Abstract
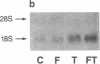
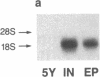
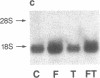
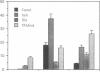
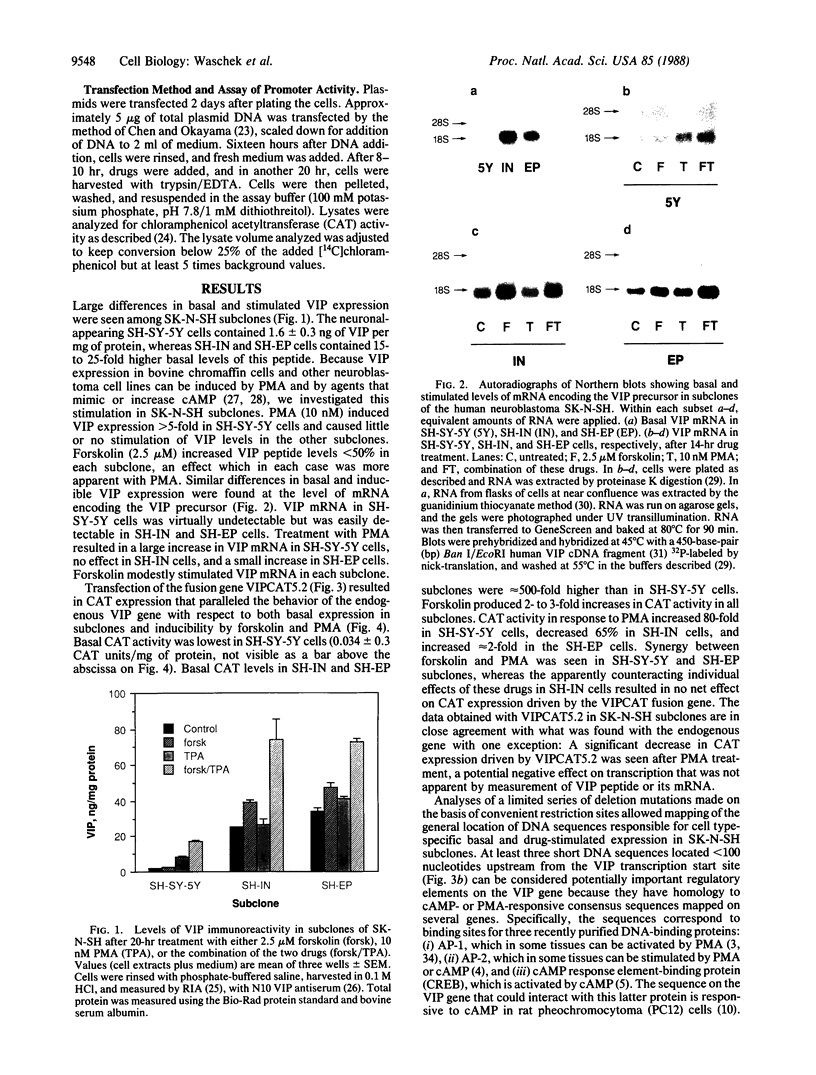
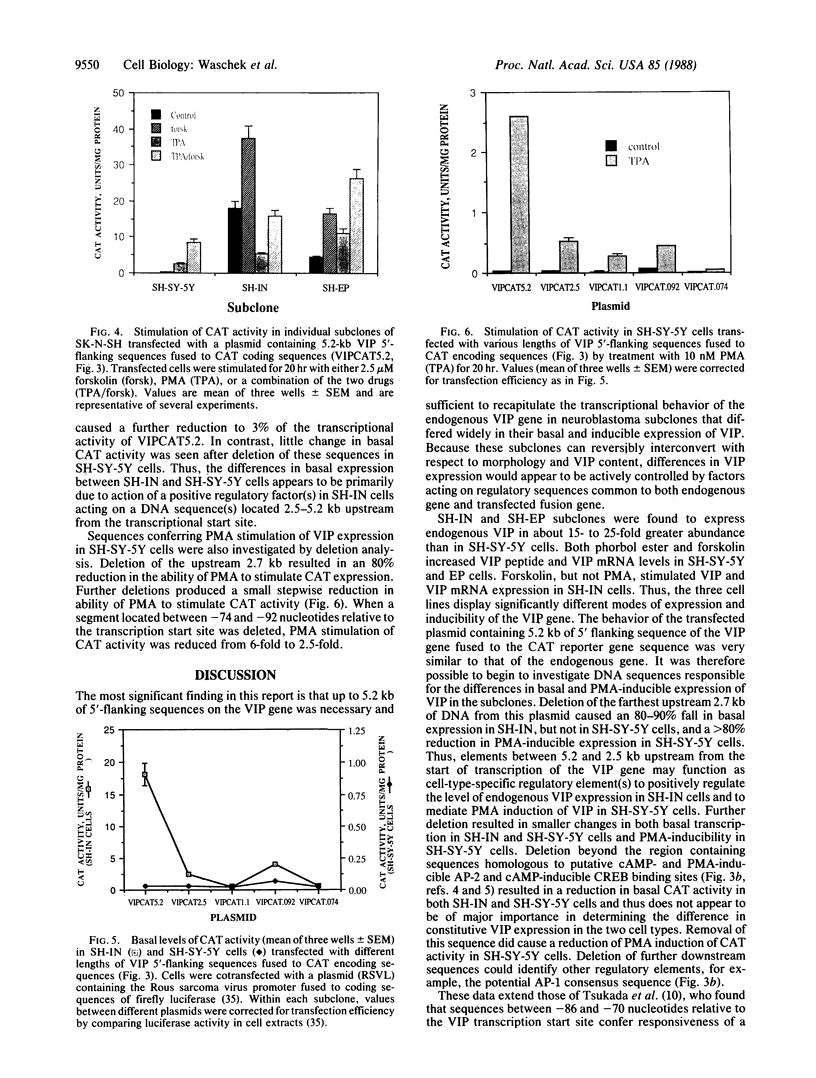
The expression of a transfected plasmid containing 5.2 kilobases (kb) of 5' regulatory DNA sequence of the human vasoactive intestinal peptide (VIP) gene attached to coding sequences of the reporter gene chloramphenicol acetyltransferase (CAT) was compared with endogenous VIP expression in subclones of the human neuroblastoma cell line SK-N-SH. These subclones vary widely in basal and inducible quantities of VIP and its precursor mRNA and can be interconverted under specified culture conditions. Endogenous VIP immunoreactivity, detectable in all subclones, was lowest in the neuronal subclone SH-SY-5Y, whereas 15- to 25-fold higher levels were observed in the epithelial-appearing SH-EP and intermediate SH-IN subclones. Treatment with 10 nM phorbol 12-myristate 13-acetate (PMA) stimulated VIP peptide levels approximately 5-fold in SH-SY-5Y cells but did not increase appreciably VIP levels in the other subclones. Treatment with 2.5 microM forskolin resulted in less than 50% stimulation of VIP expression in all subclones. Levels of mRNA encoding the VIP precursor generally paralleled these differences in VIP immunoreactivity. In cells transfected with the VIP/CAT fusion gene, CAT activity reflected closely these differences in basal VIP expression and the changes in response to PMA and forskolin. Deletion of 2.7 kb of the most upstream sequences resulted in an 80-90% reduction in basal CAT activity in SH-IN, but not SH-SY-5Y cells, and resulted in an 80% reduction in PMA stimulation in SH-SY-5Y cells. Deletion to within 74 nucleotides of the transcription start site resulted in CAT expression in SH-IN cells that was only 3% of that seen with the full 5.2-kb flanking sequences and further diminished the remaining PMA responsiveness in SH-SY-5Y cells. The data indicate that important cell-type-specific transcription regulatory sequences reside greater than 2.5 kb upstream from the VIP transcription start site.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Bloom S. R., Christofides N. D., Delamarter J., Buell G., Kawashima E., Polak J. M. Diarrhoea in vipoma patients associated with cosecretion of a second active peptide (peptide histidine isoleucine) explained by single coding gene. Lancet. 1983 Nov 19;2(8360):1163–1165. doi: 10.1016/s0140-6736(83)91215-1. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Choi O. R., Engel J. D. A 3' enhancer is required for temporal and tissue-specific transcriptional activation of the chicken adult beta-globin gene. Nature. 1986 Oct 23;323(6090):731–734. doi: 10.1038/323731a0. [DOI] [PubMed] [Google Scholar]
- Delegeane A. M., Ferland L. H., Mellon P. L. Tissue-specific enhancer of the human glycoprotein hormone alpha-subunit gene: dependence on cyclic AMP-inducible elements. Mol Cell Biol. 1987 Nov;7(11):3994–4002. doi: 10.1128/mcb.7.11.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden L. E., Eskay R. L., Scott J., Pollard H., Hotchkiss A. J. Primary cultures of bovine chromaffin cells synthesize and secrete vasoactive intestinal polypeptide (VIP). Life Sci. 1983 Aug 22;33(8):687–693. doi: 10.1016/0024-3205(83)90772-5. [DOI] [PubMed] [Google Scholar]
- Eiden L. E., Giraud P., Affolter H. U., Herbert E., Hotchkiss A. J. Alternative modes of enkephalin biosynthesis regulation by reserpine and cyclic AMP in cultured chromaffin cells. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3949–3953. doi: 10.1073/pnas.81.13.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden L. E., Nilaver G., Palkovits M. Distribution of vasoactive intestinal polypeptide (VIP) in the rat brain stem nuclei. Brain Res. 1982 Jan 14;231(2):472–477. doi: 10.1016/0006-8993(82)90386-9. [DOI] [PubMed] [Google Scholar]
- Gilman M. Z., Wilson R. N., Weinberg R. A. Multiple protein-binding sites in the 5'-flanking region regulate c-fos expression. Mol Cell Biol. 1986 Dec;6(12):4305–4316. doi: 10.1128/mcb.6.12.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imagawa M., Chiu R., Karin M. Transcription factor AP-2 mediates induction by two different signal-transduction pathways: protein kinase C and cAMP. Cell. 1987 Oct 23;51(2):251–260. doi: 10.1016/0092-8674(87)90152-8. [DOI] [PubMed] [Google Scholar]
- Itoh N., Obata K., Yanaihara N., Okamoto H. Human preprovasoactive intestinal polypeptide contains a novel PHI-27-like peptide, PHM-27. Nature. 1983 Aug 11;304(5926):547–549. doi: 10.1038/304547a0. [DOI] [PubMed] [Google Scholar]
- Jeannotte L., Trifiro M. A., Plante R. K., Chamberland M., Drouin J. Tissue-specific activity of the pro-opiomelanocortin gene promoter. Mol Cell Biol. 1987 Nov;7(11):4058–4064. doi: 10.1128/mcb.7.11.4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen P. R., Harney J. W., Moore D. D. Sequences required for cell-type specific thyroid hormone regulation of rat growth hormone promoter activity. J Biol Chem. 1986 Nov 5;261(31):14373–14376. [PubMed] [Google Scholar]
- Lawn R. M., Fritsch E. F., Parker R. C., Blake G., Maniatis T. The isolation and characterization of linked delta- and beta-globin genes from a cloned library of human DNA. Cell. 1978 Dec;15(4):1157–1174. doi: 10.1016/0092-8674(78)90043-0. [DOI] [PubMed] [Google Scholar]
- Lee W., Haslinger A., Karin M., Tjian R. Activation of transcription by two factors that bind promoter and enhancer sequences of the human metallothionein gene and SV40. Nature. 1987 Jan 22;325(6102):368–372. doi: 10.1038/325368a0. [DOI] [PubMed] [Google Scholar]
- Lee W., Mitchell P., Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987 Jun 19;49(6):741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- Linder S., Barkhem T., Norberg A., Persson H., Schalling M., Hökfelt T., Magnusson G. Structure and expression of the gene encoding the vasoactive intestinal peptide precursor. Proc Natl Acad Sci U S A. 1987 Jan;84(2):605–609. doi: 10.1073/pnas.84.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Montminy M. R., Bilezikjian L. M. Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature. 1987 Jul 9;328(6126):175–178. doi: 10.1038/328175a0. [DOI] [PubMed] [Google Scholar]
- Muglia L., Rothman-Denes L. B. Cell type-specific negative regulatory element in the control region of the rat alpha-fetoprotein gene. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7653–7657. doi: 10.1073/pnas.83.20.7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C., Albert V. R., Elsholtz H. P., Lu L. I., Rosenfeld M. G. Activation of cell-specific expression of rat growth hormone and prolactin genes by a common transcription factor. Science. 1988 Mar 18;239(4846):1400–1405. doi: 10.1126/science.2831625. [DOI] [PubMed] [Google Scholar]
- Ohsawa K., Hayakawa Y., Nishizawa M., Yamagami T., Yamamoto H., Yanaihara N., Okamoto H. Synergistic stimulation of VIP/PHM-27 gene expression by cyclic AMP and phorbol esters in human neuroblastoma cells. Biochem Biophys Res Commun. 1985 Nov 15;132(3):885–891. doi: 10.1016/0006-291x(85)91890-x. [DOI] [PubMed] [Google Scholar]
- Pruss R. M., Moskal J. R., Eiden L. E., Beinfeld M. C. Specific regulation of vasoactive intestinal polypeptide biosynthesis by phorbol ester in bovine chromaffin cells. Endocrinology. 1985 Sep;117(3):1020–1026. doi: 10.1210/endo-117-3-1020. [DOI] [PubMed] [Google Scholar]
- Rettig W. J., Spengler B. A., Chesa P. G., Old L. J., Biedler J. L. Coordinate changes in neuronal phenotype and surface antigen expression in human neuroblastoma cell variants. Cancer Res. 1987 Mar 1;47(5):1383–1389. [PubMed] [Google Scholar]
- Ross R. A., Spengler B. A., Biedler J. L. Coordinate morphological and biochemical interconversion of human neuroblastoma cells. J Natl Cancer Inst. 1983 Oct;71(4):741–747. [PubMed] [Google Scholar]
- Sastry K. N., Seedorf U., Karathanasis S. K. Different cis-acting DNA elements control expression of the human apolipoprotein AI gene in different cell types. Mol Cell Biol. 1988 Feb;8(2):605–614. doi: 10.1128/mcb.8.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986 Aug 29;46(5):705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- Tsukada T., Fink J. S., Mandel G., Goodman R. H. Identification of a region in the human vasoactive intestinal polypeptide gene responsible for regulation by cyclic AMP. J Biol Chem. 1987 Jun 25;262(18):8743–8747. [PubMed] [Google Scholar]
- Tsukada T., Horovitch S. J., Montminy M. R., Mandel G., Goodman R. H. Structure of the human vasoactive intestinal polypeptide gene. DNA. 1985 Aug;4(4):293–300. doi: 10.1089/dna.1985.4.293. [DOI] [PubMed] [Google Scholar]
- Wu L. C., Morley B. J., Campbell R. D. Cell-specific expression of the human complement protein factor B gene: evidence for the role of two distinct 5'-flanking elements. Cell. 1987 Jan 30;48(2):331–342. doi: 10.1016/0092-8674(87)90436-3. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]