Summary
We tested the involvement of cognition in adult experience-dependent neuroplasticity using primate cortical implants. In a prior study, learning an operant sensory discrimination increased cortical excitability and target selectivity. Here, the prior task was separated into three behavioral phases. First, naive animals were exposed to stimulus-reward pairings from the prior study. These yoked animals did not have to discriminate to be rewarded and did not learn the discrimination. The plasticity observed in the prior study did not occur. Second, the animals were classically conditioned to discriminate the same stimuli in a simplified format. Learning was accompanied by increased sensory response strength and an increased range of sensory inputs eliciting responses. The third study recreated the original operant discrimination, and selectivity for task targets increased. These studies demonstrate that cognitive association between sensory stimuli and reinforcers accompanies adult experience-dependent cortical plasticity and suggest that selectivity in representation and action are linked.
Introduction
Sensory-guided behavioral learning causes representational changes in primary sensory cortex (Jenkins et al., 1990; Recanzone et al., 1992a, 1992b, 1993; Xerri et al., 1994, 1999; Wang et al.,1995; Polley et al., 2004, 2006; Blake et al., 2002b, 2005). Multiple hypotheses exist to explain how behavior causes these changes. The timing hypothesis (Allard et al., 1991; Wang et al., 1995; Fu et al., 2002) explains change on the basis of the timing between different sensory stimuli. The temporal patterning of stimuli determines functional reorganization of their representations. Taken alone, the timing hypothesis is a bottom-up process, which means the sensory inputs matter, but the cognitive state does not matter. A second theory, the reward hypothesis, states that neural responses to sensory stimuli that precede reinforcers are strengthened (Kilgard and Merzenich, 1998). The reward hypothesis is not purely bottom-up, but it, as stated, does not require learning. Alternatively, by the reward-association hypothesis (Bakin and Weinberger, 1996; Bao et al., 2001), the changes seen in reinforced behaviors require animals to make a cognitive association between sensory stimuli and rewards. The animal must learn that the sensory stimuli predict a reward before the plasticity can occur. The objective of our study is to test these hypotheses in a single system.
Historically, there have been two categories of studies on the neural changes accompanying reinforced behaviors. In the first, a neuron or group of neurons is isolated and a reinforcement experiment conducted (Fritz et al., 2005; Bakin et al., 1996; Suga and Ma, 2003). This experiment can determine the effect of the reinforcement on a few neurons over a time course of a few hours. The second category of experiments has required a lengthy behavioral training period before recording a densely sampled cortical map of the relevant sensory cortex (Jenkins et al., 1990; Recanzone et al., 1992a, 1992b, 1993; Xerri et al., 1994, 1999; Wang et al., 1995; Polley et al., 2004; Beitel et al., 2003; Rutkowski and Weinberger, 2005). Upon determination of a complete map, highly detailed differences between trained and untrained animals may be defined. However, the mapping studies can be conducted at only one or two time points.
Cortical implants allow daily population measurements to be collected so that representations may be tracked throughout the learning process (Galvan and Weinberger, 2002), and micro-electrode implants additionally allow measurements of action potential output (Blake et al., 2002b, 2005), which has been closely related to perception and decisions (Mountcastle et al., 1966; Talbot et al., 1968; Newsome et al., 1989; Hernandez et al., 2000). Our long-term goal is to understand neural coding changes in perceptual learning by parallel studies of behavior, perceptual discrimination, and neural spiking in sensory cortex from before learning until steady-state task performance is reached. In the present study, we used implants to determine the representational plasticity in the cerebral cortex accompanying a cognitive association between sensation and reward.
Results
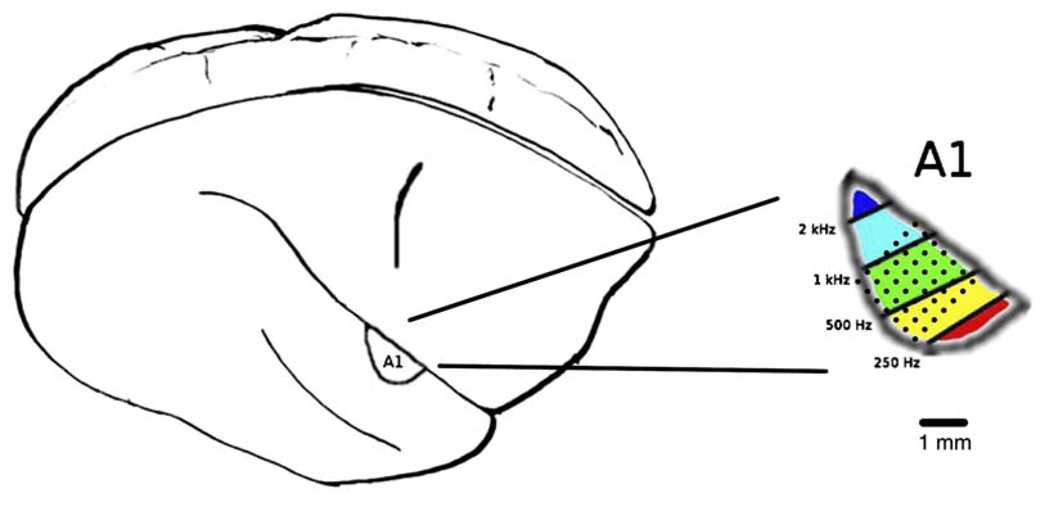
An array of 49 implant microelectrodes were positioned in primary auditory cortex, A1, of two adult owl monkeys similar to the example shown in Figure 1. Recordings from implanted microelectrodes were made before, during, and after each daily behavioral session. Sensory cortex plasticity may be defined as an altered physiological response to the same sensory stimuli. Because the learning process introduces changes in attention, motivation, and alertness which may independently alter physiological responses, receptive fields for plasticity analysis were defined using recordings made outside behavioral sessions. The passively seated animals were taught to wait through this 10 min period starting 2 months before the study was initiated. Although it is not possible to perfectly control the animal’s cognitive state while it is passively listening, animals showed little sign of changes in affect, alertness, or arousal during the prebehavioral data collection throughout the study. In contrast, the degree of motivation and determination displayed by the animals showed obvious changes during the behavioral sessions. Receptive fields were collected with sound stimuli that closely matched those in the behavioral trials in tone length, density, and intensity, as acoustic receptive fields only poorly predict responses to stimuli other than those used to measure the receptive fields (Blake and Merzenich, 2002). Animals had no laboratory experience in sensory discrimination behaviors prior to study.
Figure 1. Lateral View of Owl Monkey Brain Showing the Position of Right A1 Adjacent to the Lateral Sulcus.
The implant spans much of the portion of A1 that is exposed on the surface. The grid of dots in the right figure shows a putative overlay of electrode positions on the A1 cortical map.
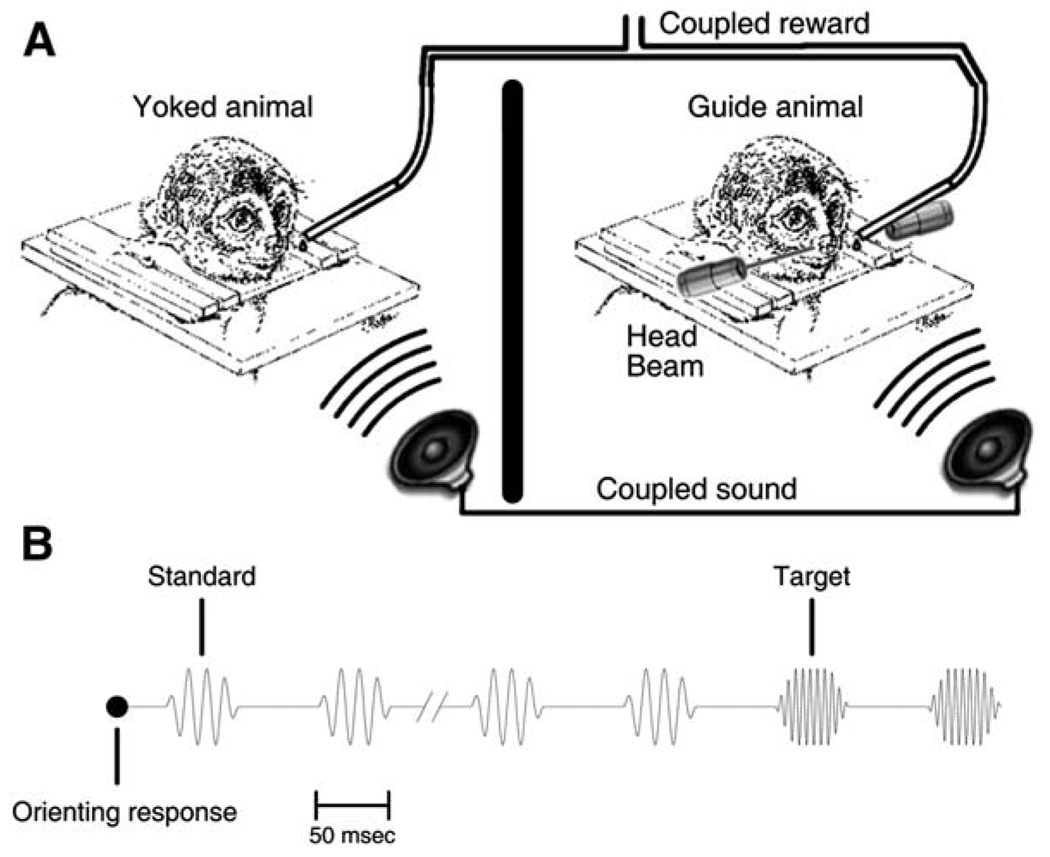
The first two behaviors test the hypothesis that pairing sounds with rewards causes plasticity. In prior work (Blake et al., 2002b), animals learned the frequency discrimination operant behavior shown in Figure 2B. If the pairing of sensory stimuli with rewards caused the observed cortical plasticity, then the same plasticity should result if naive animals are yoked to the old trials. In this behavior, the yoked animal is exposed to trials from the guide animal behavioral sessions. Within each yoked trial, all sound stimuli and rewards from the guide animal are presented with preserved temporal relations to the yoked animal. Therefore, some of the targets were presented with reward and some were not, depending on the responses of the guide animal. Furthermore, the yoked animal had no control over the trials, as seen in Figure 2A. Trial order for the yoked animal was randomized. The behavior was conducted in two phases, a before-learning phase and an after-learning phase. In the before-learning phase, the yoked animal was exposed to trials from a single behavioral session of the guide animal before it learned the frequency discrimination. In the after-learning phase, the yoked animal was exposed to trials after the guide animal learned the task. Each yoked behavioral phase lasted 2 weeks. More details are provided in the Experimental Procedures.
Figure 2. Yoked Animal Behavior.
(A) Yoked behavior. The guide animal performs an operant discrimination. The yoked animal hears the same sounds and receives the same rewards, but does not play a causal role.
(B) Behavioral task. The guide animal makes an orienting response, leaning its head forward to break an infrared beam, to initiate the trial. A series of standard frequency tone pips are played. After two to six consecutive standards, the tone pip frequency is increased to a target frequency. The guide animal is rewarded if it breaks its orienting response after the first target tone pip and within reaction time limits.
To determine whether the yoked animals learned to associate task targets with the liquid reward, conditioned responses were measured. A conditioned response is a behavioral response to the juice reward that is triggered by the predictive target tone. In this experiment, the conditioned response was licking the juice spout, and licking behavior before and after different tonal stimuli were examined to determine whether the animals were making frequency-selective conditioned responses.
During guide animal behavioral trials, as shown in Figure 2B, animals hear a series of short tonal stimuli, which increase in frequency from a standard to a target. If the animal makes an appropriate head movement shortly after the target, the trial is correct and the animal is rewarded. Errors result in short time-outs. Guide animal behavioral trials were taken from a prior study (Blake et al., 2002b), and no guide animal data are presented here.
The physiological changes observed in the yoked experiment are shown in Figure 3. The compound receptive field was measured immediately before initiating each day’s behavioral session, and examples of compound receptive fields are shown in Figures 3A and 3B. The output measures were excitability, target onset selectivity, and response range. Excitability is the integrated compound receptive field averaged across the behavioral phase. Target onset selectivity is the averaged receptive field fraction in the target frequency range and in the first 20 ms of the response (from 10 to 30 ms after stimulus onset). These onset responses were found to be most strongly plastic in our previous study (Blake et al., 2002b). The response range includes all frequencies with responses at least as strong as 25% of the maximal response, and this measurement was averaged across all days in each behavioral condition. In the transition from prelearning to postlearning yoked behavior, neither animal showed a significant change in cortical excitability or selectivity for the target stimuli, as shown in Figures 3C and 3D. One of the two yoked animals showed an increase in response range, seen in Figure 3E.
Figure 3. Yoked Behavior Physiological Plasticity.
(A) Example compound receptive field from animal one during the prelearning phase of the yoked behavior. Each row plots the firing rate changes measured in response to the corresponding frequency tone pip. The vertical bar on the ordinate covers the frequency range of the target stimuli. The horizontal bar on the abscissa shows the time at which the tone pip was on. The compound receptive field shows the sum of spiking activity from all electrodes. The color bar codes firing rate changes above and below the average prestimulus rate.
(B) Compound receptive field from animal one during yoked postlearning.
(C) Excitability during pre- (Y1) to postlearning (Y2) in animal one (A1) and animal two (A2).
(D) Target onset selectivity changes. No significant changes were found.
(E) Response range. Animal two showed a significant increase (t test, p < 0.05).
Error bars indicate standard error of the mean.
An analysis of the licking behavior during the yoked experiment was conducted to determine whether animals made frequency-discriminative licks and whether animals learned to anticipate rewards. In pre- and postlearning yoked behaviors, both animals started licking when the tone series began, increased their licking rate as the tone series continued, and stopped when the tones stopped, and did not lick again until after the reward was presented, or the next tone series began. Neither animal licked prior to the reward. Both animals showed an increase in lick rates for each consecutive tone within a trial. To test whether these increases were dependent on tone frequency, we tested whether the animals reacted differently to the first target than to the preceding standard. Two lick ratios were compared for each trial. The first was the ratio of the lick rate after the last standard divided by the rate after the second-to-last standard. The second was the lick rate after the first target divided by the lick rate after the last standard. The animals did not react differently to the first target than to the preceding standard (bootstrap test, p > 0.1 for all sessions). Although animals did make conditioned responses to tones, they did not learn to discriminate tones by frequency or that the tones could predict the occurrence of a reward.
After the yoked experiment, the behavior was changed to a simplified classical conditioning task. The targets and non-targets were separated by a larger time interval, and every target was followed by a reward with a fixed delay time. The experimental goal was for the animals to learn to make conditioned responses after the targets, and not after the non-targets, which would indicate a cognitive association between the target and the unconditioned stimulus, the juice reward. The same tonal stimuli were used. The classical conditioning behavior was run for 2 weeks.
Both animals made conditioned responses after both target and non-target stimuli and immediately prior to rewards, from the first days of the behavior. In addition, both animals learned to make conditioned responses after target stimuli more often than after non-target stimuli. Animal one demonstrated a preference to lick after targets more than after non-targets with significant differentiation of standards from targets on each day from the second to the last classical conditioning session (p < 0.05, tested using bootstrap). It made conditioned responses 4.45 times more frequently after a target than after a standard in the second week of training. In animal two, the animal’s lick patterns indicated a significant frequency discriminative anticipation of the reward (test on lick rates using bootstrap test, p < 0.05) on 6 of the last 8 days. Over the second week of training, the animal licked 1.14 times more frequently after targets than after standards. Although there are differences in lick selectivity between the two animals, the task required of each was the same.
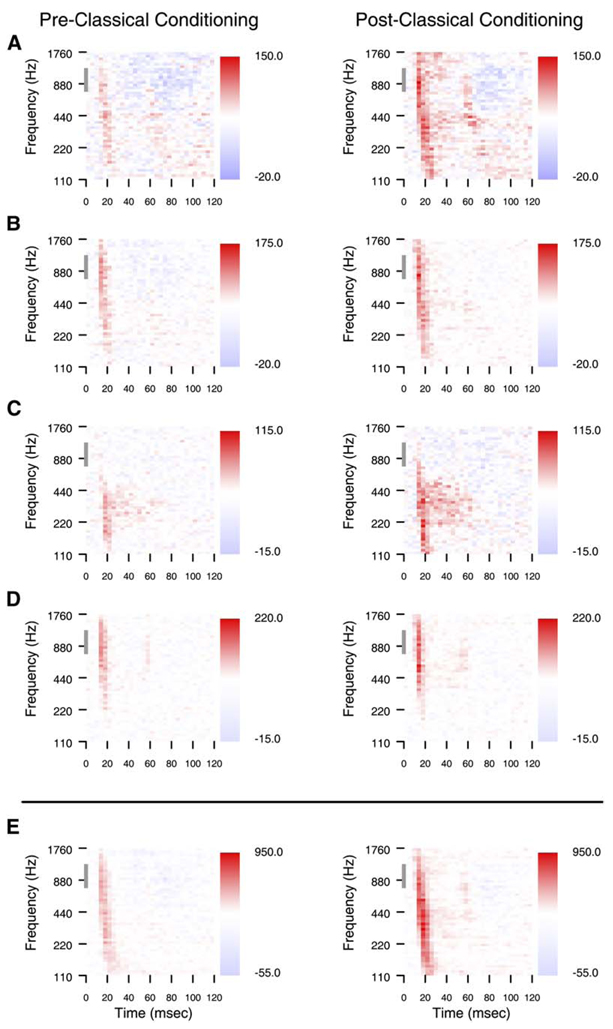
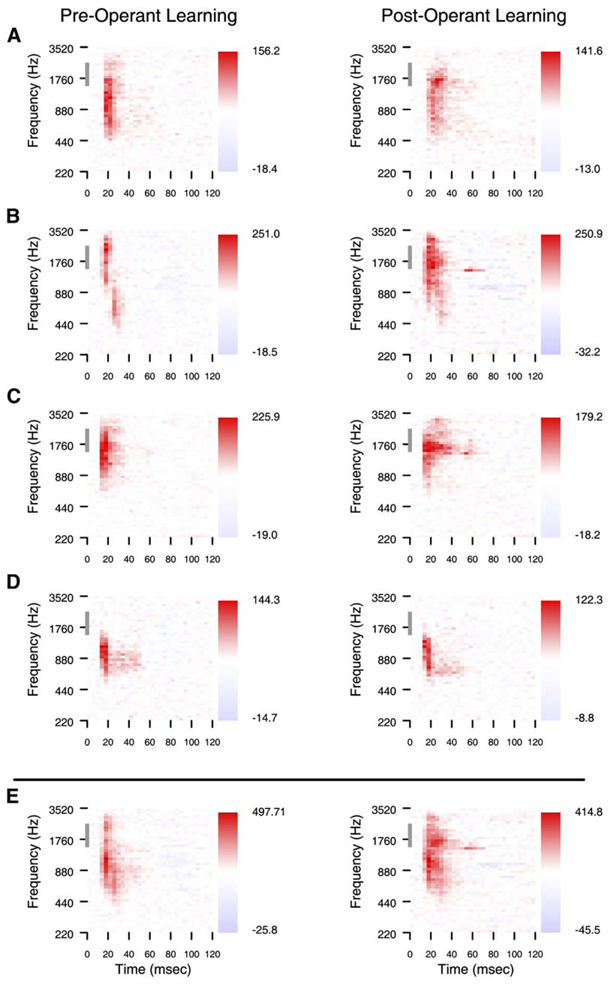
The changes in animal behavior were accompanied by changes in cortical excitability. Single-site examples chosen to demonstrate these excitability changes in animal one are shown in Figure 4. Receptive fields from four single sites, and the compound receptive fields, are shown on a day in the week prior to classical conditioning and before the fourth classical conditioning session. At each of the shown sites, receptive fields expand in spectral content and in strength, and these changes are reflected in the compound receptive field, which is the sum of all single-site receptive fields. The changes in excitability were not restricted to sites that had significant responses to the target or standard task stimuli before learning, as shown by the example in Figure 4C. Nor was the increased spectral content of receptive fields restricted to the target range. The examples in Figures 4A–4C all show enhanced responses to frequencies much lower than the task standard.
Figure 4. Matched Neural Responses before and after Classical Conditioning.
(A–D) Single-site examples of change. Four pairs of single-site examples of change from animal one are shown. The tonal receptive fields on the left are taken from one day in the week before initiating the classical conditioning behavior, and the receptive fields on the right from the same four sites are taken on the day of the fourth classical conditioning session. The gray bar on the ordinate indicates the frequency range of the conditioned stimuli. The distractor frequency was 660 Hz. Each site’s pair of receptive fields is normalized to the same maximum and minimum color scale, and changes in firing rate from the mean rate are shown next to the color scales.
(E) The compound receptive fields from the same two days. The compound receptive field is based on the sum of action potentials sampled from all active electrodes.
The number of microelectrode sites with significant tone-evoked responses ranged from 10 to 13 on different days in the classical conditioning behavior (mean 11.5) in animal one and from 0 to 12 during the postlearning yoked condition (mean 10.8). Almost half of these sites are shown in Figures 4A–4D. In both cases, all sites with consistent tone responses were from the same set of 15 sites. Electrodes that recorded inconsistently tended to be weakly responsive. Sites that responded vigorously yielded well-defined receptive fields every day. The mean number of sites in animal two were 9.7 in the second yoked condition and 9.4 in the classical conditioning condition, with similar phenomenology relating to sites that dropped in and out. There was no significant trend for changes in the distribution of sampled sites throughout the several month long period required to run all behavioral conditions.
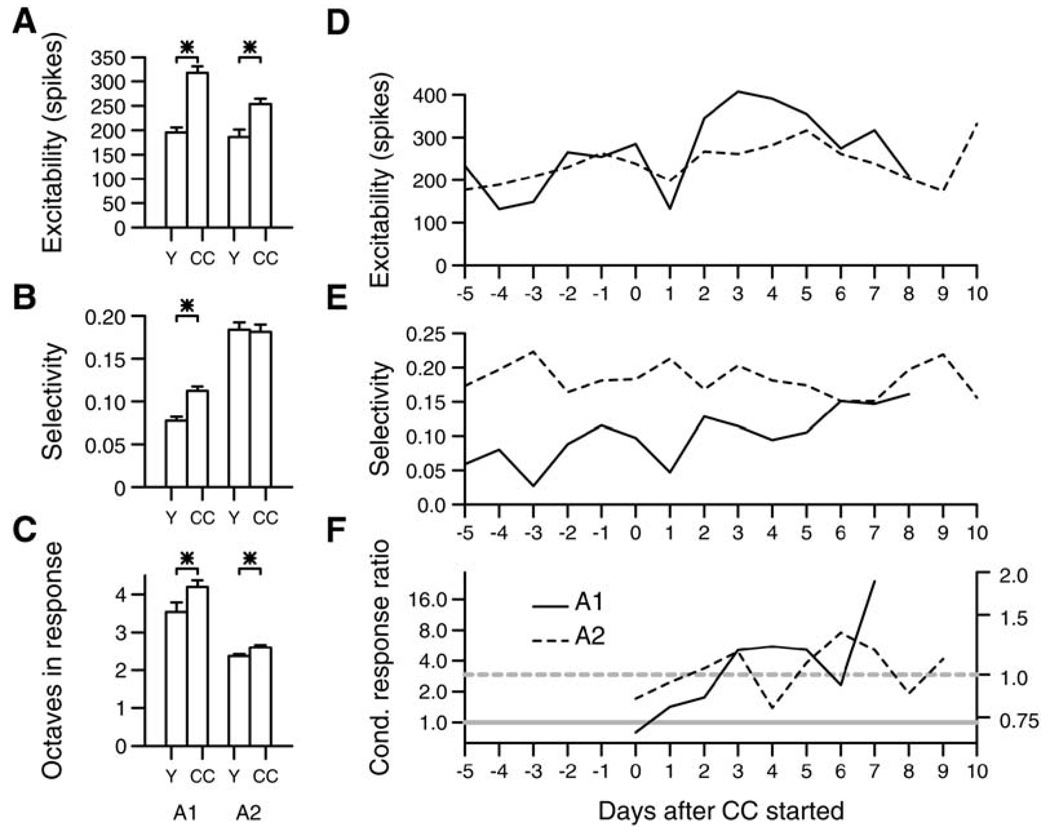
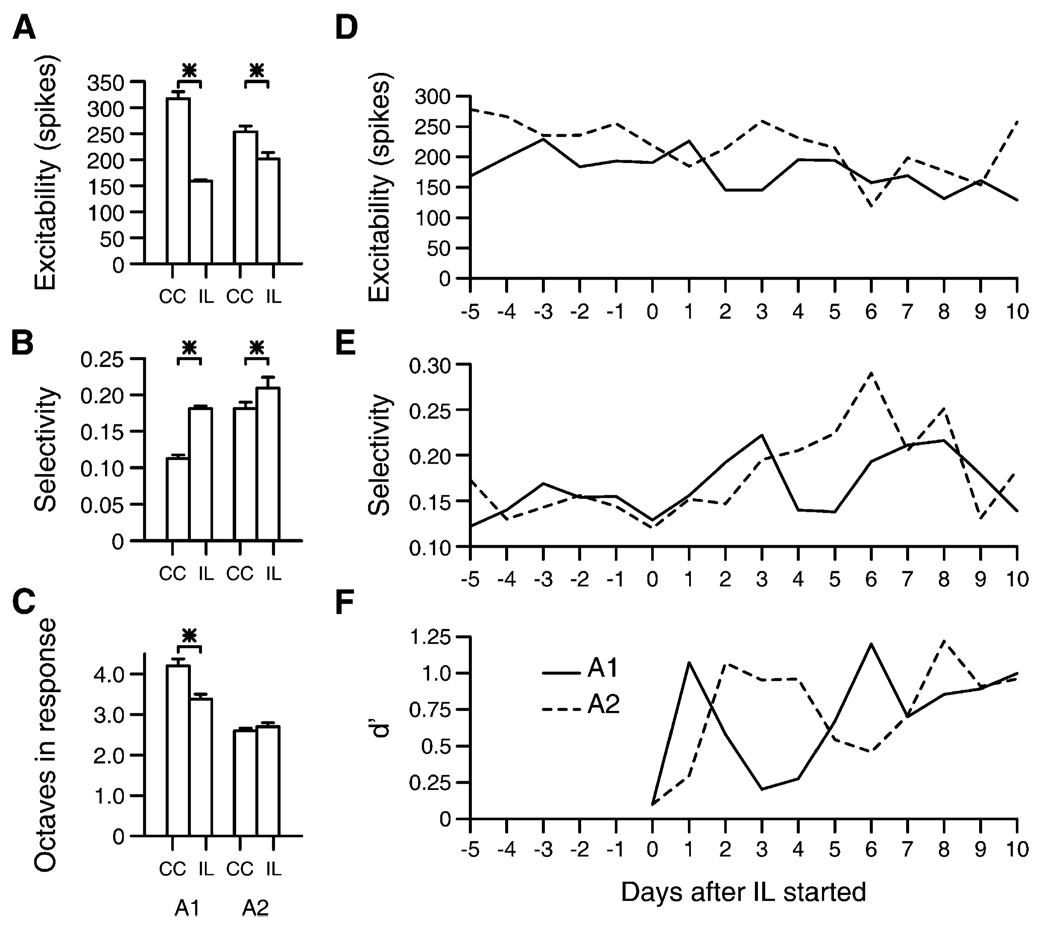
The population statistics, averaged across all sites and all days in each behavioral phase, from classical conditioning are shown in Figures 5A–5C. Cortical excitability, shown in Figure 5A, increased significantly in both animals (t test p < 0.001, both animals). Animal one had a 62% increase in responsiveness, and animal two had a 35% increase in responsiveness. However, the relative responsiveness to task target compared to non-target stimuli only increased in animal one (boot-strap test, p < 0.01), as shown in Figure 5B. In addition to these changes, both animals showed an increase in the range of frequencies that elicited a significant response in the receptive field (t test, p < 0.05), as shown in Figure 5C. The increase in animal one was 19%, and in animal two the increase was 10%.
Figure 5. Classical Conditioning Behavior Physiological Plasticity.
(A) Excitability changes. Both animals showed significant increases in excitability.
(B) Target onset selectivity changes. Animal one showed an increase in the target onset selectivity.
(C) Response range. Both animals showed a significant increase in the response range.
(D) Daily measurements of excitability changes. Day 0 was the first day of the classical conditioning behavior. Data from animal one are shown with a solid line, and data from animal two are shown with a dashed line.
(E) Daily measurements of selectivity changes.
(F) Daily measurements of the conditioned response ratio, or the ratio of the licking rate after target stimuli to the rate after non-target stimuli. The left ordinate scale applies to animal one, and the right scale applies to animal two. Gray dashed and nondashed lines show the ratio expected by chance for each animal. Data are log-scaled on the ordinates.
Error bars indicate standard error of the mean.
Daily measurements of the changes of selectivity, excitability, and conditioned responses are shown in Figures 5D–5F, to evaluate the progression of changes in the output measurements as they relate to the changes in the animal’s behavioral responses. The first physiological changes that could be attributed to a day’s behavior would occur in the next day’s recordings, as physiological measurements for each day were defined prior to behavior. The learned discrimination occurred when animals made significantly more licks after a target stimulus than after a non-target stimulus, and this occurred on the second behavioral session in animal one and the third behavioral session in animal two. A comparison of the last six recordings prior to the frequency discrimination with the six sessions after learning shows a significant increase in both animals’ excitability (t test, p < 0.02 in each animal). Animal one also shows a lag between the changes in physiologically measured selectivity and the task learning (p < 0.02, ranksum test).
In the final experiment, animals were trained in an instrumental learning task as in our previous study (Blake et al., 2002b). The instrumental learning task requires behavioral responses to task targets and suppression of behavioral responses to task standards to avoid time-outs. Whereas classical conditioning requires associating rewards with task targets, it does not require suppression of responses to standards. Both animals learned the frequency discrimination within the first three behavioral sessions, as is detailed below.
The average response selectivity and strength of AI responses changed significantly in both animals during the operant task. Four examples taken to illustrate the range of selectivity changes are shown in Figures 6A–6D. Sites typically had broad enough selectivity to respond either to both target and non-target frequencies or to only non-target frequencies. Figures 6A–6C show examples of sites selective for both target and non-target frequencies that became more strongly selective for targets only. Sites with no target selectivity, such as the example shown in Figure 6D, faded in response strength. Consistent tone-evoked responses were found on nine to ten sites each day in animal two throughout the entire experiment, which prevents a detailed analysis of the contribution of categories of single-site changes to the overall compound receptive field change. In animal one, consistent tone-evoked responses were found on 10 to 12 sites daily throughout these two behavioral conditions, and similar single-site changes were found. The compound receptive field, which is the sum of all single-site receptive fields, shown in Figure 6E, shows the shift in selectivity to a greater preference for the onset response to the target frequencies.
Figure 6. Matched Neural Responses before and after Operant Conditioning.
(A–D) Single-site examples of change. Four pairs of single-site examples of change from animal two are shown. The tonal receptive fields on the left are taken from 1 day before initiating the instrumental learning behavior, and the receptive fields on the right from the same four sites are taken 2 weeks later. The gray bar on the ordinate indicates the frequency range of the conditioned stimuli. The distractor frequency was 1109 Hz. Each receptive field is independently normalized to its maximum and minimum.
(E) The compound receptive field shows the sum of all single-site receptive fields from the same two recording days.
Population data averaged across all sites and all days in each behavioral phase and statistical comparisons of differences are shown in Figures 7A–7C. Cortical responsiveness dropped in both animals (p < 0.05, t test). The responses showed greater selectivity for the first 20 ms of the responses in the target frequency range in both animals (bootstrap test, p < 0.05 in both animals). The range of frequencies that elicited a response decreased significantly in animal one (t test, p < 0.05). In Figures 7D–7F, a comparison is made of the time course of changes in selectivity, excitability, and behavioral performance. The discrimination index is d′, a measure of the average perceptual difference between standard and targets as determined by false alarm and hit rates (McDonough and Whalen, 1995). Chance behavior would result in a d′ value averaging 0, and a d′ greater than 2 corresponds to near-perfect performance. Discrimination was significantly better than chance on each day after the first day in animal one and on each day after day two in animal two (p < 0.05 for each day comparing hit and false positive rates, assuming binomial distribution for each).
Figure 7. Instrumental Learning Physiological Plasticity.
(A) Excitability changes. Both animals showed significant decreases in excitability from CC to IL conditions.
(B) Target onset selectivity changes. Both animals showed significant increases in the target onset selectivity.
(C) Response range. Animal one showed a significant decrease in the response range.
(D) Daily measurements of excitability in the week before, and 2 weeks after, initiation of the instrumental learning condition. Data from animal one are shown with a solid line, and data from animal two are shown with a dashed line.
(E) Daily measurements of selectivity before and after instrumental learning.
(F) Daily measurements of d′, a signal detection measure indicating the discriminability shown by the behavioral choices between the task standard and targets.
Error bars indicate standard error of the mean.
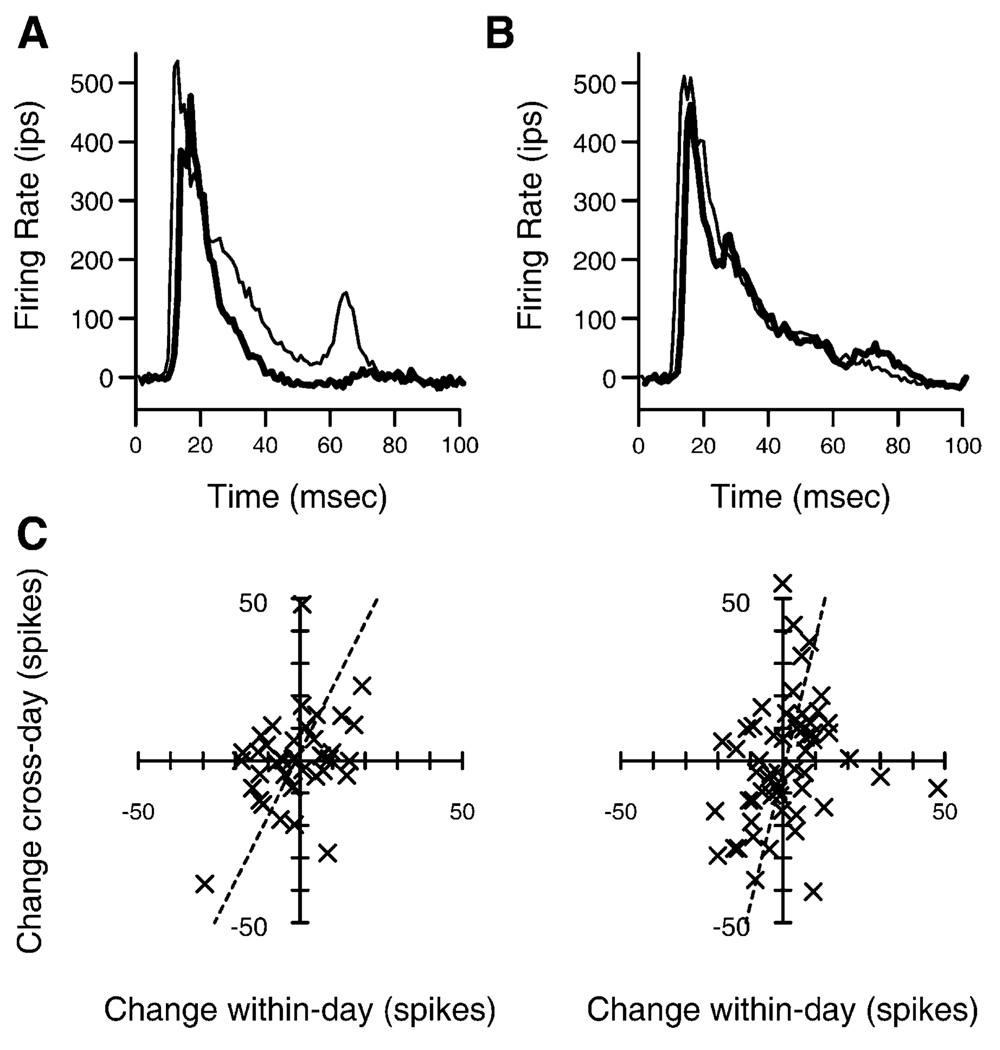
In addition to these changes in frequency selectivity and responsiveness, the temporal coherence of the target responses increased during the instrumental learning phase. Auditory cortical neurons often have both onset and sustained responses to tones (Wang et al., 2005). The response from 10 to 30 ms after stimulus onset, or onset response, was compared to the next 20 ms, or sustained response, across task phases. In both animals, the ratio of the sustained to the onset response decreased in the instrumental learning phase compared to the classical conditioning phase (ranksum test, p < 0.0001). The compound PSTHs to target and non-target frequency tone pips were compiled and averaged across each behavioral phase. These responses were then processed by subtracting the prestimulus rate and normalized by the first 20 ms of the response. The next 20 ms were then compared, datapoint by datapoint, using a ranksum test. The reduction in the later portion of the response did not occur in responses of the same neurons across a control frequency range that was never used behaviorally. Instead, within the control range, the onset and sustained responses tended to covary. Data in Figures 8A and 8B show the progression of this effect from the classical conditioning phase to the last week of instrumental learning in animal two.
Figure 8. Temporal Plasticity and Neuronal Consolidation.
(A) Population peristimulus time histogram showing the average firing rates after target tonal stimuli in the instrumental learning condition (thick line) and the classical conditioning condition (thin line). Data are from animal two.
(B) Population PSTH in response to non-target frequency stimuli in the same two behavioral conditions from the same neurons. These data show only a portion of all data considered in the cortical excitability measure.
(C) Correlation between within-day target onset response strength changes and across-day changes. Both animal one (left) and animal two (right) show significant positive correlations. A best-fit line is shown for each, and in each case the fit is significantly better than the null hypothesis of equality in change.
Another fundamental question is whether these changes are an accumulation of changes that occur within behavioral sessions or whether there are consolidative processes that occur between behavioral sessions. In addition to recording before each behavioral session, recordings were also made after each session. Within-session changes were defined as the difference between after-behavior responses and before-behavior responses. Across-day changes were defined as the difference between the next morning before-behavior responses and the current day before-behavior responses. Consistent findings in both animals occurred in the target onset responses. This measure is the integrated action potential count from 10 to 30 ms after stimulus onset in the range of the target frequencies, for example see Figures 3A and 3B. There was a significant correlation in the within-day changes and the across-day changes (r = 0.368 for animal one, r = 0.244 for animal two, p < 0.05, both animals), as shown in Figures 8C and 8D. This correlation means that if target onset excitability was greater after-behavior than before-behavior, there was a significant trend for the next day, before-behavior recording to show more target onset excitability than the previous day. A minimum perpendicular distance best-fit regression line approximated both correlations. In each case, the slope was greater than 1 (2.0 and 3.9), meaning the across-day changes were 2 to 3.9 times larger than the within-day changes. Alternately, the changes in this measure from after-behavior to the next morning were 1.0 to 2.9 times larger than the changes from before-behavior to after-behavior. To determine the significance of the >1 slope, we compared the goodness of fit of the best-fit line with the null hypothesis, a slope 1 line, and in each case the fit was significantly better with the best-fit line (ranksum test applied to distances from points to the lines, p < 0.001, both animals). This evidence is consistent with a consolidation process in which short-term changes in target onset excitability are similar in polarity and smaller than the long-term changes expressed after consolidation, when evaluated throughout the initial task-learning period.
Discussion
This work directly tests, and refutes, the hypothesis that pairing of sensory stimuli and rewards, without cognitive stimulus-reward association, is responsible for the adult experience-dependent cortical plasticity seen in reinforced learning studies. The yoked control experiment, combined with the classical conditioning study, demonstrates that cognitive association between sensory stimuli and reinforcement are required for this form of plasticity. The pairing of sound stimuli and reinforcers that caused plasticity in instrumental-learning-trained animals (Blake et al., 2002b) did not cause plasticity in yoked animals in the current study. A small change, making it easier for the animals to understand how the stimuli were related to reinforcement, led to learning and a change in cortical responsiveness during classical conditioning. The plasticity occurs only if a cognitive association between the reinforcer and the sensory stimulus forms, and not from simple stimulus-reward pairing.
Differences were also found between plasticity caused by learning in classical conditioning and instrumental learning. Once animals learned the reward association, engaging in the operant discrimination led to decreases in excitability and increases in selectivity. The obvious behavioral difference is that animals are required to withhold responses to non-target stimuli in the operant task, and their reward rate is unaffected by such responses in classical conditioning. Other work from our group (Beitel et al., 2003) has found that after a long period of operant sensory discrimination, both target and standard responses were globally suppressed relative to untrained control animals, although target responses were relatively stronger than responses to task standards. The existing evidence supports an active suppressive plasticity effect caused by some aspect of reinforced behavior. An interesting hypothesis is that behavioral plasticity mechanisms cause response decrements to task distractors or to misidentified task targets (Beitel et al., 2003). This effect would serve to complement, and compete with, the reward-association effects. Reward associations strengthen responses to targets, and the suppressive effect weakens responses to distractors, to enhance target-distractor contrast. A negative plasticity effect associated with task distractors may explain the smaller plasticity effects observed in visual orientation discrimination tasks in which stimuli are counterbalanced as targets and distractors (Ghose et al., 2002). Other orientation discrimination training (Schoups et al., 2001; Yang and Maunsell, 2004; Raiguel et al., 2006) finds increased tuning sharpness for neurons that have large firing rate changes between target and distractors, consistent with the theory that neuroplasticity serves both to strengthen target responses and to weaken distractor responses.
There are well-identified putative mechanisms that may couple the behavioral mechanisms with the plasticity observed in sensory cortex. Reward-association plasticity may be triggered by neuromodulatory nuclei (Richardson and DeLong, 1991; Schultz et al., 1997; Aston-Jones et al., 1994). The neurons in these nuclei respond phasically after primary rewards, or after sensory stimuli with a conditioned association to reward, and then release neuromodulators in sensory cortex. Some of these neuromodulatory nuclei have been linked directly to plasticity of sensory representations (Bakin and Weinberger, 1996; Kilgard and Merzenich, 1998; Bao et al., 2001). A possible cause of response decrements to task distractors is attentional activity. Such activity suppresses neural responses to non-target stimuli within the sensory receptive field of target-selective cortical neurons in the visual hierarchy (Desimone and Duncan, 1995; Reynolds and Desimone, 1999). The descending hierarchical projections that suppress non-target responses in attended behaviors may also have suppressive plasticity effects. Such effects would serve specifically to separate representations of targets and non-target stimuli that occur in the same behavior and result in plasticity that closely parallels the task demands (Polley et al., 2006; Raiguel et al., 2006).
Our results are not explainable based on changes in sampling. Prior to each day’s study, every putative auditory site was examined manually for tone-driven activity centered on, but not restricted to, the known selectivity at that site. Sites with consistent tone-evoked activity were used in that day’s recordings. Throughout the duration of our studies, every strongly tone-responsive site stayed strongly tone-responsive, and most weakly responsive sites continued to sample periodically throughout the experiment. The variability in sampling is the reason that a 2 week period was chosen for the experiments. This length of time allows enough averaging to detect the effects of the learning over the noise of the variation. Systematic alterations in sampling, such as the loss of a strongly responsive site, that accompanied changes in behavioral condition did not occur.
Our instrumental learning task causes a reduction in the later portion of the cortical response, which makes the response to the target frequencies more temporally restricted. Improved coherence in the onset response caused by operant sensory discrimination has been noted before in somatosensory cortex (Recanzone et al., 1992b). The analysis in the current study was motivated by the near complete lack of sustained responses in prior animals trained at this task (Blake et al., 2002b), in conjunction with a recent increased interest in sustained cortical responses to sound (Wang et al., 2005). Sustained responses to tone stimuli are not uncommon in awake primates (Recanzone et al., 2000), so the complete lack of sustained responses in trained animals was a potential anomaly. The reduction of this activity specific to the target frequencies indicates that the presence of sustained activity can be altered substantially by this reinforced sensory behavior, even though the behavior lacks temporal requirements in the same range of timing. Improved action potential coordination has been hypothesize to carry signal features in auditory cortex (deCharms and Merzenich, 1996).
Together, these studies (Blake et al., 2002b, 2005) suggest that formation of a cognitive association between sensory stimuli and reward, increases in cortical excitability, and spread of responses across the cortical surface (Blake et al., 2005) are complexly intertwined. With continued behavioral practice (Blake et al., 2002b), cortical responsiveness returns to its normal mode, while responses that act as reward predictors are suppressed less than other responses. The initial phase of increased excitability and spatial spread of responses may serve to greater enable modular association. Cortex typically shares responses across distances of about 0.5 mm (Mountcastle, 1957). The increase in cortical excitability and the expansion of frequency representations means that the size of the functional cortical column increases, and the number of cortical columns in any given cortical area decreases (Blake et al., 2002a). More simply, the cortex has been dedifferentiated. This initial plasticity period allows greater functional connectivity while the slower process of refining the responses to the most relevant stimuli, the targets, occurs. As the excitability and column size return to normal levels, the cortex has been functionally rewired by learning to generate more rapid, coherent responses to the task targets.
Experimental Procedures
Physiological Recordings
Data were obtained from two chronically implanted owl monkeys, Aotus trivirgatus. Microelectrodes were implanted into the presumed primary auditory cortex (A1). The A1 target was 2–3 mm anterior interaural and just lateral to the temporal:frontal fissure in the lateral bank of the lateral sulcus. Transcranial recording through burr holes confirmed A1 response characteristics and expected tonotopy (Imig et al., 1977). Surgery was performed under areflexic barbiturate anesthesia. Techniques for implantation are described in a methods paper (deCharms et al., 1999).
Recordings were made with parylene-insulated iridiummicroelectrodes (Micro Probe, MD) with tip exposures between 5 and 7 µm long, to maximize probability of sampling single units (Hubel, 1957). Implant best frequencies spanned the range of frequencies found on the exposed surface, ranging from 110 Hz to 12 kHz.
After implantation, a recovery period of several weeks ensued before recording was initiated. During training and recording sessions, the primate sat in a primate chair with its head positioned 24 inches in front of a free-field speaker. Single units were isolated online using the Rasputin system (Plexon, Inc, Dallas, TX), and 1.2 ms of spike waveform were stored for each unit discharge event, beginning 1 ms before a voltage threshold crossing. Implantation preceded behavioral study by 2–5 months.
Sound Presentation
All experiments were conducted in a double-walled anechoic chamber. Sound levels were calibrated with a Brüel and Kjær sound level meter using the “a” filter. Receptive field stimulus sounds were created digitally, recorded on an audio cd, and played through a Macintosh audio amplifier. Behavioral sounds were created using Lab-VIEW software (National Instruments) with 100 kHz sampling rates and played through the same audio amplifier. Sounds were played from a free-field speaker positioned approximately 24 inches in front of the animal. Each behavioral sound was a 50ms tone pip with 5ms raised sinusoidal ramps onset and offset ramp. The onset ramps can be described by the equation (1 − cos(2t/10ms))/2.0 for 0 < t < 5. Offset ramps are the time reverse of onset ramps. Tone pip stimuli were presented at 50 dB SPL.
Receptive field stimuli were 50 ms duration tones at 50 dB SPL, with 5 ms onset and offset ramps. Tones were played at one tone per octave per 700 ms (Blake and Merzenich, 2002), which is roughly the same presentation density used in the instrumental learning trials. The stimulus frequencies were spaced each 1/12 octave. Ten minutes of sound presentation occurred, and each tone was played approximately 70 times. Optimal linear receptive fields were reconstructed from the responses to the stimuli (Blake and Merzenich, 2002). The prestimulus mean rate was subtracted, and the integral at each frequency was determined. The single frequency integrals were thresholded at 25% of the largest integral, or strongest response. The range of frequencies sampled was the number of frequency bands that were above threshold divided by the sampling density, 1/12 octave. The integrated receptive field was the sum of all responses above threshold. The target onset response was the fraction of the total receptive field accounted for by the 10–30 ms period in the target frequency range.
The task standard was 622 Hz in animal one and 1109 Hz in animal two. Target ranges spanned from 3 to 12 semitones above the task standards. The most difficult target was a 3 semitone change, the easiest target was a 12 semitone change. For signal detection theory analysis, all targets were considered together as these animals have thresholds in the range of 0.25 to 1 semitone (Blake et al., 2002b). The control frequency range in Figures 8A and 8B is the octave below the standards.
Animal Behavior
The yoked and instrumental learning behavior were based on a limited hold reaction time behavior (Blake et al., 2002b). In this operant behavior, shown in Figure 2B, the animal initiated a trial by making an orienting response. The orienting movement was leaning the head forward to break an invisible infrared beam in front of the animal’s nose and maintaining the head in that position. After this orienting response, a series of standard stimuli, identical tone pips, were delivered. Two to six standard frequency stimuli were followed by stimuli that were higher in frequency, the target tone pips. Target tone pips repeated at the same interstimulus interval until the animal broke the orienting response. Correct responses occurred when the animal removed itself from the head beam later than 150 ms after the first target and earlier than 150 ms after the third target. Early error responses were false positives, and later errors were misses. False positives and misses resulted in 2–10 s time-outs. Rewards were followed by a dollop of orange-flavored drink (Tang).
The two yoked behaviors were based on a guide animal’s performance at the limited hold task. For a single day of performance, each guide animal trial was isolated, and the sounds within each trial were frequency shifted so the standards matched the chosen standards for the current animal. Then, all sounds and rewards were presented with preserved temporal relations to the yoked animal. In effect the yoked animal listens to another animal’s trials and gets rewarded at the same time. Trial order was randomized, and each behavioral session was terminated after the same number of liquid rewards. In all tasks, the number of daily rewards received by each animal was constant. The first yoked behavior was based on a behavioral session before the guide animal learned the frequency discrimination task (d′ = 0.10). The reward rate and relative consistency of pairing rewards with tones was low. The second yoked behavior was based on a session after the guide animal learned the task (d′ = 0.89), and reward rate was relatively high, and rewards were more consistently paired with sound stimuli. Each yoked task was run for 2 weeks, with each day’s trials drawn from the same statistical pool. The guide animal data were taken from a prior study (Blake et al., 2002b). No guide animal data are reported here.
The third task was a classical conditioning behavior. Each tone was presented at roughly 1000 ms intervals from all other tones, and every target tone was followed 500 ms later by a reward and a brief pause. The reward rate is roughly the same as the second yoked behavior, although about half as many tones are presented. The ratio of standards and targets was the same as the yoked behaviors, and each day’s behavior was terminated with the same number of rewards. The unconditioned stimulus was the liquid reward, and the unconditioned response was licking the juice spout. The conditioned stimuli were the target tones, and the standard tone stimuli were not conditioned. Comparisons were made between the animal’s conditioned response, or licking in response to tone stimuli, at comparable 400 ms periods after the target and standard stimuli. Significant discrimination was reached if the animal licked at a higher rate (using a bootstrap statistical model) after the targets than after the standards. The bootstrap statistical model (Efron and Tibshirani, 1998) assessed whether the lick rate measured in response to the target stimuli could be a result of resampling the lick responses to the standard stimuli with a probability less than 5%.
The fourth task was the operant limited hold frequency discrimination, identical to the task performed by the guide animals and described in Figure 2B. Threshold was determined with standard signal detection theory criteria (McDonough and Whalen, 1995). The probability of making a hit was compared to the false-positive probability for each of the two shorter trial lengths. The third, nonrandom, trial lengths were discarded for analysis. Hit and false-positive rates for each stimulus were used to calculate d′. The larger d′ values correspond to better discrimination performance. If animals perform at chance, d′ is distributed around zero.
Animal welfare was regulated by the Institutional Animal Care and Use Committee at the University of California, San Francisco.
Acknowledgments
D. Polley, R. Ramachandran, R. Beitel, J. Medina, L. Mundo, and E. Wong have provided useful commentary on this manuscipt and work. T. Phelps created artwork for Figure 2. The authors would like to thank K. Macleod, S. Patterson, K. McGary, L. Bocskai, M. Fong, and D. Frances for technical support. This work supported by NIH grants 5R01NS010413, 5R03DC005708, and the Coleman and Sooy funds.
References
- Allard T, Clark SA, Jenkins WM, Merzenich MM. Reorganization of somatosensory area 3b representations in adult owl monkeys after digital syndactyly. J. Neurophysiol. 1991;66:1048–1058. doi: 10.1152/jn.1991.66.3.1048. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Kubiak P, Alexinsky T. Locus coeruleus neurons in monkey are selectively activated by attended cues in a vigilance task. J. Neurosci. 1994;14:4467–4480. doi: 10.1523/JNEUROSCI.14-07-04467.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakin J, Weinberger N. Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proc. Natl. Acad. Sci. USA. 1996;93:11219–11224. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakin J, South D, Weinberger N. Induction of receptive field plasticity in the auditory cortex of the guinea pig during instrumental avoidance conditioning. Behav. Neurosci. 1996;110:905–913. doi: 10.1037//0735-7044.110.5.905. [DOI] [PubMed] [Google Scholar]
- Bao S, Chan VT, Merzenich MM. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412:79–83. doi: 10.1038/35083586. [DOI] [PubMed] [Google Scholar]
- Beitel R, Schreiner C, Cheung S, Wang X, Merzenich M. Reward-dependent plasticity in the primary auditory cortex of adult monkeys trained to discriminate temporally modulated signals. Proc. Natl. Acad. Sci. USA. 2003;100:11070–11075. doi: 10.1073/pnas.1334187100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake D, Merzenich M. Changes of AI receptive fields with sound density. J. Neurophysiol. 2002;88:3409–3420. doi: 10.1152/jn.00233.2002. [DOI] [PubMed] [Google Scholar]
- Blake D, Byl N, Merzenich M. Representation of the hand in the cerebral cortex. Behav. Brain Res. 2002a;135:179–184. doi: 10.1016/s0166-4328(02)00163-8. [DOI] [PubMed] [Google Scholar]
- Blake DT, Strata F, Churchland AK, Merzenich MM. Neural correlates of instrumental learning in primary auditory cortex. Proc. Nat. Acad. Sci. USA. 2002b;99:10114–10119. doi: 10.1073/pnas.092278099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake D, Strata F, Kempter R, Merzenich M. Experience- dependent plasticity in S1 caused by noncoincident inputs. J. Neurophysiol. 2005;94:2239–2250. doi: 10.1152/jn.00172.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deCharms RC, Merzenich MM. Primary cortical representation of sounds by the coordination of action-potential timing. Nature. 1996;381:610–613. doi: 10.1038/381610a0. [DOI] [PubMed] [Google Scholar]
- deCharms RC, Blake DT, Merzenich MM. A multielectrode implant device for the cerebral cortex. J. Neurosci. Meth. 1999;93:27–35. doi: 10.1016/s0165-0270(99)00087-4. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu. Rev. Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani R. An Introduction to the Bootstrap. Boca Raton, FL: Chapman & Hall CRC; 1998. [Google Scholar]
- Fritz J, Elhilali M, Shamma S. Differential dynamic plasticity of A1 receptive fields during multiple spectral tasks. J. Neurosci. 2005;25:7623–7635. doi: 10.1523/JNEUROSCI.1318-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Djupsund K, Gao H, Hayden B, Shen K, Dan Y. Temporal specificity in the cortical plasticity of visual space representation. Science. 2002;296:1999–2003. doi: 10.1126/science.1070521. [DOI] [PubMed] [Google Scholar]
- Galvan V, Weinberger N. Long-term consolidation and retention of learning-induced tuning plasticity in the auditory cortex of the guinea pig. Neurobiol. Learn. Mem. 2002;77:78–108. doi: 10.1006/nlme.2001.4044. [DOI] [PubMed] [Google Scholar]
- Ghose G, Yang T, Maunsell J. Physiological correlates of perceptual learning in monkey V1 and V2. J. Neurophysiol. 2002;87:1867–1888. doi: 10.1152/jn.00690.2001. [DOI] [PubMed] [Google Scholar]
- Hernandez A, Zainos A, Romo R. Neuronal correlates of sensory discrimination in the somatosensory cortex. Proc. Natl. Acad. Sci. USA. 2000;97:6191–6196. doi: 10.1073/pnas.120018597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH. Tungsten microelectrode for recording from single units. Science. 1957;125:549–550. doi: 10.1126/science.125.3247.549. [DOI] [PubMed] [Google Scholar]
- Imig TJ, Ruggero AM, Kitzes LM, Javel E, Brugge JF. Organization of auditory cortex in the owl monkey (aotus trivirgatus) J. Comp. Neurol. 1977;171:111–128. doi: 10.1002/cne.901710108. [DOI] [PubMed] [Google Scholar]
- Jenkins WM, Merzenich MM, Ochs MT, Allard T, Guic-Robles E. Functional reorganization of primary somatosensory cortex in adult owl monkeys after behaviorally controlled tactile stimulation. J. Neurophysiol. 1990;63:82–104. doi: 10.1152/jn.1990.63.1.82. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- McDonough RN, Whalen AD. Detection of Signals in Noise. San Diego, CA: Academic Press; 1995. [Google Scholar]
- Mountcastle VB. Modality and topographic properties of single neurons of the cat’s somatic sensory cortex. J. Neurophysiol. 1957;20:408–434. doi: 10.1152/jn.1957.20.4.408. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB, Talbot WH, Kornhuber HH. The neuroal transformation of mechanical stimuli delivered to the monkey’s hand. In: De Reuck AVS, Knight J, editors. Touch, Heat, and Pain. Boston, MA: Little, Brown; 1966. pp. 325–351. [Google Scholar]
- Newsome W, Britten K, Movshon J. Neuronal correlates of a perceptual decision. Nature. 1989;341:52–54. doi: 10.1038/341052a0. [DOI] [PubMed] [Google Scholar]
- Polley D, Heiser M, Blake D, Schreiner C, Merzenich M. Associative learning shapes the neural code for stimulus magnitude in primary auditory cortex. Proc. Natl. Acad. Sci. USA. 2004;101:16351–16356. doi: 10.1073/pnas.0407586101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley D, Steinberg E, Merzenich M. Perceptual learning directs auditory cortical map reorganization through top-down influences. J. Neurosci. 2006;26:4970–4982. doi: 10.1523/JNEUROSCI.3771-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiguel S, Vogels R, Mysore S, Orban G. Learning to see the difference specifically alters the most informative V4 neurons. J. Neurosci. 2006;26:6589–6602. doi: 10.1523/JNEUROSCI.0457-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanzone GH, Merzenich MM, Jenkins WM, Grajski KA, Dinse HR. Topographic reorganization of the hand representation in cortical area 3b owl monkeys trained in a frequency-discrimination task. J. Neurophysiol. 1992a;67:1031–1056. doi: 10.1152/jn.1992.67.5.1031. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Merzenich MM, Schreiner CE. Changes in the distributed temporal response properties of SI cortical neurons reflect improvements in performance on a temporally based tactile discrimination task. J. Neurophysiol. 1992b;67:1071–1091. doi: 10.1152/jn.1992.67.5.1071. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J. Neurosci. 1993;13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanzone GH, Guard DC, Phan ML. Frequency and intensity response properties of single neurons in the auditory cortex of the behaving macaque monkey. J. Neurophysiol. 2000;83:2315–2331. doi: 10.1152/jn.2000.83.4.2315. [DOI] [PubMed] [Google Scholar]
- Reynolds J, Desimone R. The role of neural mechanisms of attention in solving the binding problem. Neuron. 1999;24:19–29. doi: 10.1016/s0896-6273(00)80819-3. [DOI] [PubMed] [Google Scholar]
- Richardson RT, DeLong MR. Electrophysiological studies of the functions of the nucleus basalis in primates. Adv. Exp. Med. Biol. 1991;295:233–252. doi: 10.1007/978-1-4757-0145-6_12. [DOI] [PubMed] [Google Scholar]
- Rutkowski R, Weinberger N. Encoding of learned importance of sound by magnitude of representational area in primary auditory cortex. Proc. Natl. Acad. Sci. USA. 2005;102:13664–13669. doi: 10.1073/pnas.0506838102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoups A, Vogels R, Qian N, Orban G. Practicing orientation identification improves orientation coding in V1 neurons. Nature. 2001;412:549–553. doi: 10.1038/35087601. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Suga N, Ma X. Multiparametric corticofugal modulation and plasticity in the auditory system. Nat. Rev. Neurosci. 2003;4:783–794. doi: 10.1038/nrn1222. [DOI] [PubMed] [Google Scholar]
- Talbot WH, Darian-Smith I, Kornhuber HH, Mountcastle VB. The sense of flutter-vibration: comparison of the human capacity with response patterns of mechanoreceptive afferents from the monkey hand. J. Neurophysiol. 1968;31:301–334. doi: 10.1152/jn.1968.31.2.301. [DOI] [PubMed] [Google Scholar]
- Wang X, Merzenich MM, Sameshima K, Jenkins WM. Remodeling of hand representation in adult cortex determined by timing of tactile stimulation. Nature. 1995;378:71–75. doi: 10.1038/378071a0. [DOI] [PubMed] [Google Scholar]
- Wang X, Lu T, Snider R, Liang L. Sustained firing in auditory cortex evoked by preferred stimuli. Nature. 2005;435:341–346. doi: 10.1038/nature03565. [DOI] [PubMed] [Google Scholar]
- Xerri C, Merzenich MM, Jenkins W, Santucci S. Representational plasticity in cortical area 3b paralleling tactual-motor skill acquisition in adult monkeys. Cereb. Cortex. 1999;9:264–276. doi: 10.1093/cercor/9.3.264. [DOI] [PubMed] [Google Scholar]
- Xerri C, Stern JM, Merzenich MM. Alterations of the cortical representation of the rat ventrum induced by nursing behavior. J. Neurosci. 1994;14:1710–1721. doi: 10.1523/JNEUROSCI.14-03-01710.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Maunsell J. The effect of perceptual learning on neuronal responses in monkey visual area V4. J. Neurosci. 2004;24:1617–1626. doi: 10.1523/JNEUROSCI.4442-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]