Abstract
Volatile components, such as terpenoids, are emitted from aerial parts of plants and play a major role in the interaction between plants and their environment. Analysis of the composition and emission pattern of volatiles in the model plant Arabidopsis showed that a range of volatile components are released, primarily from flowers. Most of the volatiles detected were monoterpenes and sesquiterpenes, which in contrast to other volatiles showed a diurnal emission pattern. The active terpenoid metabolism in wild-type Arabidopsis provoked us to conduct an additional set of experiments in which transgenic Arabidopsis overexpressing two different terpene synthases were generated. Leaves of transgenic plants constitutively expressing a dual linalool/nerolidol synthase in the plastids (FaNES1) produced linalool and its glycosylated and hydroxylated derivatives. The sum of glycosylated components was in some of the transgenic lines up to 40- to 60-fold higher than the sum of the corresponding free alcohols. Surprisingly, we also detected the production and emission of nerolidol, albeit at a low level, suggesting that a small pool of its precursor farnesyl diphosphate is present in the plastids. Transgenic lines with strong transgene expression showed growth retardation, possibly as a result of the depletion of isoprenoid precursors in the plastids. In dual-choice assays with Myzus persicae, the FaNES1-expressing lines significantly repelled the aphids. Overexpression of a typical cytosolic sesquiterpene synthase resulted in the production of only trace amounts of the expected sesquiterpene, suggesting tight control of the cytosolic pool of farnesyl diphosphate, the precursor for sesquiterpenoid biosynthesis. This study further demonstrates the value of Arabidopsis for studies of the biosynthesis and ecological role of terpenoids and provides new insights into their metabolism in wild-type and transgenic plants.
INTRODUCTION
Plants produce a vast array of volatiles that mediate their interaction with the environment. A large portion of these volatiles consist of the terpenoids, also known as the isoprenoids because all are synthesized through the condensation of C5 isoprene units. Monoterpenes and sesquiterpenes represent the C10 and C15 terpene classes, respectively. These compound classes have typical characteristics, such as volatility, flavor/aroma, and toxicity, and hence play important roles in plant defense, plant-to-plant communication, and pollinator attraction (Pichersky and Gershenzon, 2002). Because of their diversity and distinct roles in plants, large efforts have been made to isolate and identify them (e.g., >1000 monoterpenes are known) and to understand their metabolism, physiological effects, and genetic regulation (Bohlmann et al., 1998).
The terpenoids, including the monoterpenes and sesquiterpenes, are derived from either the mevalonate pathway active in the cytosol/endoplasmic reticulum or the plastidial 2-C-methyl-d-erythritol 4-phosphate (MEP) pathway (Rodriguez-Concepción and Boronat, 2002). Although there is increasing evidence that there is an exchange of intermediates between compartments (Adam and Zapp, 1998; McCaskill and Croteau, 1998), the following generalizations can be made concerning the subcellular location of terpenoid biosynthesis in plants. The cytoplasmic mevalonate pathway is responsible for the production of sesquiterpenes (C15) and triterpenes (C30; e.g., sterols) and provides precursors for protein prenylation, ubiquinone, and heme A production in mitochondria. In plastids, the MEP pathway is responsible for the production of isoprene (C5), monoterpenes, diterpenes (C20; e.g., gibberellins), and tetraterpenes (C40; e.g., carotenoids). Both pathways lead to the formation of isopentenyl diphosphate (IPP) and its allylic isomer dimethylallyl diphosphate (DMAPP), the basic terpenoid biosynthesis building blocks. In each compartment, IPP and DMAPP are used by prenyltransferases in condensation reactions to produce larger prenyl diphosphates. Condensation of IPP and DMAPP catalyzed by the prenyltransferase geranyl diphosphate synthase yields geranyl diphosphate (GDP), the immediate precursor of monoterpenes. The condensation of two molecules of IPP and one of DMAPP by the action of farnesyl diphosphate (FDP) synthase generates the precursor for sesquiterpene biosynthesis. Condensation of two units of FDP produces squalene, the precursor of triterpenes and sterols. Another prenyltransferase generates geranyl geranyl diphosphate (GGDP), the precursor of diterpenes (C20) and tetraterpenes (C40). Similarly, further additions of IPP can produce longer prenyl diphosphates that can be converted subsequently to long-chain terpenoids such as rubber. After the construction of the acyclic precursors, such as GDP and FDP, the different monoterpenes and sesquiterpenes are generated through the action of terpenoid synthases (TPSs). Primary terpene skeletons formed by TPSs can be modified further by the action of various enzyme classes, such as the cytochrome P450 hydroxylases, dehydrogenases, and glycosyl and methyltransferases (Lange et al., 2000).
The development of advanced molecular tools and the existence of several conserved domains between TPS proteins gave rise to the cloning of a vast array of genes encoding TPSs from different plant species (e.g., Clarkia breweri [Dudareva et al., 1996], Lycopersicon esculentum [Colby et al., 1998], Salvia officinalis [Wise et al., 1998], Abies grandis [Bohlmann et al., 1999], Artemisia annua [Mercke et al., 1999], Zea mays [Shen et al., 2000], Elaeis oleifera [Shah and Cha, 2000], Mentha spp [Lange et al., 2000], Arabidopsis thaliana [Aubourg et al., 2002], Citrus limon [Lücker et al., 2002], and Cichorium intybus [Bouwmeester et al., 2002]; see also Bohlmann et al., 1998).
In recent years, examples of the production of transgenic plants expressing monoterpene synthases have been reported. Lücker et al. (2001) constitutively expressed the C. breweri S-linalool synthase (Lis) gene in Petunia hybrida. They demonstrated that linalool is produced in a number of organs but also that it is converted efficiently to the nonvolatile S-linalyl-β-d-glucopyranoside, probably by the action of endogenous glucosyltransferase activity. The expression of Lis in tomato under the control of a late-fruit-ripening promoter (E8 promoter) resulted in the accumulation of S-linalool and 8-hydroxy linalool in ripening fruit (Lewinsohn et al., 2001). The same gene also was expressed constitutively in carnation (Dianthus caryophyllus) flowers, and both free linalool and its cis- and trans-oxides were detected by headspace sampling of leaves and flowers of transgenic plants (Lavy et al., 2002). In an extract of transgenic flowers, only trans-linalool oxide was detected. Two sesquiterpene synthase genes also have been expressed in transgenic plants. Tobacco plants were transformed by Hohn and Ohlrogge (1991) with a fungal trichodiene synthase and by Wallaart et al. (2001) with the Artemisia annua amorpha-4,11-diene synthase. In both cases, only very low levels of the expected sesquiterpenoids were detected. The levels of trichodiene in cell-suspension cultures of the transgenic tobacco expressing trichodiene synthase could be increased 10-fold after elicitation (Zook et al., 1996). The elicited transgenic suspension culture also accumulated an oxygenated trichodiene derivative.
Complete genome sequencing and annotation revealed that the model plant Arabidopsis contains a large number of putative TPS genes. Aubourg et al. (2002) performed a detailed computer analysis of the Arabidopsis genome and reported a set of 40 terpenoid synthase homologs that cluster into five of the six phylogenetic subfamilies of the plant TPS superfamily. Recently, several of the putative TPS genes from Arabidopsis were characterized functionally. The AtTPS10 gene (At4g24210) was shown to encode a protein capable of converting GDP to the acyclic monoterpenes β-myrcene and (E)-β-ocimene (Bohlmann et al., 2000). Van Poecke et al. (2001) showed that both the expression of AtTPS10 and the production of β-myrcene were induced in Arabidopsis plants infested with Pieris rapae caterpillars. Another gene, AtTPS03 (At4g16740), was shown to encode a protein that in vitro produces almost exclusively (E)-β-ocimene (Fäldt et al., 2003). Upon mechanical wounding and treatment with jasmonic acid, headspace emission of (E)-β-ocimene was induced, and this correlated with a strong upregulation in the levels of AtTPS03 transcript. Chen et al. (2003) hypothesized that monoterpene and sesquiterpene production in Arabidopsis plants also is important for pollinator attraction. They demonstrated that >60% of the total volatiles emitted by Arabidopsis flowers are monoterpenes and sesquiterpenes (>95% of the total terpenes) and that most of them are emitted exclusively from the flowers. The recombinant protein encoded by At5g23960 was shown to be capable of generating four different products from FDP, including (−)-(E)-β-caryophyllene and α-humulene, which together account for 43% of the total volatiles emitted from flowers. Two other genes (At3g25810 and At1g61680) were shown to encode enzymes that catalyze the formation of β-myrcene and several monoterpene side products and S-linalool, respectively. Using reverse transcriptase–mediated PCR, the expression of 32 Arabidopsis genes was examined in various organs of the plants, and 6 of them showed complete or almost complete flower-specific expression.
The presence of this large family of TPS genes in Arabidopsis has made this plant species an attractive model for research on several aspects of terpenoid biosynthesis. The present study shows that Arabidopsis also can be used for the functional expression of terpene synthases and hence further demonstrates the potential of Arabidopsis for studies of the metabolism and ecological roles of terpenoids in plants.
RESULTS
Analysis of Arabidopsis Headspace Volatiles
We first examined several methods to measure volatiles, particularly terpenoids, emitted by wild-type Arabidopsis plants. The analysis of volatiles in Arabidopsis is not simple (Vainstein et al., 2001) because of the small plant size and the relatively low levels of the volatiles. The measurement of volatiles emitted by wild-type plants revealed that Arabidopsis emits an array of monoterpenes and sesquiterpenes, mainly from the flowers. The first method we used, solid-phase microextraction (SPME), allows fast, high-throughput, and semiquantitative headspace measurements. The high sensitivity of the SPME method permits the detection of volatiles from only two fully expanded, detached rosette leaves of 4-week-old Arabidopsis plants (Figure 1A). Using an autosampler that automatically exposes an SPME fiber to the sample headspace and introduces it into the injection port of the gas chromatography–mass spectrometry (GC-MS) apparatus, approximately 40 Arabidopsis lines could be screened during an overnight run. In leaves of wild-type Arabidopsis, using this method, trace amounts of limonene and β-myrcene were detected.
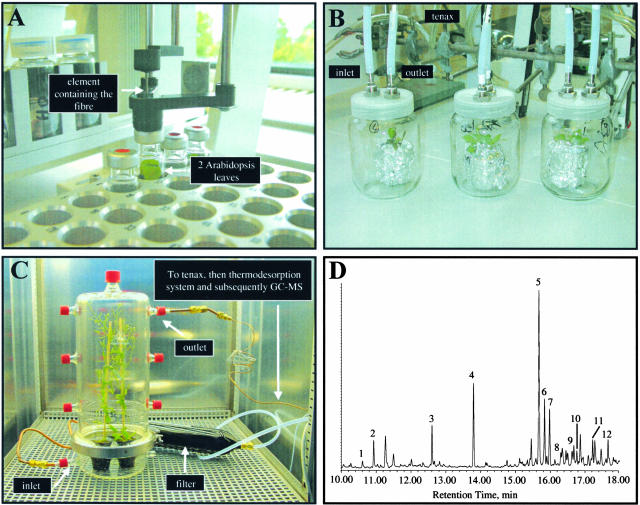
Figure 1.
Detection of Arabidopsis Headspace Volatiles by SPME, Tenax Trapping/Elution, and Tenax Trapping/Thermodesorption.
(A) The SPME method permits the detection of volatiles from only two fully expanded, detached rosette leaves of 4-week-old Arabidopsis plants. Using an autosampler that automatically exposes a fiber to the sample headspace and introduces it into the injection port of the GC-MS apparatus, 40 Arabidopsis lines could be screened during an overnight run.
(B) The Tenax system for the detection of headspace volatiles from intact Arabidopsis plants. Air is drawn through the inlet through a Tenax matrix, and the headspace volatiles emitted by the plant are absorbed at the outlet on a second Tenax matrix. Volatiles are eluted from the Tenax using pentane:diethyl ether (4:1) and are analyzed by GC-MS. In this study, headspace sampling was conducted for 24 h.
(C) Tenax trapping for the detection of headspace volatiles from intact Arabidopsis plants coupled to an automatic thermodesorption system. Filtered air is introduced to the glass container headspace, volatiles are trapped on Tenax, and after trapping, volatiles are released by thermodesorption and cryofocused before their introduction into the GC-MS apparatus. The sampling and GC-MS analysis were performed automatically, so that detailed emission patterns were obtained during a certain period of time.
(D) Typical chromatogram obtained after performing the analysis described in (C) and sampling headspace volatiles emitted by two flowering Arabidopsis plants. A subset of the volatiles are depicted: peak 1, linalool; peak 2, nonanal; peak 3, decanal; peak 4, impurity; peak 5, β-caryophyllene; peak 6, impurity; peak 7, thujopsene; peak 8, α-humulene; peak 9, α-farnesene; peak 10, β-bisabolene; peak 11, (Z)-nerolidol; and peak 12, (E)-nerolidol.
Tenax trapping, the second method we used for headspace analysis of Arabidopsis, is less sensitive compared with the SPME method, but it has the advantage that it allows quantitative analysis of volatiles emitted from an intact plant with no need to detach leaves or other tissues and that it allows for replication of the GC-MS analysis. The compactness of Arabidopsis, especially when the plant is only a few weeks old and has not produced a flowering stem, makes it most suitable for such analysis (Figure 1B). In the Tenax trapping system, headspace volatiles emitted by the plant are first trapped by a matrix of Tenax and then released from it using an organic solvent and subsequently injected into the GC-MS apparatus. In this study, headspace sampling of Arabidopsis plants using Tenax trapping was conducted for 24 h with up to eight plants analyzed simultaneously in separate cuvettes. Using Tenax trapping and subsequent elution and injection, terpenoids could be detected when sampling a number of flowering stems (∼10 enclosed in one cuvette). In this way, several sesquiterpenes were detected in the headspace, including (E)-β-caryophyllene, thujopsene, β-chamigrene, and three additional sesquiterpenes that could not be identified (data not shown). The only terpenoid detected in nonflowering plants by the Tenax trapping was traces of the monoterpene limonene.
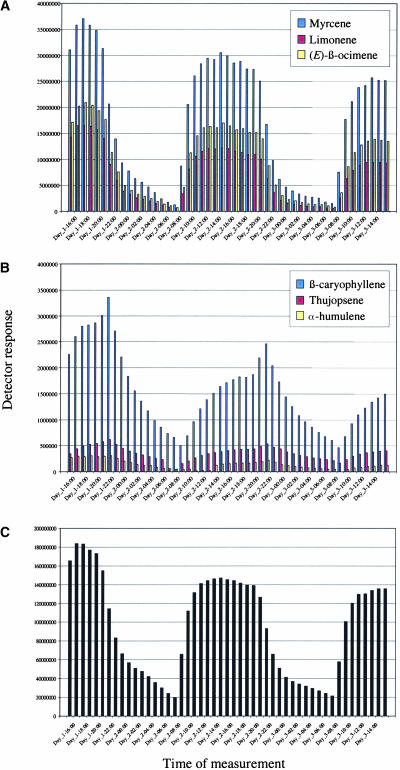
An alternative to eluting the volatiles trapped by the Tenax matrix is to concentrate them using a thermodesorption system followed by injection into the GC-MS apparatus (Figure 1C). This method allows both a quantitative and a most sensitive analysis, and an array of both monoterpenes and sesquiterpenes as well as nonterpenoids emitted from a pair of flowering Arabidopsis plants were detected in this way (Figure 1D; see also supplemental data online). Tenax trapping coupled to automatic thermodesorption analysis every hour for 2 subsequent days revealed that a large part of the Arabidopsis volatiles were emitted in a diurnal rhythm (see supplemental data online). Interestingly, nearly all monoterpenes and sesquiterpenes showed a diurnal pattern of emission, whereas other types of headspace components did not show such a rhythm (e.g., decanal, nonanal, and various alkanes). Several examples demonstrating diurnal emission of monoterpenes [myrcene, (E)-β-ocimene, and limonene] and sesquiterpenes [(E)-β-caryophyllene, thujopsene, and α-humulene] are shown in Figures 2A and 2B, respectively.
Figure 2.
Diurnal Emission of Endogenous Terpenoids and the Heterologous Linalool in Flowering Arabidopsis Plants.
Volatiles trapped on Tenax were analyzed using thermodesorption and injection into the GC-MS apparatus. Sampling was performed every hour during 48 h with two flowering FaNES1-expressing plants.
(A) Three endogenous monoterpenes showing a diurnal pattern of emission.
(B) Three endogenous sesquiterpenes showing a diurnal pattern of emission.
(C) Diurnal emission pattern of linalool produced by the expression of FaNES1.
Transgenic Arabidopsis Plants Expressing the FaNES1 Gene Produce Linalool and Nerolidol
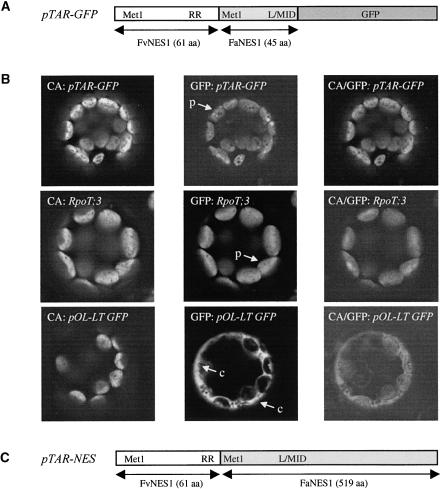
To demonstrate the potential of Arabidopsis for the heterologous expression of terpenes, the strawberry FaNES1 gene (A. Aharoni and H.J. Bouwmeester, unpublished data) was used for the transformation of Arabidopsis. Recombinant FaNES1 catalyzes the biosynthesis of the monoterpene alcohol linalool and its sesquiterpene counterpart nerolidol from GDP and FDP, respectively. FaNES1 was expressed in Arabidopsis under the control of the doubled 35S promoter of Cauliflower mosaic virus (CaMV). The protein encoded by FaNES1, normally localized to the cytosol, was directed to the plastids by the fusion of a plastid-targeting signal derived from the wild strawberry counterpart of FaNES1 (termed FvNES1; A. Aharoni and H.J. Bouwmeester, unpublished data) to its N terminus. In vivo localization experiments using green fluorescent protein (GFP) fused to a hybrid construct containing the 61–amino acid targeting signal of FvNES1 and a 45–amino acid region of the FaNES1 N terminus (pTAR-GFP; Figure 3A) showed that GFP was targeted to plastids of tobacco protoplasts (Figure 3B). Plastids are the known site for monoterpene production; therefore, we expected the production of the monoterpene linalool. However, the dual monoterpene and sesquiterpene synthase activity of the FaNES1 enzyme also would allow the production of nerolidol, if FDP is available and sesquiterpene biosynthesis could occur in Arabidopsis plastids. In addition, because linalool and nerolidol are emitted only by flowering Arabidopsis plants (see supplemental data online) and not by the rosette leaves, we anticipated a relatively easy detection of these compounds in the leaves of transgenic plants that produce and emit them. Subsequently, the pTAR-NES binary construct (Figure 3C) was used to generate transgenic Arabidopsis plants constitutively expressing the FaNES1 gene and to target the FaNES1 protein to the plastids.
Figure 3.
Transformation of Arabidopsis Plants with a Plastid-Targeted FaNES1.
(A) Fusion of the 61–amino acid (aa) N terminus encoded by the wild strawberry FvNES1 gene (A. Aharoni and H.J. Bouwmeester, unpublished data) to the coding region of the cultivated species FaNES1 gene fused to the GFP. L/MID is a conserved motif in a large number of monoterpene synthases. The RR and L/MID are conserved motifs in a large number of monoterpene synthases.
(B) Testing the GFP localization of the pTAR-GFP construct shown in (A). RpoT;3 is a positive control for targeting to plastids (Hedtke et al., 1999), whereas pOL-LT-GFP (full name, pOL-LT-GFP-L64T65; a gift from Ian Small) is the basic vector used to generate the pTAR-GFP construct in which GFP is localized to the cytosol and the nucleoplasm. GFP fluorescence was detected in plastids (p) with both pTAR-GFP and RpoT;3 and in the cytosol (c) with pOL-LT-GFP, as indicted by the arrows. CA, chlorophyll autofluorescence detected in channel 1; GFP, green fluorescent protein detected in channel 2; CA/GFP, an overlay of the images obtained from both channels.
(C) Scheme of the fusion construct harbored by the pTAR-NES binary vector that was used to transform Arabidopsis plants.
More than 120 putative transgenic plants were generated, and 4 weeks after transfer to soil, two rosette leaves from each individual line were screened for terpenoid emission by the SPME method. As expected, leaves of wild-type Arabidopsis did not produce any detectable linalool; however, transgenic lines showed varying levels of linalool emission (data not shown). Moreover, plants that emitted high levels of linalool also emitted low levels of the sesquiterpene alcohol nerolidol.
We further analyzed the headspace volatiles produced during 24 h by intact transgenic plants using the Tenax trapping/elution method (vegetative plants). The six primary transformants (T1) examined (in three separate experiments) showed a pattern of linalool and nerolidol emission similar to that shown in the SPME analysis (i.e., a high emission of linalool usually was accompanied by detectable amounts of nerolidol) (Table 1, Figure 4). Integration and copy number of the FaNES1 gene and its expression in several transgenic plants (T1 lines) were confirmed by DNA and RNA gel blot analyses (Table 1). Lines with a single T-DNA copy showed high levels of transgene expression that correlated with high levels of linalool emission. Two of the T1 lines (Tr-9 and Tr-26) containing a single T-DNA copy were selected for characterization of subsequent generations.
Table 1.
Linalool and Nerolidol Emission of Nonflowering Wild-Type and Transgenic Arabidopsis Plants
| Plant a | T-DNA Copy Number |
Transgene Expression |
Linalool and Nerolidol Emission (μg·day−1·plant−1)
|
|||||
|---|---|---|---|---|---|---|---|---|
| Day 1b
|
Day 2
|
Day 3
|
||||||
| linc | ner | lin | ner | lin | ner | |||
| Tr-4 | 7 | + | 0.17 | ndd | 0.09 | nd | 0.04 | nd |
| Tr-6 | 4 | + + | 0.56 | nd | 0.80 | nd | 0.70 | nd |
| Tr-9 | 1 | + + + | 9.59 | 0.03 | 9.90 | 0.14 | 9.49 | 0.02 |
| Tr-17 | 1 | + + | 7.67 | nd | 13.33 | 0.10 | 13.02 | 0.10 |
| Tr-19 | 5 | + | nd | nd | 0.05 | nd | 0.01 | nd |
| Tr-26 | 1 | + + + | 7.17 | 0.04 | 12.65 | 0.16 | 9.94 | 0.05 |
| Nt-4 | − | − | nd | nd | nd | nd | nd | nd |
| Nt-6 | − | − | nd | nd | nd | nd | nd | nd |
Volatiles were trapped on Tenax and eluted and analyzed using GC-MS as described in Methods. T-DNA copies and transgene expression levels (+ = low, ++ = intermediate, and +++ = high) were determined by DNA and RNA gel blot analyses, respectively. Nt, not transformed; Tr, transgenic.
Six-week-old nonflowering, plants.
Trapping was performed for 16 h.
lin, linalool; ner, nerolidol.
nd, not detected.
Figure 4.
Simultaneous Emission of Linalool and Nerolidol by FaNES1-Expressing Arabidopsis Plants.
Headspace Tenax trapping and subsequent GC-MS analysis show emission of linalool and nerolidol from vegetative parts of transgenic Arabidopsis and their absence in the headspace profile of the wild-type plant.
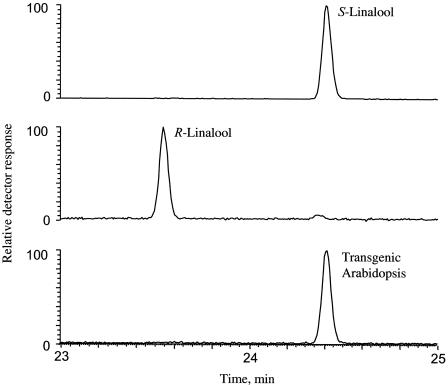
In vitro, the FaNES1 recombinant protein exhibited high enantioselectivity, producing for >99% the S-enantiomer of linalool, which is the major enantiomer produced by cultivated strawberry fruit during ripening (A. Aharoni and H.J. Bouwmeester, unpublished data). Multidimensional GC-MS analysis revealed that the transgenic Arabidopsis plants produced exclusively S-linalool (Figure 5). Remarkably, the linalool emission by transgenic plants showed a clear diurnal rhythm of emission, just like that of the endogenous terpenes (Figure 3C). Quantification of the linalool production of a number of transgenic lines using Tenax trapping/elution showed that the levels of linalool released amounted to 7.2 to 13.3 μg·day−1·plant−1 for the highest producing lines, Tr-9, Tr-26, and Tr-17 (Table 1). Levels of nerolidol emitted were 100- to 300-fold lower than those of linalool, ranging from 0.02 to 0.16 μg·day−1·plant−1 (Table 1).
Figure 5.
Enantioselective Multidimensional GC-MS Analysis of Linalool Isolated from Leaves of FaNES1-Expressing Arabidopsis Plants.
The top and middle chromatograms show the S- and R-linalool standards, respectively, and the bottom chromatogram shows linalool isolated from transgenic Arabidopsis.
Transgenic Arabidopsis Plants Expressing FaNES1 Produce Hydroxylated and/or Glycosylated Derivatives of Linalool
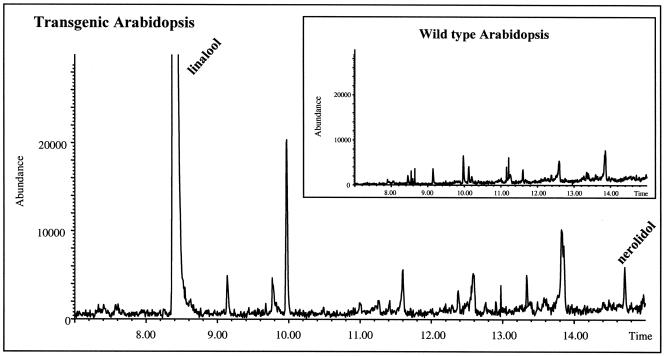
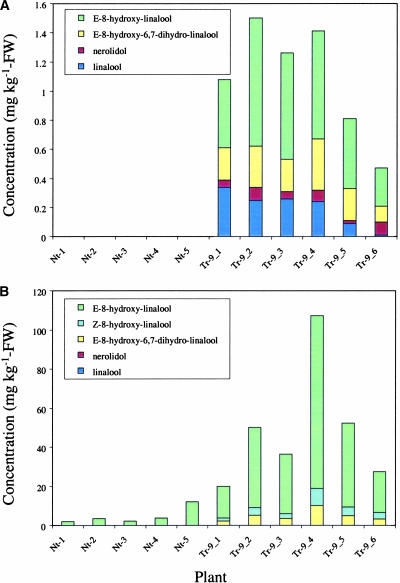
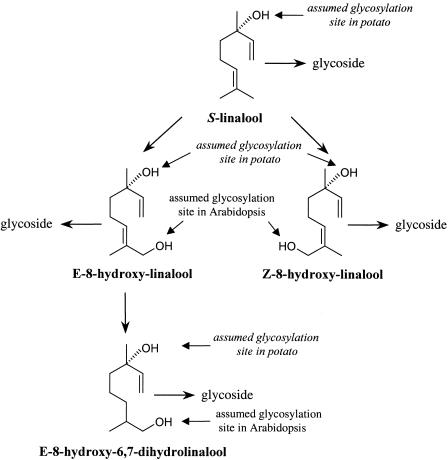
To study the fate of linalool in planta, volatiles of Arabidopsis rosette leaves were isolated by solid-phase extraction and subjected to GC-MS analysis. Linalool and nerolidol were not detected in any of the extracts obtained from wild-type plants, but transgenic plants contained up to 1.5 μg/g (fresh weight) of linalool, nerolidol, E-8-hydroxylinalool (largest amount), and E-8-hydroxy-6,7-dihydrolinalool (Figure 6A). Plants used for the analysis were derived from the progeny of the self-pollinated Tr-9 primary transformant (T1). Thus, the production of linalool and nerolidol was inherited by the next generation. The presence of glycosidically bound terpenoids was assessed after enzymatic hydrolysis of glycosides with Rohapect DL (see Methods). GC-MS analysis of liberated alcohols showed that wild-type plants contained some glycosylated E-8-hydroxylinalool, but much higher amounts of E-8-hydroxylinalool (up to 110 μg/g fresh weight) were released from the glycosidic extract obtained from transgenic plants (Figure 6B). In addition, glycosides of Z-8-hydroxylinalool (not detected as free alcohol) and E-8-hydroxy-6,7-dihydrolinalool were observed in transgenic plants but not in the wild-type plants. Interestingly, in some cases, the sum of glycosidically bound volatiles was 40- to 60-fold higher than the sum of free alcohols. Linalyl-β-d-glucopyranoside, which was demonstrated previously to be produced in transgenic petunia plants expressing the C. breweri linalool synthase gene Lis (Lücker et al., 2001), was not detected by liquid chromatography–MS analysis in either wild-type or transgenic Arabidopsis plants (data not shown). However, the ion trace m/z (mass-to-charge ratio) 391 of the glycosidic extracts obtained from transgenic Arabidopsis plants showed a compound that eluted before linalyl-β-d-glucopyranoside under reversed-phase conditions. This compound is more polar than linalyl-β-d-glucopyranoside and displays the pseudomolecular ion mass m/z 391, which makes it likely that it represents 8-hydroxylinalyl glucoside. Although linalool and nerolidol were present in transgenic Arabidopsis leaves, glycosylated tertiary alcohols were not observed. Thus, we assume that the carbohydrate moiety in 8-hydroxylinalyl glycoside is attached to the primary hydroxyl group. A scheme of the linalool derivatives detected in transgenic Arabidopsis plants is shown in Figure 7.
Figure 6.
Linalool, Linalool Derivatives, and Nerolidol Produced by Leaves of Wild-Type and FaNES1-Expressing Arabidopsis Plants.
Free (A) and glycosidically bound (B) terpenoids of Arabidopsis rosette leaves were isolated by solid-phase extraction, subjected to GC-MS analysis, and quantified. The plants are progeny (T2) of the selfed Tr-9 primary transformant (T1). FW, fresh weight; Nt, not transformed; Tr, transgenic.
Figure 7.
Linalool and Its Derivatives Produced by FaNES1-Expressing Arabidopsis Plants.
The assumed glycosylation sites in linalool and its derivatives produced by leaves of transgenic Arabidopsis and potato plants are indicated. Potato plants were transformed with the same construct used to express FaNES1 in Arabidopsis (A. Aharoni and M.A. Jongsma, unpublished data).
Transgenic Arabidopsis Plants Expressing FaNES1 Are Delayed in Growth and Development
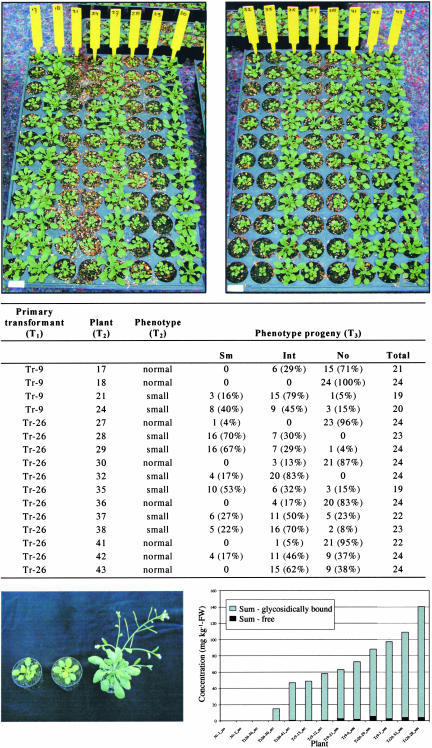
Transgenic Arabidopsis plants overexpressing FaNES1 grew less vigorously than their nontransgenic counterparts (Figure 8). Both the basal rosette and the flowering stems were retarded in growth. Because of the delayed development of the transgenic plants, they had a much younger appearance, including lighter green leaves, but eventually they produced flowering stems and viable seeds. The progeny derived from >16 selfed T2 lines showed the typical growth-retardation phenotype (e.g., T2 plant 28; Figure 8), others showed a mixture of retarded and normally growing plants (e.g., T2 plant 42; Figure 8), whereas a third group contained only normally growing plants (e.g., T2 plant 41; Figure 8). These three groups probably are the result of the self-pollination of dominant homozygous, heterozygous, and homozygous recessive T2 lines. The results demonstrate that the growth-retardation phenotype is not part of the natural variation but is inheritable through several generations of transgenic plants. We also analyzed volatile levels in transgenic lines showing growth retardation versus normally growing lines. The severity of the phenotype correlated with the levels of linalool and its derivatives that were produced, thus confirming the relationship between FaNES1 expression levels and the retarded growth of the transgenic plants (Figure 8). One of the possible explanations for the growth-retardation phenotype in the transgenic lines is the depletion of the IPP pool in the plastids, which may lead to reductions in the levels of essential isoprenoids. HPLC analysis of various plastid-derived isoprenoids such as chlorophyll a, chlorophyll b, lutein, and β-carotene revealed no significant changes in the levels of these components when FaNES1 transgenic and wild-type Arabidopsis plants were compared (data not shown).
Figure 8.
FaNES1-Expressing Arabidopsis Plants Show Growth Retardation.
T3 progeny of selfed T2 transgenic FaNES1 lines are shown at top (each column of the growth tray is labeled with the T2 line number). The T2 lines originated from either the Tr-9 or Tr-26 primary transformant (T1). In the table, 30-day-old plants were scored as either small (Sm; inflorescence stems of <5 cm), intermediate (Int; inflorescence stems of 5 to 16 cm), or normal (No; inflorescence stems of >16 cm). The percentage of lines showing one of the three phenotypes (Sm, Int, or No) among the total number of T3 plants examined per T2 line (total) is presented. At bottom left, a closer observation of two Tr-9 selfed progeny lines (left and middle) and a wild type-plant (right) of the same age is shown. The graph at bottom right shows the correlation between the size of FaNES1-expressing Arabidopsis plants and the production of free and glycosylated linalool, linalool derivatives, and nerolidol. Free and glycosidically bound terpenoids of Arabidopsis rosette leaves were isolated by solid-phase extraction, subjected to GC-MS analysis, and quantified. The plants are progeny (T2) of the selfed Tr-9 and Tr-26 primary transformants (T1). FW, fresh weight; Nt, not transformed; Tr, transgenic.
The Behavior of Myzus persicae Aphids Is Influenced by Volatiles Produced in Transgenic Plants Expressing FaNES1
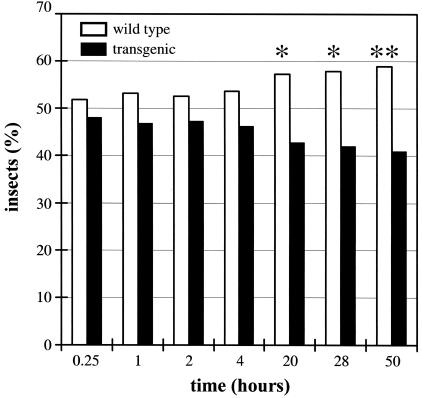
Plant volatiles, including monoterpenes such as linalool, may influence the behavior of insects, either by attracting or repelling them. Therefore, the transgenic plants generated in the course of this study, emitting volatile terpenoids that are normally not released by wild-type Arabidopsis, were a good experimental material in which to study the effect of volatiles on insect behavior. We investigated the preference of the aphid Myzus persicae (an important agricultural pest) toward detached leaves of transgenic FaNES1-expressing plants and wild-type plants in a dual-choice assay (see Methods for experimental design and statistical analysis). The results show that with time (from 20 h onward), aphids preferred leaves derived from wild-type plants (Figure 9). Linalool and its derivatives produced by the transgenic plants seemed to act as repellents of M. persicae aphids.
Figure 9.
The Behavior of M. persicae Aphids Is Influenced by Volatiles Produced in Transgenic Plants Expressing the FaNES1 Gene.
The graph shows the preference of M. persicae aphids (represented by percentage) for detached leaves of either wild-type plants (open bars) or transgenic FaNES1-expressing plants (closed bars) in a dual-choice assay. The calculated P values of the one-sided Wilcoxon signed-rank test (Hollander and Wolfe, 1973) showed significant evidence for repellence by the transgenic lines at the last three time points (*, P < 0.05; **, P < 0.01).
Transgenic Arabidopsis Plants Expressing the CiGASlo Gene Emit Low Levels of Germacrene A
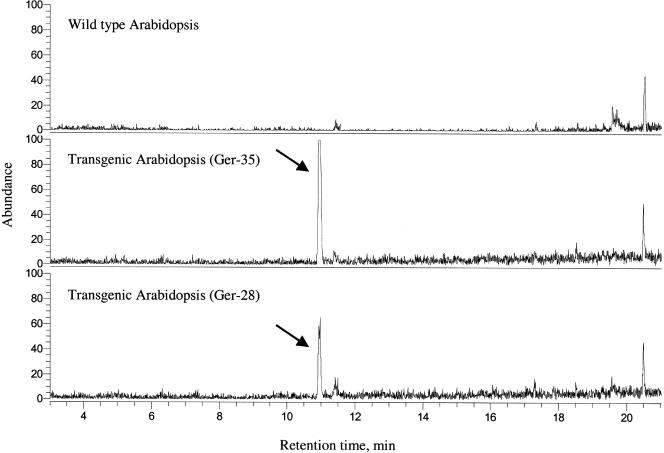
As described above, transgenic Arabidopsis plants produced only low levels of the sesquiterpene nerolidol when the FaNES1 protein was targeted to the plastids. Therefore, we analyzed whether the cytosolic expression of a sesquiterpene synthase gene would result in higher levels of sesquiterpene production. Our initial plan was to overexpress the native FaNES1 in the cytosol. However, probably as a result of the toxicity of nerolidol generated from the endogenous FDP pool by the FaNES1 enzyme in Agrobacterium tumefaciens, we could not obtain any A. tumefaciens transformants. We next decided to overexpress a different sesquiterpene synthase that produces a terpene that is less toxic and will allow us to introduce the binary construct into A. tumefaciens for plant transformation. Thus, we generated transgenic Arabidopsis plants overexpressing the germacrene A synthase gene (CiGASlo), isolated previously from chicory (Bouwmeester et al., 2002). Another advantage of using CiGASlo was that the risk is lower that it would be further converted compared with nerolidol. CiGASlo was expressed in plants under the control of the doubled 35S CaMV promoter, and transgenic plants were subjected to an initial screen of volatile production using the SPME method. This study unambiguously demonstrated the presence of β-elemene, the thermal rearrangement product of germacrene A (de Kraker et al., 1998) in the headspace of the transgenic lines (Figure 10). Based on the ratios of peak areas in the SPME analysis and on the difference in affinity of the SPME fiber, as determined using standards, we estimated that germacrene A emission from the CiGASlo-expressing lines was at least 1000-fold lower than that of linalool from the FaNES1-expressing plants.
Figure 10.
Headspace Measurements Using SPME of Detached Rosette Leaves of Transgenic Arabidopsis Plants Overexpressing the Germacrene A Synthase Gene from Chicory.
Trace amounts of β-elemene (the thermal rearrangement product of germacrene A; de Kraker et al., 1998) was detected in the headspace of the transgenic lines (Ger-28 and Ger-35) but not in wild-type control Arabidopsis. The chromatogram shows m/z 93, a typical m/z value for many monoterpenes and sesquiterpenes, but β-elemene was identified using the full mass spectrum (de Kraker et al., 1998).
DISCUSSION
To date, various aspects of terpenoid biosynthesis, such as substrate availability, the compartmentation of different metabolic branches, and the coregulation between them, are far from being completely understood. The study of terpenoid biosynthesis, particularly of monoterpenes and sesquiterpenes, traditionally has been performed in more exotic and nonmodel species, such as mint and conifers, that produce large amounts of terpenoid substances in specialized secretory cells (McConkey et al., 2000). Only in recent years has the issue of terpenoid biosynthesis been studied in more approachable plant species, such as petunia, tomato, and Arabidopsis (for review, see Mahmoud and Croteau, 2002). In such species, the terpenoid biosynthetic pathway might not be so prominent and active as in the nonmodel species mentioned above, and in some cases, it can even be localized in different cells and/or cell compartments. Arabidopsis, as a result of its known advantages, such as extensive genetic knowledge, including a sequenced genome, its short life cycle, and its compactness, also might be a suitable system for studies of terpenoid biosynthesis. It also is very easily transformed, and here, we show that transgenic Arabidopsis plants that produce terpenes could be generated relatively easily and screened efficiently for the presence of volatile terpenoids. Such plants could be used for a multitude of fundamental studies of the regulation of terpenoid metabolism.
Detection of Volatile Terpenoids in Arabidopsis
Annotation of the Arabidopsis genome revealed 40 genes that putatively encode terpenoid synthases. To date, several of these sequences have been shown experimentally to encode monoterpene or sesquiterpene synthases (Bohlmann et al., 2000; Chen et al., 2003; Fäldt et al., 2003). When we analyzed the headspace of intact flowering Arabidopsis plants using the Tenax/thermodesorption system, we detected more than two dozen monoterpenes and sesquiterpenes (see supplemental data online). As described previously (Chen et al., 2003), those components were emitted mainly from flowers, whereas only trace amounts of the monoterpenes limonene and β-myrcene were detected when the headspace of aerial vegetative tissues was analyzed using the SPME method. As shown in this study, the nearly complete absence of volatile terpenoids in the headspace of vegetative Arabidopsis tissue makes the production of newly produced terpenes easily detectable. Interestingly, nearly all terpenoids detected in the flower headspace showed a clear diurnal emission pattern, whereas other nonterpenoid components did not show such rhythmicity (Figure 2). The diurnal emission of monoterpenes and sesquiterpenes might be attributed to the role of floral scent in the attraction of insect pollinators (Raguso et al., 1996; Raguso and Pichersky, 1999), which has been suggested to occur at low frequency in wild-type Arabidopsis flowers (Snape and Lawrence, 1971). Surprisingly, linalool produced in transgenic FaNES1-expressing plants also showed a clear diurnal pattern of emission. Because the expression of FaNES1 was driven by a constitutive 35S CaMV promoter, the availability of the GDP precursor probably regulates the emission pattern. However, further experiments are needed to clarify the diurnal emission of endogenous monoterpenes and sesquiterpenes and the introduced linalool, because this observation also might be explained in other ways. These include the activities of glycosidase or glycosyltransferase enzymes making the monoterpenes more or less volatile, and in the case of the newly introduced terpene synthase FaNES1, light activation of the 35S CaMV promoter (Schnurr and Guerra, 2000) might play a role. The observation of the diurnal emission of terpenoids in Arabidopsis makes the use of transgenic Arabidopsis a perfect tool for the study of floral scent and its regulation.
Production of Monoterpenes and Sesquiterpenes in Transgenic Arabidopsis Plants
Overexpression of the strawberry FaNES1 gene in transgenic Arabidopsis plants resulted in the production of substantial levels of free, hydroxylated, and glycosylated linalool derivatives. The levels emitted by young plants (up to 13.3 μg·day−1·plant−1; Table 1) surpassed the levels produced by flowering wild-type Arabidopsis as reported by Chen et al. (2003) (0.5 μg·day−1·plant−1) by up to 27-fold (although it has to be realized that the flower volatiles are produced by only a small fraction of the total plant). As a result of the ability of the FaNES1-encoded protein to produce both a monoterpene (linalool) and a sesquiterpene (nerolidol), we could simultaneously evaluate the possibility of forming both types of terpenoids and the availability of their precursors in plastids. Rapid screening of transgenic lines for the production of linalool and nerolidol was possible because of the use of a high-throughput, automatic system for headspace measurements using SPME. Such measurements, although qualitative, permit fast screening of young transgenic lines (4 weeks old) by analyzing samples composed of only two Arabidopsis rosette leaves. The results showed that GDP, the monoterpene precursor, is available in plastids of Arabidopsis in high enough amounts to allow substantial de novo monoterpene biosynthesis. Surprisingly, trace amounts of nerolidol also were produced, suggesting that FDP also was available, albeit at low levels. The presence of FDP is surprising, because FDP production is assumed to occur either in the cytosol or in the mitochondria. The Arabidopsis proteome contains three FDP synthase isoforms: FPS1S and FPS2 encode cytosol/endoplasmic reticulum–targeted proteins, whereas FPS1L encodes a mitochondria-targeted protein (Cunillera et al., 1997). This finding suggests that either the exchange of prenyl precursors between the cytosol and the plastids, as demonstrated by several authors, also includes FDP (Adam and Zapp, 1998; Lichtenthaler, 2000) or that FDP in the plastids is released by other prenyltransferases such as GGDP synthase. The possibility that not all of the FaNES1 protein is transported into the plastids and that the small amount remaining in the cytosol has access to the cytosolic pool of FDP cannot be excluded, although we did not detect GFP expression in the cytosol of protoplasts used to examine the targeting of the FaNES1 protein (Figure 3).
In contrast to transgenic plants overexpressing monoterpene synthases, the low production of transgenic plants overexpressing sesquiterpene synthases seems to be a general phenomenon. Just as we have shown for germacrene A synthase expression in Arabidopsis (Figure 10), other authors have reported low production of sesquiterpenes from transgenic tobacco plants overexpressing trichodiene synthase and amorpha-4,11-diene synthase (Hohn and Ohlrogge, 1991; Wallaart et al., 2001). The pool of FDP in the cytosol also is used for the production of triterpenes and sterols in the same compartment. When free FDP is available in the cytosol, one would assume that the FDP pool could be shared by an introduced sesquiterpene synthase using the same substrate. Because of the importance of sterols to plant vitality, it is conceivable that the FDP pool used for the production of sterols is regulated such that it does not allow sharing by other metabolic routes, as suggested by Chappell et al. (1995). Supporting evidence for the tight control of FDP utilization in the cytosol of Arabidopsis plants was provided by Masferrer et al. (2002). Transgenic plants producing increased amounts of active FDP synthase were affected in their phenotype but did not show any change in total sterol levels or sterol profiles.
Previous studies of various transgenic plants expressing the C. breweri linalool synthase gene Lis demonstrated that the linalool formed is metabolized further to either glycosylated or hydroxylated forms. Lücker et al. (2001) showed that transgenic Lis petunia plants emit only low levels of free linalool from flowers, whereas no free linalool was detected in leaves. They observed that all of the linalool produced was converted to the nonvolatile linaloyl-β-d-glucoside. Carnation plants expressing the same gene produced and emitted linalool and its oxides from flowers (Lavy et al., 2002), whereas transgenic tomato produced linalool and 8-hydroxylinalool from ripe fruit (Lewinsohn et al., 2001). Transgenic potato plants overexpressing FaNES1 (using the same construct described in this study; A. Aharoni and M.A. Jongsma, unpublished data) also produced an array of different linalool derivatives (Figure 7).
In transgenic Arabidopsis plants, we identified a different pattern of linalool derivatization than that reported for the other species mentioned above. Both free and glycosylated linalool and linalool derivatives were identified in leaf extracts, with total levels of glycosides at least 10-fold higher (up to >60-fold in some transgenic lines) than those of the free alcohols (Figure 6). E-8-hydroxy-linalool was the predominant component in both the free alcohols as well as the glycosidic fraction. This specific pattern of linalool hydroxylation probably is the result of an endogenous Arabidopsis hydroxylase, an assumption that is supported by the presence of only E-8-hydroxy-linalool but not linalool or other linalool derivatives in leaves of wild-type Arabidopsis. Apparently, the metabolism of the monoterpene introduced in a transgenic plant is determined by the genetic background of the plant species.
The Cost of Producing High Levels of Terpenes in Arabidopsis
Transgenic Arabidopsis plants expressing FaNES1 were retarded in their growth compared with wild-type plants grown under identical greenhouse conditions. This phenotype was transmitted to subsequent generations and correlated with levels of linalool production. One major explanation for such a phenotype might be the reduction in the availability of substrates for other metabolites produced in the plastids, which play an important role in plant growth and development. These include the growth regulators gibberellin and abscisic acid and other vital components such as carotenoids, chlorophyll, and quinones. Transgenic tomato plants constitutively expressing the carotenogenic enzyme phytoene synthase showed a dwarf phenotype (Fray et al., 1995). This phenotype was shown to result from a reduction in the levels of gibberellin A1, synthesized from GGDP, the common precursor of gibberellins and carotenoids. A similar effect was reported recently for transgenic tobacco plants expressing high levels of the same enzyme (Busch et al., 2002). Overexpression of the Arabidopsis FDP synthase in transgenic Arabidopsis led to a reduction of IPP and DMAPP available for the biosynthesis of the growth regulator cytokinin (Masferrer et al., 2002). The authors suggested that the reduction in cytokinin levels could have caused, at least in part, the phenotypic alteration observed in wild-type plants (a cell death/senescence–like phenotype and less vigorous growth compared with transgenic plants). In this study, we did not detect a significant change in the levels of chlorophyll a, chlorophyll b, β-carotene, and lutein in the FaNES1-expressing plants compared with wild-type plants. It remains to be determined whether alteration in the levels of gibberellins and abscisic acid are the cause of the phenotype observed in the transgenic FaNES1-expressing plants.
A second explanation for the phenotypic alteration we observed in transgenic FaNES1 plants might be the toxic effect of the high levels of linalool produced, because several monoterpenes, including linalool, have been demonstrated to be harmful to plant cells (Muller et al., 1968; Weidenhamer et al., 1993). It should be noted that transgenic petunia, tomato, and carnation plants that produce high levels of linalool and its derivatives were not reported to show a similar phenotype, although a very similar effect to the one detected here in Arabidopsis was observed in transgenic tobacco plants and their progeny overexpressing a C. limon monoterpene synthase (J. Lücker, personal communication). In addition, transgenic potato plants that express FaNES1 and produce high levels of linalool showed yellow and damaged regions on their leaf blades (A. Aharoni and M.A. Jongsma, unpublished data). The problem we encountered in this study when attempting to transform A. tumefaciens with the construct harboring FaNES1 lacking a targeting signal (for cytosolic expression) further demonstrates the toxicity of compounds such as nerolidol, not only for plants but also for microorganisms. Both linalool and nerolidol (particularly nerolidol) have been shown to possess antimicrobial activity against gram-positive bacteria (e.g., the cariogenic Streptococcus mutans), gram-negative bacteria (e.g., Escherichia coli), yeast (e.g., Saccharomyces cerevisiae), and molds (e.g., Penicillium chrysogenum) (Kubo et al., 1993). Thus, the cost of increasing monoterpene levels should be considered when deciding on strategies for the metabolic engineering of monoterpene biosynthesis in plants.
Despite the effects on growth, it is clear from the work described here that transgenic Arabidopsis plants could serve as a valuable tool in the study of the genetic regulation of terpenoid metabolism, including its subcellular compartmentation, in plants. Such plants, if altered only in their floral scent profile, could be used for the study of pollinator attraction and the regulation of rhythmic volatile emissions in plants. In addition, monoterpenes and sesquiterpenes have been shown to be of ecological significance in plant defense (Phillips and Croteau, 1999; Degenhardt et al., 2003). In this study, we showed by dual-choice assays that the volatiles produced by the FaNES1-expressing plants (mainly linalool) influence the behavior of M. persicae aphids. The same aphid species was reported previously to be repelled by low concentrations of pure linalool when tested using an olfactometer (Hori, 1998). Chen et al. (2003) reported (and we confirmed) that flowers of Arabidopsis emit a range of monoterpenes and sesquiterpenes, including linalool. The same authors suggested a few biological roles for floral volatiles in Arabidopsis, including the attraction of pollinators, the protection of reproductive organs from oxidative damage and the defense against bacterial or fungal infection. It is tempting to hypothesize that the biological role of some of the terpenes emitted might be to repel insect herbivores, such as aphids, from feeding on the Arabidopsis inflorescence. If so, transgenic plants that produce novel terpenoids will be instrumental in understanding direct plant defense against pathogens and the altered behavior of insects caused by the production of a specific volatile. They also will be useful for studying indirect defense, as was described recently with transgenic Arabidopsis plants disturbed in jasmonic acid and salicylic acid signaling pathways (Van Poecke and Dicke, 2002). Linalool has been shown to be one of the volatile compounds released after herbivore attack in some plant species and might be involved in attracting predators of the herbivores (Gols et al., 1999; Degenhardt et al., 2003). Finally, strategies for the metabolic engineering of terpenoid biosynthesis could be evaluated rapidly in Arabidopsis and reconsidered before their application to more complex plant species.
METHODS
Reference Compounds
XAD-2 was purchased from Supelco (Bellefonte, PA). Racemic linalool, R-linalool, and valencene were from Fluka (Buchs, Switzerland). Nerolidol was purchased from Roth (Karlsruhe, Germany). R,S-8-Hydroxylinalool was synthesized from R,S-linalool according to Behr et al. (1978). R,S-Linalyl-β-d-glucopyranoside was prepared from R,S-linalool and 2,3,4,6-tetra-O-acetyl-α-d-glucopyranosyl bromide according to a modified Koenigs-Knorr synthesis (Paulsen et al., 1985).
Solid-Phase Extraction
Frozen samples were weighed and submerged in the same amount of water, homogenized by means of an Ultra-Turrax (IKA, Wilmington, NC), and centrifuged (2000g for 10 min). The residues were washed twice, and the supernatants were combined (40 mL) and subjected to solid-phase extraction on XAD-2 (20 cm, 1 cm i.d.). The column was washed successively with 50 mL of water, 50 mL of diethyl ether, and 80 mL of methanol. The diethyl ether extract was dried over sodium sulfate and concentrated to ∼100 μL. Phenol (0.1 mg/mL) was added as an internal standard. The methanol extract was concentrated in vacuo to ∼1 mL.
Enzymatic Hydrolysis and Gas Chromatography–Mass Spectrometry Analysis
Enzymatic hydrolysis was performed by dissolving an aliquot of the methanol extract, as described above, in 2 mL of 0.2 M phosphate buffer, pH 5.5. The solution was extracted twice with the same volume of diethyl ether to remove free alcohols. Subsequently, 200 μL of Rohapect D5L (Röhm, Darmstadt, Germany), a pectinolytic enzyme preparation exhibiting glycosidase activity, was added. After an incubation period of 24 h at 37°C, the liberated aglycones were extracted twice with 1 mL of diethyl ether. The combined organic layers were dried over sodium sulfate and concentrated. Capillary gas chromatography–mass spectrometry (GC-MS) analysis was performed with a Fisons Instruments (Engelsbach, Germany) GC 8000 series apparatus coupled to a Fisons Instruments MD800 quadrupole mass detector fitted with a split injector (1:20) at 230°C. A DB-Wax fused silica capillary column (30 m × 0.25 mm i.d.; df = 0.25 μm) (J&W Scientific, Folsom, CA), with a program from 50°C for 3 min to 220°C for 10 min with a temperature increase of 4°C/min, was used with a 2 mL/min flow rate of helium gas. The software Xcalibur for Windows (Thermo Finnigan, San Jose, CA) was used for data acquisition. Significant MS operating parameters were as follows: ionization voltage of 70 eV (electron impact ionization); ion source temperature of 220°C; and interface temperature of 250°C. Constituents were identified by comparison of their mass spectra and retention indices with those of authentic reference compounds. Extraction efficiency of glycosides by the XAD method was determined with p-nitrophenyl-β-d-glucoside, octyl-β-d-glucoside, linalyl-β-d-glucoside, and benzyl-β-d-glucoside (in all experiments, it was >80%). Quantification of the released alcohols was performed using phenol as the standard.
HPLC-MS
Analysis of methanol extracts was performed on a triple-stage quadrupole TSQ 7000 liquid chromatography–tandem MS system with an electrospray ionization interface (Finnigan MAT, Bremen, Germany). The temperature of the heated capillary was 240°C. The electrospray ionization capillary voltage was set to 3.5 kV, resulting in a current of 3.4 μA. Nitrogen served as both the sheath (70 p.s.i.) and the auxiliary gas (10 L/min). Data acquisition and evaluation were performed on a Personal DECstation 5000/33 (Digital Equipment, Unterföhring, Germany) with ICIS 8.1 software (Finnigan MAT). HPLC separations were performed on a Eurospher 100 C-18 apparatus (100 × 2 mm, 5 μm; Knauer, Berlin, Germany) using a linear gradient with a flow rate of 200 μL/min. Solvent A was 5 mM ammonium acetate in water; solvent B was 5 mM ammonium acetate in methanol. The gradient program was as follows: 0 to 30 min, 5 to 100% solvent B. Mass spectra were acquired in the negative mode. Product ion spectra were available by collision-induced dissociation (0.2 Pascal argon; −20 eV).
Multidimensional GC-MS
Multidimensional GC-MS analyses were performed with a Fisons 8160 gas chromatograph connected to a Fisons 8130 gas chromatograph and a Fisons MD 800 quadrupole mass spectrometer, using Fisons MASSLAB software (version 1.3). The first gas chromatograph (GC1) was equipped with a split injector (1:10 at 230°C) and a flame ionization detector (at 250°C). This gas chromatograph used a 25-m × 0.25-mm i.d. fused silica capillary column coated with a 0.25-mm film of DB-Wax 20 M (J&W Scientific) for the preseparation of the target molecule. Separation of enantiomers was achieved with the second gas chromatograph (GC2) using a 25-m × 0.25-mm i.d. fused silica capillary column coated with a 0.15-mm film of 2,3-di-O-ethyl-6-O-tert-butyl dimethylsilyl-β-cyclodextrin/PS086. A multicolumn switching system (Fisons) connected the column in GC1 to that in GC2. The retention time of the compound of interest was determined by GC separation, and the column in GC1 was connected to the flame ionization detector. Separation of the enantiomers was achieved in the second gas chromatograph after transfer of the compound of interest from the capillary column in GC1 to the column in GC2 via the switching device. The fused silica capillary column in GC1 was maintained at 60°C and then programmed to 240°C at 10°C/min with He gas flow at 3 mL/min. The fused silica capillary column in GC2 was maintained at 60°C (15 min) and then increased to 200°C at 2°C/min with He gas flow at 3 mL/min. The compound of interest was transferred from GC1 to GC2 from 9.8 to 10.3 min. The MS operating parameters were as follows: ionization voltage of 70 eV (electron impact ionization); and ion source and interface temperatures of 230 and 240°C, respectively.
Thermodesorption System
Online thermal desorption chromatography was performed using a Thermodesorption System (TDS-G) (Gerstel, Mülheim am Ruhr, Germany) coupled to a GC-MSD (HP 6890 plus 5973 mass detector; Hewlett-Packard) equipped with a split/splitless programmed temperature vaporization (PTV) injector (CIS 4; Gerstel). The TDS-G and gas chromatograph were both operated in the splitless mode. Samples were collected with the Tenax tube held at 25°C and desorbed in the following temperature conditions: 25°C (0.1-min hold) to 300°C (4-min hold) at 60°C/min. Volatiles were collected in the PTV injector at 50°C and desorbed at the following temperatures: 50 to 300°C (3-min hold) at 12°C/s. The gas chromatograph was equipped with a J&W Scientific DB-35MS column (30 m × 0.25 mm × 0.25 μm) run in constant pressure mode (0.8 bar He, 43 cm/s). The oven program was as follows: 50°C (4-min hold) to 300°C (21-min hold) at 10°C/min. To collect volatiles, plants were kept in a bell jar (15 cm diameter, 35 cm height) in a climate chamber (Elbanton, Kerkdriel, The Netherlands) at 20°C and a 12-h-light/12-h-dark photoperiod (8:00 am on, 8:00 pm off). Each hour, 5-L samples were collected automatically online into the Tenax tube at an absorption flow rate of 125 mL/min. This tube was desorbed onto the PTV injector and injected subsequently into the GC-MS apparatus and analyzed.
Tenax Trapping/Elution
Greenhouse-grown Arabidopsis thaliana plants were enclosed in 1-L glass jars closed with a Teflon-lined lid equipped with inlet and outlet. The plant pot was covered with aluminum foil to reduce the detection of soil volatiles. A vacuum pump was used to draw air through the glass jar at ∼100 mL/min, with the incoming air being purified through a glass cartridge (140 × 4 mm) containing 150 mg of Tenax TA (20/35 mesh; Alltech, Breda, The Netherlands). At the outlet, the volatiles emitted were trapped on a similar Tenax cartridge. Volatiles were sampled during 24 h. Cartridges were eluted using 3 × 1 mL of redistilled pentane:diethyl ether (4:1). Of these samples, 2 μL was analyzed by GC-MS using an HP 5890 series II gas chromatograph equipped with an HP-5MS column (30 m × 0.25 mm i.d.; df = 0.25 μm) and an HP 5972A mass selective detector as described by Bouwmeester et al. (1999). Linalool and nerolidol were quantified with authentic standards that were used to make calibration curves.
Solid-Phase Microextraction
Volatiles released by a pair of 4-week-old rosette leaves of Arabidopsis grown in the greenhouse were enclosed in a 1.5-mL autosampler GC vial containing 10 μL of water (to prevent the leaves from drying) and sampled automatically using solid-phase microextraction (SPME). The fused silica fiber of the SPME device (Supelco) coated with 100 μm of polydimethylsiloxane was inserted into the vial through an aluminum cap with a PolyTetraFluoroEthylene/butyl rubber septum, and volatiles were trapped by exposing the fiber to the headspace for 30 min. The SPME-trapped Arabidopsis volatiles were analyzed by GC-MS as described by Verhoeven et al. (1997). The difference in affinity of the SPME fiber to linalool and germacrene A was determined by analyzing a mixture of 1 μg/mL linalool and valencene (a sesquiterpene closely resembling germacrene A) in 100 μL of water (containing 1% ethanol). The fiber proved to have an eightfold preference for valencene.
Plant Material, Generation of Transgenic Arabidopsis, and Analysis of Isoprenoids
We used greenhouse-grown plants of Arabidopsis ecotype Columbia in all experiments described in the study. The complete characterization of the FaNES1 gene and the enzyme encoded will be described elsewhere (A. Aharoni and H.J. Bouwmeester, unpublished data). The FaNES1 gene was isolated from the cultivated strawberry (Fragaria × anannasa cv Elsanta) and was shown to encode a S-linalool/(3S)-E-nerolidol synthase by enzymatic assays with the recombinant FaNES1 protein produced in Escherichia coli cells. To direct the FaNES1 protein to the plastids, we fused the plastidic targeting signal of FvNES1 (the wild strawberry homolog; A. Aharoni and H.J. Bouwmeester, unpublished data) to the N terminus of the FaNES1 coding region. The fusion fragment obtained then was inserted between a doubled 35S promoter of the Cauliflower mosaic virus (CaMV) and a nopaline synthase terminator in the pBinPlus binary vector (Van Engelen et al., 1995) containing a kanamycin resistance gene inside the T-DNA for the selection of transformants generating the plant transformation vector pTAR-NES. To generate germacrene A–expressing plants, the germacrene A synthase long-form cDNA isolated from chicory (Cichorium intybus; CiGASlo) was cloned into the pBI121 binary vector as described above for the FaNES1 cDNA. Transgenic Arabidopsis ecotype Columbia was generated using the floral-dip method as described by Clough and Bent (1998). Kanamycin-resistant seedlings were transferred to soil in the greenhouse at 22°C. The analysis of carotenoids and chlorophyll was conducted according to Fraser et al. (2000) using rosette leaves of 4-week-old plants.
DNA and RNA Gel Blot Analyses
Genomic DNA was isolated from young flower buds as described by Pereira and Aarts (1998) and was used primarily for PCR screening using the FaNES1-specific primer NS600c (5′-GTGACGGTCTCCCTTGATGTCCTCACCCAGAAC-3′) and a primer in the 35S CaMV promoter, 35Score (5′-ATGACGCACAATCCCACTATC-3′). One microgram of genomic DNA from selected Arabidopsis lines was digested using the BamHI restriction enzyme and used for DNA gel blot analysis as described by Marsch-Martinez et al. (2002). To analyze the expression of FaNES1 in transgenic lines, 5 μg of total RNA isolated from rosette leaves of 4-week-old plants was used for RNA gel blot analysis as described by Aharoni et al. (2000). In both cases, the entire FaNES1 cDNA was used as a probe for hybridization.
Localization Experiments Using GFP
The hybrid fragment used for localization analysis was fused upstream of and in frame with the GFP gene in the cloning sites (SpeI and SalI) present in the cassette of pOL-LT-GFP-L64T65 using XbaI and XhoI restriction sites (at the 5′ and 3′ ends, respectively) introduced by PCR. The plasmid DNA of the pTAR-NES vector (see above) was used as template for the PCR. The pOL-LT-GFP-L64T65 vector (a kind gift of I. Small, Unité de Recherche en Genomique Végétale, Evry, France) is derived from pOL-GFPS65C (Peeters et al., 2000), which contains a modified GFP coding sequence under the control of the 35S CaMV promoter (with double enhancer). The pOL-LT-GFP-L64T65 version has no targeting sequence and labels the cytosol and the nucleoplasm. Fifty micrograms of plasmid DNA of the resulting construct (pTAR-GFP; Figure 3) was used to transform tobacco protoplasts (Nicotiana tabacum cv SRI) as described previously (Negrutiu et al., 1987). Protoplasts were examined at 24 h after transformation with a Carl Zeiss (Jena, Germany) confocal laser scanning microscope (LSM 510) with an argon ion laser. The fluorescence of GFP (absorbance, 488 nm; emission, 507 nm) was obtained using the 488-nm laserline, and the emission signal was collected using a band-pass filter (BP 505-550). For imaging, the ×40 objective was used, and the excitation intensity was set at 2 to 4% in most cases. The long-wavelength signal of the chlorophyll was collected using a long-pass filter (LP650). Optical sections were taken along the optical axis and projected into one image with the Zeiss LSM Image Browser.
Aphid Choice Assays
Adult apterous Myzus persicae aphids of mixed ages were collected with a fine brush from a rearing on Chinese cabbage (20°C, long days of 16 h of light/8 h of dark, RH of 70%). Dual-choice assays were used to test the preference of aphids for leaves from transgenic or control Arabidopsis plants. For this purpose, two mature full green leaves of similar size (one from a wild-type plant and the other from a transgenic FaNES1-expressing line) were placed abaxial side up on a thin layer of 1.5% water-agar in a Petri dish (5 cm diameter). Six separate Petri dishes were prepared as replicates for each of the six transgenic lines examined, which were selected before the experiment by confirming linalool production using the SPME method. Ten aphids were released in the lid of a Petri dish and, after closing, the Petri dishes were incubated upside down at 20°C under long-day conditions (16 h of light/8 h of dark) with a RH of 70%. Aphids could easily walk toward the leaves inside the Petri dishes. The positions of the leaves were randomized to eliminate the effect of light or temperature gradients on choice. The number of aphids on each leaf was recorded at a range of time points after the start of the experiment. At each time point, a one-sided Wilcoxon signed-rank test (Hollander and Wolfe, 1973) was used to test the null hypothesis of no repellent effect of transgenic plants expressing FaNES1 and emitting linalool against the alternative hypothesis of a repellent effect of transgenic plants. An additional experiment using four instead of six transgenic lines showed similar results.
Upon request, materials integral to the findings presented in this publication will be made available in a timely manner to all investigators on similar terms for noncommercial research purposes. To obtain materials, please contact Harro J. Bouwmeester, harro.bouwmeester@wurn.nl.
Accession Number
The GenBank accession number for CiGASlo is AF497999.
Supplementary Material
Acknowledgments
We thank Raffaella Greco, Nayelli Marsch-Martinez, and Andy Pereira for advice on the work with Arabidopsis, and Gerard Rouwendal, Iris Kappers, Bert Schipper, and Robert Sevenier for help with the analysis of transgenic plants and tobacco protoplasts. We thank Nemo Peeters, Ian Small, Maureen Hanson, and Andreas Weihe for the GFP and red fluorescent protein vectors, and Patrick Smit, Mark Hink, and Jan Willem Borst for assistance with confocal laser scanning microscopy.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.016253.
Footnotes
Online version contains Web-only data.
References
- Adam, K.P., and Zapp, J. (1998). Biosynthesis of the isoprene units of chamomile sesquiterpenes. Phytochemistry 48, 953–959. [Google Scholar]
- Aharoni, A., et al. (2000). Identification of the SAAT gene involved in strawberry flavor biogenesis by use of DNA microarrays. Plant Cell 12, 647–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubourg, S., Lecharny, A., and Bohlmann, J. (2002). Genomic analysis of the terpenoid synthase (AtTPS) gene family of Arabidopsis thaliana. Mol. Genet. Genomics 267, 730–745. [DOI] [PubMed] [Google Scholar]
- Behr, D., Wahlberg, I., Nishida, T., and Enzell, C.R. (1978). Tobacco chemistry. 45. (2E,6S)-2,6-dimethyl-2,7-octadiene-1,6-diol, a new monoterpenoid from Greek tobacco. Acta Chem. Scand. B 32, 228–234. [Google Scholar]
- Bohlmann, J., Martin, D., Oldham, N.J., and Gershenzon, J. (2000). Terpenoid secondary metabolism in Arabidopsis thaliana: cDNA cloning, characterization, and functional expression of a myrcene/(E)-β-ocimene synthase. Arch. Biochem. Biophys. 375, 261–269. [DOI] [PubMed] [Google Scholar]
- Bohlmann, J., Meyer, G.G., and Croteau, R.B. (1998). Plant terpenoid synthases: Molecular biology and phylogenetic analysis. Proc. Natl. Acad. Sci. USA 95, 4126–4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlmann, J., Phillips, M., Ramachandiran, V., Katoh, S., and Croteau, R.B. (1999). cDNA cloning, characterization, and functional expression of four new monoterpene synthase members of the Tpsd gene family from grand fir (Abies grandis). Arch. Biochem. Biophys. 368, 232–243. [DOI] [PubMed] [Google Scholar]
- Bouwmeester, H.J., Kodde, J., Verstappen, F.W.A., Altug, I.G., de Kraker, J.W., and Wallaart, T.E. (2002). Isolation and characterization of two germacrene A synthase cDNA clones from chicory. Plant Physiol. 129, 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwmeester, H.J., Verstappen, F.W.A., Posthumus, M.A., and Dicke, M. (1999). Spider mite-induced (3S)-(E)-nerolidol synthase activity in cucumber and lima bean: The first dedicated step in acyclic C11-homoterpene biosynthesis. Plant Physiol. 121, 173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch, M., Seuter, A., and Hain, R. (2002). Functional analysis of the early steps of carotenoid biosynthesis in tobacco. Plant Physiol. 128, 439–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell, J., Wolf, F., Proulx, J., Cuellar, R., and Saunders, C. (1995). Is the reaction catalyzed by 3-hydroxy-3-methylglutaryl coenzyme A reductase a rate-limiting step for isoprenoid biosynthesis in plants? Plant Physiol. 109, 1337–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, F., Tholl, D., D'Auria, J.C., Farooq, A., Pichersky, E., and Gershenzon, J. (2003). Biosynthesis and emission of terpenoid volatiles from Arabidopsis flowers. Plant Cell 15, 481–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Colby, S.M., Crock, J., Dowdle, R.B., Lemaux, P.G., and Croteau, R. (1998). Germacrene C synthase from Lycopersicon esculentum cv. VFNT Cherry tomato: cDNA isolation, characterization, and bacterial expression of the multiple product sesquiterpene cyclase. Proc. Natl. Acad. Sci. USA 95, 2216–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunillera, N., Boronat, A., and Ferrer, A. (1997). The Arabidopsis thaliana FPS1 gene generates a novel mRNA that encodes a mitochondrial farnesyl-diphosphate synthase isoform. J. Biol. Chem. 272, 15381–15388. [DOI] [PubMed] [Google Scholar]
- Degenhardt, J., Gershenzon, J., Baldwin, I.T., and Kessler, A. (2003). Attracting friends to feast on foes: Engineering terpene emission to make crop plants more attractive to herbivore enemies. Curr. Opin. Biotechnol. 14, 169–176. [DOI] [PubMed] [Google Scholar]
- de Kraker, J.W., Franssen, M.C.R., De Groot, A., Koenig, W.A., and Bouwmeester, H.J. (1998). (+)-Germacrene A biosynthesis: The committed step in the biosynthesis of bitter sesquiterpene lactones in chicory. Plant Physiol. 117, 1381–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudareva, N., Cseke, L., Blanc, V.M., and Pichersky, E. (1996). Evolution of floral scent in Clarkia: Novel patterns of S-linalool synthase gene expression in the C. breweri flower. Plant Cell 8, 1137–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fäldt, J., Arimura, G., Gershenzon, J., Takabayashi, J., and Bohlmann, J. (2003). Functional identification of AtTPS03 as (E)-β-ocimene synthase: A monoterpene synthase catalyzing jasmonate- and wound-induced volatile formation in Arabidopsis thaliana. Planta 216, 745–751. [DOI] [PubMed] [Google Scholar]
- Fraser, P.D., Pinto, M.E.S., Holloway, D.E., and Bramley, P.M. (2000). Application of high-performance liquid chromatography with photodiode array detection to the metabolic profiling of plant isoprenoids. Plant J. 24, 551–558. [DOI] [PubMed] [Google Scholar]
- Fray, R.G., Wallace, A., Fraser, P.D., Valero, D., Hedden, P., Bramley, P.M., and Grierson, D. (1995). Constitutive expression of a fruit phytoene synthase gene in transgenic tomatoes causes dwarfism by redirecting metabolites from the gibberellin pathway. Plant J. 8, 693–701. [Google Scholar]
- Gols, R., Posthumus, M.A., and Dicke, M. (1999). Jasmonic acid induces the production of gerbera volatiles that attract the biological control agent Phytoseiulus persimilis. Entomol. Exp. Appl. 93, 77–86. [Google Scholar]
- Hedtke, B., Meixner, M., Gillandt, S., Richter, E., Borner, T., and Weihe, A. (1999). Green fluorescent protein as a marker to investigate targeting of organellar RNA polymerases of higher plants in vivo. Plant J. 17, 557–561. [DOI] [PubMed] [Google Scholar]
- Hohn, T.M., and Ohlrogge, J.B. (1991). Expression of a fungal sesquiterpene cyclase gene in transgenic tobacco. Plant Physiol. 97, 460–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander, M., and Wolfe, D.A. (1973). Nonparametric Statistical Methods (New York: Wiley).
- Hori, M. (1998). Repellency of rosemary oil against Myzus persicae in a laboratory and in a screenhouse. J. Chem. Ecol. 24, 1425–1432. [Google Scholar]
- Kubo, I., Muroi, H., and Himejima, M. (1993). Antibacterial activity against Streptococcus mutans of mate tea flavor components. J. Agric. Food Chem. 41, 107–111. [Google Scholar]
- Lange, M.B., Wildung, M.R., Stauber, E.J., Sanchez, C., Pouchnik, D., and Croteau, R.B. (2000). Probing essential oil biosynthesis and secretion by functional evaluation of expressed sequence tags from mint glandular trichomes. Proc. Natl. Acad. Sci. USA 97, 2934–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavy, M., Zuker, A., Lewinsohn, E., Larkov, O., Ravid, U., Vainstein, A., and Weiss, D. (2002). Linalool and linalool oxide production in transgenic carnation flowers expressing the Clarkia breweri linalool synthase gene. Mol. Breed. 9, 103–111. [Google Scholar]
- Lewinsohn, E., et al. (2001). Enhanced levels of the aroma and flavor compound S-linalool by metabolic engineering of the terpenoid pathway in tomato fruits. Plant Physiol. 127, 1256–1265. [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler, H.K. (2000). Non-mevalonate isoprenoid biosynthesis: Enzymes, genes and inhibitors. Biochem. Soc. Trans. 28, 785–789. [PubMed] [Google Scholar]
- Lücker, J., Bouwmeester, H.J., Schwab, W., Blaas, J., van der Plas, L.H.W., and Verhoeven, H.A. (2001). Expression of Clarkia S-linalool synthase in transgenic petunia plants results in the accumulation of S-linalyl-β-d-glucopyranoside. Plant J. 27, 315–324. [DOI] [PubMed] [Google Scholar]
- Lücker, J., El-Tamer, M.K., Schwab, W., Verstappen, F.W.A., van der Plas, L.H.W., Bouwmeester, H.J., and Verhoeven, H.A. (2002). Monoterpene biosynthesis in lemon (Citrus limon): cDNA isolation and functional analysis of four monoterpene synthases. Eur. J. Biochem. 269, 3160–3171. [DOI] [PubMed] [Google Scholar]
- Mahmoud, S.S., and Croteau, R.B. (2002). Strategies for transgenic manipulation of monoterpene biosynthesis in plants. Trends Plant Sci. 7, 366–373. [DOI] [PubMed] [Google Scholar]
- Marsch-Martinez, N., Greco, R., van Arkel, G., Herrera-Estrella, L., and Pereira, A. (2002). Activation tagging using the En-I maize transposon system in Arabidopsis. Plant Physiol. 129, 1544–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masferrer, A., Arro, M., Manzano, D., Schaller, H., Fernandez-Busquets, X., Moncalean, P., Fernandez, B., Cunillera, N., Boronat, A., and Ferrer, A. (2002). Overexpression of Arabidopsis thaliana farnesyl diphosphate synthase (FPS1S) in transgenic Arabidopsis induces a cell death/senescence-like response and reduced cytokinin levels. Plant J. 30, 123–132. [DOI] [PubMed] [Google Scholar]
- McCaskill, D., and Croteau, R.B. (1998). Some caveats for bioengineering terpenoid metabolism in plants. Trends Biotechnol. 16, 349–355. [Google Scholar]
- McConkey, M.E., Gershenzon, J., and Croteau, R.B. (2000). Developmental regulation of monoterpene biosynthesis in the glandular trichomes of peppermint. Plant Physiol. 122, 215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercke, P., Crock, J., Croteau, R.B., and Brodelius, P.E. (1999). Cloning, expression, and characterization of epi-cedrol synthase, a sesquiterpene cyclase from Artemisia annua L. Arch. Biochem. Biophys. 369, 213–222. [DOI] [PubMed] [Google Scholar]
- Muller, P., Lorber, P., and Haley, B. (1968). Volatile growth inhibitors produced by Salvia leucophylla: Effect on seedling growth and respiration. Bull. Torrey Bot. Club 95, 415–422. [Google Scholar]
- Negrutiu, I., Shillito, R., Potrykus, I., Biasini, G., and Sala, F. (1987). Hybrid genes in the analysis of transformation conditions. Plant Mol. Biol. 8, 363–373. [DOI] [PubMed] [Google Scholar]
- Paulsen, H., Le-Nguyen, B., Sinnwell, V., and Seehofer, F. (1985). Synthesis of glycosides of mono-, sesqui- and diterpene alcohols. Liebigs Ann. Chem. 8, 1513–1536. [Google Scholar]
- Peeters, N.M., Chapron, A., Giritch, A., Grandjean, O., Lancelin, D., Lhomme, T., Vivrel, A., and Small, I. (2000). Duplication and quadruplication of Arabidopsis thaliana cysteinyl- and asaparaginyl-tRNA synthetase genes of organellar origin. J. Mol. Evol. 50, 413–423. [DOI] [PubMed] [Google Scholar]
- Pereira, A., and Aarts, M.G.M. (1998). Transposon tagging with the En-I system. In Arabidopsis Protocols, J.S.J. Martinez-Zapater, ed (Totowa, NJ: Humana Press), pp. 329–338.
- Phillips, M.A., and Croteau, R.B. (1999). Resin-based defenses in conifers. Trends Plant Sci. 4, 184–190. [DOI] [PubMed] [Google Scholar]
- Pichersky, E., and Gershenzon, J. (2002). The formation and function of plant volatiles: Perfumes for pollinator attraction and defense. Curr. Opin. Plant Biol. 5, 237–243. [DOI] [PubMed] [Google Scholar]
- Raguso, R.A., Light, D.M., and Pickersky, E. (1996). Electroantennogram responses of Hyles lineata (Sphingidae:Lepidoptera) to volatile compounds from Clarkia breweri (Onagraceae) and other moth-pollinated flowers. J. Chem. Ecol. 22, 1735–1766. [DOI] [PubMed] [Google Scholar]
- Raguso, R.A., and Pichersky, E. (1999). A day in the life of a linalool molecule: Chemical communication in a plant-pollinator system. Part 1. Linalool biosynthesis in flowering plants. Plant Species Biol. 14, 95–120. [Google Scholar]
- Rodriguez-Concepción, M., and Boronat, A. (2002). Elucidation of the methylerythritol phosphate pathway for isoprenoid biosynthesis in bacteria and plastids: A metabolic milestone achieved through genomics. Plant Physiol. 130, 1079–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnurr, J.A., and Guerra, D.J. (2000). The CaMV-35S promoter is sensitive to shortened photoperiod in transgenic tobacco. Plant Cell Rep. 19, 279–282. [DOI] [PubMed] [Google Scholar]
- Shah, F.H., and Cha, T.S. (2000). A mesocarp- and species-specific cDNA clone from oil palm encodes for sesquiterpene synthase. Plant Sci. 154, 153–160. [DOI] [PubMed] [Google Scholar]
- Shen, B., Zheng, Z., Dooner, H.K., Shen, B.Z., and Zheng, Z.W. (2000). A maize sesquiterpene cyclase gene induced by insect herbivory and volicitin: characterization of wild-type and mutant alleles. Proc. Natl. Acad. Sci. USA 97, 14807–14812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snape, J.W., and Lawrence, M.J. (1971). The breeding system of Arabidopsis thaliana. Heredity 27, 299–302. [Google Scholar]
- Vainstein, A., Lewinsohn, E., Pichersky, E., and Weiss, D. (2001). Floral fragrance: New inroads into an old commodity. Plant Physiol. 127, 1383–1389. [PMC free article] [PubMed] [Google Scholar]
- Van Engelen, F.A., Molthoff, J.W., Conner, A.J., Nap, J.P., Pereira, A., and Stiekema, W.J. (1995). pBINPLUS: An improved plant transformation vector based on pBIN19. Transgenic Res. 4, 288–290. [DOI] [PubMed] [Google Scholar]
- Van Poecke, R.M.P., and Dicke, M. (2002). Induced parasitoid attraction by Arabidopsis thaliana: Involvement of the octadecanoid and the salicylic acid pathway. J. Exp. Bot. 53, 1793–1799. [DOI] [PubMed] [Google Scholar]
- Van Poecke, R.M.P., Posthumus, M.A., and Dicke, M. (2001). Herbivore-induced volatile production by Arabidopsis thaliana leads to attraction of the parasitoid Cotesia rubecula: Chemical, behavioral, and gene-expression analysis. J. Chem. Ecol. 27, 1911–1928. [DOI] [PubMed] [Google Scholar]
- Verhoeven, H., Beuerle, T., and Schwab, W. (1997). Solid-phase microextraction: Artefact formation and its avoidance. Chromatographia 46, 63–66. [Google Scholar]
- Wallaart, T.E., Bouwmeester, H.J., Hille, J., Poppinga, L., and Maijers, N.C.A. (2001). Amorpha-4,11-diene synthase: Cloning and functional expression of a key enzyme in the biosynthetic pathway of the novel antimalarial drug artemisinin. Planta 212, 460–465. [DOI] [PubMed] [Google Scholar]
- Weidenhamer, J.D., Macias, F.A., Fischer, N.H., and Williamson, G.B. (1993). Just how insoluble are monoterpenes? J. Chem. Ecol. 19, 1799–1807. [DOI] [PubMed] [Google Scholar]
- Wise, M.L., Savage, T.J., Katahira, E., and Croteau, R.B. (1998). Monoterpene synthases from common sage (Salvia officinalis): cDNA isolation, characterization, and functional expression of (+)-sabinene synthase, 1,8-cineole synthase, and (+)-bornyl diphosphate synthase. J. Biol. Chem. 273, 14891–14899. [DOI] [PubMed] [Google Scholar]
- Zook, M., Hohn, T., Bonnen, A., Tsuji, J., and Hammerschmidt, R. (1996). Characterization of novel sesquiterpenoid biosynthesis in tobacco expressing a fungal sesquiterpene synthase. Plant Physiol. 112, 311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.