Abstract
Asthma is a chronic disease characterized by airway inflammation caused by the dysregulated production of cytokines secreted by allergen-specific type 2 T helper (Th2) cells. Antrodia camphorata is a commonly used fungus in Asian folk medicine, and A. camphorata polysaccharides are reported to possess anti-cancer activities. In this study, the immunomodulatory effects of purified fractionated polysaccharides (GF2) from A. camphorata on dendritic cells (DCs) and their potential preventive effects against ovalbumin (OVA) -induced asthma were investigated. In the presence of GF2, lipopolysaccharide (LPS) -activated DCs exhibited up-regulated expression of major histocompatibility complex (MHC) class II and co-stimulatory molecules, as well as enhanced interleukin-10 (IL-10) and IL-12 production. GF2 treatment on LPS-activated DCs suppressed naïve CD4+ T-cell proliferation and Th2 cell polarization with IL-10 production in an allogeneic mixed lymphocyte reaction. In animal experiments, a high dose of GF2 efficiently reduced expression levels of OVA-specific immunoglobulin G1 (IgG1) and IgE. However, lower doses of GF2 significantly enhanced OVA-specific IgG2a production. Our data also showed that administration of GF2 dose-dependently inhibited the development of airway hyperresponsiveness, airway eosinophilia and Th2 responses. OVA-specific CD4+ T cells from higher doses of GF2-treated mice had significantly lower proliferative capacities compared with control mice. Moreover, treatment with GF2 significantly increased the high levels of IL-10 and low levels of interferon-γ produced by T cells. Taken together, these data indicate that administration of A. camphorata polysaccharides (GF2) may have therapeutic potential when used as an adjuvant for the immunomodulatory treatment of allergic asthma.
Keywords: Antrodia camphorata, asthma, dendritic cells, T cells
Introduction
Antrodia camphorata is a medicinal mushroom well known in Taiwan as a folk medicine and commonly used to treat abdominal pain, diarrhoea, drug intoxication, hypertension, itchy skin and liver cancer. For both in vitro and in vivo models, the partially purified polysaccharide from A. camphorata was found to have anti-tumour effects.1 In addition, polysaccharides extracted from A. camphorata exhibited anti-hepatitis B virus activity.2 They also inhibit lipopolysaccharide (LPS)-induced inflammation in mouse macrophages.3 However, there is no report on the immunomodulatory effects of A. camphorata polysaccharides in allergic diseases.
Asthma is a global health problem that results from a complex interplay between genetic and environmental factors. It is recognized that CD4+ T-helper type 2 (Th2) cells and their cytokines [interleukin-4 (IL-4), IL-5 and IL-13] are responsible for the initiation and maintenance of allergic disorders.4–6 These Th2 cytokines induce, prolong and amplify the inflammatory response by enhancing the production of allergic-specific immunoglobulin E (IgE); enhancing recruitment, growth and differentiation of eosinophils; and directly causing airway hyperresponsiveness (AHR).7,8
It has become apparent that dendritic cells (DCs) can function both as initiators of the immune response and as inducers of T-cell tolerance.9,10 Factors that determine how a given DC functions depend on its state of maturation and the local microenvironment. To acquire naïve T-cell stimulatory ability, DCs must undergo maturation. This involves up-regulation of surface major histocompatibility complex (MHC) class II and co-stimulatory molecules during their migration from the periphery to the T-cell areas of lymphoid organs. In contrast, DCs, particularly those at an immature stage, can suppress peripheral T-cell responses to foreign antigens and induce antigen-specific tolerance.
To date, the immunomodulatory effects of A. camphorata polysaccharides on DC function and allergic asthma have never been defined. In the current study, we clarify whether treatment with purified polysaccharides from A. camphorata can alter the function of DCs and achieve protective effects to alleviate the development of airway symptoms in a mouse model of allergic asthma. Changes in serum ovalbumin (OVA) -specific antibodies, AHR, airway eosinophilia, T-cell proliferation and cytokine production by these treated mice were evaluated. Our findings provide more insights into how these polysaccharides affect the Th2-biased immune response and provide guidance on the use of immunoregulatory polysaccharides in a DC-based vaccine for asthma.
Materials and methods
Animals
Female BALB/c and C57BL/6 mice at 6–8 weeks old were obtained from the Animal Center of National Taiwan University and maintained in the Animal Center of Taipei Medical University. Animals were age-matched within each experiment. Animal care and handling protocols were approved by the Animal Committee of the College of Medicine, Taipei Medical University.
Preparation and analysis of a polysaccharide-enriched fraction from A. camphorata extracts
Antrodia camphorata (20 g) produced by a solid-state culture was provided by Well Shine Biotechnology Development (Taipei, Taiwan). Crude A. camphorata polysaccharide (AC-PS) extracts were prepared via an alkaline extraction of the solid-state culture with 1 N NaOH (400 ml) by autoclaving for 20 min at 121°, followed by neutralization and filtration to remove the residue. The filtrate was precipitated with an equal volume of 95% alcohol. Then, the precipitate (1·84 g) was collected and dissolved in 1 l of double-distilled water (ddH2O) during stirring at 4° for 24 hr. The solution was centrifuged (16 000 g) at 4° for 1 hr, and the supernatant was concentrated at 35° under a vacuum using a rotary evaporator. The slurry product was then lyophilized to obtain a water-soluble dark-brown AC-PS powder. This powder was again dissolved in 100 ml ddH2O and fractioned through a gel-filtration Fractogel TSK HW-60 (Merck, San Diego, CA) column (300 × 16 mm) with 0·1 m Tris–HCl buffer (pH 7·0) as the mobile phase. The flow rate was set to 3·5 ml/min, and fractions were collected at 14 ml/tube. Six polysaccharide-enriched fractions were collected, each dialysed to remove excess salts and lyophilized to give fractions 1 to 6 (GF1–6).
These eluted fractions were polysaccharides that did not include triterpenes, lipids, steroids, phenols or proteins. Among all fractions, the purified polysaccharide GF2 (with a 30–33% yield) was the major polysaccharide fraction and possessed optimal immunomodulatory function, therefore; it was used in the following studies.
Gel permeation chromatography analysis showed that the average molecular weight of GF2 was 2000 kDa. Moreover, GF2 contained 67% carbohydrate as determined by the phenol–sulphuric acid method. The monosaccharide composition of GF2 was further monitored by the gas chromatograph method, with glucose identified as the major monosaccharide. In addition, determination of the peptide moiety in GF2 was accomplished using the Bradford protein assay. A 33% protein content was observed in GF2. In general, GF2 belonged to the category of protein-bound polysaccharides and its ratio of carbohydrate : protein was 2 (weight/weight). GF2 preparations were tested for Gram-negative bacterial endotoxin contamination by the limulus amoebocyte lysate assay (Cambrex, Walkersville, MD). We found no detectable levels of endotoxin (< 0·10 endotoxin units/ml) in the GF2 samples.
Generation of bone-marrow-derived DCs (BMDCs) in vitro
The BMDCs were prepared as described previously.11 Briefly, tibial and femoral bone marrow from 5- to 6-week-old BALB/c mice was isolated. Bone marrow cells were cultured in RPMI-1640 and 5% fetal bovine serum complete medium with IL-4 (1000 U/ml) and granulocyte–macrophage colony-stimulating factor (GM-CSF; 500 U/ml) on day 0. Every other day, the medium was removed, and fresh medium containing GM-CSF and IL-4 was added. On day 6 of the culture, non-adherent cells were collected and washed for further assay.
Reverse transcription and quantitative polymerase chain reaction
On day 6 of the culture, bone-marrow-derived non-adherent cells were collected and 1 × 106 cells/ml were treated with RPMI-1640 medium, various concentrations of GF2 (1, 10 and 100 μg/ml), LPS (1 μg/ml) alone, or LPS plus various concentrations of GF2 for 6 hr. After incubation, the cells were collected and total RNA (2 × 106 to 4 × 106 cells) was extracted according to the TRIzol method (Invitrogen, Carlsbad, CA). Complementary DNA (cDNA) was generated from 2 μg of total RNA by using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). Four microlitres of cDNA was used with a polymerase chain reaction (PCR) master mix and TaqMan assays (Applied Biosystems). Quantitative PCR detection of mouse glyceraldehyde 3-phosphate dehydrogenase (GAPDH), IL-10 and IL-12 were conducted in triplicate using an Applied Biosystems 7900 PCR system. Cycle thresholds obtained were normalized to GAPDH. Relative fold changes were calculated by the comparative threshold cycle (CT) method, 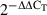 .
.
Determination of cytokine levels
Levels of IL-5, IL-10, IL-12, transforming growth factor-β (TGF-β) and interferon-γ (IFN-γ) in the culture supernatant from DCs or T cells were assayed using enzyme-linked immunosorbent assay (ELISA) kits (Duoset, R&D Systems, Minneapolis, MN).
Quantities of IL-4, IL-5, IL-10, IL-13 and IFN-γ in bronchoalveolar lavage fluid (BALF) and culture supernatants of splenocytes were also evaluated using commercially available ELISA kits (Duoset, R&D Systems).
Flow cytometric analysis of DCs
A FACSCalibur (Becton Dickinson, San Jose, CA) was used for analytical flow cytometry, and data were processed with CellQuestPro software (BD Biosciences, Mountain View, CA). The BMDCs were stained with rat anti-mouse monoclonal antibodies to CD11c, IAd (MHC class II), CD80 (B7-1) and CD86 (B7-2) (eBioscience, San Diego, CA). Staining with isotype control antibodies was performed in all experiments. The DCs were gated according to the standard forward-scatter and side-scatter profiles for CD11c+ large cells.
Isolation of allogeneic naïve CD4+ T cells
Spleens were harvested from female C57BL/6 mice and ground into single-cell suspensions. After depleting red blood cells via an ACK lysis buffer, splenocytes were washed with Hanks’ balanced salt solution (HBSS) three times and resuspended with magnetic antibody cell sorting (MACS) buffer [0·5% bovine serum albumin and 2 mm ethylenediaminetetraacetic acid in 1 × phosphate-buffered saline (PBS) solution] at 107/90 μl cells. Subsequent staining with anti-CD4 magnetic beads (Miltenyi Biotec, Auburn, CA) at 107/10 μl cells was performed for 10 min at 4°. According to the manufacturer’s instructions, cells were then washed and resuspended in MACS buffer to purify allogeneic naïve CD4+ T cells. Positively selected cells were collected for further analysis.
Allogeneic mixed lymphocyte reaction
Approximately 1 × 106 day-6 BMDCs were seeded in a 24-well plate and treated with LPS (1 μg/ml), LPS plus GF2 (100 μg/ml) or medium only. After 24 hr of culture, BMDCs were irradiated with 3000 rad (137Cs source). After washing twice with HBSS, different proportions of irradiated BMDCs (3 × 104, 7·5 × 103 and 1·87 × 103 cells/well) and allogeneic naïve CD4+ T cells (3 × 105 cells/well) were incubated at 37° in 96-well round-bottom plates. Additionally, either anti-IL-10R monoclonal antibody (10 μg/ml; R&D Systems) or an isotype-matched control monoclonal antibody was added to the cultures in the presence of LPS and GF2 stimulation. After 3 days of culture, tritiated thymidine (New England Nuclear, Boston, MA) was applied at 1 μCi/well and incubated at 37° for another 16–18 hr. Cells were then harvested onto glass filters by an automated multi-sample harvester and counted with a dry scintillation counter (Packard Instrument, Meriden, CT).
Animal models and study design
For systemic immunization, mice were immunized twice intraperitoneally with 50 μg OVA (grade V; Sigma Chemical, St Louis, MO) plus 2 mg alum (Pierce Chemical, Rockford, IL) on days 0 and 10. Subsequently, mice were exposed to OVA aerosol challenge (5% OVA in a normal saline solution) for 3 consecutive days (days 14–16). The aerosols were generated in a chamber using an ultrasonic nebulizer. To examine the effects of various dosages (1, 10 and 100 μg/mouse) of GF2, three groups of mice were simultaneously intraperitoneally injected with different doses of GF2 for 3 days each time (days − 1 to 1 and days 9 to 11) with the indicated immunization protocol. The positive control mice received PBS instead of GF2. The negative control mice were neither sensitized with OVA nor given GF2 treatment although they did receive an OVA challenge.
Serum antibody assay
Serum samples were collected from the retro-orbital venous plexus after intraperitoneal immunization with the OVA antigen. The OVA-specific IgE, IgG1 and IgG2a serum antibody titres were determined by ELISA as previously described.12 Levels of antibodies were compared with IgE, IgG1 and IgG2a standards for predetermined concentrations (immunoglobulin concentrations: IgE = 1 μg/ml, IgG2a = 15·5 μg/ml, IgG1 = 25·6 μg/ml). The concentration of standard serum was arbitrarily assigned as 1 ELISA unit (1 EU).
Measurement of AHR
Twenty-four hours after the last aerosol exposure, the airway resistance of mice was measured by a single-chamber, barometric whole-body plethysmography (Buxco Electronics, Troy, NY) as previously described.13 Briefly, mice were placed in the main chamber, and baseline readings were taken and averaged for 3 min. Aerosolized normal saline or methacholine (acetyl-β-methycholine chloride; Sigma) in increasing concentrations (6·25–100 mg/ml) were nebulized through an inlet of the main chamber for 3 min. Recordings were taken and averaged for 3 min after each nebulization. Airway reactivity was expressed as the mean enhanced pause [Penh = pause × (peak expiratory box flow/peak inspiratory box flow)], and data were expressed as the ratio of PenhMCh values compared with PenhNaCl.
Analysis of the cellular composition of BALF
One day after measuring the pulmonary function parameters, mice were killed, and their tracheas were immediately lavaged three times via a tracheal cannula with 1 ml HBSS that was free of ionized calcium and magnesium. The lavage fluid was cooled on ice and centrifuged (400 g) at 4° for 10 min. After washing, the supernatants were collected for the cytokine assay, and cell pellets were resuspended in 1 ml HBSS. The total numbers of cells in the BALF were counted with a standard haemocytometer. Differential cell counts were performed by counting at least 200 cells in the cytocentrifuged preparations, staining with Liu’s stain solution (Chi I Pao, Taipei, Taiwan), and differentiating by standard morphological criteria.
Antigen-specific proliferative assay
For the proliferation assay, 3 × 105 freshly isolated spleen cells/well were cultured with OVA (10, 50 and 100 μg/ml) and 10 μg/ml phytohaemagglutinin (PHA, Sigma), or medium only in RPMI-1640 medium containing 2% TCM (mouse serum replacement; Celox, Hopkins, MN) plus 2% fetal bovine serum. After 3–5 days of culturing, cell proliferation was estimated by methyl thiazolyl tetrazolium (MTT; Sigma) colorimetric assay. The MTT was dissolved at 5 g/l in PBS and filtered through a 0·22-μm filter. The culture supernatant was removed (100 μl/well), and the MTT solution (10 μl) was added to each well for further incubation at 37° for 4 hr. Subsequently, 100 μl of 10% sodium dodecylsulphate (SDS) in 0·01 N HCl was added to each well, and the microplates were incubated at 25° overnight, then measured with a spectrophotometer. Data were expressed as absorbance values. The stimulation index (S.I.) was calculated as the absorbance value for optical density at 590 nm (OD590) of stimulated wells divided by the OD590 absorbance value of untreated control wells.
Statistical analysis
Results are expressed as the mean ± SEM. Statistical analysis was performed using one-way analysis of variance followed by Dunnett’s post hoc test. P<0·05 was considered statistically significant.
Results
GF2 enhances IL-10 and IL-12 production in LPS-stimulated DCs
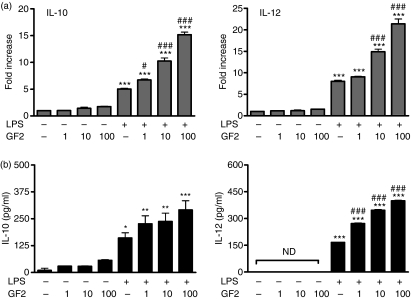
Before testing the effects of the purified GF2 fraction from A. camphorata polysaccharides on BMDCs, we examined the effects of GF2 on cell viability. After 48 hr of treatment with GF2 alone or GF2 and LPS in BMDCs, cell numbers were counted. The results showed that cell numbers were not affected by GF2 even at 200 μg/ml (Yueh-Lun Lee and Yi-Lien Chen, unpublished data). In determining whether the GF2 fraction affected cytokine production by mouse BMDCs, we compared IL-10 and IL-12 expression in LPS-stimulated DCs cultured with different doses of GF2. In the presence of GF2, increased transcription levels of IL-10 and IL-12 genes were observed in LPS-treated DCs (Fig. 1a). The protein levels of IL-10 and IL-12 also demonstrated similarly significant increases in a dose-dependent manner (Fig. 1b).
Figure 1.
GF2 up-regulated interleukin-10 (IL-10) and IL-12 expression in lipopolysaccharide (LPS) -stimulated dendritic cells (DCs). (a) IL-10 and IL-12 messenger RNA expression and (b) protein levels from different doses of GF2-treated DCs with or without LPS stimulation. On day 6 of the culture, bone marrow-derived non-adherent cells were collected and treated with RPMI-1640, various concentrations of GF2 (1, 10 and 100 μg/ml), LPS (1 μg/ml) alone, or LPS plus various concentrations of GF2 for 6 hr (or 48 hr). The transcripts and protein production for the IL-10 and IL-12 were measured by quantitative real-time reverse transcription–polymerase chain reaction and enzyme-linked immunosorbent assay, respectively. Results from triplicate experiments are shown and expressed as the mean ± SEM. *P<0·05; **P<0·01; and ***P<0·001 compared with the medium-treated DCs. #P<0·05 and ###P<0·001 compared with LPS-stimulated DCs. ND, not detectable.
GF2 enhances maturation of LPS-stimulated DCs
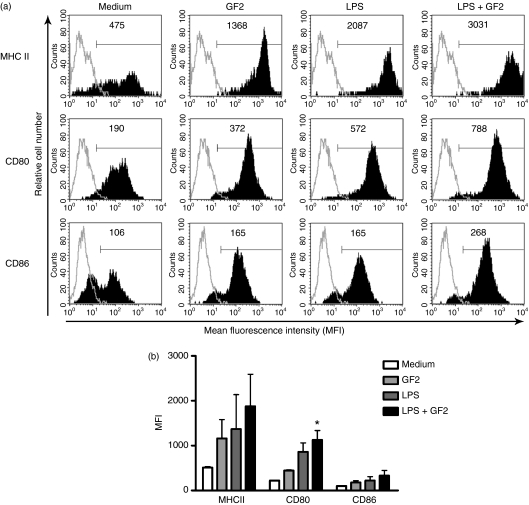
To further determine whether the GF2 fraction modulated the maturation of mouse BMDCs in vitro, we compared the phenotype of mouse DCs treated with GF2 (100 μg/ml) and those untreated in the presence of LPS stimulation. As shown in Fig. 2, the expression of the CD80 molecules in DCs stimulated with LPS plus GF2 was significantly higher than in DCs treated with only medium (Fig. 2). Although, the difference was not significant, the data demonstrate that DCs activated with LPS, GF2 or LPS plus GF2 expressed similarly increased levels of MHC class II and CD86 molecules compared with medium-treated DCs.
Figure 2.
GF2 enhanced maturation of lipopolysaccharide (LPS) -stimulated dendritic cells (DCs). On day 6 of the culture, bone marrow-derived non-adherent cells were collected and treated with RPMI-1640, GF2 (100 μg/ml), LPS (1 μg/ml), or combined GF2 and LPS for 48 hr. After incubation, cells were collected and the expressions of CD80, CD86 and major histocompatibility complex (MHC) class II by DCs were analysed by flow cytometry. LPS was used as the positive control. DCs were gated on CD11c+ large cells. (a) Values shown in the flow cytometric profiles are the mean fluorescence intensity (MFI) by CellQuestPro software. Values shown were from one representative experiment of four independent experiments performed. (b) The MFI was calculated, and results are expressed as the mean ± SEM from four experiments. *P<0·05 compared with medium-treated DCs.
GF2- and LPS-treated DCs suppress T-cell activation and Th2 cell polarization
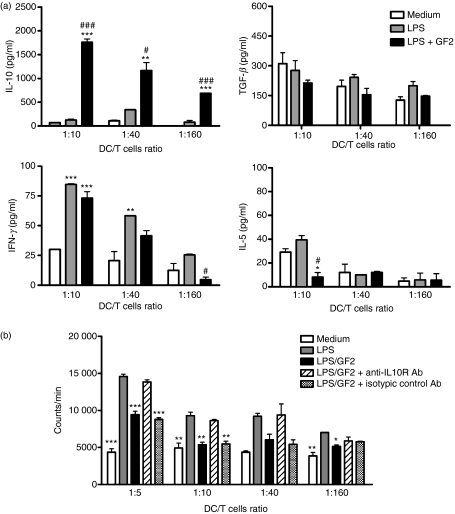
Mature DCs have the capacity to induce proliferation in allogeneic T cells at much higher levels than immature DCs.14 To determine the allogeneic mixed lymphocyte reaction, DCs were co-cultured with allogeneic naïve CD4+ T cells at different ratios (DC : T-cell ratios of 1 : 10, 1 : 40 and 1 : 160). The results showed that IL-10, not TGF-β, produced by T cells induced under GF2- and LPS-treated DC stimulation was far higher than that observed following LPS treatment of DCs (Fig. 3a). Although GF2 did not have a suppressive effect on the production of IFN-γ, there was a significant decrease in IL-5 secretion by T cells at high DC : T-cell ratios. Interestingly, LPS-activated DCs cultured in the presence of GF2 significantly suppressed T-cell proliferation and blocked IL-10-mediated signalling, markedly affecting this suppression mechanism (Fig. 3b).
Figure 3.
GF2 combined with lipopolysaccharide (LPS) -treated dendritic cells (DCs) reduced T helper type 2 (Th2) cell polarization and T-cell proliferation in an allogeneic mixed lymphocyte reaction. (a) Modulatory effects of GF2 on T-cell cytokine secretion. Approximately 1 × 106 cells/ml day-6 DCs were pretreated with medium, LPS (1 μg/ml), or LPS plus GF2 (100 μg/ml) for 24 hr. Allogeneic naïve CD4+ T cells (3 × 105 cells/well) were cultured with γ-irradiated DCs in 96-well round-bottomed plates at different DC : T (1 : 10, 1 : 40, and 1 : 160) ratios. Concentrations of interleukin-5 (IL-5), interferon-γ (IFN-γ), transforming growth factor-β (TGF-β) and IL-10 secreted by naïve CD4+ T cells, which were co-cultured with γ-irradiated DCs in 96-well plates for 3 days, were determined by enzyme-linked immunosorbent assay. (b) Modulatory effects of GF2 on T-cell proliferation. DCs were pretreated with medium, LPS (1 μg/ml), or LPS plus GF2 (100 μg/ml) for 24 hr. Allogeneic naïve CD4+ T cells were pre-incubated with or without anti-IL-10 receptor and isotype control antibodies separately for 1 hr. T cells were then cultured with γ-irradiated DCs in 96-well round-bottomed plates. After 3 days of culture, cells were pulsed with 1 μCi/well of [3H]thymidine for 16–18 hr. Specific incorporation of [3H]thymidine was determined by a β-counter, and results are expressed as counts/min. Results for all panels are expressed as the mean ± SEM of three independent experiments. *P<0·05; **P<0·01; and ***P<0·001 compared with the medium-treated DCs. #P<0·05 and ###P<0·001 compared with LPS-stimulated DCs.
High-dose GF2 treatment inhibits allergen-specific immunoglobulin production
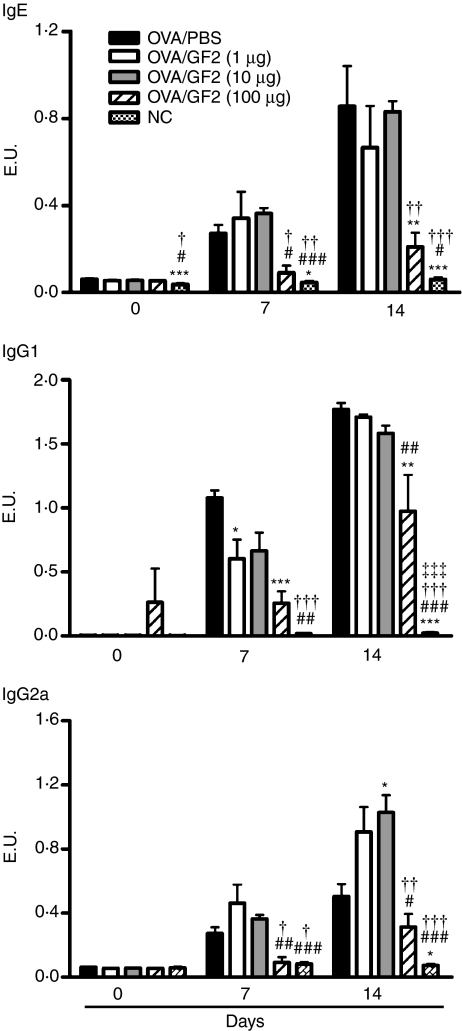
To investigate the in vivo impact of GF2 as a vaccine adjuvant for modulating the immune response of allergic diseases, groups of mice were intraperitoneally immunized with OVA plus alum on days 0 and 10, and different doses (1, 10 and 100 μg per mouse) of GF2 were also intraperitoneally injected into the mice for 3 consecutive days (days − 1 to + 1 and days 9 to 11) for each immunization. Serum samples were collected on days 0, 7 and 14. In mice, Th2 cytokine IL-4 promotes IgG1 and IgE production. However, Th1 cytokine IFN-γ induces the production of IgG2a. Therefore, the OVA-specific IgE, IgG1 and IgG2a serum antibody titres were investigated by ELISA. In the positive control mice, which received PBS treatment instead of GF2 and were sensitized with OVA, both strong IgE and IgG1 production and low IgG2a expression were induced (Fig. 4). Although the administration of GF2 at doses of 1 and 10 μg/animal did not down-regulate IgE or IgG1 production, the IgG2a synthesis was significantly up-regulated after two cycles of GF2 treatment. Notably, a high dose (100 μg) of GF2 not only efficiently inhibited OVA-specific IgE and IgG1 production, but also slightly suppressed OVA-specific IgG2a secretion.
Figure 4.
Serum anti-ovalbumin (OVA) immunoglobulin E (IgE), IgG1, and IgG2a antibody responses in GF2-treated mice. BALB/c mice were immunized twice with 50 μg/mouse OVA adsorbed on 2 mg alum on days 0 and 10. To examine the effects of GF2, three groups of mice were additionally treated with different doses (1, 10 and 100 μg/mouse) of GF2 for 3 consecutive days (days − 1 to + 1 and days 9 to 11) simultaneously with each systemic immunization. Positive control mice received phosphate-buffered saline (PBS) instead of GF2. Subsequently, sensitized mice were given aerosolized OVA three times on days 14, 15 and 16. The negative control mice were neither sensitized with OVA nor given GF2 treatment. Blood was collected on the days indicated and serum levels of anti-OVA antibodies IgE, IgG1 and IgG2a were measured by enzyme-linked immunosorbent assay. Results are expressed as the mean ± SEM of five to eight mice per group. Experiments were repeated twice with similar results. *P<0·05; **P<0·01; and ***P<0·001 compared with the control group (OVA/PBS). #P<0·05; ##P<0·01; and ###P<0·001 compared with the OVA/GF2 (1 μg) group. †P< 0·05; ††P<0·01; and †††P<0·001 compared with the OVA/GF2 (10 μg) group. ‡‡‡P<0·001 compared with the OVA/GF2 (100 μg) group.
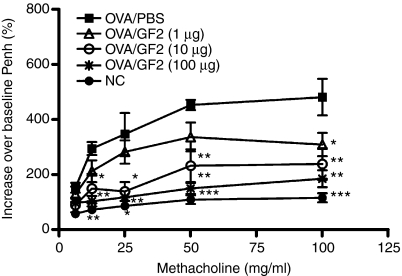
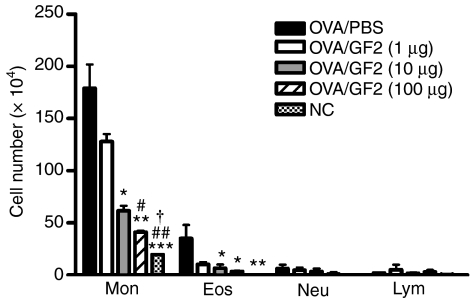
GF2 treatment ameliorates AHR and eosinophilic inflammation
To assess the protective effects of GF2 against allergic asthma, both the AHR and the accumulation of inflammatory cells in BALF were measured. On days 0 and 10, all groups of mice were immunized intraperitoneally with OVA emulsified in alum. Then the mice were exposed to OVA aerosols for 3 consecutive days (days 14–16). One day after the last OVA challenge, the airway responsiveness to aerosolized methacholine of each mouse group was measured. Positive control mice, treated with PBS instead of GF2, developed markedly increased airway responsiveness to methacholine inhalation compared with the non-allergic negative control group (Fig. 5). However, GF2 treatment dose-dependently alleviated the development of AHR compared with the positive control group. Furthermore, in positive control mice, exposure to aerosolized OVA induced a marked increase in the cell numbers of eosinophils in the BALF (Fig. 6). In contrast, mice treated with GF2 demonstrated significantly reduced increases in eosinophils.
Figure 5.
Treatment with GF2 inhibited airway hyperresponsiveness to aerosolized methacholine in ovalbumin (OVA) -sensitized and OVA-challenged mice. On days 0 and 10, all groups of mice were immunized intraperitoneally with 50 μg OVA emulsified in 2 mg alum. Then mice were exposed to OVA inhalation challenge for 3 consecutive days (days 14–16). The negative control mice were not sensitized with OVA but received OVA challenge. One day after the last OVA challenge, airway hyperresponsiveness was measured in response to increasing concentrations of methacholine (0–100 mg/ml) in conscious mice placed in a whole-body plethysmograph. Data are reported as the per cent increase in the mean enhanced pause (Penh) over baseline values. Results are expressed as the mean ± SEM of five to seven mice in each group. Experiments were repeated twice with similar results. *P<0·05; **P<0·01; and ***P<0·001 compared with the control group (OVA/phosphate-buffered saline).
Figure 6.
Changes in the cellular composition of bronchoalveolar lavage fluid (BALF) of mice exposed to an aerosolized allergen. One day after measuring the pulmonary function parameters, each group of mice was killed, and BALF was collected. Cells were counted and classified as monocytes (Mon), eosinophils (Eos), neutrophils (Neu) and lymphocytes (Lym). Results are expressed as the mean ± SEM of five to seven mice in each group. Experiments were repeated twice with similar results. *P<0·05; **P<0·01; and ***P<0·001 compared with the control group (OVA/phosphate-buffered saline). #P<0·05 and ##P<0·01 compared with the OVA/GF2 (1 μg) group. †P<0·05 compared with the OVA/GF2 (10 μg) group.
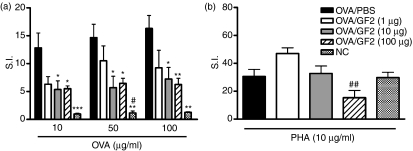
GF2 treatment reduces the allergen-specific T-cell proliferative response
To determine the proliferation of CD4+ T cells in response to an allergen, we cultured spleen CD4+ T cells with OVA. Results showed that OVA-specific CD4+ T cells from mice treated with 10 and 100 μg GF2 had significantly lower proliferation levels compared with positive control mice (Fig. 7a). Additionally, we tested PHA-induced CD4+ T-cell proliferation and observed that treatment with 100 μg GF2 slightly inhibited the proliferative capacity of CD4+ T cells, although the difference was not significant compared with positive control mice (Fig. 7b). In contrast, the 1- and 10-μg doses did not suppress the proliferative response of CD4+ T cells.
Figure 7.
Treatment with GF2 reduced the allergen-specific T-cell proliferative response in ovalbumin (OVA) -sensitized and OVA-challenged mice. One day after measuring the pulmonary function parameters, each group of mice was killed, and 3 × 105 spleen cells/well were stimulated with (a) OVA (10, 50 and 100 μg/ml) or (b) phytohaemagglutinin (PHA) (10 μg/ml) in vitro. After 3–5 days of culture, cell proliferation was estimated by methyl thiazolyl tetrazolium assay. The stimulation index (S.I.) was calculated as the OD590 absorbent value of the stimulated wells divided by the OD590 absorbent value of untreated control wells. Results are expressed as the mean ± SEM of five to seven mice in each group. Experiments were repeated twice with similar results. *P<0·05; **P<0·01; and ***P<0·001 compared with the control group (OVA/phosphate-buffered saline). #P<0·05 and ##P<0·01 compared with the OVA/GF2 (1 μg) group.
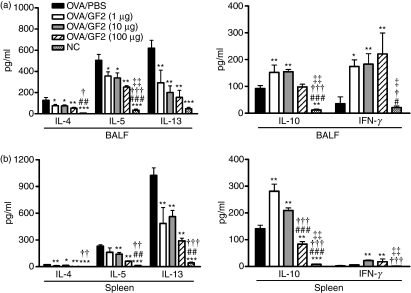
GF2 treatment suppresses the production of Th2 cytokines in BALF and the spleen
Levels of the important Th2 cytokines, IL-4, IL-5 and IL-13, in BALF and the spleen derived from each group were measured by ELISA. As shown in Fig. 8(a), levels of these Th2 cytokines in BALF significantly declined in GF2-treated mice, compared with positive control mice. Similarly, levels of Th2 cytokines in the supernatant of the splenocyte culture also strongly decreased in GF2-treated mice (Fig. 8b).
Figure 8.
Cytokine levels in GF2-treated mice. (a) Forty-eight hours after the last aerosol exposure, bronchoalveolar lavage fluid (BALF) was collected and analysed for interleukin-4 (IL-4), IL-5, IL-13, IL-10 and interferon-γ (IFN-γ) contents by enzyme-linked immunosorbent assay (ELISA). (b) Splenocytes (3 × 106 cells/ml) from GF2-treated and control groups were stimulated with 100 μg/ml ovalbumin (OVA) in 24-well plates, and culture supernatants were collected after 48 hr. Levels of cytokine production of IL-4, IL-5, IL-13, IL-10 and IFN-γ were analysed by ELISA. Results are expressed as the mean ± SEM of five to seven mice per group. Experiments were repeated twice with similar results. *P<0·05; **P<0·01; and ***P<0·001 compared with the control group (OVA/phosphate-buffered saline). #P<0·05; ##P<0·01; and ###P<0·001 compared with the OVA/GF2 (1 μg) group. †P<0·05; ††P<0·01; and †††P<0·001 compared with the OVA/GF2 (10 μg) group. ‡P<0·05 and ‡‡P<0·01 compared with the OVA/GF2 (100 μg) group.
By studying IL-10 and IFN-γ concentrations in the BALF and cell culture supernatant from the spleen, we determined whether it was possible to affect the immune response through inducing IL-10-producing or IFN-γ-producing T cells. Results showed that 1 and 10 μg GF2 treatment markedly up-regulated IL-10 production in BALF and splenocytes (Fig. 8a,b). However, mice treated with 100 μg GF2 displayed significantly inhibited expression of IL-10 in the spleen compared with positive control mice. Furthermore, the data demonstrated that treatment with all doses of GF2 enhanced a moderate level of IFN-γ production. Taken together, these findings suggest that GF2 treatment might induce protective immunity against asthma through induction of IL-10 production or Th2 skewing.
Discussion
At present, a variety of different mushroom species have been investigated, and several major substances with immunomodulatory and anti-tumour activity have been isolated from them. Previous reports indicated that most polysaccharides isolated from mushrooms induced a Th1-dominant state via induction of tumour necrosis factor-α (TNF-α), IFN-γ or IL-12.15–17 For instance, a recent study reported that A. camphorata polysaccharides are potential inducers of Th1-type cytokines such as TNF-α and IFN-γ, but not of Th2 cytokines.18 Also, A. camphorata polysaccharides inhibit inflammation by modulating pro-inflammatory cytokines.19 Current research on the immune modulation exerted by mushrooms has gone beyond the mechanisms involved in anti-tumour or anti-inflammatory activities and has ventured into their potential application in other diseases and clinical states in which modulation of certain immune parameters may be beneficial.
In recent years some researchers have focused on studying the effects of mushroom compounds on DCs.20 A protein-bound polysaccharide K (PSK) isolated from the cultured mycelium of Coriolus versicolor has been reported to promote both phenotypic and functional maturation of DCs derived from human CD14+ mononuclear cells.21 Chan et al.22 reported that crude Ganoderma lucidum spore polysaccharide (GL-S) -treated DCs suppressed T-cell proliferation and it was associated with an increased IL-10 production of the GL-S-treated DC : T-cell co-culture supernatant. By administering GF2, a protein-bound polysaccharide compound isolated from A. camphorata, we demonstrated that the production of IL-10 and IL-12 was increased in LPS-stimulated DCs in vitro. Furthermore, GF2-treated DCs caused the inhibition of T-cell proliferation by inducing IL-10 production in T cells. Therefore, we speculate that GF2 may be useful in preventing allergen-induced asthmatic disease because of its potential immunotolerant properties.
Regarding the current study, our results clearly demonstrated that multiple intraperitoneal injections of GF2 in allergen-sensitized and allergen-challenged mice strongly alleviated the severity of AHR, reduced allergen-induced eosinophilic airway inflammation, and decreased the accumulation of pro-inflammatory Th2 cytokines in the airway and spleen. How GF2 carries out its ‘regulatory’ role is not fully understood. Among the possible explanations for its negative regulatory activities is that GF2 treatment might influence DC function. Although these LPS-activated DCs with GF2 stimulation expressed high levels of MHC class II and co-stimulatory molecules and produced high levels of IL-10 and IL-12 cytokines, they are impaired in their capacity to induce T-cell responses of naïve CD4+ T cells in an allogeneic mixed lymphocyte reaction. Tolerogenic DCs have been reported to induce antigen-specific anergy in several studies.23–26 As a result, it is possible that GF2 converts OVA-pulsed DCs into tolerogenic antigen-presenting cells and might induce anergy in these antigen-specific T cells.
When the capacity of DCs to activate T cells is impaired, either as a result of incomplete maturation or the influence of specific inhibitory cytokines (e.g. IL-10), they can suppress T-cell immune reactivity. The immature developmental stages of DC differentiation are believed to induce T-cell anergy, whereas mature DCs are thought to induce strong primary T-cell responses. However, this bimodal concept of immature versus mature DCs has been challenged by studies demonstrating that a human monocyte-derived DC subset (CD123+) expressing the tryptophan-catabolizing enzyme, indoleamine 2,3 dioxygenase (IDO), inhibited T-cell proliferation in vitro.27 The IDO-mediated suppressor activity was present in both immature and mature CD123+ DCs. Some studies indicated that mature but tolerogenic DCs can be defined as being mature based on the high expression levels of MHC class II and co-stimulatory molecules; however, they produce low levels of pro-inflammatory cytokines such as IL-6, TNF-α and IL-12, and are referred to as ‘semi-mature’ DCs.28,29 Hence, based on our limited data, there are still more details requiring further clarification, such as whether GF2-treated DCs develop into the mature or semi-mature form in the presence of an antigen, how they function, and what conditions allow for the generation and use of GF2-treated DCs with defined characteristics.
A second explanation for the inhibitory effects of GF2 might be through combined GF2-activated and OVA-activated DCs inducing the expression and functional activation of regulatory T cells (Tregs). The Tregs involved in regulating allergy and asthma consist of a family of related types of T cells, including naturally occurring CD4+ CD25+ Foxp3+ Tregs as well as an inducible form of antigen-specific Tregs, such as T-regulatory 1 (Tr1) cells and Th3.30–32 These cells can suppress the proliferation of naïve and memory CD4+ and CD8+ T cells through direct cell-to-cell contact, as well as by secreting IL-10 and TGF-β. Indeed, Yamazakiet al. describe how bone marrow-derived, antigen-bearing mature DCs can expand CD4+ CD25+ Tregs both in vitro and in vivo.33 Further data from mouse experiments indicate that obviously ‘mature’ DCs induce CD4+ IL-10+ Tregs in vivo.34,35 For our study, both local (BALF) and systemic (spleen) levels of IL-10 in mice treated with 1 and 10 μg GF2 were significantly higher than in control mice. Therefore, it is possible that IL-10 may be produced by inducible Tr1 cells in these GF2-treated mice to suppress the immune response of allergen-specific Th2-cell populations and directly inhibit allergic airway inflammation.
Notably, we observed that mice treated with a high (100 μg) dose of GF2 reduced OVA-specific antibody secretion (IgG1, IgE and IgG2a) and cytokine production (IL-4, IL-5, IL-10 and IL-13). Such treatment further led to poor proliferative ability of CD4+ T cells in response to either OVA or PHA stimulation. These data indicate that high-doses of GF2 treatment might strongly inhibit both antigen-specific and non-antigen-specific T-cell immune responses via the induction of Tregs, which are known to exert suppressive activity in a non-antigen-specific manner. This means an excessive amount of a pan-immunosuppression may occur, yielding potentially dangerous conditions for the generation of these cells because an immune response against infectious pathogens might contemporarily be hampered. In this regard, GF2 must be used with great care when treating allergic disorders in the future.
We believe that controlling allergic inflammation and asthma is complex, involving several different mechanisms and several different cell types. In this study, we showed that GF2 has inductive effects on Th1 responses, including anti-OVA IgG2a and IFN-γ production in mice treated with 1 or 10 μg GF2. Recently, an inducible Th1-like Treg, which produces both IL-10 and IFN-γ, was reported to block the development of allergen-induced AHR in a murine model of asthma.36 Similarly, it was demonstrated that sublingual immunotherapy with a Dermatophagoides pteronyssinus monomeric allergoid induced clinical improvements and down-regulated allergen-specific IgE production, which is associated with a significant increase of T cells producing both IL-10 and IFN-γ.37 The same results were observed with oral probiotics, which seem to be able to alleviate the clinical symptoms of atopic dermatitis.38 Probiotics not only induce IL-10 production, but also increase the production of IFN-γ, hence supporting the concept that their preventive activities against the development of allergic reactions are mediated by a combined mechanism of immune suppression and immune deviation.
In conclusion, our findings suggest that the GF2 polysaccharide fraction of A. camphorata can be used as an adjuvant to prevent the development of allergic asthma by inducing immune tolerance. Therefore, GF2 might be a beneficial and potential partial treatment regimen to regulate host immune responses. This study provides information for the further designs of GF2-treated-DC-based immunotherapies for many diseases.
Acknowledgments
This study was supported by the National Science Council, Taiwan (NSC 95-2314-B-038-052).
Glossary
Abbreviations:
- AHR
airway hyperresponsiveness
- BALF
bronchoalveolar lavage fluid
- DC
dendritic cell
- ELISA
enzyme-linked immunosorbent assay
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- HBSS
Hanks’ balanced salt solution
- IDO
indoleamine 2,3 dioxygenase
- IFN-γ
interferon-γ
- IgG1/E
immunoglobulin G1/E
- IL-10
interleukin-10
- LPS
lipopolysaccharide
- MHC
major histocompatibility complex
- OVA
ovalbumin
- PBS
phosphate-buffered saline
- PHA
phytohaemagglutinin
- TGF-β
transforming growth factor-β
- Th2
type 2 T helper cell
- TNF-α
tumour necrosis factor-α
- Tr1
T-regulatory type 1 cells
- Treg
regulatory T cell
Disclosures
The authors have no conflicts of interests to declare.
References
- 1.Liu JJ, Huang TS, Hsu ML, Chen CC, Lin WS, Lu FJ, Chang WH. Antitumor effects of the partially purified polysaccharides from Antrodia camphorata and the mechanism of its action. Toxicol Appl Pharmacol. 2004;201:186–93. doi: 10.1016/j.taap.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 2.Lee IH, Huang RL, Chen CT, Chen HC, Hsu WC, Lu MK. Antrodia camphorata polysaccharides exhibit anti-hepatitis B virus effects. FEMS Microbiol Lett. 2002;209:63–7. doi: 10.1111/j.1574-6968.2002.tb11110.x. [DOI] [PubMed] [Google Scholar]
- 3.Wu YY, Chen CC, Chyau CC, Chung SY, Liu YW. Modulation of inflammation-related genes of polysaccharides fractionated from mycelia of medicinal basidiomycete Antrodia camphorata. Acta Pharmacol Sin. 2007;28:258–67. doi: 10.1111/j.1745-7254.2007.00500.x. [DOI] [PubMed] [Google Scholar]
- 4.Azzawi M, Bradley B, Jeffery PK, et al. Identification of activated T lymphocytes and eosinophils in bronchial biopsies in stable atopic asthma. Am Rev Respir Dis. 1990;142:1407–13. doi: 10.1164/ajrccm/142.6_Pt_1.1407. [DOI] [PubMed] [Google Scholar]
- 5.Secrist H, DeKruyff RH, Umetsu DT. Interleukin 4 production by CD4+ T cells from allergic individuals is modulated by antigen concentration and antigen-presenting cell type. J Exp Med. 1995;181:1081–9. doi: 10.1084/jem.181.3.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Del Prete G. Human Th1 and Th2 lymphocytes: their role in the pathophysiology of atopy. Allergy. 1992;47:450–5. doi: 10.1111/j.1398-9995.1992.tb00662.x. [DOI] [PubMed] [Google Scholar]
- 7.Factor P. Gene therapy for asthma. Mol Ther. 2003;7:148–52. doi: 10.1016/s1525-0016(03)00003-0. [DOI] [PubMed] [Google Scholar]
- 8.Eum SY, Haile S, Lefort J, Huerre M, Vargaftig BB. Eosinophil recruitment into the respiratory epithelium following antigenic challenge in hyper-IgE mice is accompanied by interleukin 5-dependent bronchial hyperresponsiveness. Proc Natl Acad Sci USA. 1995;92:12290–4. doi: 10.1073/pnas.92.26.12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 10.Pulendran B, Banchereau J, Maraskovsky E, Maliszewski C. Modulating the immune response with dendritic cells and their growth factors. Trends Immunol. 2001;22:41–7. doi: 10.1016/s1471-4906(00)01794-4. [DOI] [PubMed] [Google Scholar]
- 11.Fu CL, Chuang YH, Huang HY, Chiang BL. Induction of IL-10 producing CD4+ T cells with regulatory activities by stimulation with IL-10 gene-modified bone marrow derived dendritic cells. Clin Exp Immunol. 2008;153:258–68. doi: 10.1111/j.1365-2249.2008.03689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee Y, Fu C, Chiang B. Administration of interleukin-12 exerts a therapeutic instead of a long-term preventive effect on mite Der p I allergen-induced animal model of airway inflammation. Immunology. 1999;97:232–40. doi: 10.1046/j.1365-2567.1999.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiang DJ, Ye YL, Chen WL, Lee YL, Hsu NY, Chiang BL. Ribavirin or CpG DNA sequence-modulated dendritic cells decrease the IgE level and airway inflammation. Am J Respir Crit Care Med. 2003;168:575–80. doi: 10.1164/rccm.2205005. [DOI] [PubMed] [Google Scholar]
- 14.Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol. 1997;9:10–6. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- 15.Kodama N, Komuta K, Sakai N, Nanba H. Effects of D-fraction, a polysaccharide from Grifola frondosa on tumor growth involves activation of NK cells. Biol Pharm Bull. 2002;25:1647–50. doi: 10.1248/bpb.25.1647. [DOI] [PubMed] [Google Scholar]
- 16.Borchers AT, Keen CL, Gershwin ME. Mushrooms, tumors, and immunity: an update. Exp Biol Med. 2004;229:393–406. doi: 10.1177/153537020422900507. [DOI] [PubMed] [Google Scholar]
- 17.Wasser SP. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl Microbiol Biotechnol. 2002;60:258–74. doi: 10.1007/s00253-002-1076-7. [DOI] [PubMed] [Google Scholar]
- 18.Chen YJ, Cheng PC, Lin CN, Liao HF, Chen YY, Chen CC, Lee KM. Polysaccharides from Antrodia camphorata mycelia extracts possess immunomodulatory activity and inhibit infection of Schistosoma mansoni. Int Immunopharmacol. 2008;8:458–67. doi: 10.1016/j.intimp.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Shen YC, Chou CJ, Wang YH, Chen CF, Chou YC, Lu MK. Anti-inflammatory activity of the extracts from mycelia of Antrodia camphorata cultured with water-soluble fractions from five different Cinnamomum species. FEMS Microbiol Lett. 2004;231:137–43. doi: 10.1016/S0378-1097(03)00953-4. [DOI] [PubMed] [Google Scholar]
- 20.Lull C, Wichers HJ, Savelkoul HF. Antiinflammatory and immunomodulating properties of fungal metabolites. Mediators Inflamm. 2005;2:63–80. doi: 10.1155/MI.2005.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanazawa M, Mori Y, Yoshihara K, et al. Effect of PSK on the maturation of dendritic cells derived from human peripheral blood monocytes. Immunol Lett. 2004;91:229–38. doi: 10.1016/j.imlet.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Chan WK, Law HK, Lin ZB, Lau YL, Chan GC. Response of human dendritic cells to different immunomodulatory polysaccharides derived from mushroom and barley. Int Immunol. 2007;19:891–9. doi: 10.1093/intimm/dxm061. [DOI] [PubMed] [Google Scholar]
- 23.Enk AH. Dendritic cells in tolerance induction. Immunol Lett. 2005;99:8–11. doi: 10.1016/j.imlet.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Morel PA, Feili-Hariri M, Coates PT, Thomson AW. Dendritic cells, T cell tolerance and therapy of adverse immune reactions. Clin Exp Immunol. 2003;133:1–10. doi: 10.1046/j.1365-2249.2003.02161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haase C, Jorgensen TN, Michelsen BK. Both exogenous and endogenous interleukin-10 affects the maturation of bone-marrow-derived dendritic cells in vitro and strongly influences T-cell priming in vivo. Immunology. 2002;107:489–99. doi: 10.1046/j.1365-2567.2002.01529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bellinghausen I, Brand U, Steinbrink K, Enk AH, Knop J, Saloga J. Inhibition of human allergic T-cell responses by IL-10-treated dendritic cells: differences from hydrocortisone-treated dendritic cells. J Allergy Clin Immunol. 2001;108:242–9. doi: 10.1067/mai.2001.117177. [DOI] [PubMed] [Google Scholar]
- 27.Munn DH, Sharma MD, Lee JR, et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297:1867–70. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 28.Menges M, Rossner S, Voigtlander C, et al. Repetitive injections of dendritic cells matured with TNF-α induce antigen-specific protection of mice from autoimmunity. J Exp Med. 2002;195:15–21. doi: 10.1084/jem.20011341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–9. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 30.Fukaura H, Kent SC, Pietrusewicz MJ, Khoury SJ, Weiner HL, Hafler DA. Induction of circulating myelin basic protein and proteolipid protein-specific TGF-β-secreting Th3 T cells by oral administration of myelin in multiple sclerosis patients. J Clin Invest. 1996;98:70–7. doi: 10.1172/JCI118779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–40. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 32.Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–42. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 33.Yamazaki S, Iyoda T, Tarbell K, Olson K, Velinzon K, Inaba K, Steinman RM. Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen-processing dendritic cells. J Exp Med. 2003;198:235–47. doi: 10.1084/jem.20030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol. 2001;2:725–31. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- 35.McGuirk P, McCann C, Mills KH. Pathogen-specific T regulatory 1 cells induced in the respiratory tract by a bacterial molecule that stimulates interleukin 10 production by dendritic cells: a novel strategy for evasion of protective T helper type 1 responses by Bordetella pertussis. J Exp Med. 2002;195:221–31. doi: 10.1084/jem.20011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stock P, Akbari O, Berry G, Freeman GJ, Dekruyff RH, Umetsu DT. Induction of T helper type 1-like regulatory cells that express Foxp3 and protect against airway hyper-reactivity. Nat Immunol. 2004;5:1149–56. doi: 10.1038/ni1122. [DOI] [PubMed] [Google Scholar]
- 37.Cosmi L, Santarlasci V, Angeli R, et al. Sublingual immunotherapy with Dermatophagoides monomeric allergoid down-regulates allergen-specific immunoglobulin E and increases both interferon-γ- and interleukin-10-production. Clin Exp Allergy. 2006;36:261–72. doi: 10.1111/j.1365-2222.2006.02429.x. [DOI] [PubMed] [Google Scholar]
- 38.Kalliomaki M, Salminen S, Poussa T, Arvilommi H, Isolauri E. Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. Lancet. 2003;361:1869–71. doi: 10.1016/S0140-6736(03)13490-3. [DOI] [PubMed] [Google Scholar]