Abstract
OBJECTIVE
We compared the short-term efficacy of home telemonitoring coupled with active medication management by a nurse practitioner with a monthly care coordination telephone call on glycemic control in veterans with type 2 diabetes and entry A1C ≥7.5%.
RESEARCH DESIGN AND METHODS
Veterans who received primary care at the VA Pittsburgh Healthcare System from June 2004 to December 2005, who were taking oral hypoglycemic agents and/or insulin for ≥1 year, and who had A1C ≥7.5% at enrollment were randomly assigned to either active care management with home telemonitoring (ACM+HT group, n = 73) or a monthly care coordination telephone call (CC group, n = 77). Both groups received monthly calls for diabetes education and self-management review. ACM+HT group participants transmitted blood glucose, blood pressure, and weight to a nurse practitioner using the Viterion 100 TeleHealth Monitor; the nurse practitioner adjusted medications for glucose, blood pressure, and lipid control based on established American Diabetes Association targets. Measures were obtained at baseline, 3-month, and 6-month visits.
RESULTS
Baseline characteristics were similar in both groups, with mean A1C of 9.4% (CC group) and 9.6% (ACM+HT group). Compared with the CC group, the ACM+HT group demonstrated significantly larger decreases in A1C at 3 months (1.7 vs. 0.7%) and 6 months (1.7 vs. 0.8%; P < 0.001 for each), with most improvement occurring by 3 months.
CONCLUSIONS
Compared with the CC group, the ACM+HT group demonstrated significantly greater reductions in A1C by 3 and 6 months. However, both interventions improved glycemic control in primary care patients with previously inadequate control.
Within the Veterans Health Administration, ∼500,000 veterans receive care for diabetes annually; diabetes is a leading cause of morbidity and mortality and a major contributor to health care cost (1,2). Sampling data from 2009 indicate that ∼28% of veterans nationally have suboptimal glycemic control with A1C ≥8% (3). Increases in A1C levels above the normal range in patients with diabetes are associated with progressive increases in morbidity and mortality due to micro- and macrovascular disease (4). Intensive glycemic control can reduce microvascular complications in both type 1 and type 2 diabetes (5,6). However, recent studies have not demonstrated that intensive glycemic control for 3–6 years with achieved A1C targets from 6.4 to 6.9% reduces macrovascular complications in patients with long-standing type 2 diabetes (7–9). In contrast, intensive glycemic control initiated early in the course of either type 1 or type 2 diabetes appears to reduce the risk of subsequent macrovascular complications significantly even when glycemic control later deteriorates (10,11).
Home-based telemedicine has been examined as a tool for management of chronic diseases (12), including diabetes (13–19). This approach can obviate geographic barriers; provide automated education, feedback, and data transmission; and facilitate provider-to-patient communication (12). However, outcomes with home telemonitoring in diabetes and other chronic diseases have been variable (12). Of several randomized controlled trials (RCTs) using home telemonitoring in diabetes care (13–19), only two have reported significant improvement in A1C (17,18). Neither of these trials included active medication management by a provider in response to real-time transmission of self-monitored blood glucose (SMBG) data or have specifically targeted patients not meeting glycemic control goals in response to pharmacological therapy under conditions of usual care.
The present study compared the efficacy of home telemonitoring coupled with active medication management by a nurse practitioner (ACM+HT intervention) with a lower-intensity care coordination intervention (CC intervention) consisting of monthly telephone contact with a diabetes nurse educator. Our study specifically targeted veterans with A1C levels ≥8% after ≥1 year receiving pharmacological therapy under conditions of usual care.
RESEARCH DESIGN AND METHODS
The DiaTel Study was a RCT of veterans with type 2 diabetes receiving their primary care at the VA Pittsburgh Healthcare System (VAPHS) at one of the three main Pittsburgh campuses or five outlying community-based clinics. The study was approved by the VAPHS Institutional Review Board and conducted according to the principles of the Declaration of Helsinki. All participants provided signed informed consent.
Under a separate VAPHS-approved protocol, a sampling frame of potentially eligible veterans was developed from VAPHS electronic medical and pharmacy records using the following criteria: had at least one outpatient visit in a primary care clinic between 1 June 2004 and 31 December 2005, were aged <80 years, received pharmacological treatment for diabetes for ≥12 months, had no referrals to the VAPHS Diabetes Clinic in the preceding 18 months, and had a most recent A1C ≥8.0%. Approximately 20% of veterans with diabetes in our sampling frame met that A1C criterion.
After review and approval by their primary care providers (PCPs), potentially eligible veterans were invited by letter to participate. Nonresponders were contacted by primary care clinic staff to solicit their participation. The study was described to interested veterans by research staff who obtained signed consent. Eligibility was further verified by a point-of-care capillary A1C ≥7.5% at enrollment using a DCA 2000 analyzer (Bayer Healthcare). Veterans were excluded if they had a life expectancy of <6 months, were participating in another study, resided in an institutional setting, or did not have a land-based, analog home telephone line as required for the home telemonitoring device used.
Participants were randomly assigned to the ACM+HT or CC group. Randomization was stratified by quartile of capillary A1C within each site and blocked on time. The project statistician generated the random sequences, the study nurses enrolled the participants, and the study coordinator informed the nurses of the intervention assignment after each participant was enrolled. After an initial education session, participants were informed of their intervention assignments. Because of the nature of the intervention, neither participants nor study nurses could be blinded. However, primary outcomes were ascertained by personnel unconnected to this study who were unaware of intervention assignments. Recruitment started 1 October 2005; the final 6-month follow-up was 11 January 2007.
Interventions
Participants in both groups attended an initial 2-h educational session for diabetes self-management and nutrition. Participants randomly assigned to the ACM+HT group received a 6-month diabetes management support intervention using the Viterion 100 Monitor home telemonitoring device. The device permits continuous home messaging with reminders and education; ongoing monitoring of SMBG, blood pressure, and weight; and daily transmission of these data to study providers via a secure network (20). Participants were instructed to transmit uploaded measurements from Viterion-compatible peripheral devices to the study nurse practitioner daily. On Monday through Friday, the nurse practitioner reviewed SMBG, blood pressure, weight, and risk stratification reports generated by the Viterion and contacted participants as necessary. The nurse practitioner provided timely telephone follow-up, including further self-management education for participants who generated “high-risk” reports based on unacceptably high or low SMBG or blood pressure readings. Medications for glycemic, blood pressure, and lipid control were adjusted by the nurse practitioner supervised by the study endocrinologist without prior approval of the PCP who was informed retrospectively of all changes. The nurse practitioner maintained records of all medication changes made in the ACM+HT group. The nurse practitioner also called ACM+HT participants monthly to provide individualized self-management counseling tailored to specific issues, based on the status of glucose and blood pressure control from the transmitted data.
Participants randomly assigned to the CC group received monthly telephone calls from the study diabetes nurse educator regarding general health conditions, status of glycemic control, blood pressure, and weight from daily logs maintained by the participants and compliance with the prescribed diabetic regimen. Issues requiring active intervention were referred to their PCP. Participants also could initiate contact with the study diabetes nurse educator to discuss concerns related to diabetes management.
Outcomes
At baseline, 3 months, and 6 months, participants presented to VAPHS for measurement of A1C, blood pressure, and weight and a fasting lipid panel. Baseline medication regimen (dose) and changes in the regimen (dose and date) for oral hypoglycemic agents, insulin, antihypertensive medications, and lipid-lowering medications were abstracted from the electronic pharmacy records and verified by participant interview.
Statistical methods
This study was designed to detect a 1% difference in A1C with 80% power using a 0.05-level two-sided test. Improvement was defined in terms of mean differences at 3 and 6 months as well as differential change over time. The primary outcome, A1C, was specified a priori. P < 0.05 was considered to be statistically significant, with no adjustment for multiple comparisons.
Our intent-to-treat approach included all randomly assigned participants to the extent possible. Data features that mandated special methods were the laboratory reporting of a small number of A1C values exceeding some cut point (truncated values, i.e., reported as >11.5, >11.8, or >12.3%) and a few missing A1C values. A modified multiple-imputation approach was used to obtain unbiased estimates, appropriate variances, and valid tests, based on a chained-equations algorithm (21) implemented in STATA SE 9.2 (22).
Mean A1C, weight, blood pressure, and lipid values were compared for the ACM+HT and CC groups at baseline, 3 months, and 6 months. The proportions of participants in each group who reached defined clinical target values at each time point were compared using Fisher's exact tests.
For each continuous outcome, difference scores were computed between each pair of time points (baseline to 3 months, baseline to 6 months, and 3 months to 6 months). Between-group comparisons of difference scores were obtained by regressing the difference scores for each pair of time points on a dummy variable for treatment group (if necessary to accommodate multiple imputation) or using a t test. Within-group difference scores were compared with zero using linear regression including only an intercept or a t test (as appropriate). The interaction of treatment group and insulin status at baseline was assessed. In the ACM+HT group, Pearson correlations summarized associations between A1C at 6 months and the frequencies of SMBG and adjustments of insulin.
RESULTS
Of the 1,055 veterans in the initial sampling frame deemed appropriate for the study, 658 (62.4%) responded to letters of invitation to participate and 381 (57%) agreed to be contacted. Of these, 211 presented to VAPHS for signed informed consent, additional screening, and baseline measurements. The 150 consenting veterans who had a capillary A1C ≥7.5% at the baseline were randomly assigned to the ACM+HT (n = 73) or CC (n = 77) groups. Of these, 3 ACM+HT and 2 CC participants were excluded because they were subsequently found to meet baseline exclusion criteria; 2 CC participants withdrew before the initial education session and 6 ACM+HT participants withdrew afterward. This analysis includes the remaining 64 ACM+HT and 73 CC participants (supplementary Table 4A, available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc09-1012/DC1).
All participants completed the baseline assessment; 6 ACM+HT and 4 CC participants missed the 3-month assessment and 8 ACM+HT and 7 CC participants missed the 6-month assessment. A total of 8 A1C values in the ACM+HT group and 9 A1C values in the CC group were missing, and 10 A1C values were truncated.
Baseline patient characteristics
There were no significant differences by treatment group for age, sex, race, or any of the other baseline characteristics as shown in supplementary Table A1. Approximately one-third of the participants in both groups were aged ≥65 years; the vast majority were male and non-Hispanic white. The predominant comorbidities were coronary artery disease and congestive heart failure.
Medication management
Most participants in each group were taking oral hypoglycemic agents (predominantly glyburide and metformin) and antihypertensive and lipid-lowering medications at baseline, 3 months, and 6 months; >50% were using insulin (supplementary Table A2). There were no significant differences by medication class at any time point (P > 0.14 for each). By 6 months, ACM+HT participants had significantly more medication or dose changes on average involving antihypertensive agents (3.1 for ACM+HT vs. 1.9 for CC participants, P = 0.02), but not lipid-lowering agents (1.4 for ACM+HT vs. 1.1 for CC participants, P = 0.29) or oral hypoglycemic agents (1.8 for ACM+HT vs. 1.8 for CC participants, P = 0.91).
At baseline, 39 ACM+HT and 40 CC participants were using insulin. By 6 months, 1 ACM+HT and 1 CC participant had discontinued insulin, whereas 5 ACM+HT and 3 CC participants had begun insulin. Although the average daily insulin dose was similar in both groups at baseline, the average daily dose for ACM+HT participants was ∼18 IU higher than that for CC participants at 3 and 6 months (P = 0.02 and P = 0.048, respectively). The average number of adjustments in insulin dose was also higher in ACM+HT (6.6) than in CC (2.8) participants (P < 0.001). However, no significant correlation was found between the frequency of insulin adjustment and A1C at 6 months in either ACM+HT (r = 0.12; P = 0.43) or CC (r = 0.14; P = 038) participants.
Primary outcomes
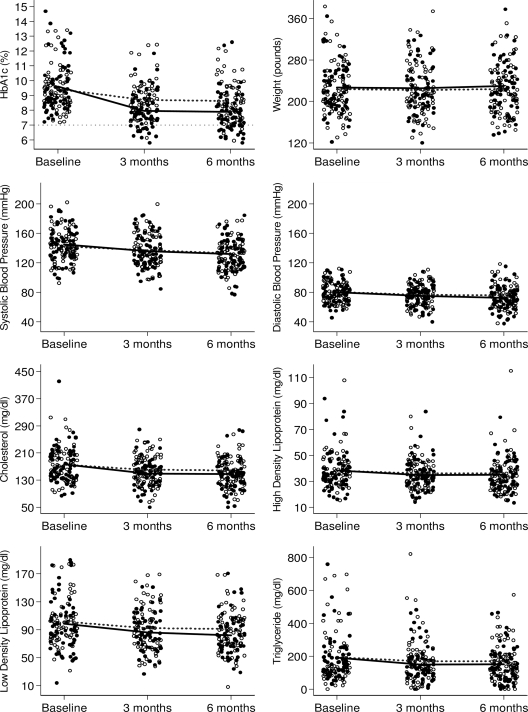
Dotplots of individual values for A1C, weight, blood pressure, and lipids are shown by treatment group for each time point in Fig. 1. Baseline values were similar for both groups (P > 0.45 for each) (Table 1). A1C was significantly lower for ACM+HT than for CC participants at both 3 and 6 months (0.7% lower at each time point, P < 0.001 for each). Significantly greater decreases in A1C were observed in the ACM+HT group relative to the CC group at 3 months (1.7 vs. 0.7%) and 6 months (1.7 vs. 0.8%), corresponding to differential decreases of ∼0.9% (P < 0.001 for each) (supplementary Table A3). There was no significant interaction between baseline insulin usage and treatment response at any time point (P > 0.39 for each) (supplementary Fig. 1).
Figure 1.
Dot plots of the primary outcome measures (A1C, weight, systolic blood pressure, diastolic blood pressure, cholesterol, HDL, LDL, and triglycerides) at baseline, 3 months, and 6 months by treatment group. ●, ACM + HT group; ○, CC group. Time-specific mean values are connected by solid black lines for the ACM + HT group and dotted lines for the CC group.
Table 1.
Time-specific primary outcomes by treatment group
| CC group | ACM + HT group | DiffCC-ACM | P | |
|---|---|---|---|---|
| n | 73 | 64 | ||
| A1C (%) | ||||
| Baseline | 9.4 ± 1.4 | 9.6 ± 1.6 | −0.2 (−0.7 to 0.3) | 0.53 |
| 3 months | 8.7 ± 1.2 | 7.9 ± 1.2 | 0.7 (0.3 to 1.2) | <0.001 |
| 6 months | 8.6 ± 1.3 | 7.9 ± 1.2 | 0.7 (0.3 to 1.2) | <0.001 |
| Weight (lb) | ||||
| Baseline | 223.5 ± 47.9 | 226.6 ± 45.4 | −3.1 (−18.9 to 12.7) | 0.70 |
| 3 months | 222.0 ± 49.6 | 225.5 ± 44.5 | −3.5 (−19.5 to 12.5) | 0.67 |
| 6 months | 223.9 ± 48.6 | 229.5 ± 47.6 | −5.7 (−21.9 to 10.6) | 0.49 |
| Systolic blood pressure (mmHg) | ||||
| Baseline | 142.3 ± 19.0 | 144.8 ± 21.7 | −2.6 (−9.5 to 4.3) | 0.46 |
| 3 months | 137.1 ± 21.4 | 135.9 ± 23.3 | 1.2 (−6.2 to 8.7) | 0.74 |
| 6 months | 133.0 ± 19.0 | 132.0 ± 24.3 | 1.0 (−6.2 to 8.2) | 0.79 |
| Diastolic blood pressure (mmHg) | ||||
| Baseline | 80.5 ± 10.1 | 79.9 ± 13.3 | 0.6 (−3.4 to 4.5) | 0.78 |
| 3 months | 76.6 ± 12.9 | 75.4 ± 12.0 | 1.3 (−2.9 to 5.4) | 0.55 |
| 6 months | 75.9 ± 13.2 | 72.4 ± 14.6 | 3.5 (−1.1 to 8.2) | 0.13 |
| Cholesterol (mg/dl) | ||||
| Baseline | 175.6 ± 43.5 | 177.3 ± 54.2 | −1.7 (−18.2 to 14.8) | 0.84 |
| 3 months | 160.8 ± 37.5 | 149.8 ± 37.2 | 11.0 (−1.7 to 23.6) | 0.09 |
| 6 months | 159.1 ± 37.2 | 148.2 ± 40.2 | 11.0 (−2.0 to 24.0) | 0.10 |
| HDL (mg/dl) | ||||
| Baseline | 38.4 ± 13.0 | 38.4 ± 13.5 | 0.0 (−4.5 to 4.5) | 0.99 |
| 3 months | 36.2 ± 11.0 | 35.0 ± 10.7 | 1.3 (−2.4 to 4.9) | 0.50 |
| 6 months | 36.4 ± 13.6 | 35.1 ± 11.3 | 1.3 (−3.0 to 5.5) | 0.55 |
| LDL (mg/dl)† | ||||
| Baseline | 101.8 ± 32.0 | 98.8 ± 36.3 | 3.0 (−8.9 to 15.0) | 0.62 |
| 3 months | 92.3 ± 32.2 | 86.3 ± 27.7 | 6.0 (−4.6 to 16.6) | 0.27 |
| 6 months | 91.2 ± 30.6 | 82.3 ± 27.9 | 8.9 (−1.6 to 19.3) | 0.10 |
| Triglycerides (mg/dl) | ||||
| Baseline | 194.1 ± 160.4 | 191.3 ± 133.3 | 2.7 (−47.5 to 53.0) | 0.92 |
| 3 months | 170.0 ± 133.6 | 149.9 ± 114.1 | 20.1 (−22.3 to 62.5) | 0.35 |
| 6 months | 170.7 ± 115.9 | 152.4 ± 99.7 | 18.3 (−18.0 to 54.6) | 0.32 |
Data are means ± SD and mean differences (CC − ACM + HT) with 95% CIs for these differences at each time point. Corresponding P values are also shown. A positive difference (DiffCC-ACM) indicates that the mean for that outcome at that time point is lower in the ACM + HT group than in the CC group. *Because measurements are rounded to one decimal place for reporting purposes, the rounded difference scores may differ slightly from the differences of the rounded means.
†CC group: n = 69; ACM + HT group: n = 59.
None of the other primary outcomes differed significantly by treatment group at either 3 or 6 months (Table 1). However, except for weight and HDL cholesterol levels, the direction of the differences favored the ACM+HT group. Within both treatment groups, A1C, blood pressure, cholesterol, and LDL improved significantly at 3 and 6 months relative to baseline, whereas HDL decreased (supplementary Table A3). Triglycerides declined significantly from baseline only in the ACM+HT group. A 4-lb mean weight increase in the ACM+HT group was the only significant within-group change between 3 and 6 months.
Similar proportions of ACM+HT and CC participants had A1C levels <8 or <9% at baseline (Table 2). However, at 6 months, 20.3% of ACM+HT and 5.5% of CC participants achieved A1C <7% (P = 0.01). Significantly more ACM+HT than CC participants also reached A1C levels of <8 and <9% at both 3 and 6 months (P ≤ 0.03 for each). Less than half of the participants had systolic blood pressure ≤130 mmHg at any time point, whereas a majority met the targets for diastolic blood pressure, LDL, and triglycerides. A higher percentage of ACM+HT than CC participants met the LDL treatment target of <100 mg/dl at 6 months (79.7 vs. 59.4%, respectively; P = 0.02).
Table 2.
Number of participants achieving each identified clinical target at baseline, 3 months, and 6 months by treatment group
| CC group | ACM + HT group | P | |
|---|---|---|---|
| n | 73 | 64 | |
| A1C <7% | |||
| Baseline | 0 | 0 | — |
| 3 months | 4 (5.5) | 9 (14.1) | 0.14 |
| 6 months | 4 (5.5) | 13 (20.3) | 0.01 |
| A1C <8% | |||
| Baseline | 6 (8.2) | 7 (10.9) | 0.77 |
| 3 months | 17 (23.3) | 34 (53.1) | <0.001 |
| 6 months | 25 (34.2) | 37 (57.8) | <0.01 |
| A1C <9% | |||
| Baseline | 29 (39.7) | 25 (39.1) | >0.99 |
| 3 months | 47 (64.4) | 54 (84.4) | 0.01 |
| 6 months | 49 (67.1) | 54 (84.4) | 0.03 |
| Systolic blood pressure ≤130 mmHg | |||
| Baseline | 19 (26.0) | 18 (28.1) | 0.85 |
| 3 months | 28 (39.7) | 29 (45.3) | 0.49 |
| 6 months | 34 (46.6) | 30 (46.9) | >0.99 |
| Diastolic blood pressure ≤80 mmHg | |||
| Baseline | 42 (57.5) | 39 (60.9) | 0.73 |
| 3 months | 46 (63.0) | 43 (71.9) | 0.72 |
| 6 months | 53 (72.6) | 50 (78.1) | 0.55 |
| LDL cholesterol <100 mg/dl* | |||
| Baseline | 36 (52.2) | 31 (52.5) | >0.99 |
| 3 months | 44 (63.8) | 43 (72.9) | 0.34 |
| 6 months | 41 (59.4) | 47 (79.7) | 0.02 |
| Triglyceride ≤150 mg/dl | |||
| Baseline | 43 (58.9) | 33 (51.6) | 0.39 |
| 3 months | 39 (53.4) | 42 (65.6) | >0.17 |
| 6 months | 42 (57.5) | 40 (62.5) | >0.60 |
Data are n (%).
*For LDL cholesterol, denominators are 69 for the CC group and 59 for the ACM + HT group.
SMBG among ACM+HT participants
Seven ACM+HT participants (10.9%) never transmitted any SMBG data after initial training. Another 9 participants (14.1%) performed SMBG on average <1 time per day, whereas 75.0% performed SMBG between 1 and 4 times per day (average 2.3 times daily) during the period in which they transmitted measurements. Among the 57 participants who transmitted measurements, 35 (61.4%) transmitted SMBG <50 mg/dl on at least 1 day (median 1 day) and 16 (28.1%) transmitted SMBG between 50 and 70 mg/dl (median 10 days). Within the ACM+HT group, the frequency of SMBG did not correlate significantly with reduction in A1C (r = −0.11; P = 0.39).
Nurse-to-participant telephone contact time was substantially greater in ACM+HT than CC participants (∼1.3 vs. 0.3 h/participant/month, respectively). In the ACM+HT group, telephone contact was triggered by transmitted suboptimal SMBG or blood pressure levels. Thus, contact time was disproportionately high in this subgroup of ACM+HT participants.
One participant in the ACM+HT group died at home suddenly 7 months after entry in the study. No postmortem examination was obtained. The participant had diabetic neuropathy, stage IV renal insufficiency, and congestive heart failure for which he had been hospitalized recently. He was treated with insulin alone and thus was taking no oral hypoglycemic agent that may have complicated his heart failure. His A1C levels fell from 11.8% at baseline to 6.5% at 3 months. Of his 468 transmitted SMBG values, 4 (0.85%) were <50 mg/dl, a frequency similar to that of ACM+HT participants overall (0.66%) and that (0.59%) of 7 other ACM+HT participants using insulin who had rapid declines in A1C (≥3%) over 3 months. No SMBG <50 mg/dl occurred during the month before his death. The relationships, if any, between the rapid decline in A1C, hypoglycemia, and sudden death in this patient are uncertain.
CONCLUSIONS
Participants in this study had suboptimal glycemic control after at least 1 year of pharmacological therapy directed by a PCP. Each of the interventions used in the study resulted in short-term improvements in A1C. However, the latter were significantly greater in ACM+HT compared with CC participants. The relative contributions of the automated messaging and monitoring capacity provided by the home telemonitoring device, the nearly fourfold greater nurse-to-participant telephone contact time, and the greater intensification of insulin therapy in the ACM+HT versus CC group to the A1C outcomes cannot be determined from our study design, which is a limitation of the design. Self-management education was an intrinsic component of the more frequent nurse-to-participant phone communications in the ACM+HT group and was additive to the educational messaging provided via the home telemonitoring device. A meta-analysis of 31 RCTs indicated an association between increased patient contact time with a diabetes nurse educator and lower A1C levels, with an estimated decrease in A1C of 1% for every additional 23.6 h of contact (23). This effect may have contributed significantly to the more marked reduction in A1C in the ACM+HT versus CC group. Greater intensification of insulin therapy in the ACM+HT group probably also contributed to the more marked declining A1C compared with that of the CC group. However, no significant correlations were found between the frequency of insulin adjustments and A1C outcomes at 6 months in either group.
A majority (75%) of ACM+HT participants performed SMBG at least daily, with a mean of 2.3 times per day. This is much more frequent than the observation in the National Health and Nutrition Examination Survey (NHANES) (24), in which 29% of patients with diabetes who were taking insulin, 65% who were taking oral agents, and 80% who managed their disease with diet alone performed SMBG less than one time per month (24). Consistent with NHANES data, which showed no correlation between the frequency of SMBG and A1C (24), we did not find a significant association between frequency of SMBG and magnitude of decline in A1C within the ACM+HT group. Lack of SMBG data for the control group is a limitation of our study, which precluded ascertainment of the relative frequency of monitoring in the CC versus ACM+HT group, the relationship of SMBG to the respective A1C outcomes, and the relative frequency of hypoglycemia in the two groups. However, multiple prior reports have indicated that the relationship between the frequency of SMBG and glycemic control is complex and inconsistent and may depend on coupling SMBG with a structured plan for treating glucose elevations (24,25). Full realization of the benefits of real-time transmission of SMBG indexes, whatever their frequency, probably depend on the prescriptive response of the provider receiving the data (25).
It is uncertain whether the improved glycemic control observed in the ACM+HT group can be sustained beyond 6 months with or without continued active care management with home telemonitoring, and if sustained, will translate into improved clinical outcomes. Recent studies have failed to demonstrate improved macrovascular outcomes with intensive glycemic control among patients with type 2 diabetes (7–9). The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial (7) reported increased mortality in a subgroup of type 2 diabetic patients subject to intensive glycemic control. The VA Diabetes Trial (9) suggested improved cardiovascular outcomes occurred only in younger patients with a shorter duration of diabetes and also raised concern about an association between hypoglycemia and cardiovascular events (9). Consistent with the VA Diabetes Trial, 10-year follow-up results from the UK Prospective Diabetes Study (10) indicated that intensive glycemic control established earlier in the course of type 2 diabetes does reduce subsequent cardiovascular events, even though the differential in A1C among patients initially treated intensively dissipated within 1 year.
By design, our study focused on patients with diabetes and suboptimal glucose control. Many of these patients did not have concurrent issues related to their blood pressure or lipid levels. Perhaps for this reason and the short duration of the trial, we did not observe large differences in these outcomes, despite continuous active medication management and self-management education for blood pressure and lipids in ACM+HT participants. A significantly higher proportion of ACM+HT (79.7%) versus CC participants (59.4%) achieved the LDL cholesterol target of <100 mg/dl at 6 months. A longer trial using HT in a different patient population might provide a better assessment of the value of this intervention in improving management of blood pressure and lipids, as suggested by the 5-year Informatics for Diabetes Education and Telemedicine (IDEATel) trial (18).
Although the present study was conducted in participants receiving care within the VA system, our findings are relevant to other patient populations. The IDEATel study, an RCT of 1,665 underserved diabetic Medicare recipients whose age, educational status, and socioeconomic status was similar to participants in the present study, compared the use of home telemonitoring combined with nurse case management under the supervision of an endocrinologist with usual care in community settings (18). Small but significant reductions in A1C, blood pressure, and LDL cholesterol favoring the intervention group were found at 5 years (18). Although IDEATel did not combine active medication management by a nurse practitioner with HT, the latter is now accepted practice in many health care organizations outside the VA. Use of only one provider in the present study may limit the ability to generalize our findings to similar interventions conducted by multiple providers. However, employment of a standardized treatment protocol supports the relevance of our results to other clinical settings. Additional research is needed to examine the questions of whether active care management with home telemonitoring is a cost-effective approach for management of patients who have not achieved adequate glycemic control with usual care and whether the short-term improvements in glycemic control observed with active care management with home telemonitoring can be sustained with less resource utilization.
Supplementary Material
Acknowledgments
This work was supported by award W81XWH-04-2-0030 from the U.S. Air Force, administered by the U.S. Army Medical Research Acquisition Activity, Fort Detrick, Maryland, and by resources and the use of facilities at the VAPHS.
No potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract form at the 25th VA Health Services Research and Development National Meeting, Washington, DC, 21–23 February 2007 and at the 67th Scientific Sessions of the American Diabetes Association, Chicago, Illinois, 22–26 June 2007.
We thank VAPHS primary care providers and clinic nurses for assistance in recruiting patients into the study; Julie Heinzl, MS, RD, CDE, for conducting nutrition education classes and consultations; and Nichole Bayliss, MS, for data management.
Footnotes
Clinical trial reg. no. NCT00245882, www.clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Maciejewski ML, Maynard C: Diabetes-related utilization and costs for inpatient and outpatient services in the Veterans Administration. Diabetes Care 2004;27:B69–B73 [DOI] [PubMed] [Google Scholar]
- 2.Miller DR, Safford MM, Pogach LM: Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care 2004;27:B10–B21 [DOI] [PubMed] [Google Scholar]
- 3.VHA Office of Quality and Performance. Unpublished data.
- 4.Stratton IM, Adler AI, Neil HAW, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holmann RR: Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. Br Med J 2000;321:405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UKPDS Group. Effect of intensive blood glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–865 [erratum 1998;352:1558] [PubMed] [Google Scholar]
- 6.Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–987 [DOI] [PubMed] [Google Scholar]
- 7.Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 9.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD: VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139 [DOI] [PubMed] [Google Scholar]
- 10.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA: 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 11.Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Currell R, Urquhart C, Wainwright P, Lewis R: Telemedicine versus face to face patient care: effects on professional practice and health care outcomes. Cochrane Database Syst Rev 2009;3:1–37 [DOI] [PubMed] [Google Scholar]
- 13.Bergenstal RM, Anderson RL, Bina DM, Johnson ML, Davidson JL, Solarz-Johnson B, Kendall DM: Impact of modem-transferred blood glucose data on clinician work efficiency and patient glycemic control. Diabetes Technol Ther 2005;7:241–247 [DOI] [PubMed] [Google Scholar]
- 14.Biermann E, Dietrich W, Rihl J, Standl E: Are there time and cost savings by using telemanagement for patients on intensified insulin therapy? A randomized, controlled trial. Comput Methods Programs Biomed 2002;69:137–146 [DOI] [PubMed] [Google Scholar]
- 15.Chase HP, Pearson JA, Wightman C, Roberts MD, Oderberg AD, Garg SK: Modem transmission of glucose values reduces the costs and need for clinic visits. Diabetes Care 2003;26:1475–1479 [DOI] [PubMed] [Google Scholar]
- 16.Marrero DG, Vandagriff JL, Kronz K, Fineberg NS, Golden MP, Gray D, Orr DP, Wright JC, Johnson NB: Using telecommunication technology to manage children with diabetes: the Computer-Linked Outpatient Clinic (CLOC) Study. Diabetes Educ 1995;21:313–319 [DOI] [PubMed] [Google Scholar]
- 17.Montori VM, Helgemoe PK, Guyatt GH, Dean DS, Leung TW, Smith SA, Kudva YC: Telecare for patients with type 1 diabetes and inadequate glycemic control: a randomized controlled trial and meta-analysis. Diabetes Care 2004;27:1088–1094 [DOI] [PubMed] [Google Scholar]
- 18.Shea S, Weinstock RS, Teresi JA, Palmas W, Starren J, Cimino JJ, Lai AM, Field L, Morin PC, Goland R, Izquierdo RE, Ebner S, Silver S, Petkova E, Kong J, Eimicke JP: IDEATel Consortium. A randomized trial comparing telemedicine case management with usual care in older, ethnically diverse medically underserved patients with diabetes mellitus: 5 year results of the IDEATel study. J Am Med Inform Assoc 2009;16:446–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vahatalo MA, Virtamo HE, Viikari JS, Ronnemaa T: Cellular phone transferred self blood glucose monitoring: prerequisites for positive outcome. Pract Diabetes Int 2004;21:192–194 [Google Scholar]
- 20.ViterionNET TeleHealthCare Network [article online]. Available from http://www.viterion.com/products_network.cfm Accessed 15 January 2009
- 21.Royston P: Multiple imputation of missing values: update of ice. Stata J 2005;5:527–536 [Google Scholar]
- 22.StataCorp. STATA Statistical Software, release 9 College Station, TX, StataCorp, 2005 [Google Scholar]
- 23.Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM: Self-management education for adults with type 2 diabetes: a meta-analysis of the effect on glycemic control. Diabetes Care 2002;25:1159–1171 [DOI] [PubMed] [Google Scholar]
- 24.Sarol JN, Jr, Nicodemus NA, Jr, Tan KM, Grava MB: Self-monitoring of blood glucose as part of a multicomponent therapy among non-insulin requiring type 2 diabetes patients: a meta-analysis (1966–2004). Curr Med Res Opin 2005;21:173–184 [DOI] [PubMed] [Google Scholar]
- 25.Welschen LM, Bloemendal E, Nijpels G, Dekker JM, Heine RJ, Stalman WA, Bouter LM: Self-monitoring of blood glucose in patients with type 2 diabetes who are not using insulin: a systematic review. Diabetes Care 2005;28:1510–1517 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.