Abstract
Background
Pulseless electrical activity is an important cause of cardiac arrest. Our purpose was to determine if induction of hypothermia with a cold perfluorocarbon-based total liquid ventilation system (TLV) would improve resuscitation success in a swine model of asphyxial cardiac arrest/PEA.
Methods
Twenty swine were randomly assigned to control (C, no ventilation, n=11) or TLV with pre-cooled PFC (n=9) groups. Asphyxia was induced by insertion of a stopper into the endotracheal tube, and continued in both groups until loss of aortic pulsations (LOAP) was reached, defined as a pulse pressure less than 2mmHg. The TLV animals underwent asphyxial arrest for an additional 2 minutes after LOAP, followed by 3 minutes of hypothermia, prior to starting CPR. The C animals underwent 5 minutes of asphyxia beyond LOAP. Both groups then underwent CPR for at least 10 minutes. The endpoint was the resumption of spontaneous circulation maintained for 10 minutes.
Results
Seven of 9 animals achieved resumption of spontaneous circulation (ROSC) in the TLV group vs. 5 of 11 in the C group (p=0.2). The mean pulmonary arterial temperature was lower in total liquid ventilation animals starting 4 minutes after induction of hypothermia (TLV 36.3 ± SE 0.2 vs. C 38.1±0.2°C, p<0.0001). Arterial pO2 was higher in total liquid ventilation animals at 2.5 minutes of CPR (TLV 76±12 vs. C 44±2 mmHg; p=0.03).
Conclusion
Induction of moderate hypothermia using perfluorocarbon-based total liquid ventilation did not improve ROSC success in this model of asphyxial cardiac arrest.
Keywords: CPR, resuscitation, cardiac arrest, asphyxia, defibrillation, perfluorocarbons, liquid ventilation
Introduction
Asphyxial cardiac arrest is an important cause of death in both pediatric and adult populations. It is increasingly being recognized as the first documented pulseless arrest rhythm within inpatient populations. It is also notable that the survival to discharge and neurologic outcomes for these patients is significantly worse than for those suffering from ventricular fibrillation (VF) arrest.1 This condition is often initiated by airway obstruction or neurologic dysfunction and leads to hypertension and tachycardia, followed by severe hypoxia and hypotension, bradycardia, and subsequent pulseless electrical activity.
Induction of hypothermia by external cooling has been shown to improve neurologic outcomes in survivors of resuscitation from ventricular fibrillation in human subjects.2,3,4 Moderate hypothermia (33°C) induced by external cooling prior to cardiac arrest in swine improved ROSC and reduced the number of defibrillation shocks compared to normothermic controls.5 The use of intra-arrest hypothermia through internal cooling also facilitated resuscitation from VF arrest in multiple swine models.6,7 The use of cold perfluorocarbons (PFC), instilled into the lungs by total liquid ventilation (TLV) in models of VF arrest, also improved the rate of ROSC. 6,8 Short-duration TLV (3 minutes) using PFCs to induce moderate hypothermia was also noted to improve resuscitation outcomes in a similar swine model.8
We hypothesized that induction of rapid intra-arrest moderate hypothermia (33°C) with the use of a cold PFC based TLV system would improve successful resuscitation in a swine model of asphyxial cardiac arrest, similar to the effect of cold TLV in VF models.
Methods
Animal Preparation
The use of animals and protocol was approved by the University of Iowa Animal Care and Use Committee. Twenty swine, nineteen female and one male between 19-26kg., were randomly assigned to a control or hypothermic group. Nine animals were assigned to TLV and eleven to the control group. The animals were first anesthetized by induction of ketamine 20mg/kg and acepromazine 0.2mg/kg administered intramuscularly followed by inhaled isoflurane by mask. The animals underwent endotracheal (ET) intubation via direct laryngoscopy and were ventilated with a positive-pressure ventilator on room air supplemented with 100% oxygen to maintain pO2 at 150 mmHg. One pentobarbital injection (100mg) was given before the experimental protocol began and isoflurane (0.5-2.5%) was given during surgery. No anesthesia was given during asphyxiation, arrest and CPR periods. Following confirmation of adequate anesthesia, bilateral femoral cut-downs were performed for placement of arterial and venous catheters. Intravenous heparin (2000 U) was given to prevent thrombosis of the indwelling catheters. Pulmonary artery (PA) temperature was measured using a Swan-Ganz thermodilution catheter. Core body temperature was measured by a temperature thermistor placed in the inferior vena cava (IVC). Intracranial and esophageal temperatures were assessed by thermistors placed into the posterior nasal cavity and distal esophagus. Defibrillation electrode pads were placed anteriorly and posteriorly on the chest wall and connected to a commercially available biphasic truncated experimental waveform defibrillator displaying a continuous ECG tracing. Arterial blood gases were monitored and adjustments to tidal volume and inspired oxygen were performed to provide adequate ventilation. Prior to induction of asphyxial arrest, measurement of baseline hemodynamics was performed. Arterial pressure was continuously monitored via an indwelling catheter in the right femoral artery. Coronary perfusion pressure, defined as the aortic diastolic pressure minus right atrial diastolic pressure during the relaxation phase of closed-chest compression, was calculated. Cardiac output at baseline and after ROSC was measured by thermodilution.
Asphyxial Arrest Protocol
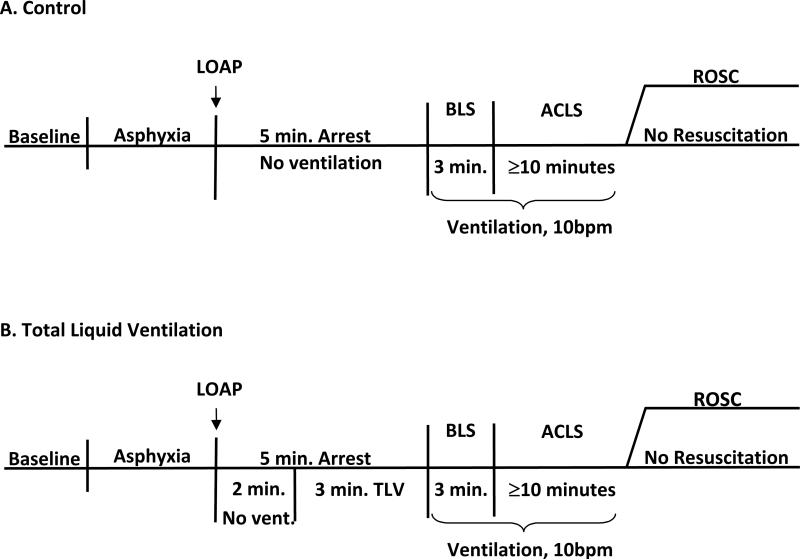
Following measurement of baseline parameters, asphyxia was induced in all animals by insertion of a rubber stopper into the end of the ET tube. This asphyxial phase was continued until loss of aortic pulsations (LOAP), defined as a pulse pressure less than 2mmHg.9 After LOAP, asphyxia was continued for an additional 5 minutes in control animals without cooling prior to the start of CPR; the stopper remained in place during this period. The TLV treated animals underwent continued asphyxial arrest for 2 minutes after LOAP, with the stopper in place, and then received the hypothermic protocol. The protocols for control and TLV treated animals are illustrated in Figures 1a and 1b.
Figure 1. Experimental protocol.
• Abbreviations: LOAP = Loss of aortic pulsations; ROSC = Resumption of spontaneous circulation; BLS = Basic life support; ACLS = Advanced cardiac life support; Min = Minutes; Vent = Ventilation
Hypothermic Protocol
Nine swine were treated with a PFC (Fluorinert™ FC-77, 3M Corp. St. Paul, MN.), a 1:1 mixture of two isomers of C8F16O, 40ml/kg. The fluid was instilled into the lungs via the endotracheal tube 2 minutes after LOAP. The liquid ventilation system consisted of a Harvard large animal respirator connected to a cold PFC reservoir which was cooled to 2°C and was pre-oxygenated with 100% oxygen beginning 2 minutes after LOAP. The system was a closed circuit in which PFC fluid was continuously circulated through the animal and cold reservoir system. The ventilator system primed with cold PFC was connected to the endotracheal tube and TLV with cold PFC; ventilation was initiated at 6 breaths per minute with a tidal volume of approximately 210 cc and maintained for 3 minutes. To minimize barotrauma during liquid ventilation, maximum intra-tracheal pressure was kept close to normal air ventilation pressures by controlling intrapulmonary PFC volume via changes in ventilation rate and suction during exhalation. The lungs were not allowed to fill with PFC above the filling volume of 40ml/kg or ventilator pressure above 20mmHg. Suction could be applied to the reservoir to increase recovery of PFC from lungs during exhalation in order to control ventilation pressure by modifying the amount of PFC in the lungs. Three minutes following PFC instillation, the TLV was stopped in the exhalation phase and the PFC was drained via suction applied to the ventilator and by placing the animal in the Trendelenburg position. Most of the PFCs were recovered before CPR began, with an additional 20-50ml expelled during the first minute of CPR. The endotracheal tube was reconnected to the air ventilator with the expiratory circuit connected to a reservoir to collect the residual PFC during the closed chest compression. The air ventilation was continued at a rate of 10 breaths/minute. During CPR the animals were ventilated with 100% oxygen (Figure 1b.).
Resuscitation Protocol
Prior to starting the resuscitation phase, intra-abdominal pressure was increased by an abdominal cuff and inflated to 35 mmHg maintained throughout the CPR period.10 CPR was performed manually by a single operator with chest compressions at 100/minute, guided by a metronome, for 3 minutes prior to the use of pharmacologic or electrical therapies. CPR was deemed adequate if performed to produce an arterial systolic blood pressure of approximately 60mmHg.11 Mechanical ventilation with 100% oxygen at a rate of 10 breaths per minute was used during the CPR phase. Chest compressions were interrupted for less than 5 seconds every 2 minutes to observe the ECG and arterial pressure.
Following the first 3 minutes of CPR, advanced cardiac life support (ACLS) was started. Epinephrine, 1 mg IV, was initially given 3 minutes after CPR was begun, and given every 4 minutes thereafter. Atropine, 1mg IV up to total dose of 3mg, was given after 3 minutes of CPR and thereafter every 2 minutes as needed for bradycardia or asystole. Sodium bicarbonate was used for pH < 7.2 as noted by arterial blood gas every 3 minutes. A biphasic direct current shock was administered if the intra-arrest rhythm indicated ventricular fibrillation. An initial shock was given at 100J, followed by immediate resumption of CPR for 2 minutes prior to reassessment of rhythm. If VF persisted, 200J was selected for successive shocks every 2 minutes until a perfusing rhythm was established.
The endpoint of resuscitation was the resumption of spontaneous circulation (ROSC), defined as peak systolic arterial pressure > 60 mmHg maintained for 10 minutes without pharmacologic support or manual or mechanical chest compression. Following ROSC, the ventilator rate was increased to 20 breaths per minute with continued 100% oxygen to correct the acidosis and hypercarbia typically demonstrated by arterial blood gases. If ROSC did not occur, CPR was continued for a minimum of 10 minutes or until success was deemed improbable.
Post-resuscitation hemodynamic parameters, temperatures, and arterial blood gases were measured 10 minutes following initial ROSC. Animals that were resuscitated were monitored for at least 10 minutes after initial ROSC and ultimately euthanized by an overdose of isoflurane followed by intravenous potassium chloride. Post-mortem autopsy was performed on all animals to determine the presence and extent of intrathoracic bleeding, lung damage, or presence of disease. The presence of significant resuscitation-induced bleeding or disease, such as pericarditis or pneumonia, excluded the animal from the study regardless of outcome.
Statistical Methods
Data are displayed as the mean ± standard error (SE), unless otherwise noted. Baseline comparisons of hemodynamics and blood gas measurements for controls and TLV treated animals were performed using a paired T-test or the Wilcoxon rank-sum test. Linear mixed model analysis for repeated measures tested for differences in blood gases over the 4 time periods (baseline, asphyxial arrest, CPR at 2.5 minutes, and CPR at 6.5 minutes) between TLV and control animals. The success rate for ROSC, and comparisons in atropine usage, were determined using Fisher's exact test. Linear mixed model analysis for repeated measures was used to compare nasal, esophageal, pulmonary artery, and inferior vena cava temperatures between TLV and control groups, as well as for differences in mean CPP in TLV and control groups. The fixed effects in the model were group, time, and group-time interaction. For all four temperatures, there was a significant group-time interaction, so that group mean comparisons were examined at each time point using test of mean contrast based on the fitted linear mixed model. The p-values obtained were adjusted using Bonferroni's method.
Results
Hemodynamic parameters showed no differences in either control or TLV animals at baseline or during the protocol (Table 1). Arterial blood gas showed a significantly higher pO2 in TLV animals (TLV 76±12 vs. CON 44±2; p=0.03) 2.5 minutes into CPR. This difference was close to significant at 6.5 minutes into CPR (p=0.08) (Table 2). Asphyxiation time, from plugging of ET tube to LOAP, was also similar between groups (TLV 391±17sec. vs. CON 370±24sec; p=0.51). The animals were not paralyzed during the arrest. The total number of gasps was insignificantly elevated in the control group (CON 26±4 vs. TLV 19±4; p=0.25), but the TLV animals were noted to have a significantly longer duration of gasping during the asphyxial phase (TLV 357±3.1sec vs. CON 319±3.4sec; p=0.02). The average pulmonary arterial (PA) and inferior vena caval (IVC) temperature during the cooling phase was 36.3±0.2°C in the TLV animals vs. 38.1+0.2°C in control animals (p<0.0001). These temperatures were significantly lower in the TLV group starting 6 minutes following induction of hypothermia and this difference persisted throughout the experiment (p<0.0001) (Figure 2). Nasal temperature averaged 36.5±0.2°C in TLV animals and was significantly lower than controls (37.8±0.2°C) at 7 minutes following hypothermic induction (p=0.0003). The average esophageal temperature in TLV animals was 34±0.3°C compared to control animals (37.3±0.3°C), which was significantly lower (p<0.0001). Resumption of spontaneous circulation (ROSC) was achieved in 7 of 9 (78%) animals in the TLV group and 5 of 11 (45%) in the control group (p=0.20). There were no differences noted in total time CPR was required in animals which achieved ROSC (TLV 6.6±1.4 min. vs. C 5.8±1.1min; p=0.22). Mean CPP during CPR did not significantly differ between the two groups (p=0.55). There were also no significant differences in the number of animals requiring epinephrine or the total amount given during arrest. There was an insignificant reduction in the use of atropine required during arrest; only 1 of 9 animals received atropine in the TLV group compared to 5 of 11 in the controls (p=0.16). Ventricular fibrillation was uncommon in both groups and occurred during the CPR phase in 2 TLV and 1 control animal, none of which achieved ROSC.
Table 1.
Hemodynamics at baseline and during arrest phase.
| Group |
Time (min. CPR) |
SAP (mmHg) |
DAP (mmHg) |
MAP (mmHg) |
HR/CR (bpm) |
CVP (mmHg) |
CO (L/min) |
CPP (mmHg) |
N |
|---|---|---|---|---|---|---|---|---|---|
| TLV | Baseline | 93±5 | 54±3 | 67±4 | 111±7 | 5.9±0.4 | 4.0±0.3 | 49±3 | 9 |
| Control |
Baseline |
87±2 |
48±1 |
61±1 |
102±3 |
6.6±0.3 |
3.7±0.1 |
41±1 |
11 |
| TLV | 1 min | 42±4 | 15±1 | 24±2 | 100±0.4 | 8.7±0.5 | 6±1 | 9 | |
| Control |
1 min |
46±1 |
16±1 |
26±1 |
100±0.4 |
8.8±0.3 |
|
7±1 |
11 |
| TLV | 2 min | 50±4 | 17±1 | 28±2 | 100±0.2 | 8.8±0.5 | 8±1 | 9 | |
| Control |
2 min |
52±2 |
18±1 |
29±1 |
100±0.1 |
9.1±0.3 |
|
9±1 |
11 |
| TLV | 3 min | 56±4 | 18±1 | 31±2 | 100±0.1 | 8.8±0.5 | 9±1 | 9 | |
| Control |
3 min |
56±2 |
19±1 |
31±1 |
100±0.1 |
8.9±0.5 |
|
10±1 |
11 |
| TLV |
4 min |
61±5 |
24±2 |
32±5 |
100±0.2 |
9.4±0.7 |
|
14±2 |
9 |
| Control | 4 min | 64±5 | 23±2 | 36±3 | 100±0.2 | 9.0±0.4 | 14±2 | 11 |
Table 2.
Arterial blood gases at baseline and during arrest phase.
| Baseline |
Arrest |
CPR |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 min. | 2.5 min. | 6.5 min. | ||||||||||
| |
pH |
pCO2 |
pO2 |
pH |
pCO2 |
pO2 |
pH |
pCO2 |
pO2 |
pH |
pCO2 |
pO2 |
| Cold TLV | 7.40 | 40 | 152 | 7.18 | 71 | 8 | 7.16 | 64 | 76* | 7.19 | 75 | 59+ |
| ±0.01 | ±1 | ±6 | ±0.02 | ±2 | ±1 | ±0.01 | ±2 | ±12 | ±0.08 | ±3 | ±5 | |
| |
n = 8 |
|
n = 9 |
n =5 |
||||||||
| Control | 7.40 | 41 | 145 | 7.21 | 71 | 6 | 7.18 | 66 | 44 | 7.19 | 77 | 43 |
| ±0.01 | ±1 | ±5 | ±0.01 | ±2 | ±1 | ±0.01 | ±2 | ±2.1 | ±0.03 | ±5 | ±2 | |
| n = 11 | n =10 | n = 8 | ||||||||||
P = 0.03, Cold TLV vs. Control
P = 0.08, Cold TLV vs. Control
Figure 2.
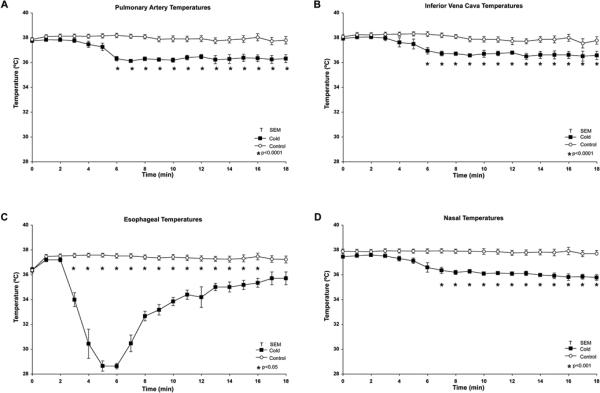
Intra-arrest pulmonary artery, inferior vena cava, nasal and esophageal temperatures.
Discussion
In this experiment, we hypothesized that intra-arrest PFC-induced moderate hypothermia would improve outcomes in this swine model of asphyxia. However, we did not find a significant difference in ROSC success between the control and TLV treated animals. These results are in contrast to Riter et al who induced VF arrest, not asphyxial arrest, and found TLV did improve ROSC success.8 This suggests an intrinsic difference based on the physiology of the arrhythmia itself.
There are important physiological differences between VF and asphyxial arrest. Asphyxial arrest causes a hypercarbic acidosis with profound hypoxia compared to VF in canine models.12 In VF arrest cardiac output ceases immediately, compared to the continued cardiac output following the initiation of asphyxia. Asphyxial arrest results in the continued delivery of CO2 to the alveolar compartment from ongoing cellular respiration and production of CO2 following the consumption of oxygen prior to pulselessness.13 The degree of acidemia was a determinant of ROSC success in a rat model of VF studied by Morimoto et al.14 In canine models of VF and asystolic arrest, myocardial oxygen consumption (MVO2) was significantly reduced in asystolic animals.15
Multiple animal studies have shown a neurologic benefit of hypothermia in both intra- and post-asphyxial arrest phases. Several authors have noted that moderate, externally-applied, hypothermia begun prior or after asphyxial arrest improved neurological outcomes in rat and swine models.16,17 Hicks et al also found reduced expression of stress-induced proteins in association with improved neurologic results in a rat model of post-asphyxial arrest resuscitation hypothermia.18 Therapeutic hypothermia has been used following asphyxial arrest in a case report following 75 minutes of prolonged cardiopulmonary resuscitation (CPR)19, but no clinical trials have examined this intervention in asphyxial arrest as opposed to VF arrest.2,3 Prior investigations in our lab have shown that TLV based PFC-induced hypothermia improved ROSC rates and reduced need for defibrillation when performed during the arrest phase in a swine model of VF6,8. Despite the lack of circulatory blood flow in the VF model successful hypothermia was achieved, presumably by physical contact of the PFC-cooled lungs with the heart during the period of cardiac arrest.
In a murine model of asystolic arrest induced by intravenous potassium, Abella et al and Zhao et al showed that intra-arrest cooling significantly improved long-term survival compared to normothermic controls.20,21 In their two studies, no significant difference in the initial ROSC rate was noted, but post-ROSC survival was significantly increased by hypothermia; survival lasted up to 7 days later. We did not study animals beyond 10 minutes and thus cannot assess long-term outcomes. Shao et al demonstrated a significant reduction in cell death following intra-arrest hypothermia prior to reperfusion; this reduction was attenuated when the hypothermia was applied late during ischemia or delayed beyond reperfusion.22
In our study, there was a notable difference in the pO2 post-asphyxiation in the TLV group versus controls (Table 2). This was due to the high oxygen tension of the PFCs and subsequent diffusion into the animal. This effect was also noted previously in our lab with the use of a PFC based TLV model of VF arrest, where it was associated with an improvement in ROSC success.6,8
Berg et al showed that bystander CPR and assisted ventilation alone improved ROSC in a piglet model of asphyxial arrest; this arrest lasted until loss of aortic pulsations was reached (mean time 6.8 min.), directly followed by intervention.23,24 The piglets Berg et al. used were smaller than the animals used in our experiment. Furthermore, we defined a LOAP point of 2mmHg pulse pressure compared to 50mmHg systolic pressure in the study of Berg et al, which substantially lengthened the total duration of the asphyxic insult in our study. In our series of experiments, the time required for the aortic pressure to fall from 50 mmHg systolic to a pulse pressure of 2mmHg ranged from 2-4 minutes in all animals. At present there is no consensus in the literature of animal models of asphyxial cardiac arrest for a specific point which would define loss of aortic pulsatile flow.
Since intranasal temperatures (reflecting intracranial temperatures) fell with cold TLV, it appears that the cold TLV technique achieves cooling of the brain as well as the heart. One manifestation of brain cooling in our TLV animals may have been the difference in gasping between the two animal groups. During the asphyxial phase in our study, gasping occurred for a significantly longer duration in the TLV animals. Gasping during cardiac arrest indicates neurologic viability and adequacy of perfusion. Bobrow et al noted the presence of gasping was directly correlated to improved survival to discharge from cardiac arrest in humans25. The induced intracranial hypothermia may have provided some neuroprotective benefits in our study by allowing TLV animals to gasp longer, thereby improving the chances for successful resuscitation. The gasping in all animals occurred during the period when the aortic pulse pressure was falling to 2mmHg; gasping ceased below 2mmHg. Delaying CPR for several minutes beyond the point where gasping ceased may have created an especially severe model of asphyxial arrest in our study.
Moderate hypothermia induced pre-arrest reduced the incidence of recurrent VF after initial ROSC in a VF arrest swine model.5 In our experiment, ventricular fibrillation only occurred in 15% of animals, none of which achieved ROSC. These results are in contrast to those of Berg et al who showed VF occurred prior to CPR initiation in 28% of piglets exposed to asphyxial arrest for 10 minutes.26 Hallstrom et al found that the conversion of asystole or pulseless electrical activity to VF significantly reduced survival to discharge and increased mortality 82% in humans.27
The exact mechanisms by which hypothermia induced pre-arrest or intra-arrest improves resuscitation outcomes are uncertain. Possible mechanisms include reduced myocardial and cerebral metabolic rate and cellular energy requirements, reduced metabolic oxygen consumption via less fatty acid catabolism, less mitochondrial dysfunction, and slowing of the enzymatic oxidative cascades responsible for cardiac and neurologic damage after ischemia-reperfusion injury6. By inducing hypothermia early in the resuscitative process – i.e., intra-arrest, and then further lowering of temperature during the post-arrest period - these beneficial mechanisms should be even more effective in reducing cardiac and cerebral injury and achieving ROSC and survival. This is the rationale for recent clinical trials of beginning hypothermia in the ambulance transporting just-resuscitated patients from the field to the hospital28,29.
Limitations
In this model of asphyxial arrest, the animals used were young healthy anesthetized pigs. This contrasts with typical patients who suffer cardiac arrest, who are not anesthetized and who have comorbid conditions which could adversely affect their survival.
Our study was performed in female pigs. We used females because our previous studies using PFC-based TLV showed significantly better ROSC in females compared to males, and we wanted to determine the maximum TLV success rate possible in this asphyxial arrest model6. However, we cannot assume that the ROSC success rate we achieved in females in this experiment will be found in male animals as well.
Our animals did not achieve the same depth of cooling as we have reached in prior studies. The average temperature at 1 minute into the CPR phase, following PFC instillation, was 36.3°C, vs. 34.8°C in the study of Riter et al.8 The PFCs in the present study were cooled prior to instillation to approximately 2°C, as opposed to our prior studies which pre-cooled the PFC's to −15°C. The ROSC rate in our study might have been higher had we achieved deeper hypothermia using colder PFC.
We used abdominal binding since this has been shown to increase coronary perfusion pressure and improve ROSC success10. However, abdominal binding is not included in the current American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiac Care30 and is not used clinically. Whether our results would be the same if we had not used abdominal binding is unknown.
The number of animals is our study was small. We observed the animals for only 10 minutes following ROSC; we have no long-term data on outcomes.
Conclusion
Induction of moderate hypothermia using a PFC-based TLV did not significantly improve ROSC success in this swine model of asphyxial cardiac arrest/PEA, unlike prior VF arrest models. Greater depth and/or duration of hypothermia might yield better results.
Abbreviations
- VF
Ventricular fibrillation
- PFC
Perfluorocarbons
- TLV
Total liquid ventilation
- ROSC
Resumption of spontaneous circulation
- ET
Endotracheal
- ABG
Arterial blood gases
- IVC
Inferior vena cava
- MAP
Mean arterial pressure
- SAP
Systemic arterial pressure
- HR
Heart rate
- PAP
Pulmonary artery pressure
- CVP
Central venous pressure
- CO
Cardiac output
- ECG
Electrocardiogram
- CPR
Cardiopulmonary resuscitation
- CPP
Coronary perfusion pressure
- LOAP
Loss of aortic pulsations
- ETCO2
End-tidal carbon dioxide
- CR
compression rate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Source of Funding: Supported in part by NHLBI grant #5 RO1 HL 71676-03.
Conflicts of Interest Statement: Dr. Kerber owns stock in 3M Corporation, none of the other authors listed have any conflicts of interest.
References
- 1.Nadkarni VM, Larkin GL, Peberdy MA, et al. National Registry of Cardiopulmonary Resuscitation Investigators. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA. 2006;295:96–8. doi: 10.1001/jama.295.1.50. [DOI] [PubMed] [Google Scholar]
- 2.The Hypothermia after Cardiac Arrest Study Group Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–56. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 3.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of outof-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–63. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 4.Nolan JP, Morley PT, VandenHoek TL, Hickey RW. Therapeutic hypothermia after cardiac arrest: an advisory statement by the advanced life support task force of the international liaison committee on resuscitation. Circulation. 2003;108:118–21. doi: 10.1161/01.CIR.0000079019.02601.90. [DOI] [PubMed] [Google Scholar]
- 5.Boddicker KA, Zhang Y, Zimmerman MB, Davies LR, Kerber RE. Hypothermia improves defibrillation success and resuscitation outcomes from ventricular fibrillation. Circulation. 2005;111:3195–201. doi: 10.1161/CIRCULATIONAHA.104.492108. [DOI] [PubMed] [Google Scholar]
- 6.Staffey KS, Dendi R, Brooks LA, et al. Liquid ventilation with perfluorocarbons facilitates resumption of spontaneous circulation in a swine cardiac arrest model. Resuscitation. 2008;78:77–84. doi: 10.1016/j.resuscitation.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menegazzi JJ, Rittenberger JC, Suffoletto BP, et al. Effects of pre-arrest and intra-arrest hypothermia on ventricular fibrillation and resuscitation. Resuscitation. 2009;80:126–32. doi: 10.1016/j.resuscitation.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riter HR, Brooks LA, Pretorius AM, Ackermann LW, Kerber RE. Intra-arrest Hypothermia: Both Cold Liquid Ventilation with Perfluorocarbons and Cold Intravenous Saline Rapidly Achieve Hypothermia, but Only Cold Liquid Ventilation Improves Resumption of Spontaneous Circulation. Resuscitation. 2009;80:561–566. doi: 10.1016/j.resuscitation.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams JA, Bassuk JA, Arias J, et al. Periodic acceleration (pGz) CPR in a swine model of asphyxia induced cardiac arrest. Short-term hemodynamic comparisons. Resuscitation. 2008;77:132–8. doi: 10.1016/j.resuscitation.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 10.Lottes AE, Rundell AE, Geddes LA, Kemeny AE, Otlewski MP, Babbs CF. Sustained abdominal compression during CPR raises coronary perfusion pressures as much as vasopressor drugs. Resuscitation. 2007;75(3):515–24. doi: 10.1016/j.resuscitation.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Idris AH, Becker LB, Ornato JP, et al. Utstein-style guidelines for uniform reporting of laboratory CPR research. A statement for healthcare professionals from a Task Force of the American Heart Association, the American College of Emergency Physicians, the American College of Cardiology. Resuscitation. 1996;33:69–84. doi: 10.1016/s0300-9572(96)01055-6. [DOI] [PubMed] [Google Scholar]
- 12.DeBehnke DJ, Hilander SJ, Dobler DW, Wickman LL, Swart GL. The hemodynamic and arterial blood gas response to asphyxiation: a canine model of pulseless electrical activity. Resuscitation. 1995;30:169–75. doi: 10.1016/0300-9572(95)00873-r. [DOI] [PubMed] [Google Scholar]
- 13.Berg RA, Henry C, Otto CW, et al. Initial end-tidal CO2 is markedly elevated during cardiopulmonary resuscitation after asphyxial cardiac arrest. Pediatr Emerg Care. 1996;12:245–8. doi: 10.1097/00006565-199608000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Morimoto Y, Kemmotsu O, Morimoto Y. Extramyocardial acidosis impairs cardiac resuscitability in isolated, perfused, rat hearts. Crit Care Med. 1996;24:1719–23. doi: 10.1097/00003246-199610000-00020. [DOI] [PubMed] [Google Scholar]
- 15.Mosca SM, Escudero E, Gelpi RJ, Kosoglov AT, Rinaldi GJ, Cingolani HE. Myocardial flow distribution. II : Empty beating heart, ventricular fibrillation and cardiac arrest. Arch Int Physiol Biochim. 1981;89:357–64. doi: 10.3109/13813458109069485. [DOI] [PubMed] [Google Scholar]
- 16.Xiao F, Safar P, Radovsky A. Mild protective and resuscitative hypothermia for asphyxial cardiac arrest in rats. Am J Emerg Med. 1998;16:17–25. doi: 10.1016/s0735-6757(98)90059-6. [DOI] [PubMed] [Google Scholar]
- 17.Agnew DM, Koehler RC, Guerguerian AM, et al. Hypothermia for 24 hours after asphyxic cardiac arrest in piglets provides striatal neuroprotection that is sustained 10 days after rewarming. Pediatr Res. 2003;52:253–62. doi: 10.1203/01.PDR.0000072783.22373.FF. [DOI] [PubMed] [Google Scholar]
- 18.Hicks SD, DeFranco DB, Callaway CW. Hypothermia during reperfusion after asphyxial cardiac arrest improves functional recovery and selectively alters stress-induced protein expression. J Cereb Blood Flow Metab. 2000;20:520–30. doi: 10.1097/00004647-200003000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Bartels M, Tjan DH, Reussen EM, van Zanten AR. Therapeutic hypothermia after prolonged cardiopulmonary resuscitation for pulseless electrical activity. Neth J Med. 2007;65:38–41. [PubMed] [Google Scholar]
- 20.Abella BS, Zhao D, Alvarado J, Hamann K, Vanden Hoek TL, Becker LB. Intra-arrest cooling improves outcomes in a murine cardiac arrest model. Circulation. 2004;109:2786–91. doi: 10.1161/01.CIR.0000131940.19833.85. [DOI] [PubMed] [Google Scholar]
- 21.Zhao D, Abella BS, Beiser DG, et al. Intra-arrest cooling with delayed reperfusion yields higher survival than earlier normothermic resuscitation in a mouse model of cardiac arrest. Resuscitation. 2008;77:242–9. doi: 10.1016/j.resuscitation.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shao ZH, Chang WT, Chan KC, et al. Hypothermia-induced cardioprotection using extended ischemia and early reperfusion cooling. Am J Physiol Heart Circ Physiol. 2007;292:H1995–2003. doi: 10.1152/ajpheart.01312.2005. [DOI] [PubMed] [Google Scholar]
- 23.Berg RA, Hilwig RW, Kern KB, Ewy GA. ‘Bystander’ chest compressions and assisted ventilation independently improve outcome from piglet asphyxial pulseless ‘cardiac arrest’. Circulation. 2000;101:1743–8. doi: 10.1161/01.cir.101.14.1743. [DOI] [PubMed] [Google Scholar]
- 24.Berg RA, Hilwig RW, Kern KB, Babar I, Ewy GA. Simulated mouth-to-mouth ventilation and chest compressions (bystander cardiopulmonary resuscitation) improves outcome in a swine model of prehospital pediatric asphyxial cardiac arrest. Crit Care Med. 1999;27:1893–9. doi: 10.1097/00003246-199909000-00030. [DOI] [PubMed] [Google Scholar]
- 25.Bobrow BJ, Zuercher M, Ewy GA, et al. Gasping during cardiac arrest in humans is frequent and associated with improved survival. Circulation. 2008;118:2550–4. doi: 10.1161/CIRCULATIONAHA.108.799940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berg RA, Kern KB, Otto CW, Samson RA, Sanders AB, Ewy GA. Ventricular fibrillation in a swine model of acute pediatric asphyxial cardiac arrest. Resuscitation. 1996;33:147–53. doi: 10.1016/s0300-9572(96)01013-1. [DOI] [PubMed] [Google Scholar]
- 27.Hallstrom A, Rea TD, Mosesso VN, Jr, et al. The relationship between shocks and survival in out-of-hospital cardiac arrest patients initially found in PEA or asystole. Resuscitation. 2007;74:418–26. doi: 10.1016/j.resuscitation.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Kim F, Olsufka M, Maynard C, et al. Pilot randomized clinical trial of pre-hospital induction of mild hypothermia in out-of-hospital cardiac arrest in patients with a rapid infusion of 4º C normal saline. Circulation. 2007;115:3064–3070. doi: 10.1161/CIRCULATIONAHA.106.655480. [DOI] [PubMed] [Google Scholar]
- 29.Menegazzi J, Rittenberger JC, Suffoletto B, et al. Effects of pre-arrest and intra-arrest hypothermia on ventricular fibrillation and resuscitation. Resuscitation. 2009;80:126–132. doi: 10.1016/j.resuscitation.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Heart Association Guidelines for CPR and Emergency Cardiac Care Circulation. 2005;112(24 Suppl):IV-47–IV-50. doi: 10.1161/CIRCULATIONAHA.105.166550. [DOI] [PubMed] [Google Scholar]