Abstract
Background
There are no prospective, multicenter double-blind placebo-controlled randomized pharmacological trials for the treatment of pain predominant functional GI disorders in children.
Aim
Evaluate the efficacy of amitriptyline in children with pain predominant functional GI disorders.
Methods
Multicenter placebo-controlled trial. Children with irritable bowel syndrome, functional abdominal pain or functional dyspepsia were randomized to 4 weeks of placebo or amitriptyline (10 mg/d <35 kg; 20 mg/d >35 kg). Assessment of GI symptoms, psychological traits and daily activities before and after intervention. Pain was assessed daily with self-report diaries. Primary outcome: overall response to treatment (child's assessment of pain relief and sense of improvement). Secondary outcomes: effect on psychosocial traits and daily functioning.
Results
90 children enrolled, 83 completed the study (placebo: 40, 30 females; drug: 43, 35 females). Patients reported feeling better 63%, worse 5% in amitriptyline arm vs. better 57.5%, worse 2.5% in placebo arm (p=0.63). Pain relief was excellent (7%), good (38%) in children on placebo and excellent (15%), good (35%) in amitriptyline (p=0.85). Logistic regression analysis of those reporting excellent or good response vs. fair, poor or failed showed no difference between amitriptyline and placebo (p=0.83). Children who had more severe pain at baseline in both groups (p=0.0065) had worse outcome. Amitriptyline reduced anxiety scores (p<0.0001).
Conclusions
Both amitriptyline and placebo were associated with excellent therapeutic response. There was no significant difference between amitriptyline and placebo after 4 weeks of treatment. Patients with mild to moderate intensity of pain responded better to treatment.
Background
Pain predominant functional GI disorders (FGIDs) are amongst the most common causes for medical consultation in children 1,2. Such disorders include three common conditions: irritable bowel syndrome (IBS), functional dyspepsia and functional abdominal pain. Pain reduces children's quality of life, psychosocial functioning, and school attendance 3-5. In children, pharmacological treatment of pain predominant FGIDs is mostly empirical and based on adult data. There have been only a few small randomized clinical trials evaluating the efficacy of drugs for the treatment of pain predominant FGIDs in children 6, 7. None of them was multicenter, double-blind, placebo-controlled and included large patient numbers. A review of the medical literature by the Subcommittee on Chronic Abdominal Pain of the American Academy of Pediatrics and the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition found limited or inconclusive evidence regarding the benefit of drugs in the treatment of pain predominant FGIDs 6-8. Similar findings were reported by the Cochrane Systematic Report 9. A common theme of both reports was the need for well-designed clinical in children. Several clinical trials have demonstrated a beneficial effect of low dose tricyclic antidepressants (TCA) and in particular amitriptyline for the treatment of IBS in adult patients 10. The Functional Bowel Disorders Working Group Report of the First World Congress of Pediatric Gastroenterology, Hepatology, and Nutrition recommended the evaluation of the role of TCAs in the treatment of pain predominant FGIDs in children 11. We designed a large prospective, multicenter, randomized clinical trial aimed at evaluating the efficacy of amitriptyline for pain relief in children with pain predominant FGIDs.
Materials and Methods
This study was designed following the methodological recommendations established by the Rome II consensus on “Design of treatment trials for gastrointestinal disorders” 12. This was a prospective, double-blind, randomized, placebo-controlled, multicenter, parallel-group study. The study consisted of a one week baseline observation period in which no amitriptyline or placebo was given, followed by a 4 week randomized, double-blind, treatment period with either placebo or amitriptyline. The study was approved by the IRB of each of the participating institutions, and informed consent was obtained from each patient.
Study Population
Children 8-17 years of age with a diagnosis of functional abdominal pain, functional dyspepsia and IBS according to the Rome II criteria were recruited from the pediatric gastroenterology clinics of six tertiary care centers geographically dispersed in the US: Children's Hospital of Pittsburgh, Pittsburgh (PA); Goryeb Children's Hospital at Atlantic Health System, Morristown, (NJ), Kansas University Medical Center, Kansas City (KS); Children's Hospital of Boston, Boston (MA); Children's Hospital of Wisconsin, Milwaukee (WI) and Children's Memorial Hospital, Chicago, (IL).
Study Protocol
Each eligible patient was evaluated by clinical history, physical examination and standardized laboratory testing across sites. Children were excluded if they were diagnosed with an organic disease, plotted below the 5th percentile for weight or height, had abnormal testing (EKG, complete blood count, erythrocyte sedimentation rate, albumin, pancreatic and liver enzymes, urine analysis, stool examination for occult blood and ova and parasites, tissue transglutaminase), they had a positive lactose breath test or had a history of symptoms resolving after 2 weeks of a lactose-free diet. Subjects entered a 1 week run-in phase during which symptoms were recorded daily basis by age-appropriate validated questions and pain scale. Children who reported a weekly average pain of more than 25 mm on a 100 mm visual analog-Likert pain scale during the run-in phase were randomized to receive either placebo or amitriptyline, 10 mg/d in patients less than 35 kg or 20 mg/day in those over 35 kg (allocation ratio placebo:amitriptyline 1:1) for 4 weeks. Thirty-five pills (5 weeks supply) of amitriptyline or placebo were provided in identical capsules to each patient (one week of extra supply was provided for the event that the last visit could not be conducted after exactly 4 weeks of treatment).
Psychological testing
All subjects completed validated, self-report and age-appropriate psychological questionnaires at the beginning and end of the study: Pain Response Inventory, Children's Depression Inventory (CDI) 13, State-Trait Anxiety Inventory for Children (STAIC) 14, 15, Children Somatization Inventory questionnaire (CSI) and Pediatric Functional Disability Inventory (PFDI) 13, 16.
Compliance and adherence to protocol
Each family was contacted twice by phone during the study. The first phone contact was scheduled within the first 3 days of the screening period to clarify any problems related to the completion of the questionnaires. The second phone contact occurred during the third week of the study to: a) assess compliance with medications and, b) assure completion of questionnaires. To assess adherence, the parent or children were requested to count the capsules left while the research assistant was on the phone. At the end of the study, the number of capsules left was subtracted from the total number of capsules (35) that were provided and the value was compared with the capsules that should have remained if capsules were taken daily. In addition, at the time of the last visit the families were asked how many times they forgot to administer the pills during the study. This response was compared with the value obtained by phone report. Children with dissimilar answers or those that missed medications for more than 20% of the days during the study were considered non-compliant. In order to assure integrity in the completion of the questionnaires, the response to the daily questions reported during the phone conversation was compared with the paper version of the same day once the questionnaires were returned. Children whose phone response and paper version did not match were considered non-compliant.
Sample size
According to the initial sample size calculation, it was expected that 70% of the subjects receiving amitriptyline would achieve adequate relief of symptoms as compared to 40% of those receiving placebo. Given these estimates, with a non-directional alpha of 0.05, and a power of 0.80, 49 evaluable patients per group were required to detect this level of difference. Our study originally planned to enroll 120 patients to compensate for an expected dropout rate of 15%. Fours years into the study, it became apparent that it was difficult to recruit enough children to reach the expected sample size and we elected to stop recruitment after 90 subjects were enrolled. Eighty-three of these subjects completed the study. Our dropout rate was lower than expected (7.8%). This final sample size provides a power of 0.72 to detect the originally proposed difference.
Data Analysis
The aim of the study was to investigate the efficacy of amitriptyline for pain relief in children with FGIDs. This aim was assessed by measuring two efficacy variables through a standardized questionnaire at the end of study (week 4 visit). The primary efficacy variable was the patient's overall assessment of satisfactory relief and satisfaction with treatment over the 4 week treatment period. This was assessed by two questions: “Overall how do you feel your problem is?” Subjects could describe their overall status as “Better”, “Same” or “Worse”; and by the question: “How did the medication relieve your pain?” Patients could describe their sense of improvement as “Excellent”, “Good”, “Fair”, “Poor” or “Failed”. Secondary efficacy variables included the effect on psychosocial traits and ability to perform daily activities. They were measured at baseline and at the end of the study by subject's response to questionnaires previously listed that assessed coping mechanisms, anxiety, depression, somatization and disability. To further investigate interference with activities, the children answered throughout the baseline and treatment period daily questions addressing their ability to conduct daily activities (sleep, play and attending school). The questions asked were based on an age-appropriate and validated pediatric questionnaires for gastrointestinal symptoms17. Symptomatic relief- Subjects recorded the presence of GI symptoms (vomiting and characteristics of the stools) and pain daily during the baseline and treatment period. Pain was assessed through an age-appropriate, self-report, validated 100 mm visual analog scale (World-Graph-Rating-Scale) 18. Additional assessment of GI symptoms and pain was conducted at the end of the study. Final questionnaire included patient's assessment of number of days of pain and location, frequency of vomiting, characteristics of the stools and use of additional medications during the previous month.
Statistical analysis
All information was coded and subjects, families and study investigators, were blinded to the randomization codes. Data were analyzed independently of the investigators at a central coordinating site, which did not have access to the code until analysis was completed. The primary end points were analyzed as binary variables. For that purpose we combined the responses to each of both questions assessing the primary outcome: a)”Failed“, “Poor” and “Fair” were combined and compared to “Good” and “Excellent” and b) “Better” was compared to “Same” and “Worse” combined. The null hypothesis was that the odds of responding to treatment were the same for the amitriptyline or placebo group at the end of 4 weeks of treatment. Fisher's Exact test was used to compare the proportion of subjects by treatment arm who reported “positive or negative” progress. The hypothesis was tested using a 2-sided test with a 5% significance level. All variables were analyzed in intention to treat analysis. Primary efficacy variables were also analyzed in per protocol analysis. Both questions assessing the primary efficacy variable were also analyzed assuming the best or worse case scenario. For this purpose we considered the seven patients that dropped from the study either as failures (“Worse” or failure to respond) or responders (“Better” or “Excellent” response) categories. To analyze potential covariates, the amitriptyline therapy and the placebo group were compared on descriptive variables. If the groups were statistically different on any of these variables, they were included as covariates in subsequent analyses. For the continuous outcomes of interest (e.g., Depression, Anxiety, Disability, Coping and Somatization), differences between the subjects given placebo and the subjects given amitriptyline therapy were compared using analysis of variance. For the categorical outcomes of interest, Fisher's Exact test or chi-square test were used. This strategy was used for the subgroup comparisons, e.g., subjects with higher depression scores compared with children with lower depression scores in regards to the outcome of the gastrointestinal symptoms. Analysis of secondary outcomes also included the change from baseline in the number of times pain interfered with sleep, play and school which were each compared between treatment groups using Wilcoxon rank-sum test. Other secondary outcomes also included daily pain diaries which were analyzed by linear mixed modeling, adjusting for the correlation between repeated measures on the same patient. Change in pain intensity throughout the study was analyzed as a dependent variable in both groups. Week 0 (average intensity of pain of the run-in phase week) was considered as baseline. Statistical significance was set at p<0.05. All analyses were conducted in SAS 9.1 (SAS Institute, Cary, N.C.).
Results
Ninety children (mean age 12.7 y, range 8-17 y) were recruited from the six sites from January 2003 thru August 2006. Randomization allocated 46 children in the amitriptyline group and 44 in the placebo group. Subjects consulted a pediatrician 300 times (148 consultations placebo group; 152 consultations amitriptyline group) prior to enrollment. This represented an average of 3.6 consultations per patient. In addition, subjects visited the ER at least once on 73 occasions. Moreover, 89% of patients had failed previous pharmacological treatment (1.4 medications per patient). Table 1 shows the demographic and clinical characteristics of subjects in the two groups. Eighty-three children completed the study (amitriptyline: 43, 35 females; placebo: 40, 30 females). Seven girls (mean age 14.1 y, range 11-17 y) discontinued the study. Three children discontinued the study for mild adverse events (amitriptyline group: fatigue 1, rash and headaches 1); (placebo group: dizziness 1). One subject discontinued due to lack of interest and three children were excluded for intercurrent viral illness, not adhering to the protocol or improper consent. All treatment-related adverse events occurred during the first two weeks of treatment.
TABLE 1. Subjects Randomization.
| Table 1 N=90 |
Placebo N= 44 |
Drug N=46 |
p-value |
|---|---|---|---|
| Age (in years) | Mean (Std) =13.0(2.7) Median = 13.0 Range = 8 to 17 |
Mean (Std) =12.5(2.9) Median = 12.5 Range = 8 to 17 |
0.39 |
| Gender: Female, (%) | 70 % | 76 % | 0.50 |
| Functional Dyspepsia | 8 % | 13 % | 0.71 |
| Functional Abdominal Pain | 31 % | 53 % | 0.07 |
| IBS | 62 % | 40 % | 0.07 |
| Compliance confirmed | 78 % | 70 % | 0.39 |
| Consultations to Pediatrician | 89% | 93% | 0.48 |
| Consultations to ER | 23% | 35% | 0.25 |
| Onset of Symptoms (months prior to study enrollment) | 24 | 27 | 0.35 |
| Baseline intensity of pain (0-100 mm) | Mean(Std) = 47.7 (14.2) Median = 45.3 Range = 24.3 to 75.3 |
Mean(Std) =48.9 (19.5) Median = 48.1 Range = 7.1 to 100.0 |
0.46 |
Compliance
Seventy-five percent of those children completing the study were considered compliant with medication intake. Timely completion of questionnaires was verified in all. There were no significant differences in age, gender, diagnosis or treatment group between or those considered compliant or non-compliant with the protocol.
Primary efficacy variable
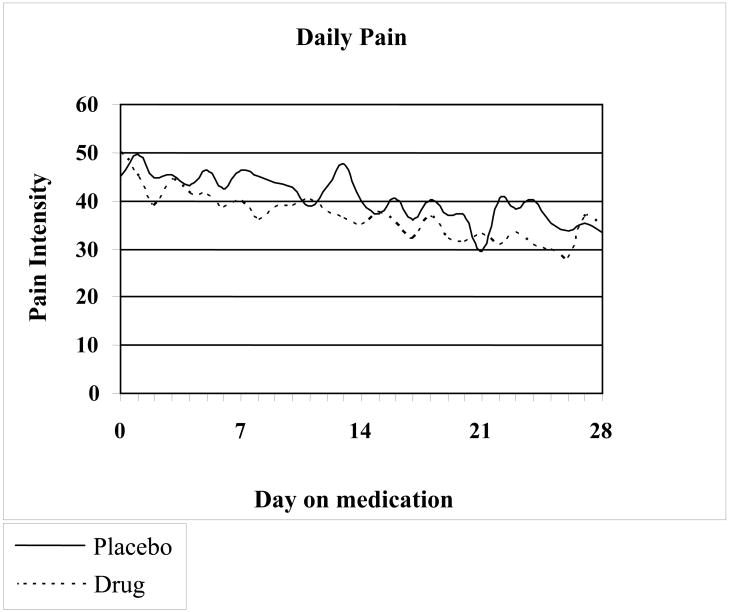
Changes in pain scores during the study period in both groups are shown in Fig. 1. In intention to treat analysis, in the amitriptyline group 59% of children reported feeling better, and 4% worse, while in the placebo group 53% of children reported feeling better, and 2% worse (p=0.81). Number needed to treat=19. Absolute risk reduction=5.3%. Treatment results were considered excellent or good in 50% of children receiving amitriptyline and excellent or good in 45% of children in the placebo group. There was no significant difference between amitriptyline and placebo (p=0.85) in subjects considering their response to treatment as excellent or good compared with those reporting fair, poor or failed. The analysis of the results per recruitment site showed no differential effect per site. Subjects were balanced between those who answered “Good to Excellent” as compared to those who answered “Failed to Fair” across six sites.
FIGURE 1.
Variation in pain intensity over time in the patients receiving amitriptyline or placebo. There is no statistically significant difference between the two groups.
Multivariate analysis showed no significant effect of gender (p=0.39), age (p=0.23) or diagnosis at baseline (IBS vs. non-IBS) (p=0.42), IBS diarrhea-predominant vs. IBS constipation-predominant (p=0.99), dyspepsia vs. non-dyspepsia (p=0.97), functional abdominal pain vs. non-functional abdominal pain (p=0.14), in the assessment of primary efficacy variables.
Per Protocol Analysis
(Table 2): When we limited our analysis to subjects who completed the study, 53% of subjects in the amitriptyline arm and 50% in the placebo arm considered the result of treatment good or excellent (p=0.83). Sixty-three percent of children in the amitriptyline arm and 57.5% in the placebo arm reported feeling better (p=0.63). Differences between both treatment arms remained non significant when non-completers were included in the analysis for each of the two questions assessing the primary efficacy variables assuming the best (p=0.99) and (p=0.70) or worse case scenario (p=0.68) and (p=0.54). Answers to both questions assessing the primary efficacy variable were also analyzed only in those children that were considered compliant with medication for drug vs. placebo and the differences were non significant (p=0.25). Fifty-five percent of those subjects considered compliant with the medication in the placebo arm and 73% in the amitriptyline reported feeling better (p=0.13). NNT=5.4.
TABLE 2. Primary Efficacy Variables.
| Intention to Treat | Total N=90 | Placebo N= 44 | Amitriptyline N= 46 | p-value | ||
|---|---|---|---|---|---|---|
| Overall how do you feel your problem is? | ||||||
| Better | 56% | 53% | 59% | p=0.81 * | ||
| Same | 33% | 36% | 30% | |||
| Worse | 3% | 2% | 4% | |||
| Dropouts | 7% | 9% | 7% | |||
| How well did the medication relieve your pain? | ||||||
| Failed | 16% | 16% | 15% | |||
| Poor | 11% | 7% | 15% | |||
| Fair | 18% | 23% | 13% | |||
| Good | 37% | 38% | 45% | 35% | 50% | p=0.85 ** |
| Excellent | 11% | 7% | 15% | |||
| Dropouts | 7% | 9% | 7% | |||
| Per Protocol | Total N=83 | Placebo N= 40 | Amitriptyline N= 43 | p-value | ||
| Overall how do you feel your problem is? | ||||||
| Better | 60% | 57.5% | 63% | p=0.63 * | ||
| Same | 36% | 40% | 32% | |||
| Worse | 4% | 2.5% | 5% | |||
| How well did the medication relieve your pain? | ||||||
| Failed | 17% | 17.5 % | 16 % | |||
| Poor | 12% | 7.5 % | 16 % | |||
| Fair | 19% | 25 % | 14 % | |||
| Good | 40% | 42.5 % | 50% | 37 % | 53% | p=0.83 ** |
| Excellent | 12% | 7.5 % | 16 % | |||
- p value of better vs. same or worse combined
- p value of combination of good or excellent vs. fair, good and excellent combined
Secondary efficacy variables
Psychological questionnaires-There was a significant overall improvement from baseline to follow-up in depression (p=0.0002), coping (p=0.02), disability (p=0.0015) and somatization (p<0.0001) in both groups (amitriptyline and placebo) but there was no significant difference between the two treatment groups. There was significant improvement in the amitriptyline arm (p<0.0001) in anxiety scores, but not in the placebo arm (p=0.40). The results of the analysis of the various domains of the CDI are provided in table 3. We were interested in assessing whether depression scores at baseline would result on a differential effect on outcome. There was no significant difference (p=0.83) at baseline in regards to depression between treatment groups. Interference with daily activities-The responses to the questions assessing interference with daily activities were consistent with the assessment of disability according to the PFDI. There was a decrease in subjects reporting interference with sleep, play and school from baseline to end of the study (p<0.0001) in both amitriptyline and placebo groups, and a tendency to more improvement in the group of children on amitriptyline. However there was not a statistically significant difference between the groups (p=0.31) when these results were analyzed by logistic regression modeling for repeated measures. Interference with activities between amitriptyline and placebo was also not significantly different when analyzed in per protocol analysis (p=0.14).
TABLE 3. Children's Depression Inventory domains by treatment group.
|
Psychological Scores N=90 Mean (std) |
Placebo N= 44 |
Drug N=46 |
p-value |
|---|---|---|---|
| CDI: score, baseline vs. follow-up | 8.2 (7.3) vs. 6.9 (6.7) | 10.0 (8.7) vs. 6.7 (6.9) | 0.31 0.91 |
| CDI: t-score, baseline vs. follow-up | 47.6 (10.9) vs. 45.8 (9.9) | 50.3 (12.5) vs. 45.5 (9.4) | 0.29 0.90 |
| CDI: Negative Mood, baseline vs. follow-up | 2.1 (2.1) vs. 1.7 (1.9) | 2.4 (2.3) vs. 1.5 (1.6) | 0.60 0.53 |
| CDI: Interpersonal Problems, baseline vs. follow-up | 0.39 (0.7) vs. 0.32 (0.7) | 0.64 (1.2) vs. 0.64 (1.1) | 0.24 0.10 |
| CDI: Ineffectiveness, baseline vs. follow-up | 1.1 (1.5) vs. 0.95 (1.3) | 1.5 (2.0) vs. 0.98 (1.4) | 0.24 0.93 |
| CDI: Anhedonia, baseline vs. follow-up | 3.4 (2.5) vs. 2.9 (2.7) | 3.9 (2.9) vs. 2.5 (2.5) | 0.44 0.41 |
| CDI: Negative Self-Esteem, baseline vs. follow-up | 1.3 (1.6) vs. 1.0 (1.5) | 1.6 (2.0) vs. 1.2 (1.8) | 0.40 0.65 |
Pain
There was a significant decrease in pain in both groups (p<0.0001). Repeated measures modeling of daily diary pain (adjusting for baseline pain) did not show a difference in the trend of pain over the study time between the treatment groups (p=0.90), nor any overall difference between treatment groups (p=0.18) (Figure 1). Baseline pain was associated with children's assessment of pain relief (p=0.0065, Cochran-Armitage test for trend). Subjects with pain <60 mm at baseline were more likely to answer ‘Good’ or ‘Excellent’ (p=0.05, OR=3.2, 95 % CI 0.99-10.1) but there was no significant differences between both treatment groups (p=0.95).
Placebo effect
Due to the large placebo response, we analyzed covariates that could predict reporting ‘good or excellent’ pain relief within the placebo group when responding to one of the questions assessing the primary efficacy variable. We failed to find any predictor with the exception of baseline anxiety scores. Age was not different between those who answered ‘good or excellent’ and those who answered ‘failed to fair’, p=0.57. Gender was not associated with ‘good or excellent’ pain relief in placebo subjects, p=0.26. Subjects were balanced between those who answered ‘Good to Excellent’ as compared to those who answered ‘Failed to Fair’ under all investigators and study centers. Logistic regression did not show an effect of diagnosis in the primary outcome within the placebo group (dyspepsia p=0.97; IBS p=0.42, functional abdominal pain p=0.14). Baseline CDI scores p=0.24 (t-test) and baseline pain intensity p=0.24 (t-test) were not different between those who answered ‘failed to fair’ and those who answered ‘good or excellent’.
To assess whether anxiety scores at baseline, diagnosis of anxiety (STAIC score >47) at baseline and changes in anxiety scores from baseline to end of the study had an effect on outcome, we conducted a logistic regression with responder status as a dependant variable. STAIC score was not associated with the outcome when both groups were analyzed combined (p=0.15). Eight children were characterized as “anxious” according to their STAIC scores. Logistic regression analysis did not show anxiety diagnosed by the STAIC predicted the primary outcome (p=0.39). There was no evidence of an association between change in anxiety score and primary outcome measures either in both treatment groups combined (p=0.29 pain relief; p=0.64 sense of improvement) or in the placebo group (p=0.43 pain relief; p=0.26 sense of improvement) or in the drug group (p=0.31 pain relief; p=0.54 sense of improvement). Based on the effect of seasonal changes in mood and global outcome, we assessed whether there was an effect of seasons. Logistic regression comparing winter to summer was not significant (p=0.17).
Discussion
To our knowledge this is the largest non-industry sponsored pharmacological clinical trial in abdominal pain associated FGIDs and the first multicenter clinical trial investigating the effect of pharmacological therapy in children and adolescents with chronic abdominal pain.
Our study followed the guidelines established by the Rome committee 19. We selected global subjective outcomes to assess treatment response and those were analyzed as binary endpoints. Patients were randomized providing a means to control for the between-patient variation. We employed validated age-appropriate psychological questionnaires and enrolled a mixed population of children with FGIDs not limiting our study only to children with IBS.
Our study showed that amitriptyline was equally effective as placebo in the treatment of pain predominant FGIDs in children. Lack of differences between treatments groups were found consistently in every outcome analyzed, at all ages, in all diagnoses and in both genders. Similar results were found in measures of global endpoints, pain relief and interference with daily activities using patient based end of study assessment and daily reports. We found improvement in all variables analyzed including psychological variables, such as depression and somatization from baseline to the end of the study without differences between treatment groups.
We found an exclusive improvement in anxiety scores in the amitriptyline group. Interestingly, amitriptyline has been associated with a reduced activation of brain areas associated with pain and emotional and cognitive function only during mental stress 10. Animal models have also shown that TCA reduce anxiety-related behaviors induced by chronic pain 20. Morgan et al. hypothesize that amitriptyline's antianxiety effects result from the inhibition of the locus coeruleus secondary to increased norepinephrine at this level 10. IBS patients respond to a painful rectal stimulus with a greater activation of the anterior cingulated cortex than controls 21-23. The antinociceptive and anxiolytic effects of amitriptyline therefore are likely to be central, possibly via noradrenergic and/or serotonergic pathways 10, 21-23.
We view our high placebo effect as an important contribution that may stimulate new studies on the placebo effect in children with gastrointestinal conditions. Combining the responses of the children's assessment of treatment success of those reporting fair, good or excellent improvement in the placebo group, the beneficial response of the placebo group in our study was 68% in ITT analysis and 75% in per protocol analysis. In order to better understand the placebo effect we reviewed the pediatric placebo literature and investigated various potential factors through logistic regression analysis. We found no studies on the placebo effect in pediatric gastroenterology. Few studies have been published on the placebo effect in the treatment of migraines in children, a condition that also subscribes to the biopsychosocial model of care. Some of the studies showed a higher placebo effect in younger children and girls 24, 25. We explored these factors, but found no effect of gender or age in our study. We also explored the role of symptom severity at enrollment that could indicate regression to the mean in one of the groups and a possible effect by study site. We did not find a relation between severity of symptoms or study site and outcome. We found a significant relation between anxiety scores and outcome within the placebo group. Children in the placebo group with higher anxiety testing scores at baseline had worse outcomes. We did not find a relation between been diagnosed with anxiety at baseline and outcome, but only 8 children in our study were diagnosed with anxiety and the study was not powered to test this hypothesis. Our results are in line with previous studies. A study on adult patients with IBS showed that the level of anxiety correlated with the placebo analgesia effect 24.
Studies comparing the placebo effect in adult and pediatric migraine studies, showed a greater placebo effect in children than adults (approximately 35% in children vs. 50% or higher in adults25). A meta-analysis reviewing the placebo effect of several clinical trials for IBS in adults, has found an average placebo response of 40% with a range of 16 to 71% 26. The high placebo response found in our study may explain the lack of difference between the amitriptyline and placebo group. A review of the factors influencing the placebo response in adult patients with IBS found an association between the use of the Rome criteria and the inclusion of a run-in phase with a lower placebo effect. Our study showed a high placebo response despite a design that included both of these factors. Studies on the placebo effect in adult patients with IBS showed that desires and expectations influenced placebo effects 24. We hypothesize that our high placebo effect may be the result of a high level of expectancy of the subjects and the parents and a strong family-doctor relationship that included frequent contact between physician and subjects and complete availability throughout the 5 weeks of the study. Parent's reassurance about the tertiary care's physician's knowledge and experience on treating functional disorders may have contributed to the placebo effect. A meta-analysis reviewing clinical trials on the efficacy of antidepressants to treat pediatric depression found that the number of sites was positively correlated with a higher placebo response 27. The study also found that a higher placebo response was negatively correlated with treatment efficacy. A pediatric study on the placebo response in children with migraines found that parallel designed studies have a higher placebo effect than in adult studies 28.
A single center study in a suburban pediatric gastroenterology practice in California conducted in 33 adolescents exclusively with IBS found a beneficial effect of amitriptyline in comparison with placebo in terms of quality of life and pain relief 29. The effect of pain relief was inconsistent in time and locations. The study found improvement of pain only in some areas of the abdomen with no relief in most of them and at certain times of follow-up but not in other times. The study found an unusual negative placebo effect in pain relief that may explain the statistical superiority of the active drug. The study used a dose of amitriptyline similar to our study and showed a beneficial effect in quality of life starting at 4 weeks.
Adequate compliance with treatment and daily completion of questionnaires is an important concern in all clinical trials. Limitation in funding precluded us from measuring amitriptyline serum concentrations or providing electronic means to record symptoms and assure daily completion of dairies. To overcome these limitations, we designed a simple mean to assess adherence. Families were called without notice at approximately study midterm and were asked to count out loud the pills left. Families unaware of the motive of our contact would be unlikely to be able to “do the math” fast enough to calculate the number of pills that should be left on that date if the patient had taken them daily. The answers to the questions from the previous day were compared at the end of the study with the paper version to assure daily completion of the diaries and identify those children planning to retrospectively fill in all diaries at one time. We found that subjects completed the dairies in timely fashion and that most but not all subjects took medication. Once non-compliant children were excluded, we found a higher efficacy of amitriptyline than in the ITT analysis. Our findings have similarities to those of the largest study on TCAs in patients with IBS. Drossman et al. found no difference between desipramine and placebo in ITT analysis, but a beneficial effect in per protocol analysis with a NNT= 5.2 30. Our study showed a NNT= 5.4 in per protocol analysis but the differences between both groups remained statistically insignificant. In the study by Drossman et al a beneficial effect was found in ITT analysis in 60% of patients in the drug group and 47% in the placebo group, while we found 50% and 59% benefit in the amitriptyline group and 46% and 52% improvement in the placebo group.
We cannot exclude that a longer period of treatment or a trial with a higher dose of TCA as used by Drossman et al. may have produced different results. However, several studies have shown both efficacy of amitriptyline at 4 weeks or earlier 10, 29, 31 and a beneficial effect of low dose amitriptyline 10, 29, 31, 32. Although we initially considered a longer follow-up we decided against it to limit the dropout rate. The four year recruitment period required to enroll 90 patients and the difficulties found in enrolling patients in trials using medications that have been associated with suicidal risks by the FDA makes conducting a lengthy, large randomized study difficult. Despite the premature termination of our study, the probability that our study incurred in type II error was 0.72, a figure that is similar to the initially intended (0.80). Based on our results, a post hoc analysis showed that a sample size of 528 patients would have been required in order to find a statistically significant difference between active drug and placebo, a value not possible to achieve in a pediatric study. Even with a much larger pool of patients available, difficulties in recruitment resulted in premature termination of a recent adult study on TCA for IBS in adult patients 33.
We did not encounter major or unexpected side effects related to amitriptyline. Limitations of the study include the possibility of selection bias. We recruited children from tertiary care sites, and the patients studied may constitute a particularly severe patient population. Patients who have mild symptoms may not seek medical attention, may be seen at the primary care office or may be less interested in participating in research studies. Therefore, we cannot exclude that amitriptyline could be more effective in children with lower pain intensity or a less chronic condition.
In conclusion, amitriptyline was equally effective as placebo in ITT analysis in pain predominant FGIDs in children. The safety profile of amitriptyline and the efficacy in the subjects using this drug, as well as the significant improvement in anxiety scores, combined with the inability to use placebo as drug in practice may justify amitriptyline treatment. The high placebo effect found in our study underscores the importance of a positive and caring therapeutic alliance between physicians, patients and families, and suggests that further studies of the use of TCA in children with functional bowel disorders are needed under different clinical conditions.
Acknowledgments
The authors thank all cardiologists who assisted in the EKG interpretation and are especially thankful to cardiologist, Dr. Lee Beerman for his services. The authors also thank Annette Langseder along with the many research assistants and nurses whose dedication and assistance to the study made everything possible. The study was supported in part by the 2003 Clinical Research Award of the American College of Gastroenterology, the CHP 19596 RA501 grant and the grants M01 RR-00048, M01 RR-00084 and MO1 RR-02172 from the National Center for Research Resources, National Institute of Health.
Abbreviations
- FGIDs
Functional Gastrointestinal Disorders
- IBS
Irritable Bowel Syndrome
- ITT
Intention to treat
- NNT
Number needed to treat
- TCA
Tricyclic Antidepressant
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Starfield B, Katz H, Gabriel A, et al. Morbidity in childhood--a longitudinal view. N Engl J Med. 1984;310(13):824–9. doi: 10.1056/NEJM198403293101305. [DOI] [PubMed] [Google Scholar]
- 2.Saps M, Seshadri R, Sztainberg M, Schaffer G, Marshall BM, Di Lorenzo C. A Prospective School-based Study of Abdominal Pain and Other Common Somatic Complaints in Children. J Pediatr. 2008 doi: 10.1016/j.jpeds.2008.09.047. [DOI] [PubMed] [Google Scholar]
- 3.Apley J. The child with abdominal pains. 2. Oxford: Blackwell Scientific Publications; 1975. [Google Scholar]
- 4.Youssef NN, Murphy TG, Langseder AL, Rosh JR. Quality of life for children with functional abdominal pain: a comparison study of patients' and parents' perceptions. Pediatrics. 2006;117(1):54–9. doi: 10.1542/peds.2005-0114. [DOI] [PubMed] [Google Scholar]
- 5.Stordal K, Nygaard EA, Bentsen BS. Recurrent abdominal pain: a five-year follow-up study. Acta Paediatr. 2005;94(2):234–6. doi: 10.1111/j.1651-2227.2005.tb01897.x. [DOI] [PubMed] [Google Scholar]
- 6.Kline RM, Kline JJ, Di Palma J, Barbero GJ. Enteric-coated, pH-dependent peppermint oil capsules for the treatment of irritable bowel syndrome in children. J Pediatr. 2001;138(1):125–8. doi: 10.1067/mpd.2001.109606. [DOI] [PubMed] [Google Scholar]
- 7.See MC, Birnbaum AH, Schechter CB, Goldenberg MM, Benkov KJ. Double-blind, placebo-controlled trial of famotidine in children with abdominal pain and dyspepsia: global and quantitative assessment. Dig Dis Sci. 2001;46(5):985–92. doi: 10.1023/a:1010793408132. [DOI] [PubMed] [Google Scholar]
- 8.Symon DN, Russell G. Double blind placebo controlled trial of pizotifen syrup in the treatment of abdominal migraine. Arch Dis Child. 1995;72(1):48–50. doi: 10.1136/adc.72.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huertas-Ceballos A, Macarthur C, Logan S. Pharmacological interventions for recurrent abdominal pain (RAP) in childhood. Cochrane database of systematic reviews (Online) 2002;(1):CD003017. doi: 10.1002/14651858.CD003017. [DOI] [PubMed] [Google Scholar]
- 10.Morgan V, Pickens D, Gautam S, Kessler R, Mertz H. Amitriptyline reduces rectal pain related activation of the anterior cingulate cortex in patients with irritable bowel syndrome. Gut. 2005;54(5):601–7. doi: 10.1136/gut.2004.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hyams J, Colletti R, Faure C, et al. Functional gastrointestinal disorders: Working Group Report of the First World Congress of Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2002;35 2:S110–7. doi: 10.1097/00005176-200208002-00008. [DOI] [PubMed] [Google Scholar]
- 12.Veldhuyzen van Zanten SJ, Talley NJ, Bytzer P, Klein KB, Whorwell PJ, Zinsmeister AR. Design of treatment trials for functional gastrointestinal disorders. Gut. 1999;45 2:II69–77. doi: 10.1136/gut.45.2008.ii69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kovacs M. The Children's Depression Inventory. Psychopharmacol Bull. 1985;21:995–8. [PubMed] [Google Scholar]
- 14.Spielberger CD. Manual for the Strait-Anxiety Inventory for children. Palo Alto: Consulting Psychologist Press; 1973. [Google Scholar]
- 15.Walker LS, Smith CA, Garber J, Van Slyke DA. Development and validation of the Pain Response Inventory for Children. Psychol Assess. 1997;9(4):392–405. [Google Scholar]
- 16.Walker LS, Greene JW. The functional disability inventory: measuring a neglected dimension of child health status. J Pediatr Psychol. 1991;16(1):39–58. doi: 10.1093/jpepsy/16.1.39. [DOI] [PubMed] [Google Scholar]
- 17.Walker LS, Sorrells SC. Brief report: Assessment of children's gastrointestinal symptoms for clinical trials. J Pediatr Psychol. 2002;27(3):303–7. doi: 10.1093/jpepsy/27.3.303. [DOI] [PubMed] [Google Scholar]
- 18.Tesler MD, Savedra MC, Holzemer WL, Wilkie DJ, Ward JA, Paul SM. The word-graphic rating scale as a measure of children's and adolescents' pain intensity. Research in nursing & health. 1991;14(5):361–71. doi: 10.1002/nur.4770140507. [DOI] [PubMed] [Google Scholar]
- 19.Camilleri M, Mangel AW, Fehnel SE, Drossman DA, Mayer EA, Talley NJ. Primary endpoints for irritable bowel syndrome trials: a review of performance of endpoints. Clin Gastroenterol Hepatol. 2007;5(5):534–40. doi: 10.1016/j.cgh.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Matsuzawa-Yanagida K, Narita M, Nakajima M, et al. Usefulness of antidepressants for improving the neuropathic pain-like state and pain-induced anxiety through actions at different brain sites. Neuropsychopharmacology. 2008;33(8):1952–65. doi: 10.1038/sj.npp.1301590. [DOI] [PubMed] [Google Scholar]
- 21.Silverman DH, Munakata JA, Ennes H, Mandelkern MA, Hoh CK, Mayer EA. Regional cerebral activity in normal and pathological perception of visceral pain. Gastroenterology. 1997;112(1):64–72. doi: 10.1016/s0016-5085(97)70220-8. [DOI] [PubMed] [Google Scholar]
- 22.Naliboff BD, Derbyshire SW, Munakata J, et al. Cerebral activation in patients with irritable bowel syndrome and control subjects during rectosigmoid stimulation. Psychosom Med. 2001;63(3):365–75. doi: 10.1097/00006842-200105000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Mertz H, Morgan V, Tanner G, et al. Regional cerebral activation in irritable bowel syndrome and control subjects with painful and nonpainful rectal distention. Gastroenterology. 2000;118(5):842–8. doi: 10.1016/s0016-5085(00)70170-3. [DOI] [PubMed] [Google Scholar]
- 24.Vase L, Robinson ME, Verne GN, Price DD. Increased placebo analgesia over time in irritable bowel syndrome (IBS) patients is associated with desire and expectation but not endogenous opioid mechanisms. Pain. 2005;115(3):338–47. doi: 10.1016/j.pain.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 25.Lewis DW, Kellstein D, Dahl G, et al. Children's ibuprofen suspension for the acute treatment of pediatric migraine. Headache. 2002;42(8):780–6. doi: 10.1046/j.1526-4610.2002.02180.x. [DOI] [PubMed] [Google Scholar]
- 26.Patel SM, Stason WB, Legedza A, et al. The placebo effect in irritable bowel syndrome trials: a meta-analysis. Neurogastroenterol Motil. 2005;17(3):332–40. doi: 10.1111/j.1365-2982.2005.00650.x. [DOI] [PubMed] [Google Scholar]
- 27.Bridge JA, Birmaher B, Iyengar S, Barbe RP, Brent DA. Placebo response in randomized controlled trials of antidepressants for pediatric major depressive disorder. Am J Psychiatry. 2009;166(1):42–9. doi: 10.1176/appi.ajp.2008.08020247. [DOI] [PubMed] [Google Scholar]
- 28.Fernandes R, Ferreira JJ, Sampaio C. The placebo response in studies of acute migraine. J Pediatr. 2008;152(4):527–33. 33 e1. doi: 10.1016/j.jpeds.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 29.Bahar RJ, Collins BS, Steinmetz B, Ament ME. Double-blind placebo-controlled trial of amitriptyline for the treatment of irritable bowel syndrome in adolescents. J Pediatr. 2008;152(5):685–9. doi: 10.1016/j.jpeds.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 30.Drossman DA, Toner BB, Whitehead WE, et al. Cognitive-behavioral therapy versus education and desipramine versus placebo for moderate to severe functional bowel disorders. Gastroenterology. 2003;125(1):19–31. doi: 10.1016/s0016-5085(03)00669-3. [DOI] [PubMed] [Google Scholar]
- 31.Otaka M, Jin M, Odashima M, et al. New strategy of therapy for functional dyspepsia using famotidine, mosapride and amitriptyline. Aliment Pharmacol Ther. 2005;21 2:42–6. doi: 10.1111/j.1365-2036.2005.02473.x. [DOI] [PubMed] [Google Scholar]
- 32.Poitras P, Riberdy Poitras M, Plourde V, Boivin M, Verrier P. Evolution of visceral sensitivity in patients with irritable bowel syndrome. Dig Dis Sci. 2002;47(4):914–20. doi: 10.1023/a:1014729125428. [DOI] [PubMed] [Google Scholar]
- 33.Talley NJ, Kellow JE, Boyce P, Tennant C, Huskic S, Jones M. Antidepressant therapy (imipramine and citalopram) for irritable bowel syndrome: a double-blind, randomized, placebo-controlled trial. Dig Dis Sci. 2008;53(1):108–15. doi: 10.1007/s10620-007-9830-4. [DOI] [PubMed] [Google Scholar]