Abstract
Through the use of chimeric CXCR4/CCR5 receptors we have previously shown that CCR5-tropic (R5) HIV-1 isolates acquire a more flexible receptor use over time, and that this links to a reduced viral susceptibility to inhibition by the CCR5 ligand RANTES. These findings may have relevance with regards to the efficacy of antiretroviral compounds that target CCR5/virus interactions. Compartmentalized discrepancies in coreceptor use may occur, which could also affect the efficacy of these compounds at specific anatomical sites, such as within the CNS. In this cross-sectional study we have used wild-type CCR5 and CXCR4 as well as chimeric CXCR4/CCR5 receptors to characterize coreceptor use by paired plasma and cerebrospinal fluid (CSF) isolates from 28 HIV-1-infected individuals. Furthermore, selected R5 isolates, with varying chimeric receptor use, were tested for sensitivity to inhibition by the CCR5 antagonist TAK-779. Discordant CSF/plasma virus coreceptor use was found in 10/28 patients. Low CD4+ T cell counts correlated strongly with a more flexible mode of R5 virus CCR5 usage, as disclosed by an increased ability to utilize chimeric CXCR4/CCR5 receptors, specifically receptor FC-2. Importantly, an elevated ability to utilize chimeric receptors correlated with a reduced susceptibility to inhibition by TAK-779. Our findings show that a discordant CSF and plasma virus coreceptor use is not uncommon. Furthermore, we provide support for an emerging paradigm, where the acquisition of a more flexible mode of CCR5 usage is a key event in R5 virus pathogenesis. This may, in turn, negatively impact the efficacy of CCR5 antagonist treatment in late stage HIV-1 disease.
Introduction
The discovery that the chemokine receptors CCR5 and CXCR4 act as essential keyholes for the entry of human immunodeficiency virus type 1 (HIV-1) into CD4-positive immune cells has increased the understanding of AIDS pathogenesis and provided the basis for new antiretroviral treatment strategies. Following viral attachment to CD4, conformational changes in the HIV envelope glycoprotein 120 (gp120) facilitate viral binding to one of these chemokine receptors with subsequent steps of membrane fusion and capsid entry.1,2 CCR5-utilizing strains (R5 viruses) are almost invariably found in HIV-1-infected individuals during the asymptomatic phase, whereas virus phenotypes with the ability to utilize CXCR4 (X4 or R5X4 virus) emerge in approximately 50% during progression to AIDS.3–6
We have previously described the use of a set of CXCR4/CCR5 chimeric receptors for studies on the evolution of coreceptor use of primary HIV-1 isolates over time.7–9 In these studies we designated R5 isolates that lacked the ability to use any of the chimeras, i.e., they are able to infect only cells expressing the CCR5 wild-type receptor, as R5narrow phenotype. R5 viruses able to use one or more chimeric receptors were designated R5broad(1), R5broad(2), or R5broad(3), depending on the number of chimeras that could support viral entry. We demonstrated that an enhanced ability of R5 isolates to utilize these chimeras and wild-type CCR5 was linked to disease progression as well as to a reduced viral susceptibility to inhibition by the CCR5 ligand RANTES. These findings imply that an important feature of R5 virus pathogenesis in progressive HIV-1 disease is the acquisition of a more flexible mode of CCR5 usage. The fact that viruses displaying the R5 broad phenotypes are less sensitive to inhibition by RANTES may also be of specific relevance in the context of antiretroviral treatment with CCR5 antagonists as it may reflect a reduced virus susceptibility to these agents.
Prior to the initiation of antiretroviral treatment with CCR5 antagonists it is mandatory to exclude the presence of X4 or R5X4 populations in plasma.10 However, information is scarce regarding possible compartmentalized discrepancies in HIV-1 coreceptor use that could impact the efficacy of CCR5 antagonism at specific anatomical sites. HIV-1 invades the central nervous system (CNS) early in the course of infection and, in the absence of antiretroviral treatment frequently causes neurological morbidity, such as AIDS dementia complex (ADC).11–13 Due to the blood–brain barrier, the CNS constitutes a restricted compartment, where the viral evolution may differ from that in peripheral blood.14–18 Within the CNS, autonomous viral production is established in local target cells, mainly comprising resident macrophages and microglial cells.19–21 A viral adaptation to replication in these target cells may include alterations in coreceptor usage. Furthermore, the mode of coreceptor use may substantially influence the pathogenic processes in the brain that are responsible for the development of neurological impairment, such as ADC.22–24 Although the cerebrospinal fluid (CSF) cannot be considered to be a compartment identical to brain tissue, it is a more readily sampled site that, due to its proximity and shared barriers, provides an important “window” into HIV CNS infection.25
In the present study we have characterized the mode of coreceptor use by paired HIV-1 plasma and CSF isolates through the use of CXCR4/CCR5 chimeric and wild-type receptors. Furthermore, the mode of coreceptor use was correlated with clinical parameters linked to disease progression, and, for selected isolates, with sensitivity to the CCR5 antagonist TAK-779.26–28
Materials and Methods
Patients
Twenty-eight HIV-1-infected patients with varying CD4+ T cell counts, varying levels of CSF and plasma viral load, and with or without ADC were retrospectively selected for participation in the study, from a longitudinal study cohort at the Department of Infectious Diseases, Sahlgrenska University Hospital, Gothenburg, Sweden.29 Informed consent was obtained from all participants. The study was approved by the Research Ethics Committee at The University of Gothenburg, Sweden. Peripheral CD4+ T cell counts and HIV-1 RNA levels in plasma and CSF were analyzed for each patient (FACS, Becton Dickinson, Mountain View, CA and Amplicor HIV Monitor, Version 1.0, Roche Diagnostic Systems, Basel, Switzerland, respectively). The patients, 22 males and six females, had CD4+ T cell counts ranging from 27 to 820 cells/μl (median 190). Fourteen patients were severely immunodeficient, with CD4+ T cell counts <200 cells/μl. Plasma viral load ranged from 1900 to 682,000 copies/ml (median 52,000) and CSF viral load ranged from 600 to >750,000 copies/ml (median 66,000).
Seven patients had ADC, as assessed by the criteria defined by the CDC and the American Academy of Neurology AIDS Task Force.30–31 Twenty-five patients were antiretroviral treatment naive, and none had received antiretroviral medication for at least 9 months prior to virus isolation.
Neopterin levels were analyzed by a commercially available radioimmunoassay (Henningtest Neopterin, BRAMS, Germany) with a upper normal reference value of 5.8 nmol/liter in CSF.32
Virus isolates
Paired plasma and CSF isolates were obtained as previously described.33 Briefly, plasma and CSF samples were centrifuged at 996 × g for 20 min in order to pellet the cells. Cell-free supernatant was centrifuged at 180,000 × g for 30 min at 4°C to pellet free virus particles. None of the CSF samples had a red cell count above 30 × 106/liter. Phytohemagglutinin (PHA)-pretreated peripheral blood mononuclear cells (PBMCs) from healthy blood donors were inoculated with the pelleted material. The cultures were grown in RPMI medium with 10% fetal calf serum and 50 units interleukin-2 (Proleukin, EuroCetus, Amsterdam, The Netherlands), in addition to 2 mg/ml polybrene, 5 mg/ml hydrocortisone acetate, and antibiotics. The supernatants of the cultures were assayed once a week for HIV-1 antigen with a p24 capture ELISA [HIVAG(a)-1, Abbott Laboratories, Chicago, IL]. Virus stocks were stored at −70°C. Prior to infection experiments, the virus was passaged once or twice in interleukin (IL)-2- and PHA-stimulated PBMCs according to standard protocols.34 The virus content was evaluated in terms of p24 assays using the Vironostika HIV-1 Antigen Microelisa system (Biomeriéux, Boxtel, The Netherlands). Selected isolates were also evaluated for the concentration of functional viral reverse transcriptase using the Cavidi HS kit (Cavidi Tech AB, Uppsala, Sweden).
Cell lines
Human glioma U87.CD4 cells were maintained in DMEM with sodium pyruvate and Glutamax-I (Invitrogen, Lidingö, Sweden), 10% fetal calf serum (FCS), 1 × MEM nonessential amino acids, 300 μg/ml G-418, and antibiotics. Stably transfected U87.CD4 cells were supplemented with 0.5 μg/ml of puromycin (Sigma, Stockholm, Sweden). All cells were grown at 37°C in 7% CO2.
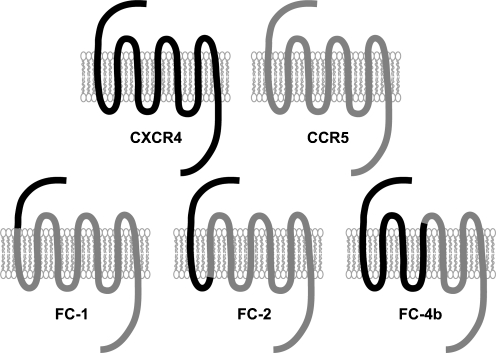
Establishment of stably transfected U87.CD4 cells expressing CCR5, CXCR4, or chimeric CXCR4/CCR5 receptors has previously been described (Fig. 1).8 Briefly, the chimeras FC-1 (CXCR4 Pro-42/Pro-35 CCR5), FC-2 (CXCR4 Asp-74/Ile-67 CCR5), and FC-4b (CXCR4 Ile-185/Cys-178 CCR5) were constructed using the single overlap and extension method.35,36 U87.CD4 cells were stably transfected and clones expressing similar levels of receptors as evaluated by flow cytometry were chosen for further experiments.
FIG. 1.
Schematic representation of the wild-type coreceptors CXCR4 (black) and CCR5 (gray) and the CXCR4/CCR5 chimeric receptors FC-1, FC-2, and FC-4b. The chimeric receptors were constructed by successively exchanging regions of CCR5 with corresponding parts of CXCR4.
Virus infections
U87.CD4 cells, stably expressing wild-type or chimeric receptors, were seeded in triplicate in 48-well plates using U87.CD4 media without G-418 or puromycin. After 3 days, cells at 20–40% confluence were infected for 2 h at 37°C with 30 ng/ml of virus (p24 concentration) in a final volume of 0.13 ml medium. After 2 h, 0.27 ml of medium was added. After incubation for 20–24 h the cells were washed twice and 1 ml of fresh medium was added. Supernatants from the infected cell cultures were collected at day 0 and day 5 of infection and assayed with p24 ELISA. Infection was defined as positive when the p24 content in the supernatant reached 100 pg/ml after p24 production by sham-transfected cells had been deducted. To semiquantitatively assess the efficiency of chimeric receptor use, we used the following grading system: 100–1000 pg p24 antigen production/ml in cell culture supernatant = low grade usage (+), 1000–10,000 pg/ml = moderate grade usage (++ ), and >10,000 pg/ml = high grade usage (+++).
The criterion used to define discordancy in chimeric receptor use was a difference in p24 production of at least log10 in parallel infection experiments. To further dissect receptor use by dual-tropic R5/X4 isolates, biological cloning, as described by Mild et al., was performed with minor modifications.37 Briefly, U87/CD4/CCR5 and U87/CD4/CXCR4 cells were inoculated with patient isolates, and infections were carried out as described above. Isolates with the ability to utilize both CCR5 and CXCR4 were characterized further where U87/CD4/CCR5 cells were inoculated with undiluted virus supernatants from infected U87/CD4/CXCR4 cells and vice versa. Following the protocol above, supernatants were analyzed on day 5 for the presence of p24 production. Infection was defined as positive when the p24 content in the supernatant reached 100 pg/ml.
TAK-779 inhibition assay
RT-normalized virus isolated from plasma and CSF was used for the experiments. PHA-activated PBMCs (105), pooled from three donors, were infected in triplicate with 0.33 ng RT/ml in the presence of TAK-779 (from Roche, obtained from the NIH Research and Reference Reagent Program, Division of AIDS, NIAID, NIH) as previously described.38 In brief, TAK-779 was serially diluted in 3-fold steps starting at the final concentration of 990 nM and simultaneously added to the cells and virus. Control cultures without inhibitor were infected in parallel. Infected PBMCs were washed with PBS on day 1 and inhibitor was added to the cultures at concentrations corresponding to the setup. Cell culture supernatants were harvested on day 7 after infection, and p24 antigen content was analyzed by Vironostika HIV-1 Antigen Microelisa system (Biomérieux, Boxtel, The Netherlands). The sensitivity to TAK-779 was evaluated as 50% or 90% inhibitory concentration (IC50 and IC90), calculated from p24 antigen release in the control cultures.
Statistics
Differences between two independent groups were assessed with the Mann–Whitney U-test. Spearman's rank correlation coefficient was used to evaluate correlations.
Results
R5 HIV-1 phenotypes predominate in CSF
Paired plasma and CSF isolates from 28 HIV-1-infected individuals were tested for their ability to infect U87.CD4 cells expressing CCR5 or CXCR4. The R5 viral phenotype predominated both in plasma and in CSF (Table 1). CXCR4 using viruses were found in plasma samples from seven patients (six R5X4 and one X4 isolate). In three of the corresponding CSF isolates only virus with the R5 phenotype was detected, and p24 production by U87.CD4.CXCR4 cells infected with one CSF isolate (R5X4) was 30 times lower than for the corresponding (X4) plasma isolate (Patient 25). Paired R5X4 isolates (three plasma and corresponding CSF isolates) were evaluated for the presence of truly dual-tropic strains and all contained true R5X4 virus subpopulations (data not shown). Taken together, HIV-1 with the R5 phenotype is commonly found in CSF even in the presence of CXCR4 using plasma virus populations.
Table 1.
Characteristics of the 28 Subjects Included in the Studya
| Patient | CDC stage | CD4+ T cell count (×106 cells/liter) | Plasma-RNA (copies/ml) | CSF-RNA (copies/ml) | CSF-neopterin (nmol/liter) | AIDS-related diseaseb | Coreceptor use in plasma | Coreceptor use in CSF |
|---|---|---|---|---|---|---|---|---|
| 1 | A1 | 820 | 2,900 | 5,100 | NA | R5 | R5 | |
| 2 | A1 | 627 | 32,000 | 2,500 | 10 | R5 | R5 | |
| 3 | B1 | 532 | 23,000 | 75,000 | 17 | R5 | R5 | |
| 4 | A1 | 530 | 8,700 | 10,500 | NA | R5 | R5 | |
| 5 | A1 | 510 | 1,900 | 28,000 | NA | R5 | R5 | |
| 6 | A1 | 510 | 70,000 | 1,450 | NA | R5X4 | R5 | |
| 7 | A1 | 505 | 56,000 | 11,000 | NA | R5 | R5 | |
| 8 | A1 | 500 | 23,000 | 600 | 6 | R5 | R5 | |
| 9 | A2 | 490 | 52,000 | 119,000 | 31 | R5 | R5 | |
| 10 | A2 | 490 | 17,000 | 49,000 | 28 | R5 | R5 | |
| 11 | A2 | 400 | 1,452 | 750,000 | NA | R5 | R5 | |
| 12 | A2 | 330 | 67,000 | 29,000 | NA | R5 | R5 | |
| 13 | C2 | 230 | 257,000 | 254,000 | 74 | ADC | R5 | R5 |
| 14 | C2 | 213 | 12,900 | 125,000 | 270 | ADC | R5 | R5 |
| 15 | C3 | 168 | 58,000 | 114,000 | 31 | KS | R5 | R5 |
| 16 | A3 | 150 | 77,000 | 118,000 | 33 | R5 | R5 | |
| 17 | C3 | 138 | 89,000 | 21,000 | 121 | ADC | R5X4 | R5 |
| 18 | A3 | 134 | 165,000 | 225,000 | 39 | R5X4 | R5X4 | |
| 19 | C3 | 87 | 36,000 | 64,000 | 102 | ADC, MAC | R5 | R5 |
| 20 | C3 | 60 | 682,000 | 750,000 | 46 | ADC | R5X4 | R5X4 |
| 21 | C3 | 49 | 534,000 | 88,000 | 50 | ADC | R5 | R5 |
| 22 | C3 | 48 | 273,000 | 14,700 | 39 | PCP | R5X4 | R5 |
| 23 | C3 | 42 | 53,000 | 70,000 | 34 | C. esophagitis | R5 | R5 |
| 24 | B3 | 40 | 15,000 | 139,000 | 21 | Cryptosporidium | R5 | R5 |
| 25 | A3 | 38 | 54,000 | 1,400 | 10 | X4 | R5X4 | |
| 26 | C3 | 36 | 52,000 | 132,000 | 42 | Lymphoma | R5 | R5 |
| 27 | C3 | 35 | 607,000 | 102,000 | 30 | ADC, PCP | R5 | R5 |
| 28 | C3 | 27 | 41,000 | 7,900 | 52 | PCP | R5X4 | R5X4 |
CDC staging, CD4+ T cell counts, HIV-RNA levels, neopterin levels, AIDS-related diseases, and viral coreceptor use. Paired isolates with discordant use of wild-type receptors or chimeric receptors are in bold.
ADC, AIDS dementia complex; KS, Kaposi sarcoma; MAC, Mycobacterium avium complex; C. esophagitis, Candida esophagitis; PCP, Pneumocystis carinii pneumonia.
Discordant R5 virus phenotypes in paired R5 plasma/CSF isolates detected through the use of CXCR4/CCR5 chimeric receptors
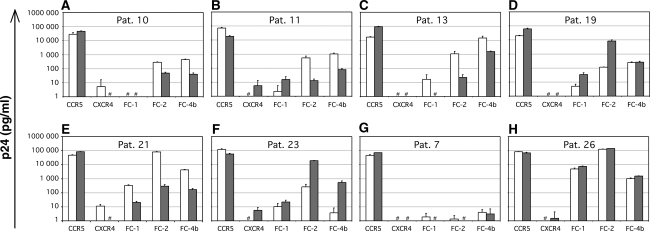
To determine if the R5 phenotypes varied among CCR5 restricted viruses isolated from plasma and CSF, chimeric receptor use was analyzed. In this analysis we were able to identify discordant plasma/CSF R5 phenotypes in 6 of 21 patients by significant discrepancies (>10-fold levels of p24 in culture supernatants) in the use of chimeric receptors (Fig. 2 and Table 1). There were no clear patterns of chimeric receptor use that could distinguish CSF from plasma isolates, since both a broader use in four of six CSF isolates and a more narrow use in two of six CSF isolates were found. Further, R5 phenotypes ranging from R5narrow to R5broad(3) were represented in both compartments in a nonspecific manner. Also, in seven patients with ADC, no specific patterns of chimeric coreceptor use by CSF or plasma isolates were found.
FIG. 2.
Receptor use of paired plasma and CSF isolates. Diagrams showing results after infection of U87.CD4 cells expressing CCR5, CXCR4, or chimeric CXCR4/CCR5 receptors, with paired plasma (gray bars) and CSF (open bars) virus isolates. (A–F) The six paired isolates with discordant use of chimeric receptors. (G) Paired isolates of concordant R5narrow phenotype. (H) Paired isolates of concordant R5broad3 phenotype. Infections are measured as p24 protein content in the cell culture supernatant. # indicates a p24 value <1. The criterium for discordant use is a >10-fold difference in p24 antigen production between the infections.
CSF neopterin levels did not correlate with the mode of coreceptor use. However, as previously shown,39–43 patients with ADC had a significantly higher mean CSF neopterin concentration (95 nmol/liter) than individuals without neurological complications (28 nmol/liter) (p = 0.03).
R5 virus ability to utilize CCR5/CXCR4 chimeric receptor FC-2 is associated with advanced disease stage and elevated CSF viral load
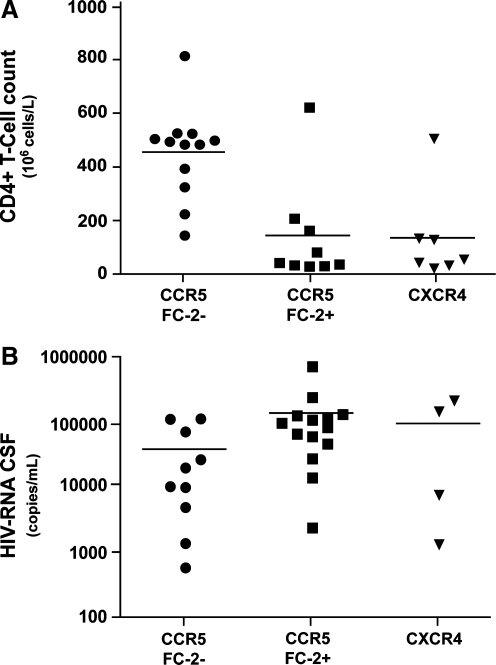
Since our previous work indicated a correlation between chimeric receptor use and degree of immunosuppression,7 the mode of CXCR4/CCR5 chimeric receptor use was correlated with CD4+ T cell counts and viral load for each individual. Individuals harboring plasma R5broad(2–3) phenotypes had significantly lower CD4+ T cell counts as compared to individuals with R5narrow or R5broad(1) phenotypes (p = 0.03). The strongest association with immune suppression was found when comparing individuals with FC-2-positive phenotypes (FC-2+) using R5 plasma isolates to those with FC-2-negative phenotypes (FC-2−) (Fig. 3). Nine patients with FC-2+ R5 isolates had a median CD4+ T cell count of 49 cells/μl, as compared to 495 cells/μl in 12 patients with FC-2− isolates (p = 0.004).
FIG. 3.
Correlations between (A) plasma virus FC-2 usage and CD4+ T cell counts and (B) CSF virus FC-2 usage and CSF HIV-RNA load. (A) Individuals harboring plasma FC-2+ R5 isolates or X4 isolates had significantly lower CD4+ T cell counts as compared to individuals with FC-2- R5 isolates (p = 0.004 and p = 0.005, respectively). (B) Individuals harboring CSF FC-2+ R5 isolates had significantly higher CSF HIV-RNA levels as compared to individuals with FC-2- R5 isolates (p = 0.02). CSF HIV-RNA levels for individuals harboring CSF X4 isolates did not significantly differ from other groups. Coreceptor use was determined as p24 antigen production >100 pg/ml in the cell culture supernatant. Horizontal lines represent mean values of CD4+ T cell counts and CSF HIV-RNA levels.
The presence of X4 or R5X4 phenotypes was, as expected, also linked to immunosuppression (median CD4+ T cell count of 60) when compared to the FC-2− group (p = 0.005), but the difference was not statistically significant when compared to R5 phenotypes in general. There was no significant correlation between FC-2 usage and plasma viral load even though there was an expected inverse correlation between CD4+ T cell counts and viral load (p = 0.01, Spearman). The presence of X4 or R5X4 phenotypes correlated significantly with a higher plasma viral load when compared to the plasma viral load in those harboring viruses with R5 phenotypes (p = 0.035). The presence of FC-2+ R5 virus phenotypes within the CSF correlated significantly with an elevated CSF viral load (p = 0.02) (Fig. 3).
Mode of coreceptor use by R5 isolates correlates with susceptibility to inhibition by the CCR5 antagonist TAK-779
To evaluate a possible relationship between mode of coreceptor use and susceptibility to inhibition by TAK-779, we selected paired R5 virus isolates from seven patients with varying degrees of immunodeficiency and chimeric receptor use, including three patients with ADC. Whereas a 50 % inhibition (IC50) was achieved for 13/14 isolates with TAK-779, at varying concentrations, a 90% inhibition (IC90) was not achieved for any of the virus isolates with an elevated usage of any chimeric receptor [designated (++) and (+++) in Table 2] even at the highest concentration of TAK-779 (990 nM). In contrast, IC90 values were achieved for all isolates that were characterized by a lack of or weak ability to utilize any chimeric receptor [designated (–) and (+), respectively, in Table 2]. Plasma and CSF isolates from the three patients with ADC were all incompletely inhibited by TAK-779. There was no correlation between sensitivity to TAK-779 and broadness in chimeric receptor use (data not shown).
Table 2.
CD4+ T-Cell Counts, ADC, Sensitivity to Inhibition by TAK-779, and Chimeric Receptor Use by Paired Plasma and CSF HIV-1 R5 Isolates
| PatientIsolate | CD4+ T Cell count (×106 cells/ml) | ADCa | TAK-779 IC50 (nM)b | TAK-779 IC90 (nM)b | Chimeric receptor usec |
|---|---|---|---|---|---|
| 2plasma | 627 | 6 | 39 | + | |
| 2csf | 6 | 32 | + | ||
| 3plasma | 532 | 70 | 235 | + | |
| 3csf | 6 | 130 | + | ||
| 16plasma | 150 | 10 | 32 | − | |
| 16csf | 15 | 140 | − | ||
| 21plasma | 49 | Yes | 60 | 860 | + |
| 21csf | 85 | >990 | +++ | ||
| 13plasma | 230 | Yes | >990 | >990 | ++ |
| 13csf | 810 | >990 | +++ | ||
| 26plasma | 36 | 225 | >990 | +++ | |
| 26csf | 183 | >990 | +++ | ||
| 27plasma | 35 | Yes | 25 | >990 | ++ |
| 27csf | 73 | >990 | ++ |
ADC, AIDS dementia complex.
Sensitivity to inhibition by TAK-779 for selected R5 HIV-1 isolates was evaluated on PHA- and IL-2-stimulated PBMCs. Maximum concentration of TAK-779 was 990 nM.
Semiquantitative analysis of the ability to utilize any of the chimeric receptors, based on the most efficient infection of either of the receptors FC-1, FC-2, or FC-4B. 100–1000 pg p24 antigen/ml (+), 1000–10,000 pg/ml (++), >10,000+pg/ml (+++).
Discussion
Previous studies have shown that CNS-derived isolates, including those from individuals with ADC, in general are R5-tropic, although exceptions do occur.44–48 However, few studies have focused on possible discrepancies in coreceptor use between peripheral virus isolates (plasma derived) and CNS virus isolates (brain or CSF derived).
In line with previous studies we also found a predominance of CCR5-using isolates within CSF in four of the seven patients who harbored X4/R5X4 plasma isolates. We believe that this R5 virus dominance in CSF isolates may be explained by a limited capacity of the studied X4/R5X4 variants to replicate in target cells within the CNS, e.g., brain macrophages and microglial cells.49
In a study of paired plasma and CSF isolates from 46 individuals, Spudich et al.,50 using a recombinant phenotypic assay, found discordant CXCR4/CCR5 usage in approximately 10% of subjects, which is similar to our findings. However, whereas they found X4-containing isolates in CSF from two patients harboring only R5 phenotypes in plasma only the opposite discordance was found in our study. Earlier studies by Di Stefano et al. used cytopathological characterization of MT-2 cells as an index of coreceptor tropism to evaluate blood and CSF isolates. It is now well established that isolates that are syncytia inducing (SI) in the MT-2 cell assay represent CXCR4-tropic isolates and non-syncytia-inducing isolates (NSI) are CCR5-tropic. In their evaluation of 22 individuals with CD4+ T cell counts <200 cells/μl, discordant phenotypes were detected in 46% of paired CSF and PBMC isolates.51 All discordant isolates were represented by NSI (R5-tropic) strains being detected in CSF in the presence of SI (X4/R5X4) strains isolated from blood, which is in compliance with our findings. In a clinical perspective, our results on wild-type coreceptor use do not support the necessity to assess CSF virus coreceptor tropism in patients with exclusive R5-tropic plasma virus that are under consideration for CCR5 antagonist treatment. Furthermore, some HIV-1-infected individuals that harbor CXCR4-using viral populations in plasma may theoretically benefit from treatment with CCR5 antagonists, as this could suppress virus replication within the CNS, thereby preventing further HIV-1-induced neurological damage in these patients.
The rationale for including our CXCR4/CCR5 chimeras in this evaluation lies within the results of our previous studies, which emphasize the heterogenic nature of R5 virus coreceptor use, and the possible implications that this may have for the pathogenesis of HIV-1 infection.7 In six patients, R5-tropic isolates with a discordant use of chimeric receptors displayed no specific patterns of receptor use that could distinguish CSF-derived isolates from plasma isolates. Also, p24 production by infected wild-type CCR5-transfected cells was similar between paired isolates (data not shown). Thus, CSF isolates were not characterized by an increased flexibility in CCR5 usage.
By assessing coreceptor use in the separate compartments we confirmed that R5 plasma isolates from HIV-infected individuals with low CD4+ T cell counts are more flexible in their use of chimeric receptors.7 In the present cross-sectional study, the strongest correlation between chimeric receptor use and immunological dysfunction was found when we specifically assessed viral ability to utilize the chimeric receptor FC-2. We found no correlation between plasma virus chimeric receptor use and plasma viral load, although CD4+ T cell counts and viral load correlated inversely as expected. However, FC-2 usage by CSF R5 isolates correlated significantly with an increased CSF viral load (Fig. 3). We believe that this may be explained by an increased ability of FC-2-utilizing R5 isolates to replicate in target cells within the CNS. This assumption is supported by results from a recent study in which we show that FC-2 usage by R5-tropic viruses correlates with an enhanced ability to infect primary macrophages in vitro (Karlsson et al., unpublished data).
Similar to previous studies, we found a correlation between CSF-neopterin levels and ADC.39–42 We did not find any significant correlation between CSF viral load and ADC, as has been suggested in some studies.52 High CSF viral loads is, however, not a uniform finding in ADC.53 High CSF HIV-RNA levels could also be found in asymptomatic patients and we showed in a previous study that 20% of untreated patients who lack neurological deficits have higher viral loads in CSF than in plasma.54 The relatively high levels of CSF viral load in the individuals presented here may in part be explained by our selection of participants, as one of the aims was to study patients with varying viral load in the two compartments.
Emerging data suggest that in individuals with AIDS who do not harbor X4/R5X4 virus phenotypes, CCR5-restricted viruses with an altered R5 phenotype may develop at immunodeficiency.38,55–59 Our study confirms the notion that R5 viruses isolated from severely immunosuppressed individuals are distinct with regards to coreceptor usage. This suggests that alterations in the mode of CCR5 use may be a key event in R5 virus pathogenesis. In a recent separate study we also showed that evolution of the R5 phenotype can be linked to adaptive molecular changes in the viral envelope glycoproteins, where viruses detected after AIDS onset display gp120 with increased net positive charge.60 These alterations may be detected in the described assay as an enhanced viral ability to utilize chimeric receptors.
HIV-1 infection in CNS may pose a specific obstacle to treatment with antiretroviral agents. Several existing antiretroviral compounds penetrate the blood–brain barrier poorly, and suboptimal CNS drug concentrations may favor the emergence of drug-resistant virus strains.61,62 A sustained low level inflammation is often detected in CSF samples from patients with undetectable plasma virus levels, even after several years of maintained antiretroviral treatment,63 and subjects with CSF viral load <2 copies/ml have lower intrathecal immunoactivation than subjects with CSF viral load between 2 and 20 copies/ml irrespective of plasma viral load.64 Together, this may indicate that full virus suppression of CNS HIV-1 infection is not always achieved with current drug regimens. Whether new compounds, such as CCR5 antagonists, would be more effective in suppressing CNS viral replication is not yet known. This provided the rationale to analyze sensitivity to CCR5 antagonism in selected R5-tropic isolates from the two compartments. For this purpose we selected paired isolates with varying chimeric receptor use, which also allowed us to correlate mode of chimeric coreceptor use with sensitivity to the CCR5 antagonist TAK-779. Although there were no general discrepancies in sensitivity to inhibition by TAK-779 between R5 isolates from the two compartments, it is important to note that the CSF isolates from all three patients with ADC were R5-tropic phenotypes that were incompletely inhibited by TAK-779 in vitro.
Although this evaluation was performed on a limited number of isolates it is striking that 90% inhibition could not be achieved for any of the seven isolates with an elevated usage of any of the chimeric receptors, whereas this was achieved for all other isolates (Table 2). Given that our CXCR4/CCR5 chimeric receptors share a common CCR5 backbone that lacks the N-terminus, it is possible that R5 isolates that are able to utilize these receptors are less dependent on interactions with the N-terminal part of CCR5. In this context it is intriguing that R5 virus resistance to the clinically available CCR5 antagonist maraviroc (Pfizer, Inc., New York, NY), has recently been explained by a reduced viral dependency on interactions with the N-terminus of CCR5.65 Furthermore, R5 virus resistance to maraviroc is also similarly characterized by a reduced maximum percentage inhibition with no change in IC50. However, the clinical relevance of reduced sensitivity to TAK-779 for the outcome of CCR5 antagonist treatment remains to be determined.
Further studies are warranted to verify the correlation found here between R5 isolate chimeric receptor utilization and viral sensitivity to inhibition by CCR5 antagonism. Nevertheless, chimeric CXCR4/CCR5 receptors may prove to be useful tools, not only in future studies of R5 virus pathogenesis, but also for optimizing antiretroviral treatment with coreceptor antagonists.
Acknowledgments
U87.CD4 cells were a kind gift from Dr. Dan Littman, Howard Hughes Medical Institute, New York. TAK-779 was obtained from the NIH Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. The expert technical assistance of Elzbieta Vincic is greatly appreciated. The help of Anna Laurén with reverse transcriptase measurements is gratefully acknowledged. The expert technical assistance of Jesper Bristulf is greatly appreciated. This work was supported by grants from the “Network for Inflammation Research” funded by the Swedish Foundation for Strategic Research (L.A.), the Royal Physiographic Society (L.A., U.K.), Faculty of Medicine, Lund University, Sweden (B.L., U.K.), the Swedish Research Council (M.J.) (2007–7092) (M.G.), the Swedish International Development Agency/Department for Research Cooperation (M.J.), the Physicians Against AIDS Research Foundation (U.K., M.J.), the National Institutes of Health (NINDS R01 NS43103-01) (M.G.), and the Medical Faculty of Gothenburg University, Sweden (ALFGBG-11067) (M.G.). U. Karlsson and L. Antonsson contributed equally to this work.
Disclosure Statement
No competing financial interests exist.
References
- 1.Feng Y. Broder CC. Kennedy PE. Berger EA. HIV-1 entry cofactor: Functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 2.Dragic T. Litwin V. Allaway GP, et al. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 3.Bieniasz PD. Cullen BR. Chemokine receptors and human immunodeficiency virus infection. Front Biosci. 1998;3:44–58. doi: 10.2741/a265. [DOI] [PubMed] [Google Scholar]
- 4.Karlsson A. Parsmyr K. Sandstrom E. Fenyo EM. Albert J. MT-2 cell tropism as prognostic marker for disease progression in human immunodeficiency virus type 1 infection. J Clin Microbiol. 1994;32:364–370. doi: 10.1128/jcm.32.2.364-370.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tersmette M. de Goede RE. Al BJ, et al. Differential syncytium-inducing capacity of human immunodeficiency virus isolates: Frequent detection of syncytium-inducing isolates in patients with acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. J Virol. 1988;62:2026–2032. doi: 10.1128/jvi.62.6.2026-2032.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koot M. Keet IP. Vos AH, et al. Prognostic value of HIV-1 syncytium-inducing phenotype for rate of CD4+ cell depletion and progression to AIDS. Ann Intern Med. 1993;118(9):681–688. doi: 10.7326/0003-4819-118-9-199305010-00004. [DOI] [PubMed] [Google Scholar]
- 7.Karlsson I. Antonsson L. Shi Y, et al. Coevolution of RANTES sensitivity and mode of CCR5 receptor use by human immunodeficiency virus type 1 of the R5 phenotype. J Virol. 2004;78:11807–11815. doi: 10.1128/JVI.78.21.11807-11815.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antonsson L. Boketoft A. Garzino-Demo A. Olde B. Owman C. Molecular mapping of epitopes for interaction of HIV-1 as well as natural ligands with the chemokine receptors, CCR5 and CXCR4. AIDS. 2003;17:2571–2579. doi: 10.1097/00002030-200312050-00004. [DOI] [PubMed] [Google Scholar]
- 9.Karlsson I. Antonsson L. Shi Y, et al. HIV biological variability unveiled: Frequent isolations and chimeric receptors reveal unprecedented variation of coreceptor use. AIDS. 2003;17:2561–2569. doi: 10.1097/01.aids.0000096853.36052.36. [DOI] [PubMed] [Google Scholar]
- 10.Whitcomb JM. Huang W. Fransen S, et al. Development and characterization of a novel single-cycle recombinant-virus assay to determine human immunodeficiency virus type 1 coreceptor tropism. Antimicrob Agents Chemother. 2007;51(2):566–575. doi: 10.1128/AAC.00853-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Price RW. Navia BA. Cho ES. AIDS encephalopathy. Neurol Clin. 1986;4(1):285–301. [PubMed] [Google Scholar]
- 12.Price RW. Brew B. Sidtis J. Rosenblum M. Scheck AC. Cleary P. The brain in AIDS: Central nervous system HIV-1 infection and AIDS dementia complex. Science. 1988;239(4840):586–592. doi: 10.1126/science.3277272. [DOI] [PubMed] [Google Scholar]
- 13.Sönnerborg AB. Ehrnst AC. Bergdahl SK. Pehrson PO. Sköldenberg BR. Strannegård OO. HIV isolation from cerebrospinal fluid in relation to immunological deficiency and neurological symptoms. AIDS. 1988;2(2):89–93. doi: 10.1097/00002030-198804000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Epstein LG. Kuiken C. Blumberg BM. Hartman S. Sharer LR. Clement M. Goudsmit J. HIV-1 V3 domain variation in brain and spleen of children with AIDS: Tissue-specific evolution within host-determined quasispecies. Virology. 1991;180:583–590. doi: 10.1016/0042-6822(91)90072-j. [DOI] [PubMed] [Google Scholar]
- 15.Korber BT. Kunstman KJ. Patterson BK. Furtado M. McEvilly MM. Levy R. Wolinsky SM. Genetic differences between blood- and brain-derived viral sequences from human immunodeficiency virus type 1-infected patients: Evidence of conserved elements in the V3 region of the envelope protein of brain-derived sequences. J Virol. 1994;68:7467–7481. doi: 10.1128/jvi.68.11.7467-7481.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohagen A. Devitt A. Kunstman KJ, et al. Genetic and functional analysis of full-length human immunodeficiency virus type 1 env genes derived from brain and blood of patients with AIDS. J Virol. 2003;77:12336–12345. doi: 10.1128/JVI.77.22.12336-12345.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong JK. Ignacio CC. Torriani F. Havlir D. Fitch NJ. Richman DD. In vivo compartmentalization of human immunodeficiency virus: Evidence from the examination of pol sequences from autopsy tissues. J Virol. 1997;71:2059–2071. doi: 10.1128/jvi.71.3.2059-2071.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiodi F. Valentin A. Keys B, et al. Biological characterization of paired human immunodeficiency virus type 1 isolates from blood and cerebrospinal fluid. Virology. 1989;173:178–187. doi: 10.1016/0042-6822(89)90233-x. [DOI] [PubMed] [Google Scholar]
- 19.Koenig S. Gendelman HE. Orenstein JM, et al. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986;233(4768):1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- 20.Watkins BA. Dorn HH. Kelly WB, et al. Specific tropism of HIV-1 for microglial cells in primary human brain cultures. Science. 1990;249(4968):549–553. doi: 10.1126/science.2200125. [DOI] [PubMed] [Google Scholar]
- 21.Ghorpade A. Nukuna A. Che M, et al. Human immunodeficiency virus neurotropism: An analysis of viral replication and cytopathicity for divergent strains in monocytes and microglia. J Virol. 1998;72(4):3340–3350. doi: 10.1128/jvi.72.4.3340-3350.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Catani MV. Corasaniti MT. Navarra M. Nistico G. Finazzi-Agro A. Melino G. gp120 induces cell death in human neuroblastoma cells through the CXCR4 and CCR5 chemokine receptors. J Neurochem. 2000;74:2373–2379. doi: 10.1046/j.1471-4159.2000.0742373.x. [DOI] [PubMed] [Google Scholar]
- 23.Hesselgesser J. Taub D. Baskar P. Greenberg M. Hoxie J. Kolson DL. Horuk R. Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1 alpha is mediated by the chemokine receptor CXCR4. Curr Biol. 1998;8:595–598. doi: 10.1016/s0960-9822(98)70230-1. [DOI] [PubMed] [Google Scholar]
- 24.Zheng J. Ghorpade A. Niemann D, et al. Lymphotropic virions affect chemokine receptor-mediated neural signaling and apoptosis: Implications for human immunodeficiency virus type 1-associated dementia. J Virol. 1999;73:8256–8267. doi: 10.1128/jvi.73.10.8256-8267.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Price RW. Staprans S. Measuring the “viral load” in cerebrospinal fluid in human immunodeficiency virus infection: Window into brain infection? Ann Neurol. 1997;42(5):675–678. doi: 10.1002/ana.410420502. [DOI] [PubMed] [Google Scholar]
- 26.Takashima K. Miyake H. Furuta RA, et al. Inhibitory effects of small-molecule CCR5 antagonists on human immunodeficiency virus type 1 envelope-mediated membrane fusion and viral replication. Antimicrob Agents Chemother. 2001;45:3538–3543. doi: 10.1128/AAC.45.12.3538-3543.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dragic T. Trkola A. Thompson DA, et al. A binding pocket for a small molecule inhibitor of HIV-1 entry within the transmembrane helices of CCR5. Proc Natl Acad Sci USA. 2000;97:5639–5644. doi: 10.1073/pnas.090576697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baba M. Nishimura O. Kanzaki N, et al. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc Natl Acad Sci USA. 1999;96:5698–5703. doi: 10.1073/pnas.96.10.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gisslén M. Hagberg L. Brew B. Cinque P. Price RW. Rosengren L. Elevated cerebrospinal fluid neurofilament protein predicts development of AIDS dementia complex. J Infect Dis. 2007;195(12):1774–1778. doi: 10.1086/518043. [DOI] [PubMed] [Google Scholar]
- 30.Nomenclature and research case definitions for neurologic manifestations of human immunodeficiency virus-type 1 (HIV-1) infection. Report of a Working Group of the American Academy of Neurology AIDS Task Force. Neurology. 1991;41:778–785. doi: 10.1212/wnl.41.6.778. [DOI] [PubMed] [Google Scholar]
- 31.Antinori A. Arendt G. Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hagberg L. Andersson L-M. Abdulle S. Gisslén M. Clinical application of CSF neopterin concentrations in HIV infection. Pteridines. 2004;15:103–107. [Google Scholar]
- 33.Andersson LM. Svennerholm B. Hagberg L. Gisslen M. Higher HIV-1 RNA cutoff level required in cerebrospinal fluid than in blood to predict positive HIV-1 isolation. J Med Virol. 2000;62:9–13. doi: 10.1002/1096-9071(200009)62:1<9::aid-jmv2>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 34.Albert J. Fenyo EM. Simple, sensitive, and specific detection of human immunodeficiency virus type 1 in clinical specimens by polymerase chain reaction with nested primers. J Clin Microbiol. 1990;28:1560–1564. doi: 10.1128/jcm.28.7.1560-1564.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olde B. Sabirsh A. Owman C. Molecular mapping of epitopes involved in ligand activation of the human receptor for the neuropeptide, VIP, based on hybrids with the human secretin receptor. J Mol Neurosci. 1998;11:127–134. doi: 10.1385/JMN:11:2:127. [DOI] [PubMed] [Google Scholar]
- 36.Lefebvre B. Formstecher P. Lefebvre P. Improvement of the gene splicing overlap (SOE) method. Biotechniques. 1995;19:186–188. [PubMed] [Google Scholar]
- 37.Mild M. Björndal A. Medstrand P. Fenyö EM. Isolation of human immunodeficiency virus-type 1 (HIV-1) clones with biological and molecular properties of the primary isolate. Virology. 2006;350(1):58–66. doi: 10.1016/j.virol.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 38.Repits J. Oberg M. Esbjornsson J, et al. Selection of human immunodeficiency virus type 1 R5 variants with augmented replicative capacity, reduced sensitivity to entry inhibitors during severe immunodeficiency. J Gen Virol. 2005;86:2859–2869. doi: 10.1099/vir.0.81111-0. [DOI] [PubMed] [Google Scholar]
- 39.Brew BJ. Bhalla RB. Paul M. Gallardo H. McArthur JC. Schwartz MK. Price RW. Cerebrospinal fluid neopterin in human immunodeficiency virus type 1 infection. Ann Neurol. 1990;28:556–560. doi: 10.1002/ana.410280413. [DOI] [PubMed] [Google Scholar]
- 40.Brew BJ. Dunbar N. Pemberton L. Kaldor J. Predictive markers of AIDS dementia complex: CD4 cell count and cerebrospinal fluid concentrations of beta 2-microglobulin and neopterin. J Infect Dis. 1996;174:294–298. doi: 10.1093/infdis/174.2.294. [DOI] [PubMed] [Google Scholar]
- 41.Fuchs D. Chiodi F. Albert J, et al. Neopterin concentrations in cerebrospinal fluid and serum of individuals infected with HIV-1. AIDS. 1989;3:285–288. doi: 10.1097/00002030-198905000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Sonnerborg AB. von Stedingk LV. Hansson LO. Strannegard OO. Elevated neopterin and beta 2-microglobulin levels in blood and cerebrospinal fluid occur early in HIV-1 infection. AIDS. 1989;3:277–283. doi: 10.1097/00002030-198905000-00005. [DOI] [PubMed] [Google Scholar]
- 43.Abdulle S. Mellgren A. Brew BJ, et al. CSF neurofilament protein (NFL)—a marker of active HIV-related neurodegeneration. J Neurol. 2007;254(8):1026–1032. doi: 10.1007/s00415-006-0481-8. [DOI] [PubMed] [Google Scholar]
- 44.Albright AV. Shieh JT. Itoh T, et al. Microglia express CCR5, CXCR4, and CCR3, but of these, CCR5 is the principal coreceptor for human immunodeficiency virus type 1 dementia isolates. J Virol. 1999;73:205–213. doi: 10.1128/jvi.73.1.205-213.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gorry PR. Bristol G. Zack JA, et al. Macrophage tropism of human immunodeficiency virus type 1 isolates from brain and lymphoid tissues predicts neurotropism independent of coreceptor specificity. J Virol. 2001;75:10073–10089. doi: 10.1128/JVI.75.21.10073-10089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He J. Chen Y. Farzan M, et al. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- 47.Shieh JT. Albright AV. Sharron M, et al. Chemokine receptor utilization by human immunodeficiency virus type 1 isolates that replicate in microglia. J Virol. 1998;72:4243–4249. doi: 10.1128/jvi.72.5.4243-4249.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smit TK. Wang B. Ng T. Osborne R. Brew B. Saksena NK. Varied tropism of HIV-1 isolates derived from different regions of adult brain cortex discriminate between patients with and without AIDS dementia complex (ADC): Evidence for neurotropic HIV variants. Virology. 2001;279:509–526. doi: 10.1006/viro.2000.0681. [DOI] [PubMed] [Google Scholar]
- 49.Schmidtmayerova H. Alfano M. Nuovo G. Bukrinsky M. Human immunodeficiency virus type 1 T-lymphotropic strains enter macrophages via a CD4-, CXCR4-mediated pathway: Replication is restricted at a postentry level. J Virol. 1998;72:4633–4642. doi: 10.1128/jvi.72.6.4633-4642.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spudich SS. Huang W. Nilsson AC, et al. HIV-1 chemokine coreceptor utilization in paired cerebrospinal fluid and plasma samples: A survey of subjects with viremia. J Infect Dis. 2005;191:890–898. doi: 10.1086/428095. [DOI] [PubMed] [Google Scholar]
- 51.Di Stefano M. Monno L. Fiore JR, et al. Neurological disorders during HIV-1 infection correlate with viral load in cerebrospinal fluid but not with virus phenotype. AIDS. 1998;12(7):737–743. doi: 10.1097/00002030-199807000-00010. [DOI] [PubMed] [Google Scholar]
- 52.McArthur JC. McClernon DR. Cronin MF, et al. Relationship between human immunodeficiency virus-associated dementia and viral load in cerebrospinal fluid and brain. Ann Neurol. 1997;42(5):689–698. doi: 10.1002/ana.410420504. [DOI] [PubMed] [Google Scholar]
- 53.Gisslen M. Hagberg L. Rosengren L, et al. Defining and evaluating HIV-related neurodegenerative disease and its treatment targets: A combinatorial approach to use of cerebrospinal fluid molecular biomarkers. J Neuroimmune Pharmacol. 2007;2:112–119. doi: 10.1007/s11481-006-9035-1. [DOI] [PubMed] [Google Scholar]
- 54.Gisslén M. Fuchs D. Svennerholm B. Hagberg L. Cerebrospinal fluid viral load, intrathecal immunoactivation, cerebrospinal fluid monocytic cell count in HIV-1 infection. J Acquir Immune Defic Syndr. 1999;21(4):271–276. doi: 10.1097/00126334-199908010-00003. [DOI] [PubMed] [Google Scholar]
- 55.Jansson M. Popovic M. Karlsson A, et al. Sensitivity to inhibition by beta-chemokines correlates with biological phenotypes of primary HIV-1 isolates. Proc Natl Acad Sci USA. 1996;93(26):15382–15387. doi: 10.1073/pnas.93.26.15382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jansson M. Backstrom E. Bjorndal A, et al. Coreceptor usage and RANTES sensitivity of non-syncytium-inducing HIV-1 isolates obtained from patients with AIDS. J Hum Virol. 1999;2:325–338. [PubMed] [Google Scholar]
- 57.Koning FA. Kwa D. Boeser-Nunnink B, et al. Decreasing sensitivity to RANTES (regulated on activation, normally T cell-expressed and -secreted) neutralization of CC chemokine receptor 5-using, non-syncytium-inducing virus variants in the course of human immunodeficiency virus type 1 infection. J Infect Dis. 2003;188:864–872. doi: 10.1086/377105. [DOI] [PubMed] [Google Scholar]
- 58.Gray L. Sterjovski J. Churchill M, et al. Uncoupling coreceptor usage of human immunodeficiency virus type 1 (HIV-1) from macrophage tropism reveals biological properties of CCR5-restricted HIV-1 isolates from patients with acquired immunodeficiency syndrome. Virology. 2005;337:384–398. doi: 10.1016/j.virol.2005.04.034. [DOI] [PubMed] [Google Scholar]
- 59.Sterjovski J. Churchill MJ. Ellett A, et al. Asn 362 in gp120 contributes to enhanced fusogenicity by CCR5-restricted HIV-1 envelope glycoprotein variants from patients with AIDS. Retrovirology. 2007;4:89. doi: 10.1186/1742-4690-4-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Repits J. Sterjovski J. Badia-Martinez D, et al. Primary HIV-1 R5 isolates from end-stage disease display enhanced viral fitness in parallel with increased gp120 net charge. Virology. 2008;379(1):125–134. doi: 10.1016/j.virol.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 61.Pierson T. McArthur J. Siliciano RF. Reservoirs for HIV-1: Mechanisms for viral persistence in the presence of antiviral immune responses and antiretroviral therapy. Annu Rev Immunol. 2000;18:665–708. doi: 10.1146/annurev.immunol.18.1.665. [DOI] [PubMed] [Google Scholar]
- 62.Letendre S. Marquie-Beck J. Capparelli E, et al. Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65(1):65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Edén A. Price RW. Spudich S, et al. Immune activation of the central nervous system is still present after >4 years of effective highly active antiretroviral therapy. J Infect Dis. 2007;196(12):1779–1783. doi: 10.1086/523648. [DOI] [PubMed] [Google Scholar]
- 64.Yilmaz A. Price RW. Spudich S, et al. Persistent intrathecal immune activation in HIV-1-infected individuals on antiretroviral therapy. J Acquir Immune Defic Syndr. 2008;47(2):168–173. doi: 10.1097/QAI.0b013e31815ace97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mori J. Mosley M. Lewis M, et al. St. Michael; Barbados: 2007. Characterization of maraviroc resistance in patients failing treatment with CCR5-tropic virus in MOTIVATE 1, MOTIVATE 2. Program and Abstracts of the XVI International HIV Drug Resistance Workshop. June 12–16. (Abstract 10). [Google Scholar]