Abstract
Tumor suppressor gene PTEN is important in the initiation and progression of human prostate carcinoma, whereas the role of TP53 remains controversial. Since Pten/Trp53 double conditional knockout mice show earlier onset and fast progression of prostate cancer when compared to Pten knockout mice, we asked whether heterozygosity of these two tumor suppressor genes was sufficient to accelerate prostatic tumorigenesis. To answer this question we examined prostatic lesion progression of Pten/Trp53 double heterozygous mice and a series of controls such as Pten heterozygous, Pten conditional knockout, Trp53 heterozygous and Trp53 knockout mice. Tissue recombination of adult prostatic epithelium coupled with embryonic rat seminal vesicle mesenchyme was used as a tool to stimulate prostatic epithelial proliferation. In our study, high-grade prostatic intraepithelial neoplasia (PIN) was found with high frequency at 8 weeks post-tissue recombination transplantation. PIN lesions in Pten/Trp53 double heterozygous mice were more severe than those seen in Pten heterozygous alone. Furthermore, morphologic features attributable to Pten or Trp53 loss appeared to be enhanced in double heterozygous tissues. LOH analysis of Pten and Trp53 in genomic DNA collected from high-grade PIN lesions in Pten heterozygous and Pten/Trp53 double heterozygous mice showed an intact wild-type allele for both genes in all samples examined. In conclusion, simultaneous heterozygosity of Pten and Trp53 accelerates prostatic tumorigenesis in this mouse model of prostate cancer independently of loss of heterozygosity of either gene.
Keywords: Prostate cancer, Tumor suppressor genes, Pten, Trp53, Mouse models, Pathology, AKT, Tissue recombinants, Embryonic mesenchyme
1. Introduction
Tumor suppressor genes are extremely important in the initiation and progression of prostate cancer (Bookstein, 1994). Among them, PTEN and TP53 are of specific interest due to the growing evidence of their role, individually and in combination, in the pathogenesis of prostate carcinoma.
PTEN encodes a dual specific phosphatase that counteracts many important cellular growth and survival pathways. One of the main substrates of this membrane phosphatase is the enzyme phosphatidyl-inositol kinase 3 (PIP3), which activates the AKT pathway and results in increased cell survival. PTEN also directly and indirectly regulates cell motility, migration and differentiation, as well as the cell cycle via interactions with focal adhesion kinase (FAK), mitogen activated protein kinases (MAPK) and cyclin D, respectively (Di Cristofano et al., 1998; Li and Sun, 1998; Mamillapalli et al., 2001; Radu et al., 2003; Sun et al., 1999; Yuan et al., 2000; Zheng et al., 2003).
In humans, loss of PTEN was identified in many primary and metastatic prostate carcinomas, as well as in prostate cancer cell lines (Cairns et al., 1997; Li et al., 1997; Suzuki et al., 1998;Wang et al., 1998). Recapitulating the human disease, Pten heterozygous mice as well as prostate conditional Pten knockout mice develop hyperplasia, PIN and invasive carcinomas with potential to metastasize (Di Cristofano et al., 1998, 2001; Kwabi-Addo et al., 2001; Podsypanina et al., 1999; Stambolic et al., 2000; Trotman et al., 2003; Wang et al., 2003).
The important tumor suppressor gene TP53 induces apoptosis in the face of cellular damage posed by intrinsic or extrinsic stressors, such as hypoxia and DNA damage. Contradictions as to the direct role of TP53 in prostate cancer abound in the literature, but TP53 mutations appear to be more common in high-grade, metastatic and androgen-independent human prostate cancer (Bookstein et al., 1993; Chi et al., 1994; Costa-Pereira and Cotter, 1999; Gumerlock et al., 1997; Meyers et al., 1998).
Aside from the tumor suppressor functions of each individual gene, numerous interactions between the gene products in vivo have been reported: PTEN regulates the transcriptional activity, DNA-binding avidity and MDM-2-mediated degradation of TP53 (Di Cristofano et al., 2001; Freeman et al., 2003; Mayo et al., 2002; Sheng et al., 2002; Stambolic et al., 2001). PTEN also mediates the inhibition of AP-1 and NF-kappa B by the tumor suppressor protein TP53 (Wang et al., 2005). In addition, TP53 regulates mTOR (target of rapamycin), an important downstream kinase in the PIP3/AKT pathway, inhibited by PTEN (Feng et al., 2005).
In vivo, lesion progression in Pten/Trp53 double prostate conditional knockout mice indicates significant cooperation between Pten and Trp53 (Chen et al., 2005). Double conditional knockout mice develop prostate cancer by 2 weeks post-puberty, with disease progression and death by 7 months of age. This is in contrast to the longer latency of prostate cancer development in Pten conditional knockout mice, and to the fact that the conditional Trp53 knockout mouse fails to develop prostatic lesions.
In light of these data, the hypothesis was that simultaneous haploinsufficiency of both PTEN and TP53 was enough to accelerate prostatic tumorigenesis. Because the latency for onset of prostate lesions in Pten/Trp53 double heterozygous mice is prohibitively long, this question was addressed by comparing prostate lesion progression in tissue recombinants made with rat seminal vesicle mesenchyme (rSVM) and mouse prostatic epithelium (mPRE) of different genetic make-ups. Our results show that simultaneous heterozygosity of Pten and Trp53 accelerates prostate tumorigenesis. In this model, the combined effect of these two genes in early lesion progression cannot be explained by LOH events since all PIN lesions examined retained their wild-type alleles for both Pten and Trp53. Finally, we discuss morphologic hallmarks of the dysfunction of each of the two genes, providing a basis for interpretation of prostatic lesions in mouse models with alterations in Pten, Trp53 or both.
2. Material and methods
2.1. Animal tissues
All prostate glands were obtained from post-pubertal mice (around 11 weeks of age) of the following genotypes: Pten+/− on a C57BL/6J background (Banach-Petrosky et al., 2006; Gao et al., 2006; Podsypanina et al., 1999), Pten prostate conditional knockout mice on a C57BL/6xDBA2-129/Balb/c background (Wang et al., 2003) and Trp53+/− and Trp53−/− on a 129 SvJ background (Mao et al., 2003). Prostatic epithelium from wild-type (WT) post-pubertal male mice was used in control tissue recombinants. Pilot studies revealed that tissue recombinants made of rSVM and Trp53+/− mouse prostatic epithelium (mPRE) were indistinguishable from WT tissue recombinants (SSC, personal communication). Thus, this group was removed from subsequent experiments.
2.2. Tissue dissections
Mice were sacrificed by CO2 asphyxiation followed by cervical dislocation. Prostate glands were removed and placed in Dulbecco’s modified Eagle’s medium (DMEM). Ducts of the anterior prostate were microdissected for the grafting experiments as described previously (Sugimura et al., 1986). Paired samples of adjacent pre-graft tissue were examined histologically and verified to be normal.
2.3. Grafting and tissue recombination
Early lesions in each genetic background were examined in tissue recombinants composed of rat seminal vesicle mesenchyme (rSVM) plus ductal segments from Pten+/−, Pten−/ −, Trp53−/ − and Pten/Trp53 double heterozygous prostates. To obtain rSVM from newborn Sprague–Dawley rat pups (Simonsen Laboratories, Inc., Gilroy, CA), seminal vesicle epitheliumwas removed with fine forceps from dissected seminal vesicle rudiments after a brief trypsin digestion. The tissue recombinants were prepared by combining rSVM with a 300–500 µm segment of mouse prostate duct (mouse prostate epithelium (mPRE)) to yield rSVM+mPRE recombinants, cultured on nutrient agar medium overnight and then grafted under the kidney capsule of 45–60-day-old, intact male nude mice (Charles River, Wilmington, MA) as described previously (Cunha and Donjacour, 1987; Donjacour and Cunha, 1995; Wang et al., 2000). The rationale for using rSVM as a prostate inductor has been previously discussed in detail (Risbridger et al., 2001). Tissue recombinant grafts were allowed to grow in vivo for 8 weeks before being harvested.
The profound proliferation elicited in adult mouse prostatic epithelium by mesenchymal prostatic inductors (such as rSVM) can be further stimulated by serial transplantation of prostatic ducts combined with fresh mesenchyme (Cunha, unpublished). Therefore, second- and third-generation tissue recombinants of Pten/Trp53 double heterozygous prostatic tissue were produced as follows: a duct was dissected from a tissue recombinant graft harvested after 8 weeks of growth and then combined with fresh rSVM. This second-generation tissue recombinant was grown on nutrient agar medium overnight and grafted under the kidney capsule of a new set of intact male nude mice. Resulting second-generation grafts were harvested after 8 weeks. Third-generation tissue recombinants were produced similarly with a fragment of prostatic duct from second-generation Pten/Trp53 double heterozygous grafts.
2.4. Histology and immunohistochemistry
All tissues were fixed in 10% neutral buffered formalin for 12 h at room temperature and then transferred to 70% ethanol until processing. For paraffin sections, tissues were dehydrated in graded ethanol and Histoclear (National Diagnostics, Atlanta, GA) and embedded. Tissues were cut 6 µm in thickness, deparaffinized, rehydrated, stained with hematoxylin and eosin, or used for immunohistochemistry.
For immunohistochemistry, antigens were unmasked by boiling sections in citrate buffer (Antigen Unmasking solution, Vector Laboratories, Burlingame, CA), pH 6, for 30 min in a microwave oven. Blocking solution (Superblock, Pierce, Rockford, IL) was applied to sections followed by the primary antibody (anti-androgen receptor, 1:50, Affinity Bioreagents, Golden, CO; anti-p63, 1:100, Santa Cruz Biotechnology, Santa Cruz, CA (#SC8431); anti-Ki67, 1:200, Novocastra Laboratories, Newcastle, UK; anti-E-cadherin, 1:200, BD Bioscience, San Jose, CA; anti-phospho-AKT (Ser473), 1:100, Cell signaling Technology, MA). Antibody binding was detected using secondary anti-rabbit or anti-mouse IgG antibodies conjugated to biotin (Amersham, Piscataway, NJ) and the ABC peroxidase method with 3′,3′-diaminobenzidine (DAB) color reagent (Vector Laboratories).
2.5. Lesion classification
Prostatic lesions of Pten heterozygous and Pten conditional knockout mice have been described previously (Podsypanina et al., 1999;Wang et al., 2003). Definition and detailed description of prostatic lesions in genetically engineered mice, including benign hyperplasias, mouse prostatic intraepithelial neoplasia (PIN) and adenocarcinoma, have been published (Park et al., 2002; Shappell et al., 2004). Briefly, PIN I has 1 or 2 layers of atypical cells; PIN II has 3 or more layers of atypical cells; PIN III normally occupies the entire glandular lumen; and PIN IV fills and expands the glandular lumen, distorting the glandular profile and often bulging into the surrounding stroma. Although PIN IV lesions can be large and show cellular pleomorphism, they can be differentiated from adenocarcinomas due to the lack of stromal invasion. Nuclear atypia, defined by variation of nuclear size, shape or both, as well as the presence of abnormal chromatin patterns (chromatin clumping, excessive or abnormal nucleoli), was also graded in all samples. Grading was performed by evaluating a total of 3 slides taken at different depths in the block (one superficial and one deeper in the block) containing 2 repeat sections (6 µm). The absolute range of lesions was determined by noting whether each type of lesion or feature was present or not in each sample. Area occupied by each type of lesion or number of lesions per sample was not evaluated.
2.6. Laser microdissection of lesions and LOH analysis of Pten and Trp53
For laser microdissection, 10 µm paraffin sections were cut and placed on uncharged glass slides. Tissues were deparaffinized with xylene and stored in a dry place until use. An Arcturus PixCell II laser capture microdissection system (Arcturus Engineering, Inc., Mountain View, CA) built into an Olympus IMT 2 inverted microscope stand was used to collect epithelium from high-grade PIN lesions (PIN III and IV) from Pten heterozygous and from first- and third-generation Pten/Trp53 double heterozygous tissue recombinants.
DNA from all samples was extracted using the HotSHOT (sodium hydroxide and Tris) solution as described previously (Truett et al., 2000). LOH analyses of Pten and Trp53 were performed as described previously (Freeman et al., 2003; Ma et al., 2005). For Pten LOH 45 PCR cycles (94 °C—3 min, 94 °C—15 s, 64 °C—30 s, 72 °C—90 s, 72 °C—7 min, 4 °C) were run using the following primers: TTG CAC AGT ATC CTT TTG AAG (WT allele—240 bp), GTC TCT GGT CCT TAC TTC C (null allele—320 bp) and ACG AGA CTA GTG AGA CGT GC (common). For Trp53 LOH 35 PCR cycles (94 °C—3 min, 94 °C—15 s, 68 °C—90 s and 72 °C—7 min, 4 °C) were run using the following primers: ATA GGT CGG CGG TTC AT (WT allele, 550 bp), CCC GAG TAG CTG GAA GAC AG (null allele, 650 bp) and CCT CGT GCT TTA CGG TAT CGC (common). PCRs were run in duplicate and using low (DNA eluted in 150 µl of buffer) and high (DNA evaporated and resuspended in 6µl of buffer) concentrations of template. Normal tissues from wild-type, Pten+/−, Pten−/−, Trp53+/− and Trp53−/− mice were used as controls.
2.7. Statistical analysis of results
The proportions of different lesion types were compared among groups using the chi-square test. The Fisher’s exact test was used when expected counts in contingency tables were less than 5. The level of significance used in all analyses was 0.05.
3. Results
3.1. Lesional incidence among experimental groups
In this study, all tissues evaluated were obtained from rSVM+mPRE tissue recombinants constructed with prostatic epithelium from mice of different genotypes. For simplicity, we will, hereafter, use the genotype of the epithelium to refer to each tissue recombinant (i.e. rSVM+ Pten+/− mPRE will be referred to as Pten+/−).
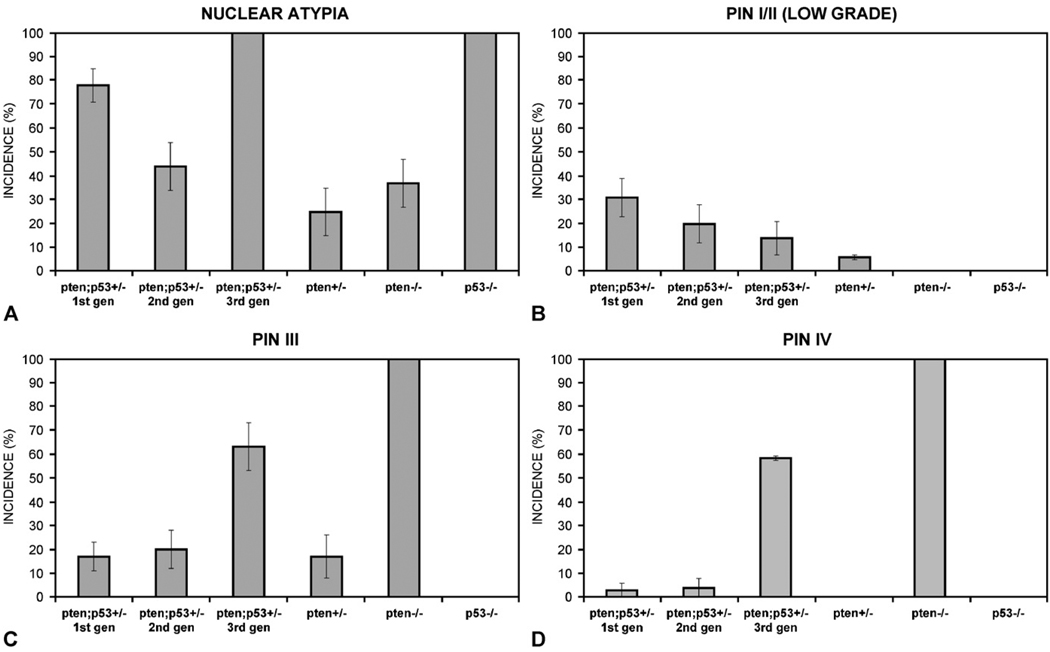
The incidence of benign hyperplasias and preneoplastic lesions (PIN) varied among the experimental groups (Table 1), with a much higher incidence of PIN in Pten/Trp53 double heterozygous tissues when compared to Pten+/−. PIN severity also varied according to the genotype of the prostatic epithelium used in tissue recombinants (Fig. 1A). Because PIN I was rare in this cohort, we combined PIN I and II (low-grade PIN) for the purpose of analysis. Invasive adenocarcinomas were not seen in our cohort. In addition to grading proliferative lesions, we also recorded the degree of nuclear atypia in all samples (Table 2). Nuclear atypia was the only significant change seen in Trp53−/− prostatic epithelium in our tissue recombinants.
Table 1.
Proportion between benign prostatic hyperplasia (BPH) and prostatic intraepithelial neoplasia (PIN) in rSVM+mPRE tissue recombinants harvested 8 weeks post-grafting.
| Prostate tissue recombinant grafts—mPRE genotypes | Sample size (n) | BPH (%) | PIN (%) |
|---|---|---|---|
| Pten+/−Trp53 +/−1st generation | 35 | 69 | 34 |
| Pten+/−Trp53 +/−2nd generation | 25 | 20 | 32 |
| Pten+/−Trp53 +/−3rd generation | 22 | 18 | 77 |
| Pten+/− | 18 | 22 | 17 |
| Pten−/− | 20 | 0 | 100 |
| Trp53−/− | 19 | 58 | 0 |
Fig. 1.
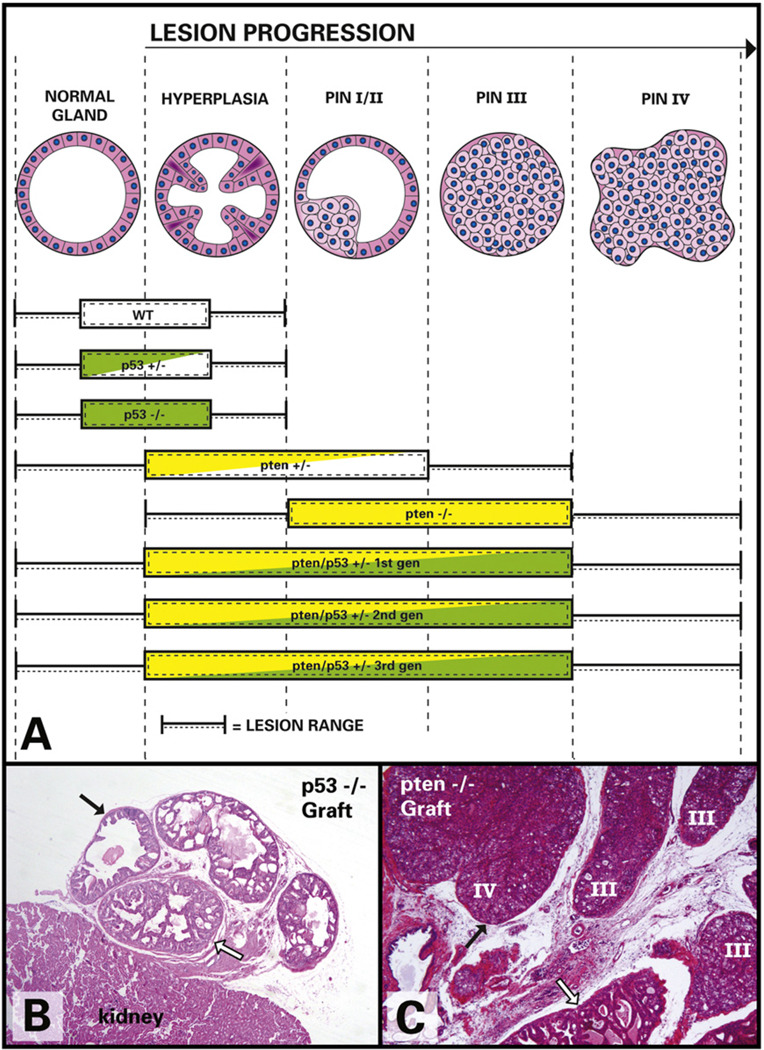
(A) Schematic illustration of lesion progression from normal gland to PIN IV (left to right) in all rSVM+mPRE tissue recombinants. Note that PIN lesions in Pten/Trp53double heterozygous tissues are more severe than in Pten+/−, but milder than in Pten−/− tissues. (B) Trp53−/− tissue recombinant graft under the kidney capsule. Note normal glands (black arrows) and mild hyperplasia (white arrow) but no PIN. (C) Pten−/− tissue recombinant show high-grade PIN (III and IV), as well as hyperplasia (white arrow). Note the distortion and bulging of glandular outline in PIN IV (black arrow).
Table 2.
Degree of severity of nuclear atypia varying from none to severe in rSVM+mPRE tissue recombinants harvested 8 weeks post-graft.
| Prostate tissue grafts—mPRE genotypes | Nuclear atypia (%) | |||
|---|---|---|---|---|
| None | Mild | Moderate | Severe | |
| Pten+/−Trp53+/−1st generation | 26 | 57 | 17 | 0 |
| Pten+/−Trp53+/− 2nd generation | 56 | 36 | 8 | 0 |
| Pten+/−Trp53+/− 3rd generation | 0 | 9 | 27 | 64 |
| Trp53−/− | 0 | 0 | 16 | 84 |
| Pten−/− | 55 | 45 | 0 | 0 |
| Pten+/− | 89 | 11 | 0 | 0 |
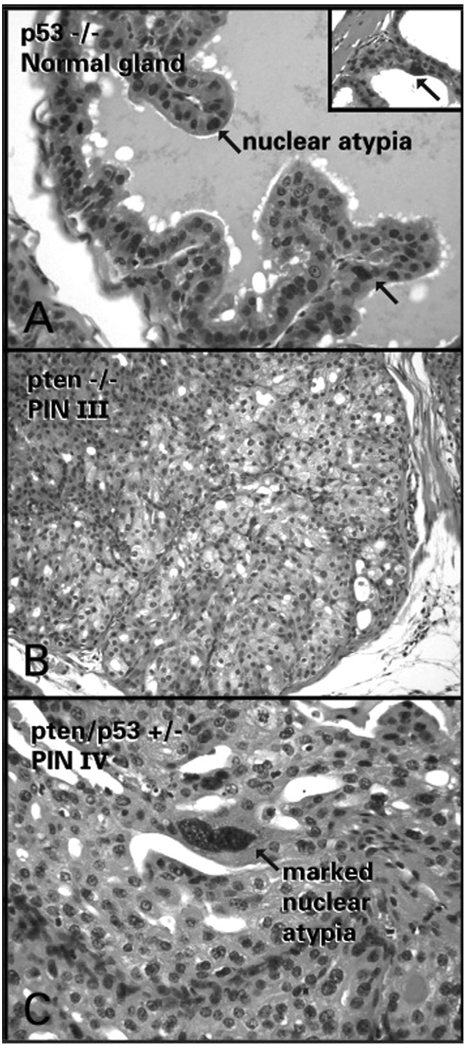
Prostatic tissues from Trp53−/−_ mice showed mild hyperplasia and no evidence of PIN (Fig. 1B). On the other hand, 100% of the tissues in this group had moderate to severe nuclear atypia (Fig. 2A), which was the only feature that differentiated these Trp53−/− tissue recombinants from those made with wild-type epithelium.
Fig. 2.
(A) Trp53−/− tissues recombinant showing normal prostate gland with moderate nuclear atypia (arrows) and severe nuclear atypia (inset). (B) PIN III in Pten−/− tissue recombinant showing large vacuolated cells with abundant cytoplasm characteristic of Pten disruption. (C) PIN IV in third-generation Pten/Trp53 double heterozygous tissues showing severe nuclear atypia characteristic of Trp53 disruption.
All of the Pten−/− tissue recombinants had areas of PIN III and IV (Fig. 1C). In this group, epithelial cells in PIN lesions tended to be large and have abundant, finely vacuolated cytoplasm (Fig. 2B). Additionally, there was mild nuclear atypia seen in the epithelium of only 45% of the Pten−/− tissue recombinants (Table 2). The difference in incidence and severity of nuclear atypia between the Pten−/− and Trp53−/− groups was highly significant (p < 0.0001).
In contrast to the Pten−/− prostatic tissues, the incidence of PIN in Pten+/− prostatic tissues was relatively low (17%, Table 1), and PIN IV was not seen. Cells with cytoplasmic vacuolation as described for Pten−/− epithelium were also found in PIN lesions in the Pten+/− group. Nuclear atypia was mild and infrequent in Pten+/− prostatic epithelium.
3.2. Multi-generation Pten/Trp53 double heterozygous tissue recombinants
The first generation of Pten/Trp53 double heterozygous tissue recombinants had a majority of benign hyperplasias (69%, Table 1) and low-grade PIN, with less frequent PIN III and rare PIN IV (Fig. 3). Overall, the lesions in this group were slightly more severe than those seen in Pten+/− tissue recombinants (p = 0.04) and milder than those seen in Pten−/ − tissue recombinants (p = 0.004). Interestingly, the incidence of nuclear atypia (Fig. 3D) in the Pten/Trp53 double heterozygous tissue recombinants of the first generation was 74% in comparison to only 11% seen in tissue recombinants made with Pten+/− mPRE (p < 0.0001).
Fig. 3.
Histograms showing the incidence of PIN lesions and nuclear atypia in all rSVM+mPRE tissue recombinants.
The second generation of Pten/Trp53 double heterozygous prostatic tissue recombinants was prepared by combining a small epithelial fragment derived from a first-generation tissue recombinant with fresh rSVM. Tissues were harvested after 8 additional weeks of growth. In spite of the resulting 9–12 additional population doublings, lesions in this second generation of tissue recombinants were unexpectedly mild. The incidences of PIN III and IV in this group were slightly higher than those of the first generation of Pten/Trp53 double heterozygous tissue recombinants (Fig. 3), but these trends were not statistically significant (p > 40.4). The proportion of nuclear atypia in the second-generation Pten/Trp53 double heterozygous tissue recombinants was, however, significantly higher than that seen in Pten+/− tissue recombinants (p = 0.02).
The third-generation Pten/Trp53 double heterozygous tissue recombinants (prepared with epithelium from the second-generation grafts) had high incidences of both PIN III (64%) and PIN IV (59%). PIN lesions were significantly more severe in this group than in the Pten+/− group (p < 0.0001), and more severe than in the first-generation Pten/Trp53 double heterozygous tissue recombinants (p = 0.0003). Nonetheless, PIN lesions were still less severe in third-generation Pten/Trp53+/− tissue recombinants than in Pten−/− tissue recombinants (p < 0.0001). Profound nuclear atypia was seen in 100% of the PIN III and IV lesions in the third generation of Pten/Trp53 double heterozygous tissue recombinants (Fig. 2C).
Overall, lesions tended to progress and become more severe as tissues were serially transplanted in second- and third-generation tissue recombinants. In addition, the simultaneous heterozygosity of Pten and Trp53 in the serially transplanted recombinants seemed to amplify morphologic features attributable to each, namely nuclear atypia (Trp53 loss) and the presence of large vacuolated cells in PIN (Pten loss).
3.3. Immunohistochemistry
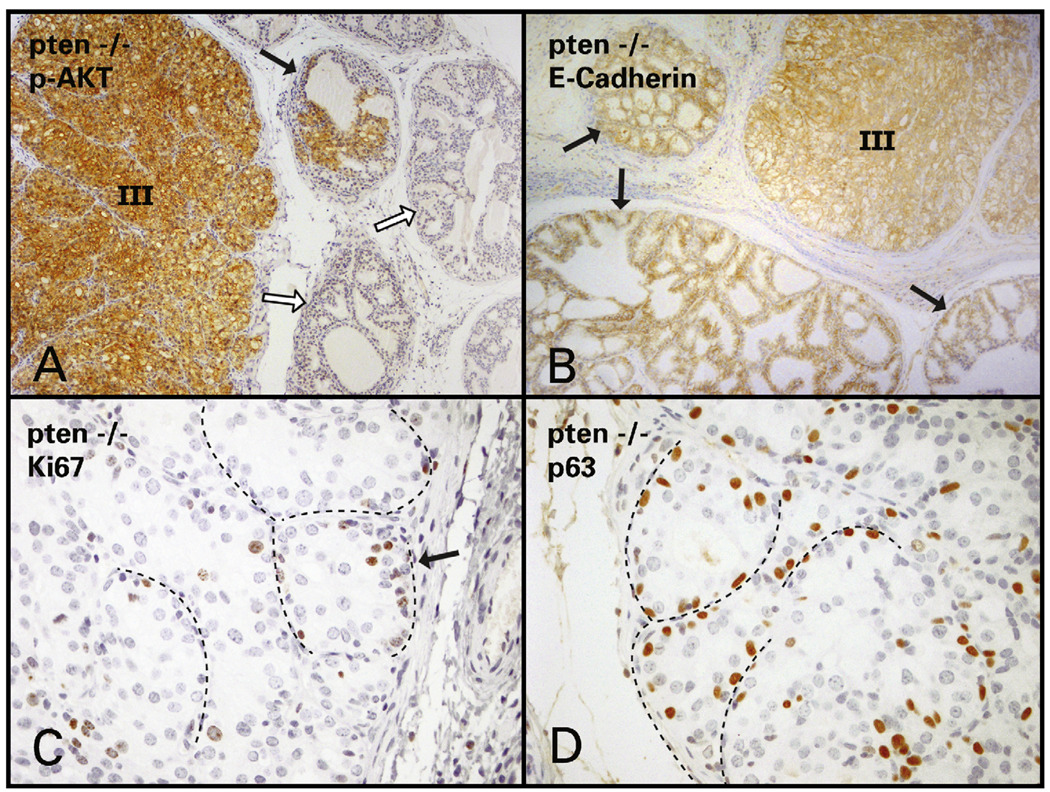
Immunohistochemical staining patterns were identical in PIN lesions in tissue recombinants made with Pten−/−, Pten+/− and Pten/Trp53 double heterozygous mPRE. PIN in these groups stained brightly for phospho-AKT, indicating the activation of the PI3K pathway (Fig. 4A). In our experimental groups E-cadherin expression was maintained by luminal epithelial cells in PIN, regardless of grade (Fig. 4B). Basal cell expression of E-cadherin was weaker or absent (data not shown). The marker Ki67 showed that proliferation was mostly confined to the basal region of the glands (Fig. 4C), which was the same area in which p63-positive cells were concentrated (Fig. 4D). Cells expressing p63 were rare towards the center or luminal aspects of PIN lesions in all groups. The majority of luminal and stromal cells and a smaller proportion of basal cells were positive for androgen receptor (AR) in PIN lesions (not illustrated).
Fig. 4.
(A) High- (III) and low-grade PIN (black arrow) stain brightly positive for phospho-AKT in Pten−/− tissue recombinant. Hyperplastic and normal gland (white arrows) do not express p-AKT. (B) PIN (III) as well as mildly hyperplastic glands (black arrows) stain brightly positive for E-cadherin. (C) In PIN III and IV, proliferating cells positive for Ki67 are concentrated at a basal location (arrow points to dotted line showing thin stromal projections). (D) p63-positive cells are also found more frequently at a basal location, where proliferating cells predominate (dotted line represents thin stromal projections).
Benign hyperplasias in Trp53−/−, Trp53+/−, wild-type and Pten+/− tissue recombinants showed immunohistochemical results identical to normal glands and were negative for phospho-AKT and positive for epithelial membrane E-cadherin and nuclear AR (both epithelium and stroma). Proliferating cells, as shown by Ki67 positivity, were rare in normal glands and randomly distributed within basal and luminal aspects of hyperplastic glandular epithelium. The antibody p63 stained a discontinuous layer of basal cells in both normal and hyperplastic glands.
3.4. LOH of Pten and Trp53 in tissue recombinants
LOH analysis of genomic DNA extracted into 150 µl of buffer from PIN III and IV lesions (high-grade PIN) from first- and third-generation Pten/Trp53 tissue recombinants revealed that 4/4 lesions in first-generation and 6/6 lesions in third-generation Pten/Trp53 double heterozygous tissues had lost their wild-type Pten allele. In contrast, all 10 lesions examined retained their Trp53 wild-type allele. Under the same conditions, LOH analysis of Pten in DNA extracted from high-grade PIN from Pten+/− tissue recombinants showed loss of the wild-type allele in 2/4 samples. However, after the samples were concentrated (150 µl to 6µl of template) we could detect the wild-type allele of Pten in all samples from Pten+/− and from first- and third-generation Pten/Trp53 double heterozygous samples. LOH analysis of Trp53 in Pten+/− tissues was also performed and showed a retained wild-type allele in all 4 samples of high-grade PIN tested.
4. Discussion
The role of the PTEN tumor suppressor gene in prostate cancer has been well documented in human prostate tumors and cell lines, as well as in several mutant mice ranging from Pten hypomorphs to Pten heterozygous and prostate conditional knockouts (Di Cristofano et al., 1998, 2001; Kwabi-Addo et al., 2001; Podsypanina et al., 1999; Stambolic et al., 2000; Trotman et al., 2003; Wang et al., 2003). Although the individual role of Trp53 in prostate cancer has been controversial, the targeted deletion of both alleles of Trp53 and Pten in the prostate was shown to result in faster prostate cancer progression (Chen et al., 2005). We showed in the present study that combined heterozygosity of Pten and Trp53 can also accelerate PIN development.
These data are in agreement with an increasing body of literature pointing towards the functional cooperation between Pten and Trp53 in mouse models of lymphoma and skin cancer (Komazawa et al., 2004; Mao et al., 2003; Freeman et al., 2003). Additionally, simultaneous LOH of PTEN and TP53 mutations were identified in a large number of human gastric tumors (Oki et al., 2005). However, simultaneous loss of both alleles of PTEN and TP53 is a rare occurrence in human cancers (Trotman and Pandolfi, 2003), suggesting that the role of these two genes in human disease may be more closely modeled in the context of heterozygosity.
Interestingly, our analysis revealed morphologic distinctions between lesions caused by Pten or Trp53 disruption. In the absence or decreased function of Pten the AKT pathway is activated, leading to a series of anabolic, growth-promoting and life-maintaining events. A morphologic consequence of this pathway is that affected epithelial cells become larger and slightly vacuolated. This appearance is characteristic of Pten disruption in many tissues and is commonly noted in metabolically active cells, such as hepatocytes and renal tubular cells of Pten+/− mice in vivo, as well as in hyperplasias or neoplasias originating in many organs of these mice, such as the thyroids glands, uterus and gallbladder. Additionally, similar cytomorphologic features can be seen in the prostate of AKT transgenic mice, which display low-grade PIN with large and vacuolated epithelial cells. This morphologic hallmark of Pten inactivation (AKT pathway) was noted in advanced PIN lesions in tissue recombinants prepared with Pten−/ −, Pten+/− and Pten/Trp53 double heterozygous prostatic epithelium.
On the other hand, prominent nuclear atypia was associated with tissues lacking Trp53 function and may reflect polyploidy and/or genetic instability resulting from Trp53 inactivation. This hallmark of Trp53 disruption was seen in tissue recombinants prepared with prostatic epithelium derived from Trp53−/− and from Pten/Trp53 double heterozygous mice, and became more severe as the double heterozygous tissues were serially transplanted. These “gene- or pathway-specific” morphologic features allow the investigator to “visualize” the genetic make-up of the lesion to a certain extent and may provide insight into the pathogenesis of prostate cancer. For example, the vacuolated epithelium in PIN lesions of Pten−/− and Pten+/− and Pten/Trp53 double heterozygous prostatic tissues indicated activation of the AKT pathway and this was confirmed by positive phospho-AKT immunohistochemistry stain in these samples.
Since LOH of Pten has been shown to play a role in prostate lesion progression by many groups (Banach-Petrosky et al., 2006; Kim et al., 2002; Kwabi-Addo et al., 2001; Ma et al., 2005; Trotman et al., 2003), we hypothesized that earlier or more frequent loss of the wild-type allele of Pten in Pten/Trp53 double heterozygous mice would help explain the increased PIN severity in this group. However, LOH analysis in our study showed retention of the wild-type allele of Pten in all high-grade PIN lesions examined. Interestingly, when PCR reactions were done with lower concentrations of template the wild-type allele of Pten could not be amplified in 50% of high-grade PIN lesions from the Pten+/− group and in 100% of the first- and third-generation Pten/Trp53 double heterozygous samples examined. These results suggest that accuracy of LOH analysis via PCR reaction is variable and seems to correlate with initial template concentration, which represents a problem in the study of laser microdissected samples. On the other hand, the apparent flaw of the first PCR experiment may suggest that there is a lower concentration of wild-type Pten in double heterozygous lesions when compared to Pten+/− lesions. Unfortunately, we could not evaluate the exact dosage of Pten in this experiment due to the small quantity of DNA obtained and relatively poor quality of RNA generated from laser microdissected PIN lesions.
The increasing incidence and severity of nuclear atypia in third-generation Pten/Trp53 double heterozygous mice mimicking Trp53−/− samples suggested that the loss of the remaining wild-type Trp53 allele might have taken place in the third-generation tissue recombinants. However, all PIN lesions in the first- and third-generation Pten/Trp53 tissues maintained a wild-type Trp53 allele, as detected by PCR analysis. An important consideration when interpreting these data is that LOH may be restricted to small groups of cells within larger lesions and may therefore be missed in the PCR analysis. This is likely since morphologically the appearance of cells within large PIN lesions was heterogeneous, and nuclear atypia was more severe in small and quite well-demarcated foci. Unfortunately, LOH analysis of such foci of increased nuclear atypia within PIN proved unrewarding due to the minute amount of DNA obtained from laser microdissected material from such small areas. In conclusion, one of the possible explanations for the lack of correlation between LOH of Pten or Trp53 with PIN progression in this study is the low sensitivity of PCR-based LOH analysis.
Conversely, the retention of the wild-type alleles of both tumor suppressor genes in our model system suggests alternative downstream pathways of cooperation between Pten and Trp53 at the pre-neoplastic phase of prostate carcinogenesis. This interaction may be at the protein level as is the case in lymphoma development in Pten/Trp53 double heterozygous mice (Freeman et al., 2003). Double heterozygous animals develop lymphoma at a similar rate as Trp53−/− mice, and much earlier than either Pten+/− or Trp53+/− control animals in spite of retention of the wild-type alleles and normal mRNA levels of both tumor suppressor genes. Although our results show that LOH does not explain the acceleration of PIN progression in Pten/Trp53 double heterozygous mice, we cannot exclude other mechanisms of loss of function of either or both genes, such as point mutations or promoter methylation, which were not addressed in this study.
A previous study has shown that loss of Rb in the prostatic epithelium results in a Trp53-mediated tumor suppressor response in both epithelial and stromal compartments (Hill et al., 2005). Whereas loss of Trp53 in the epithelium did not result in increased proliferation in this compartment, stromal loss of Trp53 resulted in formation of Phyllodes-like stromal tumors in double mutants. These data suggest that oncogenic stress in the epithelium, via Rb loss, results in negative selective pressure against Trp53 in the stromal compartment and subsequent stromal proliferation. In the present experiments we did not observe stromal proliferation consistent with LOH of Trp53 in the stroma. Furthermore, the use of rat mesenchyme in the tissue recombinant system makes the LOH analysis of the stroma difficult. Further studies utilizing Pten-deficient epithelium combined with Trp53-deficient mouse mesenchyme are granted in order to elucidate the paracrine regulation of these two tumor suppressor genes in the prostate.
It is important to mention that our results differ from those published in a recent study in which simultaneous heterozygosity of Pten and Trp53 was considered to have no effect on lesion progression (Chen et al., 2005). The difference in our results could be explained in part by the fast induction of prostatic epithelial proliferation in rSVM+mPRE tissue recombinants, which facilitates the observation of lesions that may not occur in prostate glands in vivo due to long latency. However, other important factors could have contributed to the different outcomes in both studies, namely the deletion strategies used (Cre-Lox-induced prostate conditional knockout versus regular transgenics), as well as the mixed background strains of the mice evaluated. Furthermore, criteria used to judge lesion severity and progression in Pten/Trp53 double heterozygous mice were not discussed in detail in the study mentioned above, making objective comparison of results difficult.
Background strain can play a significant role in lesion development in genetically engineered mice. For instance, it has been shown that FVB mice are more susceptible to both prostatic and skin neoplasia when compared to C57BL/6 mice, likely due to a defective sonic hedgehog signaling pathway in the C57BL/6 strain (Chiaverotti et al., 2008; Wakabayashi et al., 2007). Our cohort of double heterozygous mice was on a mixed C57BL/6J-129/SvJ background and this may help explain the absence of carcinoma progression in our tissue recombinants.
Also noteworthy is that immunohistochemical analysis revealed that proliferation in high-grade PIN lesions of all experimental groups seems to be concentrated along the p63-rich, basal region of the affected glands, which suggests a subpopulation of precursor stem cells in this location, as was described previously in Pten conditional knockout mice (Wang et al., 2006). Although decreased E-cadherin expression has been associated with prostate cancer progression in humans and in mouse models (Umbas et al., 1992, 1994, 1997; Wang et al., 2000), in our study E-cadherin expression remained unchanged in high-grade PIN lesions from all experimental groups.
Finally, the usefulness of the tissue recombinant technique in the study of slow-progressing prostate lesions in models that are otherwise extremely valuable should be underscored. Models such as the Pten+/− and Pten/Trp53 double heterozygous mice have long latency for lesion onset and slow lesion progression, which are related to the physiological growth-quiescence of the adult murine prostate. In adult mice, rates of prostatic epithelial proliferation and apoptosis are extremely low and in balance. Despite the absence of critical tumor suppressor genes such as Pten or Trp53, carcinogenesis still depends on the rate of cell proliferation, which can fix genetic lesions as they emerge.
By applying the tissue recombinant technique we take advantage of potent mesenchymal inductors, such as the rat seminal vesicle, which stimulate the mouse prostatic epithelium to undergo about 13 population doublings in 4 weeks. This high rate of prostatic epithelial proliferation exceeds the total number of prostatic epithelial population doublings that occur within the life-span of the adult mouse prostate in situ. When a very small fragment of normal murine prostatic duct from a Pten+/− adult mouse is combined with rSVM, high-grade PIN is seen as early as 4 weeks (unpublished data) and found in relatively high frequency at 8 weeks. This means high-grade PIN lesions can be studied as early as 4 months after the mutant mouse is born. In addition, the effects of the stroma in carcinogenesis can also be evaluated through the combination of epithelia and stroma of different genetic make-ups.
In short, we have shown that heterozygosity of Pten and Trp53 is sufficient to accelerate PIN progression independently of LOH of either tumor suppressor gene. Furthermore, we emphasize in this study the advantages of the tissue recombinant technology as a tool to study prostate cancer initiation and progression.
Acknowledgments
This work was supported by the MMHCC grant U01 CA084294 (CA-S) and funds from DOD PC031130 and NCI RO1 CA107166 (HW). We would like to thank Dr. Allan Balmain and Dr. Jian-Hua Mao (Comprehensive Cancer Center, UCSF) for kindly providing the Trp53+/− mice used in this study, and Sunny Yeung (Medical Media, MSKCC) for assistance with digital illustrations. We also thank Dr. Lloyd Trotman (Cold Spring Harbor Laboratory), Dr. Eva Hernando-Monge (NYU Medical Center) and Dr. William Ricke (University of Rochester) for valuable discussion.
References
- Banach-Petrosky W, Ouyang X, Gao H, Nader K, Ji Y, Suh N, DiPaola RS, Abate-Shen C. Vitamin D inhibits the formation of prostatic intraepithelial neoplasia in Nkx3.1;Pten mutant mice. Clin. Cancer Res. 2006;12:5895–5901. doi: 10.1158/1078-0432.CCR-06-1039. [DOI] [PubMed] [Google Scholar]
- Bookstein R. Tumor suppressor genes in prostatic oncogenesis. J. Cell. Biochem. 1994;19:217–223. [PubMed] [Google Scholar]
- Bookstein R, MacGrogan D, Hilsenbeck SG, Sharkey F, Allred DC. p53 is mutated in a subset of advanced-stage prostate cancers. Cancer Res. 1993;53:3369–3373. [PubMed] [Google Scholar]
- Cairns P, Okami K, Halachmi S, Halachmi N, Esteller M, Herman JG, Jen J, Isaacs WB, Bova GS, Sidransky D. Frequent inactivation of PTEN/MMAC1 in primary prostate cancer. Cancer Res. 1997;57:4997–5000. [PubMed] [Google Scholar]
- Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, Koutcher JA, Scher HI, Ludwig T, Gerald W, Cordon-Cardo C, Pandolfi PP. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi SG, deVere White RW, Meyers FJ, Siders DB, Lee F, Gumerlock PH. p53 in prostate cancer: frequent expressed transition mutations. J. Natl. Cancer Inst. 1994;86:926–933. doi: 10.1093/jnci/86.12.926. [DOI] [PubMed] [Google Scholar]
- Chiaverotti T, Couto SS, Donjacour A, Mao JH, Nagase H, Cardiff RD, Cunha GR, Balmain A. Dissociation of epithelial and neuroendocrine carcinoma lineages in the transgenic adenocarcinoma of mouse prostate model of prostate cancer. Am. J. Pathol. 2008;172:236–246. doi: 10.2353/ajpath.2008.070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Pereira AP, Cotter TG. Molecular and cellular biology of prostate cancer—the role of apoptosis as a target for therapy. Prostate Cancer Prostatic Dis. 1999;2:126–139. doi: 10.1038/sj.pcan.4500305. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Donjacour A. Mesenchymal–epithelial interactions: technical considerations. Progr. Clin. Biol. Res. 1987;239:273–282. [PubMed] [Google Scholar]
- Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumour suppression. Nat. Genet. 1998;19:348–355. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- Di Cristofano A, De Acetis M, Koff A, Cordon-Cardo C, Pandolfi PP. Pten and p27KIP1 cooperate in prostate cancer tumor suppression in the mouse. Nat. Genet. 2001;27:222–224. doi: 10.1038/84879. [DOI] [PubMed] [Google Scholar]
- Donjacour AA, Cunha GR. Development (Cambridge, England) Vol. 121. Cambridge, England: Cambridge, England; 1995. Induction of prostatic morphology and secretion in urothelium by seminal vesicle mesenchyme; pp. 2199–2207. [DOI] [PubMed] [Google Scholar]
- Feng Z, Zhang H, Levine AJ, Jin S. The coordinate regulation of the p53 and mTOR pathways in cells. Proc. Natl. Acad. Sci. USA. 2005;102:8204–8209. doi: 10.1073/pnas.0502857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman DJ, Li AG, Wei G, Li HH, Kertesz N, Lesche R, Whale AD, Martinez-Diaz H, Rozengurt N, Cardiff RD, Liu X, Wu H. PTEN tumor suppressor regulates p53 protein levels and activity through phosphatase-dependent and -independent mechanisms. Cancer Cell. 2003;3:117–130. doi: 10.1016/s1535-6108(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Gao H, Ouyang X, Banach-Petrosky WA, Gerald WL, Shen MM, Abate-Shen C. Combinatorial activities of Akt and B-Raf/Erk signaling in a mouse model of androgen-independent prostate cancer. Proc. Natl. Acad. Sci. USA. 2006;103:14477–14482. doi: 10.1073/pnas.0606836103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumerlock PH, Chi SG, Shi XB, Voeller HJ, Jacobson JW, Gelmann EP, deVere White RW The Cooperative Prostate Network. p53 abnormalities in primary prostate cancer: single-strand conformation polymorphism analysis of complementary DNA in comparison with genomic DNA. J. Natl. Cancer Inst. 1997;89:66–71. doi: 10.1093/jnci/89.1.66. [DOI] [PubMed] [Google Scholar]
- Hill R, Song Y, Cardiff RD, Van Dyke T. Selective evolution of stromal mesenchyme with p53 loss in response to epithelial tumorigenesis. Cell. 2005;123:1001–1011. doi: 10.1016/j.cell.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Cardiff RD, Desai N, Banach-Petrosky WA, Parsons R, Shen MM, Abate-Shen C. Cooperativity of Nkx3.1 and Pten loss of function in a mouse model of prostate carcinogenesis. Proc. Natl. Acad. Sci. USA. 2002;99:2884–2889. doi: 10.1073/pnas.042688999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komazawa N, Suzuki A, Sano S, Horie K, Matsuura N, Mak TW, Nakano T, Takeda J, Kondoh G. Tumorigenesis facilitated by Pten deficiency in the skin: evidence of p53-Pten complex formation on the initiation phase. Cancer Sci. 2004;95:639–643. doi: 10.1111/j.1349-7006.2004.tb03322.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kwabi-Addo B, Giri D, Schmidt K, Podsypanina K, Parsons R, Greenberg N, Ittmann M. Haploinsufficiency of the Pten tumor suppressor gene promotes prostate cancer progression. Proc. Natl. Acad. Sci. USA. 2001;98:11563–11568. doi: 10.1073/pnas.201167798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DM, Sun H. PTEN/MMAC1/TEP1 suppresses the tumorigenicity and induces G1 cell cycle arrest in human glioblastoma cells. Proc. Natl. Acad. Sci. USA. 1998;95:15406–15411. doi: 10.1073/pnas.95.26.15406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Giovanella BC, Ittmann M, Tycko B, Hibshoosh H, Wigler MH, Parsons R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science (New York, NY) 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- Ma L, Teruya-Feldstein J, Behrendt N, Chen Z, Noda T, Hino O, Cordon-Cardo C, Pandolfi PP. Genetic analysis of Pten and Tsc2 functional interactions in the mouse reveals asymmetrical haploinsufficiency in tumor suppression. Genes Dev. 2005;19:1779–1786. doi: 10.1101/gad.1314405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamillapalli R, Gavrilova N, Mihaylova VT, Tsvetkov LM, Wu H, Zhang H, Sun H. PTEN regulates the ubiquitin-dependent degradation of the CDK inhibitor p27(KIP1) through the ubiquitin E3 ligase SCF(SKP2) Curr. Biol. 2001;11:263–267. doi: 10.1016/s0960-9822(01)00065-3. [DOI] [PubMed] [Google Scholar]
- Mao JH, Wu D, Perez-Losada J, Nagase H, DelRosario R, Balmain A. Genetic interactions between Pten and p53 in radiation-induced lymphoma development. Oncogene. 2003;22:8379–8385. doi: 10.1038/sj.onc.1207083. [DOI] [PubMed] [Google Scholar]
- Mayo LD, Dixon JE, Durden DL, Tonks NK, Donner DB. PTEN protects p53 from Mdm2 and sensitizes cancer cells to chemotherapy. J. Biol. Chem. 2002;277:5484–5489. doi: 10.1074/jbc.M108302200. [DOI] [PubMed] [Google Scholar]
- Meyers FJ, Gumerlock PH, Chi SG, Borchers H, Deitch AD, deVere White RW. Very frequent p53 mutations in metastatic prostate carcinoma and in matched primary tumors. Cancer. 1998;83:2534–2539. [PubMed] [Google Scholar]
- Oki E, Tokunaga E, Nakamura T, Ueda N, Futatsugi M, Mashino K, Yamamoto M, Watanabe M, Ikebe M, Kakeji Y, Baba H, Maehara Y. Genetic mutual relationship between PTEN and p53 in gastric cancer. Cancer Lett. 2005;227:33–38. doi: 10.1016/j.canlet.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Park JH, Walls JE, Galvez JJ, Kim M, Abate-Shen C, Shen MM, Cardiff RD. Prostatic intraepithelial neoplasia in genetically engineered mice. Am. J Pathol. 2002;161:727–735. doi: 10.1016/S0002-9440(10)64228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podsypanina K, Ellenson LH, Nemes A, Gu J, Tamura M, Yamada KM, Cordon-Cardo C, Catoretti G, Fisher PE, Parsons R. Mutation of Pten/ Mmac1 in mice causes neoplasia in multiple organ systems. Proc. Natl. Acad. Sci. USA. 1999;96:1563–1568. doi: 10.1073/pnas.96.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radu A, Neubauer V, Akagi T, Hanafusa H, Georgescu MM. PTEN induces cell cycle arrest by decreasing the level and nuclear localization of cyclin D1. Mol. Cell. Biol. 2003;23:6139–6149. doi: 10.1128/MCB.23.17.6139-6149.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbridger G, Wang H, Young P, Kurita T, Wang YZ, Lubahn D, Gustafsson JA, Cunha G. Evidence that epithelial and mesenchymal estrogen receptoralpha mediates effects of estrogen on prostatic epithelium. Dev. Biol. 2001;229:432–442. doi: 10.1006/dbio.2000.9994. [DOI] [PubMed] [Google Scholar]
- Shappell SB, Thomas GV, Roberts RL, Herbert R, Ittmann MM, Rubin MA, Humphrey PA, Sundberg JP, Rozengurt N, Barrios R, Ward JM, Cardiff RD. Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor Meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee; Cancer Res; 2004. pp. 2270–2305. [DOI] [PubMed] [Google Scholar]
- Sheng X, Koul D, Liu JL, Liu TJ, Yung WK. Promoter analysis of tumor suppressor gene PTEN: identification of minimum promoter region. Biochem. Biophys. Res. Commun. 2002;292:422–426. doi: 10.1006/bbrc.2002.6662. [DOI] [PubMed] [Google Scholar]
- Stambolic V, Tsao MS, Macpherson D, Suzuki A, Chapman WB, Mak TW. High incidence of breast and endometrial neoplasia resembling human Cowden syndrome in pten+/− mice. Cancer Res. 2000;60:3605–3611. [PubMed] [Google Scholar]
- Stambolic V, MacPherson D, Sas D, Lin Y, Snow B, Jang Y, Benchimol S, Mak TW. Regulation of PTEN transcription by p53. Mol. Cell. 2001;8:317–325. doi: 10.1016/s1097-2765(01)00323-9. [DOI] [PubMed] [Google Scholar]
- Sugimura Y, Cunha GR, Donjacour AA. Morphological and histological study of castration-induced degeneration and androgen-induced regeneration in the mouse prostate. Biol. Reprod. 1986;34:973–983. doi: 10.1095/biolreprod34.5.973. [DOI] [PubMed] [Google Scholar]
- Sun H, Lesche R, Li DM, Liliental J, Zhang H, Gao J, Gavrilova N, Mueller B, Liu X, Wu H. PTEN modulates cell cycle progression and cell survival by regulating phosphatidylinositol 3,4,5,-trisphosphate and Akt/protein kinase B signaling pathway. Proc. Natl. Acad. Sci. USA. 1999;96:6199–6204. doi: 10.1073/pnas.96.11.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Freije D, Nusskern DR, Okami K, Cairns P, Sidransky D, Isaacs WB, Bova GS. Interfocal heterogeneity of PTEN/MMAC1 gene alterations in multiple metastatic prostate cancer tissues. Cancer Res. 1998;58:204–209. [PubMed] [Google Scholar]
- Trotman LC, Pandolfi PP. PTEN and p53: who will get the upper hand? Cancer Cell. 2003;3:97–99. doi: 10.1016/s1535-6108(03)00022-9. [DOI] [PubMed] [Google Scholar]
- Trotman LC, Niki M, Dotan ZA, Koutcher JA, Di Cristofano A, Xiao A, Khoo AS, Roy-Burman P, Greenberg NM, Van Dyke T, Cordon-Cardo C, Pandolfi PP. Pten dose dictates cancer progression in the prostate. PLoS Biol. 2003;1:E59. doi: 10.1371/journal.pbio.0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truett GE, Heeger P, Mynatt RL, Truett AA, Walker JA, Warman ML. Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT) BioTechniques. 2000;29:52–54. doi: 10.2144/00291bm09. [DOI] [PubMed] [Google Scholar]
- Umbas R, Schalken JA, Aalders TW, Carter BS, Karthaus HF, Schaafsma HE, Debruyne FM, Isaacs WB. Expression of the cellular adhesion molecule E-cadherin is reduced or absent in high-grade prostate cancer. Cancer Res. 1992;52:5104–5109. [PubMed] [Google Scholar]
- Umbas R, Isaacs WB, Bringuier PP, Schaafsma HE, Karthaus HF, Oosterhof GO, Debruyne FM, Schalken JA. Decreased E-cadherin expression is associated with poor prognosis in patients with prostate cancer. Cancer Res. 1994;54:3929–3933. [PubMed] [Google Scholar]
- Umbas R, Isaacs WB, Bringuier PP, Xue Y, Debruyne FM, Schalken JA. Relation between aberrant alpha-catenin expression and loss of E-cadherin function in prostate cancer. Int. J. Cancer. 1997;74:374–377. doi: 10.1002/(sici)1097-0215(19970822)74:4<374::aid-ijc2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Wakabayashi Y, Mao JH, Brown K, Girardi M, Balmain A. Promotion of Hras-induced squamous carcinomas by a polymorphic variant of the Patched gene in FVB mice. Nature. 2007;445:761–765. doi: 10.1038/nature05489. [DOI] [PubMed] [Google Scholar]
- Wang J, Ouyang W, Li J, Wei L, Ma Q, Zhang Z, Tong Q, He J, Huang C. Loss of tumor suppressor p53 decreases PTEN expression and enhances signaling pathways leading to activation of activator protein 1 and nuclear factor kappaB induced by UV radiation. Cancer Res. 2005;65:6601–6611. doi: 10.1158/0008-5472.CAN-04-4184. [DOI] [PubMed] [Google Scholar]
- Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C, Jiao J, Thomas GV, Li G, Roy-Burman P, Nelson PS, Liu X, Wu H. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4:209–221. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- Wang S, Garcia AJ, Wu M, Lawson DA, Witte ON, Wu H. Pten deletion leads to the expansion of a prostatic stem/progenitor cell subpopulation and tumor initiation. Proc. Natl. Acad. Sci. USA. 2006;103:1480–1485. doi: 10.1073/pnas.0510652103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SI, Parsons R, Ittmann M. Homozygous deletion of the PTEN tumor suppressor gene in a subset of prostate adenocarcinomas. Clin. Cancer Res. 1998;4:811–815. [PubMed] [Google Scholar]
- Wang Y, Hayward SW, Donjacour AA, Young P, Jacks T, Sage J, Dahiya R, Cardiff RD, Day ML, Cunha GR. Sex hormone-induced carcinogenesis in Rb-deficient prostate tissue. Cancer Res. 2000;60:6008–6017. [PubMed] [Google Scholar]
- Yuan ZQ, Sun M, Feldman RI, Wang G, Ma X, Jiang C, Coppola D, Nicosia SV, Cheng JQ. Frequent activation of AKT2 and induction of apoptosis by inhibition of phosphoinositide-3-OH kinase/Akt pathway in human ovarian cancer. Oncogene. 2000;19:2324–2330. doi: 10.1038/sj.onc.1203598. [DOI] [PubMed] [Google Scholar]
- Zheng HC, Sun JM, Li XH, Yang XF, Zhang YC, Xin Y. Role of PTEN and MMP-7 expression in growth, invasion, metastasis and angiogenesis of gastric carcinoma. Pathol. Int. 2003;53:659–666. doi: 10.1046/j.1440-1827.2003.01542.x. [DOI] [PubMed] [Google Scholar]