Abstract
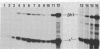
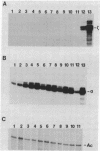
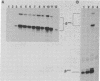
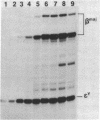
B6SUtA is a factor-dependent murine cell line of adult origin displaying the functional properties of a multipotent hematopoietic stem cell. We analyzed the globin programs of B6SUtA cells undergoing erythroid differentiation in both suspension and clonal cultures. In the absence of added erythropoietin, a small number of hemoglobinized cells were present, and these expressed predominantly embryonic globin. Addition of erythropoietin increased the number and maturation of hemoglobinized cells and led to a preferential augmentation of adult globin. Analysis of individual B6SUtA erythroid bursts showed that embryonic and adult globin can be expressed in cells derived from a single progenitor. Furthermore, by studying globin expression in cultured cells from mouse embryos, we found that the globin programs of B6SUtA cells are similar to those of erythroid progenitors at the period of transition from yolk sac to fetal liver erythropoiesis. Since B6SUtA cells are derived from adult bone marrow and they have the capacity to express embryonic globin, we speculate that the globin locus is not irreversibly modified during development and that adult cells at early stages of erythroid differentiation can transiently express ontogenetically primitive globin programs.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson J. W., Stamatoyannopoulos G. Activation of hemoglobin C synthesis in sheep marrow culture. Science. 1973 Apr 20;180(4083):310–312. doi: 10.1126/science.180.4083.310. [DOI] [PubMed] [Google Scholar]
- Al-Khatti A., Veith R. W., Papayannopoulou T., Fritsch E. F., Goldwasser E., Stamatoyannopoulos G. Stimulation of fetal hemoglobin synthesis by erythropoietin in baboons. N Engl J Med. 1987 Aug 13;317(7):415–420. doi: 10.1056/NEJM198708133170704. [DOI] [PubMed] [Google Scholar]
- Baron M. H., Maniatis T. Rapid reprogramming of globin gene expression in transient heterokaryons. Cell. 1986 Aug 15;46(4):591–602. doi: 10.1016/0092-8674(86)90885-8. [DOI] [PubMed] [Google Scholar]
- Boussios T., Bertles J. F. The globin gene expression program in the hamster embryo. Exp Hematol. 1988 Jan;16(1):1–4. [PubMed] [Google Scholar]
- Brotherton T. W., Chui D. H., Gauldie J., Patterson M. Hemoglobin ontogeny during normal mouse fetal development. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2853–2857. doi: 10.1073/pnas.76.6.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Chada K., Magram J., Costantini F. An embryonic pattern of expression of a human fetal globin gene in transgenic mice. Nature. 1986 Feb 20;319(6055):685–689. doi: 10.1038/319685a0. [DOI] [PubMed] [Google Scholar]
- Chui D. H., Wong S. C., Enkin M. W., Patterson M., Ives R. A. Proportion of fetal hemoglobin synthesis decreases during erythroid cell maturation. Proc Natl Acad Sci U S A. 1980 May;77(5):2757–2761. doi: 10.1073/pnas.77.5.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagg B. Is erythropoietin the only factor which regulates late erythroid differentiation? Nature. 1981 Jan 15;289(5794):184–186. doi: 10.1038/289184a0. [DOI] [PubMed] [Google Scholar]
- Goodman J. W., Hall E. A., Miller K. L., Shinpock S. G. Interleukin 3 promotes erythroid burst formation in "serum-free" cultures without detectable erythropoietin. Proc Natl Acad Sci U S A. 1985 May;82(10):3291–3295. doi: 10.1073/pnas.82.10.3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves M. F., Chan L. C., Furley A. J., Watt S. M., Molgaard H. V. Lineage promiscuity in hemopoietic differentiation and leukemia. Blood. 1986 Jan;67(1):1–11. [PubMed] [Google Scholar]
- Greenberger J. S., Sakakeeny M. A., Humphries R. K., Eaves C. J., Eckner R. J. Demonstration of permanent factor-dependent multipotential (erythroid/neutrophil/basophil) hematopoietic progenitor cell lines. Proc Natl Acad Sci U S A. 1983 May;80(10):2931–2935. doi: 10.1073/pnas.80.10.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K., Suda T., Suda J., Eguchi M., Ihle J. N., Nagata S., Miura Y., Saito M. Bipotential murine hemopoietic cell line (NFS-60) that is responsive to IL-3, GM-CSF, G-CSF, and erythropoietin. Exp Hematol. 1988 May;16(4):256–261. [PubMed] [Google Scholar]
- Kurtz A., Härtl W., Jelkmann W., Zapf J., Bauer C. Activity in fetal bovine serum that stimulates erythroid colony formation in fetal mouse livers is insulinlike growth factor I. J Clin Invest. 1985 Oct;76(4):1643–1648. doi: 10.1172/JCI112149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macklis R. M., Javid J., Lipton J. M., Kudisch M., Pettis P. K., Nathan D. G. Synthesis of hemoglobin F in adult simian erythroid progenitor-derived colonies. J Clin Invest. 1982 Oct;70(4):752–761. doi: 10.1172/JCI110671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magram J., Chada K., Costantini F. Developmental regulation of a cloned adult beta-globin gene in transgenic mice. Nature. 1985 May 23;315(6017):338–340. doi: 10.1038/315338a0. [DOI] [PubMed] [Google Scholar]
- Monette F. C., Sigounas G. Growth of murine multipotent stem cells in a simple "serum-free" culture system: role of interleukin-3, erythropoietin, and hemin. Exp Hematol. 1988 May;16(4):250–255. [PubMed] [Google Scholar]
- Ogawa M., Porter P. N., Nakahata T. Renewal and commitment to differentiation of hemopoietic stem cells (an interpretive review). Blood. 1983 May;61(5):823–829. [PubMed] [Google Scholar]
- Orkin S. H., Harosi F. I., Leder P. Differentiation in erythroleukemic cells and their somatic hybrids. Proc Natl Acad Sci U S A. 1975 Jan;72(1):98–102. doi: 10.1073/pnas.72.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T., Kalmantis T., Stamatoyannopoulos G. Cellular regulation of hemoglobin switching: evidence for inverse relationship between fetal hemoglobin synthesis and degree of maturity of human erythroid cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6420–6424. doi: 10.1073/pnas.76.12.6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righetti P. G., Gianazza E., Gianni A. M., Comi P., Giglioni B., Ottolenghi S., Secchi C., Rossi-Bernardi L. Human globin chain separation by isoelectric focusing. J Biochem Biophys Methods. 1979;1(1):45–57. doi: 10.1016/0165-022x(79)90045-9. [DOI] [PubMed] [Google Scholar]
- Spooncer E., Heyworth C. M., Dunn A., Dexter T. M. Self-renewal and differentiation of interleukin-3-dependent multipotent stem cells are modulated by stromal cells and serum factors. Differentiation. 1986;31(2):111–118. doi: 10.1111/j.1432-0436.1986.tb00391.x. [DOI] [PubMed] [Google Scholar]
- Suda J., Suda T., Kubota K., Ihle J. N., Saito M., Miura Y. Purified interleukin-3 and erythropoietin support the terminal differentiation of hemopoietic progenitors in serum-free culture. Blood. 1986 Apr;67(4):1002–1006. [PubMed] [Google Scholar]
- TILL J. E., MCCULLOCH E. A., SIMINOVITCH L. A STOCHASTIC MODEL OF STEM CELL PROLIFERATION, BASED ON THE GROWTH OF SPLEEN COLONY-FORMING CELLS. Proc Natl Acad Sci U S A. 1964 Jan;51:29–36. doi: 10.1073/pnas.51.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrealba de Ron A., Papayannopoulou T., Stamatoyannopoulos G. Studies of Hb F in adult nonanemic baboons: Hb F expression in erythroid colonies decreases as the level of maturation of erythroid progenitors advances. Exp Hematol. 1985 Oct;13(9):919–925. [PubMed] [Google Scholar]
- Tsapis A., Hinard N., Testa U., Dubart A., Vainchenker W., Rouyer-Fessard P., Beuzard Y., Rosa J. Globin-chain affinity chromatography on Sepharose-haptoglobin: a new method of study of hemoglobin synthesis in reticulocytes, in bone marrow and in colonies of erythroid precursors. Eur J Biochem. 1980 Dec;112(3):513–519. doi: 10.1111/j.1432-1033.1980.tb06114.x. [DOI] [PubMed] [Google Scholar]
- Valtieri M., Tweardy D. J., Caracciolo D., Johnson K., Mavilio F., Altmann S., Santoli D., Rovera G. Cytokine-dependent granulocytic differentiation. Regulation of proliferative and differentiative responses in a murine progenitor cell line. J Immunol. 1987 Jun 1;138(11):3829–3835. [PubMed] [Google Scholar]
- Wawrzyniak C. J., Popp R. A. Expression of the two adult beta-globin genes in mouse yolk sac and fetal liver erythrocytes. Dev Biol. 1987 Jan;119(1):299–301. doi: 10.1016/0012-1606(87)90231-4. [DOI] [PubMed] [Google Scholar]
- Wendling F., Shreeve M., McLeod D., Axelrad A. A self-renewing, bipotential erythroid/mast cell progenitor in continuous cultures of normal murine bone marrow. J Cell Physiol. 1985 Oct;125(1):10–18. doi: 10.1002/jcp.1041250103. [DOI] [PubMed] [Google Scholar]
- Wong P. M., Chung S. W., Chui D. H., Eaves C. J. Properties of the earliest clonogenic hemopoietic precursors to appear in the developing murine yolk sac. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3851–3854. doi: 10.1073/pnas.83.11.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. M., Chung S. W., Reicheld S. M., Chui D. H. Hemoglobin switching during murine embryonic development: evidence for two populations of embryonic erythropoietic progenitor cells. Blood. 1986 Mar;67(3):716–721. [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]