Abstract
Objective: Excisional surgery is the mainstay of treatment of Dupuytren's disease. Although outcomes are generally good, complications are common. The objective of this study was to evaluate intraoperative and postoperative complications associated with fasciectomy for Dupuytren's disease. Methods: A literature search was conducted to identify published, original research that reported surgical complications associated with fasciectomy from 1988 to 2008. Search results were manually evaluated for relevance. Complication rates according to types of disease (primary or recurrent disease) and according to time (intraoperative vs postoperative) and type were collated. Results: A total of 143 articles were identified; 41 met inclusion criteria, and of these, 28 reported overall surgical complication rates ranging from 3.6% to 39.1%. Major complications occurred in 15.7%, including digital nerve injury 3.4%, digital artery injury 2%, infection 2.4%, hematoma 2.1%, and complex regional pain syndrome 5.5%. Other common, more minor injuries included flare reaction in 9.9%, wound healing complications in 22.9%, and a range of other complications. In the few (n = 3) studies in which primary and recurrent diseases were directly compared, digital nerve injuries and digital artery injuries were approximately 10 times more common in patients with recurrent disease (˜20%) than those with primary disease (˜2%), though the numbers are too small for statistical significance. Conclusions: A review of published reports by surgeons shows that surgical fasciectomy for Dupuytren's disease has a high number of complications. Surgeons should be mindful of the potential for intraoperative and postoperative complications and counsel their patients accordingly.
Dupuytren's disease was originally noted by Plater in 16141 and carries the eponym of Baron Guillaume Dupuytren, who first lectured on the disease in 1831.2 Although Cline in 1777 and Cooper in 1822 had described the fascial contracture and its treatment by fasciotomy, they were not mentioned in Dupuytren's discussions.1 Dupuytren's disease is a genetic disorder of abnormal collagen production and deposition in the hand that is commonly characterized by metacarpophalangeal (MP) and proximal interphalangeal (PIP) joint contractures in the ring and little fingers. Dupuytren's disease can affect all races, but people of northern European descent are most commonly affected,3-5 with 3% to 6% of white adults acquiring the condition during their lifetime.3,6 Dupuytren's diathesis, which manifests as a more aggressive form of the disease, comprises a positive family history with 1 or more affected siblings or parents, male gender, age less than 50 years at onset, bilateral involvement, ectopic manifestations (particularly Garrod's pads), and Caucasian ethnicity.7 Furthermore, evidence indicates that Dupuytren's disease is more likely to occur in those with certain underlying conditions such as diabetes,8 thyroid disorders,9 alcoholism,10 and epilepsy.3 Lower incidences of Dupuytren's occur in those afflicted by rheumatoid arthritis.11
Genetic analyses show that Dupuytren's disease is an autosomal dominant disorder with variable penetrance and gene expression.12 Genetic predisposition, combined with diatheses, lifestyle choices, (eg, alcohol consumption), or trauma,13,14 can trigger micro ruptures of the collagen fibers of the palmar fascia, fibroblast proliferation, and differentiation of fibroblasts into myofibroblasts.15,16 The expanding fibroblast pool and excess collagen deposition cause nodule and cord formation in the palm or digits.
Dupuytren's disease is progressive, with onset typically occurring later in life and worsening over the course of several months to several years.17 In early stages, skin pitting and dimpling are commonly observed as pretendinous bands connected to the dermis begin to contract.18 Initially, nodules are painless and hand function is generally retained. However, as the disease progresses, cords begin to contract, causing finger flexion deformities and diminished hand function.18 The contractile properties of myofibroblasts are thought to cause the cords to shorten,15 resulting in the hallmark contractures that characterize Dupuytren's disease.
Few treatment options exist for those with Dupuytren's contracture. Surgery is currently the mainstay of treatment and is recommended for functionally impaired patients with MP joint contractures of more than 30°.18-223 Indications for the treatment of PIP joint contracture varies. Some authors recommend surgery for any degree of PIP contracture.20,22 Others feel that there should be approximately 15° (references 18, 24) or 30° (reference 25) of PIP contracture to warrant surgery. In contrast to these established guidelines, McGrouther asserts that it is better to “rely on functional difficulty and the rate of progression when deciding on surgery, rather than choosing a set amount of joint contracture.”26(p167)
Open, limited (subtotal) fasciectomy is the most commonly used surgical procedure,10,27-30 but open or closed fasciotomies, including percutaneous needle fasciotomy (ie, needle aponeurotomy), are also performed.31-35 Although surgery provides positive outcomes for most patients, extensive hand therapy is typically required after surgery. Not all patients with Dupuytren's contracture are candidates for surgery; advanced age, comorbidities, or both, often exclude patients from undergoing fasciectomy. In this circumstance, closed fasciotomy26,36 or needle aponeurtomy35 is often recommended. To date, no effective pharmacotherapy has been approved for the treatment of Dupuytren's disease,37 though an investigational procedure with Clostridium histolyticum collagenase (enzymatic fasciotomy) shows promise.38
Dupuytren's disease is not curable because it is a genetic disease and has a cellular basis. Surgeons can help improve hand impairment due to Dupuytren's disease by surgical techniques. These corrective surgical procedures improve hand function for most patients; however, intraoperative and postoperative complications are common. Recurrent disease is possible after all types of treatments, including fasciectomies.
Surgeons performing fasciectomies need to discuss potential complications and recurrence with their patients and set realistic expectations for efficacy and safety. Unfortunately, no concise source of estimated surgical complication rates exists. The purpose of this review is to provide a single resource of intraoperative and postoperative complications associated with fasciectomy for Dupuytren's disease.
METHODS
Identification of studies
Analysis of surgical complications was limited to those associated with fasciectomy and aponeuroectomy. To identify published, original research that reported surgical complications associated with surgery for Dupuytren's disease, a MEDLINE search was conducted with the following search parameters: fasciectomy[Title/Abstract] OR aponeurectomy[Title/Abstract] OR surgery[Title/Abstract] OR operate*[Title/Abstract] AND Dupuytren*[Title/Abstract] NOT review[Publication Type]. Search limitations included human subjects, English language, and dates of October 31, 1988, to October 31, 2008.
Study selection
Search results were manually evaluated for relevance. Studies that did not report complication rates associated with fasciectomy or aponeurectomy were not included in the analysis. Studies that reported complication rates associated with fasciotomy, aponeurotomy, amputation, or postsurgical application of the S-Quattro external fixation device were excluded. Case studies were also excluded.
Data analysis
Overall complication rates, complication rates according to types of disease (primary or recurrent disease), and complication rates according to time (intraoperative vs postoperative) and type were collated. Studies that did not specifically state whether patients had primary disease or recurrent disease were assumed to have had primary disease.
Averages and ranges were calculated for each complication described. The manner in which complications were reported varied from study to study (ie, by ray/finger; by hand; by patient); conversion of all surgical complication rates to a common denominator was not possible. Average rates were calculated and ranges were reported for each surgical complication across studies; the sum of all numerators was divided by the sum of all denominators and multiplied by 100.
RESULTS
Study attributes
A total of 143 articles were identified. One hundred two articles were excluded from the analysis (pathology, n = 16; treatment techniques, n = 17; postoperative care, n = 9; case studies, n = 13; long-term follow-up, n = 8; risk factors, n = 10; non-Dupuytren's disease, n = 12; surgery other than fasciectomy, n = 3; and other, n = 14). The remaining 41 articles met the inclusion criteria, reported complications associated with surgery for Dupuytren's disease, and were deemed appropriate for analysis: 27 evaluated primary (or otherwise not specified) disease, 2 evaluated recurrent disease, and 12 evaluated mixed populations (primary or recurrent disease) (Table 1).4,10,27,30,32,39-74 Of the 41 studies, 28 studies reported overall surgical complication rates ranging from 3.6% to 39.1%.
Table 1.
Studies included in the analysis*
| No. | Authors (year) | Study design | No. of patients | No. of hands | No. of joints | Joint type | Follow-up period | Surgical techniques | Disease category |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Srivastava et al (1989)39 | Retrospective | 10 | 12 | NR | MP, PIP | 1–10 y | Fasciectomy, limited or radical; Z-plasty closure or open-palm technique; amputation for advanced disease | Mixed: Primary, 70%; recurrent, 30% |
| 2 | Sennwald (1990)27 | Retrospective | 98 | 103 | NR; 239 rays | NR | 3–6 mo | Fasciectomy, radical; rotation flap or Z-plasty | Mixed: Primary, 74.8%; recurrent, 25.2% |
| 3 | Moermans (1991)40 | Prospective | 175 | 213 | 503 | MP, PIP | Mean, 2.6 ± 1.6 y; range, 0–7 y | Aponeurectomy, segmental | Mixed: Primary, 83.1%; recurrent, 16.9% |
| 4 | Foucher et al (1992)41 | Retrospective | 107 | NR | NR; 140 digits | MP, PIP | >5 y | Fasciectomy, limited; open-palm technique and/or digit | Mixed: Primary, 95%; recurrent, 5% |
| 5 | Searle and Logan (1992)42 | Retrospective | 32 | NR | NR; 40 rays | NR | Mean, 38 mo; ≥24 mo | Dermofasciectomy | Mixed: Primary, 53%; recurrent, 47% |
| 6 | Beyermann et al (2004)43 | Prospective | 43 | 43 | 43 | PIP | 24 wk | Fasciectomy (n = 32), with CLM release (n = 11) | Mixed: Primary, 67.4%; recurrent, 32.6% |
| 7 | Meathrel and Thoma (2004)44 | Retrospective | 149 | NR | NR; 261 digits | NR | NR | Fasciectomy, palmar | Mixed: Primary, 87.2%; recurrent, 12.8% |
| 8 | Kobus et al (2007)45 | Retrospective | 253 | 287 | NR | MP, PIP | Mean, 3 y | Fasciectomy, radical, with V-Y–plasty | Mixed: Primary, 86.2%; recurrent, 13.8% |
| 9 | Loos et al (2007)4 | Retrospective | 2919 | 4388 | NR | MP, PIP, DIP | NR; data span 50-y period | Fasciectomy, limited (94.8% of procedures) or total; amputation | Mixed: Primary, 88%; recurrent, 12%; data not complete |
| 10 | Bulstrode et al (2005)46 | Retrospective | 253 | NR | NR | NR | Mean, 3.6 y; range, 9 mo–11 y | Fasciectomy, modified Skoog's technique | Mixed: Primary, 75.5% (191/253); recurrent, 24.5% (62/253) |
| 11 | Ebskov et al (1997)64 | Prospective | 76 | NR | NR; mean rays involved: primary, 2.1; recurrent, 1.8 | MP, PIP | 3 wk | Fasciectomy, radical, open-palm technique | Mixed: Primary, 68.4%; recurrent, 31.6% |
| 12 | Denkler (2005)30 | Retrospective | Hospital, 26; office, 40 | NR | Hospital, 73; office, 93; digits: hospital, 42; office, 60 | MP, PIP | Hospital: mean, 10.6 ± 21.9 mo; median, 3 mo; office: 9.3 ± 9.5 mo; median, 4 mo | Fasciectomy, hospital group (traditional anesthetics with tourniquet; 43 digits) vs office group (local anesthetics with epinephrine and no tourniquet; 60 digits) | Mixed: Hospital: primary, 88.5% (23/26); 11.5% (3/26); office: primary, 95.0% (38/40); 5.0% (2/40) |
| 13 | Andrew and Kay (1991)47 | Prospective | 46 | 50 | 79 | MP, PIP | 12 mo | Aponeurectomy, segmental | Primary, 100% |
| 14 | Liu and Chen (1991)48 | Retrospective | 27 | 32 | NR; 58 digits | NR | Mean, 5.3 y; range, 1–16 y | Fasciectomy with longitudinal, lazy-s, zigzag, or transverse incision | Primary, 100% |
| 15 | Robins et al (1993)49 | Prospective | 50 | 50 | NR | NR | NR | Fasciectomy, local; usually with zigzag incision | Primary, 100% |
| 16 | Cools and Verstreken (1994)50 | Retrospective | 28 | 33 | NR | MP, PIP | Mean, 2.5 y | Fasciectomy, partial; open-palm technique | Primary, 100% |
| 17 | Citron and Nunez (2005)51 | Prospective | 79 | 79 | NR | MP, PIP | ≥2 y | Fasciectomy, modified Bruner incision (n = 47) vs longitudinal incision with Z-plasty closure (n = 33) | Primary, 100% |
| 18 | Van Giffen et al (2006)52 | Retrospective | 38 | 38 | 63 (fifth ray only) | MP, PIP | Mean, 54 mo; range, 27–75 mo | Fasciectomy, isolated limited or segmental; dermofasciectomy | Primary, 100% |
| 19 | van Rijssen et al (2006)32 | Prospective | 113 | 117 | 127† | MP, PIP, DIP | 6 wk | Limited fasciectomy | Primary, 100% |
| 20 | Skoff (2004)53 | Prospective | 30 | NR | NR | MP, PIP | Synthesis: mean, 2.7 y; range, 2.0–3.0 y; open-palm technique: mean, 3.5 y; range, 3.1–4.0 y | Fasciectomy, “synthesis” technique (n = 20) vs open-palm technique (n = 10) | Primary, 100% |
| 21 | Ritchie et al (2004)54 | Prospective | 14 | 19 | 19 | PIP | Mean, 36 mo; range, 35–39 mo | Fasciectomy (8 little fingers), with CLM release (11 little fingers) | Primary, 100% |
| 22 | Misra et al (2007)55 | Prospective | 35 | NR | 52 | MP, PIP | Mean, 1.5 y; range, 1–3 y | Fasciectomy with Z-plasty (19 joints) ± PIP joint release (33 joints) | Primary, 100% |
| 23 | Sorene et al (2007)56 | Retrospective | 19 | 22 | 44 | IP, MP, PIP, DIP | Mean, 30 mo; range, 12–118 mo | Fasciectomy, selective, through modified Bruner palmodigital incisions | Primary, 100% |
| 24 | Stahl and Calif (2008)57 | Retrospective | 23 | 26 | NR | MP, PIP, DIP | Mean, 2.5 y; range, 1.5–19 y | Fasciectomy, limited, through zigzag digitopalmar incision ± CLM release of PIP joint | NR |
| 25 | Vigroux and Valentin (1992)58 | Retrospective | 56 | 76 | NR; 137 digits | MP, PIP | Mean, 12 y, 7 mo; range, 10–22 y | Fasciectomy, regional ± PIP capsulectomy | NR |
| 26 | Foucher et al (1995)59 | Retrospective | 54 | NR | NR; 67 digits | MP, PIP | Mean, 6.6 y; ≥5 y | Fasciectomy, open-palm technique | NR |
| 27 | De Maglio et al (1996)60 | Retrospective | 124 | 145 | NR | MP, PIP | Mean, 33 mo; range, 6–59 mo | Aponeurectomy, selective; Skoog's and/or Dieckman/Iselin routes of access | NR |
| 28 | Shaw et al (1996)61 | Retrospective | 25 | 26 | NR; 39 digits | MP, PIP | 9–19 y | Fasciectomy, palmar; open-palm technique | NR |
| 29 | Weinzweig et al (1996)62 | Retrospective | 28 | 42 | 42 | PIP | Mean, F, 10.1 mo; F + C, 6.4 mo | Fasciectomy (18 patients, 27 joints); F + C; 10 patients, 15 joints) | NR |
| 30 | Citron and Messina (1998)63 | Retrospective | 13 | NR | NR; 18 digits | PIP | Mean, 18 mo; range, 2–30 mo | Preoperative traction + fasciectomy ± fasciotomy | NR |
| 31 | Gonzalez et al (1998)74 | Retrospective | 16 | 19 | 40 | IP, MP, PIP | Mean, 25 mo; range, 6–168 mo | Fasciectomy, selective, with Z-plasty; fasciectomy, segmental, with multiple curvilinear incisions or Z-plasty | NR |
| 32 | Clibbon and Logan (2001)65 | Retrospective | 56 | 67 | 67 | MP | Mean, 30 mo; range, 12–86 mo | Aponeurectomy, segmental (palmar) | NR |
| 33 | Evans et al (2002)66 | Retrospective (1983–1993; TA only); prospective (1993–1999, TA and NTA) | 268 | NR | NR; mean number of digits undergoing surgery: 1.96 (TA); 1.6 (NTA)‡ | MP, PIP | NR | Fasciectomy, with TA (n = 103) or NTA (n = 165) | NR |
| 34 | Barr et al (2003)67 | Retrospective | 5 | 5 | 14 | MP, PIP | Mean, 14 mo; range, 3–34 mo | Fasciectomy, with Z-plasty + intramuscular tenotomy of flexor digitorum superficialis in distal forearm | NR |
| 35 | Abe et al (2004)68 | Retrospective | 57 | 73 | 146 | IP, MP, PIP | Mean, 4 y; range, 2–17 y | Fasciectomy, subtotal | NR |
| 36 | Ali et al (2006)69 | Retrospective | 32 | 35 | NR | NR | Mean, 6 mo; range, 2–13 | Fasciectomy, selective regional; ulnar-based skin flap | NR |
| 37 | Coert et al (2006)10 | Retrospective | 261 (558 operations) | 556 | MP, PIP, DIP | Mean, 7.3±0.44 y; range, 0.3–48 y | Fasciectomy, partial | NR; average number of operations was 2.54 per patient over 8-y study period | |
| 38 | Reuben et al (2006)70 | Prospective | 300 | NR | NR | NR | 1, 3, 12 mo postsurgery | Fasciectomy, with general anesthesia, axillary block, or intravenous regional anesthesia with lidocaine ± clonidine | NR |
| 39 | Anwar et al (2007)71 | Retrospective | 657; 109 women, 548 men | 119 women, 589 men | 123 women, 760 men | MP, PIP, DIP | NR | Fasciectomy, fasciectomy + local flap, dermofasciectomy | NR |
| 40 | Ekerot (1995)72 | Retrospective | 15 | 16 | NR; 17 flaps | MP, PIP | ≤2 y | Fasciectomy, radical, with distally based dorsal hand flap; PIP joint capsulectomy in 4 fingers | Recurrent, 100% |
| 41 | Roush and Stern (2000)73 | Retrospective | 19 | NR | NR; 28 digits | MP, PIP, DIP | Median, 4 y; range, 1–15 y | Fasciectomy, limited, and IP arthrodesis; dermofasciectomy; fasciectomy and local flaps | Recurrent, 100% |
*NR indicates not reported; TA, tension applied; NTA, no tension applied; MP, metacarpophalangeal; PIP, proximal interphalangeal; DIP; distal interphalangeal; IP, interphalangeal; fasciectomy + capsulotomy; capsuloligamentous.
†An additional 150 joints were treated with percutaneous needle fasciotomy but were excluded from this analysis.
‡An additional 150 finger joints were treated with percutaneous needle fasciotomy but were excluded from this study since this study is discussingcomplications of surgical fasciectomy (excsion) for Dupuytren's.
Complications in patients with primary disease
Of the 27 studies that evaluated patients with primary disease,10,32,47-63,65-71,74 16 studies reported intraoperative complications. These complications included digital nerve injury (3.4%; range, 0.0%–7.7%) and digital artery injury (2.0%; range, 0.0%–2.6%) (Table 2).*
Table 2.
Reported complications* of surgery for primary Dupuytren's disease
| Complication | No. of studies reporting complications | Average, % (n/N) | Range, % |
|---|---|---|---|
| Intraoperative | |||
| Digital artery injury10,52,54,71 | 4 | 2.0 (20/989) | 0–2.6 |
| Digital nerve injury† | 15 | 3.4 (51/1510) | 0–7.7 |
| Postoperative | |||
| Amputation (classified as postoperative complication)10 | 1 | 1.5 (4/261) | … |
| Carpal tunnel syndrome56,62 | 2 | 6.4 (3/47) | 3.6–10.5 |
| Clinodactyly50 | 1 | 3.0 (1/33) | … |
| Complex regional pain syndrome (see “reflex sympathetic dystrophy”) | … | … | |
| Contracture48,63 | 2 | 6.7 (3/45) | 6.2–7.7 |
| Dysesthesia or paresthesia32,59 | 2 | 13.5 (15/111) | 3.7–22.8 |
| Edema62 | 1 | 7.1 (2/28) | … |
| Flare reaction66,71 | 2 | 9.9 (92/925) | 2.1–51.5 |
| Flexion, loss of47,49 | 2 | 4.2 (4/96) | 4.0–4.3 |
| Hematoma32,48-50,55,57,59,68,70 | 9 | 2.1 (14/657) | 0–13.0 |
| Hyperesthesia50 | 1 | 3.0 (1/33) | … |
| Hypoesthesia50,52,62 | 3 | 10.1 (10/99) | 6.0–17.9 |
| Incisional scar pain57 | 1 | 17.4 (4/23) | … |
| Infection‡ | 19 | 2.4 (44/1860) | 0–8.6 |
| Necrosis (skin, flap, or graft)10,49,50,52,53,59,60,62,68,69 | 10 | 4.3 (31/713) | 0–10 |
| Pain (not otherwise specified)50,59 | 2 | 13.8 (12/87) | 3–20.3 |
| Reflex sympathetic dystrophy (complex regional pain syndrome)10,49-53,57-63,65,70,71 | 16 | 5.8 (106/1828) | 0–69.2 |
| Stiffness62 | 1 | 3.6 (1/28) | … |
| Swan neck deformity54 | 1 | 7.1 (1/14) | … |
| Tenosynovitis50 | 1 | 3.0 (1/33) | … |
| “Trigger finger”56 | 1 | 5.3 (1/19) | … |
| Wound-healing complication32,47,49,58,60,62,66,67∥ | 8 | 22.9 (145/634) | 0–86.0. |
All 27 primary-disease studies reported postoperative complications,10,32,47-63,65-71,74 the most common being wound-healing complications (22.9%; range, 0.0%–86.0%), incisional scar pain (17.4%), dysesthesia/paresthesia (13.5%), hypoesthesia (10.1%; range, 6.0%–17.9%), flare reaction (9.9%; range, 2.1%–51.5%), reflex sympathetic dystrophy (5.8%; range, 0%–69.2%), infection (2.4%; range, 0–8.6%), and hematoma (2.1%; range, 0%–13%).
Complications in patients with recurrent disease
Only 2 studies examined patients with recurrent disease exclusively. One study did not report intraoperative complications; the other evaluated intraoperative complications and reported no digital artery injuries (Table 3).72,73 Both studies reported postoperative complications: hyperesthesia (20.0%), local cold intolerance (20.0%), hypoesthesia (15.8%), and necrosis (11.1%). No cases of bleeding, infection, graft failure, or reflex sympathetic dystrophy were observed.
Table 3.
Reported complications* of surgery for recurrent Dupuytren's disease
| Complication | No. of studies reporting complication | Average, % (n/N) |
|---|---|---|
| Intraoperative | ||
| Digital artery injury73 (anesthetic) | 1 | 0 (0/19) |
| Postoperative | ||
| Bleeding72 | 1 | 0 (0/17) |
| Graft failure73 | 1 | 0 (0/19) |
| Hyperesthesia72 | 1 | 20.0 (3/15) |
| Hypoesthesia73; poor to fair numbness noted postoperatively | 1 | 15.8 (3/19) |
| Infection72,73 | 2 | 0 (0/36) |
| Necrosis (skin, flap, or graft)72,73 | 2 | 11.1 (4/36) |
| Reflex sympathetic dystrophy (complex regional pain syndrome)73 | 1 | 0 (0/19) |
| Local cold intolerance72 | 1 | 20.0 (3/15) |
*Studies that reported no cases of a particular complication were included in calculations.
Complications in mixed populations (primary and recurrent diseases combined)
Seven studies reported intraoperative complications in a mixed population (ie, primary and recurrent disease populations combined). The overall intraoperative complications in these studies were digital nerve injury (3.6%; range, 0.6%–7.8%), digital artery injury (3.3%; range, 0.8–9.7%), and tendon injury (0.02%) (Table 4).4,27,30,40,44,46,64
Table 4.
Reported complications* of surgery for primary and recurrent Dupuytren's diseases (mixed populations)
| Complication | No. of studies reporting complication | Average, % (n/N) | Range, % |
|---|---|---|---|
| Intraoperative | |||
| Digital artery injury27,30,46 | 3 | 3.3 (14/422) | 0.8–9.7 |
| Digital nerve injury4,27,30,40,44,46,64 | 7 | 3.6 (135/3779) | 0.6–7.8 |
| Tendon injury4 | 1 | 0.2 (5/2919) | … |
| Postoperative | |||
| Bleeding4 | 1 | 1.2 (35/2919) | … |
| Complex regional pain syndrome (see “reflex sympathetic dystrophy”) | |||
| Carpal tunnel syndrome46 | 1 | 0.8 (2/253) | … |
| Severe dysesthesia leading to amputation27 | 1 | 1.0 (1/103) | … |
| Flexion, loss of30 | 1 | 1.5 (1/66) | … |
| Graft failure leading to amputation42 | 1 | 3.1 (1/32) | … |
| Hematoma27,30,40,46,64 | 5 | 1.8 (13/711) | 1.3–2.9 |
| Hypoesthesia43 | 1 | 14.0 (6/43) | … |
| Infection4,27,30,46,64 | 5 | 3.9 (134/3424) | 0.9–10.5 |
| Necrosis (skin, flap, or graft)4,30,40,45,46,64 | 6 | 2.5 (93/3780) | 0–9.2 |
| Transient paralysis27† | 1 | 0.9 (1/103) | … |
| Reflex sympathetic dystrophy (complex regional pain syndrome)27,40,41,46,64 | 5 | 4.5 (34/752) | 0–18.4 |
| Scar contracture from graft42 | 1 | 9.4 (3/32) | … |
| Scar hypertrophy39 | 1 | 10.0 (1/10) | … |
| Stiffness27,45 | 2 | 15.4 (55/356) | 1.6–51.5 |
| Vascular damage45 | 1 | 0.8 (2/253) | … |
| Wound dehiscence30 | 1 | 4.5 (3/66) | … |
| Wound-healing complications such as skin edge necrosis or slough46 | 1 | 1.2 (3/253) | … |
*Studies that reported no cases of a particular complication were included in calculations.
†Transient paralysis assumed to be caused by a tourniquet.
Eleven mixed-population studies reported overall postoperative complications: the most common were stiffness (15.4%; range, 1.6%–51.5%), hypoesthesia (14.0%), scar hypertrophy (10.0%), and scar contracture (9.4%).4,27,30,39-43,45,46,64
Comparison of complications in patients with primary or recurrent disease
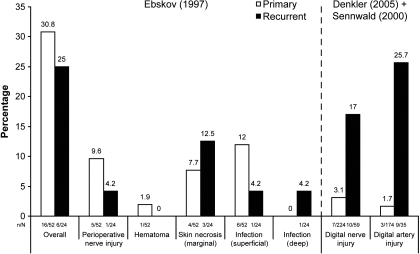
Three studies reported surgical complication rates separately for patients with primary disease and recurrent disease (Table 5 and Fig 1).27,30,64 Only one study reported overall complication rates, which were slightly higher in patients with primary disease (30.8%) than in those with recurrent disease (25.0%).64 Digital artery injury and digital nerve injury were more commonly observed in patients with recurrent disease than those with primary disease. The incidence of digital artery injury and digital nerve injury was 1.7% (3/174) and 3.1% (7/224), respectively, in patients with primary disease and 25.7% (9/35) and 17.0% (10/59), respectively, in patients with recurrent disease, indicating a approximately 10-fold difference (˜2% vs ˜20%) (Fig 1).27,30,64 However, the number of patients is too small for statistical significance.
Table 5.
Intrastudy comparison of surgical complications* in patients with primary or recurrent Dupuytren's disease
| Complication | Primary, % (n/N) | Recurrent, % (n/N) |
|---|---|---|
| Overall | 30.8 (16/52)64 | 25.0 (6/24)64 |
| Digital nerve injury | 1.3 (1/77)27 | 26.9 (7/26)27 |
| 1.5 (1/95)30 | 22.2 (2/9)30 | |
| 9.6 (5/52)64 | 4.2 (1/24)64 | |
| Digital artery injury | 2.6 (2/77)27 | 30.8 (8/26)27 |
| 1.0 (1/97)30 | 11.1 (1/9)30 | |
| Hematoma | 1.9 (1/52)64 | 0 (0/24)64 |
| 0 (0/77)27 | 7.7 (2/26)27 | |
| Skin necrosis (marginal) | 7.7 (4/52)64 | 12.5 (3/24)64 |
| Infection (superficial) | 12.0 (6/52)64 | 4.2 (1/24)64 |
| Infection (deep joint infection that led to amputation) | 0 (0/52)64 | 4.2 (1/24)64 |
*Studies that reported no cases of a particular complication were included in calculations.
Figure 1.
Surgical complications in studies (n = 3)27,30,64 that compared primary disease versus recurrent disease.
DISCUSSION
Data from this analysis clearly demonstrate that complications associated with fasciectomy for the treatment of patients with Dupuytren's disease are varied and relatively common. Data from studies that evaluated patients with primary disease showed that wound-healing complications and pain were most common. Conversely, patients with recurrent disease were more likely to experience varied types of sensory abnormalities (eg, hyperesthesia, cold intolerance, hypoesthesia) and necrosis. Data from the few studies that directly compared patients with primary and recurrent diseases showed that digital nerve injuries and digital artery injuries were much more common in patients with recurrent disease (typically ˜20%) than those with primary disease (typically ˜2%), although larger numbers of patients are needed for a valid statistical comparison. Pain was less common in patients with recurrent disease, perhaps because those who previously underwent fasciectomy and developed a pain-related complication were unlikely to undergo surgery a second time.
Surgical complication rates in the present analysis were physician reported. A large patient survey study (N = 1177) conducted by the British Society for Surgery of the Hand provides insight into patient-reported complications after Dupuytren's surgery.75 Patients with Dupuytren's disease were identified by hand surgeons throughout the United Kingdom and were invited to complete a questionnaire about surgical outcomes and complications. Patients' self-reported complications were 35.8% for numbness and 19.8% for infection.75 These values are much higher than the physician-reported complications rates provided in the current analysis.75
As with all surgeries, complication rates generally correlate with invasiveness of the procedure. Patients with severe disease often have greater tissue involvement and require more complex measures to correct the finger deformity. Consequently, patients with severe disease at the time of surgery tend to experience more complications postfasciectomy.46,75 A retrospective analysis of 253 patients with Dupuytren's disease who underwent fasciectomy showed that complication rates increased with the severity of disease, particularly when PIP joint contracture was more than 60°.46 Dias and Braybrooke75 made a similar observation, showing a clear relationship between the incidence of self-reported complications and the severity of the initial deformity, with patients who had severe disease at the time of surgery reporting more surgical complications. Loos et al4 in a large study of almost 3000 patients noted a statistically significant correlation between worsening stage of the disease and postoperative complications.
Several limitations of the present analysis must be taken into consideration when interpreting the data. First, the manner in which complication rates were reported varied from study to study and included complications per ray or finger, per patient, and per hand. Conversion of complication rates to one common denominator was not possible, so the overall rates and ranges represent blended data. Given the large number of studies (n = 41) included in the analysis, overall interpretation should not be affected, though this limitation may explain why the ranges associated with some complications are relatively broad. Second, several factors, such as patient diathesis, baseline disease severity, the type of joint affected (ie, MP or PIP), and multiple digit involvement, that can impact the frequency of surgical complications were not analyzed separately. Complication rates in the present analysis are therefore based on a heterogeneous patient population and cannot be directly compared with a specific patient subset.
In the absence of an approved pharmacotherapy, surgery provides the best opportunity for long-term functional improvement for patients with Dupuytren's disease. Although complete restoration of hand function is unlikely, most patients will experience significant gain in function. However, several drawbacks to surgery exist. First, surgery does not cure Dupuytren's disease and recurrences rates are high, ranging from 26% to 80%.37 Second, surgery in patients with recurrent disease is usually more challenging because scarring and anatomic distortion from prior procedure(s) increases the likelihood of neurovascular complications. Third, rehabilitation after open surgery may be prolonged. Finally, multiple, repetitive surgical procedures have their limitations and not all patients are good candidates for surgery.
CONCLUSIONS
This is the first report to extensively collect and analyze complications associated with surgery for Dupuytren's disease in clinical practice. Data from this study indicate that complications of surgery not only occur frequently but are also varied. Therefore, surgeons who perform fasciectomies for Dupuytren's disease should be mindful of the potential for intraoperative and postoperative complications and should counsel their patients accordingly. Furthermore, the severity of the disease and surgical history of the patient should be considered when anticipating complications. Patients undergoing fasciectomy for recurrent disease are more likely to experience either digital nerve injury or digital artery injury than patients with primary disease.
In conclusion, results of this study underscore the importance of treating Dupuytren's as an incurable genetic disease understanding that surgical excision, fasciectomy, has a high rate of major and minor complications. Surgeons must understand that while fasciectomy for Dupuytren's does offer a chance at long-term “straight” fingers, there is a high cost in terms of numbers of complications that are borne by the patient.
Acknowledgments
The author thank Maribeth Bogush, PhD, and Lynn Brown, PhD, for editorial assistance.
Footnotes
REFERENCES
- 1.Elliot D. The early history of Dupuytren's disease. Hand Clin. 1999;15:1–19. [PubMed] [Google Scholar]
- 2.Dupuytren G. De la retraction des doigts par suite d'une affection de l'aponevrose palmaire-description de la maladie-operation Chirugicale qui convient dens de cas. J Univ Hebd Méd Chir Prat Inst Med. 1831;5:349–65. [Google Scholar]
- 3.Early PF. Population studies in Dupuytren's contracture. J Bone Joint Surg Am. 1962;44B:602–13. [Google Scholar]
- 4.Loos B, Puschkin V, Horch RE. 50 years experience with Dupuytren's contracture in the Erlangen University Hospital—a retrospective analysis of 2919 operated hands from 1956 to 2006. BMC Musculoskeletal Disord. 2007;8:60. doi: 10.1186/1471-2474-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gudmundsson KG, Arngrimsson R, Sigfusson N, Bjornsson A, Jonsson T. Epidemiology of Dupuytren's disease: clinical, serological, and social assessment. The Reykjavik Study. J Clin Epidemiol. 2000;53:291–6. doi: 10.1016/s0895-4356(99)00145-6. [DOI] [PubMed] [Google Scholar]
- 6.Yost J, Winters T, Fett HC., Sr. Dupuytren's contracture; a statistical study. Am J Surg. 1955;90:568–71. doi: 10.1016/0002-9610(55)90537-7. [DOI] [PubMed] [Google Scholar]
- 7.Hindocha S, Stanley JK, Watson S, Bayat A. Dupuytren's diathesis revisited: evaluation of prognostic indicators for risk of disease recurrence. J Hand Surg [Am] 2006;31:1626–34. doi: 10.1016/j.jhsa.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Spring M, Fleck H, Cohen BD. Dupuytren's contracture. Warning of diabetes? N Y State J Med. 1970;70:1037–41. [PubMed] [Google Scholar]
- 9.Cakir M, Samanci N, Balci N, Balci MK. Musculoskeletal manifestations in patients with thyroid disease. Clin Endocrinol (Oxf) 2003;59:162–7. doi: 10.1046/j.1365-2265.2003.01786.x. [DOI] [PubMed] [Google Scholar]
- 10.Coert JH, Nerin JP, Meek MF. Results of partial fasciectomy for Dupuytren disease in 261 consecutive patients. Ann Plast Surg. 2006;57:13–17. doi: 10.1097/01.sap.0000205819.53215.52. [DOI] [PubMed] [Google Scholar]
- 11.Arafa M, Steingold RF, Noble J. The incidence of Dupuytren's disease in patients with rheumatoid arthritis. J Hand Surg [Br] 1984;9:165–6. [PubMed] [Google Scholar]
- 12.Hu FZ, Nystrom A, Ahmed A, et al. Mapping of an autosomal dominant gene for Dupuytren's contracture to chromosome 16q in a Swedish family. Clin Genet. 2005;68:424–9. doi: 10.1111/j.1399-0004.2005.00504.x. [DOI] [PubMed] [Google Scholar]
- 13.Lucas G, Brichet A, Roquelaure Y, Leclerc A, Descatha A. Dupuytren's disease: personal factors and occupational exposure. Am J Ind Med. 2008;51:9–15. doi: 10.1002/ajim.20542. [DOI] [PubMed] [Google Scholar]
- 14.Hueston JT. Dupuytren's contracture and specific injury. Med J Aust. 1968;1:1084–5. doi: 10.5694/j.1326-5377.1968.tb29329.x. [DOI] [PubMed] [Google Scholar]
- 15.Al-Qattan MM. Factors in the pathogenesis of Dupuytren's contracture. J Hand Surg [Am] 2006:31, 1527–34. doi: 10.1016/j.jhsa.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Citron N, Hearnden A. Skin tension in the aetiology of Dupuytren's disease; a prospective trial. J Hand Surg [Br] 2003;28:528–30. doi: 10.1016/s0266-7681(03)00221-3. [DOI] [PubMed] [Google Scholar]
- 17.Townley WA, Baker R, Sheppard N, Grobbelaar AO. Dupuytren's contracture unfolded. BMJ. 2006;332:397–400. doi: 10.1136/bmj.332.7538.397. doi:10.1136/bmj.332.7538.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rayan GM. Dupuytren disease: anatomy, pathology, presentation, and treatment. J Bone Joint Surg [Am] 2007;89:189–98. doi: 10.2106/00004623-200701000-00026. [DOI] [PubMed] [Google Scholar]
- 19.Reilly RM, Stern PJ, Goldfarb CA. A retrospective review of the management of Dupuytren's nodules. J Hand Surg [Am] 2005;30:1014–8. doi: 10.1016/j.jhsa.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Smith AC. Diagnosis and indications for surgical treatment. Hand Clin. 1991:7, 635–42. [PubMed] [Google Scholar]
- 21.Benson LS, Williams CS, Kahle M. Dupuytren's contracture. J Am Acad Orthop Surg. 1998(6):24–35. doi: 10.5435/00124635-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Shaw RB, Jr, Chong AKS, Zhang A, Hentz VR, Chang J. Dupuytren's disease: history, diagnosis, and treatment. Plast Reconstr Surg. 2007;120:44e–54e. doi: 10.1097/01.prs.0000278455.63546.03. [DOI] [PubMed] [Google Scholar]
- 23.Swartz WM, Lalonde DH. MOC-PSSM CME article: Dupuytren's disease. Plast Reconstr Surg. 2008;121(suppl):1–10. doi: 10.1097/01.prs.0000305932.46121.84. [DOI] [PubMed] [Google Scholar]
- 24.Hunt TR., III What is the appropriate treatment for Dupuytren contracture? Cleve Clin J Med. 2003;70:96–7. doi: 10.3949/ccjm.70.2.96. [DOI] [PubMed] [Google Scholar]
- 25.Crowley B, Tonkin MA. The proximal interphalangeal joint in Dupuytren's disease. Hand Clin. 1999;15:137–47. [PubMed] [Google Scholar]
- 26.McGrouther DA. Dupuytren's contracture. In: Green DP, Hotchkiss RN, Pederson WC, Scott ER, Wolfe W, editors. Green's Operative Hand Surgery. New York: Elsevier/Churchill Livingstone; 2005. pp. 159–85. [Google Scholar]
- 27.Sennwald GR. Fasciectomy for treatment of Dupuytren's disease and early complications. J Hand Surg [Am] 1990;15:755–61. doi: 10.1016/0363-5023(90)90151-g. [DOI] [PubMed] [Google Scholar]
- 28.Moermans JP. Long-term results after segmental aponeurectomy for Dupuytren's disease. J Hand Surg [Br] 1996;21:797–800. doi: 10.1016/s0266-7681(96)80195-1. [DOI] [PubMed] [Google Scholar]
- 29.Armstrong JR, Hurren JS, Logan AM. Dermofasciectomy in the management of Dupuytren's disease. J Bone Joint Surg [Br] 2000;82:90–4. doi: 10.1302/0301-620x.82b1.9808. [DOI] [PubMed] [Google Scholar]
- 30.Denkler K. Dupuytren's fasciectomies in 60 consecutive digits using lidocaine with epinephrine and no tourniquet. Plast Reconstr Surg. 2005;115:802–10. doi: 10.1097/01.prs.0000152420.64842.b6. [DOI] [PubMed] [Google Scholar]
- 31.Rowley DI, Couch M, Chesney RB, Norris SH. Assessment of percutaneous fasciotomy in the management of Dupuytren's contracture. J Hand Surg [Br] 1984;9:163–4. [PubMed] [Google Scholar]
- 32.van Rijssen AL, Gerbrandy FS, Ter LH, Klip H, Werker PM. A comparison of the direct outcomes of percutaneous needle fasciotomy and limited fasciectomy for Dupuytren's disease: a 6-week follow-up study. J Hand Surg [Am] 2006;31:717–25. doi: 10.1016/j.jhsa.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 33.van Rijssen AL, Werker PMN. Percutaneous needle fasciotomy in Dupuytren's disease. J Hand Surg [Br] 2006;31:498–501. doi: 10.1016/j.jhsb.2006.03.174. [DOI] [PubMed] [Google Scholar]
- 34.Lermusiaux JL, Lellouche H, Badois JF, Kuntz D. How should Dupuytren's contracture be managed in 1997? Rev Rhum Engl Ed. 1997;64:775–6. [PubMed] [Google Scholar]
- 35.Foucher G, Medina J, Navarro R. Percutaneous needle aponeurotomy: complications and results. J Hand Surg [Br] 2003;28:427–31. doi: 10.1016/s0266-7681(03)00013-5. [DOI] [PubMed] [Google Scholar]
- 36.Leclerc C. Management of Dupuytren's disease. In: Mathes SJ, Hentz VR, editors. Plastic Surgery. Philadelphia, Pa: Saunders; 2005. pp. 729–58. [Google Scholar]
- 37.Hurst LC, Badalamente MA. Nonoperative treatment of Dupuytren's disease. Hand Clin. 1999;15:97–107. [PubMed] [Google Scholar]
- 38.Badalamente MA, Hurst LC. Efficacy and safety of injectable mixed collagenase subtypes in the treatment of Dupuytren's contracture. J Hand Surg [Am] 2007;32:767–74. doi: 10.1016/j.jhsa.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 39.Srivastava S, Nancarrow JD, Cort DF. Dupuytren's disease in patients from the Indian sub-continent. Report of ten cases. J Hand Surg [Br] 1989;14:32–4. doi: 10.1016/0266-7681(89)90009-0. [DOI] [PubMed] [Google Scholar]
- 40.Moermans JP. Segmental aponeurectomy in Dupuytren's disease. J Hand Surg [Br] 1991;16:243–54. doi: 10.1016/0266-7681(91)90047-r. [DOI] [PubMed] [Google Scholar]
- 41.Foucher G, Cornil C, Lenoble E. Open palm technique for Dupuytren's disease. A five-year follow-up. Ann Chir Main Memb Super. 1992;11:362–6. doi: 10.1016/s0753-9053(05)80271-6. [DOI] [PubMed] [Google Scholar]
- 42.Searle AE, Logan AM. A mid-term review of the results of dermofasciectomy for Dupuytren's disease. Ann Chir Main Memb Super. 1992;11:375–80. doi: 10.1016/s0753-9053(05)80273-x. [DOI] [PubMed] [Google Scholar]
- 43.Beyermann K, Prommersberger KJ, Jacobs C, Lanz UB. Severe contracture of the proximal interphalangeal joint in Dupuytren's disease: does capsuloligamentous release improve outcome? J Hand Surg [Br] 2004;29:238–41. doi: 10.1016/j.jhsb.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 44.Meathrel KE, Thoma A. Abductor digiti minimi involvement in Dupuytren's contracture of the small finger. J Hand Surg [Am] 2004;29:510–3. doi: 10.1016/j.jhsa.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 45.Kobus K, Wojcicki P, Dydymski T, Wegrzyn M, Hamlawi F. Evaluation of treatment results of patients with Dupuytren's contracture—our clinical experience. Ortoped Traumatol Rehabil. 2007;9:134–40. [PubMed] [Google Scholar]
- 46.Bulstrode NW, Jemec B, Smith PJ. The complications of Dupuytren's contracture surgery. J Hand Surg [Am] 2005;30:1021–5. doi: 10.1016/j.jhsa.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 47.Andrew JG, Kay NRM. Segmental aponeurectomy for Dupuytren's disease: a prospective study. J Hand Surg [Br] 1991;16:255–7. doi: 10.1016/0266-7681(91)90048-s. [DOI] [PubMed] [Google Scholar]
- 48.Liu Y, Chen WY-K. Dupuytren's disease among the Chinese in Taiwan. J Hand Surg [Am] 1991;16:779–86. doi: 10.1016/s0363-5023(10)80135-2. [DOI] [PubMed] [Google Scholar]
- 49.Robins RHC, Scott TD, Griffiths DPG. Day care surgery for Dupuytren's contracture. J Hand Surg [Br] 1993;18:494–8. doi: 10.1016/0266-7681(93)90156-a. [DOI] [PubMed] [Google Scholar]
- 50.Cools H, Verstreken J. The open palm technique in the treatment of Dupuytren's disease. Acta Orthop Belg. 1994;60:413–20. [PubMed] [Google Scholar]
- 51.Citron ND, Nunez V. Recurrence after surgery for Dupuytren's disease: a randomized trial of two skin incisions. J Hand Surg [Br] 2005;30:563–6. doi: 10.1016/j.jhsb.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 52.Van Giffen N, Degreef I, De Smet L. Dupuytren's disease: outcome of the proximal interphalangeal joint in isolated fifth ray involvement. Acta Orthop Belg. 2006;72:671–7. [PubMed] [Google Scholar]
- 53.Skoff HD. The surgical treatment of Dupuytren's contracture: a synthesis of techniques. Plast Reconstr Surg. 2004;113:540–4. doi: 10.1097/01.PRS.0000101054.80392.88. [DOI] [PubMed] [Google Scholar]
- 54.Ritchie JFS, Venu KM, Pillai K, Yanni DH. Proximal interphalangeal joint release in Dupuytren's disease of the little finger. J Hand Surg [Br] 2004;29:15–17. doi: 10.1016/j.jhsb.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 55.Misra A, Jain A, Ghazanfar R, Johnston T, Nanchahal J. Predicting the outcome of surgery for the proximal interphalangeal joint in Dupuytren's disease. J Hand Surg [Am] 2007;32:240–5. doi: 10.1016/j.jhsa.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 56.Sorene ED, Rubinraut-Ophir E, Goodwin DR. Dupuytren's disease in Oriental Jews. J Hand Surg Eur Vol. 2007;32:543–6. doi: 10.1016/J.JHSE.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 57.Stahl S, Calif E. Dupuytren's palmar contracture in women. Israel Med Assoc J. 2008;10:445–7. [PubMed] [Google Scholar]
- 58.Vigroux JP, Valentin P. A natural history of Dupuytren's contracture treated by surgical fasciectomy: the influence of diathesis (76 hands reviewed at more than 10 years) Ann Chir Main Memb Super. 1992;11:367–74. doi: 10.1016/s0753-9053(05)80272-8. [DOI] [PubMed] [Google Scholar]
- 59.Foucher G, Cornil C, Lenoble E, Citron N. A modified open palm technique for Dupuytren's disease. Short and long term results in 54 patients. Int Orthop. 1995;19:285–8. doi: 10.1007/BF00181110. [DOI] [PubMed] [Google Scholar]
- 60.De Maglio A, Timo R, Feliziani G. Dupuytren's disease: recurrence and extension treated by selective aponeurectomy. A clinical review of 124 cases. Chir Organi Mov. 1996;81:43–8. [PubMed] [Google Scholar]
- 61.Shaw DL, Wise DI, Holms W. Dupuytren's disease treated by palmar fasciectomy and an open palm technique. J Hand Surg [Br] 1996;21:484–5. doi: 10.1016/s0266-7681(96)80051-9. [DOI] [PubMed] [Google Scholar]
- 62.Weinzweig N, Culver JE, Fleegler EJ. Severe contractures of the proximal interphalangeal joint in Dupuytren's disease: combined fasciectomy with capsuloligamentous release versus fasciectomy alone. Plast Reconstr Surg. 1996;97:560–6. doi: 10.1097/00006534-199603000-00011. [DOI] [PubMed] [Google Scholar]
- 63.Citron N, Messina JC. The use of skeletal traction in the treatment of severe primary Dupuytren's disease. J Bone Joint Surg [Br] 1998;80:126–9. doi: 10.1302/0301-620x.80b1.8019. [DOI] [PubMed] [Google Scholar]
- 64.Ebskov LB, Boeckstyns MEH, Sorensen AI, Haugegaard M. Day care surgery for advanced Dupuytren's contracture. J Hand Surg [Br] 1997;22:191–2. doi: 10.1016/s0266-7681(97)80060-5. [DOI] [PubMed] [Google Scholar]
- 65.Clibbon JJ, Logan AM. Palmar segmental aponeurectomy for Dupuytren's disease with metacarpophalangeal flexion contracture. J Hand Surg [Br] 2001;26:360–1. doi: 10.1054/jhsb.2001.0602. [DOI] [PubMed] [Google Scholar]
- 66.Evans RB, Dell PC, Fiolkowski P. A clinical report of the effect of mechanical stress on functional results after fasciectomy for Dupuytren's contracture. J Hand Ther. 2002;15:331–9. doi: 10.1016/s0894-1130(02)80004-7. [DOI] [PubMed] [Google Scholar]
- 67.Barr V, Bhatia R, Hawkins P, Savage R. Intramuscular tenotomy of flexor digitorum superficialis in the distal forearm after surgical excision of Dupuytren's disease. J Hand Surg [Br] 2003;28:37–9. doi: 10.1054/jhsb.2002.0860. [DOI] [PubMed] [Google Scholar]
- 68.Abe Y, Rokkaku T, Ofuchi S, Tokunaga S, Takahashi K, Moriya H. Surgery for Dupuytren's disease in Japanese patients and a new preoperative classification. J Hand Surg [Br] 2004;29:235–9. doi: 10.1016/j.jhsb.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 69.Ali SN, McMurtrie A, Rayatt S, Roberts JO. Ulnar-based skin flap for Dupuytren's fasciectomy. Scand J Plast Reconstr Surg Hand Surg. 2006;40:307–10. doi: 10.1080/02844310600836794. [DOI] [PubMed] [Google Scholar]
- 70.Reuben SS, Pristas R, Dixon D, Faruqi S, Madabhushi L, Wenner S. The incidence of complex regional pain syndrome after fasciectomy for Dupuytren's contracture: a prospective observational study of four anesthetic techniques. Anesth Analg. 2006;102:499–503. doi: 10.1213/01.ane.0000194879.85643.ff. [DOI] [PubMed] [Google Scholar]
- 71.Anwar MU, Al Ghazal SK, Boome RS. Results of surgical treatment of Dupuytren's disease in women: a review of 109 consecutive patients. J Hand Surg [Am] 2007;32:1423–8. doi: 10.1016/j.jhsa.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 72.Ekerot L. The distally-based dorsal hand flap for resurfacing skin defects in Dupuytren's contracture. J Hand Surg [Br] 1995;20:111–4. doi: 10.1016/s0266-7681(05)80028-2. [DOI] [PubMed] [Google Scholar]
- 73.Roush TF, Stern PJ. Results following surgery for recurrent Dupuytren's disease. J Hand Surg [Am] 2000;25:291–6. doi: 10.1053/jhsu.2000.jhsu25a0291. [DOI] [PubMed] [Google Scholar]
- 74.Gonzalez MH, Sobeski J, Grindel S, Chunprapaph B, Weinzweig N. Dupuytren's disease in African-Americans. J Hand Surg [Br] 1998;23:306–7. doi: 10.1016/s0266-7681(98)80046-6. [DOI] [PubMed] [Google Scholar]
- 75.Dias JJ, Braybrooke J. Dupuytren's contracture: an audit of the outcomes of surgery. J Hand Surg [Br] 2006;31:514–21. doi: 10.1016/j.jhsb.2006.05.005. [DOI] [PubMed] [Google Scholar]