Hypothesis
Impaired wound healing in diabetics is due to pathological angiogenesis, which is a result of aberrant sphingosine-1-phosphate (S1P) signaling. Pharmacological modulation of sphingosine-1-phosphate-dependent signaling normalizes healing in diabetic wounds.
Sphingosine-1-phosphate (S1P) is a bioactive lysophospholipid that is produced in all cells and is enriched in blood and lymph. Sources of circulating S1P include platelets, erythrocytes and probably vascular endothelium.
S1P activates signaling pathways controlling cell proliferation, cell survival and cell migration, primarily by ligating five plasma membrane G protein-coupled receptors, S1P1-5 (1). Previously known as the endothelial differentiation gene (EDG) receptors, the S1P receptors are expressed in various combinations on different cell types (2). Through their effects on cells, S1P-mediated signals contribute to cell survival and migration, organ development, angiogenesis, vascular integrity and lymphocyte trafficking. S1P signaling through the S1P1 receptor is essential for vascular maturation during embryogenesis, whereas S1P signaling through the S1P2 receptor may contribute to pathological angiogenesis in the adult (3, 4). Intracelluarly, S1P regulates calcium mobilization through receptor-dependent and possibly also receptor-independent mechanisms. Indirect effects of S1P signaling on nitric oxide synthesis, AKT and PDGF signaling expand the influence of S1P to the regulation of vascular tone, wound healing and protein synthesis.
Pathogenesis of the diabetic wound
Normal wound repair is coordinated by cytokines and growth factors in healthy tissue healing and includes the migration of mature fibroblasts and keratinocytes to a temporary granulation tissue matrix, differentiation of skin progenitor cells, and angiogenesis. Chronically increased levels of blood glucose in diabetic patients lead to impaired wound healing, decreased wound strength and impaired wound-related angiogenesis.
Pathological analysis of diabetic wounds demonstrates malformation of the extra cellular matrix likely through abnormal fibroblasts, which show impaired migration and proliferation when exposed to high glucose concentrations in vitro (5, 6). Defective release of cytokines and growth factors, such as PDGF, leads to aberrant response of inflammatory cells to the wound site (7). These factors along with impaired macro- and microvascular circulation and decreased oxygen to the wounded tissues result in poor wound healing in diabetic patients.
Interestingly, preclinical studies employing a combination of subcutaneously administered S1P and the S1P2 antagonist JTE013 (to block pathological angiogenesis) reportedly increased formation of granular tissue in diabetic mice (7).
Sphingosine-1-phosphate metabolism
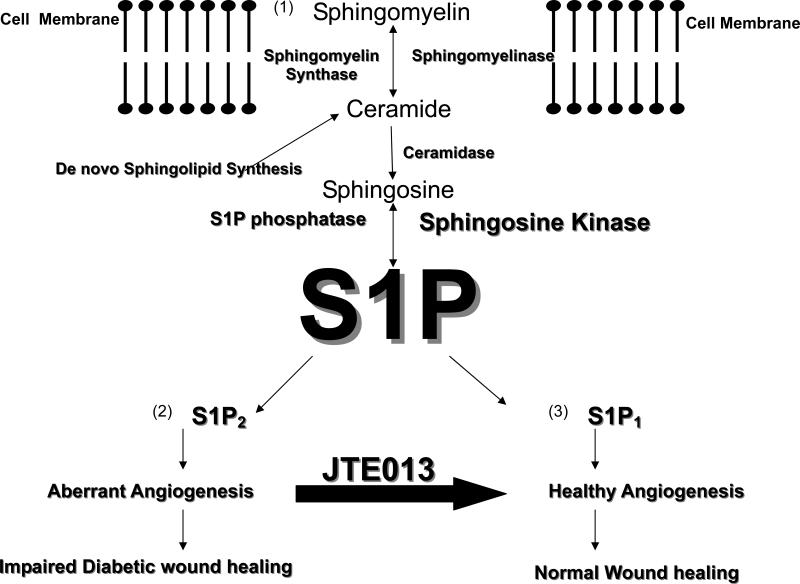
S1P is derived from the enzymatic breakdown of membrane sphingomyelin (Figure 1) and the turnover of other higher order sphingolipids enriched in lipid rafts. In response to stress conditions including hypoxia, radiation, chemotherapy or stimulation with TNFα, sphingomyelinases hydrolyze sphingomyelin, generating the pro-apoptotic molecule ceramide. This biochemical step is referred to as the “sphingomyelin cycle”. Ceramide can also be generated in cells de novo. Ceramide generated from either pathway can be deacylated by ceramidases to form the long chain base, sphingosine. Sphingosine is phosphorylated by sphingosine kinase, thereby generating active S1P (1). Metabolism of S1P is accomplished by sphingosine phosphate lyase and lipid phosphatases.
Fig 1. S1P generation and actions in wound healing.
S1P acts as a ligand for five differentially expressed G protein coupled cell surface receptors. Expression of S1P1 on endothelial cells is required to promote the migration of pericytes and smooth muscle cells to nascent blood vessels, a critical step in healthy blood vessel maturation. In contrast, S1P2 activation promotes pathological angiogenesis, an undesirable feature of diabetic wounds, retinopathy of prematurity and other diseases. Subcutaneous administration of S1P or the S1P2 antagonist JTE013 stimulate the development of healthy granular tissue and enhance angiogenesis in mouse models of diabetes. We hypothesize that administration of receptor type selective S1P1 agonists and/or S1P2 antagonists should shift the balance from aberrant angiogenesis to healthy angiogenesis, thereby improving diabetic wound healing.
Although this degradative pathway was previously considered an inefficient cellular mechanism for recycling, it is now clear that many intermediates generated in this pathway are bioactive.
Angiogenesis and vascular maturation are essential for wound healing
Angiogenesis and vascular maturation are pivotal for wound healing. The S1P receptor is expressed in abundance on endothelial cells, vascular smooth muscle cells, and pericytes. Activation of S1P1 directs cortical actin filament formation and other cytoskeletal proteins needed for endothelial cell migration. The recruitment of vascular smooth muscle cells and pericytes to the nascent blood vessel is also communicated through the S1P1 receptor (3). Through these effects, S1P promotes endothelial tube formation, vascular maturation and reduces vascular permeability.
The role of S1P1 receptors in angiogenesis was discovered with the initial characterization of homozygous S1P1 knockout mice. These mice die at post-gestational day 12.5-14.5 due to massive internal hemorrhage (3). While they do exhibit angiogenesis, the vasculature of the mice does not mature. The lack of S1P1 receptors in the endothelium is associated with impaired migration of pericytes and smooth muscle cells to the developing blood vessels, with loss of adhesion junctions between pericytes and endothelial cells, leading to vascular instability. Thus the S1P1 receptor is pivotal to angiogenic maturation and mediates endothelial cell survival, migration cell-cell interactions and adhesion.
Aberrant S1P signaling results in pathological angiogenesis
Pathologic angiogenesis, or the rapid proliferation of abnormal blood vessels, underlies the pathogenesis of proliferative diabetic retinopathy, retinopathy of prematurity (ROP), sickle cell retinopathy, age-related macular degeneration, psoriasis, and tumor angiogenesis. Studies involving S1P2 knock out mice confirm that the activation of the S1P2 receptor is necessary for the growth of abnormal retinal vessels in response to hypoxia, that induces ROP (4). S1P promotes tumor angiogenesis, which is essential for tumor progression (8). This observation is consistent with findings of increased sphingosine kinase expression in human cancers of the brain, breast, colon, kidney, lung, ovary, rectum, small intestine, stomach and uterus. Thus, activation of S1P pathways can induce desirable angiogenesis, whereas inhibition of the same pathways, and in particular those mediated by S1P2, represent a novel therapeutic approach to prevent pathological angiogenesis.
S1P and wound healing
Wound healing involves migration of fibroblasts and keratinocytes to the site of injury. Lysophospholipids including S1P are enriched in acute wound fluid (9). Laminin 5 is a small protein that induces adhesion and migration of human keratinocytes during wound healing. S1P enhances production of laminin 5, promoting the connection between the epithelial hemidesmosomes and the underlying basement membrane in human keratinocytes (9). In response to injury, thrombin promotes activation of S1P, which stimulates angiogenesis. S1P further promotes proliferation and migration of human keratinocytes and the formation of a fibronectin matrix at the dermal-epidermal junction (10).
In addition to its effects on superficial wound healing, S1P-mediated signaling through S1P1 and S1P3 appear to contribute to deep tissue repair. S1P1-3 are expressed on hepatic myofibroblasts, cells which promote remodeling of the liver matrix in acute injury (11). Acute liver injury in mice induced with carbon tetrachloride increased expression of sphingosine kinase and upregulation of S1P2,3 . Liver injury was also associated with increased production of S1P and activation of S1P mitogenic effects on the hepatic myofibroblasts (11).
Pharmacological modulation of S1P signaling
S1P receptors are considered excellent targets for pharmacological modulation, because they reside on the cell surface, are differentially expressed in tissues and signal through different G protein-coupled pathways. This situation creates the potential for specificity with regard to response and tissue, as well as the potential to generate agonists and antagonists for each receptor. A number of S1P receptor ligands have been identified in chemical library screens or developed through rational drug design approaches (12). These include agents such as FTY720, an immunomodulatory drug that induces a reversible, transient lymphopenia and diminishes lymphocyte interaction with solid organ grafts after oral administration. FTY720 is a prodrug that is phosphorylated in vivo to FTY720-phosphate. FTY720-phosphate acts as an S1P analog and “super-ligand” that binds to receptors S1P1,3,4,5 resulting in downregulation of the S1P1 receptor . Although it has shown promise as an immune modulator, the lack of specificity of FTY720 exemplifies the major drawback of currently available S1P receptor ligands. Newer agents such as KRP-203 and SEW2871 are more selective for S1P1 and appear to exhibit better toxicity profiles. Drugs with receptor-inhibitory activity such as the S1P2 antagonist JTE-013 also appear promising, although only a few preclinical studies have been undertaken with this agent to date. As more selective agents become available, it should become possible to maximize the clinical utility and minimize off-target effects of S1P receptor targeting.
Summary
S1P signaling through the S1P1 receptor is essential for vascular maturation and contributes to healthy angiogenesis, whereas the fundamental lesion in diabetic wound healing is pathological angiogenesis, a process that is regulated by S1P signaling through S1P2 receptors. Pharmacological modulation of S1P-dependent signaling pathways may become useful for management of diabetic wounds, particularly by employing strategies of S1P1 agonism, S1P2 antagonism or a combination of the two. S1P receptor drug targeting is already being investigated in the clinical setting and has shown promising therapeutic results for several diseases. However, the current armamentarium of ligands for modulating S1P signaling suffers from lack of receptor type specificity. Eventually, as more specific agonists and antagonists of the five S1P receptors become available and are evaluated in clinical trials, the ability to harness S1P signals to promote angiogenesis, wound healing and influence other aspects of perioperative physiology should become possible. Depending upon the toxicity profile of these new drugs, they could be employed for prevention and/or treatment of diabetic wounds.
Acknowledgements
This work was supported by National Institute of Diabetic and Digestive and Kidney Diseases Grant 9T32DK078514-06A2 and National Institutes of Health Grant CA77528.
Financial Support: This work is being supported by National Institute of Diabetic and Digestive and Kidney Diseases Grant 9T32DK078514-06A2 (KNG) and National Institutes of Health Grant CA77528 (JDS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Saba J, Hla T. Point-counterpoint of sphingosine 1-phosphate metabolism. Circ Res. 2004;94(6):724–734. doi: 10.1161/01.RES.0000122383.60368.24. [DOI] [PubMed] [Google Scholar]
- 2.Rosen H, Goetzl E. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat Rev Immunol. 2005;5:560–570. doi: 10.1038/nri1650. [DOI] [PubMed] [Google Scholar]
- 3.Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest. 2000;106(8):951–61. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skoura A, Sanchez T, Claffey K, Mandala SM, Proia RL, Hla T. Essential role of sphingosine 1-phosphate receptor 2 in pathological angiogenesis of the mouse retina. J Clin Invest. 2007;117(9):2506–16. doi: 10.1172/JCI31123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lerman O, Galiano R, Armour M, Levine J, Gurtner G. Cellular dysfunction in the diabetic fibroblast: impairment in migration, vascular endothelial growth factor production, and response to hypoxia. Am J Pathol. 2003;162:303–312. doi: 10.1016/S0002-9440(10)63821-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Velander P, Theopold C, Hirsch T, Bleiziffer O, Zuhaili B, Fossum M, et al. Impaired wound healing in an acute diabetic pig model and the effects of local hyperglycemia. Wound Repair Regen. 2008;16:288–293. doi: 10.1111/j.1524-475X.2008.00367.x. [DOI] [PubMed] [Google Scholar]
- 7.Kawanabe T, Kawakami T, Yatomi Y, Shimada S, Soma Y. Sphingosine-1-phosphate accelerates wound healing in diabetic mice. J Dermatol Sci. 2007;48:53–60. doi: 10.1016/j.jdermsci.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Visentin B, Vekich J, Sibbald B, Cavalli A, Moreno K, Matteo R, et al. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell. 2006;9:225–238. doi: 10.1016/j.ccr.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 9.Amano S, Akutsu N, Ogura Y, Nishiyama T. Increase of laminin 5 synthesis in human keratinocytes by acute wound fluid, inflammatory cytokines and growth factors, and lysophospholipids. Br J Dermatol. 2004;151:961–970. doi: 10.1111/j.1365-2133.2004.06175.x. [DOI] [PubMed] [Google Scholar]
- 10.Watterson K, Lanning D, Diegelmann R, Spiegel S. Regulation of fibroblast functions by lysophospholipid mediators: potential roles in wound healing. Wound Repair Regen. 2007;15:607–616. doi: 10.1111/j.1524-475X.2007.00292.x. [DOI] [PubMed] [Google Scholar]
- 11.Serriere-Lanneau V, Teixeira-Clerc F, Li L, Schippers M, de Wries W, Julien B, et al. The sphingosine 1-phosphate receptor S1P2 triggers hepatic wound healing. FASEB J. 2007;21:2005–2013. doi: 10.1096/fj.06-6889com. [DOI] [PubMed] [Google Scholar]
- 12.Takabe K, Paugh SW, Milstien S, Spiegel S. “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev. 2008;60(2):181–95. doi: 10.1124/pr.107.07113. [DOI] [PMC free article] [PubMed] [Google Scholar]