Abstract
Study Objectives
The purpose of this study was to determine if asthma with and without rhinitis represent distinct forms of disease.
Design
A prospective cross-sectional study.
Participants
Healthy controls, participants with asthma without rhinitis, and participants with both asthma and rhinitis
Interventions
We compared lung function and airway inflammation between the three groups of participants.
Results
We recruited 32 participants: 12 normals, 8 asthmatics without, and 12 with rhinitis. Compared to asthmatics with rhinitis, asthmatics without rhinitis had more severe airflow limitation (FEV1/FVC 60.6 [IQR 22.8] versus 74.8 % [IQR 7.8] and fewer induced sputum eosinophils (2.8 [IQR 5.8] and 9.6 [IQR 23.8], respectively). Sputum interleukin 6 correlated inversely with lung function measured by post-bronchodilator FEV1 in the study cohort (Spearman correlation coefficient −0.55, p<0.01).
Conclusions
Asthmatics without rhinitis tend to have lower lung function and less eosinophilic inflammation in the lung. This study suggests that asthmatics without rhinitis represent a distinct phenotype of asthma in which low lung function is dissociated from eosinophilic cellular inflammation.
Keywords: rhinitis, asthma pathogenesis, exhaled nitric oxide, interleukin-6, lung function tests, eosinophilic airway inflammation
Introduction
Rhinitis and sinusitis are very common co-morbidities in asthma. Data from the European Community Respiratory Health Survey show that rhinitis is reported in 74–81% of asthmatics[1]. Asthma and rhinitis are likely part of the same disease process, with common inflammatory pathways implicated in the pathogenesis of both conditions. This has led to the concept of a common disease affecting a unified airway[2].
However, not all asthmatic patients have rhinitis (or sinusitis). In a retrospective study, we previously reported that asthmatics without rhinitis and sinusitis have lower lung function than asthmatics with rhinitis and sinusitis[3], others have also shown that asthmatics without rhinitis tend to have lower lung function, but may have increased symptoms, particularly cough[4]. Taken together, this suggests that asthmatics with and without rhinitis may represent distinct phenotypes of asthma.
The purpose of this study was to determine if asthmatic patients with and without rhinitis had distinct features of inflammation in the lower airway, as this would be further evidence that they represent two distinct entities, and that these patients may require different treatment options. This was a prospective cross-sectional study of three groups of participants: normal control participants, asthmatic participants without rhinitis and asthmatics with rhinitis. We compared symptoms, lung function and inflammation, as assessed by induced sputum, exhaled nitric oxide and nasal lavage, in the three groups of participants.
Materials and Methods
Participants
The study was approved by the local Institutional Review Board and informed consent was obtained from all participants. We enrolled three groups of participants: normal controls, asthmatics without rhinitis and asthmatics with perennial rhinitis. Participants were recruited from an outpatient pulmonary clinic and local advertising. All participants were age 18 years or older. Normal control participants had no history of asthma, no nasal symptoms and a negative methacholine challenge test. All asthmatic participants had a physician diagnosis of asthma and physiological evidence of asthma either a significant response to bronchodilator or a positive methacholine challenge tests (PC20 < 8 mg/ml to methacholine). All asthmatics had poorly controlled asthma asascertained by a score of 1.5 or higher on the Juniper Asthma Control Questionnaire [5] and asthmatic participants without rhinitis denied having any history of nasal symptoms except during upper respiratory tract infections. Asthmatic participants with rhinitis reported chronic nasal symptoms (greater that 12 weeks). We excluded any participants that had used nasal or systemic steroids in the last 6 weeks, but included participants on inhaled corticosteroids. Participants with a greater than 20 pack year smoking history, and anyone who had smoked within the last 6 months were also excluded. All testing was done over a 2 week period.
Pulmonary Function tests
Spirometry was performed according to ATS guidelines. An improvement in FEV1 and/or FVC of greater than 12% and greater than 200 ml established reversibility. If an asthmatic participant did not have evidence of bronchodilator responsiveness, they had a methacholine challenge test, and all normal participants underwent methacholine challenge test. Methacholine challenge was performed according to ATS guidelines[6].
Exhaled Nitric Oxide (NO)
Nitric Oxide was collected offline according to procedures recommended by the American Thoracic Society[7]. Participants were asked to inhale orally to total lung capacity, and then immediately perform a slow vital capacity maneuver against an expiratory resistance of 12 cm H2O into a reservoir bag without a breath-hold. The reservoir was immediately sealed, and analyzed within 24 hours by offline chemiluminescence (Sievers Instruments, Boulder, CO).
Induced Sputum
Induced sputum was performed after bronchodilation with albuterol. Sputum was collected after the inhalation of increasing concentrations of saline (3, 4 and 5% saline for 7 minutes each). Sputum was kept at 4°C, and processed within 2 hours of collection. Sputum plugs were separated from saliva within 2 hours. Dithiotreitol (DTT; Sputolysin; Calbiochem Corporation, San Diego, CA) was freshly prepared in a 1:10 dilution with phosphate buffered saline and added to the plugs in a volume (in µl) equal to four times the weight of the selected plugs (in mg). Samples were incubated on a roller at room temperature for 15 minutes, then 4 volumes of phosphate-buffered saline were added and the samples were filtered through 48-µm nylon gauze. Cytospins were prepared and stained with DiffQuik. Differential cell counts were determined by two observers masked to the participants’ identity. Slides with greater that 20% squamous epithelial cells were not included in the analysis, 500 non-squamous cells were counted on each slide.
Sputum supernatant was collected after centrifugation at 400 × g for 10 minutes and frozen at −80°C for later analysis For the simultaneous quantitation of multiple analytes, sputum supernatant was analyzed in duplicate using the Bio-Plex suspension array system (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions. Standards were diluted in 0.9% NaCl plus protease inhibitor mixture. Standard curves were calculated and samples were analyzed using the Bio-Plex Manager software (Bio-Rad).
Nasal Procedures
Participants answered standardized questionnaires assessing their nasal symptoms[8, 9].
For nasal lavage with saline five milliliters of saline (0.9%) was instilled into each nostril, after 10 seconds the lavage fluid was expelled and collected for analysis of cytokines. Nasal lavage was frozen at −80°C for later analysis using the Bioplex suspension array system as described above.
Statistical Analysis
Descriptive statistics were used to summarize the baseline demographics for the study population. The population was divided into three groups: normals, asthmatics without rhinitis and asthmatics with rhinitis. Data were compared using one way analysis of variance for normally distributed values, Kruskal Wallis rank sum test for non-normally distributed values and chi-squared test for proportions. We determined correlations between non-normally distributed variables using Spearman’s rank correlation. All analyses were performed using STATA 8.2 (StataCorp, College Station, TX).
Results
A total of 12 normal controls, 8 asthmatics without rhinitis and 12 asthmatics with rhinitis were recruited to the study (Table 1). The study population was predominantly female. Questionnaires pertaining to nasal symptoms confirmed the reliability of the categorization of our groups, as asthmatics without rhinitis and normal controls had minimal nasal symptoms as measured by validated rhinitis and sinusitis questionnaires.
Table 1.
Demographics and nasal symptoms of Study Participants1
| normal | asthma only | asthma-rhinitis | P2 | |
|---|---|---|---|---|
| n | 12 | 8 | 12 | |
| female (n) | 10 | 6 | 7 | 0.4 |
| age (mean and SD) | 32 ± 20 | 36 ± 15 | 30 ± 12 | 0.7 |
| allergy history3 (n) | 3 | 4 | 11 | 0.7 |
| age asthma onset | NA | 18 ± 16 | 10 ± 3 | 0.2 |
| ever smoked | 2 | 1 | 3 | |
| pack year smoking history (median and range) |
0 (0–10) | 0 (0–9) | 0 (0–10) | |
| Inhaled corticosteroid (n) | NA | 6 | 9 | |
| Sinonasal Characteristics | ||||
| Sinus Symptom Score4 | 0(1.65) | 1.64(3.8) | 23.5(14.5) | 0.0001 |
| SNOT-205 | 0.02(0.08) | 0.45(0.7) | 1.8(1.4) | 0.0001 |
| RQLQ - nose domain6 | 0(0) | 0.15(0.53) | 1.8(1.3 | 0.0001 |
values reported are median and interquartile range unless otherwise stated.
P value for one way analysis of variance for normally distributed values, kruskal wallis for non-normally distributed values and chi-squared test for proportions.
Proportion of participants responding “yes” to the question “Do you have any history of allergies or hay fever”
Sinus Symptom Score, seven item visual analogue scale 0-60, higher score indicative of more severe symptoms
Sino-Nasal Outcomes Test-20: 0-5, higher score indicative of more severe impairment [8]
Rhinitis Quality of Life Questionnaire scale: 0-6, higher score indicative of more severe impairment. Value shown for nose domain of RQLQ [9].
Asthmatic participants with rhinitis reported slightly earlier onset of their asthma. Other demographics variables were similar between the groups. Participants were predominantly Caucasian.
The asthmatic participants were selected to have symptomatic asthma, as we reasoned that symptomatic asthmatics would have active airway inflammation. Both the asthmatics with and without rhinitis reported similar symptoms of poorly controlled asthma with a mean Juniper Asthma Control score of greater than 2 in both groups (Table 2). Although severity of symptoms was similar in both groups, asthmatics without rhinitis tended to have lower lung function as measured by both pre- and post-bronchodilator FEV1, and had significantly worse airflow limitation as measured by the absolute ratio of FEV1/FVC (Table 2).
Table 2.
Lung Function and Asthma Symptom Scores
| normal | asthma only | asthma-rhinitis | p1 | |
|---|---|---|---|---|
| n | 12 | 8 | 12 | |
| FEV1 pre bronchodilator % predicted |
90.6(9.7) | 64.1(40.8) | 80.4(12.2) | 0.08 |
| FEV1 post bronchodilator % predicted |
100(16) | 78.1(29) | 89.3(7.3) | < 0.05 |
| FVC pre-bronchodilator % predicted |
87.3(9.8) | 86.2(29.1) | 92.4(13.3) | 0.55 |
| FEV1/FVC pre bronchodilator | 81.5(12.1) | 60.6(22.8) | 74.8(7.8) | 0.01 |
| PEFR (% predicted) | 123(30) | 96.8(50) | 110(24) | 0.21 |
| ACQ2 | 0 | 2.3(1.3) | 2.1(0.4) | 0.3 |
values reported for median and interquartile range, Kruskal Wallis for non-normally distributed values. For PC20 and ACQ, p value is reported for comparison between two asthmatic groups.
ACQ = Juniper Asthma Control Questionnaire[5]
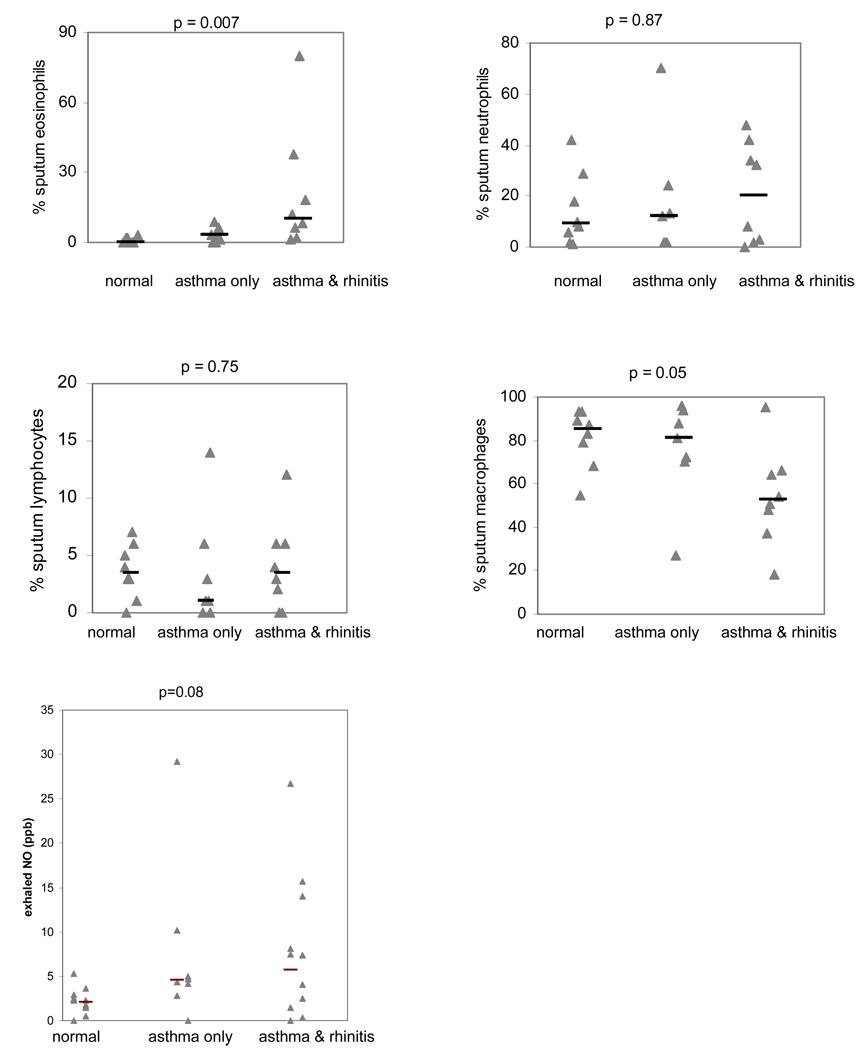
Asthmatics without rhinitis had fewer eosinophils in their induced sputum compared with asthmatics with rhinitis, though more than normal control participants (Figure 1), and this trend persisted when we excluded participants not on inhaled corticosteroids (median sputum eosinophils in asthmatics without rhinitis not on inhaled corticosteroids 2.1, IQR 4.2, those with rhinitis 7.7, IQR 16.0, p = 0.06). Mean exhaled nitric oxide levels measured by the offline method were slightly lower in the asthmatic participants without rhinitis (figure 1), and there was a significant correlation between sputum eosinophilia and exhaled nitric oxide in asthmatic participants (Spearman rank correlation coefficient = 0.52, p < 0.05).
Figure 1.
Induced sputum cell counts and exhaled nitric oxide levels in normal controls, asthmatics without rhinitis (asthma only) and asthmatics with rhinitis. Actual and median values are shown. P value reported for Kruskal Wallis test. Only data from induced sputum slides with less than 20% squamous epithelial cells are shown.
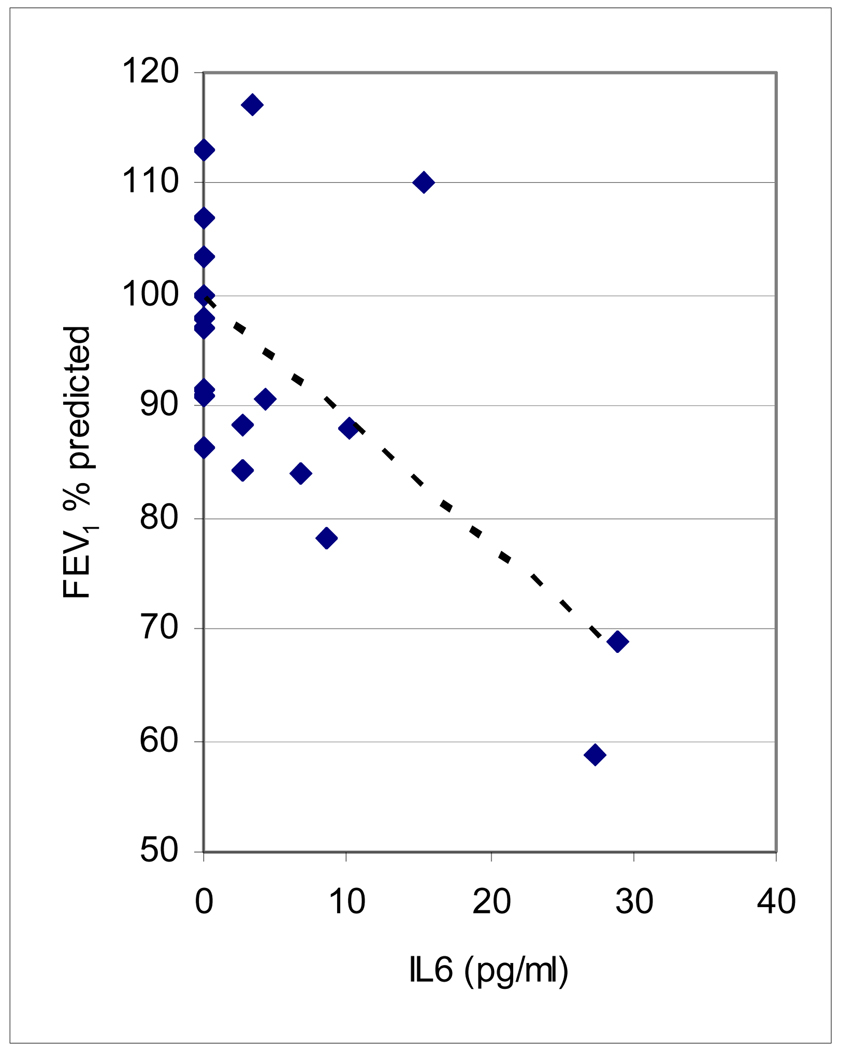
Levels of IL-4, IL-5 and TNFα in induced sputum supernatant were below the detection limit of our assay (1.8 pg/ml). Overall, we did not find significant differences in differences in sputum cytokines between the three groups (Table 3), though sputum IL-6 was inversely associated with lower levels of post-bronchodilator FEV1 (Figure 2, Spearman correlation coefficient = −0.55, p < 0.01) in the study cohort as a whole.
Table 3.
Induced Sputum Cytokine levels
| Normal | Asthma only | Asthma and rhinitis | P1 | |
|---|---|---|---|---|
| n | 8 | 5 | 7 | |
| IL-8 (pg/ml) | 499 (594) | 884(1009) | 158(1203)2 | 0.61 |
| IFN-γ (pg/ml) | 16.2(49.8) | 72.8(83.3) | 0 (62.58) | 0.22 |
| IL-6 (pg/ml) | 1.8(2.4) | 4.4(7.6) | 2.7(5.7) | 0.70 |
Values shown are for median and interquartile range Levels of IL-4, IL-5 and TNFα were below detectable limit, p value reported for Krukall Wallis test.
Figure 2.
FEV1 % predicted post bronchodilator and induced sputum IL-6 levels in study cohort
Nasal lavage cytokines showed no difference in Th2 (IL-4 and IL-5) or Th1 cytokines (TNFα and interferon-γ) between the two groups of participants with asthma. There were, however significantly lower levels of IL-8 in asthmatic participants with rhinitis compared to participants without rhinitis (Table 4).
Table 4.
Nasal Lavage Cytokine levels:
| Normal | Asthma only | Asthma and rhinitis |
p1 | |
|---|---|---|---|---|
| n | 9 | 6 | 9 | |
| IL-8 (ng/ml) | 4.7(1.5) | 4.1(2.1) | 2.4(1.9) | 0.112 |
| IL-4 (pg/ml) | 27.0(6.0) | 39.5(28.0) | 37.5(35.8) | 0.36 |
| IL-5 (pg/ml) | 4.8 (2.5) | 4.3 (2.8) | 5.0(2.0) | 0.95 |
| IFN-γ (pg/ml) | 13.8(5.5) | 10.4(2.5) | 10.5(2.5) | 0.14 |
| TNF-α (pg/ml) | 11.0(5.0) | 8.8(3.3) | 8.0 (2.5) | 0.52 |
Values shown are for median and interquartile range, p value reported for Kruskall Wallis test.
p value for comparison of participants with perennial rhinitis compared to those without rhinitis < 0.05
Discussion
This study supports the concept that asthmatics without rhinitis represent a separate phenotype of asthma with distinct features in terms of symptoms, spirometric lung function and cellular inflammatory markers in induced sputum. This prospective trial supports our previous retrospective observation that asthmatics without rhinitis tend to have lower lung function than asthmatics with rhinitis, and suggests that further investigation into the causes of loss of lung function in these patients is warranted.
Many studies have been published that support the concept that rhinitis in patients with asthma likely represents part of a disease continuum with manifestations at different anatomic sites.[2] In asthmatics with rhinitis, the inflammation in the upper and lower airway is closely linked. Bonay recently showed that nasal allergen challenge in patients with allergic rhinitis led to eosinophilia in induced sputum[10]. Conversely, segmental allergen challenge through a bronchoscope leads to increased interleukin-5 and eosinophils in the nose[11]. Allergen challenge can lead to a systemic response: inhalational challenge leads to the mobilization of cells from the bone marrow[12], so rhinitis and asthma in these patients are likely part of the same disease affecting the entire respiratory system. These elegant studies support the concept of a link between the upper and lower airway, but few studies have focused on asthmatic patients without rhinitis, which likely represents approximately 20% of the asthmatic population[2, 3].
In a retrospective study of participants in a large clinical trial, we reported that asthmatics without rhinitis had lower lung function compared to asthmatics with rhinitis[3], supporting the observations of other investigators[4]. The purpose of our current study was to determine if there were pathophysiological differences in these two groups of asthmatics.
We found distinct features of cellular inflammation in the lower airway of these two groups of asthmatics, who had similar symptoms and a similar prevalence of allergy (by history). Asthmatics without rhinitis had lower levels of eosinophils, with similar levels of neutrophils and increased levels of macrophages compared to those with rhinitis. Our results differ from that reported by Gaga et al in which they compared eosinophils in bronchial mucosa of asthmatics with and without rhinitis and reported finding no difference in those with and without rhinitis: as they only reported levels of bronchial eosinophils in 9 participants, the may have been underpowered to detect a difference between the two groups of asthmatics[13]. However, others have also reported a direct correlation between induced sputum eosinophils and sino-nasal disease: sinus CT scores correlate with induced sputum eosinophils in patients with chronic rhinosinusitis, (and this does not appear to be related to atopic status)[14, 15]. Asthma without rhinitis appears to be characterized by fewer eosinophils. Other investigators have described sub-groups of asthmatics with low levels of eosinophils in induced sputum[16, 17], they appear less likely to respond to inhaled corticosteroid therapy[18, 19]. Inferring from these previous studies, asthmatics without rhinitis may be less likely to respond to inhaled corticosteroid therapy, though this would require further investigation.
We did find a significant relationship between sputum eosinophils and exhaled nitric oxide levels, as has been reported in previous studies[21]. We did not find any correlation between lung function and cellular composition of induced sputum, though our small study was likely underpowered to detect this. However the trend that we detected, low lung function in patients with fewer eosinophils and neutrophils in induced sputum, contrasts to some recent reports which have found that induced sputum eosinophilia and/neutrophilia correlate with low lung function[22, 23]. Our study population differs from these other studies, which did not divide patients according to the presence/ absence of rhinitis, the majority of asthma patients in these previous studies likely are similar to our group of asthmatic patients with rhinitis which represent approximately 80% of the asthmatic population.
Although we did not find any differences in sputum cytokine expression between the two groups of asthmatics that would explain their different lung function, we speculate that IL-6 may be involved in the pathogenesis of low lung function in both groups of asthmatics, as when considering the entire cohort, interleukin-6 was inversely associated with post-bronchodilator FEV1. IL-6 is produced by epithelial cells, macrophages, and possibly airway smooth muscle cells, in response to a range of environmental exposures including tobacco smoke [24] and occupational hazards such as diesel exhaust[25], organic dusts[26], and other airborne particulates[27]. Interleukin-6 is also strongly induced by airway infection with viral pathogens such as influenza, RSV, and rhinovirus[28–30] and is a potent stimulant of neutrophil production and recruitment, leading to non-eosinophilic airway inflammation. In addition, IL-6 may have effects on remodeling of the airways: IL-6 is a smooth muscle mitogen[14], and over-expression of IL-6 in the murine airway causes subepithelial fibrosis with collagen deposition[31]. Sputum IL-6 levels have been associated with severity of disease and accelerated decline in FEV1 in COPD patients [32, 33] suggesting that asthmatics without rhinitis may share some features of patients with COPD. It is important to note that no patient in this study had a greater than 10 pack year smoking history, though we did not obtain a history regarding second hand smoke exposure.
In nasal lavage, we did not find significant differences between any of the groups in terms of either Th1 cytokines (tumor necrosis factor-α and interferon-γ) or Th2 cytokines (Interleukins -4 and -5). We did find lower levels of IL-8 in the nasal lavage of asthmatics with perennial rhinitis, this may be related to the chronic nasal disease: although acute nasal allergen challenge increases IL-8 in nasal lavage fluid [34] chronically, IL-8 levels decrease in nasal lavage fluid of patients with seasonal allergic rhinitis[35]. These observations suggest that there are differences in nasal inflammation in asthmatics with rhinitis and those without rhinitis.
One of the major limitations of our study is that it is a small study, and our observations will been to be confirmed in a larger study population. We were limited in our ability to recruit asthmatics without rhinitis, as we were only able to recruit 8 participants over a three year period (on careful questioning we found almost all asthmatics will report some history of allergic or perennial nasal symptoms). We did not perform allergy testing in this study, which would have been an interesting covariate. However, previous studies suggest that lower airway eosinophilia in adults is not dependant on atopic status[36–38], and one recent publication showed that bronchial eosinophilia was higher in non-atopic than atopic asthmatics with nasal polyposis[39], suggesting that induced sputum eosinophils in asthmatics with sino-nasal disease is not related to atopic status.
In conclusion, our data suggests that asthmatics without rhinitis appear to have distinct features compared to asthmatics with rhinitis. Asthmatics with rhinitis tend to have more severe airflow limitation, along with fewer eosinophils in their induced sputum. This suggests they may be less responsive to intervention with inhaled corticosteroids, and that loss of lung function in these patients may not be related to eosinophilic inflammation, but may be related to altered cytokine expression in the airway. This suggests that future studies are required to confirm these phenotypes of asthma and investigate if those without rhinitis respond differently to medical treatments.
Acknowledgements
Supported by grants RR019965 & MO1RR00109
Footnotes
Conflicts of Interest
The authors report that they have no conflicts of interest pertaining to the work reported in this manuscript.
References
- 1.Leynaert B, Neukirch C, Kony S, Guenegou A, Bousquet J, Aubier M, et al. Association between asthma and rhinitis according to atopic sensitization in a populationbased study. JAllergy ClinImmunol. 2004;113(1):86–93. doi: 10.1016/j.jaci.2003.10.010. 1/2004. [DOI] [PubMed] [Google Scholar]
- 2.Togias A. Rhinitis and asthma: evidence for respiratory system integration. JAllergy ClinImmunol. 2003;111(6):1171–1183. doi: 10.1067/mai.2003.1592. 6/2003. [DOI] [PubMed] [Google Scholar]
- 3.Dixon AE, Kaminsky DA, Holbrook JT, Wise RA, Shade DM, Irvin CG. Allergic rhinitis and sinusitis in asthma: differential effects on symptoms and pulmonary function. Chest. 2006 Aug;130(2):429–435. doi: 10.1378/chest.130.2.429. [DOI] [PubMed] [Google Scholar]
- 4.Bousquet J, Boushey HA, Busse WW, Canonica GW, Durham SR, Irvin CG, et al. Characteristics of patients with seasonal allergic rhinitis and concomitant asthma. ClinExpAllergy. 2004;34(6):897–903. doi: 10.1111/j.1365-2222.2004.01969.x. 6/2004. [DOI] [PubMed] [Google Scholar]
- 5.Juniper EF, Bousquet J, Abetz L, Bateman ED. Identifying 'well-controlled' and 'not well-controlled' asthma using the Asthma Control Questionnaire. Respir Med. 2006 Apr;100(4):616–621. doi: 10.1016/j.rmed.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. AmJRespirCrit Care Med. 2000;161(1):309–329. doi: 10.1164/ajrccm.161.1.ats11-99. 1/2000. [DOI] [PubMed] [Google Scholar]
- 7.Recommendations for standardized procedures for the on-line and off-line measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide in adults and children-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. AmJRespirCrit Care Med. 1999;160(6):2104–2117. doi: 10.1164/ajrccm.160.6.ats8-99. 12/1999. [DOI] [PubMed] [Google Scholar]
- 8.Piccirillo JF, Merritt MG, Jr, Richards ML. Psychometric and clinimetric validity of the 20-Item Sino-Nasal Outcome Test (SNOT-20) OtolaryngolHead Neck Surg. 2002;126(1):41–47. doi: 10.1067/mhn.2002.121022. 1/2002. [DOI] [PubMed] [Google Scholar]
- 9.Juniper EF. Quality of life in adults and children with asthma and rhinitis. Allergy. 1997;52(10):971–977. doi: 10.1111/j.1398-9995.1997.tb02416.x. 10/1997. [DOI] [PubMed] [Google Scholar]
- 10.Bonay M, Neukirch C, Grandsaigne M, Lecon-Malas V, Ravaud P, Dehoux M, et al. Changes in airway inflammation following nasal allergic challenge in patients with seasonal rhinitis. Allergy. 2006 Jan;61(1):111–118. doi: 10.1111/j.1398-9995.2006.00967.x. [DOI] [PubMed] [Google Scholar]
- 11.Braunstahl GJ, KleinJan A, Overbeek SE, Prins JB, Hoogsteden HC, Fokkens WJ. Segmental bronchial provocation induces nasal inflammation in allergic rhinitis patients. AmJRespirCrit Care Med. 2000;161(6):2051–2057. doi: 10.1164/ajrccm.161.6.9906121. 6/2000. [DOI] [PubMed] [Google Scholar]
- 12.Dorman SC, Babirad I, Post J, Watson RM, Foley R, Jones GL, et al. Progenitor egress from the bone marrow after allergen challenge: role of stromal cell-derived factor 1alpha and eotaxin. J Allergy Clin Immunol. 2005 Mar;115(3):501–507. doi: 10.1016/j.jaci.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 13.Gaga M, Lambrou P, Papageorgiou N, Koulouris NG, Kosmas E, Fragakis S, et al. Eosinophils are a feature of upper and lower airway pathology in non-atopic asthma, irrespective of the presence of rhinitis. ClinExpAllergy. 2000;30(5):663–669. doi: 10.1046/j.1365-2222.2000.00804.x. 5/2000. [DOI] [PubMed] [Google Scholar]
- 14.ten Brinke A, Grootendorst DC, Schmidt JT, de Bruine FT, van Buchem MA, Sterk PJ, et al. Chronic sinusitis in severe asthma is related to sputum eosinophilia. JAllergy ClinImmunol. 2002;109(4):621–626. doi: 10.1067/mai.2002.122458. 4/2002. [DOI] [PubMed] [Google Scholar]
- 15.Staikuniene J, Vaitkus S, Japertiene LM, Ryskiene S. Association of chronic rhinosinusitis with nasal polyps and asthma: clinical and radiological features, allergy and inflammation markers. Medicina (Kaunas, Lithuania) 2008;44(4):257–265. [PubMed] [Google Scholar]
- 16.Tsoumakidou M, Papadopouli E, Tzanakis N, Siafakas NM. Airway inflammation and cellular stress in noneosinophilic atopic asthma. Chest. 2006 May;129(5):1194–1202. doi: 10.1378/chest.129.5.1194. [DOI] [PubMed] [Google Scholar]
- 17.Kikuchi S, Nagata M, Kikuchi I, Hagiwara K, Kanazawa M. Association between neutrophilic and eosinophilic inflammation in patients with severe persistent asthma. International archives of allergy and immunology. 2005;137 Suppl 1:7–11. doi: 10.1159/000085425. [DOI] [PubMed] [Google Scholar]
- 18.Bacci E, Cianchetti S, Bartoli M, Dente FL, Di Franco A, Vagaggini B, et al. Low sputum eosinophils predict the lack of response to beclomethasone in symptomatic asthmatic patients. Chest. 2006 Mar;129(3):565–572. doi: 10.1378/chest.129.3.565. [DOI] [PubMed] [Google Scholar]
- 19.Berry M, Morgan A, Shaw DE, Parker D, Green R, Brightling C, et al. Pathological features and inhaled corticosteroid response of eosinophilic and non-eosinophilic asthma. Thorax. 2007 Dec;62(12):1043–1049. doi: 10.1136/thx.2006.073429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lemiere C, Ernst P, Olivenstein R, Yamauchi Y, Govindaraju K, Ludwig MS, et al. Airway inflammation assessed by invasive and noninvasive means in severe asthma: eosinophilic and noneosinophilic phenotypes. J Allergy Clin Immunol. 2006 Nov;118(5):1033–1039. doi: 10.1016/j.jaci.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Berry MA, Shaw DE, Green RH, Brightling CE, Wardlaw AJ, Pavord ID. The use of exhaled nitric oxide concentration to identify eosinophilic airway inflammation: an observational study in adults with asthma. Clin Exp Allergy. 2005 Sep;35(9):1175–1179. doi: 10.1111/j.1365-2222.2005.02314.x. [DOI] [PubMed] [Google Scholar]
- 22.Shaw DE, Berry MA, Hargadon B, McKenna S, Shelley MJ, Green RH, et al. Association between neutrophilic airway inflammation and airflow limitation in adults with asthma. Chest. 2007 Dec;132(6):1871–1875. doi: 10.1378/chest.07-1047. [DOI] [PubMed] [Google Scholar]
- 23.ten Brinke A, Zwinderman AH, Sterk PJ, Rabe KF, Bel EH. Factors associated with persistent airflow limitation in severe asthma. Am J Respir Crit Care Med. 2001 Sep 1;164(5):744–748. doi: 10.1164/ajrccm.164.5.2011026. [DOI] [PubMed] [Google Scholar]
- 24.Torres A, Utell MJ, Morow PE, Voter KZ, Whitin JC, Cox C, et al. Airway inflammation in smokers and nonsmokers with varying responsiveness to ozone. Am J Respir Crit Care Med. 1997 Sep;156(3 Pt 1):728–736. doi: 10.1164/ajrccm.156.3.9601054. [DOI] [PubMed] [Google Scholar]
- 25.Nordenhall C, Pourazar J, Ledin MC, Levin JO, Sandstrom T, Adelroth E. Diesel exhaust enhances airway responsiveness in asthmatic subjects. Eur Respir J. 2001 May;17(5):909–915. doi: 10.1183/09031936.01.17509090. [DOI] [PubMed] [Google Scholar]
- 26.Deetz DC, Jagielo PJ, Quinn TJ, Thorne PS, Bleuer SA, Schwartz DA. The kinetics of grain dust-induced inflammation of the lower respiratory tract. Am J Respir Crit Care Med. 1997 Jan;155(1):254–259. doi: 10.1164/ajrccm.155.1.9001321. [DOI] [PubMed] [Google Scholar]
- 27.Raulf-Heimsoth M, Pesch B, Schott K, Kappler M, Preuss R, Marczynski B, et al. Irritative effects of fumes and aerosols of bitumen on the airways: results of a cross-shift study. Archives of toxicology. 2007 Jan;81(1):35–44. doi: 10.1007/s00204-006-0115-z. [DOI] [PubMed] [Google Scholar]
- 28.Matsukura S, Kokubu F, Noda H, Tokunaga H, Adachi M. Expression of IL-6, IL-8, and RANTES on human bronchial epithelial cells, NCI-H292, induced by influenza virus A. J Allergy Clin Immunol. 1996 Dec;98(6 Pt 1):1080–1087. doi: 10.1016/s0091-6749(96)80195-3. [DOI] [PubMed] [Google Scholar]
- 29.Yoon JS, Kim HH, Lee Y, Lee JS. Cytokine induction by respiratory syncytial virus and adenovirus in bronchial epithelial cells. Pediatr Pulmonol. 2007 Mar;42(3):277–282. doi: 10.1002/ppul.20574. [DOI] [PubMed] [Google Scholar]
- 30.Oliver BG, Johnston SL, Baraket M, Burgess JK, King NJ, Roth M, et al. Increased proinflammatory responses from asthmatic human airway smooth muscle cells in response to rhinovirus infection. Respiratory research. 2006;7:71. doi: 10.1186/1465-9921-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DiCosmo B, Geba G, Picarella D, Elias JA, Rankin JA, Stripp B, et al. Expression of interleukin-6 by airway epithelial cells. Effects on airway inflammation and hyperreactivity in transgenic mice. Chest. 1995 Mar;107(3 Suppl):131S. doi: 10.1378/chest.107.3_supplement.131s. [DOI] [PubMed] [Google Scholar]
- 32.Hacievliyagil SS, Gunen H, Mutlu LC, Karabulut AB, Temel I. Association between cytokines in induced sputum and severity of chronic obstructive pulmonary disease. Respir Med. 2006 May;100(5):846–854. doi: 10.1016/j.rmed.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 33.Donaldson GC, Seemungal TA, Patel IS, Bhowmik A, Wilkinson TM, Hurst JR, et al. Airway and systemic inflammation and decline in lung function in patients with COPD. Chest. 2005 Oct;128(4):1995–2004. doi: 10.1378/chest.128.4.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raulf-Heimsoth M, Wirtz C, Papenfuss F, Baur X. Nasal lavage mediator profile and cellular composition of nasal brushing material during latex challenge tests. Clin Exp Allergy. 2000 Jan;30(1):110–121. doi: 10.1046/j.1365-2222.2000.00690.x. [DOI] [PubMed] [Google Scholar]
- 35.Kuna P, Lazarovich M, Kaplan AP. Chemokines in seasonal allergic rhinitis. J Allergy Clin Immunol. 1996 Jan;97(1 Pt 1):104–121. doi: 10.1016/s0091-6749(96)70288-9. [DOI] [PubMed] [Google Scholar]
- 36.Anees W, Huggins V, Pavord ID, Robertson AS, Burge PS. Occupational asthma due to low molecular weight agents: eosinophilic and non-eosinophilic variants. Thorax. 2002 Mar;57(3):231–236. doi: 10.1136/thorax.57.3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ackerman V, Marini M, Vittori E, Bellini A, Vassali G, Mattoli S. Detection of cytokines and their cell sources in bronchial biopsy specimens from asthmatic patients. Relationship to atopic status, symptoms, and level of airway hyperresponsiveness. Chest. 1994 Mar;105(3):687–696. doi: 10.1378/chest.105.3.687. [DOI] [PubMed] [Google Scholar]
- 38.Louis R, Van Tulder L, Poncelet M, Corhay JL, Mendez P, Radermecker M. Correlation between bronchoalveolar lavage (BAL) fluid cell lysate histamine content and BAL fluid eosinophil count in atopic and nonatopic asthmatics. International archives of allergy and immunology. 1997 Mar;112(3):309–312. doi: 10.1159/000237471. [DOI] [PubMed] [Google Scholar]
- 39.Ediger D, Sin BA, Heper A, Anadolu Y, Misirligil Z. Airway inflammation in nasal polyposis: immunopathological aspects of relation to asthma. Clin Exp Allergy. 2005 Mar;35(3):319–326. doi: 10.1111/j.1365-2222.2005.02194.x. [DOI] [PubMed] [Google Scholar]