Abstract
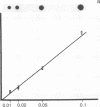
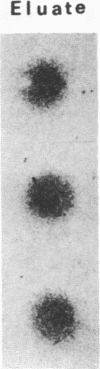
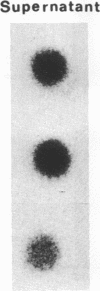
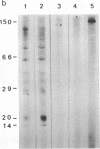
Helper T cells with receptors specific for IgD have immunoaugmenting properties. We have now detected soluble IgD-binding factor in cell supernatants immobilized on nitrocellulose paper by their ability to bind 125I-labeled IgD. IgD-binding factor is released by normal splenic T cells stimulated with recombinant interleukin 2, recombinant interleukin 4, or crosslinked IgD in amounts paralleling the induction of IgD receptors on the cells. IgD receptors are constitutively produced by antigen-specific helper T-cell hybridomas 2H10 and A3.4C6. Incubation of these hybridoma cells with recombinant interleukin 2 increases release of IgD-binding factor while reducing expression of IgD receptors. Specificity of the binding factor for IgD is established by (i) competitive inhibition; (ii) the ability of the binding factor to bind radiolabeled IgD and not monoclonal IgE, IgG2a, or polyclonal IgG; and (iii) the removal of the binding factor on passage through an IgD-Sepharose column and recovery in a subsequent acid eluate.
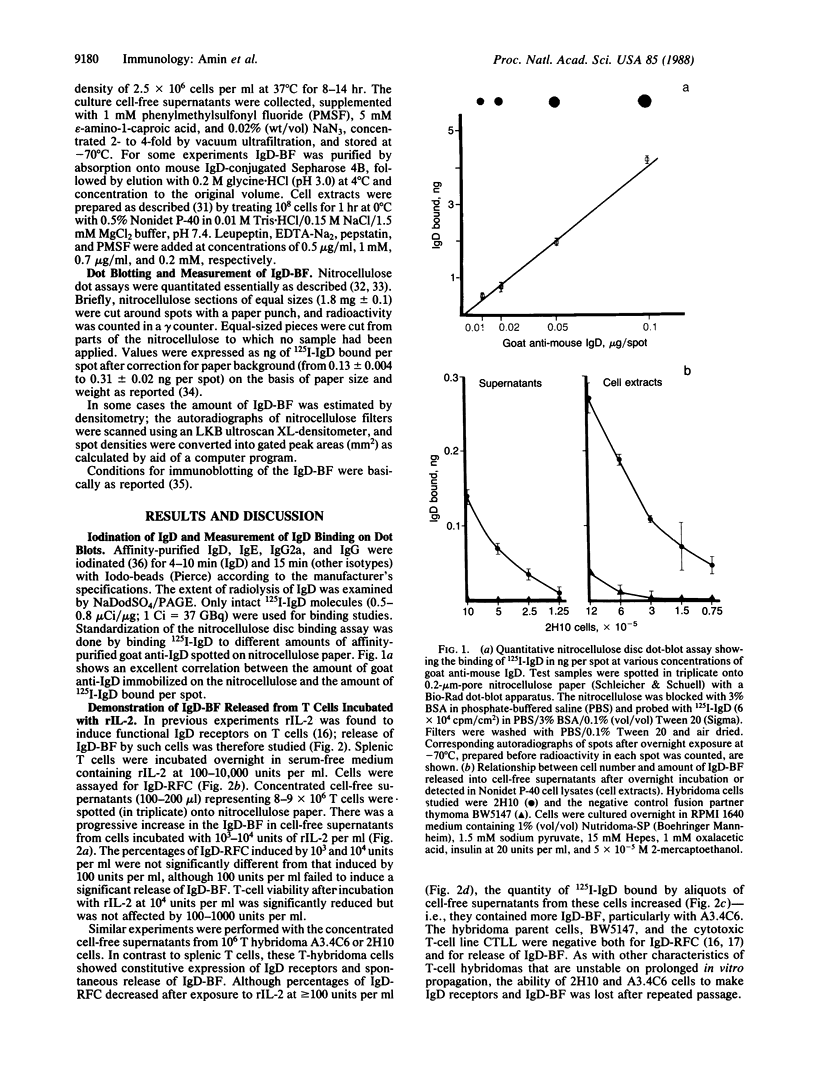
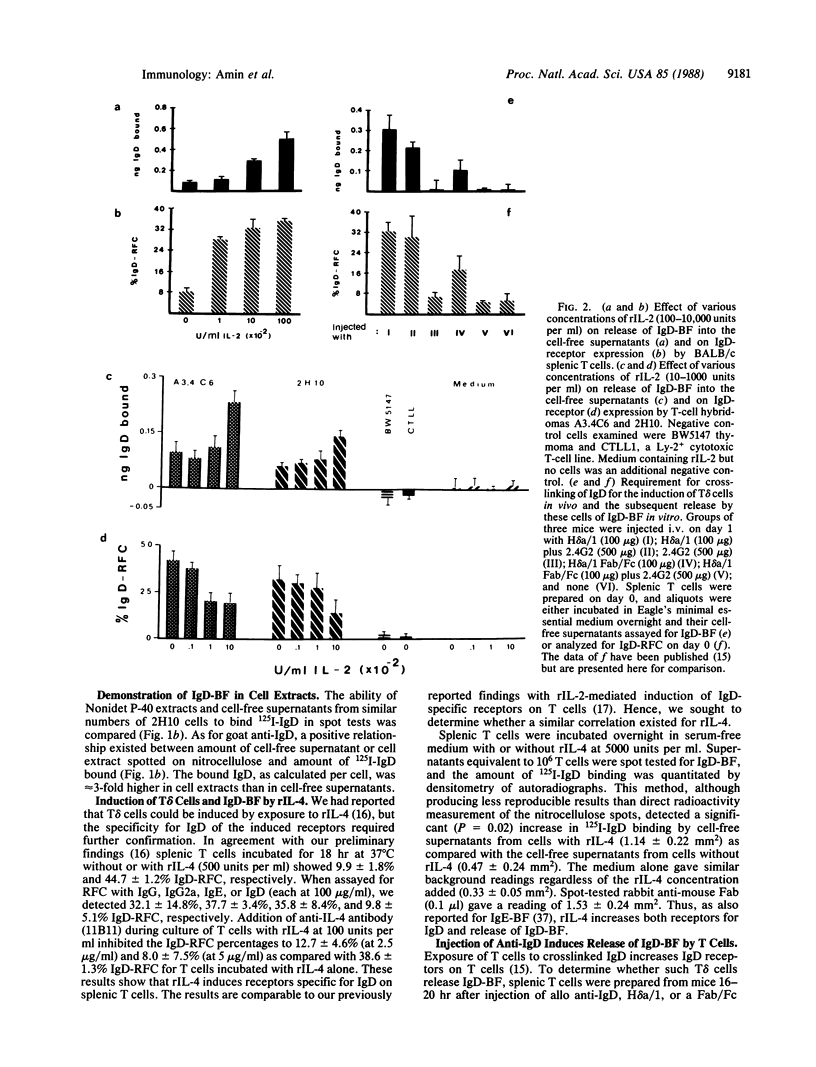
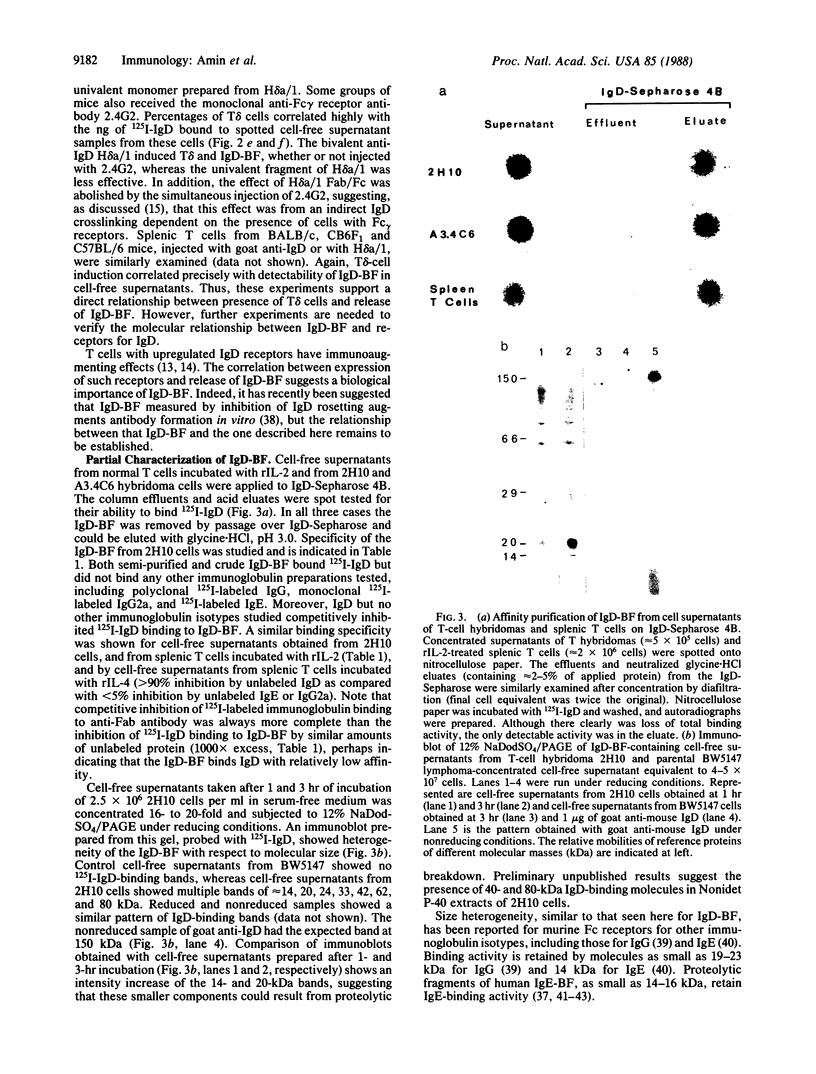
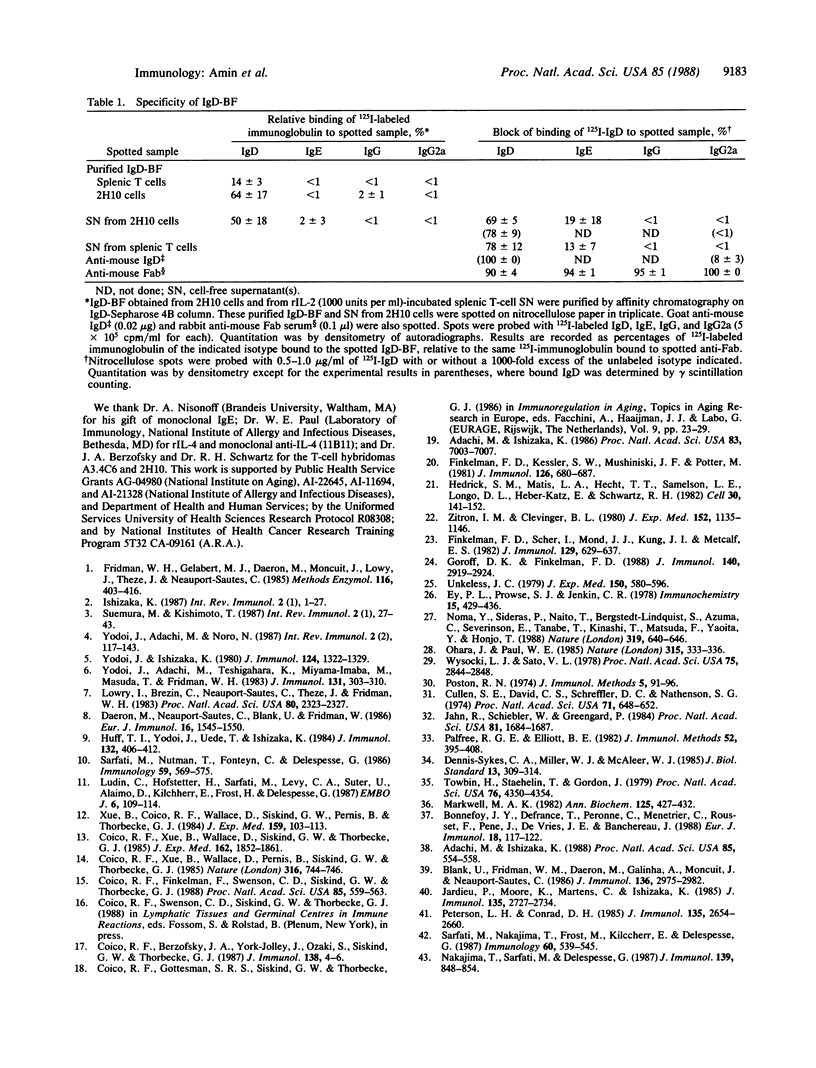
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi M., Ishizaka K. Enhancement of antibody responses by IgD-binding factors induced by anti-IgD treatment of spleen cells. Proc Natl Acad Sci U S A. 1988 Jan;85(2):554–558. doi: 10.1073/pnas.85.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi M., Ishizaka K. IgD-binding factors from mouse T lymphocytes. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7003–7007. doi: 10.1073/pnas.83.18.7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank U., Fridman W. H., Daëron M., Galinha A., Moncuit J., Néauport-Sautès C. Size and charge heterogeneity of murine IgG-binding factors (IgG-BF). J Immunol. 1986 Apr 15;136(8):2975–2982. [PubMed] [Google Scholar]
- Bonnefoy J. Y., Defrance T., Peronne C., Menetrier C., Rousset F., Pène J., De Vries J. E., Banchereau J. Human recombinant interleukin 4 induces normal B cells to produce soluble CD23/IgE-binding factor analogous to that spontaneously released by lymphoblastoid B cell lines. Eur J Immunol. 1988 Jan;18(1):117–122. doi: 10.1002/eji.1830180118. [DOI] [PubMed] [Google Scholar]
- Coico R. F., Berzofsky J. A., York-Jolley J., Ozaki S., Siskind G. W., Thorbecke G. J. Physiology of IgD. VII. Induction of receptors for IgD on cloned T cells by IgD and interleukin 2. J Immunol. 1987 Jan 1;138(1):4–6. [PubMed] [Google Scholar]
- Coico R. F., Finkelman F., Swenson C. D., Siskind G. W., Thorbecke G. J. Exposure to crosslinked IgD induces receptors for IgD on T cells in vivo and in vitro. Proc Natl Acad Sci U S A. 1988 Jan;85(2):559–563. doi: 10.1073/pnas.85.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coico R. F., Xue B., Wallace D., Pernis B., Siskind G. W., Thorbecke G. J. T cells with receptors for IgD. Nature. 1985 Aug 22;316(6030):744–746. doi: 10.1038/316744a0. [DOI] [PubMed] [Google Scholar]
- Coico R. F., Xue B., Wallace D., Siskind G. W., Thorbecke G. J. Physiology of IgD. VI. Transfer of the immunoaugmenting effect of IgD with T delta-containing helper cell populations. J Exp Med. 1985 Dec 1;162(6):1852–1861. doi: 10.1084/jem.162.6.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen S. E., David C. S., Shreffler D. C., Nathenson S. G. Membrane molecules determined by the H-2 associated immune response region: isolation and some properties. Proc Natl Acad Sci U S A. 1974 Mar;71(3):648–652. doi: 10.1073/pnas.71.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daëron M., Néauport-Sautès C., Blank U., Fridman W. H. 2.4G2, a monoclonal antibody to macrophage Fc gamma receptors, reacts with murine T cell Fc gamma receptors and IgG-binding factors. Eur J Immunol. 1986 Dec;16(12):1545–1550. doi: 10.1002/eji.1830161213. [DOI] [PubMed] [Google Scholar]
- Dennis-Sykes C. A., Miller W. J., McAleer W. J. A quantitative Western Blot method for protein measurement. J Biol Stand. 1985 Oct;13(4):309–314. doi: 10.1016/s0092-1157(85)80044-5. [DOI] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Finkelman F. D., Kessler S. W., Mushinski J. F., Potter M. IgD-secreting murine plasmacytomas: identification and partial characterization of two IgD myeloma proteins. J Immunol. 1981 Feb;126(2):680–687. [PubMed] [Google Scholar]
- Finkelman F. D., Scher I., Mond J. J., Kung J. T., Metcalf E. S. Polyclonal activation of the murine immune system by an antibody to IgD. I. Increase in cell size and DNA synthesis. J Immunol. 1982 Aug;129(2):629–637. [PubMed] [Google Scholar]
- Fridman W. H., Gelabert M. J., Daëron M., Moncuit J., Löwy I., Thèze J., Néauport-Sautès C. IgG-binding factors. Methods Enzymol. 1985;116:403–416. doi: 10.1016/s0076-6879(85)16032-5. [DOI] [PubMed] [Google Scholar]
- Goroff D. K., Finkelman F. D. Activation of B cells in vivo by a Fab/Fc fragment of a monoclonal anti-IgD antibody requires an interaction between the antibody fragment and a cellular IgG Fc receptor. J Immunol. 1988 May 1;140(9):2919–2924. [PubMed] [Google Scholar]
- Hedrick S. M., Matis L. A., Hecht T. T., Samelson L. E., Longo D. L., Heber-Katz E., Schwartz R. H. The fine specificity of antigen and Ia determinant recognition by T cell hybridoma clones specific for pigeon cytochrome c. Cell. 1982 Aug;30(1):141–152. doi: 10.1016/0092-8674(82)90020-4. [DOI] [PubMed] [Google Scholar]
- Huff T. F., Yodoi J., Uede T., Ishizaka K. Presence of an antigenic determinant common to rat IgE-potentiating factor, IgE-suppressive factor, and Fc epsilon receptors on T and B lymphocytes. J Immunol. 1984 Jan;132(1):406–412. [PubMed] [Google Scholar]
- Ishizaka K. T cell factors involved in the isotype-specific regulation of the IgE antibody response. Int Rev Immunol. 1987 Jan;2(1):1–26. doi: 10.3109/08830188709044744. [DOI] [PubMed] [Google Scholar]
- Jahn R., Schiebler W., Greengard P. A quantitative dot-immunobinding assay for proteins using nitrocellulose membrane filters. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1684–1687. doi: 10.1073/pnas.81.6.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardieu P., Moore K., Martens C., Ishizaka K. Relationship among IgE-binding factors with various molecular weights. J Immunol. 1985 Oct;135(4):2727–2734. [PubMed] [Google Scholar]
- Löwy I., Brezin C., Neauport-Sautes C., Theze J., Fridman W. H. Isotype regulation of antibody production: T-cell hybrids can be selectively induced to produce IgG1 and IgG2 subclass-specific suppressive immunoglobulin-binding factors. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2323–2327. doi: 10.1073/pnas.80.8.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüdin C., Hofstetter H., Sarfati M., Levy C. A., Suter U., Alaimo D., Kilchherr E., Frost H., Delespesse G. Cloning and expression of the cDNA coding for a human lymphocyte IgE receptor. EMBO J. 1987 Jan;6(1):109–114. doi: 10.1002/j.1460-2075.1987.tb04726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A. A new solid-state reagent to iodinate proteins. I. Conditions for the efficient labeling of antiserum. Anal Biochem. 1982 Sep 15;125(2):427–432. doi: 10.1016/0003-2697(82)90025-2. [DOI] [PubMed] [Google Scholar]
- Nakajima T., Sarfati M., Delespesse G. Relationship between human IgE-binding factors (IgE-BF) and lymphocyte receptors for IgE. J Immunol. 1987 Aug 1;139(3):848–854. [PubMed] [Google Scholar]
- Noma Y., Sideras P., Naito T., Bergstedt-Lindquist S., Azuma C., Severinson E., Tanabe T., Kinashi T., Matsuda F., Yaoita Y. Cloning of cDNA encoding the murine IgG1 induction factor by a novel strategy using SP6 promoter. Nature. 1986 Feb 20;319(6055):640–646. doi: 10.1038/319640a0. [DOI] [PubMed] [Google Scholar]
- Ohara J., Paul W. E. Production of a monoclonal antibody to and molecular characterization of B-cell stimulatory factor-1. Nature. 1985 May 23;315(6017):333–336. doi: 10.1038/315333a0. [DOI] [PubMed] [Google Scholar]
- Palfree R. G., Elliott B. E. An enzyme-linked immunosorbent assay (ELISA) for detergent solubilized Ia glycoproteins using nitrocellulose membrane discs. J Immunol Methods. 1982 Aug 13;52(3):395–408. doi: 10.1016/0022-1759(82)90010-2. [DOI] [PubMed] [Google Scholar]
- Peterson L. H., Conrad D. H. Fine specificity, structure, and proteolytic susceptibility of the human lymphocyte receptor for IgE. J Immunol. 1985 Oct;135(4):2654–2660. [PubMed] [Google Scholar]
- Poston R. N. A buffered chromic chloride method of attaching antigens to red cells: use in haemagglutination. J Immunol Methods. 1974 May;5(1):91–96. doi: 10.1016/0022-1759(74)90050-7. [DOI] [PubMed] [Google Scholar]
- Sarfati M., Nakajima T., Frost H., Kilccherr E., Delespesse G. Purification and partial biochemical characterization of IgE-binding factors secreted by a human B lymphoblastoid cell line. Immunology. 1987 Apr;60(4):539–545. [PMC free article] [PubMed] [Google Scholar]
- Sarfati M., Nutman T., Fonteyn C., Delespesse G. Presence of antigenic determinants common to Fc IgE receptors on human macrophages, T and B lymphocytes and IgE-binding factors. Immunology. 1986 Dec;59(4):569–575. [PMC free article] [PubMed] [Google Scholar]
- Suemura M., Kishimoto T. IgE class-specific regulatory factor(s) and Fc epsilon receptors on lymphocytes. Int Rev Immunol. 1987 Jan;2(1):27–42. doi: 10.3109/08830188709044745. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkeless J. C. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979 Sep 19;150(3):580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue B., Coico R., Wallace D., Siskind G. W., Pernis B., Thorbecke G. J. Physiology of IgD. IV. Enhancement of antibody production in mice bearing IgD-secreting plasmacytomas. J Exp Med. 1984 Jan 1;159(1):103–113. doi: 10.1084/jem.159.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yodoi J., Adachi M., Noro N. IgA binding factors and Fc receptors for IgA: comparative studies between IgA and IgE Fc receptor systems. Int Rev Immunol. 1987 Jan;2(2):117–141. doi: 10.3109/08830188709044750. [DOI] [PubMed] [Google Scholar]
- Yodoi J., Adachi M., Teshigawara K., Miyamainaba M., Masuda T., Fridman W. H. T cell hybridomas coexpressing Fc receptors (FcR) for different isotypes. II. IgA-induced formation of suppressive IgA binding factor(s) by a murine T hybridoma bearing Fc gamma R and Fc alpha R. J Immunol. 1983 Jul;131(1):303–310. [PubMed] [Google Scholar]
- Yodoi J., Ishizaka K. Lymphocytes bearing Fc receptors for IgE. IV. Formation of IgE-binding factor by rat T lymphocytes. J Immunol. 1980 Mar;124(3):1322–1329. [PubMed] [Google Scholar]
- Zitron I. M., Clevinger B. L. Regulation of murine B cells through surface immunoglobulin. I. Monoclonal anti-delta antibody that induces allotype-specific proliferation. J Exp Med. 1980 Nov 1;152(5):1135–1146. doi: 10.1084/jem.152.5.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]