Abstract
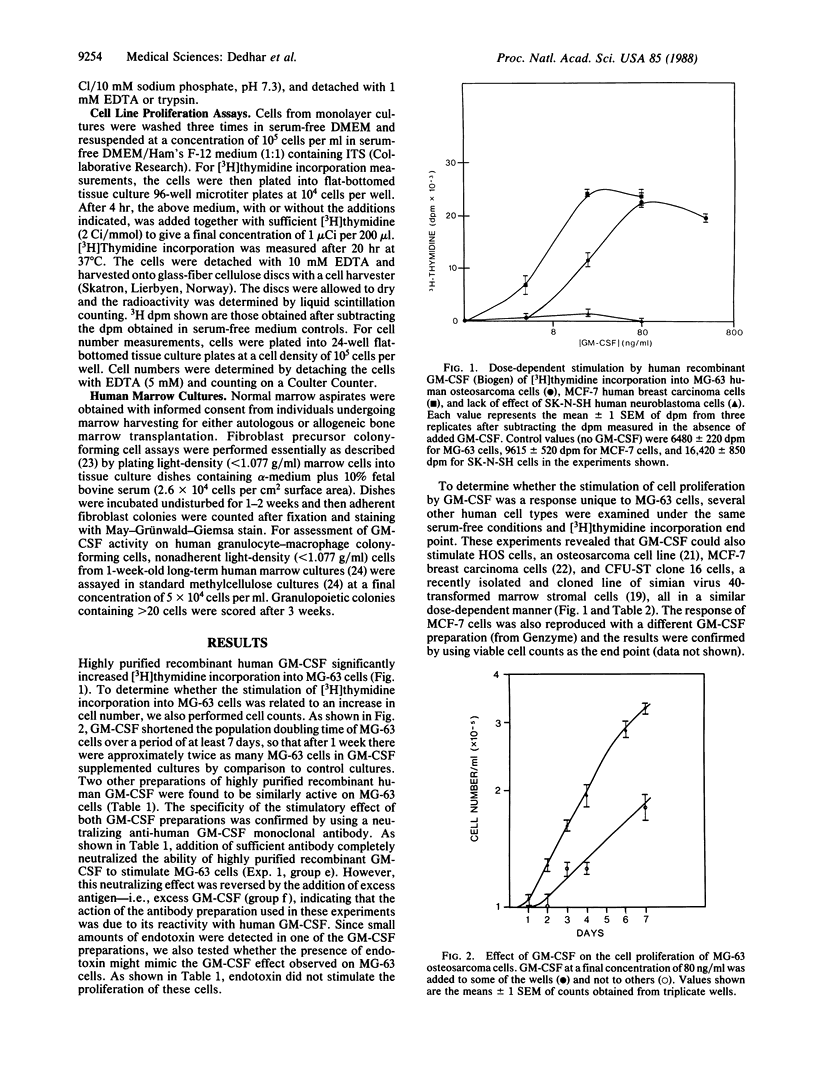
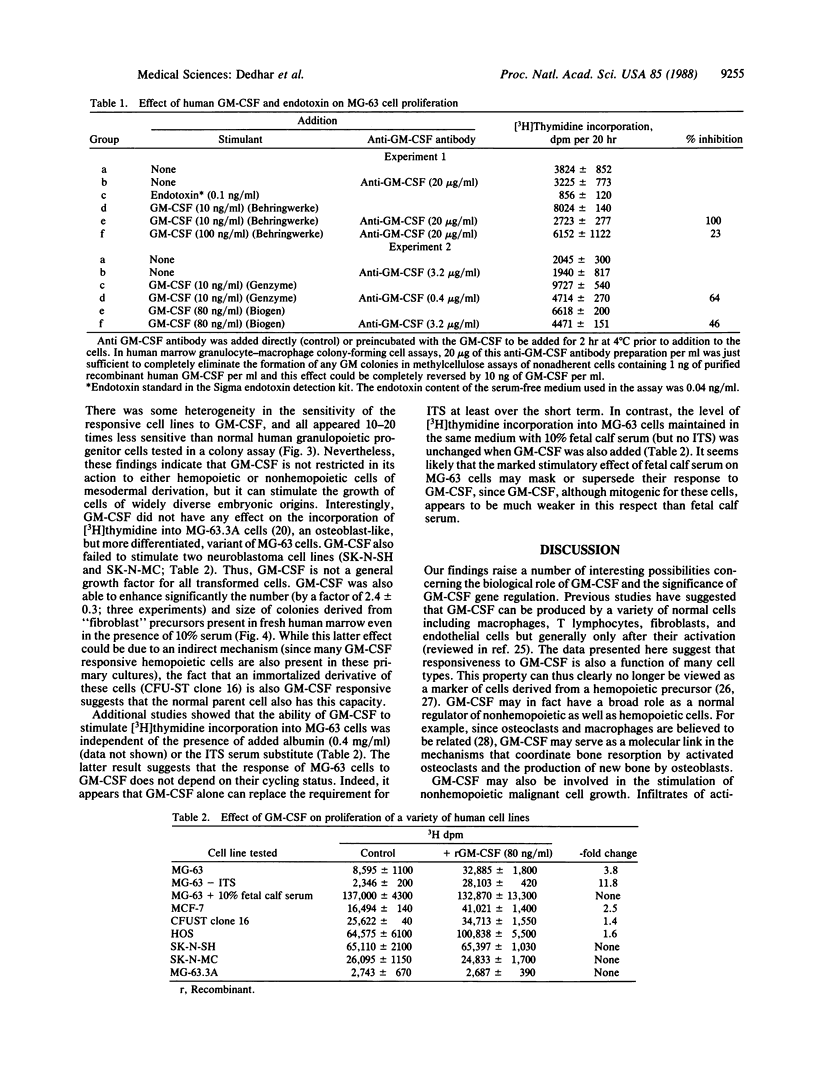
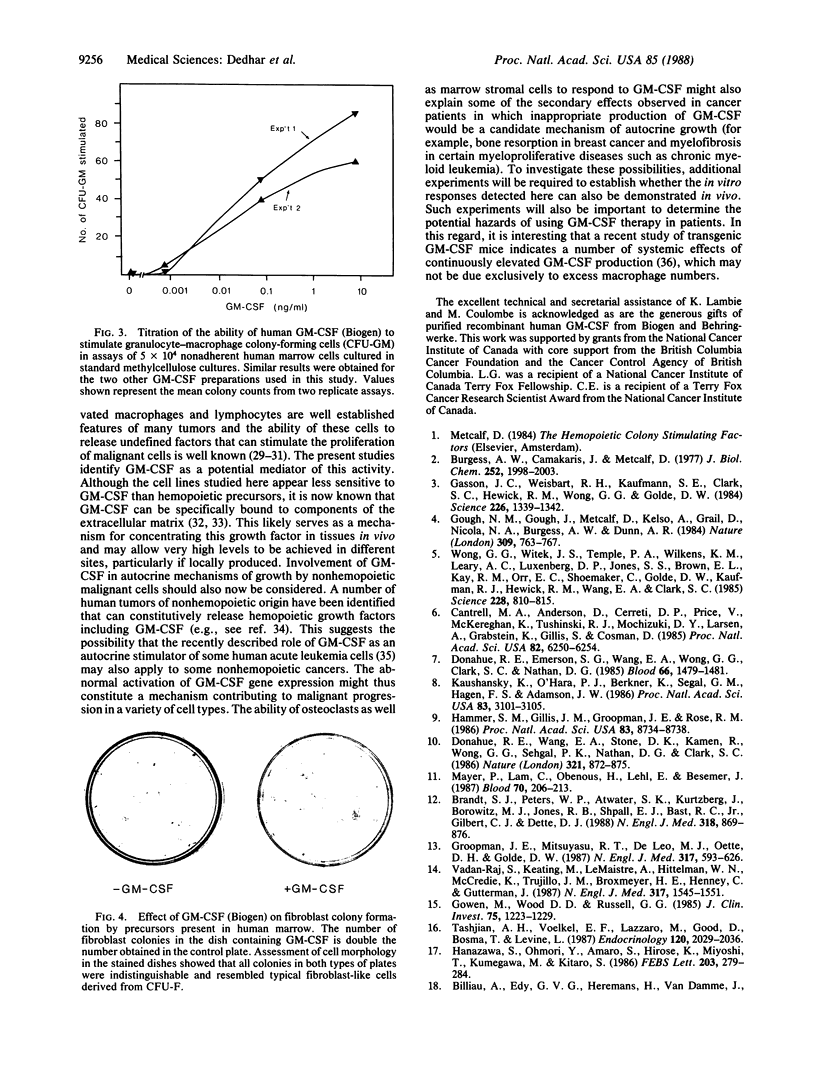
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a member of a family of glycoprotein hormones that stimulate the proliferation and differentiation of hemopoietic cells in vitro and in vivo. We now report that human GM-CSF can also stimulate the proliferation of two osteogenic sarcoma cell lines, a breast carcinoma cell line, a simian virus 40-transformed marrow stromal cell line, and normal marrow fibroblast precursors. These findings suggest a more general regulatory function of GM-CSF on nonhemopoietic cell types than previously anticipated. They also raise the possibility of adverse side effects of GM-CSF therapy in patients whose malignant cells may be directly stimulated by this molecule and suggest a previously unanticipated role of GM-CSF gene activation in the evolution of solid tumors and in the pathogenesis of myelofibrosis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billiau A., Edy V. G., Heremans H., Van Damme J., Desmyter J., Georgiades J. A., De Somer P. Human interferon: mass production in a newly established cell line, MG-63. Antimicrob Agents Chemother. 1977 Jul;12(1):11–15. doi: 10.1128/aac.12.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt S. J., Peters W. P., Atwater S. K., Kurtzberg J., Borowitz M. J., Jones R. B., Shpall E. J., Bast R. C., Jr, Gilbert C. J., Oette D. H. Effect of recombinant human granulocyte-macrophage colony-stimulating factor on hematopoietic reconstitution after high-dose chemotherapy and autologous bone marrow transplantation. N Engl J Med. 1988 Apr 7;318(14):869–876. doi: 10.1056/NEJM198804073181401. [DOI] [PubMed] [Google Scholar]
- Burgess A. W., Camakaris J., Metcalf D. Purification and properties of colony-stimulating factor from mouse lung-conditioned medium. J Biol Chem. 1977 Mar 25;252(6):1998–2003. [PubMed] [Google Scholar]
- Cantrell M. A., Anderson D., Cerretti D. P., Price V., McKereghan K., Tushinski R. J., Mochizuki D. Y., Larsen A., Grabstein K., Gillis S. Cloning, sequence, and expression of a human granulocyte/macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6250–6254. doi: 10.1073/pnas.82.18.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Malaspina H., Gay R. E., Resnick G., Kapoor N., Meyers P., Chiarieri D., McKenzie S., Broxmeyer H. E., Moore M. A. Characterization of human bone marrow fibroblast colony-forming cells (CFU-F) and their progeny. Blood. 1980 Aug;56(2):289–301. [PubMed] [Google Scholar]
- Clark S. C., Kamen R. The human hematopoietic colony-stimulating factors. Science. 1987 Jun 5;236(4806):1229–1237. doi: 10.1126/science.3296190. [DOI] [PubMed] [Google Scholar]
- Coulombel L., Eaves A. C., Eaves C. J. Enzymatic treatment of long-term human marrow cultures reveals the preferential location of primitive hemopoietic progenitors in the adherent layer. Blood. 1983 Aug;62(2):291–297. [PubMed] [Google Scholar]
- Dedhar S., Argraves W. S., Suzuki S., Ruoslahti E., Pierschbacher M. D. Human osteosarcoma cells resistant to detachment by an Arg-Gly-Asp-containing peptide overproduce the fibronectin receptor. J Cell Biol. 1987 Sep;105(3):1175–1182. doi: 10.1083/jcb.105.3.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue R. E., Emerson S. G., Wang E. A., Wong G. G., Clark S. C., Nathan D. G. Demonstration of burst-promoting activity of recombinant human GM-CSF on circulating erythroid progenitors using an assay involving the delayed addition of erythropoietin. Blood. 1985 Dec;66(6):1479–1481. [PubMed] [Google Scholar]
- Donahue R. E., Wang E. A., Stone D. K., Kamen R., Wong G. G., Sehgal P. K., Nathan D. G., Clark S. C. Stimulation of haematopoiesis in primates by continuous infusion of recombinant human GM-CSF. 1986 Jun 26-Jul 2Nature. 321(6073):872–875. doi: 10.1038/321872a0. [DOI] [PubMed] [Google Scholar]
- Gabrilove J. L., Welte K., Harris P., Platzer E., Lu L., Levi E., Mertelsmann R., Moore M. A. Pluripoietin alpha: a second human hematopoietic colony-stimulating factor produced by the human bladder carcinoma cell line 5637. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2478–2482. doi: 10.1073/pnas.83.8.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasson J. C., Weisbart R. H., Kaufman S. E., Clark S. C., Hewick R. M., Wong G. G., Golde D. W. Purified human granulocyte-macrophage colony-stimulating factor: direct action on neutrophils. Science. 1984 Dec 14;226(4680):1339–1342. doi: 10.1126/science.6390681. [DOI] [PubMed] [Google Scholar]
- Gordon M. Y., Riley G. P., Watt S. M., Greaves M. F. Compartmentalization of a haematopoietic growth factor (GM-CSF) by glycosaminoglycans in the bone marrow microenvironment. 1987 Mar 26-Apr 1Nature. 326(6111):403–405. doi: 10.1038/326403a0. [DOI] [PubMed] [Google Scholar]
- Gough N. M., Gough J., Metcalf D., Kelso A., Grail D., Nicola N. A., Burgess A. W., Dunn A. R. Molecular cloning of cDNA encoding a murine haematopoietic growth regulator, granulocyte-macrophage colony stimulating factor. 1984 Jun 28-Jul 4Nature. 309(5971):763–767. doi: 10.1038/309763a0. [DOI] [PubMed] [Google Scholar]
- Gowen M., Wood D. D., Russell R. G. Stimulation of the proliferation of human bone cells in vitro by human monocyte products with interleukin-1 activity. J Clin Invest. 1985 Apr;75(4):1223–1229. doi: 10.1172/JCI111819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groopman J. E., Mitsuyasu R. T., DeLeo M. J., Oette D. H., Golde D. W. Effect of recombinant human granulocyte-macrophage colony-stimulating factor on myelopoiesis in the acquired immunodeficiency syndrome. N Engl J Med. 1987 Sep 3;317(10):593–598. doi: 10.1056/NEJM198709033171003. [DOI] [PubMed] [Google Scholar]
- Hamburger A. W., White C. P. Growth factors for human tumor clonogenic cells elaborated by macrophages isolated from human malignant effusions. Cancer Immunol Immunother. 1986;22(3):186–190. doi: 10.1007/BF00200031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer S. M., Gillis J. M., Groopman J. E., Rose R. M. In vitro modification of human immunodeficiency virus infection by granulocyte-macrophage colony-stimulating factor and gamma interferon. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8734–8738. doi: 10.1073/pnas.83.22.8734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanazawa S., Ohmori Y., Amano S., Hirose K., Miyoshi T., Kumegawa M., Kitano S. Human purified interleukin-1 inhibits DNA synthesis and cell growth of osteoblastic cell line (MC3T3-E1), but enhances alkaline phosphatase activity in the cells. FEBS Lett. 1986 Jul 28;203(2):279–284. doi: 10.1016/0014-5793(86)80758-x. [DOI] [PubMed] [Google Scholar]
- Kaushansky K., O'Hara P. J., Berkner K., Segal G. M., Hagen F. S., Adamson J. W. Genomic cloning, characterization, and multilineage growth-promoting activity of human granulocyte-macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1986 May;83(10):3101–3105. doi: 10.1073/pnas.83.10.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang R. A., Metcalf D., Cuthbertson R. A., Lyons I., Stanley E., Kelso A., Kannourakis G., Williamson D. J., Klintworth G. K., Gonda T. J. Transgenic mice expressing a hemopoietic growth factor gene (GM-CSF) develop accumulations of macrophages, blindness, and a fatal syndrome of tissue damage. Cell. 1987 Nov 20;51(4):675–686. doi: 10.1016/0092-8674(87)90136-x. [DOI] [PubMed] [Google Scholar]
- Mayer P., Lam C., Obenaus H., Liehl E., Besemer J. Recombinant human GM-CSF induces leukocytosis and activates peripheral blood polymorphonuclear neutrophils in nonhuman primates. Blood. 1987 Jul;70(1):206–213. [PubMed] [Google Scholar]
- Nijweide P. J., Burger E. H., Feyen J. H. Cells of bone: proliferation, differentiation, and hormonal regulation. Physiol Rev. 1986 Oct;66(4):855–886. doi: 10.1152/physrev.1986.66.4.855. [DOI] [PubMed] [Google Scholar]
- Rhim J. S., Cho H. Y., Huebner R. J. Non-producer human cells induced by murine sarcoma virus. Int J Cancer. 1975 Jan 15;15(1):23–29. doi: 10.1002/ijc.2910150104. [DOI] [PubMed] [Google Scholar]
- Roberts R., Gallagher J., Spooncer E., Allen T. D., Bloomfield F., Dexter T. M. Heparan sulphate bound growth factors: a mechanism for stromal cell mediated haemopoiesis. Nature. 1988 Mar 24;332(6162):376–378. doi: 10.1038/332376a0. [DOI] [PubMed] [Google Scholar]
- Ruff M. R., Farrar W. L., Pert C. B. Interferon gamma and granulocyte/macrophage colony-stimulating factor inhibit growth and induce antigens characteristic of myeloid differentiation in small-cell lung cancer cell lines. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6613–6617. doi: 10.1073/pnas.83.17.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon S. E., Hamburger A. W. Immunoproliferation and cancer: a common macrophage-derived promoter substance. Lancet. 1978 Jun 17;1(8077):1289–1290. doi: 10.1016/s0140-6736(78)91270-9. [DOI] [PubMed] [Google Scholar]
- Singer J. W., Charbord P., Keating A., Nemunaitis J., Raugi G., Wight T. N., Lopez J. A., Roth G. J., Dow L. W., Fialkow P. J. Simian virus 40-transformed adherent cells from human long-term marrow cultures: cloned cell lines produce cells with stromal and hematopoietic characteristics. Blood. 1987 Aug;70(2):464–474. [PubMed] [Google Scholar]
- Soule H. D., Vazguez J., Long A., Albert S., Brennan M. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst. 1973 Nov;51(5):1409–1416. doi: 10.1093/jnci/51.5.1409. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Voelkel E. F., Lazzaro M., Goad D., Bosma T., Levine L. Tumor necrosis factor-alpha (cachectin) stimulates bone resorption in mouse calvaria via a prostaglandin-mediated mechanism. Endocrinology. 1987 May;120(5):2029–2036. doi: 10.1210/endo-120-5-2029. [DOI] [PubMed] [Google Scholar]
- Vadhan-Raj S., Keating M., LeMaistre A., Hittelman W. N., McCredie K., Trujillo J. M., Broxmeyer H. E., Henney C., Gutterman J. U. Effects of recombinant human granulocyte-macrophage colony-stimulating factor in patients with myelodysplastic syndromes. N Engl J Med. 1987 Dec 17;317(25):1545–1552. doi: 10.1056/NEJM198712173172501. [DOI] [PubMed] [Google Scholar]
- Wong G. G., Witek J. S., Temple P. A., Wilkens K. M., Leary A. C., Luxenberg D. P., Jones S. S., Brown E. L., Kay R. M., Orr E. C. Human GM-CSF: molecular cloning of the complementary DNA and purification of the natural and recombinant proteins. Science. 1985 May 17;228(4701):810–815. doi: 10.1126/science.3923623. [DOI] [PubMed] [Google Scholar]
- Young D. C., Griffin J. D. Autocrine secretion of GM-CSF in acute myeloblastic leukemia. Blood. 1986 Nov;68(5):1178–1181. [PubMed] [Google Scholar]