Abstract
Background and Purpose
Functional electrical stimulation (FES) is a popular post-stroke gait rehabilitation intervention. Although stroke causes multi-joint gait deficits, FES is commonly used only for the correction of swing phase foot drop. Ankle plantarflexor muscles play an important role during gait. The aim of the current study is to test the immediate effects of delivering FES to both ankle plantarflexors and dorsiflexors on post-stroke gait.
Methods
Gait analysis was performed as subjects (N=13) with chronic post-stroke hemiparesis walked at their self-selected walking speeds during walking with and without FES.
Results
Compared to delivering FES to only the ankle dorsiflexor muscles during the swing phase, delivering FES to both the paretic ankle plantarflexors during terminal stance and dorsiflexors during swing phase provided the advantage of greater swing phase knee flexion, greater ankle plantarflexion angle at toe-off, and greater forward propulsion. Although FES of both the dorsi- and plantar-flexor muscles improved swing phase ankle dorsiflexion compared to noFES, the improvement was less than that observed by stimulating the dorsiflexors alone, suggesting the need to further optimize stimulation parameters and timing for the dorsiflexor muscles during gait.
Conclusions
In contrast to the typical FES approach of only stimulating ankle dorsiflexor muscles during the swing phase, delivering FES to both the plantar- and dorsi-flexor muscles can help to correct post-stroke gait deficits at multiple joints (ankle and knee) during both the swing and stance phases of gait. Our study shows the feasibility and advantages of stimulating the ankle plantarflexors during FES for post-stroke gait.
Keywords: Functional Electrical Stimulation, Variable-frequency trains, Ankle plantarflexors
INTRODUCTION
The combination of functional electrical stimulation (FES) and treadmill training is a novel and effective intervention for post-stroke gait rehabilitation 1, 2. In a recent randomized controlled trial, the FES approach of stimulating multiple muscles provided a significant therapeutic benefit when added to a comprehensive post-stroke gait training program1. However, in post-stroke individuals, typically FES is delivered only to ankle dorsiflexors to correct ‘foot drop’ during the swing phase 3–5, thereby failing to address other important stance and swing phase post-stroke gait deficits at the hip and knee. We recently showed that the traditional approach of delivering FES to ankle dorsiflexor muscles during gait successfully corrects swing phase foot drop but also produces ‘adverse effects’ on post-stroke gait such as decreased swing phase knee flexion and decreased ankle plantarflexion at toe-off 6. Also, recently, decreased propulsive force generation at transition from paretic stance to swing has been shown to represent a critical deficit in post-stroke gait, contributing to a greater energy cost of walking, and shown to be correlated with hemiparetic severity, walking speed, and gait asymmetry in individuals post-stroke 7, 8. Dorsiflexor FES does not address this critical deficit of decreased propulsive force generation during paretic terminal stance 7–9.
The ankle plantarflexor muscles, which play an important role in the gait cycle 10, demonstrate decreased force generating ability in individuals with hemiparesis following stroke. Decreased force generation by the ankle plantarflexors is related to deficits during both the swing and stance phases of gait 9, 11 and most notably, to slow walking speeds post-stroke 9. Forward dynamic simulations of healthy gait predict that decreased forward propulsive force generation by the ankle plantarflexors during terminal stance can lead to decreased ipsilateral leg kinetic energy at toe-off, thereby resulting in decreased swing phase knee flexion 10, 12. Although predictions from gait simulations 10, 12 purport the relationships between ankle plantarflexor muscle activation, propulsive force generation, and swing phase knee flexion, no previous study has provided direct experimental evidence demonstrating these relationships. Also, in the current study, we used novel stimulation patterns called variable-frequency trains (VFTs) which have been shown to enhance isometric muscle performance 13, and more recently, gait performance 6, compared to typically used stimulation patterns (constant-frequency trains, CFTs) (Figure 1).
Figure 1.
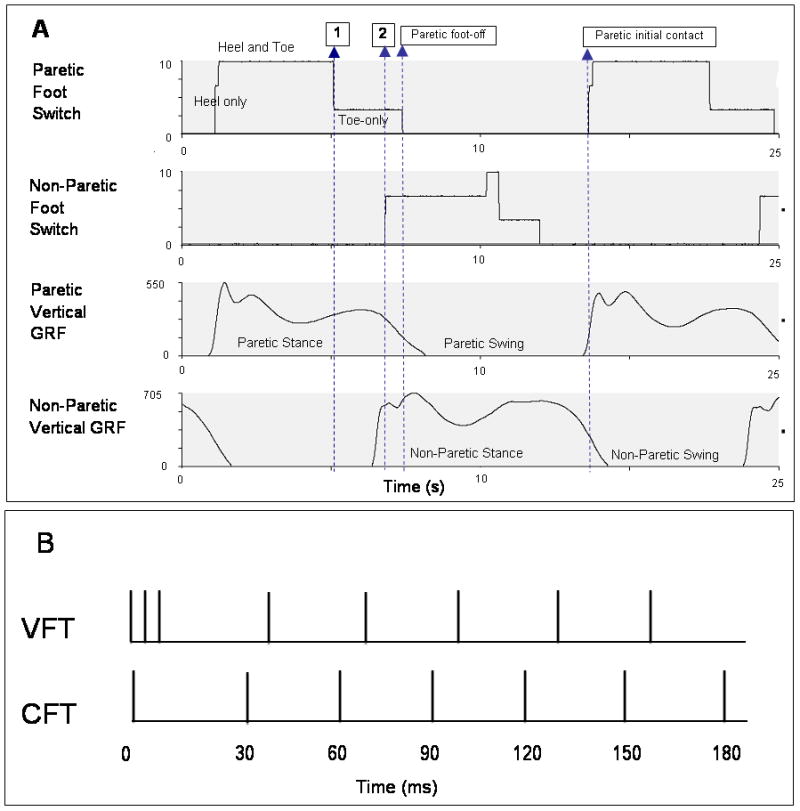
(A) Foot switch and vertical ground reaction force (GRF) data from the paretic and non-paretic leg of one subject. There was considerable variability in the relative timing of the events shown in this figure across subjects. Vertical GRFs were used to identify swing and stance phases of gait. Footswitch signals were used to trigger FES during gait. During each gait cycle, dorsiflexor FES was started at paretic foot-off and terminated at paretic initial contact. Plantarflexor FES was started at paretic heel-off (Arrow 1) for Logic 1 and at non-paretic initial contact (Arrow 2) for Logic 2; and terminated at paretic foot-off. (B) Stimulation train patterns used for FES. VFTs consisting of a three-pulse 200-Hz burst at the start of a 30-Hz CFT were used in this study. Traditionally-used CFTs are shown for comparison.
The aim of the this study was to compare post-stroke walking patterns during walking with FES delivered to both the plantar- and dorsi-flexor muscles versus walking with FES delivered only to the ankle dorsiflexors.
METHODS
Thirteen subjects (age =49 to 72 years; 9 males) with post-stroke hemiparesis participated in this study (Table 1). Inclusion criteria were: 6 months following a stroke involving cerebral cortical regions, able to walk for 5 minutes at their self-selected walking speed, and sufficient passive ankle dorsiflexion range of motion to enable the paretic ankle joint to reach either neutral ankle angle (0°) or a minimum of 5° of plantarflexion with the knee flexed. Exclusion criteria were: substantial cognitive deficits, severe aphasia, cerebellar involvement, and any pre-existing conditions affecting walking function. All subjects signed informed consent forms approved by the Human Subjects Review Board of the University of Delaware.
Table 1.
Demographic and clinical information about the 13 subjects tested in the present study. Data for Subject 13 were not included in the results due to technical problems during gait analysis. The last column states whether Logic 1 (L1) or Logic 2 (L2) was used for plantarflexor stimulation during gait (see Methods for details).
| Subject (No.) | Gender (M/F) | Age (yrs.) | Time since stroke (yrs.) | Side of Hemiparesis (L/R) | Gait Speed (m/s) | FuglMeyer Score (max score 34) | PDF Logic |
|---|---|---|---|---|---|---|---|
| 1 | M | 66 | 2.4 | L | 0.9 | 24 | L1 |
| 2 | M | 52 | 6.3 | L | 0.6 | 20 | L1 |
| 3 | F | 58 | 21.3 | L | 0.2 | 23 | - |
| 4 | F | 51 | 1.9 | L | 0.3 | 20 | L2 |
| 5 | M | 49 | 9.3 | R | 0.9 | 28 | L2 |
| 6 | M | 72 | 6.1 | R | 0.5 | 18 | L2 |
| 7 | M | 57 | 2.7 | R | 0.7 | 22 | L1 |
| 8 | M | 58 | 9.9 | R | 0.7 | 21 | L2 |
| 9 | M | 60 | 5.8 | R | 0.8 | 25 | L1 |
| 10 | M | 74 | 4.7 | R | 0.7 | 31 | L2 |
| 11 | M | 56 | 9.8 | R | 1.2 | 25 | L2 |
| 12 | F | 46 | 2.2 | L | 0.9 | 23 | L2 |
| 13 | F | 66 | 1.4 | R | 0.3 | 18 | - |
| Average | 58.8 | 6.4 | 0.7 | 23 | |||
| StErr | 2.5 | 1.6 | 0.1 | 1 | |||
Self-adhesive surface electrical stimulation electrodes were attached over the ankle dorsiflexor (2′X2′, TENS Products, Grand Lake, CO) and plantarflexor (2′X5′, ConMed Corp, New York) muscles. A Grass S8800 stimulator in combination with a Grass Model SIU8TB stimulus isolation unit was used to deliver electrical stimulation (Grass Instrument Company, Quincy, MA). For both the dorsi- and plantar-flexor muscles, stimulation amplitude was set using a 300-ms long, 30-Hz train with pulse duration of 300-μs.
For the ankle dorsiflexor muscles, stimulation amplitude was set with subjects seated to achieve a neutral ankle joint position (0°) with minimal ankle eversion or inversion. For the ankle plantarflexor muscles, stimulation amplitude was set with the subjects standing in a position similar to terminal double support of the paretic leg during gait to achieve lifting of the paretic heel off the ground or until the subject’s maximal tolerance was reached, whichever occurred first. Two compression closing foot switches (25-mm diameter MA-153, Motion Lab Systems Inc., Baton Rouge, LA) were attached bilaterally to the soles of each subject’s shoes-one under the forefoot and the second under hindfoot.
A customized, real-time, FES system (CompactRIO, National Instruments, TX) was used to control the Grass stimulator and deliver stimulation during the gait cycle. The FES system delivered stimulation to the ankle dorsiflexor muscles from the time the forefoot foot switch of the paretic leg was off the ground until the hindfoot foot switch of the paretic leg contacted the ground (Figure 1A). Plantarflexor FES was triggered using two different timing logics (Logics 1 and 2) to stimulate the ankle plantarflexors during the paretic terminal double support phase of gait. Logic 1 delivered stimulation from the time the paretic hindfoot switch was off the ground up to the time the paretic forefoot switch was off the ground. Logic 2 delivered stimulation from the time the non-paretic forefoot switch contacted the ground upto the time when the paretic forefoot switch of the paretic leg was off the ground. The FES stimulation pattern comprised a high-frequency (200-Hz) 3-pulse burst 13 followed by a lower frequency (30-Hz) constant frequency train (Figure 1B).
Gait analysis was performed as subjects walked on a split-belt treadmill instrumented with two, 6-degree of freedom force platforms (AMTI, Watertown, MA). For safety, subjects held on to a front handrail during walking, and all subjects wore a harness that was attached to an overhead support. No body weight was supported by the harness. Marker data were collected at 100-Hz using an 8-camera motion analyses system (Vicon 5.2, Oxford, UK) that was synchronized with the force plates that measured ground reaction forces from each leg separately at 2000-Hz.
The data presented in this report are a subset of the data collected during one testing session for each subject; multiple walking trials were collected within the session 6. Rest intervals of 5–10 min were provided between consecutive walking trials; each trial was 20- to 40-s long 6. The current study reports data from 3 walking trials tested at the subject’s self-selected walking speeds: (1) walking without FES (noFES), (2) walking with FES delivered to the ankle dorsiflexor muscles during swing phase using VFTs (DF), (3) walking with FES delivered to both the ankle dorsi- and plantar-flexor muscles using VFTs (PDF). The noFES walking trials were collected at the beginning, middle, and end of the testing session. The order of testing of each of the 3 walking conditions with FES was randomly assigned to each subject.
Data Processing
Marker trajectories and ground reaction force data were low-pass filtered (Butterworth fourth order, phase lag) at 6- and 30-Hz, respectively, using commercial software (Visual 3D; C-Motion, Rockville, MD). Vertical ground reaction forces were used to determine gait events. The noFES data were obtained by averaging the 3 noFES trials from the beginning, middle, and end of the session. For the PDF condition, for each subject, the timing logic (Logic 1 versus 2) that generated greater peak anterior ground reaction force was included in the analysis.
Dependent Variables
Peak Anterior GRF during Paretic Terminal Stance was defined as the maximum anterior GRF (AGRF) between the onset of the propulsion (anteriorly-directed) phase of antero-posterior GRFs and the end of the stance phase.
Percent Paretic Propulsion was defined as the ratio of the integral of the AGRF from the onset of propulsion through the end of stance phase for the paretic leg versus the total AGRF integral for the paretic and non-paretic legs 7.
Peak Knee Flexion during Swing phase was determined for the paretic leg.
Ankle angle at paretic toe-off and peak ankle angle during the paretic swing phase were also measured for the paretic leg.
Statistical Analysis
Paired t-tests were performed to detect differences between noFES versus PDF, DF versus PDF, and when applicable, between noFES versus DF. Gait data for the DF walking condition have been reported previously6, but are included in the present study for comparison with PDF.
A one-way ANOVA with post-hoc paired comparisons compared peak AGRF and peak swing phase knee flexion angles during the noFES walking condition at the beginning, middle, and end of the session was performed to assess for presence of muscle fatigue or potentiation in ankle plantarflexor muscles.
All statistical analyses were performed using SPSS 16.0 (SPSS Inc., Chicago, IL). Descriptive statistics are presented as Mean ± Standard errors.
RESULTS
Of the 13 subjects tested in this study, one subject’s data (Subject 13) were excluded from the analyses because of technical problems during data-collection. Results are presented for the remaining 12 subjects (see Table 1). GRF data for 1 of the remaining 12 subjects (Subject 4) were not included in the analyses because the subject showed no forward propulsion (anteriorly-directed GRFs) during gait. We found that accurate timing of delivery of plantarflexor FES during gait was challenging; having two timing logic options for plantarflexor FES enabled us to deliver plantarflexor FES to all the subjects included in our study. For each subject, data for all outcome variables represent the mean of data from 6 consecutive strides.
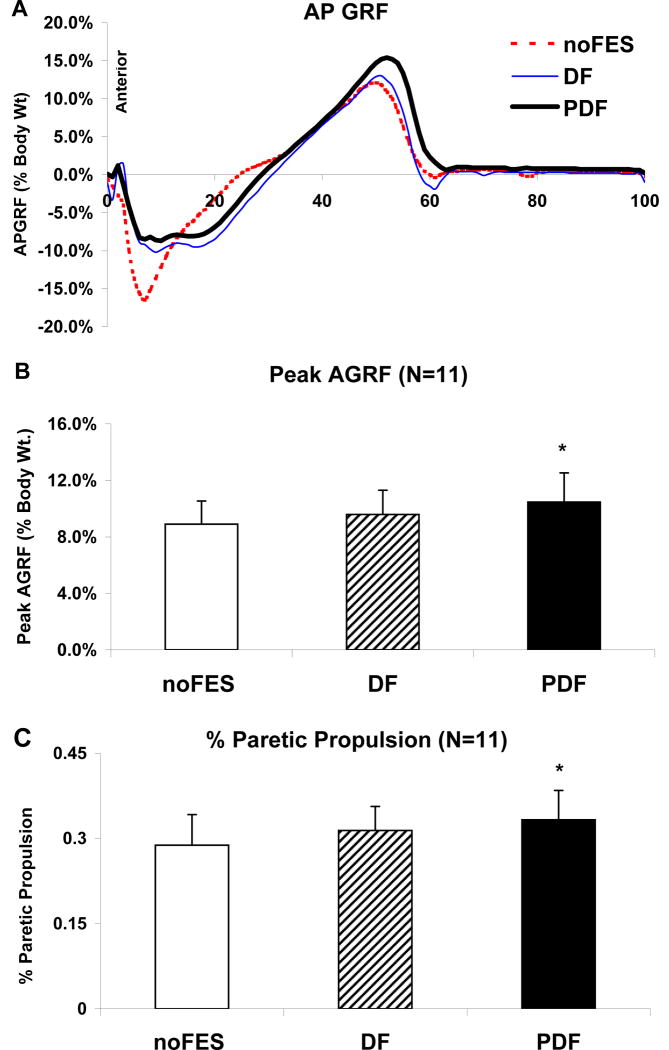
Compared to walking without FES (8.9±1.6 % body weight), the peak AGRF for the paretic leg showed an ~18% increase during walking with PDF (p=0.025) (Figure 2). There was no difference in peak AGRF between DF and PDF (p=0.25). Peak AGRF values for the non-paretic extremity were 15.9±2.2%.
Figure 2.
(A) Antero-posterior GRF during the gait cycle for one representative subject. (B) and (C) Means (N=11) and standard errors for (B) peak AGRF and (C) % paretic propulsion during paretic stance. Walking conditions presented are noFES, DF, and PDF. * Significant difference from noFES (p 0.05). † Significant difference from DF (p≤0.05).
During walking without FES, the average contribution of the paretic leg to total propulsion was 28.8±5.4 %. In normal gait, each leg would contribute equally (i.e., 50%) to total propulsion. Compared to noFES, the % paretic propulsion increased to 33.1±5.2 % during PDF (p=0.02) (See Table 2 and Figure 2). There was no difference in % propulsion between PDF and DF (31.1±4.2 %) (p=0.047). The values for paretic push-off integrals during noFES, DF, and PDF were 2.1±0.4, 2.3±0.4, and 2.7±0.5 % body weight seconds, respectively. The values for the non-paretic push-off integrals during noFES, DF, and PDF were 5.1±0.7, 5.0±0.8, and 5.4±0.9 % body weight seconds, respectively.
We detected a significant reduction in peak swing phase knee flexion angles during DF (40.8±4.2°) compared to each of the remaining 2 walking conditions (both p-values ≤0.01) (Figure 3). There were no differences in peak swing knee flexion between noFES (44.1±4.2°) versus PDF (44.3±4.6°) (p=0.81). The peak swing phase knee flexion angles for the non-paretic extremity were 68.3±2.0°.
Figure 3.
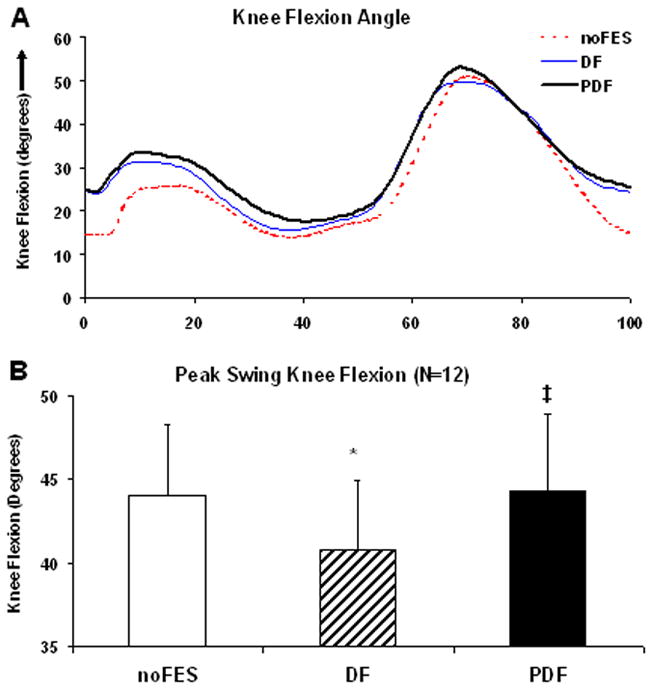
(A) Sagittal plane knee angles during the gait cycle for one representative subject. (B) Means (N=12) and standard errors for peak swing phase knee flexion angles during noFES, DF, and PDF. * Significant difference from noFES (p≤0.05). ‡ Significant difference from DF (p≤0.05).
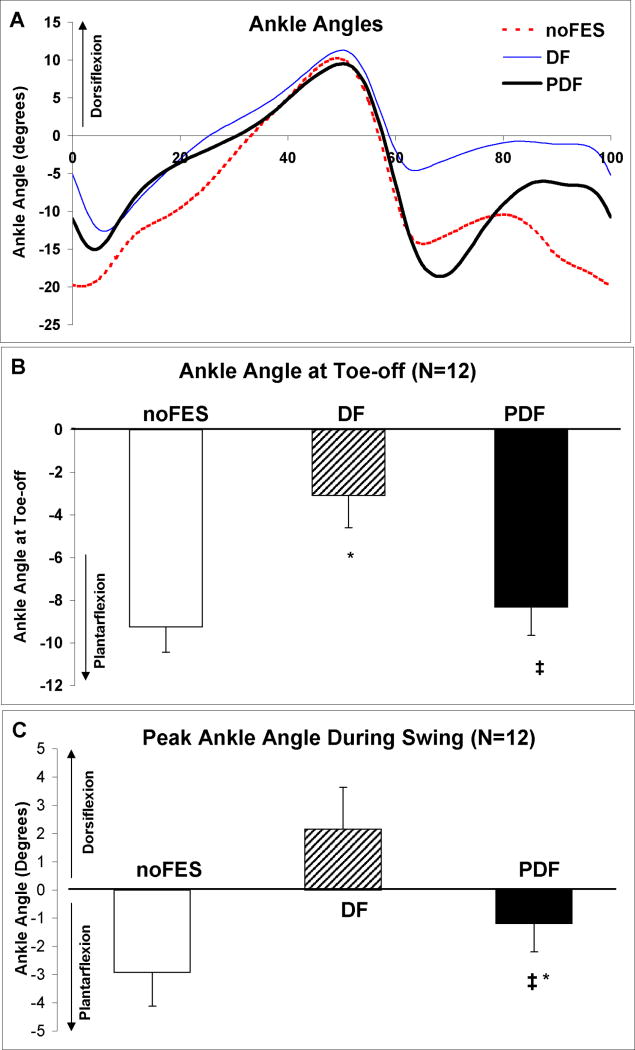
During walking without FES, the average ankle plantarflexion angles at paretic toe-off were −9.2±1.2°. There was significant reduction in ankle plantarflexion at toe-off during DF stimulation6 (−3.1±1.5°) compared to each of the other 2 walking conditions (both p-values ≤0.01) (Figure 4). There were no differences in ankle angle at toe-off between noFES versus PDF (−8.3±1.3°) (p=0.41). The ankle angles at toe-off for the non-paretic extremity were 13.4±2.1°.
Figure 4.
(A) Sagittal plane ankle angles during the gait cycle for one representative subject. Means (N=12 subjects) and standard errors for ankle angles at paretic toe-off (B) and peak swing phase ankle dorsiflexion (C) during noFES, DF, and PDF. * Significant difference from noFES (p≤0.05). ‡ Significant difference from DF (p≤0.05). Negative angles represent plantarflexion.
Without FES, subjects walked with their paretic ankle angles in a slightly plantarflexed position during the swing phase (−2.9±1.2°) (Figure 4). Dorsiflexor FES brought the paretic ankle into a dorsiflexed position during swing phase (2.1±1.5°). Compared to noFES, the paretic ankle demonstrated significantly greater peak swing phase ankle dorsiflexion during walking with PDF (−1.2±1.0°) (p =0.04). PDF produced a significant reduction in ankle dorsiflexion compared to DF (2.1±1.5°) (p≤0.01). The peak ankle swing phase ankle angles for the non-paretic extremity were 5.2±2.0°.
Effects of fatigue were tested by comparing noFES trials collected at the beginning, middle, and end of the testing session. There were no significant differences in Peak AGRF among the 3 noFES walking trials collected at the beginning, middle, and end of the testing session (8.5±.1.3%, 8.3±.1.2%, and 8.7±.1.3%, respectively) (F=0.26, p=0.77). Similarly, there were no significant differences in peak swing phase knee flexion angles among the noFES walk trials collected at these three time points (48.7±.3.2°, 49.7±.3.6°, and 50.3±.3.6°, respectively) (F=0.96, p=0.41).
DISCUSSION
Our results showed that delivering FES to both the paretic ankle plantarflexor muscles during terminal stance and to the ankle dorsiflexor muscles during swing phase provided the advantage of increased swing phase knee flexion and increased plantarflexion angles at toe-off compared to the traditional approach of stimulating the ankle dorsiflexors alone. Stimulating both the plantar- and dorsi-flexor muscles also resulted in greater peak anterior GRFs, % contribution of the paretic leg to total propulsion, and swing phase ankle dorsiflexion angles compared to noFES. Although FES of both the dorsi- and plantar-flexor muscles was able to improve ankle dorsiflexion during swing phase compared to noFES, stimulating both the dorsi-and plantar-flexor muscles produced lesser peak dorsiflexion during swing phase compared to stimulating the dorsiflexors alone at both these phases of the gait cycle. Additionally, we showed the feasibility of using novel physiologically-based variable-frequency train patterns13 during FES.
Forward-dynamic gait simulations of able-bodied individuals predict that during terminal stance, the gastrocnemius muscle plays an important role in generating push-off force, thereby providing the critical propulsion needed for initiation of the swing phase of gait 9, 10, 14. In support of previous findings by Bowden et. al 7, our current results showed that during walking without FES, the average contribution of the paretic leg towards total propulsion was 28.1±5.5 %, indicating marked asymmetry in the amount of propulsion generated by the paretic compared to the non-paretic leg (perfect symmetry would result in 50% contribution by each leg). Previous researchers have suggested that decreased paretic plantarflexion muscle force generation at terminal stance is related to the inadequate paretic leg propulsion during walking after stroke, though this has never been directly tested 9, 15. Thus, we hypothesized that FES delivered to the paretic plantarflexor muscles during terminal stance would increase propulsive force generation. Our results demonstrated that, as hypothesized, adding FES to the ankle plantarflexor muscles in post-stroke individuals who were receiving dorsiflexor FES resulted in an ~18% increase in both the peak AGRF and % paretic propulsion compared to walking without FES. Our present results provide the first direct evidence in post-stroke individuals that forward propulsion produced by the paretic leg during walking can be positively influenced by electrically stimulating the paretic plantarflexor muscles during terminal stance.
Based on predictions of forward dynamic gait simulations,10, 12 we hypothesized that delivering FES to the paretic ankle plantarflexors during terminal stance would generate greater forward propulsive forces, which in turn would provide the paretic leg with greater kinetic energy at toe-off, thereby increasing paretic knee flexion during swing phase10, 12. No previous studies reported changes in swing phase knee flexion as a result of plantar- or dorsi-flexor FES. In the present study, because of foot drop during the swing phase, we were unable to assess the effects of delivering FES to only the plantarflexor muscles on gait performance; both the ankle plantar- and dorsi-flexor muscles were stimulated. Much to our surprise, the traditional approach of delivering FES to only the ankle dorsiflexor muscles during swing (DF condition) resulted in an ~8 % (3.3°) decrease in mean swing knee flexion compared to walking without FES6. However, delivering FES to both the ankle plantar- and dorsi-flexor muscles resulted in an 8.6% increase in average swing knee flexion compared to delivering FES to ankle dorsiflexor muscles alone, thus counteracting the effects of DF stimulation. Thus, the increase in swing knee flexion produced by plantarflexor FES was able to counteract the decrease in swing knee flexion produced by dorsiflexor FES. This is a critically important finding, given that chronic stroke survivors generally show decreased knee flexion in the paretic leg during swing compared to the non-paretic leg and compared to neurologically unimpaired control subjects walking at matched speeds 16, 17.
Another advantage of delivering FES to both the plantar-and dorsi-flexor muscles versus to the dorsiflexors alone was observed at the ankle joint as the paretic leg transitioned from stance to swing phase. As shown in our previous study6 and as reported by others (18), compared to noFES, dorsiflexor FES reduced ankle plantarflexion angles at toe-off. However, in the present study, just as was found for peak knee flexion, delivering plantar-and dorsi-flexor FES together counteracted the decreased ankle plantarflexion caused by dorsiflexor FES alone at toe-off. These results, together with the results for peak knee flexion during swing, suggest that the application of DF stimulation alone may negatively impact important aspects of the gait pattern in chronic stroke survivors, but that this impact may be overcome by with the addition of plantarflexion stimulation.
In contrast to the improvements in AGRF during terminal stance, knee flexion during swing phase, and ankle plantarflexion at toe-off with plantar- and dorsi-flexor FES, plantar- and dorsi-flexor FES (PDF) resulted in lesser ankle dorsiflexion during the swing phase compared to dorsiflexor FES. The dorsiflexor muscles were stimulated with the same stimulation intensity during the PDF and DF walking conditions. Thus, given the increased plantarflexion at toe-off facilitated by the plantarflexion stimulation during PDF versus DF, the same dorsiflexor stimulation would have to generate a greater ankle excursion to achieve the same peak swing phase ankle angle. Greater dorsiflexor stimulation intensity would therefore be needed to generate the same peak swing phase dorsiflexion during PDF versus during DF. Increasing the dorsiflexor muscle’s stimulation intensity when both the plantar- and dorsi-flexors are being stimulated can help the dorsiflexor muscles to overcome the opposing plantarflexor force and thereby increase the degree of swing phase dorsiflexion produced during plantar- and dorsi-flexor FES. Furthermore, the timing of onset of dorsiflexor FES during gait can be modified to minimize plantarflexor-dorsiflexor muscle co-contraction during FES.
In our current study, the participants held onto a handrail during treadmill walking, Only two recent studies investigated the effect of handrail hold during treadmill walking 15, 19. In a group of able-bodied individuals, Siler and colleagues 19 showed no effect of handrail hold on sagittal plane gait kinematics during treadmill walking. In a group of individuals with post-stroke hemiparesis, Chen and colleagues15 showed that while handrail hold did not significantly affect paretic swing time, swing knee flexion, and leg kinetic energy at toe-off, , it resulted in decreased leg kinetic energy at toe-off for the non-paretic leg15. Thus, based on limited evidence, we can infer that % propulsion, which is computed using push-off integral values for both the paretic and non-paretic legs, may be a variable influenced by handrail hold. However, in the current study, all data were collected within one testing session, and we attempted to keep the type and strength of handrail hold constant throughout the testing session. Also, given that all the data were collected within one testing session, it is difficult to envision that changes in the non-paretic leg propulsion due to handrail hold would be differentially affected by FES. Nevertheless, the handrail-hold maybe a potential limitation in our study. Future studies are needed to systematically study the effect of handrails on gait kinetics during treadmill walking 15, 19.
Recent randomized controlled trials and meta-analyses of literature concluded that dorsiflexor FES can produce improvement in walking speed 5, 20 and energy efficiency 21, 22. It is noteworthy, however, that with the exception of a recent study by Daly and colleagues1, the majority of the studies and controlled trials that reported improvements in post-stroke walking speed with FES stimulated only the dorsiflexors during swing 4, 5, 20, 21 and did not investigate the effect of DF stimulation on other swing and stance phase post-stroke gait deficits. Our results show that the traditional FES approach of stimulating ankle dorsiflexor muscles during swing phase achieves correction of only the ankle dorsiflexor deficit during the swing phase, and can result in reduced swing phase knee flexion, which is already reduced post-stroke. In contrast, delivering FES to both the plantar- and dorsi-flexor muscles can help to correct post-stroke gait deficits at both the ankle and knee joints and during both the swing (knee flexion, ankle dorsiflexion) and stance phases (propulsive force generation, ankle plantarflexion at toe-off) of gait. The immediate effects of FES shown in our study suggest that FES strategies, similar to the one used in the present study, when used as a gait training intervention, may produce even greater improvements in gait performance compared to those obtained by stimulating the dorsiflexors alone.
Acknowledgments
Funding Sources: National Institutes of Nursing Research R01 grants # NR010786 and HD038582; NIH Shared Instrumentation Grant S10 RR022396-01; DOD Grant W911NF-05-1-0097. University of Delaware Dissertation Fellowship.
The authors thank Ms. Margie Roos, PT, NCS for clinical testing and subject recruitment, Ms. Leigh Shrewsbury for scheduling and recruitment, and Ms. Sarah Flynn for assisting with data-analyses.
References
- 1.Daly JJ, Roenigk K, Holcomb J, Rogers JM, Butler K, Gansen J, McCabe J, Fredrickson E, Marsolais EB, Ruff RL. A randomized controlled trial of functional neuromuscular stimulation in chronic stroke subjects. Stroke. 2006;37:172–178. doi: 10.1161/01.STR.0000195129.95220.77. [DOI] [PubMed] [Google Scholar]
- 2.Dickstein R. Rehabilitation of gait speed after stroke: A critical review of intervention approaches. Neurorehabil Neural Repair. 2008;22:649–660. doi: 10.1177/1545968308315997. [DOI] [PubMed] [Google Scholar]
- 3.Burridge JH, Haugland M, Larsen B, Pickering RM, Svaneborg N, Iversen HK, Christensen PB, Haase J, Brennum J, Sinkjaer T. Phase ii trial to evaluate the actigait implanted drop-foot stimulator in established hemiplegia. J Rehabil Med. 2007;39:212–218. doi: 10.2340/16501977-0039. [DOI] [PubMed] [Google Scholar]
- 4.Kottink AI, Oostendorp LJ, Buurke JH, Nene AV, Hermens HJ, MJIJ The orthotic effect of functional electrical stimulation on the improvement of walking in stroke patients with a dropped foot: A systematic review. Artif Organs. 2004;28:577–586. doi: 10.1111/j.1525-1594.2004.07310.x. [DOI] [PubMed] [Google Scholar]
- 5.Robbins SM, Houghton PE, Woodbury MG, Brown JL. The therapeutic effect of functional and transcutaneous electric stimulation on improving gait speed in stroke patients: A meta-analysis. Arch Phys Med Rehabil. 2006;87:853–859. doi: 10.1016/j.apmr.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 6.Kesar TM, Perumal R, Jancosko A, Reisman DS, Rudolph KS, Higginson JS, Binder-Macleod SA. Novel Patterns of Functional Electrical Stimulation Have an Immediate Effect on Dorsiflexor Muscle Function During Gait for People Poststroke. Phys Ther. 2010 doi: 10.2522/ptj.20090140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowden MG, Balasubramanian CK, Neptune RR, Kautz SA. Anterior-posterior ground reaction forces as a measure of paretic leg contribution in hemiparetic walking. Stroke. 2006;37:872–876. doi: 10.1161/01.STR.0000204063.75779.8d. [DOI] [PubMed] [Google Scholar]
- 8.Balasubramanian CK, Bowden MG, Neptune RR, Kautz SA. Relationship between step length asymmetry and walking performance in subjects with chronic hemiparesis. Arch Phys Med Rehabil. 2007;88:43–49. doi: 10.1016/j.apmr.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Nadeau S, Gravel D, Arsenault AB, Bourbonnais D. Plantarflexor weakness as a limiting factor of gait speed in stroke subjects and the compensating role of hip flexors. Clin Biomech (Bristol, Avon) 1999;14:125–135. doi: 10.1016/s0268-0033(98)00062-x. [DOI] [PubMed] [Google Scholar]
- 10.Neptune RR, Kautz SA, Zajac FE. Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking. J Biomech. 2001;34:1387–1398. doi: 10.1016/s0021-9290(01)00105-1. [DOI] [PubMed] [Google Scholar]
- 11.Higginson JS, Zajac FE, Neptune RR, Kautz SA, Delp SL. Muscle contributions to support during gait in an individual with post-stroke hemiparesis. J Biomech. 2006a;39:1769–1777. doi: 10.1016/j.jbiomech.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 12.Anderson FC, Goldberg SR, Pandy MG, Delp SL. Contributions of muscle forces and toe-off kinematics to peak knee flexion during the swing phase of normal gait: An induced position analysis. J Biomech. 2004;37:731–737. doi: 10.1016/j.jbiomech.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 13.Binder-Macleod S, Kesar T. Catchlike property of skeletal muscle: Recent findings and clinical implications. Muscle Nerve. 2005;31:681–693. doi: 10.1002/mus.20290. [DOI] [PubMed] [Google Scholar]
- 14.Jonkers I, Delp S, Patten C. Capacity to increase walking speed is limited by impaired hip and ankle power generation in lower functioning persons post-stroke. Gait Posture. 2009;29:129–137. doi: 10.1016/j.gaitpost.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen G, Patten C, Kothari DH, Zajac FE. Gait deviations associated with post-stroke hemiparesis: Improvement during treadmill walking using weight support, speed, support stiffness, and handrail hold. Gait Posture. 2005b;22:57–62. doi: 10.1016/j.gaitpost.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Chen G, Patten C, Kothari DH, Zajac FE. Gait differences between individuals with post-stroke hemiparesis and non-disabled controls at matched speeds. Gait Posture. 2005a;22:51–56. doi: 10.1016/j.gaitpost.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Olney SJ, Richards C. Hemiparetic gait following stroke. Part i: Characteristics. Gait Posture. 1996;4:136–148. [Google Scholar]
- 18.Voigt M, Sinkjaer T. Kinematic and kinetic analysis of the walking pattern in hemiplegic patients with foot-drop using a peroneal nerve stimulator. Clin Biomech (Bristol, Avon) 2000;15:340–351. doi: 10.1016/s0268-0033(99)00082-0. [DOI] [PubMed] [Google Scholar]
- 19.Siler WL, Jorgensen AL, Norris RA. Grasping the handrails during treadmill walking does not alter sagittal plane kinematics of walking. Arch Phys Med Rehabil. 1997;78:393–398. doi: 10.1016/s0003-9993(97)90231-8. [DOI] [PubMed] [Google Scholar]
- 20.Kottink AI, Hermens HJ, Nene AV, Tenniglo MJ, van der Aa HE, Buschman HP, Ijzerman MJ. A randomized controlled trial of an implantable 2-channel peroneal nerve stimulator on walking speed and activity in poststroke hemiplegia. Arch Phys Med Rehabil. 2007;88:971–978. doi: 10.1016/j.apmr.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Burridge JH, Taylor PN, Hagan SA, Wood DE, Swain ID. The effects of common peroneal stimulation on the effort and speed of walking: A randomized controlled trial with chronic hemiplegic patients. Clin Rehabil. 1997;11:201–210. doi: 10.1177/026921559701100303. [DOI] [PubMed] [Google Scholar]
- 22.Weber DJ, Stein RB, Chan KM, Loeb G, Richmond F, Rolf R, James K, Chong SL. Bionic walkaide for correcting foot drop. IEEE Trans Neural Syst Rehabil Eng. 2005;13:242–246. doi: 10.1109/TNSRE.2005.847385. [DOI] [PubMed] [Google Scholar]