supplemental Fig.S1); the unmodified and MGO-modified proteins showed similar amounts of α-helix, β-sheet and random coil (∼6%, ∼39% and ∼54%, respectively). The near-UV CD spectrum showed minor alterations in the tertiary structure in MGO-modified proteins. The signal intensity for Phe in the 250-270 nm region was unaffected at up to a 50 μM concentration of MGO. However, the intensity was slightly decreased in protein modified with 100 μM or 500 μM MGO relative to unmodified αA-crystallin. There were also minor differences in the intensity and position of peaks beyond 270 nm (Tyr and Trp) for the mildly modified α-crystallin relative to the unmodified protein. These data suggest that while the secondary structure is unaffected, the tertiary structure is slightly altered by mild modification with MGO.
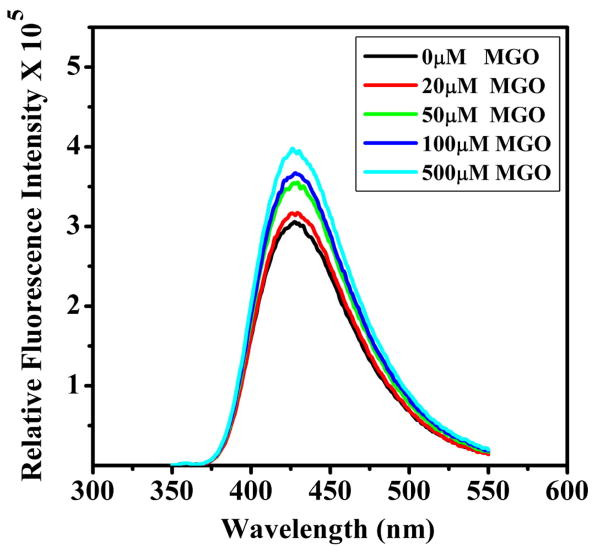
Changes in tertiary structure could potentially expose additional hydrophobic sites on the protein. TNS is a hydrophobic ligand (with a low fluorescence quantum yield in water) which binds to hydrophobic sites on protein molecules. Binding of TNS to hydrophobic sites dramatically increases its quantum yield [42]. This reagent has been widely used for probing the surface hydrophobicity of proteins, including α-crystallin [43, 44]. It is evident from the results in Fig. 2 that mild modification by MGO causes an increase in the surface hydrophobicity of αA-crystallin. This increase is dependent on MGO concentration. The surface hydrophobicity increased by 4% and 30% with 20 and 500 μM MGO, respectively.
Figure 2. Surface hydrophobicity increases in mildly modified αA-crystallin.
Protein concentration was 0.1 mg/ml and TNS concentration was 100 μM, in 50 mM phosphate buffer (pH 7.4). The fluorescence spectrum of the samples at 25°C was recorded between 350-520 nm. The excitation wavelength was 320 nm.
3.3. Chaperone function of mildly modified α-crystallin
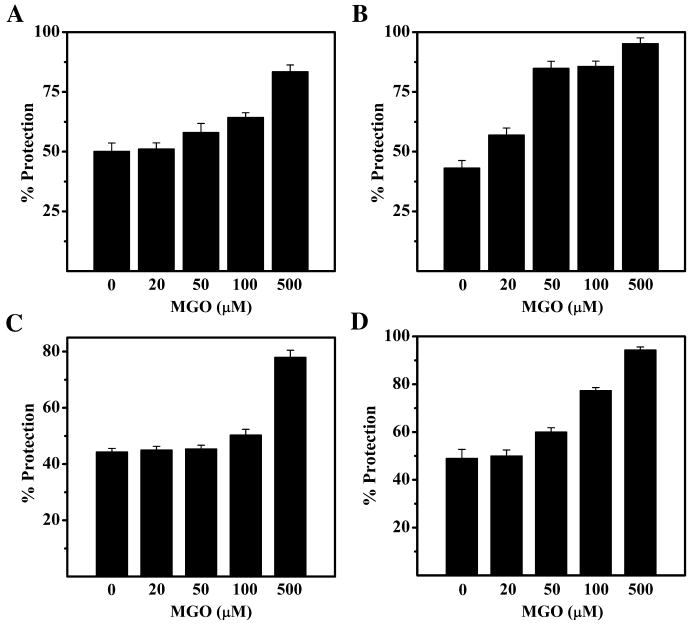
To determine if the increase in surface hydrophobicity resulted in improvement in the chaperone function of αA-crystallin, we assessed the chaperone function using six client proteins βL-crystallin, γ-crystallin, MDH, LDH, CS and insulin. Figure 3A shows the profile of aggregation of βL-crystallin in the presence of αA-crystallin. At a 1:40 (w/w) ratio of αA-crystallin to βL-crystallin, we observed nearly 50% protection with unmodified αA-crystallin. However, this protection increased to ∼64 and ∼83% with 100 and 500 μM MGO-modified αA-crystallin. At a 1:20 ratio of αA-crystallin to γ-crystallin, we observed nearly 43% protection against thermal aggregation of γ-crystallin (Fig. 3B) and it improved to 57%, 84%, 86 and 95% with 20, 50, 100 and 500 μM MGO-modified αA-crystallin, respectively.
Figure 3. Chaperone function is increased in mildly modified human αA-crystallin.
A. Thermal aggregation of 0.25 mg/ml of βL-crystallin at 60 °C. B. Thermal aggregation of 0.125 mg/ml of γ-crystallin at 65°C. C. Thermal aggregation of 0.25 mg/ml of MDH at 50°C. D. Thermal aggregation of 0.25 mg/ml of LDH at 60°C. Each data point is the average of three independent measurements.
Using MDH as the client protein, at a ratio of nearly 1:1 (w/w) we observed an enhancement in chaperone function at MGO concentrations >100 μM (Fig. 3C). About a 6% improvement in chaperone function was seen with αA-crystallin modified with 100 μM MGO and this increased to about 34% with αA-crystallin modified with 500 μM MGO, in comparison to unmodified αA-crystallin. With LDH as the client protein, at a αA-crystallin:LDH ratio of 1:20, a ∼30% enhancement of chaperone function was apparent at a MGO concentration of 100 μM (Fig. 3D). This increased to nearly 45% with 500 μM MGO modified protein in comparison to unmodified αA-crystallin. Such improvement in the chaperone function with mildly modified αA-crystallin was also seen when insulin and citrate synthase were used as client proteins (Supplemental Fig. S2).
Together, these data suggest that mild modification by MGO is sufficient to increase the chaperone function, although the increase in the chaperone function is not uniform across client proteins. There was no linear relationship between the extent of hydroimidazolone modification and the improvement in the chaperone function. One explanation could be that since alphaA-crystallin exists as a poydisperse oligomer with 4-30 subunits that can exchange subunits between oligomers, it could contribute to the lack of a liner relationship. Moreover, the lack of a crystal structure for αA-crystallin makes it difficult to predict how hydroimidazolone modification brings about changes in the chaperone function. Further work with crystallizable sHsps (such as, Hsp16.9) might shed light on this.
The data also suggest that mild modification by MGO increases the surface hydrophobicity of αA-crystallin, and that may have resulted in an enhancement of chaperone function. However, it should be pointed out that change in surface hydrophobicity does not always correlate with change in chaperone function. Studies have shown that there is no strict correlation between surface hydrophobicity and chaperone function in α-crystallin [45]. Furthermore, modification of α-crystallin with high concentrations of MGO (1-10 mM) results in a dramatic decrease in surface hydrophobicity, while the chaperone function is still elevated compared to the unmodified protein [25].
3.4. Effect of mild modification by MGO on the protein refolding ability of αA-crystallin
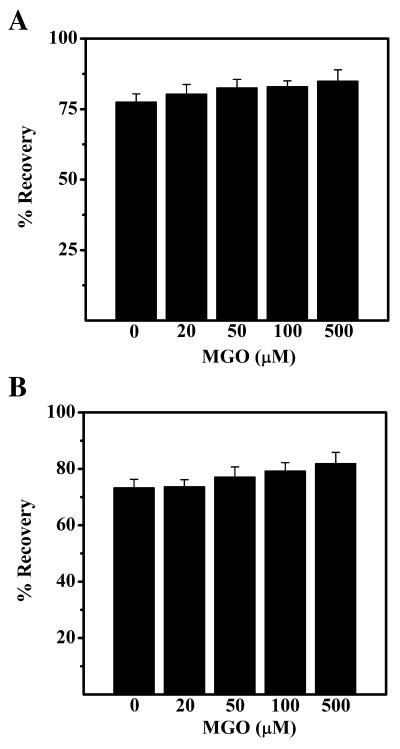
Although it is now well established that MGO modification enhances the chaperone function of αA-crystallin, it is not known whether it would result in the enhancement of its ability to refold denatured proteins. This was assessed using two human lens proteins. βL-Crystallin and γ-crystallin in the presence of unmodified or MGO-modified αA-crystallin were denatured by incubating in 8M urea. Rapid refolding was done by diluting the denatured proteins in the refolding buffer. It has been shown that refolding of βL-crystallin and γ-crystallin is very efficient when αA-crystallin is present during the denaturation [39]. αA-Crystallin was able to refold βL-crystallin, as 77% of βL-crystallin was recovered in the soluble form (Fig. 4A). MGO (20-100 μM) modification did not change this property, although at 500 μM MGO, there was a slight increase in the recovery (additional 8%). Similarly, αA-crystallin was also able to efficiently refold γ-crystallin with a recovery of 73% in the soluble form (Fig. 4B). The refolding ability of MGO-modified αA-crystallin was similar to unmodified protein, except in 500 μM MGO-modified protein, where a ∼7 % increase was observed.
Figure 4. The ability of αA-crystallin to refold denatured human lens proteins is unchanged by mild MGO modification.
βL-crystallin (A) and γ-crystallin (B) (300 μg protein each) were denatured in 20 mM sodium phosphate buffer containing 8M urea along with 60 μg of native and MGO-modified αA-crystallin for 5 hr at room temperature. Rapid refolding was done by diluting 50 μl of incubated crystallins with 950 μl of 10 mM phosphate buffer (pH 7.4) containing 100 mM NaCl. The mixture was incubated for 1 hr at room temperature.
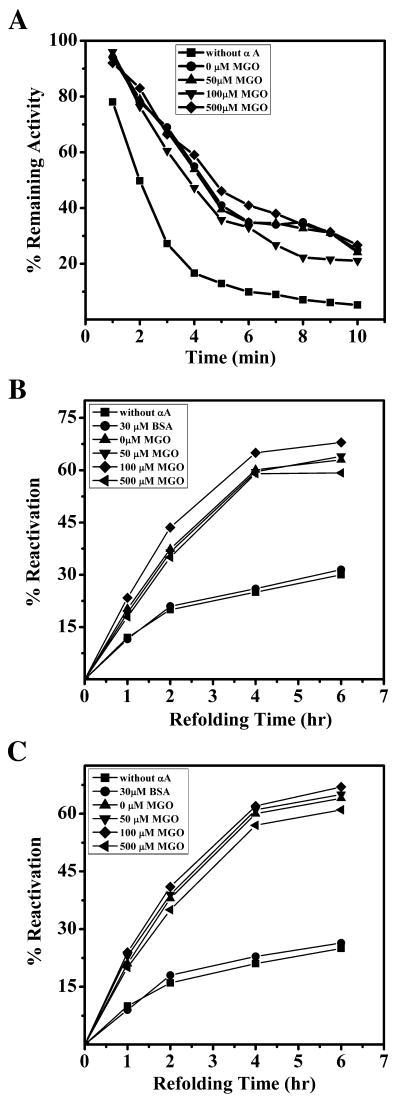
Alpha-crystallin has been shown to prevent thermal inactivation of several enzymes [46-48]. We tested if this property is affected by MGO modification using CS as the client protein. CS loses its activity when incubated at 43°C. αA-Crystallin has the ability to protect CS from thermal inactivation [49]. MGO modified αA-crystallin, which has the ability to increase protection of CS during thermal aggregation (Fig. 2S) and higher surface hydrophobicity (Fig. 2), failed to enhance thermal inactivation of CS, and acted in a fashion similar to unmodified αA-crystallin, even when modified with 500 μM MGO (Fig. 5A). These data imply that hydrophobic sites that are exposed as a result of MGO-modification neither participate in nor cause a decrease in inhibition of αA-crystallin-mediated thermal inactivation of CS.
Figure 5. Inhibition of thermal inactivation of CS and assistance in refolding of chemically denatured enzymes by MGO-modified αA-crystallin are similar to unmodified αA-crystallin.
A. CS (15 μM) was diluted 100-fold in 40 mM HEPES buffer pH 7.4 (to a final volume of 300 μl) in the presence or absence of 30 μM αA-crystallin (native and MGO modified) and incubated at 43°C. Residual CS activity was measured as described in Methods. CS diluted as above and kept on ice served as the control. Each data point is the average of three independent measurements. B. MDH (1 μM) was denatured in 6M GdCl for 8 h at 25°C. Refolding was initiated by diluting the denatured MDH 100-fold in a refolding buffer with or without 30 μM αA-crystallin (native and MGO-modified) or BSA. Enzyme concentration during refolding was 10 nM. The activity of the refolded enzyme was assayed by adding 20 μl of refolding mixture at various time intervals to 480 μl of a refolding buffer that contained 0.1 mM NADH and 0.4 mM oxaloacetate. The decrease in absorbance at 340 nm with time was recorded. MDH (1 μM) kept on ice served as the control. Each data point is the average of three independent measurements. C. LDH (1 μM) was denatured in 6M GdCl for 8 h at 25°C. Refolding was performed as described for the MDH assay above, with the exception that 0.4 mM of sodium pyruvate was used as the substrate and the assay was performed at 37°C. The concentration of αA-crystallin (native and MGO modified) and BSA was 30 μM. LDH (1 μM) kept on ice served as the control. Each data point is the average of three independent measurements.
Alpha-crystallin helps in the in vitro refolding of many enzymes [50, 51]. To determine if MGO-modification would alter the ability of αA-crystallin protect to assist in the refolding of denatured enzymes, we used denatured LDH and MDH and assayed the level of reactivation as measure of their refolding to their native states.
LDH and MDH were inactivated (denatured) by treating them with GdHCl. Refolding to the native state was accomplished by diluting GdHCl with buffer, in the presence or absence of unmodified or MGO-modified αA-crystallin. In the absence of αA-crystallin, both MDH and LDH regained approximately 30% and 20% of the level of activity they have in the native state, respectively (Fig 5B and 5C). In the presence of unmodified and MGO-modified αA-crystallin, both MDH and LDH regained approximately 60% of the level of activity they have in the native state. These results suggest that mild modification by MGO has no affect on the protein refolding ability of αA-crystallin. To determine if the effects of αA-crystallin on refolding of proteins is specific, we used BSA (30 μM) in the place of αA-crystallin in the refolding assays. BSA did not show any effect in either MDH or LDH refolding assay (Fig. 5B and C). This suggests that the improvement of the chaperone function of αA-crystallin by MGO-modification is specific.
The above observations are in contrast to a previously published report [32], where it was found that MGO-modification diminishes α-crystallin's ability to refold (activate) denatured enzymes. In that study, α-crystallin was modified with high concentrations of MGO (10 mM). At such high concentrations, α-crystallin is likely to be extensively crosslinked and therefore might lose its ability to refold proteins that have been denatured by heat or chaotropic agents.
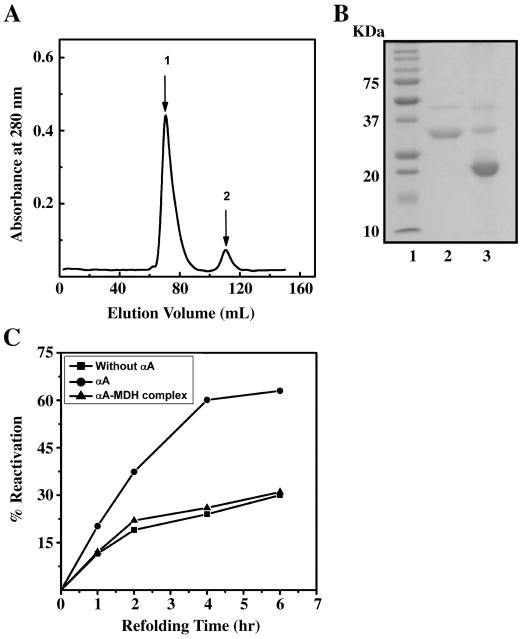
Further, we determined whether binding of catalytically inactivate MDH reduces the refolding potential of αA-crystallin. This experiment was conducted to test if the binding sites responsible for chaperone function are the same as those that participate in refolding of denatured proteins. First, we purified the αA-crystallin-MDH complex (generated by incubating αA-crystallin with MDH at 50°C) by gel filtration (Fig. 6A) and confirmed that the purified protein had both αA-crystallin and MDH by SDS-PAGE (Fig. 6B). We then determined αA-crystallin content in the complex by an ELISA and used 30 μM αA-crystallin from both free and αA-crystallin-MDH complex in the MDH refolding assay. The αA-crystallin-MDH complex did not show chaperone function in the MDH assay (data not shown) and lost the ability to refold denatured MDH (Fig. 6C). These data demonstrate that the sites responsible for the chaperone and refolding functions are the same, as the αA-crystallin-MDH complex completely lost its refolding ability.
Figure 6. αA-Crystallin-MDH complex loses the ability to refold denatured MDH.
A. Purification of αA-crystallin-MDH complex. Catalytically inactive MDH was incubated with αA-crystallin at 50°C for 1 hr. The complex was centrifuged and the clear supernatant was chromatographed on Sephacryl S-300. Peak 1 is the αA-crystallin-MDH complex and peak 2 is free αA-crystallin. MDH from Sigma (cat #M9004) had the expected molecular weight (monomer=35 kDa), but had a minor protein at Mr=46 kDa and that protein also bound to αA-crystallin. B. SDS-PAGE of αA-crystallin-MDH complex. The samples were run on a 15 % gel and stained with Bio-safe Coomassie brilliant blue G 250 (Bio-Rad). Lane 1 is molecular weight markers, lane 2 is MDH alone and lane 3 is αA-crystallin-MDH complex C. MDH refolding assay. The refolding assay details were similar to those described in the legend for Fig. 5. Each data point is the average of three independent measurements.
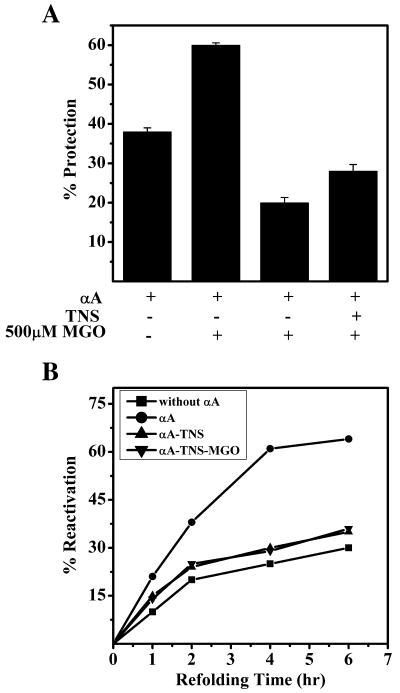
Our data show that mild modification by MGO exposes additional hydrophobic sites in αA-crystallin, and that MGO modified αA-crystallin exhibits a refolding ability similar to that of unmodified αA-crystallin. To determine if MGO-exposed hydrophobic sites participate in the chaperone function and refolding of proteins, we first generated a αA-crystallin-TNS complex by incubating αA-crystallin with TNS and dialyzing this complex against 0.1 M sodium phosphate buffer (pH 7.4), to remove unbound TNS. At this concentration we expected TNS to bind most hydrophobic patches in αA-crystallin and block the chaperone function. As a control, we subjected native αA-crystallin to similar conditions. As expected, chaperone function was drastically reduced after TNS binding (Fig. 7A). A portion of both the native αA-crystallin and the αA-crystallin-TNS complex was then modified with 500 μM MGO and their chaperone function was assessed. Upon modification with MGO, the chaperone functions of αA-crystallin and αA-crystallin-TNS complex increased by 23% and 8%, respectively. This shows that MGO modification exposes additional hydrophobic sites on αA-crystallin.
Figure 7.
A. Prior TNS binding significantly reduces the chaperone function of αA-crystallin but subsequent MGO modification improves the function. The ability of the αA-crystallin-TNS complex (unmodified and MGO modified) to chaperone CS was assayed. The reaction conditions are similar to those described in the legend for Fig 5. B. Prior TNS-binding abolishes the MDH refolding ability of MGO-modified αA-crystallin. The assay details were similar to those described in the legend for Fig. 5. Each data point is the average of three independent measurements.
We then assessed the capacity of MGO-modified and unmodified αA-crystallin-TNS complexes to refold GdHCl denatured MDH. Our results show that the αA-crystallin-TNS complex failed to reactivate denatured MDH, unlike the native αA-crystallin (Fig. 7B). MGO modified αA-crystallin-TNS complex did not show refolding ability either, suggesting that additional hydrophobic sites exposed upon MGO modification do not participate in the refolding of denatured proteins. Together these data (Figs. 6 and 7) demonstrate that the sites responsible for chaperone function and protein refolding are the same, and MGO modification exposes new hydrophobic sites that interact with client proteins during chaperone function, but these newly exposed sites do not take part in the refolding of denatured proteins. Past studies have shown that surface hydrophobicity has a strong influence on the ability of α-crystallin to refold denatured proteins [26, 30], and thus our conclusion is in line with these findings. However, our study deviates from a previous study that established that an increase in chaperone function leads to an increase in refolding ability [31]. However, in that study, metal ions were used to enhance the chaperone function, not MGO, as in the present study.
There are several possibilities as to why hydroimidazolone-modification enhances the chaperone function of αA-crystallin. First, hydroimidazolone-modification imparts mild changes in tertiary structure, which results in the exposure of additional hydrophobic sites that participate in chaperone function. Second, it could convert the dimeric substructure to the monomeric substructure of the oligomer, based on a recently proposed concept [52]. This shift may expose additional sites with chaperone function. Finally, hydroimidazolone-modification may stabilize the oligomeric state of αA-crystallin and improve chaperone function.
Our findings from this study could have significance for cellular homeostasis. When cells are subjected to stress, a significant loss of enzyme activity and serious damage to other components could occur and compromise cellular functions. A considerable amount of energy must be spent to repair such damage to proteins. Renaturation of denatured proteins, assisted by α-crystallin, could be a mechanism to thwart cellular damage by stress. Since MGO modification enhances the chaperone function without compromising the refolding ability of αA-crystallin, we propose that MGO modification of αA-crystallin may be beneficial to cells experiencing stress.
Supplementary Material
Figure S1. A. Far-UV circular dichroic spectra of mildly modified αA-crystallin. The spectra were recorded between 250-195 nm in a cell with a 1mm path length, at 25 °C. Protein concentration was 0.2 mg/ml in 10 mM sodium phosphate buffer (pH 7.4). The mean residue ellipticity, [θ] MRW was calculated, using a value of 110, and each spectrum is the average of three scans. B. Near-UV circular dichroic spectra of mildly modified αA-crystallin. The spectra were recorded between 350-250 nm in a cell with a 10 mm path length, at 25°C. The protein concentration was 1.0 mg/ml in 10 mM sodium phosphate buffer (pH 7.4). Each spectrum is the average of three scans.
A. Chemical aggregation of 0.32 mg/ml of insulin at 25 °C. B. Thermal aggregation of 0.060 mg/ml of CS at 43°C.
Acknowledgments
This study was supported by NIH grants R01EY-016219 and R01EY-09912, by grant P30EY-11373 to the Visual Sciences Research Center of CWRU and by the Ohio Lions Eye Research Foundation. RHN is recipient of a Senior Scientific Investigator Award from the Research to Prevent Blindness, New York. We thank Dr. Ashis Biswas for critical reading of the manuscript.
Abbreviations
- sHSP
Small heat shock protein
- DTT
Dithiothreitol
- GdHCl
Guanidine hydrochloride
- CS
Citrate synthase
- MDH
Malate dehydrogenase
- LDH
Lactate dehydrogenase
- MGO
Methylglyoxal
- NADH
β-nicotinamide adenine nucleotide reduced dipotassium salt
- TNS
2-(p-toluidinyl) naphthalene-6-sulfonic acid sodium salt
- HI
Hydroimidazolone
- DTNB
5,5′-Dithiobis (2-nitro-benzoic acid)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Harding JJ. Cataract: Biochemistry, Epidemiology and Pharmacology. Chapman and Hall; London: 1991. [Google Scholar]
- 2.Bloemendal H. Lens proteins. CRC Crit Rev Biochem. 1982;12:1–38. doi: 10.3109/10409238209105849. [DOI] [PubMed] [Google Scholar]
- 3.Ingolia TD, Craig EA. Four small Drosophila heat shock proteins are related to each other and to mammalian alpha-crystallin. Proc Natl Acad Sci U S A. 1982;79:2360–2364. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Narberhaus F. Alpha-crystallin-type heat shock proteins: socializing minichaperones in the context of a multichaperone network. Microbiol Mol Biol Rev. 2002;66:64–93. doi: 10.1128/MMBR.66.1.64-93.2002. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Derham BK, Harding JJ. Alpha-crystallin as a molecular chaperone. Prog Retin Eye Res. 1999;18:463–509. doi: 10.1016/s1350-9462(98)00030-5. [DOI] [PubMed] [Google Scholar]
- 6.Horwitz J. Alpha-crystallin. Exp Eye Res. 2003;76:145–153. doi: 10.1016/s0014-4835(02)00278-6. [DOI] [PubMed] [Google Scholar]
- 7.Horwitz J. Alpha crystallin: The quest for a homogeneous quaternary structure. Exp Eye Res. 2008 doi: 10.1016/j.exer.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horwitz J, Huang Q, Ding L. The native oligomeric organization of alpha-crystallin, is it necessary for its chaperone function? Exp Eye Res. 2004;79:817–821. doi: 10.1016/j.exer.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Sharma KK, Kumar RS, Kumar GS, Quinn PT. Synthesis and characterization of a peptide identified as a functional element in alphaA-crystallin. J Biol Chem. 2000;275:3767–3771. doi: 10.1074/jbc.275.6.3767. [DOI] [PubMed] [Google Scholar]
- 10.Brady JP, Garland D, Duglas-Tabor Y, Robison WG, Jr, Groome A, Wawrousek EF. Targeted disruption of the mouse alpha A-crystallin gene induces cataract and cytoplasmic inclusion bodies containing the small heat shock protein alpha B-crystallin. Proc Natl Acad Sci U S A. 1997;94:884–889. doi: 10.1073/pnas.94.3.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyle DL, Takemoto L, Brady JP, Wawrousek EF. Morphological characterization of the Alpha A- and Alpha B-crystallin double knockout mouse lens. BMC Ophthalmol. 2003;3:3. doi: 10.1186/1471-2415-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shroff NP, Cherian-Shaw M, Bera S, Abraham EC. Mutation of R116C results in highly oligomerized alpha A-crystallin with modified structure and defective chaperone-like function. Biochemistry. 2000;39:1420–1426. doi: 10.1021/bi991656b. [DOI] [PubMed] [Google Scholar]
- 13.Treweek TM, Rekas A, Lindner RA, Walker MJ, Aquilina JA, Robinson CV, Horwitz J, Perng MD, Quinlan RA, Carver JA. R120G alphaB-crystallin promotes the unfolding of reduced alpha-lactalbumin and is inherently unstable. Febs J. 2005;272:711–724. doi: 10.1111/j.1742-4658.2004.04507.x. [DOI] [PubMed] [Google Scholar]
- 14.Xi JH, Bai F, Gross J, Townsend RR, Menko AS, Andley UP. Mechanism of small heat shock protein function in vivo: a knock-in mouse model demonstrates that the R49C mutation in alpha A-crystallin enhances protein insolubility and cell death. J Biol Chem. 2008;283:5801–5814. doi: 10.1074/jbc.M708704200. [DOI] [PubMed] [Google Scholar]
- 15.Hsu CD, Kymes S, Petrash JM. A transgenic mouse model for human autosomal dominant cataract. Invest Ophthalmol Vis Sci. 2006;47:2036–2044. doi: 10.1167/iovs.05-0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cherian M, Abraham EC. Decreased molecular chaperone property of alpha-crystallins due to posttranslational modifications. Biochem Biophys Res Commun. 1995;208:675–679. doi: 10.1006/bbrc.1995.1391. [DOI] [PubMed] [Google Scholar]
- 17.Thampi P, Hassan A, Smith JB, Abraham EC. Enhanced C-terminal truncation of alphaA- and alphaB-crystallins in diabetic lenses. Invest Ophthalmol Vis Sci. 2002;43:3265–3272. [PubMed] [Google Scholar]
- 18.Thornalley PJ. The glyoxalase system in health and disease. Mol Aspects Med. 1993;14:287–371. doi: 10.1016/0098-2997(93)90002-u. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed MU, Brinkmann Frye E, Degenhardt TP, Thorpe SR, Baynes JW. N-epsilon-(carboxyethyl)lysine, a product of the chemical modification of proteins by methylglyoxal, increases with age in human lens proteins. Biochem J. 1997;324(Pt 2):565–570. doi: 10.1042/bj3240565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chellan P, Nagaraj RH. Protein crosslinking by the Maillard reaction: dicarbonyl-derived imidazolium crosslinks in aging and diabetes. Arch Biochem Biophys. 1999;368:98–104. doi: 10.1006/abbi.1999.1291. [DOI] [PubMed] [Google Scholar]
- 21.Biemel KM, Friedl DA, Lederer MO. Identification and quantification of major maillard cross-links in human serum albumin and lens protein. Evidence for glucosepane as the dominant compound. J Biol Chem. 2002;277:24907–24915. doi: 10.1074/jbc.M202681200. [DOI] [PubMed] [Google Scholar]
- 22.Ahmed N, Thornalley PJ, Dawczynski J, Franke S, Strobel J, Stein G, Haik GM. Methylglyoxal-derived hydroimidazolone advanced glycation end-products of human lens proteins. Invest Ophthalmol Vis Sci. 2003;44:5287–5292. doi: 10.1167/iovs.03-0573. [DOI] [PubMed] [Google Scholar]
- 23.Wilker SC, Chellan P, Arnold BM, Nagaraj RH. Chromatographic quantification of argpyrimidine, a methylglyoxal-derived product in tissue proteins: comparison with pentosidine. Anal Biochem. 2001;290:353–358. doi: 10.1006/abio.2001.4992. [DOI] [PubMed] [Google Scholar]
- 24.Kumar MS, Reddy PY, Kumar PA, Surolia I, Reddy GB. Effect of dicarbonyl-induced browning on alpha-crystallin chaperone-like activity: physiological significance and caveats of in vitro aggregation assays. Biochem J. 2004;379:273–282. doi: 10.1042/BJ20031633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagaraj RH, Oya-Ito T, Padayatti PS, Kumar R, Mehta S, West K, Levison B, Sun J, Crabb JW, Padival AK. Enhancement of chaperone function of alpha-crystallin by methylglyoxal modification. Biochemistry. 2003;42:10746–10755. doi: 10.1021/bi034541n. [DOI] [PubMed] [Google Scholar]
- 26.Biswas A, Miller A, Oya-Ito T, Santhoshkumar P, Bhat M, Nagaraj RH. Effect of site-directed mutagenesis of methylglyoxal-modifiable arginine residues on the structure and chaperone function of human alphaA-crystallin. Biochemistry. 2006;45:4569–4577. doi: 10.1021/bi052574s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biswas A, Lewis S, Wang B, Miyagi M, Santoshkumar P, Gangadhariah MH, Nagaraj RH. Chemical Modulation of the Chaperone Function of Human {alpha}A-Crystallin. J Biochem. 2008;144:21–32. doi: 10.1093/jb/mvn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmed N, Dobler D, Dean M, Thornalley PJ. Peptide mapping identifies hotspot site of modification in human serum albumin by methylglyoxal involved in ligand binding and esterase activity. J Biol Chem. 2005;280:5724–5732. doi: 10.1074/jbc.M410973200. [DOI] [PubMed] [Google Scholar]
- 29.Ahmed N, Thornalley PJ. Chromatographic assay of glycation adducts in human serum albumin glycated in vitro by derivatization with 6-aminoquinolyl-N-hydroxysuccinimidyl-carbamate and intrinsic fluorescence. Biochem J. 2002;364:15–24. doi: 10.1042/bj3640015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biswas A, Das KP. Alpha-crystallin assisted refolding of enzyme substrates: optimization of external parameters. Protein J. 2007;26:247–255. doi: 10.1007/s10930-006-9066-8. [DOI] [PubMed] [Google Scholar]
- 31.Biswas A, Das KP. Zn2+ enhances the molecular chaperone function and stability of alpha-crystallin. Biochemistry. 2008;47:804–816. doi: 10.1021/bi7011965. [DOI] [PubMed] [Google Scholar]
- 32.Kumar MS, Reddy PY, Sreedhar B, Reddy GB. Alphab-crystallin-assisted reactivation of glucose-6-phosphate dehydrogenase upon refolding. Biochem J. 2005;391:335–341. doi: 10.1042/BJ20050506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henle TH, Walter AW, Haessner R, Klostermeyer H. Detection and identification of a protein-bound imidazolone resulting from the reaction of arginine residues and methylglyoxal. Z Lebensm Unters Forsch. 1994;199:55–58. [Google Scholar]
- 34.Ahmed N, Argirov OK, Minhas HS, Cordeiro CA, Thornalley PJ. Assay of advanced glycation endproducts (AGEs): surveying AGEs by chromatographic assay with derivatization by 6-aminoquinolyl-N-hydroxysuccinimidyl-carbamate and application to Nepsilon-carboxymethyl-lysine- and Nepsilon-(1-carboxyethyl)lysine-modified albumin. Biochem J. 2002;364:1–14. doi: 10.1042/bj3640001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santhoshkumar P, Sharma KK. Phe71 is essential for chaperone-like function in alpha A-crystallin. J Biol Chem. 2001;276:47094–47099. doi: 10.1074/jbc.M107737200. [DOI] [PubMed] [Google Scholar]
- 36.Derham BK, Harding JJ. Effect of aging on the chaperone-like function of human alpha-crystallin assessed by three methods. Biochem J. 1997;328(Pt 3):763–768. doi: 10.1042/bj3280763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhattacharyya J, Das KP. Alpha-crystallin does not require temperature activation for its chaperone-like activity. Biochem Mol Biol Int. 1998;46:249–258. doi: 10.1080/15216549800203762. [DOI] [PubMed] [Google Scholar]
- 38.Zhi W, Landry SJ, Gierasch LM, Srere PA. Renaturation of citrate synthase: influence of denaturant and folding assistants. Protein Sci. 1992;1:522–529. doi: 10.1002/pro.5560010407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raman B, Ramakrishna T, Rao CM. Rapid refolding studies on the chaperone-like alpha-crystallin. Effect of alpha-crystallin on refolding of beta- and gamma-crystallins. J Biol Chem. 1995;270:19888–19892. doi: 10.1074/jbc.270.34.19888. [DOI] [PubMed] [Google Scholar]
- 40.Ahmed N, Thornalley PJ. Peptide mapping of human serum albumin modified minimally by methylglyoxal in vitro and in vivo. Ann N Y Acad Sci. 2005;1043:260–266. doi: 10.1196/annals.1333.031. [DOI] [PubMed] [Google Scholar]
- 41.Haik GM, Jr, Lo TW, Thornalley PJ. Methylglyoxal concentration and glyoxalase activities in the human lens. Exp Eye Res. 1994;59:497–500. doi: 10.1006/exer.1994.1135. [DOI] [PubMed] [Google Scholar]
- 42.McClure WO, Edelman GM. Fluorescent probes for conformational states of proteins. I. Mechanism of fluorescence of 2-p-toluidinylnaphthalene-6-sulfonate, a hydrophobic probe. Biochemistry. 1966;5:1908–1919. doi: 10.1021/bi00870a018. [DOI] [PubMed] [Google Scholar]
- 43.Bera S, Thampi P, Cho WJ, Abraham EC. A positive charge preservation at position 116 of alpha A-crystallin is critical for its structural and functional integrity. Biochemistry. 2002;41:12421–12426. doi: 10.1021/bi0204140. [DOI] [PubMed] [Google Scholar]
- 44.Shroff NP, Bera S, Cherian-Shaw M, Abraham EC. Substituted hydrophobic and hydrophilic residues at methionine-68 influence the chaperone-like function of alphaB-crystallin. Mol Cell Biochem. 2001;220:127–133. doi: 10.1023/a:1010834107809. [DOI] [PubMed] [Google Scholar]
- 45.Reddy GB, Kumar PA, Kumar MS. Chaperone-like activity and hydrophobicity of alpha-crystallin. IUBMB Life. 2006;58:632–641. doi: 10.1080/15216540601010096. [DOI] [PubMed] [Google Scholar]
- 46.Khanova HA, Markossian KA, Kleimenov SY, Levitsky DI, Chebotareva NA, Golub NV, Asryants RA, Muronetz VI, Saso L, Yudin IK, Muranov KO, Ostrovsky MA, Kurganov BI. Effect of alpha-crystallin on thermal denaturation and aggregation of rabbit muscle glyceraldehyde-3-phosphate dehydrogenase. Biophys Chem. 2007;125:521–531. doi: 10.1016/j.bpc.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 47.Rajaraman K, Raman B, Rao CM. Molten-globule state of carbonic anhydrase binds to the chaperone-like alpha-crystallin. J Biol Chem. 1996;271:27595–27600. doi: 10.1074/jbc.271.44.27595. [DOI] [PubMed] [Google Scholar]
- 48.Hess JF, FitzGerald PG. Protection of a restriction enzyme from heat inactivation by [alpha]-crystallin. Mol Vis. 1998;4:29. [PubMed] [Google Scholar]
- 49.Rajaraman K, Raman B, Ramakrishna T, Rao CM. Interaction of human recombinant alphaA- and alphaB-crystallins with early and late unfolding intermediates of citrate synthase on its thermal denaturation. FEBS Lett. 2001;497:118–123. doi: 10.1016/s0014-5793(01)02451-6. [DOI] [PubMed] [Google Scholar]
- 50.Raman B, Ramakrishna T, Rao CM. Effect of the chaperone-like alpha-crystallin on the refolding of lysozyme and ribonuclease A. FEBS Lett. 1997;416:369–372. doi: 10.1016/s0014-5793(97)01240-4. [DOI] [PubMed] [Google Scholar]
- 51.Ganea E, Harding JJ. alpha-crystallin assists the renaturation of glyceraldehyde-3-phosphate dehydrogenase. Biochem J. 2000;345(Pt 3):467–472. [PMC free article] [PubMed] [Google Scholar]
- 52.Benesch JL, Ayoub M, Robinson CV, Aquilina JA. Small heat shock protein activity is regulated by variable oligomeric substructure. J Biol Chem. 2008;283:28513–28517. doi: 10.1074/jbc.M804729200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. A. Far-UV circular dichroic spectra of mildly modified αA-crystallin. The spectra were recorded between 250-195 nm in a cell with a 1mm path length, at 25 °C. Protein concentration was 0.2 mg/ml in 10 mM sodium phosphate buffer (pH 7.4). The mean residue ellipticity, [θ] MRW was calculated, using a value of 110, and each spectrum is the average of three scans. B. Near-UV circular dichroic spectra of mildly modified αA-crystallin. The spectra were recorded between 350-250 nm in a cell with a 10 mm path length, at 25°C. The protein concentration was 1.0 mg/ml in 10 mM sodium phosphate buffer (pH 7.4). Each spectrum is the average of three scans.
A. Chemical aggregation of 0.32 mg/ml of insulin at 25 °C. B. Thermal aggregation of 0.060 mg/ml of CS at 43°C.