Abstract
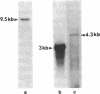
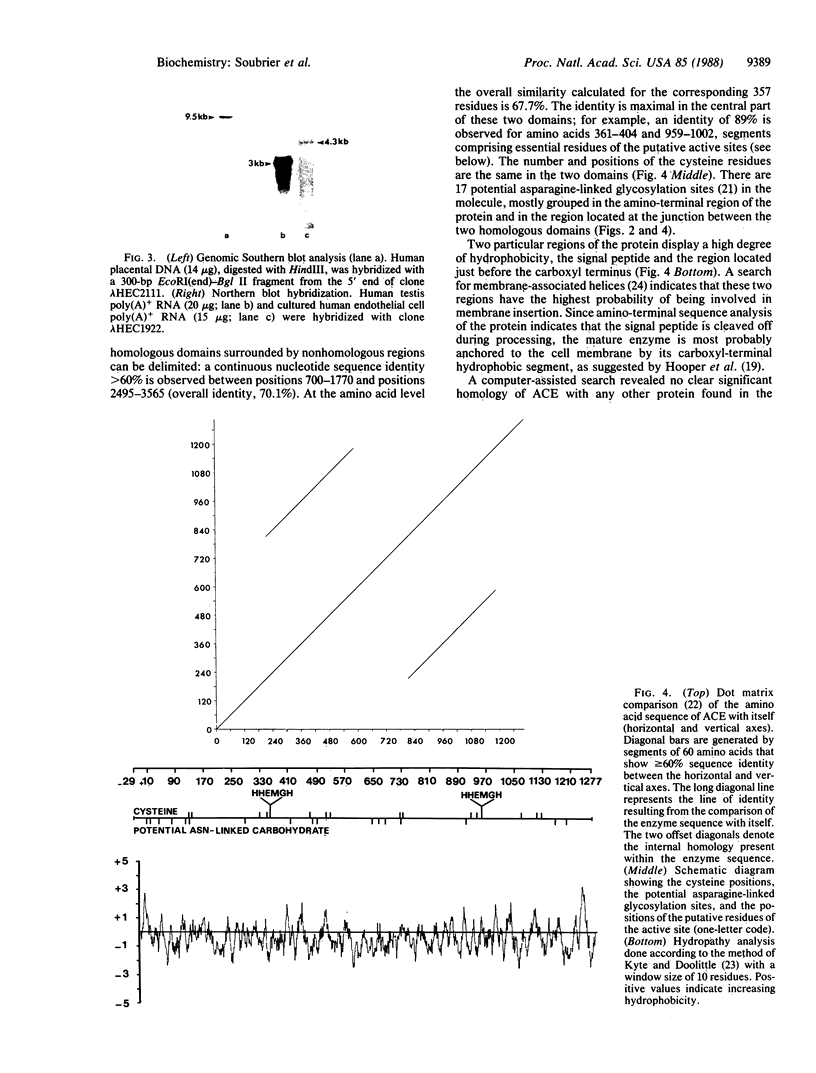
The amino-terminal amino acid sequence and several internal peptide sequences of angiotensin I-converting enzyme (ACE; peptidyl-dipeptidase A, kininase II; EC 3.4.15.1) purified from human kidney were used to design oligonucleotide probes. The nucleotide sequence of ACE mRNA was determined by molecular cloning of the DNA complementary to the human vascular endothelial cell ACE mRNA. The complete amino acid sequence deduced from the cDNA contains 1306 residues, beginning with a signal peptide of 29 amino acids. A highly hydrophobic sequence located near the carboxyl-terminal extremity of the molecule most likely constitutes the anchor to the plasma membrane. The sequence of ACE reveals a high degree of internal homology between two large domains, suggesting that the molecule resulted from a gene duplication. Each of these two domains contains short amino acid sequences identical to those located around critical residues of the active site of other metallopeptidases (thermolysin, neutral endopeptidase, and collagenase) and therefore bears a putative active site. Since earlier experiments suggested that a single Zn atom was bound per molecule of ACE, only one of the two domains should be catalytically active. The results of genomic DNA analysis with the cDNA probe are consistent with the presence of a single gene for ACE in the haploid human genome. Whereas the ACE gene is transcribed as a 4.3-kilobase mRNA in vascular endothelial cells, a 3.0-kilobase transcript was detected in the testis, where a shorter form of ACE is synthesized.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alhenc-Gelas F., Weare J. A., Johnson R. L., Jr, Erdös E. G. Measurement of human converting enzyme level by direct radioimmunoassay. J Lab Clin Med. 1983 Jan;101(1):83–96. [PubMed] [Google Scholar]
- Bause E. Structural requirements of N-glycosylation of proteins. Studies with proline peptides as conformational probes. Biochem J. 1983 Feb 1;209(2):331–336. doi: 10.1042/bj2090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein K. E., Martin B. M., Striker L., Striker G. Partial protein sequence of mouse and bovine kidney angiotensin converting enzyme. Kidney Int. 1988 Mar;33(3):652–655. doi: 10.1038/ki.1988.48. [DOI] [PubMed] [Google Scholar]
- Bull H. G., Thornberry N. A., Cordes E. H. Purification of angiotensin-converting enzyme from rabbit lung and human plasma by affinity chromatography. J Biol Chem. 1985 Mar 10;260(5):2963–2972. [PubMed] [Google Scholar]
- Bünning P., Riordan J. F. The functional role of zinc in angiotensin converting enzyme: implications for the enzyme mechanism. J Inorg Biochem. 1985 Jul;24(3):183–198. doi: 10.1016/0162-0134(85)85002-9. [DOI] [PubMed] [Google Scholar]
- Caldwell P. R., Seegal B. C., Hsu K. C., Das M., Soffer R. L. Angiotensin-converting enzyme: vascular endothelial localization. Science. 1976 Mar 12;191(4231):1050–1051. doi: 10.1126/science.175444. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Das M., Hartley J. L., Soffers R. L. Serum angiotensin-converting enzyme. Isolation and relationship to the pulmonary enzyme. J Biol Chem. 1977 Feb 25;252(4):1316–1319. [PubMed] [Google Scholar]
- Das M., Soffer R. L. Pulmonary angiotensin-converting enzyme. Structural and catalytic properties. J Biol Chem. 1975 Sep 10;250(17):6762–6768. [PubMed] [Google Scholar]
- Devault A., Lazure C., Nault C., Le Moual H., Seidah N. G., Chrétien M., Kahn P., Powell J., Mallet J., Beaumont A. Amino acid sequence of rabbit kidney neutral endopeptidase 24.11 (enkephalinase) deduced from a complementary DNA. EMBO J. 1987 May;6(5):1317–1322. doi: 10.1002/j.1460-2075.1987.tb02370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D., Weiss R. M., Terwilliger T. C. The helical hydrophobic moment: a measure of the amphiphilicity of a helix. Nature. 1982 Sep 23;299(5881):371–374. doi: 10.1038/299371a0. [DOI] [PubMed] [Google Scholar]
- El-Dorry H. A., Pickett C. B., MacGregor J. S., Soffer R. L. Tissue-specific expression of mRNAs for dipeptidyl carboxypeptidase isoenzymes. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4295–4297. doi: 10.1073/pnas.79.14.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdös E. G., Skidgel R. A. The angiotensin I-converting enzyme. Lab Invest. 1987 Apr;56(4):345–348. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Goldberg G. I., Wilhelm S. M., Kronberger A., Bauer E. A., Grant G. A., Eisen A. Z. Human fibroblast collagenase. Complete primary structure and homology to an oncogene transformation-induced rat protein. J Biol Chem. 1986 May 15;261(14):6600–6605. [PubMed] [Google Scholar]
- Gospodarowicz D., Cheng J., Lirette M. Bovine brain and pituitary fibroblast growth factors: comparison of their abilities to support the proliferation of human and bovine vascular endothelial cells. J Cell Biol. 1983 Dec;97(6):1677–1685. doi: 10.1083/jcb.97.6.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmquist B., Bünning P., Riordan J. F. A continuous spectrophotometric assay for angiotensin converting enzyme. Anal Biochem. 1979 Jun;95(2):540–548. doi: 10.1016/0003-2697(79)90769-3. [DOI] [PubMed] [Google Scholar]
- Hooper N. M., Keen J., Pappin D. J., Turner A. J. Pig kidney angiotensin converting enzyme. Purification and characterization of amphipathic and hydrophilic forms of the enzyme establishes C-terminal anchorage to the plasma membrane. Biochem J. 1987 Oct 1;247(1):85–93. doi: 10.1042/bj2470085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata K., Blacher R., Soffer R. L., Lai C. Y. Rabbit pulmonary angiotensin-converting enzyme: the NH2-terminal fragment with enzymatic activity and its formation from the native enzyme by NH4OH treatment. Arch Biochem Biophys. 1983 Nov;227(1):188–201. doi: 10.1016/0003-9861(83)90362-4. [DOI] [PubMed] [Google Scholar]
- Kester W. R., Matthews B. W. Crystallographic study of the binding of dipeptide inhibitors to thermolysin: implications for the mechanism of catalysis. Biochemistry. 1977 May 31;16(11):2506–2516. doi: 10.1021/bi00630a030. [DOI] [PubMed] [Google Scholar]
- Koenig M., Hoffman E. P., Bertelson C. J., Monaco A. P., Feener C., Kunkel L. M. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987 Jul 31;50(3):509–517. doi: 10.1016/0092-8674(87)90504-6. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lathe R. Synthetic oligonucleotide probes deduced from amino acid sequence data. Theoretical and practical considerations. J Mol Biol. 1985 May 5;183(1):1–12. doi: 10.1016/0022-2836(85)90276-1. [DOI] [PubMed] [Google Scholar]
- Malfroy B., Schofield P. R., Kuang W. J., Seeburg P. H., Mason A. J., Henzel W. J. Molecular cloning and amino acid sequence of rat enkephalinase. Biochem Biophys Res Commun. 1987 Apr 14;144(1):59–66. doi: 10.1016/s0006-291x(87)80475-8. [DOI] [PubMed] [Google Scholar]
- Matsas R., Kenny A. J., Turner A. J. The metabolism of neuropeptides. The hydrolysis of peptides, including enkephalins, tachykinins and their analogues, by endopeptidase-24.11. Biochem J. 1984 Oct 15;223(2):433–440. doi: 10.1042/bj2230433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan U. S., Ryan J. W., Whitaker C., Chiu A. Localization of angiotensin converting enzyme (kininase II). II. Immunocytochemistry and immunofluorescence. Tissue Cell. 1976;8(1):125–145. doi: 10.1016/0040-8166(76)90025-2. [DOI] [PubMed] [Google Scholar]
- SKEGGS L. T., Jr, KAHN J. R., SHUMWAY N. P. The preparation and function of the hypertensin-converting enzyme. J Exp Med. 1956 Mar 1;103(3):295–299. doi: 10.1084/jem.103.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- St Clair D. K., Presper K. A., Smith P. L., Stump D. C., Heath E. C. Bovine angiotensin-converting enzyme: amino-terminal sequence analysis and preliminary characterization of a hybridization-selected primary translation product. Biochem Biophys Res Commun. 1986 Dec 30;141(3):968–972. doi: 10.1016/s0006-291x(86)80138-3. [DOI] [PubMed] [Google Scholar]
- Staden R. An interactive graphics program for comparing and aligning nucleic acid and amino acid sequences. Nucleic Acids Res. 1982 May 11;10(9):2951–2961. doi: 10.1093/nar/10.9.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter S. M., Snyder S. H. Characterization of angiotensin converting enzyme by [3H]captopril binding. Mol Pharmacol. 1986 Feb;29(2):142–148. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Yang H. Y., Erdös E. G., Levin Y. A dipeptidyl carboxypeptidase that converts angiotensin I and inactivates bradykinin. Biochim Biophys Acta. 1970 Aug 21;214(2):374–376. doi: 10.1016/0005-2795(70)90017-6. [DOI] [PubMed] [Google Scholar]