Abstract
Transient increases in cytosolic free calcium concentration ([Ca2+]cyt) are essential for plant responses to a variety of environmental stimuli, including low temperature. Subsequent reestablishment of [Ca2+]cyt to resting levels by Ca2+ pumps and C-REPEAT BINDING FACTOR/DEHYDRATION RESPONSIVE ELEMENT BINDING FACTOR 1 (Ca2+/H+) antiporters is required for the correct transduction of the signal. We have isolated a cDNA from Arabidopsis that corresponds to a new cold-inducible gene, RARE COLD INDUCIBLE4 (RCI4), which was identical to CALCIUM EXCHANGER 1 (CAX1), a gene that encodes a vacuolar Ca2+/H+ antiporter involved in the regulation of intracellular Ca2+ levels. The expression of CAX1 was induced in response to low temperature through an abscisic acid–independent pathway. To determine the function of CAX1 in Arabidopsis stress tolerance, we identified two T-DNA insertion mutants, cax1-3 and cax1-4, that display reduced tonoplast Ca2+/H+ antiport activity. The mutants showed no significant differences with respect to the wild type when analyzed for dehydration, high-salt, chilling, or constitutive freezing tolerance. However, they exhibited increased freezing tolerance after cold acclimation, demonstrating that CAX1 plays an important role in this adaptive response. This phenotype correlates with the enhanced expression of CBF/DREB1 genes and their corresponding targets in response to low temperature. Our results indicate that CAX1 ensures the accurate development of the cold-acclimation response in Arabidopsis by controlling the induction of CBF/DREB1 and downstream genes.
INTRODUCTION
Ca2+ is used by most cells to convert external signals into cytosolic information, which can drive processes that are required for full responses to a particular stimulus. Change in the cytosolic concentration of free Ca2+ ([Ca2+]cyt) is the basis for Ca2+ serving as a second messenger (Sze et al., 2000). Transient increases in [Ca2+]cyt are assumed to mediate a wide variety of biotic and abiotic signals. Biotic stimuli include the hormones abscisic acid (ABA) and gibberellins and fungal elicitors (Bush and Jones, 1988; Knight et al., 1991; McAinsh and Hetherington, 1998). Abiotic signals include red, blue, and UV/B light, each acting via different transduction pathways (Shacklock et al., 1992; Frohnmeyer et al., 1998; Baum et al., 1999), touching, hyperosmotic stress, high salt, and high and low temperatures (Knight et al., 1991, 1997; Gong et al., 1998). Different messages can be encoded by changing the magnitude, duration, localization, or frequency of the [Ca2+]cyt spike (Ghosh and Greenberg, 1995; Sanders et al., 1999). Precise regulation of [Ca2+]cyt is essential to develop adequate responses to specific stimuli (Sze et al., 2000). Increases in [Ca2+]cyt result mainly from Ca2+ influx through permeable channels in the plasma membrane and/or Ca2+ discharge from internal stores (Piñeros and Tester, 1997; White, 1998). After Ca2+ influx, efflux systems to internal stores and out of the cell restore [Ca2+]cyt to unstimulated levels via Ca2+ pumps and C-REPEAT BINDING FACTOR/DEHYDRATION RESPONSIVE ELEMENT BINDING FACTOR 1 (Ca2+/H+) exchangers (Knight, 2000).
Influx mechanisms have received considerable attention because their regulation is of primary importance to initiate a Ca2+ signal (Sanders et al., 2002). By contrast, less consideration has been given to the role of efflux systems in Ca2+ signaling. An important question is whether efflux systems help to shape the dynamic form of a Ca2+ spike and, thereby, help to define the information encoded in the signal. The diversity of Ca2+ pumps and H+-coupled Ca2+ cotransporters suggests that these transporters could participate in determining the overall amplitude, duration, and frequency of Ca2+ signals. In the case of Ca2+ pumps, some studies have provided indications of their role in Ca2+ signaling. For instance, the frequency of repetitive Ca2+ waves induced by inositol triphosphate increased when the SERCA (sarcoplasmic/endoplasmic reticulum Ca2+-ATPase) pump was overexpressed in Xenopus oocytes (Camacho and Lechleiter, 1993). Furthermore, the overexpression of SERCA1, -2a, and -2b isoforms in oocytes provoked the dispersion of Ca2+ waves, limiting their propagation in the cytoplasm (Lechleiter et al., 1998). In addition, a deregulated form of ACA4, a vacuolar Ca2+-ATPase from Arabidopsis, has been reported to confer increased tolerance to salt stress when expressed in a yeast mutant with nonfunctioning endogenous Ca2+ pumps and grown on Ca2+-depleted medium (Geisler et al., 2000). Regarding Ca2+/H+ antiporters, their role in Ca2+ signaling (decoding the appropriate response) has not been the subject of many studies and requires further attention.
CALCIUM EXCHANGER 1 (CAX1) was the first plant gene encoding a Ca2+/H+ antiporter to be cloned. It was identified by screening a cDNA library from Arabidopsis for clones able to complement a yeast mutant defective in vacuolar Ca2+ transport (Hirschi et al., 1996). A cDNA from mung bean (VCAX1) that showed high sequence identity with CAX1 was cloned subsequently (Ueoka-Nakanishi et al., 1999). Arabidopsis appears to have up to 10 genes that encode cation/H+ antiporters closely related to CAX1 (Maser et al., 2001), although the functions of most of them remain unknown. CAX1 seems to be localized in the vacuolar membrane (Cheng et al., 2003) and has high Ca2+ transport capacity and low Ca2+ affinity (Shigaki et al., 2001). It has been proposed that CAX1 may play a role in reducing cytosolic Ca2+ concentration to resting levels after a [Ca2+]cyt increase in response to external stimuli (Hirschi, 1999). Transgenic tobacco plants overexpressing CAX1 show increased sensitivity to chilling temperatures (Hirschi, 1999), suggesting that it can play a role in plant adaptation to this environmental condition. Mutations in CAX1 reduce tonoplast Ca2+/H+ antiport activity and vacuolar-type H+-translocating ATPase activity while increasing tonoplast Ca2+-ATPase activity (Cheng et al., 2003). cax1 mutants (cax1-1 and cax1-2) are not affected in their sensitivity to chilling temperatures, but they exhibit altered plant development and perturbed hormone sensitivities (Cheng et al., 2003). The expression of CAX1 is highly induced in response to exogenous Ca2+ and nitrate (Hirschi, 1999; Wang et al., 2000).
By screening a cDNA library from cold-acclimated etiolated seedlings of Arabidopsis with a subtracted probe enriched in cold-induced transcripts (Jarillo et al., 1994), we identified different RARE COLD INDUCIBLE (RCI) genes (Jarillo et al., 1994; Capel et al., 1997; Llorente et al., 2002). One of these genes, RCI4, was identical to CAX1. Here, we report that CAX1 is induced in response to low temperature and that this regulation is mediated through an ABA-independent pathway. The characterization of two T-DNA insertion mutants, cax1-3 and cax1-4, demonstrated that they are not affected in their constitutive capacity to tolerate freezing temperature, dehydration, chilling, or high salt. Interestingly, however, they exhibit an increased ability to cold acclimate, which correlates with an enhanced expression of CBF/DREB1 genes and downstream targets in response to low temperature. These results indicate that CAX1 plays an essential role in the cold-acclimation response by controlling CBF/DREB1 expression, likely by ensuring the proper control of Ca2+ homeostasis under low-temperature conditions.
RESULTS
The Expression of CAX1 Is Induced Transiently in Leaves in Response to Low Temperature
RCI4 was isolated by screening a cDNA library prepared from cold-acclimated (4°C, 7 days) etiolated seedlings of Arabidopsis with a subtracted cDNA probe enriched in cold-induced transcripts. Comparison of the nucleotide sequence of RCI4 with sequences available in the databases revealed 100% identity with CAX1, a gene that encodes a vacuolar Ca2+/H+ antiporter (Hirschi et al., 1996; Cheng et al., 2003).
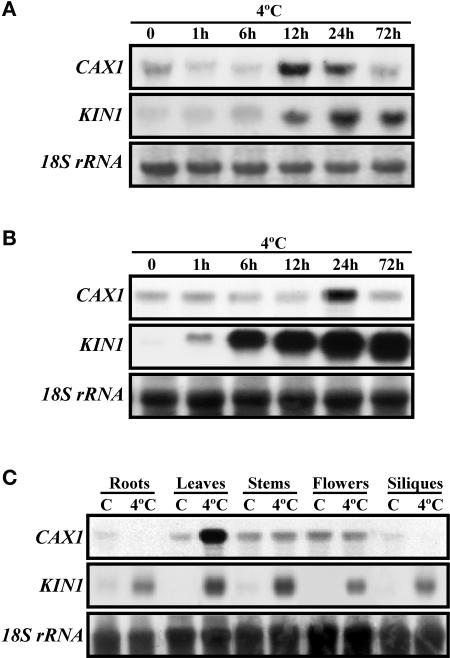
The expression of CAX1 in response to low temperature was first characterized in etiolated seedlings of Arabidopsis. Because CAX1 is a component of a gene family (Maser et al., 2001), a specific CAX1 probe was obtained to avoid cross-hybridizations. The specificity of this probe was determined by DNA gel blot analysis (data not shown). RNA gel blot hybridizations indicated that CAX1 expression was induced transiently in etiolated seedlings, reaching a maximum level after 12 to 24 h of cold treatment (Figure 1A). Expression analysis performed in Arabidopsis plants showed that CAX1 transcripts also were induced transiently in leaves when exposed to low temperature (Figure 1B). To determine the expression pattern of CAX1 in different organs of Arabidopsis, total RNA from roots, leaves, stems, flowers, and siliques from unstressed or stressed (4°C, 24 h) plants were subjected to RNA gel blot hybridizations with the specific CAX1 probe. Results revealed that under unstressed conditions, CAX1 transcripts were present at low levels in all organs analyzed. In response to low temperature, CAX1 transcripts did not experience apparent changes in stems and flowers, whereas they increased in leaves and decreased in roots and siliques (Figure 1C). A probe that recognizes the KIN1 gene from Arabidopsis, the expression of which is induced by cold, dehydration, high-salt, and ABA treatments (Kurkela and Borg-Franck, 1992), was used as a positive control for low-temperature treatments. These data indicate that the expression of CAX1 is induced specifically in leaves in response to low temperature and that this induction is regulated transiently.
Figure 1.
Accumulation of CAX1 Transcripts in Response to Low Temperature.
(A) RNA gel blot hybridization with total RNA (10 μg) from 4-day-old etiolated seedlings exposed to 4°C for the indicated times.
(B) RNA gel blot hybridization with total RNA (10 μg) from leaves of 3-week-old plants exposed to 4°C for the indicated times.
(C) RNA gel blot hybridization with total RNA (10 μg) from roots, rosette leaves, stems, flowers, and siliques of 8-week-old plants exposed to 4°C for 1 day.
Cold treatment efficacy and equal RNA loading were controlled with probes for KIN1 and 18S rRNA, respectively.
CAX1 Expression Is Regulated Negatively by Dehydration and Not Affected by High Salt or ABA
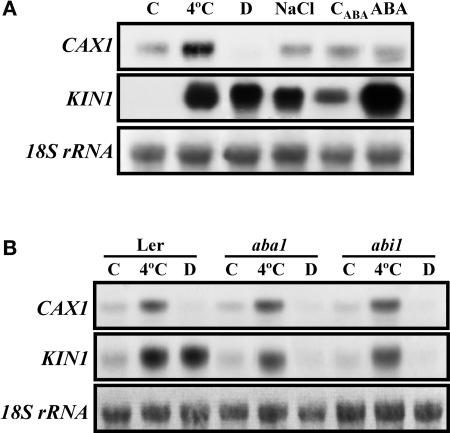
Because many cold-inducible genes also are responsive to exogenous ABA and osmotic stresses (Thomashow, 1999), the effect of dehydration, high salt, and ABA on CAX1 mRNA accumulation was analyzed. Given the expression of CAX1 in response to low temperature (see above), this study was performed in leaves. Although CAX1 transcript accumulation was unaffected by NaCl and ABA treatments, it was reduced notably below control levels by dehydration (Figure 2A). KIN1 was used as a positive control for these treatments.
Figure 2.
Accumulation of CAX1 Transcripts in Response to Different Treatments and in ABA-Deficient and ABA-Insensitive Mutants.
(A) RNA gel blot hybridization with total RNA (10 μg) obtained from 3-week-old leaves grown at control temperature (C), exposed for 1 day at 4°C (4°C), dehydrated until they lost 50% of their fresh weight (D), or treated with 250 mM NaCl (NaCl), with 100 μM ABA (ABA), or with the ABA solvent (CABA).
(B) RNA gel blot hybridization with total RNA (10 μg) obtained from 3-week-old leaves of Landsberg erecta (Ler), aba1, and abi1 plants grown at control temperature (C), exposed for 1 day at 4°C (4°C), or dehydrated until they lost 50% of their fresh weight (D).
Cold treatment efficacy and equal RNA loading were controlled with probes for KIN1 and 18S rRNA, respectively.
To determine whether the regulation of CAX1 expression by cold and dehydration is mediated by ABA, the accumulation of corresponding mRNAs in response to both stresses was analyzed in ABA-deficient (aba1) and ABA-insensitive (abi1) mutants. Figure 2B shows that the expression of CAX1 in response to low temperature and dehydration was identical in the mutants and in the wild type. Thus, the regulation of CAX1 expression by low temperature and dehydration was not mediated by ABA. KIN1 was used as a positive control for these experiments.
Identification of T-DNA Insertion Mutants in CAX1
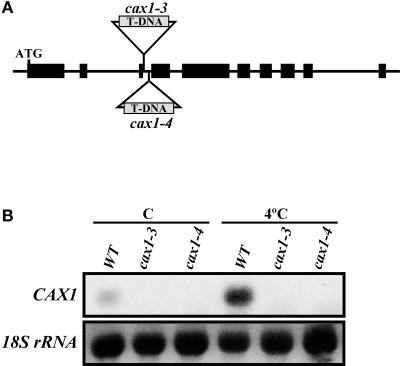
To define the function of CAX1, a reverse-genetics approach was used. Two different transgenic lines containing T-DNA insertions in the third intron of CAX1 were identified (Figure 3A). In both cases, they had single T-DNA insertions (data not shown) that were predicted to disrupt CAX1 expression. This finding was confirmed by RNA gel blot hybridization (Figure 3B), indicating that these new CAX1 alleles (cax1-3 and cax1-4) are null or severely hypomorphic. No obvious morphological differences were found between wild-type and mutant plants.
Figure 3.
Localization of T-DNAs and Accumulation of CAX1 Transcripts in cax1-3 and cax1-4.
(A) Scheme of the CAX1 gene. Large and small boxes represent exons and introns, respectively. ATG indicates the start codon. T-DNA insertions corresponding to cax1-3 and cax1-4 are shown. The scheme is not drawn to scale.
(B) RNA gel blot hybridization with total RNA (10 μg) obtained from 3-week-old leaves of Col wild-type (WT), cax1-3, and cax1-4 plants grown at 20°C (C) or exposed for 1 day at 4°C (4°C). Equal RNA loading was controlled with a probe for 18S rRNA.
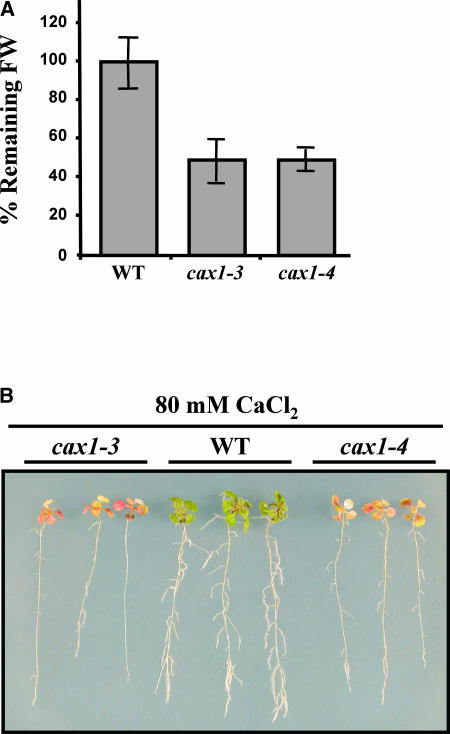
To verify that cax1-3 and cax1-4 mutants had reduced CAX1 activity, three different sets of experiments were performed. First, we measured the tolerance of the mutants to Ca2+. It has been reported that yeast mutants defective in VCX1, an antiporter homologous with CAX1 (Hirschi et al., 1996), are more sensitive to Ca2+ in the growing medium than is the wild type (Cunningham and Fink, 1996). We reasoned that the absence of CAX1 also might enhance the Ca2+ sensitivity of our mutants. Wild-type and cax1 plants grown on standard medium were transferred to medium supplemented with increasing concentrations of CaCl2 (20 to 100 mM) for 7 days. Ca2+ sensitivity was estimated as the percentage of initial fresh weight remaining after treatment. Wild-type and cax1 plants showed no significant differences in their initial fresh weight values (data not shown). After 7 days, the levels of added CaCl2 that caused a fresh weight loss of 50% in the cax1 mutants was 80 mM. At this [Ca2+], wild-type plants experienced no loss of fresh weight (Figure 4A), demonstrating that the mutants have enhanced sensitivity to Ca2+. These significant differences were very apparent at the morphological level, with cax1 mutants displaying a highly stressed phenotype compared with the wild type (Figure 4B).
Figure 4.
Ca2+ Tolerance in cax1-3 and cax1-4.
(A) Tolerance to Ca2+ was estimated as the percentage of initial fresh weight (FW) that remained after transferring 3-week-old Col wild-type (WT), cax1-3, and cax1-4 plants to a medium containing 80 mM CaCl2 for 7 days. Data are expressed as means of three independent experiments with 20 plants each. Bars indicate standard errors. In all cases, values obtained from wild-type and mutant plants were significantly different (P < 0.05) as determined by Student's t test.
(B) Representative wild-type and mutant plants after CaCl2 treatment.
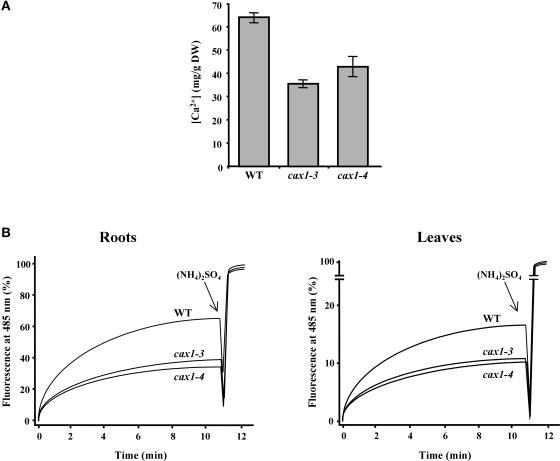
We also determined the accumulation of total Ca2+ in cax1 mutants. Because the CAX1 antiporter is involved in Ca2+ homeostasis (Hirschi, 1999), alterations in its activity are expected to change Ca2+ partitioning. As shown in Figure 5A, cax1-3 and cax1-4 accumulate 46 and 35% less Ca2+ in their leaves, respectively, than does the wild type. Given the high [Ca2+] in the central vacuole and the fact that this occupies 80 to 90% of the whole plant cell (Marty, 1999; Ueoka-Nakanishi et al., 1999), total Ca2+ accumulation can be considered an indirect measure of the vacuolar Ca2+ content. Therefore, it can be assumed that cax1 mutants have a lower level of vacuolar Ca2+ than do wild-type plants, which suggests that Ca2+ partitioning is affected by cax1 mutations.
Figure 5.
Ca2+ Concentration and Ca2+/H+ Antiport Activity in cax1-3 and cax1-4.
(A) Ca2+ content of leaves from 3-week-old Col wild-type (WT), cax1-3, and cax1-4 plants was determined by atomic absorption spectrophotometry. Data are expressed as means of three independent experiments. Bars indicate standard errors. In all cases, values obtained from wild-type and mutant plants were significantly different (P < 0.05) as determined by Student's t test. DW, dry weight.
(B) Ca2+/H+ antiport activity was measured in vacuole-enriched membrane vesicles from roots and leaves of Col wild-type, cax1-3, and cax1-4 plants pretreated with 100 mM CaCl2 by monitoring the quenching of 9-amino-6-chloro-2-methoxyacridine fluorescence. The percentage of fluorescence recovered during a 12-min time course is represented. At 11 min, (NH4)2SO4 (25 mM) was added for full recovery of fluorescence. Representative results from five replicate experiments are shown. Each replicate experiment was performed using independent membrane vesicle preparations.
Finally, we measured Ca2+/H+ antiport activity in vacuole-enriched membrane vesicles isolated from roots of wild-type and cax1 plants treated previously with 100 mM CaCl2. Figure 5B shows that the activity detected in vesicles prepared from cax1-3 and cax1-4 plants is ~50% lower than the activity found in wild-type vesicles. Similar results were obtained with vacuole-enriched membrane vesicles isolated from leaves of plants pretreated with 100 mM CaCl2 (Figure 5B). Together, these results demonstrate that the loss of the CAX1 transcripts in cax1-3 and cax1-4 causes a reduction in Ca2+/H+ antiport activity.
cax1 Mutants Show an Enhanced Cold-Acclimation Response
Ca2+ is a second messenger in plant responses to different abiotic stresses, including high salt, dehydration, and low temperatures (Knight et al., 1991, 1997), and changes in [Ca2+]cyt constitute the basis for encoding such responses (Knight, 2000). To explore a possible role of Ca2+/H+ antiporters in Ca2+-mediated stress responses, cax1-3 and cax1-4 were analyzed for salt, dehydration, chilling, and freezing tolerance.
Salt tolerance was estimated by determining the fresh weight of 3-week-old wild-type and cax1 plants after growing for 2 weeks in a medium containing 100 mM NaCl. Dehydration was induced by maintaining 3-week-old plants on dry filter paper for 1 day without watering. The rate of dehydration was established as the percentage of initial fresh weight that remained after treatment. Chilling injury was assessed by placing 4-day-old wild-type and mutant seedlings at 4°C for 5 weeks. No significant differences between cax1 and wild-type plants were found in any case (data not shown).
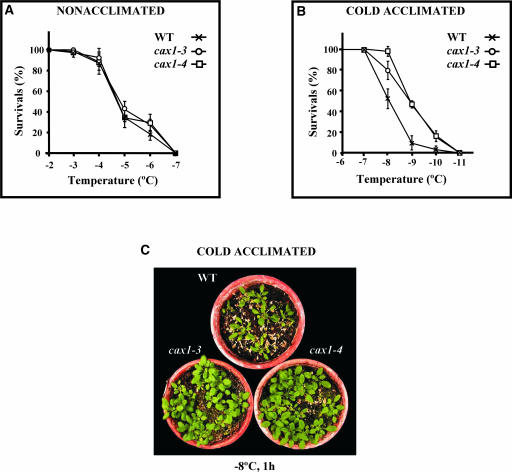
Tolerance to freezing was examined in 3-week-old wild-type and cax1 plants before and after cold acclimation (4°C, 7 days). In all cases, freezing tolerance was determined as the percentage of surviving plants after exposure to different freezing temperatures for 1 h. Figure 6A shows that nonacclimated cax1 and wild-type plants had similar capacities to tolerate freezing. The LT50 (temperature that causes 50% lethality) value was −4.6°C in both cases. By contrast, cax1-3 and cax1-4 plants displayed significantly greater freezing tolerance than did wild-type plants after cold acclimation (Figure 6B). The LT50 values of acclimated wild-type and cax1 plants were estimated to be −8.0 and −8.9°C, respectively. The increased freezing tolerance manifested by the mutants with respect to the wild type after cold acclimation was very apparent (Figure 6C). Therefore, cax1 mutations enhanced the freezing tolerance of Arabidopsis after cold acclimation. These data indicate that CAX1 negatively controls the cold-acclimation response.
Figure 6.
Freezing Tolerance of cax1-3 and cax1-4.
Three-week-old Col wild-type (WT), cax1-3, and cax1-4 plants were exposed to different freezing temperatures for 1 h. Freezing tolerance was estimated as the percentage of plants surviving each specific temperature after 2 weeks of recovery under unstressed conditions. In (A) and (B), data are expressed as means of three independent experiments with 50 plants each. Bars indicate standard errors.
(A) Freezing tolerance of nonacclimated wild-type and cax1 plants.
(B) Freezing tolerance of cold-acclimated (4°C, 7 days) wild-type and cax1 plants.
(C) Representative cold-acclimated wild-type and cax1 plants after being exposed at −8°C for 1 h and recovering for 2 weeks at 20°C.
cax1 Mutants Show Enhanced Induction of CBF/DREB1 Genes in Response to Low Temperatures
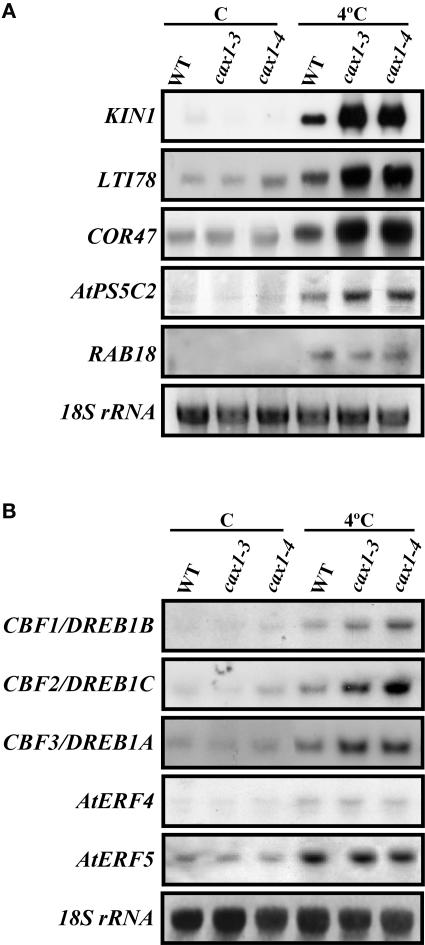
Because cax1 mutations affected the ability of Arabidopsis to cold acclimate, we investigated whether they also could influence cold-induced gene expression. The accumulation of transcripts corresponding to several genes whose expression is regulated by low temperatures through different pathways (KIN1 [Kurkela and Franck, 1990], COR47 [Gilmour et al., 1992], RAB18 [Lang and Palva, 1992], LTI78 [Nordin et al., 1993], and AtP5CS2 [Strizhov et al., 1997]) was examined by RNA gel blot analysis. Under control conditions, wild-type and mutant plants always showed very similar expression patterns (Figure 7A). In response to cold, the levels of all messengers increased in both wild-type and mutant plants (Figure 7A). However, in KIN1, LTI78, COR47, and AtP5CS2, these levels were considerably higher in cax1 mutants than in the wild type (Figure 7A). Interestingly, all of these genes are considered to be targets of the CBF/DREB1 transcriptional activators (Gilmour et al., 2000). The cold induction of RAB18, which has not been described as a CBF/DREB1 target, was similar in mutants and the wild type (Figure 7A).
Figure 7.
Transcript Levels of Cold-Inducible Genes in cax1-3 and cax1-4.
(A) RNA gel blot hybridization with total RNA (10 μg) obtained from 3-week-old Col wild-type (WT), cax1-3, and cax1-4 plants grown under control conditions (C) or exposed for 1 day at 4°C (4°C).
(B) RNA gel blot hybridization with total RNA (20 μg) obtained from 3-week-old Col wild-type, cax1-3, and cax1-4 plants grown under control conditions (C) or exposed for 1 h at 4°C (4°C).
Equal RNA loading was controlled with a probe for 18S rRNA.
The overexpression of CBF/DREB1 genes in transgenic Arabidopsis activates the expression of downstream CBF/DREB1 targets, which in turn promotes freezing tolerance (Jaglo-Ottosen et al., 1998; Liu et al., 1998; Kasuga et al., 1999; Gilmour et al., 2000). Thus, one hypothesis is that the increased induction of CBF/DREB1 target genes displayed by cax1 mutants in response to low temperature may be caused by an increased induction of the CBF/DREB1 genes themselves. RNA gel blot experiments using CBF1/DREB1B-, CBF2/DREB1C-, and CBF3/DREB1A-specific probes showed that the cold induction of the three genes was enhanced by the cax1 mutations (Figure 7B). The cold induction of AtERF4 and AtERF5, two genes that encode transcription factors that are regulated by low temperature and ethylene (Fujimoto et al., 2000), was not affected in cax1 plants (Figure 7B). Together, these results indicate that CAX1 acts to negatively control the expression of CBF/DREB1 genes and their corresponding downstream targets.
DISCUSSION
This work describes the functional characterization of CAX1, a vacuolar Ca2+/H+ antiporter, in response to abiotic stresses. Expression analysis revealed that CAX1 was subjected to an intricate regulation. Under control conditions, CAX1 transcripts accumulated at basal levels in all organs analyzed. In response to low temperature, these levels increased transiently in etiolated seedlings and leaves of adult plants and decreased in roots and siliques. Furthermore, CAX1 expression was inhibited by dehydration but was not affected by ABA and high-salt treatments. Although the significance of this expression pattern is not clear at present, it indicates that during Arabidopsis development and in response to different adverse environmental situations, the regulation of CAX1 is accomplished through several ABA-independent signal transduction pathways. In addition, CAX1 has been reported to be induced in response to exogenous Ca2+ (Hirschi, 1999) and nitrate (Wang et al., 2000). Recent studies have shown that CAX1 also is regulated at the post-transcriptional level by an autoinhibitory N-terminal region (Pittman et al., 2002). The implication of this regulation in CAX1 function remains to be determined.
We have identified two T-DNA insertion mutants, cax1-3 and cax1-4, that are null or highly hypomorphic for CAX1 expression as judged by mRNA levels. Both mutants accumulated lower levels of total Ca2+ than did wild-type plants and were hypersensitive to Ca2+ in the growth medium, and their Ca2+/H+ antiport activity was reduced by 50%. cax1-3 and cax1-4 plants responded differentially to salt, dehydration, chilling, and freezing treatments. In fact, after being exposed to high salt, dehydration, or chilling temperatures, they showed no significant differences with respect to the wild type. These results are identical to those reported by Cheng et al. (2003) after characterizing the cax1-1 and cax1-2 alleles. Compared with the wild type, cax1-3 and cax1-4 displayed no differences in their constitutive freezing tolerance. However, their freezing tolerance after cold acclimation was significantly greater than that of cold-acclimated wild-type plants, indicating that CAX1 negatively controls the cold-acclimation response. Interestingly, it was reported that transgenic tobacco plants overexpressing CAX1 are hypersensitive to chilling stress, suggesting a role for this Ca2+/H+ antiporter in the plant response to low temperatures (Hirschi, 1999). Our results demonstrate that CAX1 does not play a general role in the development of Arabidopsis tolerance to abiotic stresses but has a specific function in cold acclimation, which is consistent with the fact that CAX1 expression is induced specifically in response to low temperature. Although the origin of this specificity remains unknown, it is tempting to speculate that it is related to the cold-induced Ca2+ signature.
The question arises of why cax1 mutants have an increased ability to cold acclimate. Expression analysis revealed that this capability is correlated with an enhanced expression of the CBF/DREB1 genes and their corresponding downstream targets in response to low temperature. The expression of other cold-inducible genes that have not been reported to be regulated by CBF/DREB1 transcriptional activators was not altered in cold-treated cax1 plants. The CBF/DREB1 genes encode a small family of transcriptional activators that have been reported to play important roles in freezing tolerance and cold acclimation in Arabidopsis (Jaglo-Ottosen et al., 1998; Liu et al., 1998; Kasuga et al., 1999; Gilmour et al., 2000). They are induced specifically and transiently by low temperature, and this induction precedes that of downstream cold-inducible genes, which results in an increase in freezing tolerance (Gilmour et al., 1998; Liu et al., 1998; Medina et al., 1999). The data presented here indicate that CAX1 negatively controls the cold induction of the CBF/DREB1 regulon and, thereby, the cold-acclimation response.
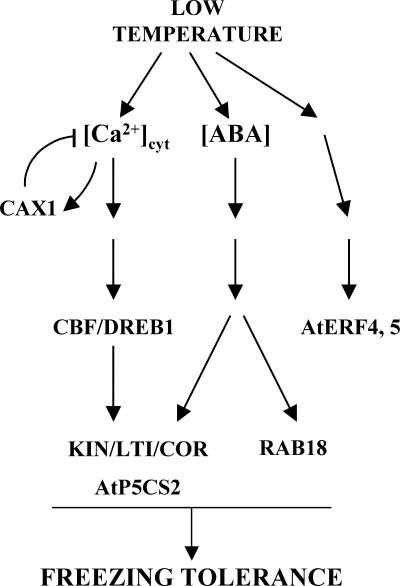
A working model for CAX1 function in low-temperature signaling during cold acclimation is presented in Figure 8. When plants are exposed to low temperature, a transient increase in [Ca2+]cyt is induced, generating a Ca2+ signal (Knight et al., 1991). This signal, conveniently decoded and transduced by downstream effectors such as AtCBL1 (Kudla et al., 1999), CDPKs (Martin and Busconi, 2001), or CIPK3 (Kim et al., 2003), induces the expression of the CBF/DREB1 genes as well as that of CAX1. Subsequently, CBF/DREB1 transcription factors activate the expression of downstream target genes, which in turn, together with other cold-inducible genes regulated through additional signaling pathways, promote freezing tolerance. Consistent with this part of the model, the expression of some CBF/DREB1 target genes under low-temperature conditions has been reported to be mediated by the cold-induced increase in [Ca2+]cyt (Knight et al., 1996; Tähtiharju et al., 1997; Townley and Knight, 2002). Moreover, the induction of CBF/DREB1 genes in response to low temperature is inhibited in the presence of the Ca2+ chelator EGTA, suggesting that their expression also is dependent on cold-induced [Ca2+]cyt increases (R. Catalá and J. Salinas, unpublished results). The expression of CAX1, which has been reported to be induced by Ca2+ (Hirschi, 1999) in cooperation with other Ca2+ transporters (i.e., Ca2+/H+ exchangers and Ca2+-ATPases), would contribute to the reestablishment of resting levels of cytosolic Ca2+ and CBF/DREB1 expression. In agreement with this notion, Townley and Knight (2002) reported that the overexpression of CaM3, an Arabidopsis gene that encodes the calmodulin CaM3, inhibits the cold induction of LTI78 and KIN1, and they suggested that this inhibition is caused by the activation of a Ca2+-ATPase by CaM3. Thus, we propose that CAX1 plays a role in controlling the correct restoration of [Ca2+]cyt levels after the transient increase induced by the cold signal, which is essential for an adequate response, including the accurate expression of CBF/DREB1. It is conceivable that mutations that affect CAX1 activity may perturb that restoration, leading to an increase in [Ca2+]cyt that under cold conditions would result in the overaccumulation of CBF/DREB1 transcripts and the subsequent increase in the induction of target genes and freezing tolerance.
Figure 8.
Hypothetical Model for CAX1 Function in Low-Temperature Signaling.
The model presented suggests that Ca2+/H+ antiport activity may increase in Arabidopsis in response to low temperature. If so, this increase would be reduced in cax1 mutants. The possibility exists, however, that CAX1 is induced in response to low temperature just to maintain steady state levels of CAX1 activity when Arabidopsis copes with this environmental constraint. Under our experimental conditions, no significant differences in Ca2+/H+ antiport activity were detected among vacuole-enriched membrane vesicles from wild-type plants grown under control conditions and vesicles isolated from wild-type plants exposed to 4°C (Figure 9A). Similarly, we detected no differences between vesicles isolated from the cax1 mutants before and after low-temperature treatment (Figure 9A). Nevertheless, these results cannot exclude the possibility that Ca2+/H+ antiport activity could be induced during cold acclimation. In fact, Ca2+/H+ antiport activity can be measured directly in vacuole-enriched membrane vesicles from Arabidopsis only after pretreatment of the plants with 100 mM CaCl2 (Pittman et al., 2002), which could mask the cold induction. When wild-type or mutant plants were pretreated with 50 mM CaCl2, the Ca2+/H+ antiport activity measured was lower in all cases, but no cold induction was detected (Figure 9B).
Figure 9.
Ca2+/H+ Antiporter Activity in Wild-Type, cax1-3, and cax1-4 Plants in Response to Low Temperature.
Ca2+/H+ antiport activity was measured in vacuole-enriched membrane vesicles from roots and leaves of Ca2+-treated Col wild-type (WT), cax1-3, and cax1-4 plants grown at 20°C (C) or exposed for 1 day at 4°C (4°C) by monitoring the quenching of 9-amino-6-chloro-2-methoxyacridine fluorescence. The percentage of fluorescence recovered during a 12-min time course is represented. At 11 min, (NH4)2SO4 (25 mM) was added for full recovery of fluorescence. Representative results from three replicate experiments are shown. Each replicate experiment was performed using independent membrane vesicle preparations.
(A) Antiport activity in vesicles from roots and leaves of plants pretreated with 100 mM CaCl2.
(B) Antiport activity in vesicles from roots of plants pretreated with 50 mM CaCl2.
In summary, we demonstrate that CAX1 participates in the development of the cold-acclimation response in Arabidopsis by negatively controlling the induction of CBF/DREB1 and downstream genes, probably by ensuring the proper control of Ca2+ homeostasis in response to low temperature. Two other negative effectors of the cold-acclimation response, PP2C and HOS1, were identified recently (Lee et al., 2001; Tähtiharju and Palva, 2001), indicating that this adaptive process is subject to a precise control that includes active negative regulation. Although further studies are needed to address the participation of other Ca2+ transporters in low-temperature signaling and the mechanisms by which [Ca2+]cyt regulates CBF/DREB1 expression, our findings provide new insights to advance our understanding of the molecular basis of the cold-acclimation response.
METHODS
Plant Materials and Growth Conditions
Arabidopsis thaliana ecotypes Columbia (Col) and Landsberg erecta and abscisic acid (ABA)–deficient (aba1-1) and ABA-insensitive (abi1-1) mutants were used in this study. Etiolated seedlings were obtained from seeds sown under sterile conditions in Petri dishes containing mineral nutrient solution (Haughn and Somerville, 1986) solidified with 0.8% (w/v) agar. Etiolation was achieved by covering plates with aluminum foil. Plants for Ca2+-, dehydration-, chilling-, and salt-tolerance assays, as well as for the preparation of vacuole-enriched membrane vesicles, were obtained from seeds sown under sterile conditions in Petri dishes containing GM medium (MS medium [Murashige and Skoog, 1962] supplemented with 1% sucrose) solidified with 0.8% (w/v) agar. Soil-grown plants were obtained by sowing seeds in pots containing a mixture of organic substrate and vermiculite (3:1, v/v) and irrigating the pot with water and mineral nutrient solution medium once per week. Unless specified otherwise, in all cases, 3-week-old plants grown at 20°C under long-day photoperiods (16 h of cool-white fluorescent light; PPFD of 70 μmol·m−2·s−1) were used for experiments.
Low-temperature treatments were performed by transferring 4-day-old etiolated seedlings and plants to a growth chamber set to 4°C for different periods in the dark or under the light and photoperiodic conditions described above. Low-temperature treatments in different organs were performed in 8-week-old plants. Dehydration was induced by removing plants from soil and allowing them to lose 50% of their initial fresh weight. Salt stress was accomplished by watering plants with 250 mM NaCl for 24 h. For ABA treatment, plants were sprayed with 100 μM ABA. The ABA stock solution (100 mM) was prepared in DMSO, and control treatments were given with water containing the same final concentration as the ABA solvent. After the treatments, seedlings and plants used for RNA gel blot hybridizations were frozen immediately in liquid N2 and stored at −80°C until their use.
Tolerance to Ca2+ and NaCl was checked by transferring plants to new Petri dishes containing agar medium supplemented with different concentrations (20, 40, 60, 80, and 100 mM) of CaCl2 or 100 mM NaCl. Ca2+ and NaCl tolerance were estimated based on the percentage of initial fresh weight remaining after 7 and 14 days of treatment, respectively. Dehydration tolerance was investigated by removing plants from the medium, placing them on dry filter paper, and allowing them to develop for 1 day without watering. The rate of dehydration was estimated as the percentage of initial fresh weight remaining after treatment. Chilling was imposed by placing Petri dishes containing 4-day-old seedlings at 4°C under long-day photoperiods (35 μmol·m−2·s−1) for 5 weeks. Tolerance to chilling was determined as the percentage of plants surviving after the treatment. Freezing tolerance was analyzed by exposing nonacclimated and cold-acclimated (4°C, 7 days) plants to 4°C for 30 min in darkness and subsequently allowing the temperature to decrease at a rate of 2°C/h. The final desired freezing temperature was maintained for 1 h, and then the temperature was increased again to 4°C at the same rate. After thawing at 4°C for 12 h in the dark, plants were returned to the original growth conditions. Tolerance to freezing was determined as the percentage of plants surviving after 2 weeks of recovery in control conditions.
Preparation of Vacuole-Enriched Membrane Vesicles and Ca2+/H+ Antiport Activity Assays
Vacuole-enriched membrane vesicles were prepared from root or leaf tissues obtained from plants pretreated with 50 or 100 mM CaCl2 for 18 h before harvest, according to Cheng et al. (2003). Ca2+/H+ antiport activity was measured by monitoring the formation and dissipation of pH gradients across membrane vesicles, essentially as described by Schumaker and Sze (1986). Vesicles (50 μg) were incubated in a buffer containing 0.3 M sorbitol, 5 mM 1,3-bis(Tris[hydroxymethyl]methylamino) propane–Mes, pH 7.6, 1 mM Mg2+-ATP, 100 mM KCl, 3 mM MgSO4, and 100 nM validomycin. Ca2+-dependent pH gradient dissipation was initiated by the addition of 10 μM CaCl2 and measured by monitoring the fluorescence quenching of 9-amino-6-chloro-2-methoxyacridine (Molecular Probes, Eugene, OR) for 12 min. Fluorescence quenching was monitored in a thermostatted cell at 25°C using a fluorescence spectrometer (model RF-540; Shimadzu, Kyoto, Japan) at excitation and emission wavelengths of 415 and 485 nm, respectively. After 11 min, (NH4)2SO4 was added to a final concentration of 25 mM to dissipate the pH gradient and determine total fluorescence recovery.
Molecular Biology Methods
CAX1 was isolated by screening a cDNA library prepared from cold-acclimated etiolated seedlings of Arabidopsis with a subtracted cDNA probe enriched in cold-induced transcripts as described by Jarillo et al. (1994). Databases were searched for sequence similarities using the Basic Local Alignment Search Tool (BLAST) program of the National Centre for Biotechnology Information (Altschul et al., 1997).
Total RNA was isolated as described by Logemann et al. (1987). Restriction digestion, cloning, and DNA and RNA gel blot hybridization were performed according to standard procedures (Sambrook et al., 1989). The CAX1-specific probe was obtained by PCR from genomic DNA of Arabidopsis ecotype Col using the primers 5′-TGTCGTCACTGCAACAGGAGGA-3′ and 5′-GACATTCATAGATAGTTCATTGC-3′. The specific probes for KIN1 (Kurkela and Borg-Franck, 1992), LTI78 (Nordin et al., 1993), COR47 (Gilmour et al., 1992), AtP5CS2 (Strizhov et al., 1997), RAB18 (Lang and Palva, 1992), CBF1/DREB1B, CBF2/DREB1C, and CBF3/DREB1A (Medina et al., 1999), and AtERF4 and AtERF5 (Fujimoto et al., 2000) were obtained by PCR from genomic DNA of ecotype Col with the following primers: KIN1 (5′-GGCACCACACTCCCTTTAG-3′ and 5′-GAATATAAGTTTGGCTCGTC-3′); LTI78 (5′-ACCATAATACATCAAAGAC-3′ and 5′-CGGGATTTGACGGAGAACC-3′); COR47 (5′-TGGCTGAGGAGTACAAGAACAACG-3′ and 5′-CTTCACCGATCCAACAGCTCTTCT-3′); AtP5CS2 (5′-CGCGGATCCCTCGTTCTCTCGTGTTTTCG-3′ and 5′-GCGGATCCGACATCAGCAGAGAAAGAGAG-3′); RAB18 (5′-CCCCTGCAGTCCATATCCGAAACCGGA-3′ and 5′-GGGGAATTCACGTACCGAGCTAGAGCTGG-3′); CBF1/DREB1B (5′-GTGACGTGTCGCTTTGGAGTTAC-3′ and 5′-GTGAAGCAAAGAAGTAGAAAACG-3′); CBF2/DREB1C (5′-TCGAGGGAGATGATGACGTGTCCT-3′ and 5′-TATTTTGATTTGTTGCTTATGG-3′), CBF3/DREB1A (5′-CGACGGCGATGATGACGACGTA-3′ and 5′-GCATTTAAGAATAGCCCACACT-3′); AtERF4 (5′-TATCCGAGAATGGCCAAG-3′ and 5′-GACTGAGAGAGAGAGAGAGG-3′); and AtERF5 (5′-ATGGCGACTCCTAACGAAG-3′ and 5′-CGTCAGCATACACATCGTTC-3′). Equal RNA loading was monitored using an EcoRI fragment from the 18S rRNA as a probe (Tremousaygue et al., 1992). RNA samples from each experiment were analyzed on at least two independent blots, and each experiment was repeated at least twice.
Isolation of T-DNA Insertion Mutants in CAX1
A total of 60,000 Arabidopsis T-DNA insertion lines (J.M. Alonso and J.R. Ecker, unpublished data) were screened for cax1 mutants by PCR. Specific primers for the left and right borders of the T-DNA and for CAX1 (5′-CTTTGGGGATTCGGCAATGCATGAT-3′ and 5′-CGGATATTAGCGATTCCTCCACAGAA-3′) were used to identify mutant lines.
Total Ca2+ Content Analysis
Leaves from 3-week-old Col, cax1-3, and cax1-4 plants watered with 2 mM CaCl2 for 2 days were frozen in liquid N2 and lyophilized. At least 0.25 mg of these materials was treated with 0.5 mL of 1 M HCl for 1 day. Supernatants were collected after centrifugation at 13,000g for 10 min, and total Ca2+ content was determined by atomic absorption spectrophotometry (model 2380; Perkin-Elmer, Norwalk, CT).
Upon request, materials integral to the findings presented in this publication will be made available in a timely manner to all investigators on similar terms for noncommercial research purposes. To obtain materials, please contact J. Salinas, salinas@inia.es.
Acknowledgments
We thank J. Capel and J.A. Jarillo for assistance with the RCI clones, J.P. Donaire and K. Venema for assistance with the Ca2+/H+ antiporter activity assays, and E. Rodriguez and A. Redondo for technical support. We also are grateful to A. Rodríguez-Navarro, G. Salcedo, and J.J. Sánchez-Serrano for critical reading of the manuscript. This work was funded by grants from the Comisión Interministerial de Ciencia y Tecnología (Grants BIO98-0189 and BIO01-0344) and the European Union (Grant QLK3-CT00-328) to J.S.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.015248.
References
- Altschul, S.F., Madden, T.L., Schaffer, A.A., Zhang, J., Zhang, Z., Miller, W., and Lipman, D.J. (1997). Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum, G., Long, J.C., Jenkins, G.I., and Trewavas, A.J. (1999). Stimulation of the blue light phototropic receptor NPH1 causes a transient increase in cytosolic Ca2+. Proc. Natl. Acad. Sci. USA 96, 13554–13559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush, D.S., and Jones, R.L. (1988). Cytoplasmic calcium and α-amylase secretion from barley aleurone protoplasts. Eur. J. Cell Biol. 46, 466–469. [Google Scholar]
- Camacho, P., and Lechleiter, J.D. (1993). Increased frequency of calcium waves in Xenopus laevis oocytes that express a calcium-ATPase. Science 260, 226–229. [DOI] [PubMed] [Google Scholar]
- Capel, J., Jarillo, J.A., Salinas, J., and Martinez-Zapater, J.M. (1997). Two homologous low-temperature-inducible genes from Arabidopsis encode highly hydrophobic proteins. Plant Physiol. 115, 569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, N.H., Pittman, J.K., Barkla, B.J., Shigaki, T., and Hirschi, K.D. (2003). The Arabidopsis cax1 mutant exhibits impaired ion homeostasis, development, and hormonal responses and reveals interplay among vacuolar transporters. Plant Cell 15, 347.–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham, K.W., and Fink, G.R. (1996). Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+-ATPases in Saccharomyces cerevisiae. Mol. Cell. Biol. 16, 2226–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohnmeyer, H., Bowler, C., Zhu, J.-K., Yamagata, H., Schäfer, E., and Chua, N.-H. (1998). Different roles for calcium and calmodulin in phytochrome and UV-regulated expression of chalcone synthase. Plant J. 13, 763–772. [Google Scholar]
- Fujimoto, S.Y., Ohta, M., Usui, A., Shinshi, H., and Ohme-Takagi, M. (2000). Arabidopsis ethylene-responsive element binding factors act as transcriptional activators or repressors of GCC box–mediated gene expression. Plant Cell 12, 393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler, M., Frangne, N., Gomes, E., Martinoia, E., and Palmgren, M.G. (2000). The ACA4 gene of Arabidopsis encodes a vacuolar membrane calcium pump that improves salt tolerance in yeast. Plant Physiol. 124, 1814–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh, A., and Greenberg, M.E. (1995). Calcium signaling in neurons: Molecular mechanisms and cellular consequences. Science 268, 239–247. [DOI] [PubMed] [Google Scholar]
- Gilmour, S.J., Artus, N.N., and Thomashow, M.F. (1992). cDNA sequence analysis and expression of two cold-regulated genes of Arabidopsis thaliana. Plant Mol. Biol. 18, 13–21. [DOI] [PubMed] [Google Scholar]
- Gilmour, S.J., Sebolt, A.M., Salazar, M.P., Everard, J.D., and Thomashow, M.F. (2000). Overexpression of the Arabidopsis CBF3 transcriptional activator mimics multiple biochemical changes associated with cold acclimation. Plant Physiol. 124, 1854–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour, S.J., Zarka, D.G., Stockinger, E.J., Salazar, M.P., Houghton, J.M., and Thomashow, M.F. (1998). Low temperature regulation of the Arabidopsis CBF family of AP2 transcriptional activators as an early step in cold-induced COR gene expression. Plant J. 16, 433–442. [DOI] [PubMed] [Google Scholar]
- Gong, M., Van de Luit, A.H., Knight, M.R., and Trewavas, A.J. (1998). Heat-shock-induced changes in intracellular Ca2+ level in tobacco seedlings in relation to thermotolerance. Plant Physiol. 116, 429–437. [PMC free article] [Google Scholar]
- Haughn, G., and Somerville, C. (1986). Sulfonylurea-resistant mutants of Arabidopsis thaliana. Mol. Gen. Genet. 204, 430–434. [Google Scholar]
- Hirschi, K.D. (1999). Expression of Arabidopsis CAX1 in tobacco: Altered calcium homeostasis and increased stress sensitivity. Plant Cell 11, 2113–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi, K.D., Zhen, R.G., Cunningham, K.W., Rea, P.A., and Fink, G.R. (1996). CAX1, an H+/Ca2+ antiporter from Arabidopsis. Proc. Natl. Acad. Sci. USA 93, 8782–8786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaglo-Ottosen, K.R., Gilmour, S.J., Zarka, D.G., Schabenberger, O., and Thomashow, M.F. (1998). Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science 280, 104–106. [DOI] [PubMed] [Google Scholar]
- Jarillo, J.A., Capel, J., Leyva, A., Martinez-Zapater, J.M., and Salinas, J. (1994). Two related low-temperature-inducible genes of Arabidopsis encode proteins showing high homology to 14-3-3 proteins, a family of putative kinase regulators. Plant Mol. Biol. 25, 693–704. [DOI] [PubMed] [Google Scholar]
- Kasuga, M., Liu, Q., Miura, S., Yamaguchi-Shinozaki, K., and Shinozaki, K. (1999). Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat. Biotechnol. 17, 287–291. [DOI] [PubMed] [Google Scholar]
- Kim, K.N., Cheong, Y.H., Grant, J.J., Pandey, G.K., and Luan, S. (2003). CIPK3, a calcium sensor-associated protein kinase that regulates abscisic acid and cold signal transduction in Arabidopsis. Plant Cell 15, 411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight, H. (2000). Calcium signaling during abiotic stress in plants. Int. Rev. Cytol. 195, 269–324. [DOI] [PubMed] [Google Scholar]
- Knight, H., Trewavas, A.J., and Knight, M.R. (1996). Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell 8, 489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight, H., Trewavas, A.J., and Knight, M.R. (1997). Calcium signaling in Arabidopsis thaliana responding to drought and salinity. Plant J. 12, 1067–1078. [DOI] [PubMed] [Google Scholar]
- Knight, M.R., Campbell, A.K., Smith, S.M., and Trewavas, A.J. (1991). Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature 352, 524–526. [DOI] [PubMed] [Google Scholar]
- Kudla, J., Xu, Q., Harter, K., Gruissem, W., and Luan, S. (1999). Genes for calcineurin B-like proteins in Arabidopsis are differentially regulated by stress signals. Proc. Natl. Acad. Sci. USA 96, 4718–4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurkela, S., and Borg-Franck, M. (1992). Structure and expression of kin2, one of two cold- and ABA-induced genes of Arabidopsis thaliana. Plant Mol. Biol. 19, 689–692. [DOI] [PubMed] [Google Scholar]
- Kurkela, S., and Franck, M. (1990). Cloning and characterization of a cold- and ABA-inducible Arabidopsis gene. Plant Mol. Biol. 15, 137–144. [DOI] [PubMed] [Google Scholar]
- Lang, V., and Palva, E.T. (1992). The expression of a rab-related gene, rab18, is induced by abscisic acid during the cold acclimation process of Arabidopsis thaliana (L.) Heynh. Plant Mol. Biol. 20, 951–962. [DOI] [PubMed] [Google Scholar]
- Lechleiter, J.D., John, L.M., and Camacho, P. (1998). Ca2+ wave dispersion and spiral wave entrainment in Xenopus laevis oocytes overexpressing Ca2+ ATPases. Biophys. Chem. 72, 123–129. [DOI] [PubMed] [Google Scholar]
- Lee, H., Xiong, L., Gong, Z., Ishitani, M., Stevenson, B., and Zhu, J.K. (2001). The Arabidopsis HOS1 gene negatively regulates cold signal transduction and encodes a RING finger protein that displays cold-regulated nucleo-cytoplasmic partitioning. Genes Dev. 15, 912–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Q., Kasuga, M., Sakuma, Y., Abe, H., Miura, S., Yamaguchi-Shinozaki, K., and Shinozaki, K. (1998). Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 10, 1391–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente, F., Lopez-Cobollo, R.M., Catala, R., Martinez-Zapater, J.M., and Salinas, J. (2002). A novel cold-inducible gene from Arabidopsis, RCI3, encodes a peroxidase that constitutes a component for stress tolerance. Plant J. 32, 13–24. [DOI] [PubMed] [Google Scholar]
- Logemann, J., Schell, J., and Willmitzer, L. (1987). Improved method for the isolation of RNA from plant tissues. Anal. Biochem. 163, 16–20. [DOI] [PubMed] [Google Scholar]
- Martin, M.L., and Busconi, L. (2001). A rice membrane-bound calcium-dependent protein kinase is activated in response to low temperature. Plant Physiol. 125, 1442–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty, F. (1999). Plant vacuoles. Plant Cell 11, 587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maser, P., et al. (2001). Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol. 126, 1646–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinsh, M.R., and Hetherington, A.M. (1998). Encoding specificity in Ca2+ signaling systems. Trends Plant Sci. 3, 32–36. [Google Scholar]
- Medina, J., Bargues, M., Terol, J., Perez-Alonso, M., and Salinas, J. (1999). The Arabidopsis CBF gene family is composed of three genes encoding AP2 domain-containing proteins whose expression is regulated by low temperature but not by abscisic acid or dehydration. Plant Physiol. 119, 463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige, Y., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15, 473–497. [Google Scholar]
- Nordin, K., Vahala, T., and Palva, E.T. (1993). Differential expression of two related, low-temperature-induced genes in Arabidopsis thaliana (L.) Heynh. Plant Mol. Biol. 21, 641–653. [DOI] [PubMed] [Google Scholar]
- Piñeros, M., and Tester, M. (1997). Characterization of the high-affinity verapamil binding site in a plant plasma membrane Ca2+-selective channel. J. Membr. Biol. 157, 139–145. [DOI] [PubMed] [Google Scholar]
- Pittman, J.K., Sreevidya, C.S., Shigaki, T., Ueoka-Nakanishi, H., and Hirschi, K.D. (2002). Distinct N-terminal regulatory domains of Ca2+/H+ antiporters. Plant Physiol. 130, 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas, J. (2002). Molecular mechanisms of signal transduction in cold acclimation. In Plant Signal Transduction, D. Scheel and C. Wasternack, eds (Oxford, UK: Oxford University Press), pp. 116–139.
- Sambrook, J., Fritsch, E.F., and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Sanders, D., Brownlee, C., and Harper, J.F. (1999). Communicating with calcium. Plant Cell 11, 691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders, D., Pelloux, J., Brownlee, C., and Harper, J.F. (2002). Calcium at the crossroads of signaling. Plant Cell 14 (suppl.), S401.–S417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumaker, K.S., and Sze, H. (1986). Calcium transport into the vacuole of oat roots: Characterization of H+/Ca2+ exchange activity. J. Biol. Chem. 261, 12172–12178. [PubMed] [Google Scholar]
- Shacklock, P.S., Read, N.D., and Trewavas, A.J. (1992). Cytosolic free calcium mediates red light induced photomorphogenesis. Nature 358, 153–155. [Google Scholar]
- Shigaki, T., Cheng, N.H., Pittman, J.K., and Hirschi, K. (2001). Structural determinants of Ca2+ transport in the Arabidopsis H+/Ca2+ antiporter CAX1. J. Biol. Chem. 276, 43152–43159. [DOI] [PubMed] [Google Scholar]
- Strizhov, N., Abraham, E., Okresz, L., Blickling, S., Zilberstein, A., Schell, J., Koncz, C., and Szabados, L. (1997). Differential expression of two P5CS genes controlling proline accumulation during salt-stress requires ABA and is regulated by ABA1, ABI1 and AXR2 in Arabidopsis. Plant J. 12, 557–569. [DOI] [PubMed] [Google Scholar]
- Sze, H., Liang, F., Hwang, I., Curran, A.C., and Harper, J.F. (2000). Diversity and regulation of plant Ca2+ pumps: Insights from expression in yeast. Annu. Rev. Plant Physiol. Plant Mol. Biol. 51, 433–462. [DOI] [PubMed] [Google Scholar]
- Tähtiharju, S., and Palva, T. (2001). Antisense inhibition of protein phosphatase 2C accelerates cold acclimation in Arabidopsis thaliana. Plant J. 26, 461–470. [DOI] [PubMed] [Google Scholar]
- Tähtiharju, S., Sangwan, V., Monroy, A.F., Dhindsa, R.S., and Borg, M. (1997). The induction of kin genes in cold-acclimating Arabidopsis thaliana: Evidence of a role for calcium. Planta 203, 442–447. [DOI] [PubMed] [Google Scholar]
- Thomashow, M.F. (1999). Plant cold acclimation: Freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 571–599. [DOI] [PubMed] [Google Scholar]
- Townley, H.E., and Knight, M.R. (2002). Calmodulin as a potential negative regulator of Arabidopsis COP gene expression. Plant Physiol. 128, 1169–1172. [DOI] [PubMed] [Google Scholar]
- Tremousaygue, D., Laudie, M., Grellet, F., and Delseny, M. (1992). The Brassica oleracea rDNA spacer revisited. Plant Mol. Biol. 18, 1013–1018. [DOI] [PubMed] [Google Scholar]
- Ueoka-Nakanishi, H., Nakanishi, Y., Tanaka, Y., and Maeshima, M. (1999). Properties and molecular cloning of Ca2+/H+ antiporter in the vacuolar membrane of mung bean. Eur. J. Biochem. 262, 417–425. [DOI] [PubMed] [Google Scholar]
- Wang, R., Guegler, K., LaBrie, S.T., and Crawford, N.M. (2000). Genomic analysis of a nutrient response in Arabidopsis reveals diverse expression patterns and novel metabolic and potential regulatory genes induced by nitrate. Plant Cell 12, 1491–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, P.J. (1998). Calcium channels in the plasma membrane of root cells. Ann Bot. 81, 173–183. [Google Scholar]