Abstract
Objective
The ability to sustain and grow portions of human tumors as xenografts in SCID mice provides a valuable preclinical opportunity to test the response of human tumors to treatments, both individually and in combination. Using this model, our laboratory has previously demonstrated that the growth of several human adenocarcinomas can be inhibited by Apo2L/TRAIL. Apo2L/TRAIL triggers apoptosis in many types of tumor cells, and when combined with various chemotherapeutic agents results in enhanced inhibition of tumor growth in many xenograft models.
Methods
To gain further insight into the antitumor potential of Apo2L/TRAIL in combination with chemotherapy, we compared the responses of 2 human colon adenocarcinomas, both of which were sensitive to CPT-11 while one was sensitive and the other comparatively resistant to Apo2L/TRAIL.
Results
In both cases, a greater degree of growth inhibition was achieved when these agents were used in combination. Western blot analysis demonstrated that in the Apo2L/TRAIL-sensitive tumor total cellular expression of Apo2L/TRAIL death receptors (DR4 and DR5) as well as protein expression of the pro-apoptotic molecule BAX were higher and the anti-apoptotic molecule Bcl-2 was lower in comparison to the Apo2L/TRAIL-resistant tumor.
Conclusion
These results indicate that both Apo2L/TRAIL-sensitive and -resistant colon tumors will respond to a combination of CPT-11 and Apo2L/TRAIL and predict that this will be useful in the treatment of human colon cancers in a clinical setting.
Key Words: Apo2L/TRAIL, Apoptosis, Colon cancer, CPT-11, Xenograft
Introduction
Successful development of anti-cancer therapies relies on demonstrating and optimizing the anti-tumor efficacy of therapeutics in a spectrum of preclinical models, each of which has strengths and weaknesses. Often, the response of human cell lines and xenografts has not correlated with the clinical efficacy observed in clinical trials. In one NIH study, for example, it was found that only cell line xenografts of non-small lung cancer correlated with clinical activity in tumors of the same histology [1]. A recent retrospective analysis found that although the cell line xenograft model was predictive for non-small cell lung and ovarian cancers, panels of colon or breast tumor cell line xenografts failed to predict clinical performance in phase II studies for these tumors [2]. For this reason, investigators continue to explore the relevance of new models. We and others [3, 4] have developed and applied a patient tumor/SCID mouse xenograft model to evaluate the effect(s) of novel therapies and treatment regimens on actual patient tumors [5,6,7] in order to extend observations made with human cell lines. These human tumor xenografts recapitulate both the range of histology and diversity of the original tumors and have the advantage of using low-passage human tumors which have never been cultured in vitro. Thus they are a valuable preclinical bridge to the clinic.
Apo2L/TRAIL (tumor-necrosis factor apoptosis-inducing ligand) is a member of the TNF family [8,9,10]. Similar to TNF and FasL, Apo2L/TRAIL is a type II transmembrane protein that can induce apoptosis in malignant cells [11, 12] and does not exhibit the normal tissue or systemic toxicity of TNF or anti-Fas antibodies or FasL [13, 14]. Apo2L/TRAIL induces apoptosis through its interaction with two death domain-containing receptors, DR4 [15, 16] and DR5 [16, 17]. Following Apo2L/TRAIL engagement in these receptors, caspase-8 is activated and triggers cell death, either by directly activating downstream effector caspases, e.g. caspase-3, and/or by recruiting the mitochondrial pathway to cell death [9, 10]. Not all cancer cells undergo apoptosis when treated with Apo2L/TRAIL and it has been shown that combination of Apo2L/TRAIL with a variety of chemotherapeutic agents can sensitize resistant cells and result in an improved anti-tumor effect [9].
Previous work on the effects of Apo2L/TRAIL has been largely carried out on long-established cell lines either in vitro or in vivo. We have previously shown, using the SCID mouse/human tumor xenograft model to evaluate the effects of Apo2L/TRAIL on human colon cancer, that three different human tumors show varying degrees of sensitivity to Apo2L/TRAIL [7]. In this study, we have identified a human colon tumor that is inherently resistant to Apo2L/TRAIL and compared it to a tumor that is sensitive to the same dose of Apo2L/TRAIL. We found a correlation between total expression levels of BAX and Bcl-2, and sensitivity of these two tumors to apoptosis induced by ApoL/TRAIL alone. Furthermore, we found that combination therapy with CPT-11 enhanced the anti-tumor effect of Apo2L/TRAIL, resulting in increased tumor growth inhibition than was achieved with either agent alone in both the Apo2L/TRAIL-sensitive and -resistant tumors. These results predict that both Apo2L/TRAIL-sensitive and -resistant tumors will respond to treatment with Apo2L/TRAIL in combination with CPT-11 and thus this validates what has been observed for some cell lines and supports the development of this therapeutic strategy.
Material and Methods
Colon Tumor Model
The SCID mouse-human tumor xenograft model used in these experiments has been described previously [7]. Pieces of human colon tumors obtained from surgical specimens (provided by the Tissue Procurement Facility at the Roswell Park Cancer Institute) were cut into 2 × 2 mm pieces in tissue culture medium and implanted subcutaneously into 2–3 SCID mice. Tumors that grew to 1 cm in diameter were resected and serially passaged. Tumors that grew in the second passage were considered to have successfully engrafted. Tumors that successfully engrafted were subsequently serially passaged into several mice thereby providing sufficient tumor material for experiments with large numbers of mice.
SCID Mice
CB-17 SCID/SCID mice were used at 6–8 weeks of age and kept under sterile conditions in pathogen-free environment. Mice were provided with sterile water and food ad libitum, and all manipulations were carried out aseptically inside a laminar flow hood.
Materials
Apo2L/TRAIL was generously provided by Genentech (San Francisco, Calif., USA). Irinotecan hydrochloride (CPT-11) was obtained from Upjohn (Kalamazoo, Mich., USA). Antibodies used were rabbit polyclonal antibodies for DR4 (PC397) and DR5 (PC392) from Oncogene Research Products (Cambridge, Mass., USA). Rabbit polyclonal antibodies for DcR1 (No. 550622) and DcR2 (No. 559684) were obtained from BD PharMingen (San Diego, Calif., USA). Mouse monoclonal antibodies for BAX (No. 610982) were obtained from BD Transduction Laboratories (San Diego, Calif., USA) and reported to be reactive to dogs and humans. Mouse monoclonal antibodies to Bcl-2 (No. 556354) and rabbit polyclonal antibody for cFLIP (No. 556567) were obtained from BD PharMingen. All antibodies were reported to be against human proteins.
Experimental Design
Engrafted tumors were used as donor tumors to implant experimental groups of mice all bearing the same patient's tumor. Mice were randomly divided into control or treatment groups of 6 mice each. Treatment was initiated when the tumors reached approximately 4–5 mm in diameter (a mean volume of 50 mm3). To assess sensitivity to Apo2L/TRAIL alone, Apo2L/TRAIL (500 μg/200 μl) was administered daily for 14 days by intraperitoneal injection. To investigate the effects of Apo2L/TRAIL and CPT-11 in combination, Apo2L/TRAIL (500 μg/200 μl) was given daily by intraperitoneal injection for 14 days, and CPT-11 (10 or 20 mg/kg) was administered three times per week by intravenous injection. Animals (5 or 6 mice/group) received two of these treatment cycles separated by a 2-week interval [7]. Tumors were measured three times a week and the relative tumor volumes were calculated. Mice were sacrificed by cervical dislocation and the tumors were removed, fixed in neutral buffered formalin, and either embedded in paraffin and/or snap-frozen for further study.
Western Blot Analysis
One mouse was removed from each group on day 3 of treatment. Each tumor was resected and pieces of the tumor xenografts were homogenized in lysis buffer (containing 20 mM Tris, pH 7.5, 120 mM NaCl, 100 mM NaF, 0.5% nonionic P40,200 μM Na3VO4, 50 mM β-glycerophosphate, 10 mM NaPPi, 4 mM PMSF, 10 μg/ml leupeptin, 2 mM benzamidine and 10 μg/ml aprotinin). Protein concentration of the lysates was assessed using a colorometric assay (BioRad, Hercules, Calif., USA). Equal amounts of protein were separated by SDS-PAGE and transferred to polyvinylidene fluoride membranes (Millipore, Bedford, Mass., USA). After blocking with 5% nonfat milk in PBST (PBS with 0.1% Tween) for 1 h at room temperature, membranes were incubated overnight at 4°C with specific antibodies. Membranes were then washed with PBST and incubated further at room temperature for 1 h with horseradish peroxidase-conjugated goat anti-mouse or anti-rabbit antibodies. The integrated pixel densities of immunoreactive electrophoretic bands were calculated using Image Quant software from Molecular Dynamics (Sunnyvale, Calif., USA), normalized to the corresponding density of the actin bands, and calculated as a factor of the control.
Immunohistochemistry
TUNEL Assay. To detect apoptotic cells in tumor sections, the ApopTag Plus peroxidase in situ apoptosis detection kit (Intergen, Purchase, N.Y., USA) was used. Five-micrometer-thick paraffin-embedded tumor sections were deparaffinized in xylene and rehydrated in decreasing concentrations of ethanol. Tissue sections were incubated in 20 μg/ml proteinase K (Dako, Carpinteria, Calif., USA) at room temperature for 15 min. Following rinsing in distilled water, endogenous peroxidase was blocked by incubating slides in 3% hydrogen peroxide in 0.05 M phosphate buffer containing 0.145 M sodium chloride (PBS), pH 7.4, for 5 min. After washing with PBS, the sections were incubated with equilibration buffer and then TdT enzyme in a humidified chamber at 37°C for 60 min. Slides were subsequently put into prewarmed working strength stop buffer for 10 min. After rinsing in PBS, the sections were incubated with anti-digoxigenin-peroxidase conjugate for 30 min. Bound antibody was localized with diaminobenzamidine (Peroxidase Substrate Kit; Vector Laboratories, Burlingame, Calif., USA) and slides were counterstained with hematoxylin. To quantify apoptosis, at least 2,000 cells were counted under the microscope in several random fields of each section. The number of TUNEL staining-positive cells was divided by the total number of cells counted, and the result was expressed as percentage of apoptotic cells. Evaluation of the p53 status was accomplished using monoclonal antibody clone DO7 (Novocastra) and antigen retrieval with steam and antigen retrieval buffer (Dako).
Sequencing of Human p53 Genes
Total RNA from 11501 and 11124 tumors was isolated using TRIzol (Invitrogen, Carlsbad, Calif., USA) according to the manufacturer's recommendations. Two micrograms of RNA were reverse transcribed to generate the first cDNA strand using the Superscript II system (Invitrogen).
To distinguish murine from human p53, primers were designed 5′- and 3′- in non-conserved regions of the DNA binding domain (DBD) of the p53 gene. The primer sets used for the amplification are forward primer, 5′-GCCGTCCCAAGCAATGGATGA-3′ and reverse primer, 5′-AGCTCGTGGTGAGGCTCCCCTTTCTT-3′. To test the specificity of the primers, p53 was amplified in gradient PCR reactions (increasing annealing temperature) using p53+/+ cDNA from both murine and human cell lines. The determined optimal conditions were used in a PCR reaction using the Accuprime enzyme (Invitrogen) and the 11124 and 11501 cDNA. Several amplicons from different PCR reactions were then gel purified and cloned into pDrive (Qiagen, Valencia, Calif., USA) before sequencing by the Roswell Park Cancer Institute Biopolymer Facility.
Statistical Analysis
Statistical significance was determined by comparing the final relative volumes of tumors. Student's t test was used for experiments evaluating the efficacy of Apo2L/TRAIL alone. One-way ANOVA followed by Tukey's test was used for experiments evaluating the efficacy of Apo2L/TRAIL in combination with CPT-11. Differences were considered significant if p < 0.05.
Results
Characterization of the Responses of Colon Tumors to Apo2L/TRAIL Alone
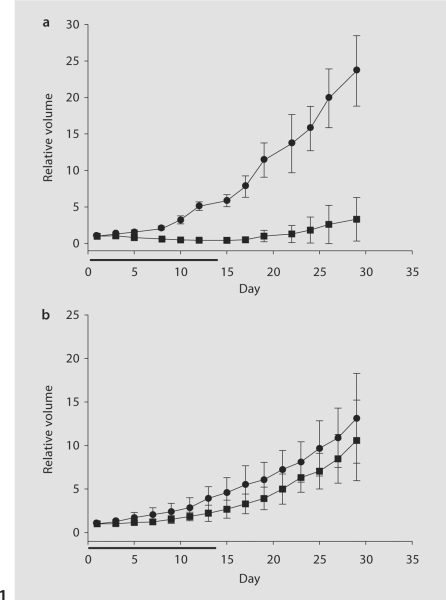
We have previously reported the responses of three different human colon tumors to Apo2L/TRAIL alone using a human tumor-SCID model, and varying degrees of sensitivity were noted [7]. Characterization of a fourth colon tumor led to the identification of a tumor that is comparatively resistant to Apo2L/TRAIL. As seen in figure 1a, tumor 11124 is highly sensitive to Apo2L/TRAIL and the growth rate was significantly reduced in the Apo2L/TRAIL-treated group compared with that of the control group (p < 0.001). Although tumor growth resumed following cessation of treatment, the difference in tumor size was still significant on day 29 (p < 0.001). In contrast, tumor 11501 was resistant to Apo2L/TRAIL and, while treated tumors were slightly smaller than those of the control group, this difference was not statistically significant at either time point (fig. 1b). These results demonstrate that human colon cancers which are directly derived from patients display both sensitivity and resistance to Apo2L/TRAIL.
Fig. 1.
The effects of Apo2L/TRAIL alone on surgical specimens of 2 patients’ colon adenocarcinomas grown in SCID mice. Mice bearing tumors of 50 mm3 starting volume were treated with intraperitoneal injections of 500 μg Apo2L/TRAIL daily for 14 days. a The growth of tumor 11124 was significantly inhibited by Apo2L/TRAIL (• = Control; ▪= Apo2L/TRAIL; p < 0.001). b The growth of tumor 11501 was not significantly inhibited (• = Control; ▪ = Apo2L/TRAIL). Horizontal bar indicates one cycle of treatment. Error bars = standard deviation, n = 6.
The CPT-11 and Apo2L/TRAIL Combination Enhanced Anti-Tumor Efficacy
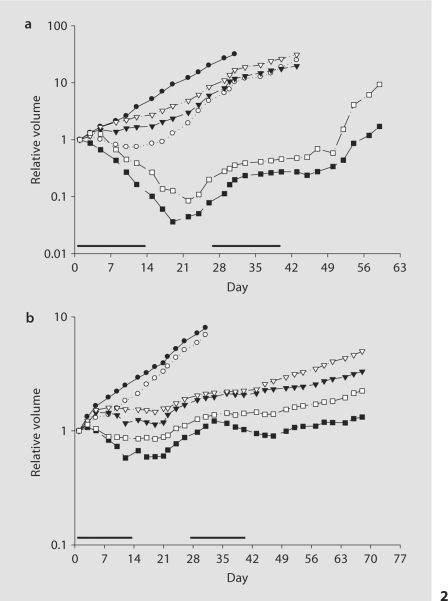
Because the dose and schedule of Apo2L/TRAIL evaluated here did not suppress tumor growth completely, the response of these tumors to combination treatment with Apo2L/TRAIL and the chemotherapeutic drug CPT-11 was evaluated. To extend our earlier study on tumor-bearing mice which were treated for only 14 days, we wanted to evaluate whether tumor growth could be more effectively controlled with a longer duration of treatment. Therefore, mice were treated with 500 μg Apo2L/TRAIL alone and in combination with either 10 or 20 mg/kg CPT-11 for 14 days, and after a 2-week rest, treatment was resumed for 2 weeks. Evaluation of the tumors on day 14 after the first treatment cycle shows that administration of either Apo2L/TRAIL or CPT-11 (10 or 20 mg/kg) alone significantly suppressed the growth of tumor 11124 (fig. 2a: p < 0.05 in all cases), although this effect was not complete. More effective tumor inhibition was achieved with both combination therapies: both 20 mg/kg CPT- 11 + Apo2L/TRAIL and 10 mg/kg CPT-11 + Apo2L/TRAIL treatment inhibited tumor growth more effectively than each treatment alone (each p < 0.01), and this significantly improved efficacy was still evident on day 42. In each group, 2 of the 5 tumors treated with combination therapy underwent regression and were completely eliminated.
Fig. 2.
The effect of Apo2L/TRAIL alone and in combination with CPT-11 on 2 patients’ colon tumors grown in SCID mice: tumor 11124 (a) and 11501 (b). a On day 14, at the end of the first cycle, the tumors treated with Apo2L/TRAIL alone (○) were significantly smaller than those of the control (•; p < 0.05). A dose-dependent inhibition of tumor growth was observed with CPT-11 alone. The treatment with either 20 mg/kg (▾) or 10 mg/kg (▿) CPT-11 also inhibited tumor growth, and the difference was statistically significant (p < 0.05). The combination treatment with Apo2L/TRAIL and either 20 (▪) or 10 mg/kg (□) CPT-11 resulted not only in growth inhibition but also in tumor regression (p < 0.01 and p < 0.001, respectively). b On day 14, the tumors treated with Apo2L/TRAIL alone (○) were slightly smaller than those of the control (•), but the difference was not statistically significant. A dose-dependent inhibition of tumor growth was observed with CPT-11 alone at either 20 (▾) or 10 mg/kg (▿; p < 0.01 for both). Combination treatment with Apo2L/TRAIL and either 20 (▪) or 10 mg/kg (□) CPT-11 initially resulted in significant inhibition (p < 0.01 for both compared to control) and in slight tumor regression. Four of 5 tumors remained palpable in each group on day 43, however there was a significant difference in size between the tumors treated with CPT-11 alone and those that received combination treatment. Bars indicate cycles of treatment. n = 5.
Although tumor 11501 was resistant to Apo2L/TRAIL alone, tumor 11501 was sensitive to CPT-11 and administration of CPT-11 alone at these doses resulted in a dose-dependent inhibition of growth. After one treatment cycle, the tumors in the CPT-11 (both 20 and 10 mg/kg)-treated groups were significantly smaller than those of the control group (p < 0.01). This inhibition of tumor growth was enhanced with combination therapy. Apo2L/TRAIL combined with either 20 or 10 mg/kg CPT-11 inhibited tumor growth more effectively than either agent alone. At the end of the second cycle of treatment (day 43), 1 tumor in each group had completely regressed. Comparison of the palpable tumors in each group showed a statistically significant difference in tumor inhibition between the CPT-11 monotherapy at each dose and the corresponding combination therapy (fig. 2b, p < 0.05 in each case). However, no treatment resulted in complete, long-lasting elimination of all tumors.
While the improved effect of combination therapy over single therapy was found to be synergistic for tumor 11124 but not for tumor 11501, these data indicate that, in either the case of a tumor that is sensitive to Apo2L/TRAIL alone or in one that is resistant, the combination of CPT-11 and Apo2L/TRAIL leads to enhanced tumor growth inhibition.
Histology of Tumors Treated with Apo2L/TRAIL
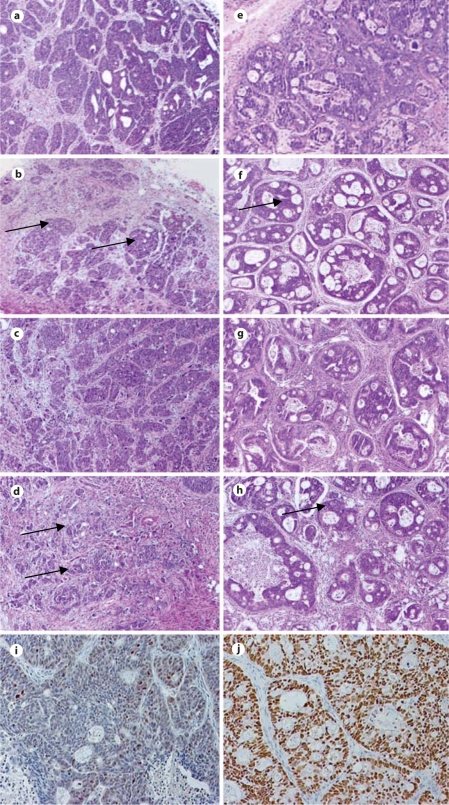
Histological analysis of tumor 11124 indicated that this is a moderately to poorly differentiated adenocarcinoma (fig. 3a); tumor 11501 is a moderately differentiated adenocarcinoma (fig. 3e). To investigate the effect of treatment on these tumors further, representative tumors were removed from each group after 3 days of treatment. Even at this early time point, the Apo2L/TRAIL-sensitive tumor that had been treated with Apo2L/TRAIL consisted of comparatively more stromal cells and connective tissue than an untreated control tumor (fig. 3b), while a tumor treated with CPT-11 alone (20 mg/kg), although slightly smaller, resembled the control (fig. 3c). In comparison, a tumor treated with CPT-11 (20 mg/kg) in combination with Apo2L/TRAIL is significantly smaller and contains smaller, more scattered groups of viable tumor cells and proportionately more stromal cells (fig. 3d). While the resistant tumors treated with either Apo2L/TRAIL or CPT-11 alone have slightly fewer tumor cells (fig. 3f, g), the tumor receiving combination therapy contains a noticeably higher proportion of connective tissue with smaller glands (fig. 3h). Because sensitivity to Apo2L/TRAIL is reportedly p53 independent (see reviews [9, 18]), we performed an immunohistochemical analysis of p53 expression in these two tumors. Results show moderate overexpression of p53 in the sensitive tumor 11124 (fig. 3i) and a high degree of overexpression in the resistant tumor 11501 (fig. 3j). These results suggested that the Apo2L/TRAIL-resistant tumor might have a p53 mutation which resulted in accumulation of the abnormal protein. To further investigate this question, the human p53 DBD, where most of the p53 functional mutations have been described [19], was amplified and sequenced. In both tumors, however, the p53 DBD sequence was wild type and no mutations were identified (data not shown).
Fig. 3.
Histological features of tumor 11124 (a–d) and tumor 11501 (e–h) following treatment for 3 days. a Untreated tumor: extensive tumor growth is seen. b Apo2L/TRAIL-treated tumor: areas of viable tumor cells (arrows) are surrounded by proportionately more stromal cells and connective tissue compared with the untreated tumor. c Cpt-11 (20 mg/kg)-treated tumor appears similar to the control. d Apo2L/TRAIL + CPT-11 (20 mg/kg)-treated tumor: smaller than the control and with greatly reduced numbers of viable tumor cells (arrows) and an increased proportion of stromal cells and connective tissue on day 3. e Untreated tumor: extensive tumor growth is seen. f Apo2L/TRAIL-treated tumor: the tumor appears histologically similar to the untreated tumor and viable tumor cells (arrow) are surrounded by a slightly increased amount of stroma. g CPT-11 (20 mg/kg)-treated tumor has slightly greater proportion of stroma than the control. h Apo2L/TRAIL + CPT-11 (20mg /kg)- treated tumor: these tumors are smaller with much smaller ducts (arrow) and more connective tissue. i, j Immunohistochemical localization of p53. i Nuclear staining of p53 is heterogeneous and low in tumor 11124. j Nuclear staining of p53 is visible in the majority of nuclei in tumor 11501.
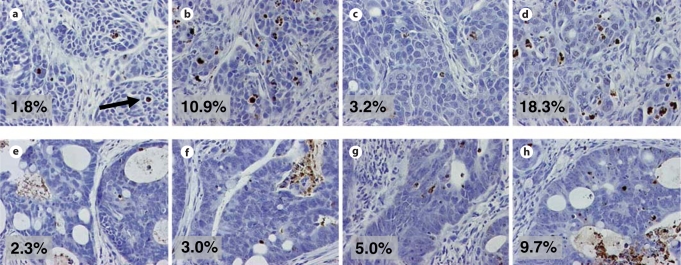
To investigate the degree of apoptosis induced by treatment, TUNEL staining was performed on tumors following 3 days of treatment, and analysis was performed on a representative tumor from each group (for both tumors: 11124 and 11501) in the combination studies. On day 3, a tumor was removed from each experimental group, and the percentage of TUNEL-positive cells was enumerated over several microscopic fields (at least 2,000 cells were counted). Representative sections are shown in figure 4. Tumor 11124 treated with Apo2L/TRAIL alone or in combination with CPT-11 (20 mg/kg) had more apoptotic cells than a control tumor or a tumor treated with CPT-11 alone: control 1.8%, Apo2L/TRAIL 10.9%, 20 mg/kg CPT-11 3.2% and the combination 18.3% apoptotic cells. Tumor 11501 treated with Apo2L/TRAIL and CPT-11 (20 mg/kg) also showed an increase in the number of apoptotic cells: control 2.3%, Apo2L/TRAIL 3.0%, 20 mg/kg CPT-11 5.0% and the combination therapy had 9.7% apoptotic cells.
Fig. 4.
TUNEL staining of tumors collected on day 3 of treatment shows increased apoptosis (arrow indicates an apoptotic cell) in tumors treated with Apo2L/TRAIL in combination with CPT-11 over either treatment alone. a–d Tumor 11124. a Control = 1.8%. b Apo2L/TRAIL = 10.9%. c CPT-11 = 3.2%. d Apo2L/TRAIL + CPT-11 = 18.3%. e–h Tumor 11501. e Control = 2.3%. f Apo2L/TRAIL = 3.0%. g CPT-11 = 5.0%. h Apo2L/TRAIL + CPT-11 = 9.7%.
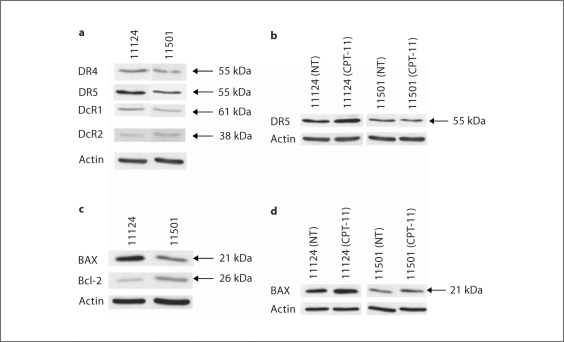
Western Blot Analysis of the Expression of DR4 and DR5
To determine whether there is a difference between the expression of death receptors in the Apo2L/TRAIL-sensitive tumor (11124) and the Apo2L/TRAIL-resistant tumor (11501), tumor samples from mice in each treated group were removed on day 3 and total cellular expression of death receptors (DR4, DR5, DcR1 and DcR2) was assessed by Western blot analysis.
Both tumors were seen to express all four death receptors. However, slightly higher levels of both DR4 (1.9-fold) and DR5 (2.1-fold) were detected in the Apo2L/TRAIL-sensitive tumor compared to the resistant tumor (fig. 5a). This analysis also demonstrated that exposure to CPT-11 leads to modestly increased expression of DR5 in the Apo2L/TRAIL-sensitive tumor (1.5-fold), but not the Apo2L/TRAIL-resistant tumor (fig. 5b). We also investigated the protein expression of BAX and Bcl-2, two members of the Bcl-2 family that have been reported to play a role in the sensitivity of colon carcinoma cells to Apo2L/TRAIL [20]. The sensitive tumor was found to express a higher level of the pro-apoptotic protein BAX (3.5-fold) and a lower level of the anti-apoptotic molecule Bcl-2 (0.5-fold) compared to the Apo2L/TRAIL-resistant tumor (fig. 5c). Furthermore, CPT-11 (20 mg/kg) treatment slightly increased BAX expression in both the Apo2L/TRAIL-sensitive tumor (1.5-fold) and Apo2L/TRAIL-resistant tumor (1.7-fold; fig. 5d). Additionally, these tumors expressed equivalent levels of cFLIP, an important inhibitor of death receptor-mediated apoptosis, and CPT-11 treatment did not detectably affect this expression (data not shown).
Fig. 5.
a Protein expression of Apo2L/TRAIL receptors. The expression of DR4, DR5, DcR1 and DcR2 was assessed by Western blot analysis. Both tumors expressed each of the Apo2L/TRAIL receptors. The Apo2L/TRAIL-sensitive tumor 11124 has higher levels of DR4 (1.9-fold) and DR5 (2.1-fold) than the Apo2L/TRAIL-resistant tumor 11501. b Following treatment with CPT-11, tumor 11124 expressed a higher level of DR5 (1.5-fold) than the control tumor (NT = No treatment). In contrast, following CPT-11 treatment of tumor 11501, the expression of DR5 was unchanged. c The Apo2L/TRAIL-sensitive tumor 11124 has a higher BAX (3.5-fold) and lower Bcl-2 (0.5-fold) expression than the Apo2L/TRAIL-resistant tumor 11501. d CPT-11 treatment increased the expression of BAX in both tumors (11124: 1.5-fold and 11501: 1.7-fold). Actin is shown as a loading control.
Discussion
Previously, we have demonstrated that colon tumor xenografts derived from human tumors naturally exhibit sensitivity (to varying degrees) to Apo2L/TRAIL alone [7]. In this study, we have extended these observations and described the characterization of a human colon tumor that is inherently resistant to Apo2L/TRAIL, suggesting that, as previously observed in human pancreatic tumor xenografts [6], human colon tumors can be expected to exhibit a full range of sensitivity to Apo2L/TRAIL in a clinical setting. Various chemotherapeutic drugs have been shown to sensitize tumor cells to members of the TNF family and, furthermore, it has been shown that chemotherapeutic drugs can sensitize even Apo2L/TRAIL-resistant cancer cells to Apo2L/TRAIL [14, 21, 22]. Additionally, since there have been many reports demonstrating an enhanced efficacy of Apo2L/TRAIL in combination with CPT-11 in colon cell lines both in vitro and in vivo [23,24,25,26] and we have previously reported that combination therapy improved the response in a different Apo2L/TRAIL-sensitive human colon xenograft [7], we aimed to compare the anti-tumor effects of Apo2L/TRAIL alone and in combination with CPT-11 on these two different human colon tumors, particularly to determine if this approach would be beneficial in the treatment of an Apo2L/TRAIL-resistant tumor. In this study, Apo2L/TRAIL treatment in combination with CPT-11 showed the greatest tumor growth inhibition not only against an Apo2L/TRAIL-sensitive tumor but, encouragingly, also against an Apo2L/TRAIL-resistant tumor. In fact, in the sensitive tumor, Apo2L/TRAIL in combination with either dose of CPT-11 significantly inhibited tumor growth and this effect was synergistic. These results suggest that CPT-11 levels could be reduced if used in combination with Apo2L/TRAIL. This has the potential to reduce the toxic side effects of chemotherapeutics and may retard development of drug resistance. Following cessation of treatment, however, tumors treated with either Apo2L/TRAIL and/or CPT-11 re-grew and it remains to be investigated whether higher doses or different treatment schedules can prolong tumor growth inhibition.
Histological analysis in combination with the TUNEL assay confirms that the antitumor activity of Apo2L/TRAIL can be attributed to the induction of apoptosis. Interestingly, although immunohistochemical visualization of p53 accumulation might suggest the presence of a p53 mutation in the Apo2L/TRAIL-resistant tumor (11501), sequencing of DBD, which is the region most often mutated [19], did not identify a mutation. This disparity has been reported previously for colon tumors [27] and indicates that immunohistochemistry cannot reliably predict the p53 functional status. The presence of wild-type DBD correlates well with the observed sensitivity of both tumors to CPT-11, as previously reported to be required for chemosensitization to death ligand therapy [28].
Our results predict that this inherent range of sensitivity to Apo2L/TRAIL is likely to be faced in a clinical situation. Previous reports have described multiple mechanisms that affect sensitivity to Apo2L/TRAIL-mediated apoptosis in various cell lines. These include expression levels of Apo2L/TRAIL receptors [22, 29], the expression of inhibitory downstream molecules such as FLIPs [30] and IAPs [31], and the activation of anti-apoptotic transcription factors such as NFκB [32]. Additionally, some reports have demonstrated that Apo2L/TRAIL-induced apoptosis requires BAX for t-BID-mediated mitochondrial activation of caspase-9 [25, 33], and loss of BAX results in resistance to death receptor-mediated apoptosis [20]. Another study suggests that Bcl-2 can inhibit Apo2L/TRAIL-induced apoptosis [34]. We investigated the expression of Apo2L/TRAIL receptors and comparison of these two tumors shows that the Apo2L/TRAIL-resistant tumor has slightly lower expression of total cellular DR4 and DR5 by Western blot analysis than the sensitive tumor. However, a direct relationship to cell surface expression may not exist [35, 36], and an analysis of total cellular expression and surface expression of Apo2L/TRAIL receptors needs to be carried out in a larger panel of tumors. We also investigated the protein expression of BAX and Bcl-2 and found that the Apo2L/TRAIL-sensitive tumor has a higher level of BAX and lower Bcl-2 expression than the Apo2L/TRAIL-resistant tumor. In order to reach any broad conclusions about the role of these factors in sensitivity and resistance, a larger panel of tumors needs to be investigated.
Some reports have shown that chemotherapeutic drugs can increase DR5 expression [37] and we examined this possibility in these tumors. Our results show a modest increase in DR5 expression in the Apo2L/TRAIL-sensitive human tumor following CPT-11 treatment, confirming our earlier observations in a different sensitive human colon tumor [7]. BAX expression was slightly increased in both tumors following CPT-11 treatment, in agreement with previous observations in a prostate tumor xenograft in vivo [26]. Again, further study in a larger number of tumors is needed to elucidate the mechanism(s) by which the CPT-11-Apo2L/TRAIL combination produces the enhanced anti-tumor effect as well as to more clearly define the differences in the response of a sensitive versus a resistant tumor.
In conclusion, the present study suggests that in a clinical setting, human colon tumors will inherently exhibit a wide range of sensitivity to Apo2L/TRAIL and some may even be comparatively resistant. However, our results demonstrate that Apo2L/TRAIL can augment the anti-tumor effect of CPT-11, even in Apo2L/TRAIL-resistant tumors. In fact, our results suggest that combined with Apo2L/TRAIL, doses of CPT-11 may be lowered, thus reducing toxicity and improving the quality of life of patients.
Acknowledgements
This project was supported by grant numbers CA108888-01A1 and P30CA016056 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. The authors thank Rose Pitoniak for her assistance in these studies.
References
- 1.Johnson JI, Decker S, Zaharevitz D, Rubinstein LV, Venditti JM, Schepartz S, Kalyandrug S, Christian M, Arbuck S, Hollingshead M, Sausville EA. Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br J Cancer. 2001;84:1424–1431. doi: 10.1054/bjoc.2001.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Voskoglou-Nomikos T, Pater JL, Seymour L. Clinical predictive value of the in vitro cell line, human xenograft, and mouse allograft preclinical cancer models. Clin Cancer Res. 2003;9:4227–4239. [PubMed] [Google Scholar]
- 3.Fu XY, Besterman JM, Monosov A, Hoffman RM. Models of human metastatic colon cancer in nude mice orthotopically constructed by using histologically intact patient specimens. Proc Natl Acad Sci USA. 1991;88:9345–9349. doi: 10.1073/pnas.88.20.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubio-Viqueira B, Jimeno A, Cusatis G, Zhang X, Iacobuzio-Donahue C, Karikari C, Shi C, Danenberg K, Danenberg PV, Kuramochi H, Tanaka K, Singh S, Salimi-Moosavi H, Bouraoud N, Amador ML, Altiok S, Kulesza P, Yeo C, Messersmith W, Eshleman J, Hruban RH, Maitra A, Hidalgo M. An in vivo platform for translational drug development in pancreatic cancer. Clin Cancer Res. 2006;12:4652–4661. doi: 10.1158/1078-0432.CCR-06-0113. [DOI] [PubMed] [Google Scholar]
- 5.Ghamande S, Hylander BL, Oflazoglu E, Lele S, Fanslow W, Repasky EA. Recombinant CD40 ligand therapy has significant antitumor effects on CD40-positive ovarian tumor xenografts grown in SCID mice and demonstrates an augmented effect with cisplatin. Cancer Res. 2001;61:7556–7562. [PubMed] [Google Scholar]
- 6.Hylander BL, Pitoniak R, Penetrante RB, Gibbs JF, Oktay D, Cheng J, Repasky EA. The anti-tumor effect of Apo2L/TRAIL on patient pancreatic adenocarcinomas grown as xenografts in SCID mice. J Transl Med. 2005;3:22. doi: 10.1186/1479-5876-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naka T, Sugamura K, Hylander BL, Widmer MB, Rustum YM, Repasky EA. Effects of tumor necrosis factor-related apoptosis-inducing ligand alone and in combination with chemotherapeutic agents on patients' colon tumors grown in SCID mice. Cancer Res. 2002;62:5800–5806. [PubMed] [Google Scholar]
- 8.Kelley SK, Ashkenazi A. Targeting death receptors in cancer with Apo2L/TRAIL. Curr Opin Pharmacol. 2004;4:333–339. doi: 10.1016/j.coph.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Ashkenazi A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat Rev Cancer. 2002;2:420–430. doi: 10.1038/nrc821. [DOI] [PubMed] [Google Scholar]
- 10.Almasan A, Ashkenazi A. Apo2L/TRAIL: apoptosis signaling, biology, and potential for cancer therapy. Cytokine Growth Factor Rev. 2003;14:337–348. doi: 10.1016/s1359-6101(03)00029-7. [DOI] [PubMed] [Google Scholar]
- 11.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 12.Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem. 1996;271:12687–12690. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- 13.Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, Smith C, Smolak P, Goodwin RG, Rauch CT, Schuh JC, Lynch DH. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 14.Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, DeForge L, Koumenis IL, Lewis D, Harris L, Bussiere J, Koeppen H, Shahrokh Z, Schwall RH. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan G, O'Rourke K, Chinnaiyan AM, Gentz R, Ebner R, Ni J, Dixit VM. The receptor for the cytotoxic ligand TRAIL. Science. 1997;276:111–113. doi: 10.1126/science.276.5309.111. [DOI] [PubMed] [Google Scholar]
- 16.Schneider P, Thome M, Burns K, Bodmer JL, Hofmann K, Kataoka T, Holler N, Tschopp J. TRAIL receptors 1 (DR4) and 2 (DR5) signal FADD-dependent apoptosis and activate NF-κB. Immunity. 1997;7:831–836. doi: 10.1016/s1074-7613(00)80401-x. [DOI] [PubMed] [Google Scholar]
- 17.Walczak H, Degli-Esposti MA, Johnson RS, Smolak PJ, Waugh JY, Boiani N, Timour MS, Gerhart MJ, Schooley KA, Smith CA, Goodwin RG, Rauch CT. TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. EMBO J. 1997;16:5386–5397. doi: 10.1093/emboj/16.17.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galligan L, Longley DB, McEwan M, Wilson TR, McLaughlin K, Johnston PG. Chemotherapy and TRAIL-mediated colon cancer cell death: the roles of p53, TRAIL receptors, and c-FLIP. Mol Cancer Ther. 2005;4:2026–2036. doi: 10.1158/1535-7163.MCT-05-0262. [DOI] [PubMed] [Google Scholar]
- 19.Walker DR, Bond JP, Tarone RE, Harris CC, Makalowski W, Boguski MS, Greenblatt MS. Evolutionary conservation and somatic mutation hotspot maps of p53: correlation with p53 protein structural and functional features. Oncogene. 1999;18:211–218. doi: 10.1038/sj.onc.1202298. [DOI] [PubMed] [Google Scholar]
- 20.LeBlanc H, Lawrence D, Varfolomeev E, Totpal K, Morlan J, Schow P, Fong S, Schwall R, Sinicropi D, Ashkenazi A. Tumor-cell resistance to death receptor-induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog Bax. Nat Med. 2002;8:274–281. doi: 10.1038/nm0302-274. [DOI] [PubMed] [Google Scholar]
- 21.Jazirehi AR, Ng CP, Gan XH, Schiller G, Bonavida B. Adriamycin sensitizes the Adriamycin-resistant 8226/Dox40 human multiple myeloma cells to Apo2L/tumor necrosis factor-related apoptosis-inducing ligand-mediated (TRAIL) apoptosis. Clin Cancer Res. 2001;7:3874–3883. [PubMed] [Google Scholar]
- 22.Lacour S, Hammann A, Wotawa A, Corcos L, Solary E, Dimanche-Boitrel MT. Anticancer agents sensitize tumor cells to tumor necrosis factor-related apoptosis-inducing ligand-mediated caspase-8 activation and apoptosis. Cancer Res. 2001;61:1645–1651. [PubMed] [Google Scholar]
- 23.Gliniak B, Le T. Tumor necrosis factor-related apoptosis-inducing ligand's antitumor activity in vivo is enhanced by the chemotherapeutic agent CPT-11. Cancer Res. 1999;59:6153–6158. [PubMed] [Google Scholar]
- 24.Jin H, Yang R, Fong S, Totpal K, Lawrence D, Zheng Z, Ross J, Koeppen H, Schwall R, Ashkenazi A. Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand cooperates with chemotherapy to inhibit orthotopic lung tumor growth and improve survival. Cancer Res. 2004;64:4900–4905. doi: 10.1158/0008-5472.CAN-04-0408. [DOI] [PubMed] [Google Scholar]
- 25.Ravi R, Bedi A. Requirement of BAX for TRAIL/Apo2L-induced apoptosis of colorectal cancers: synergism with sulindac-mediated inhibition of Bcl-x(L) Cancer Res. 2002;62:1583–1587. [PubMed] [Google Scholar]
- 26.Ray S, Almasan A. Apoptosis induction in prostate cancer cells and xenografts by combined treatment with Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand and CPT-11. Cancer Res. 2003;63:4713–4723. [PubMed] [Google Scholar]
- 27.Kaserer K, Schmaus J, Bethge U, Migschitz B, Fasching S, Walch A, Herbst F, Teleky B, Wrba F. Staining patterns of p53 immunohistochemistry and their biological significance in colorectal cancer. The J Pathol. 2000;190:450–456. doi: 10.1002/(SICI)1096-9896(200003)190:4<450::AID-PATH545>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 28.Wang S, El-Deiry WS. Requirement of p53 targets in chemosensitization of colonic carcinoma to death ligand therapy. Proc Natl Acad Sci USA. 2003;100:15095–15100. doi: 10.1073/pnas.2435285100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheridan JP, Marsters SA, Pitti RM, Gurney A, Skubatch M, Baldwin D, Ramakrishnan L, Gray CL, Baker K, Wood WI, Goddard AD, Godowski P, Ashkenazi A. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science. 1997;277:818–821. doi: 10.1126/science.277.5327.818. [DOI] [PubMed] [Google Scholar]
- 30.Kim K, Fisher MJ, Xu SQ, el-Deiry WS. Molecular determinants of response to TRAIL in killing of normal and cancer cells. Clin Cancer Res. 2000;6:335–346. [PubMed] [Google Scholar]
- 31.Deveraux QL, Reed JC. IAP family proteins – suppressors of apoptosis. Genes Dev. 1999;13:239–252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 32.Goke R, Goke A, Goke B, Chen Y. Regulation of TRAIL-induced apoptosis by transcription factors. Cell Immunol. 2000;201:77–82. doi: 10.1006/cimm.2000.1650. [DOI] [PubMed] [Google Scholar]
- 33.Roth W, Reed JC. Apoptosis and cancer: when BAX is TRAILing away. Nat Med. 2002;8:216–218. doi: 10.1038/nm0302-216. [DOI] [PubMed] [Google Scholar]
- 34.Munshi A, Pappas G, Honda T, McDonnell TJ, Younes A, Li Y, Meyn RE. TRAIL (APO-2L) induces apoptosis in human prostate cancer cells that is inhibitable by Bcl-2. Oncogene. 2001;20:3757–3765. doi: 10.1038/sj.onc.1204504. [DOI] [PubMed] [Google Scholar]
- 35.Lincz LF, Yeh TX, Spencer A. TRAIL-induced eradication of primary tumour cells from multiple myeloma patient bone marrows is not related to TRAIL receptor expression or prior chemotherapy. Leukemia. 2001;15:1650–1657. doi: 10.1038/sj.leu.2402251. [DOI] [PubMed] [Google Scholar]
- 36.Zhang XD, Franco AV, Nguyen T, Gray CP, Hersey P. Differential localization and regulation of death and decoy receptors for TNF-related apoptosis-inducing ligand (TRAIL) in human melanoma cells. J Immunol. 2000;164:3961–3970. doi: 10.4049/jimmunol.164.8.3961. [DOI] [PubMed] [Google Scholar]
- 37.Nagane M, Pan G, Weddle JJ, Dixit VM, Cavenee WK, Huang HJ. Increased death receptor 5 expression by chemotherapeutic agents in human gliomas causes synergistic cytotoxicity with tumor necrosis factor-related apoptosis-inducing ligand in vitro and in vivo. Cancer Res. 2000;60:847–853. [PubMed] [Google Scholar]