Abstract
The highly related transcription factors Sox4 and Sox11 are expressed in the developing sympathetic nervous system. In the mouse, Sox11 appears first, whereas Sox4 is prevalent later. Using mouse mutagenesis and overexpression strategies in chicken, we studied the role of both SoxC proteins in this tissue. Neither Sox4 nor Sox11 predominantly functioned by promoting pan-neuronal or noradrenergic differentiation of sympathetic neurons as might have been expected from studies in neuronal precursors of the central nervous system. The transcriptional network that regulates the differentiation of sympathetic neurons remained intact and expression of noradrenergic markers showed only minor alterations. Instead, Sox11 was required in early sympathetic ganglia for proliferation of tyrosine hydroxylase-expressing cells, whereas Sox4 ensured the survival of these cells at later stages. In the absence of both Sox4 and Sox11, sympathetic ganglia remained hypoplastic throughout embryogenesis because of consecutive proliferation and survival defects. As a consequence, sympathetic ganglia were rudimentary in the adult and sympathetic innervation of target tissues was impaired leading to severe dysautonomia.
Keywords: Apoptosis, Proliferation, Sympathoadrenal lineage, Conditional mutagenesis, Noradrenergic differentiation, Mouse, Chicken
INTRODUCTION
Transcription factors of the Sox family are important developmental regulators (Bowles et al., 2000; Wegner, 1999). However, not all of the twenty mammalian Sox proteins (Schepers et al., 2002) have been analyzed in similar depth. Whereas proteins of the SoxB1 and SoxE subgroups have been the subject of many studies (Guth and Wegner, 2008), SoxC proteins have received relatively little attention so far.
Mammalian SoxC proteins comprise Sox4, Sox11 and Sox12 (Schepers et al., 2002). These three proteins are highly homologous and show similar DNA-binding properties and transactivation potential in vitro (Dy et al., 2008; Hoser et al., 2008). All SoxC proteins are widely and dynamically expressed during mouse embryogenesis (Dy et al., 2008; Hargrave et al., 1997; Hoser et al., 2008; Kuhlbrodt et al., 1998). Expression patterns show extensive overlap, complicating determination of their developmental roles.
All three SoxC genes have been deleted in mice. The corresponding mutants have revealed essential developmental functions for Sox4 and Sox11. Sox4 is required for heart and outflow tract formation, B cell development, T cell differentiation, pancreas and skeletal development (Nissen-Meyer et al., 2007; Schilham et al., 1996; Wilson et al., 2005). Sox11 is likewise involved in heart and outflow tract formation and has additional roles in skeletal development, spleen formation and in the developing anterior eye segment (Sock et al., 2004; Wurm et al., 2008). Both deficiencies are lethal. Sox12-deficient mice, by contrast, do not show overt phenotypic abnormalities (Hoser et al., 2008). SoxC-specific expression patterns and functions appear furthermore conserved in other vertebrates (Maschhoff et al., 2003; Mavropoulos et al., 2005).
No nervous system phenotype has been reported in SoxC-deficient mice, despite the fact that Sox4, Sox11 and Sox12 are strongly expressed throughout the developing central (CNS) and peripheral nervous systems (Dy et al., 2008; Hargrave et al., 1997; Hoser et al., 2008; Kuhlbrodt et al., 1998). Overexpression of SoxC proteins and knockdown by siRNA nevertheless suggest that neural functions exist. Neural tube electroporations in chicken embryos indicated that Sox4 and Sox11 promote the acquisition of pan-neuronal properties in precursors of CNS neurons (Bergsland et al., 2006), and transgenic overexpression in the mouse has provided evidence for a negative influence on the differentiation of CNS glia (Hoser et al., 2007; Potzner et al., 2007). Available data thus point to an influence of SoxC proteins on differentiation events.
Here, we used constitutive and conditional mutagenesis to delete both Sox4 and Sox11 in the developing sympathetic nervous system of compound mouse mutants. Our studies showed that Sox4 and Sox11 are essential for the development of this compartment of the autonomic nervous system. The underlying mechanism, however, was much more complex than expected. SoxC proteins predominantly influenced proliferation in sympathetic ganglia at early stages and survival at late stages, whereas they had only mild effects on the noradrenergic differentiation of sympathetic neurons. In addition to their role as differentiation factors, SoxC proteins thus have other essential functions in nervous system development.
MATERIALS AND METHODS
Mouse husbandry, genotyping, BrdU labeling and dissections
Mice used in this study carried the following Sox4 or Sox11 alleles on a mixed 129SvJ/C57Bl6J background: Sox4loxP (Penzo-Mendez et al., 2007), Sox11lacZ (Sock et al., 2004), Sox11loxP (P. Bhattaram, A.P.-M., E.S., C. Colmenares, K. J. Kaneko, M. L. DePamphilis, M.W. and V.L., unpublished). For conditional deletion of the Sox4loxP and the Sox11loxP alleles, Dbh::Cre and Wnt1::Cre transgenes were used (Danielian et al., 1998; Parlato et al., 2007). Genotyping was performed by PCR using published primers for Sox4loxP and Sox11lacZ (Penzo-Mendez et al., 2007; Sock et al., 2004), 5′-TTCGTGATTGCAACAAAGGCGGAG-3′ and 5′-GCTCCCTGCAGTTTAAGAAATCGG-3′ for Sox11loxP, 5′-GACAGGCAGGCCTTCTCTGAA-3′ and 5′-CTTCTCCACACCAGCTGTGGA-3′ for Dbh::Cre, and 5′-ATGCTGTTTCACTGGTTATG-3′ and 5′-ATTGCCCCTGTTTCACTATC-3′ for Wnt1::Cre. For BrdU labeling, pregnant mice were injected intraperitoneally with BrdU (Sigma) at 100 μg/g body weight 1 hour before dissection (Stolt et al., 2003). Embryos were obtained at 11.5, 12.5, 14.5, 16.5 and 18.5 dpc from staged pregnancies, were fixed in 4% paraformaldehyde and frozen at –80°C in Jung Tissue Freezing Medium (Leica, Nussloch, Germany) after cryoprotection. Tissue from adult animals was similarly treated.
Plasmids and retroviral constructs
The complete open reading frames of mouse Sox4 and Sox11 (NM_009238 and NM_009234) were inserted into the pCAGGS expression plasmid, and the coding sequences of rat Sox4 and Sox11 (XM_344594 and NM_053349) into the NotI site of RCAS-BP(B)-CNS (Tsarovina et al., 2004). Chicken embryo fibroblasts were transfected with the retroviral DNA and maintained in culture until complete viral infection was achieved.
Viral infection of chicken embryos and transfection of chicken dorsal root ganglia cultures
Developing chicken embryos were staged according to Hamburger and Hamilton (Hamburger and Hamilton, 1992) before fixation in 4% paraformaldehyde for 2-18 hours and freezing. For RCAS viral infection, fertilized virus-free chicken eggs (Charles River, Sulzfeld, Germany) were incubated for 2 days. Cell aggregates of chicken fibroblasts infected with Sox4-RCAS and Sox11-RCAS were implanted into the right side of chicken embryos at brachial levels between the neural tube and the last somites formed (Reissmann et al., 1996). Embryos were allowed to further develop until E6-8.
Dorsal root ganglia were isolated from E5 chicken embryos (Rüdiger et al., 2009). After trypsinization and trituration, cells were electroporated with 3 μg of pCAGGS-GFP for controls or 1 μg of pCAGGS-GFP combined with 2 μg pCAGGS-Sox4, pCAGGS-Sox11 or pCAGGS-Phox2a using Nucleofactor II (Amaxa). Transfected cells were plated on collagen-coated four-well culture dishes (50,000 cells/well) and incubated in 10% CO2 at 37°C in MEM containing 15% chicken embryo extract, 15% horse serum and 1% penicillin/streptomycin for 5 days.
Immunohistochemistry, in situ hybridization, proliferation assay and TUNEL
Frozen embryos and tissues were transversally sectioned on a cryotome at 10 μm for immunohistochemistry, proliferation studies and TUNEL, and at 14 μm for in situ hybridizations.
For immunochemistry, the following primary antibodies were used in various combinations: guinea pig antisera against Sox4 (1:1500 dilution) (Hoser et al., 2008), Sox10 (1:1000) (Maka et al., 2005), Sox11 (1:1000) (Hoser et al., 2008), Insm1 (1:5000, gift of C. Birchmeier, MDC, Berlin, Germany), mouse monoclonals against Tubb3 (Tuj1; 1:4000, Covance), HuC/D (1:500, Developmental Studies Hybridoma Bank), Th (1:500) (Rohrer et al., 1986), rabbit antisera against Th (1:1000, Biomol), Phox2a (1:500, gift of C. Goridis, Ecole Normale Superieure, Paris, France), Phox2b (1:500, gift of C. Goridis), GFP (1:1000, Invitrogen), β-galactosidase (1:500, ICN) and anti-β-galactosidase goat antiserum (1:500, Biotrend). Secondary antibodies conjugated to Alexa Fluor 488, Cy2 or Cy3 immunofluorescent dyes (Dianova and Molecular Probes) were used for detection. Incorporated BrdU was visualized by Alexa Fluor 488-coupled mouse monoclonals against BrdU (1:20, Molecular Probes). TUNEL was performed according to the manufacturer's protocol (Chemicon).
In situ hybridizations employed DIG-labeled antisense riboprobes and horseradish peroxidase-coupled anti-DIG antibodies (Hoser et al., 2008). Riboprobes recognized Mash1 (gift of F. Guillemot, National Institute of Medical Research, Mill Hill, London, UK), Gata3 (gift of C. Goridis), Hand2 (NM_010402, position 925-1578), Sox4 and Sox11 (Hoser et al., 2008) in mouse and RCAS RT (Stanke et al., 1999), Scg10, Tubb3, MAP2, Th (Ernsberger et al., 1995), Sox4 (Maschhoff et al., 2003) and Sox11 (gift of P. Scotting, University of Nottingham, UK) in chicken.
Thoracic-level sections caudal to the stellate ganglion (corresponding to the mouse heart and chicken wing bud regions) were microscopically analyzed and documented as described (Stolt et al., 2003). For figures, sections were selected in which ganglia exhibited their largest size unless stated otherwise.
Quantifications
Numbers of immunoreactive cells or DAPI-positive nuclei per section in sympathetic ganglia were counted. Alternatively, immunoreactive areas were determined morphometrically using ImageJ software (NIH) by summarizing suprathreshold zones regardless of variations in brightness. Data were obtained from 12 or more sections in which the ganglia exhibited their largest size. Sections were generated from at least three different embryos for each genotype and embryonic age. Diagrams show mean values ± s.e.m. Statistical significance was determined by unpaired two-tailed Student's t-test using Prism4 software (GraphPad) and P-values are provided in the figure legends.
RESULTS
Sox4 and Sox11 are expressed in the developing sympathetic nervous system
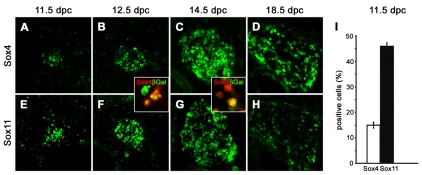
A previous study had detected Sox4 and Sox11 transcripts in developing mouse sympathetic ganglia (SG) (Hoser et al., 2008). To study their expression patterns in greater detail, we performed immunohistochemistry on mouse embryos from 11.5 until 18.5 days post-coitum (dpc). We focused on thoracic levels caudal to the stellate ganglion, but observed similar expression patterns at lumbar levels. In forming SG, Sox4 expression was low at 11.5 dpc, with only 15% of cells showing detectable Sox4 staining (Fig. 1A,I). One day later, the number of Sox4-positive cells had significantly increased (Fig. 1B) and reached its peak at 14.5 dpc (Fig. 1C). Even at 18.5 dpc, a substantial number of cells was still labeled by the Sox4 antibody, although the signal had started to decline (Fig. 1D). Adult SG no longer exhibited Sox4 staining (data not shown).
Fig. 1.
The occurrence of SoxC proteins in mouse SG. (A-H) Immunohistochemistry was performed with antibodies directed against Sox4 (A-D) and Sox11 (E-H) on transverse thoracic-level sections of wild-type embryos caudal to the stellate ganglion at 11.5 (A,E), 12.5 (B,F), 14.5 (C,G) and 18.5 (D,H) dpc. Sympathetic chain ganglia are shown. The insets show magnifications of SG from Sox11+/lacZ embryos at 12.5 and 14.5 dpc double labeled with antibodies against Sox4 (red) and β-galactosidase (green). (I)The number of Sox4-positive (white) and Sox11-positive (black) cells was determined in SG at 11.5 dpc relative to the total number of DAPI-positive nuclei.
In contrast to Sox4, Sox11 was already highly expressed in SG at 11.5 dpc, with 46% of all cells exhibiting nuclear staining (Fig. 1E,I). Most cells within the ganglia remained Sox11 positive at 12.5 dpc (Fig. 1F). Sox11 protein levels had decreased by 14.5 dpc, so that fewer cells were intensely stained (Fig. 1G). The decline continued as Sox11 protein had disappeared from most cells at 18.5 dpc (Fig. 1H). Sox11 protein was absent from adult SG (data not shown).
Similar results were obtained when transcripts were analyzed instead of proteins. Sox11 transcript levels were higher than those of Sox4 at early embryonic stages (see Fig. S1A,D in the supplementary material) and lower at late embryonic stages (see Fig. S1C,F in the supplementary material), with robust expression of both genes at 14.5 dpc (see Fig. S1B,E in the supplementary material).
Sox4 and Sox11 were also expressed in developing chicken SG (see Fig. S2A,B,F,G,K,L in the supplementary material). SoxC transcripts became detectable at embryonic day (E) 3, between the 33- and 35-somite stages, after the onset of Phox2b expression (see Fig. S2C,H,M in the supplementary material) but before the expression of the terminal differentiation genes Th and Scg10 (see Fig. S2D,E,I,J,N,O in the supplementary material). This is approximately the same time at which Gata2 expression is initiated, but slightly later than that of Hand2 (see Table S1 in the supplementary material) (Tsarovina et al., 2004). Sox4 and Sox11 were detectable throughout the analyzed period of chicken development, including E10 as the latest stage (see Fig. S2K,L in the supplementary material). Sox4 and Sox11 are thus strongly expressed in developing mouse and chicken SG, with slight species-specific differences in their temporal expression profiles.
Because Sox4 and Sox11 expression strongly overlapped in developing SG, we analyzed whether they were co-expressed on a cellular level. As the anti-Sox4 and anti-Sox11 antibodies were both raised in guinea pig, we could not perform co-immunohistochemistry and so shifted our analysis from wild-type to Sox11+/lacZ embryos, where β-galactosidase can be used as a surrogate marker for Sox11 expression (Sock et al., 2004). In these animals, we detected an extensive overlap between β-galactosidase and Sox4 expression on the cellular level (Fig. 1, insets). At 12.5 dpc, almost all Sox4-positive cells also expressed β-galactosidase, but additional β-galactosidase-positive cells did not contain Sox4 (Fig. 1, inset between B and F). At 14.5 dpc the situation was reversed. Now, β-galactosidase-positive cells were generally Sox4 positive, with additional cells only labeled by the Sox4 antibody (Fig. 1, inset between C and G).
Sox4 and Sox11 are expressed in immature cells of the sympathoadrenal lineage
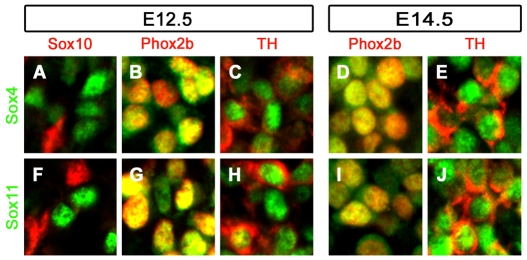
Developing SG contain different cell types that can be distinguished by marker gene expression. Some cells express the SoxE protein Sox10 (Britsch et al., 2001; Kim et al., 2003; Reiprich et al., 2008), which marks undifferentiated neural crest cells at early stages and glial cells at later stages. At no time did we detect a significant overlap between Sox10 and Sox4 or Sox11 in developing SG, arguing that neural crest stem cells and glia are not the main expression sites for both SoxC proteins (Fig. 2A,F and data not shown). Instead, Sox4 and Sox11 colocalized with the marker for sympathoadrenal precursor cells, Phox2b, at 12.5 and 14.5 dpc (Fig. 2B,D,G,I). There was also an extensive overlap at both stages with tyrosine hydroxylase (Th), a marker for immature and mature sympathetic neurons (Fig. 2C,E,H,J). Some cells, however, were stained for Th only (e.g. Fig. 2C,E). As their number increased with development (data not shown), we assume that cells that are only positive for Th represent sympathetic neurons at a more differentiated stage and that SoxC proteins are downregulated during the maturation of noradrenergic neurons.
Fig. 2.
Cell type-specific expression of Sox4 and Sox11 in mouse SG. (A-J) Antibodies directed against Sox4 (A-E) or Sox11 (F-J) (both green) were combined with antibodies directed against the neural crest stem cell and glial cell marker Sox10 (A,F), the sympathoadrenal precursor cell marker Phox2b (B,D,G,I) and the noradrenergic neuronal marker Th (C,E,H,J) (all red) in co-immunohistochemistry on transverse sections of wild-type embryos at 12.5 (A-C,F-H) and 14.5 (D,E,I,J) dpc.
Sox11 influences Sox4 expression in sympathetic ganglia at early stages
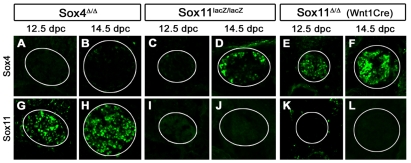
Considering the overlapping occurrence of Sox4 and Sox11 in developing SG, we tested whether the two SoxC proteins influence each other's expression. We first tested mice in which Sox4 was specifically ablated in the sympathoadrenal lineage using a Dbh::Cre transgene (Parlato et al., 2007). Immunohistochemical analysis of Sox4loxP/loxP; Dbh::Cre (henceforth called Sox4Δ/Δ) mice at 12.5 and 14.5 dpc showed that Sox4 was efficiently deleted in SG (Fig. 3A,B). Nevertheless, Sox11 expression remained unchanged (compare Fig. 3G,H with Fig. 1F,G), arguing against a direct influence of Sox4 on Sox11 expression or a compensatory upregulation of Sox11 in the absence of Sox4.
Fig. 3.
Occurrence of Sox4 and Sox11 in SG of mice with SoxC deficiencies. (A-L) Immunohistochemistry was performed with antibodies directed against Sox4 (A-F) and Sox11 (G-L) on transverse thoracic-level sections of Sox4loxP/loxP; Dbh::Cre (Sox4Δ/Δ) (A,B,G,H), Sox11lacZ/lacZ (C,D,I,J) and Sox11loxP/loxP; Wnt1::Cre (Sox11Δ/Δ) (E,F,K,L) embryos at 12.5 (A,C,E,G,I,K) and 14.5 (B,D,F,H,J,L) dpc. Sympathetic chain ganglia were detected by Th co-immunohistochemistry and are circled.
By contrast, alterations in the Sox4 expression pattern were apparent in embryos with a constitutive Sox11 deletion (Fig. 3I,J). Unlike wild-type ganglia, the SG of these Sox11lacZ/lacZ embryos did not exhibit Sox4-specific immunoreactivity at 12.5 dpc (compare Fig. 3C with Fig. 1B). Sox4 became detectable only at 14.5 dpc, with a few Sox4-positive cells present at the outer rim of the SG (Fig. 3D). At later stages, Sox4 expression normalized (data not shown). When the same analysis was carried out in mice in which Sox11 was selectively deleted in the neural crest (using Wnt1::Cre) or sympathoadrenal lineage (using Dbh::Cre), Sox4 expression was comparable to that in the wild type (Fig. 3E,F,K,L and data not shown). This suggests that Sox11 in non-neural-crest-derived cells outside the SG transiently influences Sox4 expression within the ganglion. At 11.5 and 12.5 dpc, Sox11 was detected in the surrounding mesenchymal cells and in some cells in the wall of the dorsal aorta (data not shown). Which cell type and signaling pathway influence ganglionic Sox4 expression are currently unknown.
SG development is impaired in the absence of SoxC proteins
To study the role of Sox4 and Sox11 in SG development, we analyzed mice with SoxC deficiencies. As compensatory effects between the two SoxC genes appeared possible, we included not only Sox4Δ/Δ and Sox11lacZ/lacZ mice, but also mice with combined deficiencies.
When SG were visualized by Th immunoreactivity, alterations were already evident at 11.5 dpc (Fig. 4A-D,M). The SG in Sox11lacZ/lacZ mice were significantly smaller and the mean area of Th-positive cells comprised only one-third of that in the wild type. Nearly identical size reductions were recorded for ganglia in double-deficient mice, in agreement with the predominance of Sox11 and the lack of Sox4 expression in Sox11lacZ/lacZ embryos at this early stage. SG from Sox4Δ/Δ mice, by contrast, showed only mild size reductions.
Fig. 4.
SG development in mice with SoxC deficiencies. (A-L) Immunohistochemistry was performed with antibodies directed against Th on transverse thoracic-level sections of wild-type (A,E,I), Sox4Δ/Δ (B,F,J), Sox11lacZ/lacZ (C,G,K) and Sox4Δ/Δ; Sox11lacZ/lacZ (dko) (D,H,L) embryos at 11.5 (A-D), 14.5 (E-H) and 18.5 (I-L) dpc. (M) The average Th-positive SG area per section was determined from immunohistochemical stainings in wild-type (black), Sox4Δ/Δ (blue), Sox11lacZ/lacZ (green) and Sox4Δ/Δ; Sox11lacZ/lacZ (dko, red) embryos at 11.5, 14.5 and 18.5 dpc. Area sizes are presented relative to the wild-type size at 18.5 dpc, which was set to 100. Reductions in size relative to the age-matched wild type were statistically significant (P<0.001, Student's t-test) for all ganglia except for Sox11lacZ/lacZ ganglia at 18.5 dpc. (N) Average cell numbers within the ganglia per section were determined by counting DAPI-positive nuclei. Reductions in cell number relative to the age-matched wild type were statistically significant (P<0.001, Student's t-test) for all ganglia except Sox4Δ/Δ ganglia at 11.5 and 14.5 dpc, and Sox11lacZ/lacZ ganglia at 18.5 dpc.
At 14.5 dpc, SG size reductions in Sox11lacZ/lacZ and double-deficient mice were still very similar, with ganglia being reduced to less than half the wild-type size (Fig. 4E,G,H,M). Consistent with the increased Sox4 expression in the wild type at this stage, the impact of Sox4 on SG development was now more obvious. Ganglia in Sox4Δ/Δ mice were on average three-quarters the wild-type size (Fig. 4E,F,M).
By 18.5 dpc, SG in double-deficient mice were only one-quarter the size of wild-type ganglia (Fig. 4I,L,M) and, compared with 14.5 dpc, their size had even decreased. By contrast, SG of Sox11lacZ/lacZ mice had recovered almost to the size of wild-type ganglia (Fig. 4I,K,M). The SG of Sox4Δ/Δ mice were now clearly affected (Fig. 4I,J), being two-thirds the size of wild-type SG (Fig. 4M). Mutant SG size had furthermore not significantly increased between 14.5 dpc and 18.5 dpc.
These size differences in mutant ganglia were paralleled by changes in cell number (Fig. 4N). When compared with the wild type, cell numbers were already reduced in Sox11-deficient and double-deficient ganglia at 11.5 dpc and were decreased in all three mutant genotypes at 14.5 dpc. Similar to the overall size, cell numbers had normalized in the SG of Sox11lacZ/lacZ mice by 18.5 dpc and were now exclusively lower in Sox4Δ/Δ and double-deficient mice.
Thus, Sox4 and Sox11 both influence SG development, although predominantly in different time windows: whereas Sox11 was essential for early stages, Sox4 took over during later stages.
Sox4 and Sox11 have only a mild influence on noradrenergic differentiation
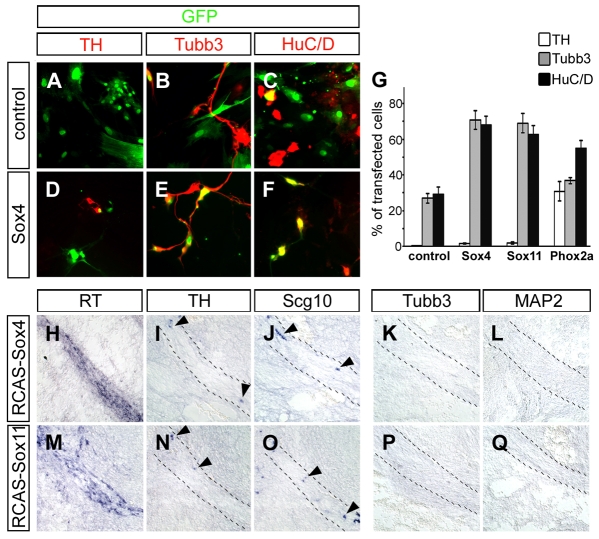
We first analyzed the impact of Sox4 and Sox11 on the differentiation of sympathetic neurons. Previous studies in chicken embryos had shown that overexpression of Phox2a/b in cultured neural crest cells (Stanke et al., 1999) and progenitor cells from E5 dorsal root ganglia (DRG) (Rüdiger et al., 2009) is sufficient to induce pan-neuronal markers and markers of noradrenergic differentiation. This was reproduced in the current study, where 31% of all Phox2a-transfected cells initiated expression of the noradrenergic marker Th (Fig. 5G). However, when Sox4 and Sox11 were overexpressed in E5 DRG cultures, Th expression was not increased above background levels (Fig. 5A,D,G). What was increased in cells transfected with Sox4 or Sox11 were the pan-neuronal markers HuC/D (Elavl3/4 – Mouse Genome Informatics) and Tubb3 (62-71% compared with 27-29% in GFP control transfections, see Fig. 5B,C,E-G). Co-expression of Phox2a with Sox4 or Sox11 did not increase the expression rate of pan-neuronal or noradrenergic markers above the level obtained with Phox2a alone (data not shown).
Fig. 5.
The influence of Sox4 and Sox11 on neuronal differentiation in vitro and in vivo. (A-F) E5 chicken DRG cells were transfected before plating with expression plasmids for GFP (A-C) or chicken Sox4 and GFP (D-F). After 5 days in culture, cells were fixed and immunostained for GFP (green) in combination with Th (A,D) as a noradrenergic marker, or Tubb3 (B,E) or HuC/D (C,F) as pan-neuronal markers (all red). (G) Neuronal differentiation of cultured chicken DRG precursor cells transfected with GFP (control), Sox4/GFP, Sox11/GFP or Phox2a/GFP was quantified from immunostainings similar to those shown in A-F. Increases relative to control GFP transfections were statistically significant (P<0.002, Student's t-test) for the pan-neuronal markers Tubb3 and HuC/D after transfection of Sox4 and Sox11, and for the noradrenergic marker Th after Phox2a transfection. (H-Q) Chicken embryos were infected at E2 with RCAS virus expressing Sox4 (H-L) or Sox11 (M-Q) and analyzed at E6 by in situ hybridization for expression of reverse transcriptase (RT, indicative of infected cells) (H,M), Th (I,N), Scg10 (J,O), Tubb3 (K,P), or MAP2 (L,Q) in the brachial nerve. The nerve area is marked by dashed lines and cells ectopically expressing neuronal markers by arrowheads.
In an alternative approach, we infected chicken neural crest cells in vivo shortly after their emigration at brachial levels with RCAS viruses expressing either Sox4 or Sox11 and analyzed their development 4 and 6 days later in the brachial nerve (Fig. 5H-Q and data not shown). It had previously been shown that infections with control RCAS virus do not induce neuronal markers. By contrast, after infection with Phox2a-expressing RCAS viruses, the brachial nerve abounded with Scg10- and Th-positive cells (Stanke et al., 1999). When Sox4- or Sox11-expressing RCAS viruses were used, a few cells were detected along the brachial nerve that ectopically expressed Scg10 or Th (Fig. 5I,J,N,O) and almost none expressed substantial levels of Tubb3 or microtubule-associated protein 2 (MAP2) (Fig. 5K,L,P,Q), despite good infection rates as evidenced by strong expression of the viral reverse transcriptase (Fig. 5H,M). In contrast to the results from DRG cultures, not even pan-neuronal markers were reliably induced by Sox4 or Sox11 in this in vivo paradigm. This suggests that SoxC proteins are unlikely to exert their influence on sympathetic nervous system development primarily by altering neuronal differentiation.
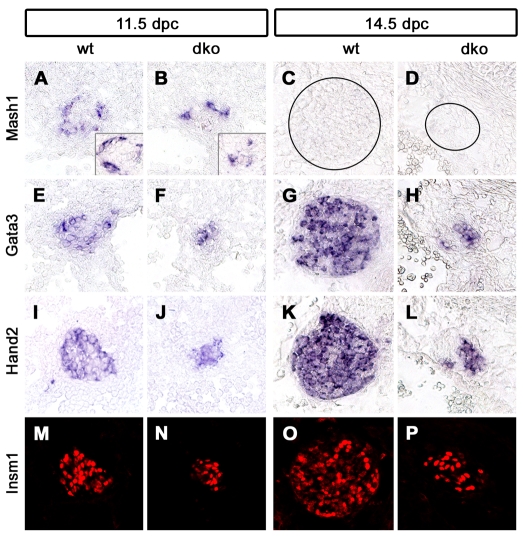
To substantiate this conclusion, Sox4Δ/Δ and Sox11lacZ/lacZ mice were analyzed for the expression of several transcription factors that are part of the regulatory network that drives noradrenergic differentiation during sympathetic nervous system development (Goridis and Rohrer, 2002). Expression of Phox2b, Mash1 (Ascl1 – Mouse Genome Informatics), Gata3, Hand2 and Insm1 was detected in the small SG of Sox4Δ/Δ; Sox11lacZ/lacZ mice at 11.5 dpc (Fig. 6A,B,E,F,I,J,M,N and see Fig. S3A-D in the supplementary material), 12.5 dpc (insets in Fig. 6A,B, and data not shown) and, with the exception of Mash1, also at 14.5 dpc (Fig. 6C,D,G,H,K,L,O,P and Fig. 7A-D). Considering that Mash1 expression was similarly downregulated in the wild type by 14.5 dpc, the noradrenergic regulatory network appears intact and functional in the SG of double-deficient mice.
Fig. 6.
Analysis of components of the noradrenergic gene regulatory network in mice with combined Sox4 and Sox11 deficiencies. (A-P) In situ hybridizations and immunohistochemical stainings were performed on transverse thoracic-level sections of wild-type (A,C,E,G,I,K,M,O) and Sox4Δ/Δ; Sox11lacZ/lacZ (dko) (B,D,F,H,J,L,N,P) embryos at 11.5 (A,B,E,F,I,J,M,N), 12.5 (insets in A,B) and 14.5 (C,D,G,H,K,L,O,P) dpc caudal to the stellate ganglion using riboprobes specific for Mash1 (A-D), Gata3 (E-H), Hand2 (I-L) and antibodies to Insm1 (M-P).
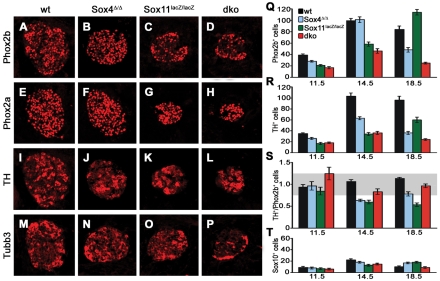
Fig. 7.
SG marker gene expression in mice with SoxC deficiencies. (A-P) Immunohistochemistry was performed on transverse thoracic-level sections of wild-type (A,E,I,M), Sox4Δ/Δ (B,F,J,N), Sox11lacZ/lacZ (C,G,K,O) and Sox4Δ/Δ; Sox11lacZ/lacZ (dko) (D,H,L,P) embryos caudal to the stellate ganglion at 14.5 dpc using antibodies directed against Phox2b (A-D), Phox2a (E-H), Th (I-L) and Tubb3 (M-P). (Q-T) The numbers of Phox2b-positive cells (Q), Th-positive cells (R), the ratio of Th- to Phox2b-positive cells (S) and the number of Sox10-positive cells (T) were quantified in wild-type (black), Sox4Δ/Δ (blue), Sox11lacZ/lacZ (green) and Sox4Δ/Δ; Sox11lacZ/lacZ (dko, red) embryos at 11.5, 14.5 and 18.5 dpc. Reductions in the number of Phox2b- and Th-positive cells relative to the age-matched wild type were statistically significant (P<0.001, Student's t-test) except for Phox2b-positive cells in Sox4Δ/Δ ganglia at 14.5 dpc and Sox11lacZ/lacZ ganglia at 18.5 dpc, and except for Th-positive cells in Sox4Δ/Δ ganglia at 11.5 dpc. Numbers of Sox10-positive cells, by contrast, were comparable among genotypes. The gray area in S marks the range in which the ratio of Th- to Phox2b-positive cells is not significantly different from that of the wild type.
We also analyzed markers that are indicative of consecutive steps of noradrenergic differentiation, starting with Phox2b as the earliest protein to appear in the sympathoadrenal precursor and proceeding via Phox2a to Th. Additionally, we included Tubb3 as a marker for pan-neuronal differentiation. All markers were detectable by immunohistochemistry at 11.5 dpc (see Fig. S3 in the supplementary material) and 14.5 dpc (Fig. 7). By visual inspection, the number of cells expressing each marker was reduced in the SG of all three mutant genotypes as compared with the wild type. These reductions, however, appeared to be proportional to the overall reductions in SG size and cell number in the respective mutants (compare Fig. 7 with Fig. 4).
For double-deficient embryos, this impression was confirmed by quantification (Fig. 7 and data not shown). The numbers of Phox2b- and Th-positive cells were reduced at 11.5, 14.5 and 18.5 dpc (Fig. 7Q,R), but the ratio of Th-positive cells to Phox2b-positive cells remained in a range in which differences to the wild type were not statistically significant (Fig. 7S). In Sox4Δ/Δ embryos the situation was more complex. Whereas the ratio of Th- to Phox2b-positive cells was unaltered at 11.5 dpc, Th-positive cells were selectively reduced at 14.5 dpc, leading to a lower ratio of Th- to Phox2b-positive cells (Fig. 7Q-S). At 18.5 dpc, the ratio was again similar to that of the wild type (Fig. 7Q-S).
Ganglia of Sox11lacZ/lacZ embryos displayed reduced numbers of Phox2b-positive cells at 11.5 dpc, but a normal ratio of Th- to Phox2b-positive cells (Fig. 7Q-S). At 14.5 dpc, Phox2b-positive cells were still reduced and, because of an even lower number of Th-positive cells, the ratio of Th- to Phox2b-positive cells was significantly decreased. Although the number of Phox2b-positive cells had recovered to wild-type levels at 18.5 dpc, the number of Th-expressing cells, as well as the ratio of Th- to Phox2b-positive cells, remained significantly lower than in the wild type (Fig. 7Q-S). At this time, Sox11-deficient SG normalize in size and cell number. However, most Phox2b-positive cells have not yet undergone maturation. The over-representation of Phox2b-positive cells and the reciprocal under-representation of Th-positive cells therefore support the hypothesis of a transient delay of sympathetic nervous system development in vivo. However, all of the observed influences on noradrenergic differentiation in the single mutants are mild and transient.
In contrast to the reduction of cells expressing noradrenergic and pan-neuronal markers in mutant genotypes, there was no significant difference in the number of Sox10-expressing cells compared with the wild type (Fig. 7T). Sox10-expressing neural crest stem cells and glia are thus unaffected in developing SG by the loss of Sox4 and Sox11.
SoxC proteins influence proliferation during early stages and survival during late stages of sympathetic nervous system development
The reduced SG size in SoxC mutants could be explained by an influence of SoxC proteins on proliferation. As a measure of proliferative activity, we analyzed BrdU incorporation during a 1-hour period at various times of embryogenesis. BrdU-labeled cells were detected in significant numbers in wild-type SG when the nucleoside analog was administered between 11.5 and 16.5 dpc, with a maximum at 14.5 dpc (Fig. 8A-F,M). This leads to an increase in SG size from 11.5 dpc to 18.5 dpc (see Fig. 4). However, the rate of proliferation, determined as the fraction of BrdU-labeled cells in the ganglion, continuously decreased from a peak at 11.5 dpc (Fig. 8N). Throughout SG development, most BrdU-incorporating cells expressed Th (Fig. 8B,D,F). Sox10-positive cells were only occasionally BrdU labeled (Fig. 8A,C,E), indicating that the majority of proliferating cells are immature sympathetic neurons.
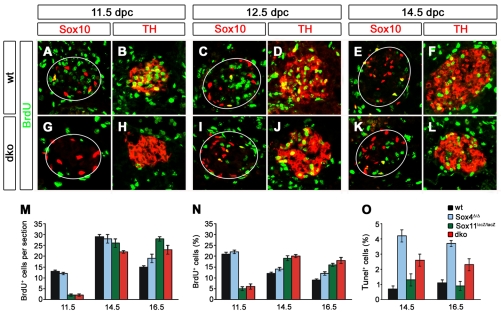
Fig. 8.
Proliferation and apoptosis in SG of mice with SoxC deficiencies. (A-L) Co-immunohistochemistry was performed on transverse thoracic-level sections of wild-type (A-F) and Sox4Δ/Δ; Sox11lacZ/lacZ (dko) (G-L) embryos at 11.5 (A,B,G,H), 12.5 (C,D,I,J) and 14.5 (E,F,K,L) dpc using antibodies directed against BrdU (green) in combination with antibodies to Sox10 (A,C,E,G,I,K) or Th (B,D,F,H,J,L). For better comparison of proliferation rates in both genotypes, sections with similar SG sizes are shown. (M,N) For quantifications, absolute numbers of BrdU-positive cells (M) and proliferation rates (percentage of ganglion cells labeled with BrdU; N) were determined in wild-type (black), Sox4Δ/Δ (blue), Sox11lacZ/lacZ (green) and Sox4Δ/Δ; Sox11lacZ/lacZ (dko, red) embryos at 11.5, 14.5 and 16.5 dpc. (O) In the same genotypes, apoptosis rates were determined by TUNEL at 14.5 and 16.5 dpc. For each determination, at least 12 sections were used per genotype and embryonic age.
When BrdU incorporation was analyzed in Sox4Δ/Δ; Sox11lacZ/lacZ embryos, the SG was almost devoid of proliferating cells at 11.5 dpc (Fig. 8G,H,M,N) and the number of BrdU-positive cells was still dramatically reduced at 12.5 dpc (Fig. 8I,J). However, proliferation had started by 12.5 dpc, and by 14.5 dpc the absolute number of BrdU-labeled cells was only slightly lower than in the wild type (Fig. 8K-M). Taking the smaller SG size into consideration, proliferation rates exceeded those in the wild type at 14.5 dpc and remained higher in Sox4Δ/Δ; Sox11lacZ/lacZ embryos even at 16.5 dpc (Fig. 8M,N). Most proliferating cells in the SG of double-deficient embryos were Th positive (Fig. 8G-L).
We also analyzed proliferation in the SG of mice with single SoxC gene deficiencies. BrdU incorporation in the SG of Sox4Δ/Δ embryos was very similar to that in the wild type regarding absolute cell numbers and proliferation rates, which continuously declined from a peak at early times (Fig. 8M,N). Proliferation rates of Sox11lacZ/lacZ embryos, by contrast, resembled those in double-deficient embryos, with almost no BrdU incorporation at 11.5 dpc and high numbers of BrdU-labeled cells at 14.5 and 16.5 dpc (Fig. 8M,N). Our results indicate that Sox11, especially, has a significant impact on proliferation until 12.5 dpc, which explains the early SG size reductions in mice with Sox11 deficiency. Proliferation defects are, however, overcome at later stages of development even in the absence of both Sox4 and Sox11, indicating that the influence of SoxC proteins on proliferation is restricted to early stages of sympathetic nervous system development.
Why then did the SG of double-deficient embryos fail to increase in size during later stages of embryogenesis? To address the possibility of increased apoptotic cell death, we performed TUNEL (Fig. 8O and data not shown). At 12.5 dpc, SG only rarely contained apoptotic cells and there was no difference between wild-type and mutant ganglia (data not shown). From 14.5 dpc onwards, apoptotic cells were reproducibly detected in SG. When apoptotic cells were quantified, we detected a 2.5- to 4-fold increase in Sox4Δ/Δ embryos and Sox4Δ/Δ; Sox11lacZ/lacZ embryos at 14.5 and 16.5 dpc (Fig. 8O). Sox11lacZ/lacZ embryos, by contrast, exhibited wild-type apoptotic rates in their SG. SoxC proteins thus influence survival from 14.5 dpc onwards. Survival is furthermore predominantly a function of Sox4, as the main SoxC protein in late embryonic SG.
Embryonic deletion of Sox4 and Sox11 impairs the function of the adult sympathetic nervous system
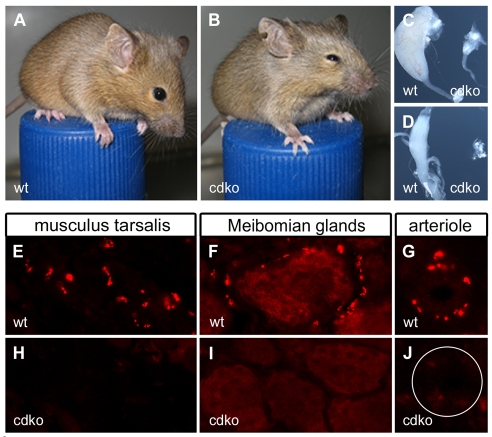
In Sox4Δ/Δ; Sox11lacZ/lacZ embryos, SG are severely growth retarded by the time of birth. As these mice die at birth because of unrelated defects resulting from the homozygous constitutive Sox11 deletion (Sock et al., 2004), it is impossible to clarify whether the defect in the sympathetic nervous system is permanent and leads to functional impairments in the adult. To address these issues, we deleted both Sox4 and Sox11 selectively in the sympathoadrenal lineage using conditional SoxC alleles and the Dbh::Cre transgene. The resulting Sox4Δ/Δ; Sox11Δ/Δ mice were viable and reached adulthood, but were unable to fully open their eyes (Fig. 9A,B). Such ptosis is also observed in humans with syndromic defects of the sympathetic nervous system and is caused by a missing sympathetic innervation of the musculus tarsalis of the eyelid. Indeed, Sox4Δ/Δ; Sox11Δ/Δ mice also lacked such innervation as shown by Th immunohistochemistry (Fig. 9E,H). The lack of sympathetic innervation was not restricted to eyelid muscle, but also affected other target areas in the eye such as the Meibomian glands (Fig. 9F,I) and arterioles (Fig. 9G,J). The sympathetic nervous system defect is thus likely to be global. In agreement, Sox4Δ/Δ; Sox11Δ/Δ mice possess only small rudiments of the superior cervical ganglia and stellate ganglia (Fig. 9C,D).
Fig. 9.
Sympathetic nervous system defects in adult mice with loss of Sox4 and Sox11. (A,B) A phenotypic comparison of wild-type mice (wt, A) and Sox4Δ/Δ; Sox11Δ/Δ mice with a conditional deletion of Sox4 and Sox11 in the sympathetic nervous system (cdko, B) at 3 months of age revealed blepharoptosis (drooping of the upper eyelid) in the mutant. (C,D) Superior cervical ganglia (C) and stellate ganglia (D) were dramatically reduced in size in Sox4Δ/Δ; Sox11Δ/Δ (cdko) mice. (E-J) Sympathetic innervation of musculus tarsalis (E,H), Meibomian glands (F,I) and arterioles (G,J) was readily detected in the eye of wild-type mice (E,F,G), but missing in Sox4Δ/Δ; Sox11Δ/Δ (cdko) mice (H,I,J), as evident from immunohistochemistry with antibodies against Th. The arteriole is circled in J.
DISCUSSION
SoxC proteins are strongly expressed in the mouse developing nervous system in an overlapping pattern (Dy et al., 2008; Hargrave et al., 1997; Hoser et al., 2008; Kuhlbrodt et al., 1998). Despite this, little evidence has so far been obtained from targeted deletion studies in the mouse to indicate that these proteins actually have crucial functions in nervous system development (Cheung et al., 2000). Functional redundancy between SoxC proteins offered a plausible explanation for the lack of overt neural defects. Here, we present data for an essential role of SoxC proteins in sympathetic nervous system development from loss-of-function studies in the mouse, uncovering important functions in the proliferation and survival of developing sympathetic neurons. Although we focused our analysis on ganglia of the sympathetic chain at thoracic levels, hypoplasia was also observed for ganglia at lumbar levels and superior cervical ganglia, arguing that SoxC proteins function throughout the whole developing sympathetic nervous system.
Most of the experiments presented were performed on mice in which Sox11 was constitutively deleted in addition to a conditional deletion of Sox4 using a Dbh::Cre transgene. Despite the relatively late deletion via Dbh::Cre, the absence of Sox4 caused developmental defects in the sympathoadrenal lineage. Comparable results were furthermore obtained when Sox4 was deleted using a Wnt1::Cre transgene that initiates expression of the recombinase already in neural crest cells at the time of their emigration and long before ganglia formation (data not shown).
Taking the constitutive Sox11 deletion into account, some of the observed changes in sympathetic neuron development might not be cell-intrinsic, but rather caused by loss of Sox11 in surrounding cells. The loss of early Sox4 expression in the SG of embryos with constitutive Sox11 deletion, but not in embryos with selective neural crest or sympathoadrenal Sox11 deletion, illustrates this. Despite this caveat, available evidence clearly shows that both Sox4 and Sox11 have essential cell-intrinsic tasks in developing sympathetic neurons and their precursors. Otherwise, it cannot be explained how mice in which both Sox4 and Sox11 were selectively deleted in the sympathoadrenal lineage exhibited strong sympathetic nervous system defects and severe dysautonomia as adults. This was not observed in mice with conditional loss of Sox4 only.
In recent years, the transcriptional network has been identified that drives pan-neuronal as well as noradrenergic differentiation of sympathetic neurons (Goridis and Rohrer, 2002; Huber, 2006). In vivo, the bHLH transcription factor Mash1 and the homeodomain protein Phox2b are first induced in sympathoadrenal precursor cells by extracellular signals, such as bone morphogenetic proteins (Schneider et al., 1999), and then turn on a host of additional transcription factors, including Phox2a, Gata3, Hand2 and Insm1, to jointly drive differentiation (Lim et al., 2000; Lucas et al., 2006; Tsarovina et al., 2004; Wildner et al., 2008).
Intriguingly, expression of these transcriptional regulators was not altered in Sox4/Sox11-deficient mice. Late noradrenergic markers such as Th were also expressed in significant amounts. The only detectable differentiation effect was a delay in Th expression in Sox4- or Sox11-deficient precursors that was restricted to defined developmental periods and not observed in embryos deficient for both SoxC proteins. We thus consider it unlikely that SoxC proteins primarily function as maturation factors during sympathetic neuron development. In agreement with this conclusion, overexpression of Sox4 and Sox11 caused only minimal effects on noradrenergic differentiation in the chicken embryo and in cultured chicken DRG progenitor cells.
This contrasts with previous studies on SoxC functions in the CNS. Neural tube electroporations in chicken embryos had implicated SoxC proteins in neuronal precursor maturation (Bergsland et al., 2006). In these experiments, neuronal specification remained unaltered, but specified precursors prematurely expressed pan-neuronal differentiation markers. Our own transgenic overexpression studies of Sox4 in different glial lineages of the CNS had pointed to a role of SoxC proteins in maintenance of the glial precursor state and/or prevention of terminal differentiation (Hoser et al., 2007; Potzner et al., 2007).
Instead of differentiation defects, we found major alterations in cell proliferation and survival in the sympathoadrenal lineage of Sox4/Sox11-deficient mice. These effects occurred at different times of development, with proliferation defects being restricted to earlier times (11.5-12.5 dpc) and increased apoptosis visible only at later times (14.5-16.5 dpc). Taking into account that Sox11 expression was much stronger at early times and that Sox4 expression dominated at later times, it is not surprising that the early requirement for proliferation segregates with Sox11 and the later survival function with Sox4. The different temporal expression profiles clearly present one major factor that prevents a full functional redundancy between Sox4 and Sox11. The increased apoptosis in Sox4-deficient SG at 14.5 dpc may, however, also be taken as evidence that the survival function is specific to Sox4. After all, Sox11 is still expressed in significant amounts at this stage and Sox11-deficient ganglia did not exhibit increased apoptosis.
Most other transcription factors that have been identified as regulators in the sympathoadrenal lineage primarily exert their effect on differentiation rather than proliferation. Early proliferative defects, similar to those found in Sox11-deficient as well as in Sox4 and Sox11 double-deficient SG, have been observed in mice deficient for Insm1 (Wildner et al., 2008), Hand2 (Hendershot et al., 2008; Schmidt et al., 2009) and Mash1 (Morikawa et al., 2009). In Sox4/Sox11-deficient and Insm1-deficient mice, proliferation rates were reduced until 12.5 dpc but had normalized or even increased above controls by 14.5 dpc. This phenotypic similarity might indicate that Insm1 and Sox11 are involved in the same pathway. Taking into account that Insm1 expression is not dramatically altered in SoxC-deficient mice, Insm1 and Sox11 either cooperate or Sox11 might be downstream of Insm1. In contrast to the transient roles of Sox4/Sox11 and Insm1, Hand2 and Mash1 are required for the proliferation of progenitors and immature sympathetic neurons at least up to 14.5 dpc (Hendershot et al., 2008; Morikawa et al., 2009; Schmidt et al., 2009).
Late survival defects in SG have so far primarily been observed in mice with deficiencies in the Ngf-TrkA signaling system (Crowley et al., 1994; Fagan et al., 1996) and in NT3 (Ntf3) (Farinas et al., 1994; Francis et al., 1999; Wyatt et al., 1997). Target-derived Ngf promotes survival via TrkA (Ntrk1) receptors on sympathetic neurons, whereas NT3 is believed to function indirectly and to promote access of growing nerve fibers to target-derived Ngf. Since increased rates of apoptosis in SoxC-deficient mice overlap temporally with those in Ngf-, TrkA- or NT3-deficient mice, it is possible that Sox4 is involved in mediating Ngf-dependent survival defects. Sympathetic neurons, however, rely longer on Ngf for survival than SoxC expression lasts in SG (Glebova and Ginty, 2005), indicating that the relationship might not be as straightforward.
It is intriguing that SoxC function varies between different neural lineages and different developmental stages. Considering the different effects of ectopic SoxC expression in the spinal cord (Bergsland et al., 2006), in cultured DRG progenitors and in neural crest cells in the embryo, SoxC function appears strongly context dependent. It will be interesting to see whether this context-dependent function is predominantly caused by interactions with different partner proteins, as generally postulated for Sox proteins (Kamachi et al., 2000; Wegner, 2005), or whether other mechanisms, such as differential post-translational modifications, also play a role.
Supplementary Material
Acknowledgments
We thank Anna Hartwig for technical assistance; Simone Reiprich for help with statistical analysis; Afsahneh Majdazari and Ernst Tamm for advice; and C. Birchmeier, C. Goridis, F. Guillemot, K. Maschhoff and P. Scotting for the gift of antibodies and plasmids. This work was supported by grants from the DFG to E.S. (So251/3-1) and H.R. (RO2551/1-1) and the NIH to V.L. (R01-AR54153). H.R. is also supported by grants from the Schram-Stiftung and Sander-Stiftung. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.042101/-/DC1
References
- Bergsland M., Werme M., Malewicz M., Perlmann T., Muhr J. (2006). The establishment of neuronal properties is controlled by Sox4 and Sox11. Genes Dev. 20, 3475-3486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles J., Schepers G., Koopman P. (2000). Phylogeny of the SOX family of developmental transcription factors based on sequence and structural indicators. Dev. Biol. 227, 239-255 [DOI] [PubMed] [Google Scholar]
- Britsch S., Goerich D. E., Riethmacher D., Peirano R. I., Rossner M., Nave K. A., Birchmeier C., Wegner M. (2001). The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev. 15, 66-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung M., Abu-Elmagd M., Clevers H., Scotting P. J. (2000). Roles of Sox4 in central nervous system development. Mol. Brain Res. 79, 180-191 [DOI] [PubMed] [Google Scholar]
- Crowley C., Spencer S. D., Nishimura M. C., Chen K. S., Pitts-Meek S., Armanini M. P., Ling L. H., McMahon S. B., Shelton D. L., Levinson A. D., et al. (1994). Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell 76, 1001-1011 [DOI] [PubMed] [Google Scholar]
- Danielian P. S., Muccino D., Rowitch D. H., Michael S. K., McMahon A. P. (1998). Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr. Biol. 8, 1323-1326 [DOI] [PubMed] [Google Scholar]
- Dy P., Penzo-Mendez A., Wang H., Pedraza C. E., Macklin W. B., Lefebvre V. (2008). The three SoxC proteins-Sox4, Sox11 and Sox12-exhibit overlapping expression patterns and molecular properties. Nucleic Acids Res. 36, 3101-3117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernsberger U., Patzke H., Tissier-Seta J. P., Reh T., Goridis C., Rohrer H. (1995). The expression of tyrosine hydroxylase and the transcription factors cPhox-2 and Cash-1: evidence for distinct inductive steps in the differentiation of chick sympathetic precursor cells. Mech. Dev. 52, 125-136 [DOI] [PubMed] [Google Scholar]
- Fagan A. M., Zhang H., Landis S., Smeyne R. J., Silos-Santiago I., Barbacid M. (1996). TrkA, but not TrkC, receptors are essential for survival of sympathetic neurons in vivo. J. Neurosci. 16, 6208-6218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinas I., Jones K. R., Backus C., Wang X. Y., Reichardt L. F. (1994). Severe sensory and sympathetic deficits in mice lacking neurotrophin-3. Nature 369, 658-661 [DOI] [PubMed] [Google Scholar]
- Francis N., Farinas I., Brennan C., Rivas-Plata K., Backus C., Reichardt L., Landis S. (1999). NT-3, like NGF, is required for survival of sympathetic neurons, but not their precursors. Dev. Biol. 210, 411-427 [DOI] [PubMed] [Google Scholar]
- Glebova N. O., Ginty D. D. (2005). Growth and survival signals controlling sympathetic nervous system development. Annu. Rev. Neurosci. 28, 191-222 [DOI] [PubMed] [Google Scholar]
- Goridis C., Rohrer H. (2002). Specification of catecholaminergic and serotonergic neurons. Nat. Rev. Neurosci. 3, 531-541 [DOI] [PubMed] [Google Scholar]
- Guth S. I. E., Wegner M. (2008). Having it both ways: Sox protein function between conservation and innovation. Cell Mol. Life Sci. 65, 3000-3018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V., Hamilton H. L. (1992). A series of normal stages in the development of the chick embryo. 1951. Dev. Dyn. 195, 231-272 [DOI] [PubMed] [Google Scholar]
- Hargrave M., Wright E., Kun J., Emery J., Cooper L., Koopman P. (1997). Expression of the Sox11 gene in mouse embryos suggests roles in neuronal maturation and epithelio-mesenchymal induction. Dev. Dyn. 210, 79-86 [DOI] [PubMed] [Google Scholar]
- Hendershot T. J., Liu H., Clouthier D. E., Shepherd I. T., Coppola E., Studer M., Firulli A. B., Pittman D. L., Howard M. J. (2008). Conditional deletion of Hand2 reveals critical functions in neurogenesis and cell type-specific gene expression for development of neural crest-derived noradrenergic sympathetic ganglion neurons. Dev. Biol. 319, 179-191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoser M., Baader S. L., Bosl M. R., Ihmer A., Wegner M., Sock E. (2007). Prolonged glial expression of Sox4 in the CNS leads to architectural cerebellar defects and ataxia. J. Neurosci. 27, 5495-5505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoser M., Potzner M. R., Koch J. M. C., Bösl M. R., Wegner M., Sock E. (2008). Sox12 deletion in the mouse reveals non-reciprocal redundancy with the related Sox4 and Sox11 transcription factors. Mol. Cell. Biol. 28, 4675-4687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber K. (2006). The sympathoadrenal cell lineage: specification, diversification, and new perspectives. Dev. Biol. 298, 335-343 [DOI] [PubMed] [Google Scholar]
- Kamachi Y., Uchikawa M., Kondoh H. (2000). Pairing SOX off: with partners in the regulation of embryonic development. Trends Genet. 16, 182-187 [DOI] [PubMed] [Google Scholar]
- Kim J., Lo L., Dormand E., Anderson D. J. (2003). SOX10 maintains multipotency and inhibits neuronal differentiation of neural crest stem cells. Neuron 38, 17-31 [DOI] [PubMed] [Google Scholar]
- Kuhlbrodt K., Herbarth B., Sock E., Enderich J., Hermans-Borgmeyer I., Wegner M. (1998). Cooperative function of POU proteins and Sox proteins in glial cells. J. Biol. Chem. 273, 16050-16057 [DOI] [PubMed] [Google Scholar]
- Lim K.-C., Lakshmanan G., Crawford S. E., Gu Y., Grosveld F., Engel J. D. (2000). Gata3 loss leads to embryonic lethality due to noradrenaline deficiency of the sympathetic nervous system. Nature 25, 209-212 [DOI] [PubMed] [Google Scholar]
- Lucas M. E., Muller F., Rudiger R., Henion P. D., Rohrer H. (2006). The bHLH transcription factor hand2 is essential for noradrenergic differentiation of sympathetic neurons. Development 133, 4015-4024 [DOI] [PubMed] [Google Scholar]
- Maka M., Stolt C. C., Wegner M. (2005). Identification of Sox8 as a modifier gene in a mouse model of Hirschsprung disease reveals underlying molecular defect. Dev. Biol. 277, 155-169 [DOI] [PubMed] [Google Scholar]
- Maschhoff K., Anziano P., Ward P., Baldwin H. (2003). Conservation of Sox4 gene structure and expression during chicken embryogenesis. Gene 320, 23-30 [DOI] [PubMed] [Google Scholar]
- Mavropoulos A., Devos N., Biemar F., Zecchin E., Argenton F., Edlund H., Motte P., Martial J., Peers B. (2005). sox4b is a key player of pancreatic alpha cell differentiation in zebrafish. Dev. Biol. 285, 211-223 [DOI] [PubMed] [Google Scholar]
- Morikawa Y., Zehir A., Maska E., Deng C., Schneider M. D., Mishina Y., Cserjesi P. (2009). BMP signaling regulates sympathetic nervous system development through Smad4-dependent and -independent pathways. Development 136, 3575-3584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen-Meyer L. S., Jemtland R., Gautvik V. T., Pedersen M. E., Paro R., Fortunati D., Pierroz D. D., Stadelmann V. A., Reppe S., Reinholt F. P., et al. (2007). Osteopenia, decreased bone formation and impaired osteoblast development in Sox4 heterozygous mice. J. Cell Sci. 120, 2785-2795 [DOI] [PubMed] [Google Scholar]
- Parlato R., Otto C., Begus Y., Stotz S., Schutz G. (2007). Specific ablation of the transcription factor CREB in sympathetic neurons surprisingly protects against developmentally regulated apoptosis. Development 134, 1663-1670 [DOI] [PubMed] [Google Scholar]
- Penzo-Mendez A., Dy P., Pallavi B., Lefebvre V. (2007). Generation of mice harboring a Sox4 conditional null allele. Genesis 45, 776-780 [DOI] [PubMed] [Google Scholar]
- Potzner M., Griffel C., Lütjen-Drecoll E., Bösl M. R., Wegner M., Sock E. (2007). Prolonged Sox4 expression in oligodendrocytes interferes with normal myelination in the central nervous system Mol. Cell. Biol. 27, 5316-5326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiprich S., Stolt C. C., Schreiner S., Parlato R., Wegner M. (2008). SoxE proteins are differentially required in mouse adrenal gland development. Mol. Biol. Cell 19, 1575-1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissmann E., Ernsberger U., Francis-West P. H., Rueger D., Brickell P. M., Rohrer H. (1996). Involvement of bone morphogenetic protein-4 and bone morphogenetic protein-7 in the differentiation of the adrenergic phenotype in developing sympathetic neurons. Development 122, 2079-2088 [DOI] [PubMed] [Google Scholar]
- Rohrer H., Acheson A. L., Thibault J., Thoenen H. (1986). Developmental potential of quail dorsal root ganglion cells analyzed in vitro and in vivo. J. Neurosci. 6, 2616-2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüdiger R., Binder E., Tsarovina K., Schmidt M., Reiff T., Stubbusch J., Rohrer H. (2009). In vivo role for CREB signaling in the noradrenergic differentiation of sympathetic neurons. Mol. Cell. Neurosci. 42, 142-151 [DOI] [PubMed] [Google Scholar]
- Schepers G. E., Taesdale R. D., Koopman P. (2002). Twenty pairs of Sox: extent, homology, and nomenclature of the mouse and human Sox transcription factor families. Dev. Cell 3, 167-170 [DOI] [PubMed] [Google Scholar]
- Schilham M. W., Oosterwegel M. A., Moerer P., Ya J., Deboer P. A. J., Vandewetering M., Verbeek S., Lamers W. H., Kruisbeek A. M., Cumano A., et al. (1996). Defects in cardiac outflow tract formation and pro-B-lymphocyte expansion in mice lacking Sox-4. Nature 380, 711-714 [DOI] [PubMed] [Google Scholar]
- Schmidt M., Lin S., Pape M., Ernsberger U., Stanke M., Kobayashi K., Howard M. J., Rohrer H. (2009). The bHLH transcription factor Hand2 is essential for the maintenance of noradrenergic properties in differentiated sympathetic neurons. Dev. Biol. 329, 191-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C., Wicht H., Enderich J., Wegner M., Rohrer H. (1999). Bone morphogenetic proteins are required in vivo for the generation of sympathetic neurons. Neuron 24, 861-870 [DOI] [PubMed] [Google Scholar]
- Sock E., Rettig S. D., Enderich J., Bösl M. R., Tamm E. R., Wegner M. (2004). Gene targeting reveals a widespread role for the high-mobility-group transcription factor Sox11 in tissue remodeling. Mol. Cell. Biol. 24, 6635-6644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanke M., Junghans D., Geissen M., Goridis C., Ernsberger U., Rohrer H. (1999). The Phox2 homeodomain proteins are sufficient to promote the development of sympathetic neurons. Development 126, 4087-4094 [DOI] [PubMed] [Google Scholar]
- Stolt C. C., Lommes P., Sock E., Chaboissier M.-C., Schedl A., Wegner M. (2003). The Sox9 transcription factor determines glial fate choice in the developing spinal cord. Genes Dev. 17, 1677-1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsarovina K., Pattyn A., Stubbusch J., Muller F., van der Wees J., Schneider C., Brunet J. F., Rohrer H. (2004). Essential role of Gata transcription factors in sympathetic neuron development. Development 131, 4775-4786 [DOI] [PubMed] [Google Scholar]
- Wegner M. (1999). From head to toes: the multiple facets of Sox proteins. Nucleic Acids Res. 27, 1409-1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner M. (2005). Secrets to a healthy Sox life: Lessons for melanocytes. Pigment Cell Res. 18, 74-85 [DOI] [PubMed] [Google Scholar]
- Wildner H., Gierl M. S., Strehle M., Pla P., Birchmeier C. (2008). Insm1 (IA-1) is a crucial component of the transcriptional network that controls differentiation of the sympatho-adrenal lineage. Development 135, 473-481 [DOI] [PubMed] [Google Scholar]
- Wilson M. E., Yang K. Y., Kalousova A., Lau J., Kosaka Y., Lynn F. C., Wang J., Mrejen C., Episkopou V., Clevers H. C., et al. (2005). The HMG box transcription factor Sox4 contributes to the development of the endocrine pancreas. Diabetes 54, 3402-3409 [DOI] [PubMed] [Google Scholar]
- Wurm A., Sock E., Fuchshofer R., Wegner M., Tamm E. R. (2008). Anterior segment dysgenesis in the eyes of mice deficient for the high-mobility-group transcription factor Sox11. Exp. Eye Res. 86, 895-907 [DOI] [PubMed] [Google Scholar]
- Wyatt S., Pinon L. G., Ernfors P., Davies A. M. (1997). Sympathetic neuron survival and TrkA expression in NT3-deficient mouse embryos. EMBO J. 16, 3115-3123 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.