Abstract
Nicotinic acetylcholine receptors (nAChRs) are in the superfamily of cys-loop receptors, and are widely expressed in the nervous system where they participate in a variety of physiological functions, including regulating excitability and neurotransmitter release, as well as neuromuscular contraction. Members of the cys-loop family of receptors, which also includes the molluscan ACh-binding protein (AChBP), a soluble protein that is analogous to the extracellular ligand-binding domain of the cys-loop receptors, are pentameric assemblies of five subunits, with each subunit arranged around a central pore. The binding of ACh to the extracellular interface between two subunits induces channel opening. With the recent 4 Å resolution of the Torpedo nAChR, and the crystal structure of the AChBP, much has been learned about the structure of the ligand-binding domain and the channel pore, as well as major structural rearrangements that may confer channel opening, including a major rearrangement of the C-loop within the ligand binding pocket, and perhaps other regions including the F-loop (the β8–β9 linker), the β1–β2 linker and the cys-loop. Here I will review the latest findings from my lab aimed at a further understanding of the function of the neuronal nAChR channels (and in particular the role of desensitization), and our search for novel AChBP species that may lead to a further understanding of the function of the cys-loop receptor family.

Jerrel Yakel received his BS from Oregon State University, and his PhD from the University of California, Los Angeles, where he studied ligand-gated ion channels and serotonin receptors in cultured hippocampal neurons and cell lines with Meyer Jackson. During a postdoctoral fellowship with Hersch Gerschenfeld at the Ecole Normale Superieure (Paris, France), he investigated the regulation of voltage-gated calcium channels by G protein-coupled receptors. During a second postdoctoral stage at the Vollum Institute with Alan North and Tom Soderling, he studied the function of regulation of ligand-gated ion channels. He joined NIEHS as an investigator in 1993, and is currently a Senior Investigator in the Laboratory of Neurobiology. His laboratory explores the function and regulation of ligand-gated ion channels, in particular the neuronal nicotinic receptor channels, in the hippocampus.
Structure and function of the nAChR
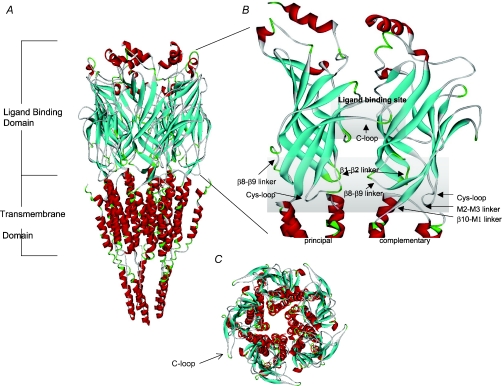
The superfamily of cys-loop neurotransmitter-gated ion channels includes the nAChRs, the serotonin 5-HT3, GABAA and GABAC, and glycine receptors. These channels are either homo- or heteromeric assemblies of five subunits, with each subunit arranged around a central pore (for review see Corringer et al. 2000; Giniatullin et al. 2005; Unwin, 2005; Sine & Engel, 2006). To understand how these receptor channels function, it is important to know the structure and transition of the receptor in its various states, including the closed (in the absence of agonist), the open (in the presence of agonist) and the desensitized states (high-affinity ligand-bound but non-conducting state of the channel). Using a variety of techniques, including (but not limited to) electron microscopy, biochemistry, chemical labelling, site-directed mutagenesis and electrophysiology, much has been learned about the structure and function of various members of the cys-loop receptor family (Lester et al. 2004; Unwin, 2005). However, since 2001, two major discoveries have increased our understanding of the structure and function of nAChRs and related proteins. The cloning and characterization of the molluscan ACh-binding protein (AChBP, Brejc et al. 2001; Smit et al. 2001) was one of these occasions since this protein was found to be a soluble pentameric protein analogous to the extracellular ligand-binding domain of the cys-loop family of receptors. The other major advancement was the refined 4 Å resolution electron microscopy structure of the Torpedo nAChR (Unwin, 2005). This structure provided a complete picture of the nAChR, including not only the ligand-binding domain, but also the pore and intracellular region in near-physiological conditions (Fig. 1).
Figure 1. Molecular model of the rat α7 nAChR.
A, a side view of the pentameric α7 receptor model is shown. The model was developed based on sequence alignment with the cryoelectron microscopy structure of the T. marmorata nAChR as indicated in Gay et al. (2007). The α helices are shown in red and the β strands in blue. The extracellular ligand binding domain is shown close up in B. The ligand binding site is composed of a cluster of aromatic residues from both the principal and complementary subunits and is capped by the C-loop. The transition domain consists of several loops including: Cys-loop, β1–β2 linker, β8–β9 linker, β10–M1 linker and the M2–M3 linker. C, a top-down view of the α7 receptor model.
Structural transitions during desensitization
Neuronal nAChRs are broken down into two major subtypes, the α7 and the non-α7 subtypes. Most of our recent work has focused on understanding the role of the α7 nAChR subtype on regulating excitability in the hippocampus since this is the predominant subtype on rat hippocampal interneurons (Jones & Yakel, 1997; Sudweeks & Yakel, 2000; Khiroug et al. 2003). Besides being highly permeable to calcium (with permeability ratios (versus sodium) of 7–20 for expressed α7 receptors; Fucile, 2004), the α7 nAChR can undergo rapid onset of desensitization, the process whereby the channel closes even in the continued presence of agonist. Although the mechanism of desensitization is not completely understood, it is thought to be an important factor in controlling cholinergic signalling and perhaps in certain nAChR-related diseases (Giniatullin et al. 2005). For example, for the congenital myasthenic syndromes (CMS), a heterogeneous group of disorders caused by genetic defects affecting neuromuscular transmission, there are mutations of the endplate nAChRs that result in abnormal synaptic responses by altering desensitization (Giniatullin et al. 2005). In addition, α7 nAChR-selective positive allosteric modulators (α7-PAMs) are being developed as a possible therapeutic strategy in the treatment of Alzheimer's disease and other neurological disorders (e.g. schizophrenia; Young et al. 2008). Type II α7-PAMs (e.g. PNU-120596; Bertrand & Gopalakrishnan, 2007) dramatically potentiate α7 nAChR-mediated responses by removing desensitization (Hurst et al. 2005); however, this removal of desensitization may cause toxicity due to the excessive influx of calcium through the α7 receptors (Ng et al. 2007). Therefore it is critical to have a greater understanding of the desensitization of the α7 nAChRs and how ligands may affect this process.
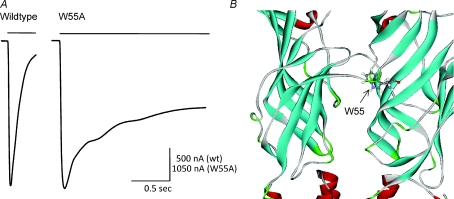
There has been much work that has focused on identifying key residues within the pore domain of nAChRs involved in desensitization (Giniatullin et al. 2005). However, regions in the extracellular domain of nAChRs might also be involved in receptor desensitization, and thus could be the target for development of therapeutic ligands. Recently we found that the tryptophan residue at position 55 of the rat α7 nAChR (W55) within the β2 strand was the site where synthetic peptides derived from apolipoprotein E non-competitively inhibited α7 receptors through hydrophobic interactions (Gay et al. 2007). In addition, when W55 was mutated to alanine, there was a dramatic slowing in the rate of onset of desensitization (Fig. 2); kinetic modelling indicated that the rate of transition of the receptor from the open to the desensitized state decreased by >30-fold, and the rate of recovery from desensitization increased by ∼2-fold (Gay et al. 2008). For the related 5-HT3 receptor channel, Reeves et al. (2005) proposed that recovery from desensitization may require reformation of an interaction between the β1–β2 linker and the M2–M3 linker (Fig. 1B). Perhaps any structural change that interferes with the reformation of this β1–β2 linker/M2–M3 linker interaction might have an effect on the kinetics of desensitization. Our data suggested that aromatic residues at position 55 of the rat α7 nAChR are critical for maintaining rapid desensitization, and that W55 may be a potential target for modulatory agents operating via hydrophobic interactions.
Figure 2.
The rate of α7 nAChR desensitization is slowed by the W55A mutation A, representative inward current responses due to the rapid and sustained application of ACh (1 mm; indicated by the horizontal bar) for wildtype (left) and W55A mutant α7 receptor (right) expressed in Xenopus oocytes. B, a close-up of the ligand binding domain of the α7 receptor highlighting the tryptophan 55 (W55) that is within the β2 strand across from the C-loop.
Recently we have identified another region of the extracellular domain of the α7 receptor that has a dramatic effect on desensitization, a proline residue near the middle of the β9 strand (P180). We found that the substitution of P180 to either threonine or serine slowed the onset of desensitization, nearly as much as with the W55A mutation mentioned above (McCormack et al. 2008). We demonstrated that the P180 sidechain hydroxyl group was an important determinant of the rate of desensitization of the α7 nAChR, and possibly that the rapid desensitization of the α7 nAChR may in part be due to the disruption of hydrogen bond interactions in the backbone of the outer β-sheet (McCormack et al. 2008).
The superposition of many crystal structures of AChBP with a variety of different ligands (including both agonists and antagonists) falls into two groups (Hansen et al. 2005; Dutertre & Lewis, 2006; Ulens et al. 2006); the first configuration seen with antagonists (and some buffer molecules) has the C-loop in an ‘open’ configuration (corresponding to the closed or resting state of the channel; Fig. 1C), and the second configuration seen with agonists has the C-loop in a ‘closed’ configuration (presumably corresponding to either the open or desensitized state of the receptor). When comparing these two configurations, it appears that movement may occur in regions other than just the C-loop, including the β1–β2 linker, the cys-loop and the β8–β9 linker (Fig. 1B), suggesting that these changes may be associated with receptor activation. The inactive state of the Torpedo nAChR resembles the antagonist-bound AChBP structures, with the C-loop in an open position, and similar relative locations of the β1–β2 linker and cys-loop (Unwin, 2005). Comparison of αversus non-α subunits indicated a clockwise rotation of ∼10 deg of the inner β strands. The two α subunits appear to be in a ‘distorted’ conformation, while the non-α subunits are in a more ‘relaxed’ conformation. Upon agonist binding, the α subunits rotate to the non-α subunit conformation, and this movement may lead to gating of the channel by displacing the β1–β2 linker, thereby affecting M2 and the channel pore (Unwin, 2005). Agonist binding models for the non-α7 neuronal nAChRs (Le Novere et al. 2002; Costa et al. 2003) also provide useful information into interactions and conformational changes induced by ligand binding.
As noted above for the AChBP structures with agonist bound, these are thought to be in configurations akin to either the open or desensitized receptor state. It has been difficult to gain structural information for the nAChRs in the open versus desensitized state because after the addition of agonist, receptors open and then desensitize quickly (depending on the subtype of nAChR involved; Giniatullin et al. 2005). Dutertre & Lewis (2006) compared multiple AChBP crystal structures and suggested that there are no noticeable structural differences between open and desensitized states. Ulens et al. (2006) attempted to distinguish the desensitized state of the AChBP by evaluating crystal structures containing α conotoxins that, according to functional data, were thought to favour the desensitized state of the nAChRs. However, crystals of these toxins with AChBP did not indicate significant differences from other antagonist-bound structures. Therefore, we still lack definitive structures for the desensitized states of the nAChR and other members of the cys-loop ligand-gated ion channel family.
Cloning of novel species of the AChBPs
Although the previously characterized molluscan AChBPs do not contain an ion channel pore or intracellular domains, they do bind nAChR ligands. Furthermore, when attached to the pore domain of the serotonin 5-HT3A receptor, ACh can activate the opening of this hybrid channel (Bouzat et al. 2004). Thus, the crystal structure of AChBP may help in elucidating the tertiary structure of the extracellular ligand-binding domain of nAChRs and transitions that occur in response to ligand binding.
We searched for novel AChBP homologues in different species by looking for cys-loop domains with a hydrophilic characteristic since the absence of transmembrane domains would not require the characteristic hydrophobic cys-loop found in functional receptors. Using this approach, we identified a homologue of the mollusc AChBP in the marine polychaete Capitella teleta (previously referred to as Capitella capitata), from the annelid phylum (McCormack et al. 2009). The Capitella teleta AChBP (ct-AChBP) has ∼20–30% amino acid identity with known molluscan AChBPs, a shortened cys-loop compared to other cys-loop receptors and AChBPs, and a variation on a conserved triad found near the C-loop, which is associated with ligand binding in other AChBPs and nAChRs. Mass spectrometry results indicate that Asn122 of ct-AChBP is glycosylated when expressed using HEK293 cells. Small-angle X-ray scattering (SAXS) data are consistent with ct-AChBP existing as a soluble pentamer in solution. NMR experiments conducted to determine binding affinities show that acetylcholine and nicotine bind to ct-AChBP with dissociation constant (KD) values of 28 μm and 200 nm, respectively. The α7 nAChR subtype-selective agonist choline bound with a lower affinity, having a KD value of 164 μm. Our finding of a functional AChBP in a marine annelid demonstrates that AChBPs may possess variation in hallmark motifs such as ligand binding residues and cys-loop length, and shows conclusively that this neurotransmitter-binding protein is not limited to the mollusc phylum. The cloning of new AChBP molecules (that have some structural differences between known AChBP and nAChR sequences) should aid in the understanding of what structural motifs are required for ligand binding and gating of the nAChRs, as well as in the design of therapeutic ligands.
Linking ligand binding to function
Much has been learned about the structural mechanisms of receptor transitions during gating from an array of functional studies on nAChRs or other members in the cys-loop ligand-gated ion channel family. More recently, understanding the structure of AChBPs has greatly aided in understanding some of the changes that result due to ligand binding. It is important to understand that functional studies give us a framework within which the structural data of various states of the receptor can be understood. For example, the extensive work of Auerbach and colleagues has provided an overall picture of the sequential nature of receptor gating for muscle nAChRs. By measuring the rate-equilibrium free energy relationships in combination with point mutations in both the ligand binding and pore domains (Zhou et al. 2005), they have been able to suggest blocks of coordinated motions starting with the β4–β5 linker, the β7–β8 linker and the C-loop (which increases affinity for agonists), through the transition zone (the cys-loop and the β1–β2 linker), to the pore region (M2) and gating of the channel (Grosman et al. 2000; Chakrapani et al. 2004; Purohit et al. 2007). This conformational wave propagates throughout the nAChR via Brownian motion in ∼1 μs (Grosman et al. 2000; Chakrapani & Auerbach, 2005).
More recently Lape et al. (2008) have proposed a new functional state of the nAChR and glycine receptor, the flip state, to help explain partial agonism of these receptors. The flip state is a new state of the receptor (one of a number of brief intermediate closed states) immediately preceding channel opening, with a higher affinity for agonist than the resting (closed) state of the receptor. In their model, partial agonism is the result of the reduced ability (versus full agonists) to achieve the flipped state (Lape et al. 2008). Therefore, functional studies such as these, when combined with the high resolution crystal structures, will undoubtedly lead to further dramatic advances in the near future in our understanding of the inner workings of the cys-loop receptor family.
In conclusion, the exact nature of the structure of the cys-loop ligand-gated ion channel subunits and the movements observed during and after ligand binding, gating and desensitization, are still unknown. Nevertheless, a general hypothesis has emerged that indicates agonist binding induces closure of the C-loop, which is conveyed to the M2 pore region, resulting in channel opening. Thus, the transduction pathway involves many regions of the channel. With the continued use of a variety of experimental, structural and modelling techniques, including the recent crystallization of the extracellular domain of the mouse muscle α1 nAChR subunit (Dellisanti et al. 2007), major advances await in the future. These studies probably will continue to elucidate the various steps linking ligand binding to gating and desensitization, as well as how we might be able to affect function through external ligands that might be useful in treating various neurological disorders and diseases.
Acknowledgments
Research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
References
- Bertrand D, Gopalakrishnan M. Allosteric modulation of nicotinic acetylcholine receptors. Biochem Pharmacol. 2007;74:1155–1163. doi: 10.1016/j.bcp.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Bouzat C, Gumilar F, Spitzmaul G, Wang HL, Rayes D, Hansen SB, Taylor P, Sine SM. Coupling of agonist binding to channel gating in an ACh-binding protein linked to an ion channel. Nature. 2004;430:896–900. doi: 10.1038/nature02753. [DOI] [PubMed] [Google Scholar]
- Brejc K, Van Dijk WJ, Klaassen RV, Schuurmans M, Van Der Oost J, Smit AB, Sixma TK. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001;411:269–276. doi: 10.1038/35077011. [DOI] [PubMed] [Google Scholar]
- Chakrapani S, Auerbach A. A speed limit for conformational change of an allosteric membrane protein. Proc Natl Acad Sci U S A. 2005;102:87–92. doi: 10.1073/pnas.0406777102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrapani S, Bailey TD, Auerbach A. Gating dynamics of the acetylcholine receptor extracellular domain. J Gen Physiol. 2004;123:341–356. doi: 10.1085/jgp.200309004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corringer PJ, Le Novere N, Changeux JP. Nicotinic receptors at the amino acid level. Annu Rev Pharmacol Toxicol. 2000;40:431–458. doi: 10.1146/annurev.pharmtox.40.1.431. [DOI] [PubMed] [Google Scholar]
- Costa V, Nistri A, Cavalli A, Carloni P. A structural model of agonist binding to the α3β4 neuronal nicotinic receptor. Brit J Pharmacol. 2003;140:921–931. doi: 10.1038/sj.bjp.0705498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellisanti CD, Yao Y, Stroud JC, Wang ZZ, Chen L. Crystal structure of the extracellular domain of nAChR α1 bound to α-bungarotoxin at 1.94 A resolution. Nat Neurosci. 2007;10:953–962. doi: 10.1038/nn1942. [DOI] [PubMed] [Google Scholar]
- Dutertre S, Lewis RJ. Toxin insights into nicotinic acetylcholine receptors. Biochem Pharmacol. 2006;72:661–670. doi: 10.1016/j.bcp.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Fucile S. Ca2+permeability of nicotinic acetylcholine receptors. Cell Calcium. 2004;35:1–8. doi: 10.1016/j.ceca.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Gay EA, Bienstock RJ, Lamb PW, Yakel JL. Structural determinates for apolipoprotein E-derived peptide interaction with the α7 nicotinic acetylcholine receptor. Mol Pharmacol. 2007;72:838–849. doi: 10.1124/mol.107.035527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay EA, Giniatullin R, Skorinkin A, Yakel JL. Aromatic residues at position 55 of rat α7 nicotinic acetylcholine receptors are critical for maintaining rapid desensitization. J Physiol. 2008;586:1105–1115. doi: 10.1113/jphysiol.2007.149492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giniatullin R, Nistri A, Yakel JL. Desensitization of nicotinic ACh receptors: shaping cholinergic signalling. Trends Neurosci. 2005;28:371–378. doi: 10.1016/j.tins.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Grosman C, Zhou M, Auerbach A. Mapping the conformational wave of acetylcholine receptor channel gating. Nature. 2000;403:773–776. doi: 10.1038/35001586. [DOI] [PubMed] [Google Scholar]
- Hansen SB, Sulzenbacher G, Huxford T, Marchot P, Taylor P, Bourne Y. Structures of Aplysia AChBP complexes with agonists and antagonists reveal distinctive binding interfaces and conformations. Embo Journal. 2005;24:3635–3646. doi: 10.1038/sj.emboj.7600828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst RS, Hajós M, Raggenbass M, Wall TM, Higdon NR, Lawson JA, et al. A novel positive allosteric modulator of the α7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. J Neurosci. 2005;25:4396–4405. doi: 10.1523/JNEUROSCI.5269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Yakel JL. Functional nicotinic ACh receptors on interneurons in the rat hippocampus. J Physiol. 1997;504:603–610. doi: 10.1111/j.1469-7793.1997.603bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khiroug L, Giniatullin R, Klein RC, Fayuk D, Yakel JL. Functional mapping and Ca2+regulation of nicotinic acetylcholine receptor channels in rat hippocampal CA1 neurons. J Neurosci. 2003;23:9024–9031. doi: 10.1523/JNEUROSCI.23-27-09024.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lape R, Colquhoun D, Sivilotti LG. On the nature of partial agonism in the nicotinic receptor superfamily. Nature. 2008;454:722–727. doi: 10.1038/nature07139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Novere N, Grutter T, Changeux JP. Models of the extracellular domain of the nicotinic receptors and of agonist- and Ca2+-binding sites. Proc Natl Acad Sci U S A. 2002;99:3210–3215. doi: 10.1073/pnas.042699699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester HA, Dibas MI, Dahan DS, Leite JF, Dougherty DA. Cys-loop receptors: new twists and turns. Trends Neurosci. 2004;27:329–336. doi: 10.1016/j.tins.2004.04.002. [DOI] [PubMed] [Google Scholar]
- McCormack T, Gay EA, Yakel JL. A single amino acid substitution in the β9 strand of rat α7 nicotinic receptor which slows desensitization. 2008. Abstr Soc Neurosci.
- McCormack T, Petrovich RM, Mercier KA, DeRose EF, Cuneo MJ, Williams J, London RE, Yakel JL. Identification and functional characterization of a novel acetylcholine-binding protein from the marine annelid Capitella capitata. Abstr Soc Neurosci. 2009. [DOI] [PMC free article] [PubMed]
- Ng HJ, Whittemore ER, Tran MB, Hogenkamp DJ, Broide RS, Johnstone TB, Zheng L, Stevens KE, Gee KW. Nootropic α7 nicotinic receptor allosteric modulator derived from GABAA receptor modulators. Proc Natl Acad Sci U S A. 2007;104:8059–8064. doi: 10.1073/pnas.0701321104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit P, Mitra A, Auerbach A. A stepwise mechanism for acetylcholine receptor channel gating. Nature. 2007;446:930–933. doi: 10.1038/nature05721. [DOI] [PubMed] [Google Scholar]
- Reeves DC, Jansen M, Bali M, Lemster T, Akabas MH. A role for the β1-β2 loop in the gating of 5-HT3 receptors. J Neurosci. 2005;25:9358–9366. doi: 10.1523/JNEUROSCI.1045-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine SM, Engel AG. Recent advances in Cys-loop receptor structure and function. Nature. 2006;440:448–455. doi: 10.1038/nature04708. [DOI] [PubMed] [Google Scholar]
- Smit AB, Syed NI, Schaap D, Van Minnen J, Klumperman J, Kits KS, et al. A glia-derived acetylcholine-binding protein that modulates synaptic transmission. Nature. 2001;411:261–268. doi: 10.1038/35077000. [DOI] [PubMed] [Google Scholar]
- Sudweeks SN, Yakel JL. Functional and molecular characterization of neuronal nicotinic ACh receptors in rat CA1 hippocampal neurons. J Physiol. 2000;527:515–528. doi: 10.1111/j.1469-7793.2000.00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulens C, Hogg RC, Celie PH, Bertrand D, Tsetlin V, Smit AB, Sixma TK. Structural determinants of selective α-conotoxin binding to a nicotinic acetylcholine receptor homolog AChBP. Proc Natl Acad Sci U S A. 2006;103:3615–3620. doi: 10.1073/pnas.0507889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J Mol Biol. 2005;346:967–989. doi: 10.1016/j.jmb.2004.12.031. [DOI] [PubMed] [Google Scholar]
- Young GT, Zwart R, Walker AS, Sher E, Millar NS. Potentiation of α7 nicotinic acetylcholine receptors via an allosteric transmembrane site. Proc Natl Acad Sci U S A. 2008;105:14686–14691. doi: 10.1073/pnas.0804372105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Pearson JE, Auerbach A. Φ-Value analysis of a linear, sequential reaction mechanism: theory and application to ion channel gating. Biophys J. 2005;89:3680–3685. doi: 10.1529/biophysj.105.067215. [DOI] [PMC free article] [PubMed] [Google Scholar]