Abstract
Background
The potential benefit in preventing pelvic floor disorders (PFDs) is a frequently cited reason for requesting or performing cesarean delivery on maternal request (CDMR). However, for primigravid women without medical/obstetric indications, the lifetime cost-effectiveness of CDMR remains unknown, particularly with regard to lifelong pelvic floor consequences. Our objective was to assess the cost-effectiveness of CDMR in comparison to trial of labor (TOL) for primigravid women without medical/obstetric indications with a single childbirth over their lifetime, while explicitly accounting for the management of PFD throughout the lifetime.
Methods
We used Monte Carlo simulation of a decision model containing 249 chance events and 101 parameters depicting lifelong maternal and neonatal outcomes in the following domains: actual mode of delivery, emergency hysterectomy, transient maternal morbidity and mortality, perinatal morbidity and mortality, and the lifelong management of PFDs. Parameter estimates were obtained from published literature. The analysis was conducted from a societal perspective. All costs and quality-adjusted life-years (QALYs) were discounted to the present value at childbirth.
Results
The estimated mean cost and QALYs were $14,259 (95% confidence interval [CI] $8,964-$24,002) and 58.21 (95% CI 57.43-58.67) for CDMR and $13,283 (95% CI $7,861-$23,829) and 57.87 (95% CI 56.97-58.46) for TOL over the combined lifetime of the mother and the child. Parameters related to PFDs play an important role in determining cost and quality of life.
Conclusions
When a woman without medical/obstetric indications has only one childbirth in her lifetime, cost-effectiveness analysis does not reveal a clearly preferable mode of delivery.
Introduction
Cesarean delivery is the most commonly performed operating room procedure in the United States.1 In addition to being major abdominal surgery, cesarean delivery is associated with increased risk of neonatal respiratory morbidity and can cause complications in subsequent pregnancies, such as uterine rupture, placenta previa, and placenta accreta.2 Cesarean delivery on maternal request (CDMR), defined as cesarean delivery for a singleton pregnancy on maternal request at term in the absence of any medical or obstetric indications,3 has generated nationwide debate as the cesarean delivery rate reaches its highest level (31.1% of all births in 2006).4 The widespread concern that some of this increase may be attributable to an increase in CDMR5,6 provided impetus for the National Institutes of Health (NIH) 2006 State-of-the-Science Conference on CDMR.3
Patients and healthcare providers frequently report prevention of pelvic floor disorders (PFDs) as the primary reason for choosing cesarean delivery.6,7 PFDs include several clinical conditions, such as urinary incontinence (UI), fecal incontinence (FI), and pelvic organ prolapse (POP). Among women 20 and older in the United States, the prevalences of UI, FI, and POP are 15.7%, 9.0%, and 2.9%, respectively.8 These debilitating conditions significantly impact women's quality of life (QOL) and increase healthcare costs. Each year in the United States, UI costs $19.5 billion,9 and over 200,000 women undergo inpatient surgery for POP.10,11
Although there is evidence supporting an association between vaginal birth and the development of PFDs,3 only two cost-effectiveness studies12,13 comparing modes of delivery have incorporated PFDs as a maternal outcome. One examined vaginal birth after a previous cesarean delivery,12 and the other compared planned cesarean delivery with trial of labor (TOL) for primigravid women with macrosomic infants.13 For primigravid women without medical or obstetric indications, however, the lifetime cost-effectiveness of CDMR vs. TOL remains unknown, particularly with regard to lifelong pelvic floor consequences. This subgroup of pregnant women is at the center of the CDMR debate.
The choice between CDMR and TOL is complex, involving both maternal and neonatal factors with short-term and long-term implications. Drawing on the advantage of decision analysis, which allows for multiple, often conflicting, factors to be analyzed in a single model, this study makes an important first step toward addressing this complex question by investigating the lifelong cost-effectiveness of CDMR vs. TOL for primigravid women without medical or obstetric indications having only one childbirth in their lifetime. A single birth model provides important insights for future studies of two or more childbirths.
Materials and Methods
Decision tree model
Cost-effectiveness analysis is a method designed to assess the comparative impacts (i.e., cost and effectiveness) of different health interventions.14 It involves estimating the incremental costs and effects of an intervention compared with some alternatives.14 The method has been widely applied to inform difficult clinical and public health decisions and has been used to assess the relative cost and benefit trade-offs of alternative healthcare interventions in gynecologic oncology, gynecologic surgery, maternal-fetal medicine, infertility treatment, and other subspecialties in the field of obstetrics and gynecology.15–17
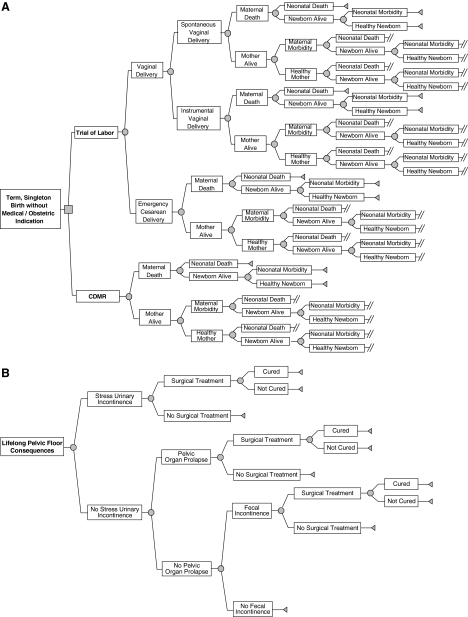
A decision tree is a visual tool to illustrate how each compared intervention relates to the possible outcomes.18 In this study, a decision tree model containing 249 chance events and 101 parameters was constructed mapping the sequence of most relevant clinical outcomes after each delivery management scheme (CDMR or TOL) throughout the mother and the newborn's lifetime (Fig. 1).
FIG. 1.
Decision tree model for one term singleton birth without medical or obstetric indications. Part A of the figure illustrates the portion of the decision tree related to short-term maternal and neonatal outcomes. Part B of the figure depicts the portion of the decision tree related to the long-term pelvic floor consequences. Square, decision node; circle, chance node; triangle, end node; double slash, branch continues with the Lifelong Pelvic Floor Consequences subtree.
The patient in this analysis was defined as a 25-year-old (i.e., the mean age of American women at first childbirth19), primigravid woman with a term singleton birth without medical or obstetric indications (e.g., known fetal or maternal risk factors) favoring either management strategy. The woman has no history of PFD before delivery. We assumed that women who opt for CDMR undergo a cesarean delivery before onset of labor and that women undergoing TOL could not have a cesarean delivery without medical reasons. The analysis was conducted from a societal perspective, including all costs to the healthcare system and the patient.
Our analysis focused on women who actually have only one birth over their lifetime, who account for 21.6% of parous women in the United States.20 We limited our analysis to a single birth because of lack of data on the differential risk of PFD consequences in women with different delivery modes at successive childbirths (e.g., an instrumental vaginal delivery followed by a CDMR vs. a CDMR followed by a spontaneous vaginal delivery). Single birth is also the scenario most favorable to CDMR and often is discussed as a specific situation in which it might be appropriate.
Our model incorporated the following PFDs: stress UI (SUI), FI, and POP. SUI is believed to be primarily a result of the childbirth experience, which can cause injury to muscles, connective tissues, and nerves.16 In contrast, the etiology of urge UI, the other main type of UI in women, is less well characterized and, therefore, was not included in our model.21 The definition of FI varies in the literature, with some studies including flatus incontinence whereas others consider involuntary loss of stool only.22 Because prior studies show that flatal incontinence has less impact on QOL and there is confusion in women reporting voluntary vs. involuntary passing of flatus,23 we defined FI as involuntary leakage of liquid/solid stool in our analysis. We did not include female sexual dysfunction (FSD)5 because the published data on FSD do not provide sufficient detail to suit our analysis.
Our selection of other maternal and perinatal morbidities was guided by the 2006 Visco et al.24 systematic review of research comparing outcomes of CDMR and TOL and supplemented by our review of more recent studies. Visco et al.24 indicated moderate quality or some limited evidence for differential incidence of infection, hemorrhage/blood transfusion, and surgical complications between CDMR and TOL, as well as differences in the rate of neonatal respiratory morbidity. More recent studies also suggested evidence of differential rates of peripartum hysterectomy between CDMR and TOL.25–27
Data sources
Estimates of the base value and plausible ranges for the probability, cost, and utility parameters associated with each health state were obtained from the published literature (Tables 1, 2, 3, and 4). A utility value, ranging from 0 (death) to 1 (perfect health), quantifies an individual's perception and preference for a health state. All parameter estimates were reviewed by an expert panel, including two obstetricians and the two clinical authors with considerable clinical and research experience (D.F. and J.O.L.D). Because this study analyzed published data, it was not human subject research and, hence, did not require Institutional Review Board oversight.
Table 1.
Estimates of Probability Parameters in Model
| Parameter | Base value | Rangea | References |
|---|---|---|---|
| Trial of labor | |||
| Probability of having a vaginal delivery | 90% | 84.4%–97.0% | 25, 28–30 |
| If vaginal delivery | |||
| Probability of spontaneous vaginal delivery | 90% | 85.0%–95.0% | 25, 31, 32 |
| If spontaneous vaginal delivery | |||
| Probability of maternal death | 0.0% | 0.0%–0.0020% | 33, 34b |
| Probability of neonatal death | 0.02% | 0.018%–0.063% | 35–38 |
| If spontaneous vaginal delivery and mother alive | |||
| Probability of composite maternal morbidityc | 1.22% | 0.94%–1.35% | 31, 39–44 |
| Probability of having stress urinary incontinence (SUI) | 19.90% | 14.93%–40.80% | 45–49 |
| Probability of having pelvic organ prolapse (POP) | 8.9% | 7%–9.79% | 47, 50 |
| Probability of having fecal incontinence (FI) | 2.65% | 2.13%–6.9% | 47, 48, 51–53 |
| If spontaneous vaginal delivery and baby alive | |||
| Probability of neonatal morbidityd | 1.0% | 0.75%–1.25% | 54 |
| If instrumental vaginal delivery | |||
| Probability of maternal death | 0.0028% | 0.0024%–0.0028% | 29, 33, 34b |
| Probability of neonatal death | 0.0352% | 0.030%–0.063% | 35–38 |
| If instrumental vaginal delivery and mother alive | |||
| Probability of composite maternal morbidityc | 3.81% | 2.58%–4.19% | 29, 31, 39–44 |
| Probability of having SUI | 21.80% | 16.35%–43.50% | 45–47, 49 |
| Probability of having POP | 12.0% | 7%–13.2% | 47, 50 |
| Probability of having FI | 8.0% | 3.9%–18.8% | 47, 51–53, 55, 56 |
| If instrumental vaginal delivery and baby alive | |||
| Probability of neonatal morbidityd | 0.9% | 0.68%–1.13% | 54 |
| If emergency cesarean delivery | |||
| Probability of maternal death | 0.0097% | 0.0097%–0.0250% | 25, 33, 34, 57 |
| Probability of neonatal death | 0.08% | 0.06%–0.169% | 36, 38 |
| If emergency cesarean delivery and mother alive | |||
| Probability of composite maternal morbidityc | 13.69% | 10.04%–17.01% | 31, 39–42, 44, 58–61 |
| Probability of having SUI | 11.50% | 7.00%–33.00% | 45, 47, 49, 50, 62 |
| Probability of having POP | 7% | 0.00%–11.0% | 47, 50 |
| Probability of having FI | 5% | 4.0%–8.47% | 47, 52, 55, 56, 63 |
| If emergency cesarean delivery and baby alive | |||
| Probability of neonatal morbidityd | 4.5% | 3.55%–4.95% | 64, 65 |
| Cesarean delivery on maternal request (CDMR) | |||
| Probability of maternal death | 0.0059% | 0.0%–0.0148% | 26, 33, 34, 57 |
| Probability of neonatal death | 0.047% | 0.0%–0.173% | 36, 38, 66–69 |
| If CDMR and mother alive | |||
| Probability of composite maternal morbidityc | 5.01% | 2.47%–9.39% | 25, 26, 31, 39–44, 59, 61, 70 |
| Probability of having SUI | 10.00% | 0.0%–33.00% | 45–47, 49, 50, 62 |
| Probability of having POP | 1% | 0.0%–6% | 47, 50 |
| Probability of having FI | 1.78% | 0.0%–7.7% | 47, 52, 55, 56, 63, 71 |
| If CDMR and baby alive: | |||
| Probability of neonatal morbidityd | 3.3% | 2.9%–3.63% | 54, 64, 65, 72 |
| Treatment of pelvic floor disorders | |||
| SUI | |||
| Proportion of SUI symptomatic women seeking healthcare for SUI | 61% | 45.75%–76.25% | 73 |
| Proportion of women seeking healthcare for SUI who receive surgical treatment | 29.8% | 22.35%–37.25% | 74 |
| Success rate of SUI surgeries | 81.3% | 63%–93% | 75–82 |
| POP | |||
| Proportion of POP symptomatic women seeking healthcare for POP | 73% | 54.75%–91.25% | 73 |
| Proportion of women seeking healthcare for POP who receive surgical treatment | 75% | 56.25%–93.75% | Authors' assumption |
| Success rate of surgical treatment for POP | 71% | 58%–100% | 83–89 |
| FI | |||
| Proportion of FI symptomatic women seeking healthcare for FI | 33.8% | 20.5%–43% | 73, 90, 91 |
| Proportion of women seeking healthcare for FI who receive surgical treatment | 17.46% | 13.10%–34.50% | 92e |
| Success rate of FI surgeries | 25% | 10%–54% | 93–97 |
Plausible ranges of the parameters were determined based on data from the literature or ±25% of the base value if no range was available from the literature.
Supplemented by authors' analysis of the 2004 National Hospital Discharge Survey data.
Including any blood transfusion, wound infection and endometritis, peripartum hysterectomy, and surgical injury of the uterine, bladder, or bowel (see Table 2 for more details).
Including respiratory distress syndrome (RDS) and transient tachypnea (TTN).
Supplemented by unpublished research data from the Michigan Bowel Control Program.
Table 2.
Probability Parameters Associated with Composite Maternal Morbidity
| Parameter | Base value | Rangea | References |
|---|---|---|---|
| Trial of labor | |||
| Spontaneous vaginal delivery | |||
| Maternal: Any blood transfusion | 0.3% | 0.11%–0.33% | 31, 39, 40, 44 |
| Maternal: Infection (including wound infection and endometritis) | 0.8% | 0.72%–0.88% | 39–41 |
| Maternal: Peripartum hysterectomy | 0.023% | 0.0207%–0.03% | 27, 42, 43 |
| Maternal: Surgical injury (including uterine, bladder, or bowel injuries) | 0.1% | 0.09%–0.11% | 39, 40 |
| Instrumental vaginal delivery | |||
| Maternal: Any blood transfusion | 1.0% | 0.12%–1.1% | 31, 39, 40, 44 |
| Maternal: Infection (including wound infection and endometritis) | 2.6% | 2.34%–2.86% | 39–41 |
| Maternal: Peripartum hysterectomy | 0.05% | 0.03%–0.055% | 29, 42, 43 |
| Maternal: Surgical injury (including uterine, bladder, or bowel injuries) | 0.2% | 0.1%–0.22% | 39, 40 |
| Emergency cesarean delivery | |||
| Maternal: Any blood transfusion | 0.6% | 0.37%–1.1% | 31, 39, 40, 44, 58, 59 |
| Maternal: Infection (including wound infection and endometritis) | 11.2% | 9.45%–13.14% | 39–41, 58, 61 |
| Maternal: Peripartum hysterectomy | 0.12% | 0.108%–0.38% | 42, 58, 59 |
| Maternal: Surgical injury (including uterine, bladder, or bowel injuries) | 2.1% | 0.17%–3.02% | 39, 40, 58, 60 |
| Cesarean delivery on maternal request | |||
| Maternal: Any blood transfusion | 0.3% | 0.07%–4.455% | 26, 31, 39, 40, 44, 59, 70 |
| Maternal: Infection (including wound infection and endometritis) | 4.53% | 2.3%–8.261% | 26, 39–41, 61 |
| Maternal: Peripartum hysterectomy | 0.06% | 0.0%–0.715% | 25–27, 39, 42, 43, 59 |
| Maternal: Surgical injury (including uterine, bladder, or bowel injuries) | 0.14% | 0.1%–0.17% | 26, 39, 40 |
Plausible ranges of the parameters were determined based on data from the literature or ±25% of the base value if no range was available from the literature.
Table 3.
Estimates of Cost-related Parameters, in 2007 U.S. Dollars
| Parameter | Base value | Rangea | References |
|---|---|---|---|
| Obstetric care (including delivery and postpartum care) | |||
| Spontaneous vaginal delivery | $3,520 | $2,640–$4,400 | 98–100 |
| Instrumental vaginal delivery | $3,569 | $2,677–$4,461 | 98–101 |
| Emergency cesarean delivery | $6,513 | $4,885–$8,141 | 98–100, 102 |
| Cesarean delivery on maternal request | $4,735 | $3,666–$6,110 | 30, 98, 102 |
| Maternal outcomes | |||
| Composite maternal morbidity after spontaneous vaginal deliveryb | $1,308 | $981–$1,635 | 12 |
| Composite maternal morbidity after instrumental vaginal deliveryb | $1,283 | $962–$1,604 | 12 |
| Composite maternal morbidity after emergency cesarean deliveryb | $313 | $235–$391 | 12 |
| Composite maternal morbidity after planned cesarean deliveryb | $219 | $164–$274 | 12 |
| Maternal death | $2,589 | $1,942–$3,236 | 12 |
| Neonatal outcomes | |||
| Neonatal morbidity (postbirth respiratory problem) | $62,843 | $24,082–$180,611 | 12 |
| Neonatal death | $48,662 | $24,082–$72,245 | 12 |
| National mean age of women at first childbirth | 25.2 years | – | 19 |
| Additional productivity loss of cesarean delivery compared with vaginal delivery | 2 weeks | – | Authors' assumption |
| National median wage rate/week for women aged ≥25 | $654 | $491–$818 | 103 |
| National median wage rate/week for workers (regardless of gender and age) | $695 | $521–$869 | 103 |
| Female life expectancy at age of first childbirth (i.e., 25.2 years of age) | 56 years | – | 104 |
| Life expectancy at birth (regardless of gender) | 77.5 years | – | 104 |
| Pelvic floor disorders (PFDs) | |||
| Time of PFD onset (measured as number of years after delivery) | 10 years | 0–20 years | Authors' assumption |
| Stress urinary incontinence (SUI) | |||
| Annual cost of routine care for SUI (for community dwelling adult, mainly absorbent materials and cleaning) | $595 | $129–$2,185 | 105, 106 |
| Annual productivity loss associated with SUI when age <65 years | 104 hours | 52–192.4 hours | 9 |
| Age at which women undergo SUI surgeries | 54 years | 49–59 years | 107, 108 |
| Cost of diagnosis evaluation of SUI | $239 | $120–$479 | 105 |
| Cost of SUI surgery | $9,849 | $5,396–$19,296 | 105 |
| Pelvic organ prolapse (POP) | |||
| Annual cost of routine care for POP | $0 | – | Authors' assumption |
| Annual productivity loss associated with POP when age <65 years | 0 hours | – | Authors' assumption |
| Age at which women undergo POP surgeries | 59 years | 49–60 years | 108–110 |
| Cost of diagnosis evaluation of POP | $230 | $173–$288 | 98 |
| Cost of POP surgery | $5,787 | $4,340–$7,234 | 109 |
| Fecal incontinence (FI) | |||
| Annual cost of routine care (primarily absorbent materials and cleaning) | $241 | $24–$1,009 | 111, 112 |
| Annual productivity loss associated with FI when age <65 years | 37.44 hours | 28.08–46.8 hours | 113 |
| Age at which women undergo FI surgeries | 55 years | 49–62 years | 114, 115 |
| Cost of diagnosis evaluation of FI | $659 | $494–$824 | 111 |
| Cost of FI surgery | $7,868 | $5,901–$9,835 | 116 |
Plausible ranges of the parameters were determined based on data from the literature or ±25% of the base value if no range was available from the literature.
The cost of the composite maternal morbidity was derived based on the following cost estimates (adjusted to 2007 U.S. dollars) from Chung et al.12 weighted by the distribution of the probability of each individual morbidity subsequent to different modes of delivery: peripartum hysterectomy after vaginal delivery ($5,710), infection after vaginal delivery ($1,562), blood transfusion after vaginal delivery ($395), surgical injury ($997), emergency hysterectomy after cesarean delivery ($1,547), infection after cesarean delivery ($171), and blood transfusion after cesarean delivery ($321).
Table 4.
Estimates of Utility-Related Parameters
| Parameter | Base value | Rangea | References |
|---|---|---|---|
| Obstetric events | |||
| Spontaneous vaginal delivery | 0.92 | 0.69–1.00 | 117, 118 |
| Instrumental vaginal delivery | 0.76 | 0.57–0.95 | 117, 118 |
| Emergency cesarean delivery | 0.59 | 0.44–0.74 | 117, 118 |
| Cesarean delivery on maternal request | 0.91 | 0.50–0.99 | 117, 118 |
| Maternal outcomes | |||
| Peripartum hysterectomyb (regardless of mode of delivery) | 0.605 | 0.3–0.81 | 13, 119 |
| Infectionc (regardless of mode of delivery) (first year after delivery) | 0.995 | 0.972–0.999 | 12 |
| Blood transfusionc (regardless of mode of delivery) (first year after delivery) | 0.995 | 0.972–0.999 | 12 |
| Surgical injury after cesarean deliveryc (first year after delivery) | 0.972 | 0.945–0.995 | 12 |
| Maternal death | 0 | – | Authors' assumption |
| Neonatal outcomes | |||
| Postbirth respiratory problemd | 0.99 | 0.70–0.99 | 120 |
| Neonatal death | 0.01 | 0.–0.02 | 117, 120 |
| Pelvic floor disorderse | |||
| Stress urinary incontinence (SUI) | 0.81 | 0.60–1 | 106 |
| After successful surgical treatment for SUI | 0.870 | 0.689–1 | 121 |
| Pelvic organ prolapse (POP) | 0.7067 | 0.4677–0.9457 | 122 |
| After successful surgical treatment for POP | 0.949 | 0.782–1 | 121 |
| Fecal incontinence (FI) | 0.50 | 0.40–0.65 | 13 |
| After successful surgical treatment for FI | 0.943 | 0.821–1 | 121 |
Plausible ranges of the parameters were determined based on data from the literature or ±25% of the base value if no range was available from the literature.
We assumed that the disutility of hysterectomy lasts for the woman's entire lifetime.
Chung et al.12 estimated the per diem disutility of infection to be 0.48 and a duration of disutility for 4 days. We assumed the same per diem disutility value (i.e., 0.48) for infection, blood transfusion, and surgical injury. For infection and blood transfusion, we varied the event duration from 1 to 21 days and use 4 days as base case. For surgical injury, we varied the duration of disutility from 4 to 42 days and use 21 days as base case.
We used the disutility of admission to neonatal nursery as a proxy measure for the disutility associated with postbirth respiratory problem.
When data on the Pelvic Floor Impact Questionnaire (PFIQ) were used, we assumed linear relationship between the FPIQ score and utility score.
We accounted for the major cost items during and after childbirth over the course of the woman's and newborn's lifetimes, including delivery, maternal and neonatal mortality and morbidity, management and treatment of PFDs, and productivity loss (Table 3). Hospital facility costs and physicians' professional fees associated with the delivery were estimated using the Medicare fee schedules,98 which are developed to measure the costs of providing medical services to Medicare patients and widely used in economic evaluations to represent the societal cost of healthcare.109 Because the medically recommended period of recovery for cesarean delivery is usually 2 weeks longer than that for vaginal delivery, we incorporated a 2-week difference in productivity loss between these delivery modes.123,124 (A sensitivity analysis was conducted varying the difference in this recovery period between vaginal and cesarean delivery. There was no statistically meaningful difference in our findings.) Lost productivity in the event of maternal and neonatal death was estimated based on a work life from age 25 to 65 and 18 to 65 for the mother and the child, respectively. For PFDs, we only modeled the routine care cost, diagnostic evaluation and surgery costs, and productivity loss for the subset of women who actively seek healthcare for the condition (used as an indicator for having bothersome symptoms) because some women may not have symptoms bothersome enough to entail such costs. Behavioral and pharmacological therapies of PFDs were not included in the model because of their relatively lower cost compared with surgical treatment and the limited availability of such data for FI and POP. Moreover, costs associated with reoperations for PFDs were not considered because of a lack of quality data on the timing of such reoperations. All cost estimates were adjusted to 2007 U.S. dollars.125 Future costs were discounted to the time at childbirth using a 3% discount rate.
Effectiveness was measured by quality-adjusted life-years (QALYs) over the combined lifetime of the mother and the newborn. QALYs were computed by multiplying the number of expected life-years in each health state by the utility associated with that health state. Our model assumed the utility for maternal outcomes was independent from the utility for neonatal outcomes and the QALYs were additive. For example, we assumed the utility of maternal death and the utility of a healthy neonate after cesarean delivery were independent, such that each future year in the woman's and the newborn's life was counted as 0 and 1 QALY, respectively, with the overall QALYs being the sum of the numbers. For concurrent maternal outcomes (e.g., maternal infection and surgical injury), we assumed the utilities were independent and multiplicative. Only women actively seeking healthcare for a PFD condition were assumed to incur disutility. QALY estimates later in life were discounted to the time at childbirth using a 3% discount rate.
Data analysis
We used Monte Carlo simulation (n = 5000 iterations) to determine the expected cost and expected QALY throughout the lifetime of the woman and newborn for CDMR and TOL, respectively. Monte Carlo simulation is a method of using repeated random sampling to compute the results. Possible values of each input parameter were defined by a prespecified distribution. In each iteration, a random set of values for all input parameters was drawn from such prespecified distributions, entered in the model, and used to calculate the outcome measures. By doing so, the simulation accounted for the variability of parameter values and identified important factors influencing the cost-effectiveness outcome.
We simultaneously varied the value of 79 parameters from five domains: actual mode of delivery, transient maternal morbidity and mortality, paripartum hysterectomy, perinatal morbidity and mortality, and the lifelong management of PFDs (i.e., all parameters with a specified range in Tables 1, 2, 3, and 4). We assumed a RiskPert distribution for the probability and utility parameters (a special form of beta distribution).126 The base value identified for each parameter (Tables 1 and 4) corresponded to the mode of the RiskPert distribution. We also assumed that the lower and upper bounds of the parameters covered 95% of the values for the underlying distribution. For cost parameters, we drew on the desirable property of lognormal distributions (e.g., skewed distribution, positive and unbounded range).127 The parameter base value (Table 3) corresponded to the mode of the lognormal distribution, and the natural logs of the lower and upper bounds of the parameter were assumed to cover 95% of the values for the underlying normal distribution. We applied truncated lognormal distributions to age parameters in a similar manner except they were subject to certain minimum and maximum values, such as minimum age of 25 (i.e., age at delivery) and maximum age of 81 (i.e., 25 plus the life expectancy of American women at age 25) for onset of PFDs.
With 5000 iterations of data, the mean and 95% confidence intervals (CIs) for the expected cost and QALY associated with CDMR and TOL, respectively, were calculated. We also estimated the mean and 95% CIs for the expected incremental cost (i.e., CDMR cost − TOL cost), incremental QALY (i.e., CDMR QALY − TOL QALY), the average cost-effectiveness ratios (i.e., CDMR cost/CDMR QALY, TOL cost/TOL QALY), and the incremental cost-effectiveness ratio (ICER) (i.e., incremental cost divided by incremental QALY). An incremental cost-effectiveness plane and cost-effectiveness acceptability curve also were constructed to assess the probability distribution of the ICER. Because cost and ICER measures are typically not normally distributed and our results also suggested skewed distribution of QALY data, we estimated the 95% CIs based on a nonparametric method using the 2.5th and 97.5th percentiles.128 DecisionTools Suite® software (Palisade Corporation, Ithaca, NY) and SAS 9.1 (SAS Institute Inc., Cary, NC) were used for data analysis.
Results
For a primigravid woman without medical or obstetric indications having only one childbirth in her lifetime, Monte Carlo simulation suggested that, on average, CDMR would cost $14,259 (95% CI $8,964-$24,002) over the combined lifetime of the mother and newborn, whereas TOL would cost $13,283 (95% CI $7,861-$23,829) (Table 5). The estimated mean incremental cost of CDMR (compared with TOL) was $976 (95% CI −$7,863-$7,935). In terms of QALY, undergoing CDMR would result in 58.21 QALYs (95% CI 57.43-58.67) over the lifetime of the mother and the newborn, and TOL was expected to generate 57.87 QALYs (95% CI 56.97-58.46). The estimated mean incremental QALY of CDMR (compared with TOL) was 0.35 (95% CI −0.24-1.10). Because the confidence intervals of both the estimated mean incremental cost and incremental QALY contain zero, there was no statistically significant difference in the expected cost or expected QALY between CDMR and TOL at the 0.05 level.
Table 5.
Summary of Results from Monte Carlo Simulation
| Monte Carlo simulation | Trial of labor | Cesarean delivery on maternal request (CDMR)a |
|---|---|---|
| Cost, mean (95% CIa) | $13,283 ($7,861-$23,829) | $14,259 ($8,964-$24,002) |
| Quality-adjusted life-years (QALY), mean (95% CI) | 57.87 (56.97-58.46) | 58.21 (57.43-58.67) |
| Average cost-effectiveness ratio, mean (95% CI) | $230/QALY ($135/QALY-$414/QALY) | $245/QALY ($153/QALY-$417/QALY) |
| ΔCost,b mean (95% CI) | $976 (−$7,863-$7,935) | |
| ΔQALY,c mean (95% CI) | 0.35 (−0.24-1.10) | |
| Most significant parametersd | Effect of 1 SDe increase in parameter on ΔCostb | Effect of 1 SDe increase in parameter on ΔQALYc |
|---|---|---|
| Probability of stress urinary incontinence (SUI) after CDMR (SDe = 0.09) | $2,509 | −0.17 |
| Probability of SUI after spontaneous vaginal delivery (SDe = 0.07) | −$1,711 | 0.10 |
| Cost of neonatal morbidity (SDe = 47,589) | $924 | n/a |
| Cost of CDMR (SDe = 625) | $625 | n/a |
| Annual routine care cost for SUI (SDe = 1,009) | −$1,080 | n/a |
| Age of pelvic floor disorder onset (SDe = 5.20) | $817 | −0.11 |
| Utility of CDMR (SDe = 0.13) | n/a | 0.13 |
| Utility of pelvic organ prolapse (SDe = 0.13) | n/a | −0.09 |
| Utility of SUI (SDe = 0.10) | n/a | −0.10 |
CI, confidence interval.
ΔCost, cost of CDMR − cost of trial of labor.
ΔQALY, QALY of CDMR − QALY of trial of labor.
All these parameters are statistically significant at 0.05 level.
SD, standard deviation. These are estimated SDs of the parameters based on the simulation (n = 5000 iterations).
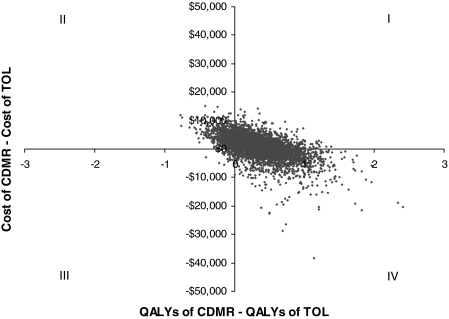
Figure 2 shows the incremental cost-effectiveness plane, which plots the joint distribution of the incremental cost and incremental QALY. Each dot on the plane corresponds to one incremental cost and QALY pair resulting from one iteration of the simulation. The incremental cost and QALY pairs were largely distributed across each quadrant of the incremental cost-effectiveness plane, primarily in the first, second, and fourth quadrants. In 12.14% of the iterations, CDMR was dominated by TOL (i.e., CDMR was more costly and less effective than TOL), whereas in 33.32% of the iterations, CDMR was the dominant strategy, with higher QALY and lower cost. In the other 54.54% of the iterations, one delivery scheme was less costly and the other generated higher QALY, with significant variability in the magnitude of the ICER (95% CI $352/QALY-$220,496/QALY).
FIG. 2.
Incremental cost-effectiveness plane. The horizontal axis represents the difference in quality-adjusted life-years (QALYs) between cesarean delivery on maternal request (CDMR) and trial of labor (TOL) (i.e., incremental QALY). The vertical axis represents the difference in the costs between CDMR and TOL (i.e., incremental cost). Each dot in the figure corresponds to one incremental cost and incremental QALY pair resulting from one iteration of the Monte Carlo simulation.
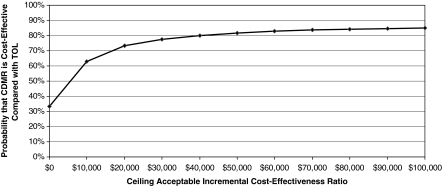
Figure 3 illustrates the probability that CDMR is cost-effective compared with TOL for a given cutoff cost-effectiveness ratio that a society is willing to pay. For example, if a society is willing to pay $50,000 for one QALY, there is an 82% chance that undergoing CDMR is cost-effective compared with TOL (i.e., there is an 82% chance that the additional cost of CDMR is < $50,000 for each additional QALY gained). The null hypothesis that there is no net benefit of CDMR is rejected only when the cost-effectiveness acceptability curve is above 95%.129 In our simulation, the probability of CDMR being cost-effective never exceeded 88% for any cutoff cost-effectiveness ratio. Therefore, we could not reject the null hypothesis that there was no net benefit of CDMR in comparison to TOL.
FIG. 3.
Cost-effectiveness acceptability curve. This figure illustrates the probability that cesarean delivery on maternal request (CDMR) is cost-effective compared with trial of labor (TOL) for a given cutoff cost-effectiveness ratio that a society is willing to pay. For example, if a society is willing to pay $50,000 for one QALY, there is an 82% chance that undergoing CDMR is cost-effective (i.e., there is an 82% chance that the additional cost of CDMR is < $50,000 for each additional QALY gained).
Table 5 reports the factors identified as most influential in the simulation and their estimated effects on the incremental cost and QALY. The cost of CDMR and neonatal morbidity significantly affected the incremental cost between CDMR and TOL. PFD-related parameters also were found important. For example, based on the simulation data, a 1 standard deviation (SD) increase in the probability of developing SUI after CDMR was associated with a $2,509 increase in the incremental cost and a 0.17 reduction in incremental QALY. In contrast, a 1 SD increase in the probability of developing SUI after spontaneous vaginal delivery resulted in a $1,711 reduction in the incremental cost and a 0.10 increase in incremental QALY. In addition, the age of PFD onset, women's perceived quality of life when having POP or SUI, and the annual routine care cost for SUI all influenced the expected incremental cost and QALY between CDMR and TOL.
Discussion
There has been a growing debate surrounding the appropriateness of CDMR, especially in view of the recent substantial increase in cesarean delivery rate in the United States. Using currently available evidence, we assessed the lifetime cost-effectiveness of CDMR compared with TOL from a societal perspective. Our results showed that for primigravid women without medical or obstetric indications and with only one childbirth over their lifetime, CDMR and TOL are associated with comparable costs and QALYs. Moreover, PFD-related parameters were found to be important factors in the cost-effectiveness assessment.
By modeling the impact of delivery mode on lifelong PFD outcomes, this study provides a more comprehensive view of the long-term cost and quality of life consequences of CDMR among primigravid women who have no medical or obstetric indications and do not go on to have future deliveries. The model structure developed in this analysis can be used as a basis for future cost-effectiveness analyses of CDMR that include multiple deliveries.
Although prevention of PFDs is a frequently cited reason for requesting or performing CDMR, our analyses suggest that even after considering the long-term pelvic floor consequences, CDMR is not superior to TOL in terms of lifelong cost and quality of life for a primigravid woman without medical or obstetric indications having only one childbirth over her lifetime. However, our findings also imply that CDMR is not worse than TOL in this subpopulation of women. This is consistent with the NIH State-of-the-Science statement that there are “relatively similar degrees of risk from both pathways in women intending to limit their childbearing to one or two children.”3
Our finding, however, should not be generalized to women with multiple childbirths. Women with a primary cesarean delivery face increased risk for complications in subsequent pregnancies.2 This could substantially increase the cost while reducing QOL for women undergoing CDMR. Similarly, women with more than one vaginal delivery are at higher risk for PFDs, which could increase the lifetime cost while reducing the QALY for TOL patients. The overall impact of additional childbirths on the cost-effectiveness of CDMR will require further investigation as more data become available about maternal and neonatal outcomes associated with different modes of delivery at successive childbirths. Further, the cost-effectiveness of CDMR will likely differ for women with medical indications or in certain high-risk situations. For example, a planned cesarean delivery may prove beneficial for preterm or postterm births by preventing serious morbidity and mortality during labor. Further research analyzing this issue for subsets of women with certain indications would inform whether there are specific subpopulations who may benefit from CDMR.
The framework of cost-effectiveness analysis offers a unique opportunity to identify gaps in the current literature about CDMR and its relationship with PFDs. For example, we located only two studies examining women's utility related to their delivery experience and outcomes.117,118 This makes it difficult to evaluate the implications of CDMR for women's quality of life. In addition, the PFD-related parameters were found to be significant factors in our model; yet there is a fair amount of uncertainty surrounding these parameters. Future research providing better estimates of these parameters would facilitate more elaborated comparisons between CDMR and TOL.
Several additional factors must be kept in mind when interpreting our findings. First, to streamline the analytical model, we did not consider coexisting PFDs. Consequently, we might have overestimated the cost related to the care of PFDs, biasing our results in favor of CDMR. This could also cause underestimation of disutility associated with PFDs, however, biasing the QALY estimates in favor of TOL. Second, although there is evidence that forceps delivery may be more likely than vacuum delivery to cause PFDs,130 our analysis could not stratify on these two types of instrumental vaginal delivery because of a lack of detailed data.
Conclusions
This study makes an important first step toward addressing a complicated question: Is cesarean delivery on maternal request more cost-effective than trial of labor when lifelong pelvic floor consequences are considered? Our results suggest that in the absence of medical and obstetric indications, CDMR and TOL are not significantly different from each other in terms of lifelong cost and QALY for primigravid women having only one childbirth over their lifetime. Women's QOL related to delivery experience and the PFD-related factors should be studied more closely and incorporated in future decision analyses. This will help advance the understanding of the complex relationship between childbirth and long-term maternal outcomes and allow for more informed clinical and policy recommendations.
Acknowledgments
This work was supported in part by the Office for Research on Women's Health SCOR on Sex and Gender Factors Affecting Women's Health (National Institute of Child Health and Human Development grant number 1 P50 HD044406). D.A.P. is supported by a grant from the National Cancer Institute/National Institutes of Health (1 K07 CA120040-01). Preliminary results from this study were presented at the Institute for Operations Research and the Management Sciences (INFORMS) annual meeting in Pittsburgh, PA, November 5–8, 2006; the Healthcare Engineering Alliance (HEA)'s first annual Healthcare Engineering Symposium in Research Triangle Park, NC, April 6–8, 2008; and the 34th annual Operational Research Applied to Health Services (ORAHS) conference in Toronto, Ontario, Canada, July 28–August 1, 2008.
Disclosure Statement
The authors have no conflicts of interest to report.
References
- 1.Sakala C. Corry MP. Evidence-based maternity care: What it is and what it can achieve. New York: Childbirth Connection, Reforming States Group, Milbank Memorial Fund; 2008. [Nov 5;2008 ]. [Google Scholar]
- 2.ACOG Committee Opinion No. 386: Cesarean delivery on maternal request. Obstet Gynecol. 2007;110:1209–1212. doi: 10.1097/01.AOG.0000291562.48203.fc. [DOI] [PubMed] [Google Scholar]
- 3.The National Institutes of Health. National Institutes of Health State-of-the-Science Conference statement: Cesarean delivery on maternal request March 27–29, 2006. Obstet Gynecol. 2006;107:1386–1397. [PubMed] [Google Scholar]
- 4.Hamilton BE. Martin JA. Ventura SJ. National Vital Statistics Reports, vol 56, No. 7. Hyattsville, MD: National Center for Health Statistics; 2007. Births: Preliminary data for 2006. [Google Scholar]
- 5.Buhling KJ. Schmidt S. Robinson JN. Klapp C. Siebert G. Dudenhausen JW. Rate of dyspareunia after delivery in primiparae according to mode of delivery. Eur J Obstet Gynecol Reprod Biol. 2006;124:42–46. doi: 10.1016/j.ejogrb.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Wax JR. Cartin A. Pinette MG. Blackstone J. Patient choice cesarean: An evidence-based review. Obstet Gynecol Surv. 2004;59:601–616. doi: 10.1097/01.ogx.0000133942.76239.57. [DOI] [PubMed] [Google Scholar]
- 7.Penna L. Arulkumaran S. Cesarean section for non-medical reasons. Int J Gynaecol Obstet. 2003;82:399–409. doi: 10.1016/s0020-7292(03)00217-0. [DOI] [PubMed] [Google Scholar]
- 8.Nygaard I. Barber MD. Burgio KL, et al. Prevalence of symptomatic pelvic floor disorders in U.S. women. JAMA. 2008;300:1311–1316. doi: 10.1001/jama.300.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu TW. Wagner TH. Bentkover JD. Leblanc K. Zhou SZ. Hunt T. Costs of urinary incontinence and overactive bladder in the United States: A comparative study. Urology. 2004;63:461–465. doi: 10.1016/j.urology.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 10.Brown JS. Waetjen LE. Subak LL. Thom DH. Van den Eeden S. Vittinghoff E. Pelvic organ prolapse surgery in the United States, 1997. Am J Obstet Gynecol. 2002;186:712–716. doi: 10.1067/mob.2002.121897. [DOI] [PubMed] [Google Scholar]
- 11.Boyles SH. Weber AM. Meyn L. Procedures for pelvic organ prolapse in the United States, 1979–1997. Am J Obstet Gynecol. 2003;188:108–115. doi: 10.1067/mob.2003.101. [DOI] [PubMed] [Google Scholar]
- 12.Chung A. Macario A. El-Sayed YY. Riley ET. Duncan B. Druzin ML. Cost-effectiveness of a trial of labor after previous cesarean. Obstet Gynecol. 2001;97:932–941. doi: 10.1016/s0029-7844(01)01355-2. [DOI] [PubMed] [Google Scholar]
- 13.Culligan PJ. Myers JA. Goldberg RP. Blackwell L. Gohmann SF. Abell TD. Elective cesarean section to prevent anal incontinence and brachial plexus injuries associated with macrosomia—A decision analysis. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:19–28. doi: 10.1007/s00192-004-1203-3. [DOI] [PubMed] [Google Scholar]
- 14.Gold MR. Siegel JE. Russell LB. Weinstein MC. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 15.Manuel MR. Chen LM. Caughey AB, et al. Cost-effectiveness analyses in gynecologic oncology: Methodological quality and trends. Gynecol Oncol. 2004;93:1–8. doi: 10.1016/j.ygyno.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 16.Van Voorhis BJ. Evaluating economic studies in reproductive medicine. Semin Reprod Med. 2003;21:85–93. doi: 10.1055/s-2003-39998. [DOI] [PubMed] [Google Scholar]
- 17.Grobman WA. Decision analysis in obstetrics and gynecology. Obstet Gynecol Surv. 2006;61:602–607. doi: 10.1097/01.ogx.0000234860.76274.19. [DOI] [PubMed] [Google Scholar]
- 18.Haddix AC. Teutsch SM. Corso PS. Prevention effectiveness: A guide to decision analysis and economic evaluation. New York: Oxford University Press; 2003. [Google Scholar]
- 19.Martin J. Hamilton B. Sutton P. Ventura SJ. Menacker F. Munson ML. National Vital Statistics Reports, vol 54 No. 2. Hyattsville, MD: National Center for Health Statistics; 2005. Births: Final data for 2003. [PubMed] [Google Scholar]
- 20.Dye J. Fertility of American women (data from the June 2004 Supplement of the CPS) Washington, DC: U.S. Census Bureau; 2005. [Google Scholar]
- 21.Norton P. Brubaker L. Urinary incontinence in women. Lancet. 2006;367:57–67. doi: 10.1016/S0140-6736(06)67925-7. [DOI] [PubMed] [Google Scholar]
- 22.Landefeld CS. Bowers BJ. Feld AD, et al. National Institutes of Health state-of-the-science conference statement: Prevention of fecal and urinary incontinence in adults. Ann Intern Med. 2008;148:449–458. doi: 10.7326/0003-4819-148-6-200803180-00210. [DOI] [PubMed] [Google Scholar]
- 23.Boreham MK. Richter HE. Kenton KS, et al. Anal incontinence in women presenting for gynecologic care: Prevalence, risk factors, and impact upon quality of life. Am J Obstet Gynecol. 2005;192:1637–1642. doi: 10.1016/j.ajog.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 24.Visco AG. Viswanathan M. Lohr KN, et al. Cesarean delivery on maternal request: Maternal and neonatal outcomes. Obstet Gynecol. 2006;108:1517–1529. doi: 10.1097/01.AOG.0000241092.79282.87. [DOI] [PubMed] [Google Scholar]
- 25.Liu S. Liston RM. Joseph KS. Heaman M. Sauve R. Kramer MS. Maternal mortality and severe morbidity associated with low-risk planned cesarean delivery versus planned vaginal delivery at term. Can Med Assoc J. 2007;176:455–460. doi: 10.1503/cmaj.060870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silver RM. Landon MB. Rouse DJ, et al. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstet Gynecol. 2006;107:1226–1232. doi: 10.1097/01.AOG.0000219750.79480.84. [DOI] [PubMed] [Google Scholar]
- 27.Whiteman MK. Kuklina E. Hillis SD, et al. Incidence and determinants of peripartum hysterectomy. Obstet Gynecol. 2006;108:1486–1492. doi: 10.1097/01.AOG.0000245445.36116.c6. [DOI] [PubMed] [Google Scholar]
- 28.Menacker F. Declercq E. Macdorman MF. Cesarean delivery: Background, trends, and epidemiology. Semin Perinatol. 2006;30:235–241. doi: 10.1053/j.semperi.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Smith GC. Pell JP. Cameron AD, et al. Risk of perinatal death associated with labor after previous cesarean delivery in uncomplicated term pregnancies. JAMA. 2002;287:2684–2690. doi: 10.1001/jama.287.20.2684. [DOI] [PubMed] [Google Scholar]
- 30.Declercq E. Barger M. Cabral HJ, et al. Maternal outcomes associated with planned primary cesarean births compared with planned vaginal births. Obstet Gynecol. 2007;109:669–677. doi: 10.1097/01.AOG.0000255668.20639.40. [DOI] [PubMed] [Google Scholar]
- 31.Koroukian SM. Relative risk of postpartum complications in the Ohio Medicaid population: Vaginal versus cesarean delivery. Med Care Res Rev. 2004;61:203–224. doi: 10.1177/1077558703260123. [DOI] [PubMed] [Google Scholar]
- 32.Kozak LJ. Weeks JD. U.S. trends in obstetric procedures, 1990–2000. Birth. 2002;29:157–161. doi: 10.1046/j.1523-536x.2002.00182.x. [DOI] [PubMed] [Google Scholar]
- 33.Hall MH. Variation in caesarean section rate. Maternal mortality higher after caesarean section. BMJ. 1994;308:654–655. [PMC free article] [PubMed] [Google Scholar]
- 34.Hall MH. Bewley S. Maternal mortality and mode of delivery. Lancet. 1999;354:776. doi: 10.1016/S0140-6736(05)76016-5. [DOI] [PubMed] [Google Scholar]
- 35.Demissie K. Rhoads GG. Smulian JC, et al. Operative vaginal delivery and neonatal and infant adverse outcomes: Population-based retrospective analysis. BMJ. 2004;329:24–29. doi: 10.1136/bmj.329.7456.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Towner D. Castro MA. Eby-Wilkens E. Gilbert WM. Effect of mode of delivery in nulliparous women on neonatal intracranial injury. N Engl J Med. 1999;341:1709–1714. doi: 10.1056/NEJM199912023412301. [DOI] [PubMed] [Google Scholar]
- 37.Wen SW. Liu S. Kramer MS, et al. Comparison of maternal and infant outcomes between vacuum extraction and forceps deliveries. Am J Epidemiol. 2001;153:103–107. doi: 10.1093/aje/153.2.103. [DOI] [PubMed] [Google Scholar]
- 38.MacDorman MF. Declercq E. Menacker F. Malloy MH. Neonatal mortality for primary cesarean and vaginal births to low-risk women: Application of an “intention-to-treat” model. Birth. 2008;35:3–8. doi: 10.1111/j.1523-536X.2007.00205.x. [DOI] [PubMed] [Google Scholar]
- 39.Allen V. O'Connell C. Baskett T. Maternal morbidity associated with cesarean delivery without labor compared with induction of labor at term. Obstet Gynecol. 2006;108:286–294. doi: 10.1097/01.AOG.0000215988.23224.e4. [DOI] [PubMed] [Google Scholar]
- 40.Allen VM. O'Connell CM. Liston RM. Baskett TF. Maternal morbidity associated with cesarean delivery without labor compared with spontaneous onset of labor at term. Obstet Gynecol. 2003;102:477–482. doi: 10.1016/s0029-7844(03)00570-2. [DOI] [PubMed] [Google Scholar]
- 41.Burrows LJ. Meyn LA. Weber AM. Maternal morbidity associated with vaginal versus cesarean delivery. Obstet Gynecol. 2004;103:907–912. doi: 10.1097/01.AOG.0000124568.71597.ce. [DOI] [PubMed] [Google Scholar]
- 42.Forna F. Miles AM. Jamieson DJ. Emergency peripartum hysterectomy: A comparison of cesarean and postpartum hysterectomy. Am J Obstet Gynecol. 2004;190:1440–1444. doi: 10.1016/j.ajog.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 43.Simoes E. Kunz S. Bosing-Schwenkglenks M. Schmahl FM. Association between method of delivery, puerperal complication rate and postpartum hysterectomy. Arch Gynecol Obstet. 2005;272:43–47. doi: 10.1007/s00404-004-0692-0. [DOI] [PubMed] [Google Scholar]
- 44.Lydon-Rochelle M. Holt VL. Martin DP. Easterling TR. Association between method of delivery and maternal rehospitalization. JAMA. 2000;283:2411–2416. doi: 10.1001/jama.283.18.2411. [DOI] [PubMed] [Google Scholar]
- 45.Farrell SA. Allen VM. Baskett TF. Parturition and urinary incontinence in primiparas. Obstet Gynecol. 2001;97:350–356. doi: 10.1016/s0029-7844(00)01164-9. [DOI] [PubMed] [Google Scholar]
- 46.Fritel X. Fauconnier A. Levet C. Benifla JL. Stress urinary incontinence 4 years after the first delivery: A retrospective cohort survey. Acta Obstet Gynecol Scand. 2004;83:941–945. doi: 10.1111/j.0001-6349.2004.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.MacLennan AH. Taylor AW. Wilson DH. Wilson D. The prevalence of pelvic floor disorders and their relationship to gender, age, parity and mode of delivery. Br J Obstet Gynaecol. 2000;107:1460–1470. doi: 10.1111/j.1471-0528.2000.tb11669.x. [DOI] [PubMed] [Google Scholar]
- 48.Peschers UM. Sultan AH. Jundt K. Mayer A. Drinovac V. Dimpfl T. Urinary and anal incontinence after vacuum delivery. Eur J Obstet Gynecol Reprod Biol. 2003;110:39–42. doi: 10.1016/s0301-2115(03)00111-8. [DOI] [PubMed] [Google Scholar]
- 49.Schytt E. Lindmark G. Waldenstrom U. Symptoms of stress incontinence 1 year after childbirth: Prevalence and predictors in a national Swedish sample. Acta Obstet Gynecol Scand. 2004;83:928–936. doi: 10.1111/j.0001-6349.2004.00431.x. [DOI] [PubMed] [Google Scholar]
- 50.Lukacz ES. Lawrence JM. Contreras R. Nager CW. Luber KM. Parity, mode of delivery, and pelvic floor disorders. Obstet Gynecol. 2006;107:1253–1260. doi: 10.1097/01.AOG.0000218096.54169.34. [DOI] [PubMed] [Google Scholar]
- 51.Faltin DL. Sangalli MR. Roche B. Floris L. Boulvain M. Weil A. Does a second delivery increase the risk of anal incontinence? Br J Obstet Gynaecol. 2001;108:684–688. doi: 10.1111/j.1471-0528.2001.00185.x. [DOI] [PubMed] [Google Scholar]
- 52.MacArthur C. Bick DE. Keighley MR. Faecal incontinence after childbirth. Br J Obstet Gynaecol. 1997;104:46–50. doi: 10.1111/j.1471-0528.1997.tb10648.x. [DOI] [PubMed] [Google Scholar]
- 53.Sultan AH. Kamm MA. Bartram CI. Hudson CN. Anal sphincter trauma during instrumental delivery. Int J Gynaecol Obstet. 1993;43:263–270. doi: 10.1016/0020-7292(93)90514-w. [DOI] [PubMed] [Google Scholar]
- 54.Dani C. Reali MF. Bertini G, et al. Risk factors for the development of respiratory distress syndrome and transient tachypnoea in newborn infants. Italian Group of Neonatal Pneumology. Eur Respir J. 1999;14:155–159. doi: 10.1034/j.1399-3003.1999.14a26.x. [DOI] [PubMed] [Google Scholar]
- 55.Abramov Y. Sand PK. Botros SM, et al. Risk factors for female anal incontinence: New insight through the Evanston-Northwestern twin sisters study. Obstet Gynecol. 2005;106:726–732. doi: 10.1097/01.AOG.0000161367.65261.16. [DOI] [PubMed] [Google Scholar]
- 56.Bahl R. Strachan B. Murphy DJ. Pelvic floor morbidity at 3 years after instrumental delivery and cesarean delivery in the second stage of labor and the impact of a subsequent delivery. Am J Obstet Gynecol. 2005;192:789–794. doi: 10.1016/j.ajog.2004.10.601. [DOI] [PubMed] [Google Scholar]
- 57.Lydon-Rochelle M. Holt VL. Easterling TR. Martin DP. Cesarean delivery and postpartum mortality among primiparas in Washington State, 1987–1996(1) Obstet Gynecol. 2001;97:169–174. doi: 10.1016/s0029-7844(00)01119-4. [DOI] [PubMed] [Google Scholar]
- 58.Allen VM. O'Connell CM. Baskett TF. Maternal and perinatal morbidity of caesarean delivery at full cervical dilatation compared with caesarean delivery in the first stage of labour. Br J Obstet Gynaecol. 2005;112:986–990. doi: 10.1111/j.1471-0528.2005.00615.x. [DOI] [PubMed] [Google Scholar]
- 59.Bergholt T. Stenderup JK. Vedsted-Jakobsen A. Helm P. Lenstrup C. Intraoperative surgical complication during cesarean section: An observational study of the incidence and risk factors. Acta Obstet Gynecol Scand. 2003;82:251–256. doi: 10.1034/j.1600-0412.2003.00095.x. [DOI] [PubMed] [Google Scholar]
- 60.Gregory KD. Henry OA. Ramicone E. Chan LS. Platt LD. Maternal and infant complications in high and normal weight infants by method of delivery. Obstet Gynecol. 1998;92:507–513. doi: 10.1016/s0029-7844(98)00224-5. [DOI] [PubMed] [Google Scholar]
- 61.van Ham MA. van Dongen PW. Mulder J. Maternal consequences of caesarean section. A retrospective study of intra-operative and postoperative maternal complications of caesarean section during a 10-year period. Eur J Obstet Gynecol Reprod Biol. 1997;74:1–6. doi: 10.1016/s0301-2115(97)02725-5. [DOI] [PubMed] [Google Scholar]
- 62.Rortveit G. Daltveit AK. Hannestad YS. Hunskaar S. Urinary incontinence after vaginal delivery or cesarean section. N Engl J Med. 2003;348:900–907. doi: 10.1056/NEJMoa021788. [DOI] [PubMed] [Google Scholar]
- 63.Lal M.Mann CH.Callender R.Radley S.Does cesarean delivery prevent anal incontinence? Obstet Gynecol 2003101305–312.12576254 [Google Scholar]
- 64.Levine EM. Ghai V. Barton JJ. Strom CM. Mode of delivery and risk of respiratory diseases in newborns. Obstet Gynecol. 2001;97:439–442. doi: 10.1016/s0029-7844(00)01150-9. [DOI] [PubMed] [Google Scholar]
- 65.Morrison JJ. Rennie JM. Milton PJ. Neonatal respiratory morbidity and mode of delivery at term: Influence of timing of elective caesarean section. Br J Obstet Gynaecol. 1995;102:101–106. doi: 10.1111/j.1471-0528.1995.tb09060.x. [DOI] [PubMed] [Google Scholar]
- 66.Foley ME. Alarab M. Daly L. Keane D. Rath A. O'Herlihy C. The continuing effectiveness of active management of first labor, despite a doubling in overall nulliparous cesarean delivery. Am J Obstet Gynecol. 2004;191:891–895. doi: 10.1016/j.ajog.2004.05.072. [DOI] [PubMed] [Google Scholar]
- 67.Gilbert WM. Hicks SM. Boe NM. Danielsen B. Vaginal versus cesarean delivery for breech presentation in California: A population-based study. Obstet Gynecol. 2003;102:911–917. doi: 10.1016/s0029-7844(03)00809-3. [DOI] [PubMed] [Google Scholar]
- 68.Gould JB. Danielsen B. Korst LM, et al. Cesarean delivery rates and neonatal morbidity in a low-risk population. Obstet Gynecol. 2004;104:11–19. doi: 10.1097/01.AOG.0000127035.64602.97. [DOI] [PubMed] [Google Scholar]
- 69.MacDorman MF. Declercq E. Menacker F. Malloy MH. Infant and neonatal mortality for primary cesarean and vaginal births to women with “no indicated risk,” United States, 1998–2001 Birth Cohorts. Birth. 2006;33:175–182. doi: 10.1111/j.1523-536X.2006.00102.x. [DOI] [PubMed] [Google Scholar]
- 70.Allen VM. O'Connell CM. Farrell SA. Baskett TF. Economic implications of method of delivery. Am J Obstet Gynecol. 2005;193:192–197. doi: 10.1016/j.ajog.2004.10.635. [DOI] [PubMed] [Google Scholar]
- 71.McKinnie V. Swift SE. Wang W, et al. The effect of pregnancy and mode of delivery on the prevalence of urinary and fecal incontinence. Am J Obstet Gynecol. 2005;193:512–517. doi: 10.1016/j.ajog.2005.03.056. [DOI] [PubMed] [Google Scholar]
- 72.Zanardo V. Simbi AK. Franzoi M. Solda G. Salvadori A. Trevisanuto D. Neonatal respiratory morbidity risk and mode of delivery at term: Influence of timing of elective caesarean delivery. Acta Paediatr. 2004;93:643–647. doi: 10.1111/j.1651-2227.2004.tb02990.x. [DOI] [PubMed] [Google Scholar]
- 73.Morrill M. Lukacz ES. Lawrence JM. Nager CW. Contreras R. Luber KM. Seeking healthcare for pelvic floor disorders: A population-based study. Am J Obstet Gynecol. 2007;197:6. doi: 10.1016/j.ajog.2007.02.051. e1–86.e6. [DOI] [PubMed] [Google Scholar]
- 74.Kinchen KS. Long S. Orsini L. Crown W. Bump RC. Healthcare utilization among women who undergo surgery for stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15:154–159. doi: 10.1007/s00192-004-1133-0. [DOI] [PubMed] [Google Scholar]
- 75.Chene G. Amblard J. Tardieu AS, et al. Long-term results of tension-free vaginal tape (TVT) for the treatment of female urinary stress incontinence. Eur J Obstet Gynecol Reprod Biol. 2007;134:87–94. doi: 10.1016/j.ejogrb.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 76.Mammen C. Chilaka V. Cust MP. Urological surgical techniques. Best Pract Res Clin Obstet Gynaecol. 2006;20:139–156. doi: 10.1016/j.bpobgyn.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 77.Ministry of Health. Long-term Care. Tension-free vaginal tape for stress urinary incontinence. Health technology literature review. www.health.gov.on.ca/english/providers/program/mas/tech/reviews/sum_tvt_020104.html#2. [May 25;2006 ]. www.health.gov.on.ca/english/providers/program/mas/tech/reviews/sum_tvt_020104.html#2
- 78.Nilsson CG. Kuuva N. Falconer C. Rezapour M. Ulmsten U. Long-term results of the tension-free vaginal tape (TVT) procedure for surgical treatment of female stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12(Suppl 2):S5–8. doi: 10.1007/s001920170003. [DOI] [PubMed] [Google Scholar]
- 79.Schraffordt Koops SE. Bisseling TM. Heintz AP. Vervest HA. The effectiveness of tension-free vaginal tape (TVT) and quality of life measured in women with previous urogynecologic surgery: Analysis from The Netherlands TVT database. Am J Obstet Gynecol. 2006;195:439–444. doi: 10.1016/j.ajog.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 80.Ulmsten U. Henriksson L. Johnson P. Varhos G. An ambulatory surgical procedure under local anesthesia for treatment of female urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 1996;7:81–85. doi: 10.1007/BF01902378. [DOI] [PubMed] [Google Scholar]
- 81.Valpas A. Kivela A. Penttinen J, et al. Tension-free vaginal tape and laparoscopic mesh colposuspension in the treatment of stress urinary incontinence: Immediate outcome and complications—A randomized clinical trial. Acta Obstet Gynecol Scand. 2003;82:665–671. [PubMed] [Google Scholar]
- 82.Ward KL. Hilton P. A prospective multicenter randomized trial of tension-free vaginal tape and colposuspension for primary urodynamic stress incontinence: two-year follow-up. Am J Obstet Gynecol. 2004;190:324–331. doi: 10.1016/j.ajog.2003.07.029. [DOI] [PubMed] [Google Scholar]
- 83.Carey MP. Slack MC. Transvaginal sacrospinous colpopexy for vault and marked uterovaginal prolapse. Br J Obstet Gynaecol. 1994;101:536–540. doi: 10.1111/j.1471-0528.1994.tb13158.x. [DOI] [PubMed] [Google Scholar]
- 84.Hefni MA. El-Toukhy TA. Long-term outcome of vaginal sacrospinous colpopexy for marked uterovaginal and vault prolapse. Eur J Obstet Gynecol Reprod Biol. 2006;127:257–263. doi: 10.1016/j.ejogrb.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 85.Juma S. Anterior vaginal suspension for vaginal vault prolapse. Tech Urol. 1995;1:150–156. [PubMed] [Google Scholar]
- 86.Lovatsis D. Drutz HP. Safety and efficacy of sacrospinous vault suspension. Int Urogynecol J Pelvic Floor Dysfunct. 2002;13:308–313. doi: 10.1007/s001920200067. [DOI] [PubMed] [Google Scholar]
- 87.Maher CF. Cary MP. Slack MC. Murray CJ. Milligan M. Schluter P. Uterine preservation or hysterectomy at sacrospinous colpopexy for uterovaginal prolapse? Int Urogynecol J Pelvic Floor Dysfunct. 2001;12:381–384. doi: 10.1007/s001920170017. [DOI] [PubMed] [Google Scholar]
- 88.Nygaard IE. McCreery R. Brubaker L, et al. Abdominal sacrocolpopexy: A comprehensive review. Obstet Gynecol. 2004;104:805–823. doi: 10.1097/01.AOG.0000139514.90897.07. [DOI] [PubMed] [Google Scholar]
- 89.Olsen AL. Smith VJ. Bergstrom JO. Colling JC. Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501–506. doi: 10.1016/S0029-7844(97)00058-6. [DOI] [PubMed] [Google Scholar]
- 90.Johanson JF. Lafferty J. Epidemiology of fecal incontinence: The silent affliction. Am J Gastroenterol. 1996;91:33–36. [PubMed] [Google Scholar]
- 91.Bliss DZ. Fischer LR. Savik K. Managing fecal incontinence: Self-care practices of older adults. J Gerontol Nurs. 2005;31:35–44. doi: 10.3928/0098-9134-20050701-08. [DOI] [PubMed] [Google Scholar]
- 92.Demirci S. Gallas S. Bertot-Sassigneux P. Michot F. Denis P. Leroi AM. Anal incontinence: The role of medical management. Gastroenterol Clin Biol. 2006;30:954–960. doi: 10.1016/s0399-8320(06)73356-5. [DOI] [PubMed] [Google Scholar]
- 93.Bravo Gutierrez A. Madoff RD. Lowry AC. Parker SC. Buie WD. Baxter NN. Long-term results of anterior sphincteroplasty. Dis Colon Rectum. 2004;47:727–731. doi: 10.1007/s10350-003-0114-6. [DOI] [PubMed] [Google Scholar]
- 94.Halverson AL. Hull TL. Long-term outcome of overlapping anal sphincter repair. Dis Colon Rectum. 2002;45:345–348. doi: 10.1007/s10350-004-6180-6. [DOI] [PubMed] [Google Scholar]
- 95.Karoui S. Leroi AM. Koning E. Menard JF. Michot F. Denis P. Results of sphincteroplasty in 86 patients with anal incontinence. Dis Colon Rectum. 2000;43:813–820. doi: 10.1007/BF02238020. [DOI] [PubMed] [Google Scholar]
- 96.Malouf AJ. Norton CS. Engel AF. Nicholls RJ. Kamm MA. Long-term results of overlapping anterior anal-sphincter repair for obstetric trauma. Lancet. 2000;355:260–265. doi: 10.1016/S0140-6736(99)05218-6. [DOI] [PubMed] [Google Scholar]
- 97.Trowbridge ER. Morgan D. Trowbridge MJ. DeLancey JO. Fenner DE. Sexual function, quality of life, and severity of anal incontinence after anal sphincteroplasty. Am J Obstet Gynecol. 2006;195:1753–1757. doi: 10.1016/j.ajog.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 98.Centers for Medicare. Medicaid Services. 2007 Medicare payment schedule. www.cms.hhs.gov/home/medicare.asp. [May 30;2007 ]. www.cms.hhs.gov/home/medicare.asp
- 99.Vahratian A. Zhang J. Hasling J. Troendle JF. Klebanoff MA. Thorp JM., Jr The effect of early epidural versus early intravenous analgesia use on labor progression: A natural experiment. Am J Obstet Gynecol. 2004;191:259–265. doi: 10.1016/j.ajog.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 100.Vahratian A. Zhang J. Troendle JF. Sciscione AC. Hoffman MK. Labor progression and risk of cesarean delivery in electively induced nulliparas. Obstet Gynecol. 2005;105:698–704. doi: 10.1097/01.AOG.0000157436.68847.3b. [DOI] [PubMed] [Google Scholar]
- 101.Guillemette J. Fraser WD. Differences between obstetricians in caesarean section rates and the management of labour. Br J Obstet Gynaecol. 1992;99:105–108. doi: 10.1111/j.1471-0528.1992.tb14464.x. [DOI] [PubMed] [Google Scholar]
- 102.Riley ET. Cohen SE. Macario A. Desai JB. Ratner EF. Spinal versus epidural anesthesia for cesarean section: A comparison of time efficiency, costs, charges, and complications. Anesth Analg. 1995;80:709–712. doi: 10.1097/00000539-199504000-00010. [DOI] [PubMed] [Google Scholar]
- 103.Bureau of Labor Statistics. Weekly and hourly earnings data from the Current Population Survey. www.bls.gov/data/ [May 29;2007 ]. www.bls.gov/data/
- 104.Arias E United States life tables. National Vital Statistics Reports, vol 54, No. 14. Hyattsville, MD: National Center for Health Statistics; 2003. 2006. [PubMed] [Google Scholar]
- 105.Wilson L. Brown JS. Shin GP. Luc KO. Subak LL. Annual direct cost of urinary incontinence. Obstet Gynecol. 2001;98:398–406. doi: 10.1016/s0029-7844(01)01464-8. [DOI] [PubMed] [Google Scholar]
- 106.Subak LL. Brown J. Kraus S, et al. The “cost” of urinary incontinence for women. Obstet Gynecol. 2006;107:908–916. doi: 10.1097/01.AOG.0000206213.48334.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Waetjen LE. Subak LL. Shen H, et al. Stress urinary incontinence surgery in the United States. Obstet Gynecol. 2003;101:671–676. doi: 10.1016/s0029-7844(02)03124-1. [DOI] [PubMed] [Google Scholar]
- 108.Fialkow MF. Newton KM. Lentz GM. Weiss NS. Lifetime risk of surgical management for pelvic organ prolapse or urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:437–440. doi: 10.1007/s00192-007-0459-9. [DOI] [PubMed] [Google Scholar]
- 109.Subak LL. Waetjen LE. van den Eeden S. Thom DH. Vittinghoff E. Brown JS. Cost of pelvic organ prolapse surgery in the United States. Obstet Gynecol. 2001;98:646–651. doi: 10.1016/s0029-7844(01)01472-7. [DOI] [PubMed] [Google Scholar]
- 110.Uma R. Libby G. Murphy DJ. Obstetric management of a woman's first delivery and the implications for pelvic floor surgery in later life. Br J Obstet Gynaecol. 2005;112:1043–1046. doi: 10.1111/j.1471-0528.2005.00641.x. [DOI] [PubMed] [Google Scholar]
- 111.Mellgren A. Jensen LL. Zetterstrom JP. Wong WD. Hofmeister JH. Lowry AC. Long-term cost of fecal incontinence secondary to obstetric injuries. Dis Colon Rectum. 1999;42:857–865. doi: 10.1007/BF02237089. [DOI] [PubMed] [Google Scholar]
- 112.Adang EM. Engel GL. Rutten FF. Geerdes BP. Baeten CG. Cost-effectiveness of dynamic graciloplasty in patients with fecal incontinence. Dis Colon Rectum. 1998;41:725–733. doi: 10.1007/BF02236259. [DOI] [PubMed] [Google Scholar]
- 113.Deutekom M. Dobben AC. Dijkgraaf MG. Terra MP. Stoker J. Bossuyt PM. Costs of outpatients with fecal incontinence. Scand J Gastroenterol. 2005;40:552–558. doi: 10.1080/00365520510012172. [DOI] [PubMed] [Google Scholar]
- 114.Halverson AL. Hull TL. Paraiso MF. Floruta C. Outcome of sphincteroplasty combined with surgery for urinary incontinence and pelvic organ prolapse. Dis Colon Rectum. 2001;44:1421–1426. doi: 10.1007/BF02234592. [DOI] [PubMed] [Google Scholar]
- 115.Deutekom M. Terra MP. Dobben AC, et al. Selecting an outcome measure for evaluating treatment in fecal incontinence. Dis Colon Rectum. 2005;48:2294–2301. doi: 10.1007/s10350-005-0162-1. [DOI] [PubMed] [Google Scholar]
- 116.Sung VW. Rogers ML. Myers DL. Akbari HM. Clark MA. National trends and costs of surgical treatment for female fecal incontinence. Am J Obstet Gynecol. 2007;197:652–e1–5. doi: 10.1016/j.ajog.2007.08.058. [DOI] [PubMed] [Google Scholar]
- 117.Vandenbussche FP. De Jong-Potjer LC. Stiggelbout AM, et al. Differences in the valuation of birth outcomes among pregnant women, mothers, and obstetricians. Birth. 1999;26:178–183. doi: 10.1046/j.1523-536x.1999.00178.x. [DOI] [PubMed] [Google Scholar]
- 118.Turner CE. Young JM. Solomon MJ, et al. Vaginal delivery compared with elective caesarean section: The views of pregnant women and clinicians. Br J Obstet Gynaecol. 2008;115:1494–1502. doi: 10.1111/j.1471-0528.2008.01892.x. [DOI] [PubMed] [Google Scholar]
- 119.Mankuta DD. Leshno MM. Menasche MM. Brezis MM. Vaginal birth after cesarean section: Trial of labor or repeat cesarean section? A decision analysis. Am J Obstet Gynecol. 2003;189:714–719. doi: 10.1067/s0002-9378(03)00833-0. [DOI] [PubMed] [Google Scholar]
- 120.Pham CT. Crowther CA. Birth outcomes: Utility values that postnatal women, midwives and medical staff express. Br J Obstet Gynaecol. 2003;110:121–127. [PubMed] [Google Scholar]
- 121.Barber MD. Walters MD. Bump RC. Short forms of two condition-specific quality-of-life questionnaires for women with pelvic floor disorders (PFDI-20 and PFIQ-7) Am J Obstet Gynecol. 2005;193:103–113. doi: 10.1016/j.ajog.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 122.Wren PA. Janz NK. Brubaker L, et al. Reliability of health-related quality-of-life measures 1 year after surgical procedures for pelvic floor disorders. Am J Obstet Gynecol. 2005;192:780–788. doi: 10.1016/j.ajog.2004.10.603. [DOI] [PubMed] [Google Scholar]
- 123.Sale JS. Kollenberg K. New York: Simon and Schuster; 1996. The working parents handbook. [Google Scholar]
- 124.The U.S. Equal Employment Opportunity Commission. Pregnancy discrimination. www.eeoc.gov/types/pregnancy.html. [May 20;2009 ]. www.eeoc.gov/types/pregnancy.html
- 125.Bureau of Labor Statistics. Consumer price index—All urban consumers. www.bls.gov/data/home.htm. [Dec 31;2007 ]. www.bls.gov/data/home.htm
- 126.Palisade Corporation. Guide to using @RISK: Risk analysis and simulation add-in for Microsoft Excel. Ithaca, NY: Microsoft; 2008. [Google Scholar]
- 127.Doubilet P. Begg CB. Weinstein MC, et al. Probabilistic sensitivity analysis using Monte Carlo simulation. A practical approach. Med Decis Making. 1985;5:157–177. doi: 10.1177/0272989X8500500205. [DOI] [PubMed] [Google Scholar]
- 128.Lord J. Asante MA. Estimating uncertainty ranges for costs by the bootstrap procedure combined with probabilistic sensitivity analysis. Health Econ. 1999;8:323–333. doi: 10.1002/(sici)1099-1050(199906)8:4<323::aid-hec431>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 129.Lothgren M. Zethraeus N. Definition, interpretation and calculation of cost-effectiveness acceptability curves. Health Econ. 2000;9:623–630. doi: 10.1002/1099-1050(200010)9:7<623::aid-hec539>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 130.Kearney R. Miller JM. Ashton-Miller JA, et al. Obstetric factors associated with levator ani muscle injury after vaginal birth. Obstet Gynecol. 2006;107:144–149. doi: 10.1097/01.AOG.0000194063.63206.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]