Abstract
Streptococcus mitis is the closest relative of the major human pathogen S. pneumoniae. The 2,15 Mb sequence of the Streptococcus mitis B6 chromosome, an unusually high-level beta-lactam resistant and multiple antibiotic resistant strain, has now been determined to encode 2100 genes. The accessory genome is estimated to represent over 40%, including 75 mostly novel transposases and IS, the prophage φB6 and another seven phage related regions. Tetracycline resistance mediated by Tn5801, and an unusual and large gene cluster containing three aminoglycoside resistance determinants have not been described in other Streptococcus spp. Comparative genomic analyses including hybridization experiments on a S. mitis B6 specific microarray reveal that individual S. mitis strains are almost as distantly related to the B6 strain as S. pneumoniae. Both species share a core of over 900 genes. Most proteins described as pneumococcal virulence factors are present in S. mitis B6, but the three choline binding proteins PcpA, PspA and PspC, and three gene clusters containing the hyaluronidase gene, ply and lytA, and the capsular genes are absent in S. mitis B6 and other S. mitis as well and confirm their importance for the pathogenetic potential of S. pneumoniae. Despite the close relatedness between the two species, the S. mitis B6 genome reveals a striking X-alignment when compared with S. pneumoniae.
Introduction
Streptococcus mitis, a commensal resident of the upper respiratory tract, is part of the Mitis group of Gram positive bacteria that include one of the major human pathogens Streptococcus pneumoniae. S. mitis rarely causes disease such as endocarditis [1]–[3]. In contrast, the pathogenicity potential of S. pneumoniae is high, leading to pneumonia, meningitis, otitis media, sepsis and bronchitis. The capsule is essential for virulence in S. pneumoniae, and 91 capsular types unique to the pneumococcus are known [4], [5]. Major pneumococcal virulence factors include pneumolysin, a hemolytic cytolysin (Ply), the autolysin LytA, and a variety of surface proteins implicated in host cell interaction [6], [7]. Moreover, all S. pneumoniae isolates possess choline containing teichoic acids which are the anchor structure of choline binding proteins (CBPs) known to express important functions in murein metabolism and host-pathogen interactions [8]. Most of these genes appear to be absent from S. mitis although occasional isolates containing these genes have been described [8]–[10].
S. mitis consists of many unrelated lineages according to comparative sequence analysis of selected genes, whereas S. pneumoniae strains form a tight cluster of clonal groups [11]–[13]. Each one of these lineages is as distant from a putative ancestor as is S. pneumoniae, suggesting that S. pneumoniae might be a specialized S. mitis clone that has evolved as a residence of the upper respiratory tract. It is a general hypothesis that pathogenic bacteria have evolved from commensal species by the acquisition of virulence genes [14], but this concept has been questioned for S. pneumoniae based on the finding that over 700 genes extracted from a comparative analysis of three pneumococcal genomes have no homologous counterpart in other bacteria, whereas this number appears to be marginal in S. mitis [12]. Therefore it has been postulated that S. mitis has evolved from a pathogenic population as a result of loss of virulence genes. However, only one unfinished genome of an S. mitis type strain NCTC12261 has been available for such analyses.
Members of the Mitis group are naturally transformable. This property is reflected by a high degree of variability between S. pneumoniae clones on the genomic level. Genomic comparison using microarray technology and calculations based on in silico data indicate that different S. pneumoniae clones differ from each other by over ten percent of their genes [15]–[17] and that only 46% of all genes might be conserved within the species [18]. Transformation of S. pneumoniae with S. mitis DNA can easily be achieved in the laboratory, but it is not clear as to what extent this occurs in the natural habitat.
In order to investigate the relationship between the two species, we have analyzed the genome sequence of a representative S. mitis in detail. S. mitis B6 was chosen for several reasons. It clusters within the group of S. mitis according to genomic hybridization data [15], and it has been verified as S. mitis by MLST analysis [11]. It is a high level penicillin and multiple antibiotic resistant isolate [19], [20]. The S. mitis B6 genome was investigated with emphasis on cell surface components and elements involved in the mobility of genomic material. A detailed comparative analysis was performed with six finished S. pneumoniae genomes in order to gain insights into interspecies gene transfer. Moreover, a B6-specific oligonucleotide microarray was designed for comparative hybridization analyses.
Results and Discussion
Genome Sequence of S. mitis B6: General Features
General features of the S. mitis genome are listed in Table 1. The sequence of the circular genome covers 2,146,611 bp with an average GC content of 39.98% (40.74% for coding sequences) which is similar to the features of finished S. pneumoniae genomes (between 2.04 and 2.24 Mb, and around 40% GC) (Fig. 1A). The first base of the dnaA gene represents the genome start point, and the putative terminus is located downstream from xerC. Both, XerC and XerD bare the unusual conserved sequence motifs described for S. pneumoniae [21] involved in an unconventional recombination machinery [22]. There are four rRNA operons. The first 16S rRNA differs from the other three genes in one nucleotide, and there are six respectively seven differences to the 16S rRNA sequences of S. pneumoniae R6. Two of the 23S rRNA (smi_0018 and smi_0492) differ from the other two 23S rRNA genes by one nucleotide. Out of the 2018 predicted proteins, biological functions were assigned to 1362 (67%), and 84 had no database match (4%).
Table 1. General features of the S. mitis B6 genome and comparison with S. pneumoniae R6.
| S. mitis B6 | S. pneumoniae R6a | |
| total number of bases | 2,146,611b | 2,038,615 |
| GC % | 39.98 | 39 |
| genes (total) | 2100 | 2115 |
| density | 0.98 genes per kb | |
| average length (nt) | 908 | |
| coding percentage | 87.4 | |
| rRNA | 12 | 12 |
| tRNA | 61 | 58 |
| RNA coding genes | 8 | 3 |
| CDS | 2018 | 2042 |
| average length (aa) | 310 | |
| hypothetical proteins | 83 | |
| conserved hypothetical proteins | 570 |
Only major features are listed. Numbers that are related to the gene number and annotation (hypothetical etc.) are not included due to the early time of annotation in 2001.
the number refers to the sequenced genome. An additional 7.8 kb are present in monX as confirmed by Southern hybridization data as described in the methods section.
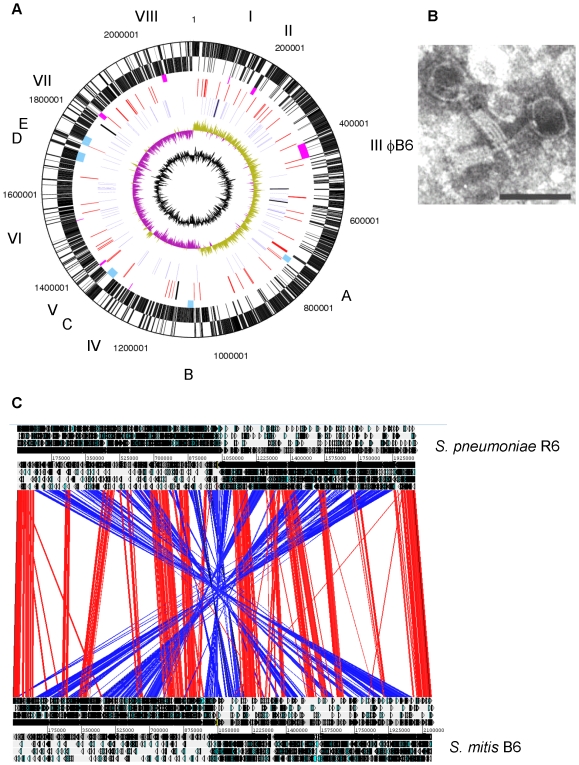
Figure 1. The S. mitis B6 genome.
A. Circular display of the S. mitis B6 chromosome. Black dots mark clusters larger than 15 kb which are absent in most or all S. pneumoniae and S. mitis; open circles indicate phages related islands. The outer two circles show open reading frames oriented in the forward and reverse direction, respectively. The third circle marks phage related elements including φB6 (pink; roman numbers) and gene clusters >15 kb of the accessory genome which are absent in most or all S. pneumoniae and S. mitis (blue; A: ntp cluster; B: unknown function; C: Tn5801; D: monX cluster; E: aminoglycoside resistance). The fourth circle shows IS (red) and the two group II introns (black), the fifth circle BOX elements (blue) and RUP (black). The sixth circle shows GC skew, purple indicating negative values; the sixth circle indicates the G+C content. B. Electron micrograph of φB6. Phage particles were purified from mitomycin C-induced S. mitis B6 cultures (0.2 µg/ml). The bar reprents 100 nm. C. Genome alignment of S. mitis B6 with S. pneumoniae R6. In the display using ACT, red areas mark regions of the same orientation in both species, blue indicates regions implicated in the X-alignment. Only regions >1 kb are shown.
Despite the fact that the S. pneumoniae R6 genome could be used in an alignment strategy for the assembly of the contigs generated after the shot gun sequencing, the overall arrangement of the S. mitis B6 genome reveals a striking arrangement termed X-alignment [23] when compared to S. pneumoniae genomes. Fifteen major regions can be recognized where the alignment between S. pneumoniae R6 and the S. mitis B6 genomes is conserved, interspersed with regions that are symmetrically inverted in respect to the position of the replication origin or terminus (Fig. 1B). At the same time, the preferred location of genes on the leading strand is maintained. Comparison with other S. mitis genome sequences suggests that this feature might be common among this species (unpublished results). The reason for this phenomenon is not clear. It has been discussed that the splitting of tandemly repeated sequences by inversion about the origin causes such X-alignment stabilizing the coexistence of duplicated genes [23]. Inversion events have been linked to replication, and the termination process may also contribute to the chromosome architecture [23]–[25]. In this context it should be noted that also in S. pneumoniae the genomic synteny is not always maintained: there is a large inversion across the terminus of S. pneumoniae CGSP14, where the breakpoints are located within IS elements [26]. However, no repeat sequences are apparent in the S. mitis B6 genome, although in several breakpoints defined according to the S. pneumoniae R6 genome backbone the insertion element ISSmi1 (see below) is closely associated with these positions on the corresponding sites of the S. mitis B6 genome, or IS elements are found in the S. pneumoniae genome.
Mobile and Repeat Elements
S. mitis harbors a large number of elements that are putatively mobile. Among these are 63 recognizable insertion sequences (IS) (Fig. 1 and 2; Table 2): five novel elements, but also some described in other streptococci. The known IS include IS1381, IS861, ISSpn2, ISSsu4 and ISSmu1 from S. pneumoniae, S. suis and S. mutans. The majority of IS in S. mitis B6 is made up of the novel element ISSmi1 which is present in 42 complete copies and in one internally deleted variant. ISSmi1 belongs to the IS30 family and is related to another new IS detected in S. mitis B6, ISSmi3. The transposases of these elements both consisting of 388 aa share 56% identical residues. The third novel IS found in this genome is ISSmi2, which is peculiar as its transposase gene has no stop codon. Consequently, transposase proteins of varying length are produced upon integration at different sites. It may be worth mentioning that one of the ISSmi2 copies resides within bacteriophage ΦB6 DNA. The other new IS, ISSmi4 and ISSmi5, belong to the IS66 and ISL3 family, respectively. Target duplications are found at the insertion sites of ISSmi1 and ISSmi3, while no target duplication appears to be produced upon integration of the other new IS.
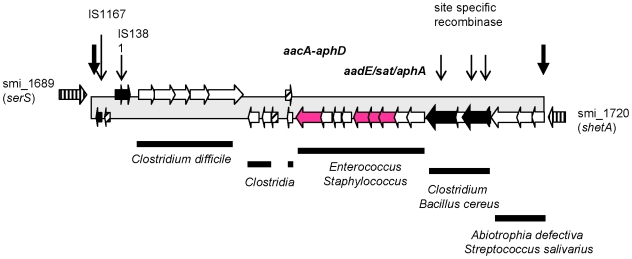
Figure 2. The aminoglycoside resistance gene cluster.
The two genes smi_1689 and smi_1720 flanking the cluster are conserved in S. pneumoniae and S. mitis. Red: genes implicated in antibiotic resistance as indicated above the genes; black: IS and recombinases; hatched: S. mitis B6 specific hypothetical genes. Thick lines below mark regions with homology to genes in other species as indicated. The fat arrows left and right mark repeat sequences.
Table 2. Mobile genetic elements in S. mitis B6.
| designation | element | copy number | Reference |
| ISSmi1 | insertion sequence | 43 | this work |
| ISSmi2 | insertion sequence | 4 | this work |
| ISSmi3 | insertion sequence | 2 | this work |
| ISSmi4 | insertion sequence | 1 | this work |
| ISSmi5 | insertion sequence | 1 | this work |
| IS1167 | insertion sequence | 4 | [99] |
| IS1381 | insertion sequence | 2 | [100] |
| ISSsu4 | insertion sequence | 4 | [101] |
| ISSmu1 | insertion sequence | 1 | [102] |
| ISSpn2 | insertion sequence | 1 | http://www-is.biotoul.fr/is |
| Tn5801 | conjugative transposon | 1 | [27] |
| S.m.I.1 | group II intron | 2 | this work |
| BOX | intergenic repeat unit | 103 | [30] |
| RUP | intergenic repeat unit | 3 | [31], [103] |
There may be three additional novel IS in the genome. However we were not able to clearly identify the boundaries of these elements. The remarkable number of IS make up close to 90 kb in the S. mitis B6 genome. Curiously, relics of ISSmi1 consisting mainly of the terminal inverted repeats are present in all S. pneumoniae genomes sequenced so far. These elements could perhaps be mobilized with a functional ISSmi1 transposase.
A large mobile element originally described in Staphylococcus aureus Mu50 as conjugative transposon Tn5801 [27] is present in S. mitis showing an identity of 99%. Tn5801 carries a tetM resistance gene (see below) and belongs to an increasing group of elements classified as integrative and conjugative elements (ICE) [28]. S. mitis B6 is the first streptococcal strain to harbor Tn5801. Tn5801 apparently integrated into a 20 bp sequence downstream of guaA encoding a GMP-synthase. This short sequence is not present in pneumococcal genomes sequenced so far perhaps explaining the absence of this element in these organisms.
In addition to mobile DNA elements, two copies of group II introns, self-splicing RNAs and retroelements, are contained in the S. mitis genome. The S. mitis B6 group II intron S.m.I1 belongs to the bacterial class C introns with a group II C structure according to the classification by Dai et al. [29]. S.m.I1 is nearly identical to the pneumococcal S.p.I1 except for a 62 bp deletion removing two variable stem-loops that are not considered functionally important. Like most bacterial group II introns, both S.m.I1 copies are inserted between genes.
The intergenic repeat elements BOX [30] and RUP which are frequently found in S. pneumoniae genomes [31] are also present in B6 (Fig. 1 and 2). Whereas BOX elements are abundant, there are only three RUP elements. None of the RUP elements is present at the equivalent position in the R6 genome and two are located at the end of a B6-specific region. This could mean that RUP elements are active elements likely to be associated with gene acquisition in agreement with the proposal that RUP could still be mobile [31]. BOX elements are often present at the same position in both genomes, but examples of extra BOX elements in B6 or S. pneumoniae R6, and inversion or expansion/reduction of BOX elements located at the corresponding positions can also be found. Altogether, the mobile elements described above represent over 6% of the S. mitis B6 genome.
The Prophage φB6 and Phage Related Islands
S. mitis B6 contains one complete prophage of 44 kb which yields morphologically intact particles of the Myoviridae type after induction with mitomycin C (details will be described elsewhere). This phage was named φB6 by Romero et al. who received the S. mitis B6 strain for analysis of the phage-encoded amidase [32]. Remarkably, so far only one other Myovirus EJ-1 has been described in streptococci isolated from an atypical S. pneumoniae [33] whose sequence has been reported recently [32]. The φB6 lytA allele which differs from the S. pneumoniae lytA sequence in 20% (17% aa) is followed by one copy of ISSmi2 which is part of the prophage. One gene specifies a tyrosine-specific tRNA which may be beneficial to the translational efficiency of the bacterial host. φB6 particles contain circularly permuted, terminally redundant genomes which are likely generated from concatemers by the pac mechanism of DNA packaging [34]. Similar to other pac phages, the pac site is located within the gene for a small subunit of the terminase protein.
Integration of the circularized phage DNA into the host genome occurs within a 9-bp core sequence, common to the attachment sites attP and attB [35]. The core of attP is located 36 bp downstream of the φB6 integrase gene. Prophage integration at attB leads to disruption of ssbB near its 5′-terminal end. SsbB, a single-stranded DNA binding protein, binds to the incoming donor DNA strand during transformation [36]. Since deletion mutants of SsbB in S. pneumoniae have a reduced transformation efficiency, the integration of φB6 into this gene might contribute to the low transformability observed with S. mitis B6.
There are another seven phage related gene clusters, six of which are associated with genes encoding complete and degenerate integrases/recombinases (Table 3). Although temperate phages are common in S. pneumoniae, the presence of so many relics of phage origin is unusual. φB6 and the other phage remnants of S. mitis B6 are unrelated to pneumophages such as the temperate phage ΦMM1 present in the Spain23F-1 clone {Croucher, 2009 2435/id}, or other phage remnants of S. pneumoniae TIGR4 or Hu19A_6. However, remnants of the phage integrase associated with cluster 7 are found in TIGR4 elsewhere in the genome. Acquisition of MM1 was associated with increased adhesion to eucaryotic cells [38], and it is possible that also oral streptococci benefit from the presence of some phage products. No paralogues of apparent virulence genes are associated with the phage clusters unlike the situation in Staphylococcus aureus where prophages might contribute substantially to the virulence potential [39]. The phage-related sequences constitute over 4% of the S. mitis B6 genome, confirming that temperate bacteriophages contribute significantly to genome variability in human streptococci as previously postulated for dairy lactic acid bacteria [40].
Table 3. Phage related gene clusters.
| No | genes | smi | size (kb) | GC % |
| 1a | 5 | 0096–0100 | 3.2 | 31 |
| 2a | 15 | 0177–0191 | 10 | 32.5 |
| 3 φB6a | 74 | 0407–0479 | 44.3 | 39.8 |
| 4a | 4 | 1260–1263 | 1.6 | 29 |
| 5a | 7 | 1366–1372 | 5.3 | 34.9 |
| 6 | 2 | 1505–1506 | 1.1 | 28 |
| 7a | 16 | 1781–1795 | 13.8 | 31.3 |
| 8a | 18 | 2000–2017 | 12.5 | 38.1 |
Phage clusters were identified by the presence of two or more coding regions specifying products with significant homology to proteins of known bacteriophages. Size was calculated according to the positions of repetitive sequences (possible integration sites), distinct shifts in the G+C content, and shifts between phage-related and host-related gene functions.
includes phage related integrase or fragments thereof.
Antibiotic Resistance
As mentioned above, the tetracycline resistance determinant tetM is located on the 25 kb Tn5801. The TetM gene is widespread in S. pneumoniae but is not associated with this element. In the multiple antibiotic resistant clone Spain23F-1, tetM is located on Tn916 part of which is similar to Tn5801, and only tetM and short flanking regions are identical to S. mitis B6 sequences.
S. mitis B6 harbours a remarkable number of genes associated with aminoglycoside resistance, all of which are common among Gram positive cocci. There is a ∼25 kb cluster which includes three such resistance determinants (Fig. 2). It contains genes of the bifunctional enzyme AacA-AphD (aminoglycoside acetyltransferase and phosphotransferase) present in Tn4001-like elements in the genomes or plasmids of Gram-positive cocci [41]. In the near vicinity the three genes aphA, sat and aadE (aminoglycoside 3′-phosphotransferase, streptothricin acetyltransferase, and aminoglycoside 6-adenylyltransferase) are clustered which are also frequently found in Staphylococcus and Enterococcus as part of Tn5405 and pJH1, respectively. In addition, mainly Clostridium and Streptococcus spp. homologues are located in this region (Fig. 2).
The origin of this cluster is obscure. Among 28 finished and unfinished genomes of S. pneumoniae listed in the NCBI data base, only strain CGSP14 contains aphA and sat together with a truncated version of aadE. None of the current finished microbial genomes contains these genes in the combination found in S. mitis B6. The three genes aphA, sat and aadE have been identified in viridans streptococci [42]. There are reports where aacA-aphD as well as aphA and aadE were found by PCR analysis within the same S. aureus strain [43], [44], but the genomic context is not clear.
S. mitis B6 is also rifampicin resistant due to a mutation in RpoB H486N which has frequently been identified in S. pneumoniae [45]. It should be noted that in most S. pneumoniae, rpoB has been annotated differently starting 13 codons upstream and therefore this mutation has been defined as H499N, but in the other Gram positive cocci the size of RpoB corresponds to that in S. mitis B6.
All five high molecular weight PBPs of S. mitis B6 have been implicated in beta-lactam resistance, but for pbp1b and pbp2a only partial sequences were described [20]. Surprisingly, pbp1b contains an authentic stop codon at position 567 within the penicillin-binding/transpeptidase domain. This explains why PBP1b could not be visualized as beta-lactam complex on SDS-gels [20]. PBP1b is dispensable under laboratory conditions [46], [47], and apparently at least its transpeptidase activity in vivo as well. PBP2a is also modified due to the integration of an ISSmi1 element at the very end of pbp2a resulting in a two amino acid extension of the C-terminus. However, no impact on protein function is to be expected by this modification.
When compared to PBP genes of penicillin sensitive S. pneumoniae, S. mitis B6 pbp1a and pbp2b show a mosaic structure diverging between 3–29%, and pbp2x and pbp1b diverge by 19–25% throughout. In contrast, flanking regions and pbp3 differ from the S. pneumoniae genes by only 2–10%. The only exception is pbp2x where ftsL upstream of pbp2x also diverges from the S. pneumoniae sequence by 23%. This could mean that also in S. mitis B6 some PBP genes are the product of gene transfer which occurred during the acquisition of penicillin resistance. However, PBP2x genes are highly variable among penicillin sensitive S. mitis ([48] and unpublished results), and thus the evolutionary history of PBP alterations in S. mitis B6 is not clear.
Cell Wall Associated Proteins
Prominent members of cell surface proteins that have been implicated in virulence and host cell interactions of S. pneumoniae belong to the families of choline-binding proteins (CBPs) [8] and cell wall bound proteins containing the characteristic LPXTG motif [49]. Members of these two protein families are abundant in S. mitis B6, and were therefore analyzed in detail.
S. mitis B6 harbors 22 choline binding proteins (CBPs) which exceeds the numbers found in S. pneumoniae genomes by far; e.g. there are 12 CBPs in S. pneumoniae R6, and 14 in S. pneumoniae TIGR4 (Table 4). The presence of CBPs is not surprising, since S. mitis B6 contains the licD1 and licD2 operons known to be responsible for the choline decoration of the pneumococcal teichoic acid, and a licD3 homologue as well. This strongly indicates that also in S. mitis B6 choline-containing teichoic acids are present. In agreement with the formation of long chains in medium supplemented with 2% choline, S. mitis B6 contains homologues of all six CBPs implicated in murein hydrolysis and cell separation in S. pneumoniae: LytB, LytC, Pce, CbpD, and the φB6-associated LytA; also a CbpF homologue which has recently been shown to inhibit LytC in vitro and in vivo [50] is present. Only Cbp12/13 contain a putative endo-beta-N-acetylglucosaminidase domain, whereas the function of the other S. mitis B6 CBPs cannot be deduced.
Table 4. CBPs in S. mitis and S. pneumoniae.
| S. mitis B6 | S. mitis a | S. m. NCTC12261 | S. p TIGR4. | S. p. R6 | ||
| smi | gene | aa | (aa) | (aa) | (aa) | |
| 0037 | cbp1 | 432 | 0 | − | − | − |
| 0038 | cbp2 | 500 | 0 | − | − | − |
| 0055 | cbp3 | 393 | 4 | + | − | − |
| 0057 | cbp4 | 389 | 2 | + | − | − |
| 0086 | cbpI | 395 | 5 | + | (+) | − |
| 0402 | cbp5 | 304 | 10 | − | (+) | (+) |
| 0478 | lytA d | 318 | 4 | − | + | + |
| 0725 | cbp6 b,c | 352 | 1 | − | − | − |
| 0726 | cbp7 b | 366 | 1 | − | − | − |
| 0933 | pce2 | 330 | 1 | − | − | − |
| 0934 | pce1 | 627 | 6 | + | + | + |
| 0966 | lytB | 570 | 6 | + (568) | + (658) | + (721) |
| 1280 | cbp8 | 304 | 3 | − | − | − |
| 1348 | cbp9 | 339 | 5 | − | − | − |
| 1467 | cbp10 b | 324 | 3 | − | − | − |
| 1479 | cbp11 b | 190 | 9 | + (190) | + (332) | + (329) |
| 1563 | lytC | 536 | 5 | + | + | + (501) |
| 1724 | cbp12 b,d | 356 | 5 | − | − | − |
| 1725 | cbp13 b,d | 404 | 5 | − | − | − |
| 1748 | cbpF e | 313 | 6 | + | + (340) | + (338) |
| 1875 | cbp14 b | 498 | 0 | − | − | − |
| 2051 | cbpD | 372 | 10 | + (369) | + (448) | + (448) |
The nomenclature follows that of S. pneumoniae TIGR4. The number of amino acids is indicated in brackets if significantly distinct from the B6 sequence. +: present; −: absent; (+) three frames (TIGR4 cbpI) or remnants (cbp5).
genomic hybridization with ten S. mitis strains using a S. mitis B6 specific microarray; the number refers to S. mitis strains giving positive signals.
40mer repeat.
phage associated.
present in S. pneumoniae CDC3059-06.
position of SP0377 (cbpC) but more similar to SP0391 (cbpF).
CBPs are a family of modular proteins with a mostly C-terminal choline binding domain (CBD) responsible for the interaction with the choline-containing teichoic acids. The CBD consists of repetitive choline binding motif (COG5263; glucan-binding domain; YG repeat) with characteristic conserved residues (Pfam accession PF01473). At least two groups of CBPs can be recognized in S. mitis B6 on the basis of the ‘repeat’ sequences: those which contain the ‘classical’ 20mer modules (15 CBPs), and a second group (7 CBPs) where only 40mer repeats can easily be aligned instead (Fig. 3) [8]; the ‘repeats’ of LytA, LytB and Pce are highly modified and thus cannot be aligned easily. Only one CBP (CBP11) of the 40mer repeat family is conserved in almost all S. pneumoniae (spr0583/SP0677 in R6 and TIGR4, respectively).
Figure 3. Alignment of the choline-binding modules with 40mer repeat sequences.
The S. pneumoniae R6 Spr583 (CBP11 homologue) is included.
There are three examples where the S. mitis B6 CBPs are shorter compared to the S. pneumoniae homologues, due to variations in the repeat module (CBP11 and CbpD), or the SH3 domain in LytB probably representing another cell-wall interacting domain [51] (see Table 4). CBP11 contains two 40mer repeats in S. mitis B6, whereas in most S. pneumoniae there are four 40mers and an additional C-terminal extension, and in the S. pneumoniae Spain23F-1 this C-terminal extension is associated with a protein unrelated to CBPs. Interestingly, genes encoding these CBPs are also present in the S. mitis NCTC12261 sequence and they are as small as in S. mitis B6, suggesting that diversification of CBPs by duplication of repeat modules and recombination events has occurred in S. pneumoniae. There are remnants of CBP5 in several S. pneumoniae genomes, and its N-terminal region is highly related to PcpA of S. pneumoniae CGSP14.
Tandem arrangement of CBPs is common in S. mitis B6: there are four pairs of CBPs in S. mitis B6. It is tempting to speculate that tandem rearrangement is the result of gene duplication, and indeed the functional domains of Pce2 and Pce1 are appr. 26% identical to each other and not to other CBPs. However, the situation is more complicated, since the non-repeat modules of the CBPs 1/2, CBP6/7, and CBP12/13 are not related pairwise except for very short sequences. All these data document that duplication and recombination events resulting in functional diversification of CBPs are important mechanisms during the evolution of oral streptococci.
There are 18 cell surface proteins bearing the cell wall attachment motif LPXTG [52], covering almost 7% of the coding sequences. Nine of them are homologues to proteins of S. pneumoniae R6 which contains only 12 LPXTG proteins (Table 5). Two of the eight B6-specific LPXTG proteins have predicted functions. At the position equivalent to the S. pneumoniae IgA protease, a gene encoding another LPXTG-containing protein (smi_1064) is located in B6. Relics of smi_1064 can be found in S. pneumoniae in another genomic region (spr0346–0348). One cell surface protein NanA2 contains a central NanA domain which is also present in most S. pneumoniae, but in addition it contains B6-specific N- and C-terminal domains of unknown function. The Ser-rich protein MonX (“monster”) and associated genes encoding compounds involved in export and glycosylation of MonX are representatives of the S. pneumoniae accessory genome. The cluster differs from that in S. pneumoniae TIGR4 by an additional putative glycosyltransferase, and a putative acetyltransferase which however is found in other S. pneumoniae strains. It is common among other Streptococcus spp.; the S. gordonii protein has been described as a platelet binding protein which may be important for oral colonization [53], [54]. MonX and Smi1002 are among the largest proteins of B6, both covering over 4000 amino acid residues.
Table 5. Cell wall surface anchor family proteins in S. mitis B6 (LPXTG).
| S. mitis a | S. mitis | S. pneumoniae | homology/featuresb | |||
| smi (size, aa) | gene | NCTC 12261 | R6 gene (aa) | TIGR4 gene (aa) | ||
| 0091 (899) | 0 | + | spr0075 (1171) | SP0082 (857) | hypo, 152mer repeat (4) | |
| 0345 (1757) | 4 | + | spr0440 (1659) | SP0498 (1659) | hypo | |
| 0601 (1907) | nanA | 2 | − | spr1536 (1035) | SP1693 (962 stop) | sialidase A domain (neuraminidase A) |
| 0705 (2183) | prtA | 4 | − | spr0561 (2144) | SP0641 (2140) | cell wall-associated serine proteinase PrtA |
| 0810 (979) | 0 | − | − | − | hypo, coiled-coil domain; KA-rich 77mer repeats (6, deg.) | |
| 0979 (1218) | 1 | − | − | − | hypo, coiled-coil domain; KA-rich 77mer repeats (6) | |
| 1002 (4138) | 2 | (+) | − | − | hypo, Pro-rich; interspersed repetitive domains (95mers) | |
| 1064 (1702) | 2 | − | (−) | (−) | hypo, Pro-rich; interspersed repetitive domains (95mers) | |
| 1306 (2474) | 1 | (+) | − | − | hypo, coiled-coil domain; KA-rich 77mer repeats (8) | |
| 1317 (779) | 2 | (+) | − | − | hypo, Pro-rich; 36mer repeat (4) | |
| 1398 (1699) | 2 | − | − | − | serine protease | |
| 1482 (1969) | zmpB | 4 | + | spr0581 (1876) | SP0664 (1881) | zinc metalloprotease |
| 1531 (2997) | 6 | − | spr1403 (2551) | − | glycine rich protein (87mer repeat, 7) | |
| 1534 (2391) | bgaA | 0 | − | spr0565 (2228) | SP0648 (2233) | beta-galactosidase |
| 1537 (2770) | 0 | − | − | − | N-acetyl-beta-hexosaminidase | |
| 1538 (2322) | 0 | − | spr0328 (1767) | SP0368 (1767 fs) | hypo | |
| 1662 (1591)c | monX | 5 | − | − | SP1772 (4776) | hypo, Ser-rich repeats |
| 1848 (1298) | pulA | 9 | + | spr0247 (1256) | SP0268 (1280) | alkaline amylopullulanase |
aa: amino acids; + homologue present; - absent; (+) variable sequence; (−) fragments.
genomic hybridization with ten S. mitis strains using a S. mitis B6 specific microarray; the number refers to S. mitis strains giving positive signals.
hypo: hypothetical; the presence of large coiled-coil domains is indicated; the number in brackets refers to the number of repeats; deg.: degenerate.
the number corresponds to the sequenced region; the estimated size according to Southern hybridization is approximately 4,200 aa.
Remarkably, several of the LPXTG proteins contain novel repeat sequences of various length. Three B6-specific LPXTG proteins are predicted to be arranged in prominent coiled-coil structures [55] all of which have KA-rich repeats (smi_0810, 0979 and 1306, Fig. S1). Similar proteins are present in S. thermophilus, S. hemolyticus, and Lactococcus reuteri. Related prolin-rich degenerate repeats are present in two proteins (smi_1002 and 1064), and again homologues are found in other lactic acid bacteria.
The genes encoding B6-specific LPXTG proteins are frequently associated with transposases or IS: smi_0810 is flanked by ISSsu4, smi_1317 is adjacent to Tn5801, and smi_1306 and smi_1534 are next to IS1167. Curious is also the accumulation of four LPXTG protein encoding genes in one region: smi_1531, smi_1534, smi_1537 and smi_1538. Three of them are homologues to R6 proteins; however the genes are located at distinct regions of the R6 genome.
Bacteriocins and Competence
Competence for genetic transformation plays a major role for gene acquisition in S. pneumoniae and oral streptococci as well. The regulation of competence and bacteriocin genes involves similar components, and curiously some bacteriocins are part of the competence regulon. In both cases, a two component systems (TCS) which responds to a specific peptide pheromone is responsible for the induction of competence genes or bacteriocins. The peptide pheromones as well as the group II bacteriocins contain a double glycine (GG) N-terminal leader peptide.
Group II bacteriocins are common among Gram positive bacteria. A highly variable bacteriocin gene cluster has been described in S. pneumoniae containing a set of blp/pnc genes encoding bacteriocins and immunity proteins as well as CAAX proteases [56]–[59]. Adjacent are a regulatory system responsible for induction of the bacteriocins (TCS13; BlpRS/SpiRH), and a peptide pheromone of the GG-family as well as the dedicated ABC transporter involved in export of the pheromone; its role in bacteriocin export is not clear (Fig. 4).
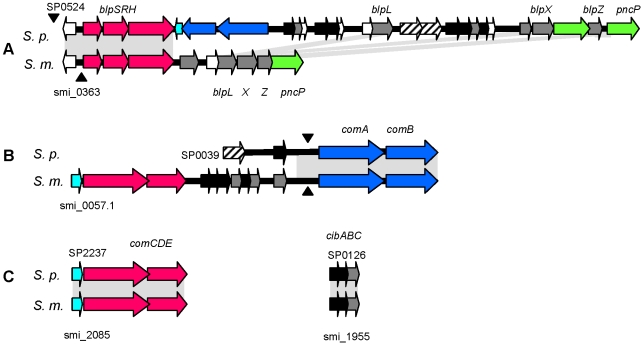
Figure 4. Bacteriocin clusters in S. mitis B6.
A: the blp/pnc cluster; B: cluster II upstream comAB; C: components implicated in competence regulation. The gene designation of TIGR4 is given above, and S. mitis B6 gene numbers are indicated below. Grey areas indicate regions of >80% identity. Red: Response regulators and histidine kinases; dark blue: ABC peptide transporter; light blue: peptide pheromone; green: CAAX proteases; black: bacteriocins; grey: immunity proteins; striped: IS; white: hypothetical proteins. Black triangles mark the position of BOX elements.
There are two bacteriocin clusters in S. mitis which differ substantially from S. pneumoniae (Fig. 4). One is related to the blp/pnc cluster, but although BlpRS are conserved, the pheromone/ABC transporter region is missing, and there are only immunity proteins and a CAAX protease. The second cluster is located upstream of comAB encoding the transporter for the competence pheromone peptide CSP, and includes genes encoding bacteriocins/immunity proteins and a TCS with a putative pheromone peptide as well. At this position, a single unrelated bacteriocin gene blpU is present in S. pneumoniae, and a BOX element upstream of comAB is conserved in both species (Fig. 4). In S. mitis NCTC12261, variations at this locus compared to B6 concern only number and sequence of the bacteriocins/immunity proteins [51].
Competence is induced via the two component system ComDE in response to high concentrations of CSP, the comC product. ComE controls early competence genes including the alternative sigma-factor ComX which in turn regulates late competence genes. Among the ComX regulated proteins are the bacteriocins CibAB, and the immunity protein CibC [60]. CibAB and the ComX responsive cell wall hydrolysase CbpD [61] allow lysis (allolysis) and thus DNA release of non-competent cells of the same strain [61], [62], a process termed fractricide. Since cibABC are absent in the S. mitis NCTC12261 sequence it has been suggested that fractricide in S. pneumoniae has evolved independently from S. mitis [62]. In S. mitis B6, however, the full repertoire of genes implicated in competence induced lysis including cibABC, and other relevant competence genes comM and comW. All genes are located at the position equivalent to that of the S. pneumoniae genome, documenting that this system has evolved prior to the separation of the two species.
It is curious that S. mitis B6 contains only one ABC transporter of the peptide-processing family, ComAB, suggesting that it might be involved in the export not only of peptide pheromones but for bacteriocins as well. In fact, S. mitis B6 shows bacteriocin activity against several strains of S. pneumoniae, S. mitis and S. oralis, and is competent for genetic transformation albeit only to low transformation efficiency (see below). Alternatively, the CAAX proteases might be involved in bacteriocin/immunity protein processing. A CAAX protease is important to express bacteriocin activity in S. pneumoniae [58], and the Enterococcus faecalis CAAX protease Eep is required for the processing of a pheromone precursor cAD1 [63]. In this context it should be noted that there are another two bacteriocin genes and one putative immunity protein gene in the S. mitis B6 genome, and all three are flanked by a CAAX protease gene. Moreover, B6 contains a third TCS of the Agr family with homology to SarRK involved in lantibiotic production in S. salivarius, but no bacteriocin related genes are associated with it. Experimental evidence will be required to understand export and processing of pheromone and bacteriocin precursors.
Unfortunately, we were unable to genetically manipulate S. mitis B6 so far in order to investigate gene function via the isolation of non-functional mutants by gene disruption. Due the multiple-antibiotic resistant phenotype, spectinomycin resistance would represent an ideal marker. However, attempts to integrate the spectinomycin resistance gene aad9 into several loci have failed completely. Only by using DNA of a spontaneous spectinomycin resistant mutant of S. mitis B6 containing a mutation in rpsE (C70A resulting in Thr21Pro), spectinomycin resistant transformants were obtained with a frequency of ∼10−4. The development of suitable genetic tools is subject of current investigations.
Genomic Comparison with S. mitis and S. pneumoniae
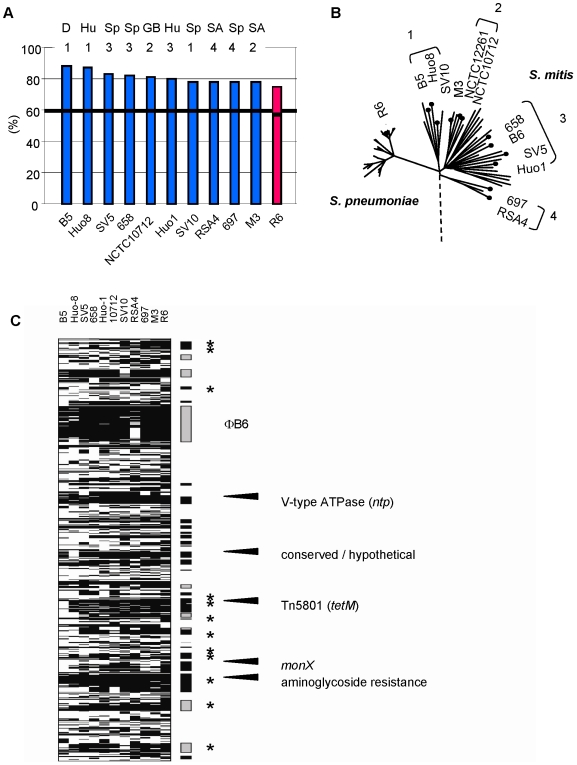
A comparative genomic hybridization analysis (CGH) of S. mitis B6 with other S. mitis strains was performed using a B6-specific oligonucleotide microarray. Ten genetically diverse S. mitis strains were chosen representing four deep routed branches of the S. mitis group [11], [15], and S. pneumoniae strain R6 was included as a reference S. pneumoniae genome. (Fig. 5A and B). The S. mitis strains originated from different geographic areas including East and West European countries and South Africa in order to ensure a broad diversity of the genomes investigated.
Figure 5. Genomic comparison of S. mitis B6.
A. Genomic hybridization analysis of S. mitis strains using a S. mitis B6 specific microarray. Mobile elements and phage related gene clusters, and ambiguous signals were not considered. The percentage of positive hybridization signals is indicated on the left. The vertical fat black line indicates positive genes common to all S. mitis strains. The values for S. pneumoniae R6 are shown in red. The roman numbers specify the S. mitis groups as shown in (B) based on the MLST-derived tree [11]. B. Genetic relationship of S. mitis. Geographic origin of the strains: D, Germany; GB: Great Britain; Hu: Hungary; SA: South Africa; Sp: Spain. C. Gene clusters of S. mitis B6 as detected by genomic hybridization of S. mitis on the B6-specific oligonucleotide microarray. The genes are arranged according to the annotated genome with the replication start on top. Low hybridization signals are indicated by black lines; genes that hybridized with DNA of all strains are not shown. Clusters that are not contained in the B6 strain are marked by boxes on the right, grey boxes indicate phage related gene clusters. * mark the presence of transposases/recombinases. Arrows indicate gene clusters >15 kb.
Most of the IS elements, recombinases and transposases gave signals in at least one strain. Exceptions were ISSmi1 which hybridized with no S. mitis, and large parts of Tn5801 were present only in S. mitis B5. Also all of the phage related gene clusters including φB6 hybridized with at least one S. mitis. Whereas BOX sequences appeared to be present in all S. mitis, some strains failed to hybridize with RUP features (Table S2).
Mobile elements and phage related gene clusters were excluded in the following quantitative analysis, leaving 1760 microarray features to be considered. Altogether, 95% of these B6 features hybridized with at least one of the ten S. mitis. The presence of several CBPs including CbpD and the lic1 operon in all S. mitis strongly suggested that a choline decorated cell wall and associated proteins are widespread in this species [8], [12]. Individual S. mitis hybridized with 88–78% of these features (Fig. 5A). 59% (1039 features) were recognized by all S. mitis thus representing the core genome of this strain collection (Fig. 5A; and Table S3), i.e. between 17.5 and 22% of the B6 genes were variably present in individual S. mitis. The variation between individual S. mitis appears to be not necessarily due to the genetic distance from S. mitis B6 or the geographic region of the isolate (Fig. 5B), and indicates that frequent gene transfer results in a highly variable accessory genome among S. mitis. Most of the genes that failed to hybridize with the B6 genes are arranged in clusters/regions >4 kb in S. mitis (Fig. 5C; and Table S2 and S3).
93 (5%) B6 specific genes remained, including three CBPs (CBP1, 2 and 14), two LPXTG-proteins (smi_1537 and smi_1538) and a bacteriocin gene with associated CAAX protease (Table S2); also the aminoglycoside resistance gene cluster was absent in all other S. mitis.
The results concerning S. pneumoniae R6 confirm a very close relatedness between the two species: it hybridized with almost all S. mitis core genes (973 features), and the same clusters that are variably absent in S. mitis can be recognized in S. pneumoniae R6 (Fig. 5A and C). The number of features not hybridizing with the mitis core genes is as high as in the two South African S. mitis strains (17.5%; Fig. 5A).
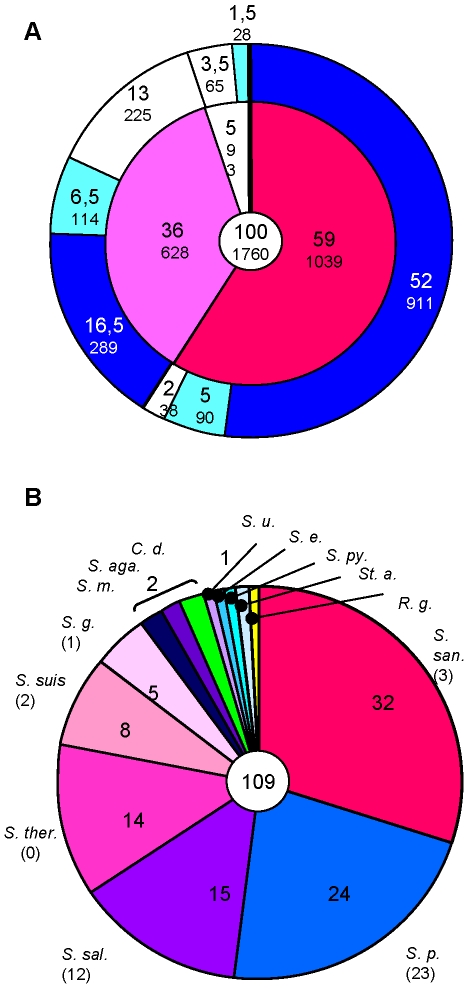
The data obtained from the CGH were complemented by a comparative in silico analysis on the protein level using six finished S. pneumoniae genomes of different serotype and MLST sequence type: R6, TIGR4, ATCC700699, G54, CGSP14 and U19A_6 (Fig. 6), in order to see which of the mitis core genes are shared by S. pneumoniae, and which genes remain S. mitis specific. Altogether, 1432 (81%) out of 1760 S. mitis B6 gene products had homologues in S. pneumoniae, including 911 (52%) of the S. mitis core. On the other hand, over 16% of the accessory S. mitis genome was present in all six S. pneumoniae strains. These numbers should be taken as a minimum, since the protein homology search used in the in silico analysis is less stringent compared to the hybridization results (Table S3).
Figure 6. Genomic comparison of S. mitis and S. pneumoniae.
Genes represented on the oligonucleotide microarray (1760 features) excluding mobile elements and phage related gene clusters were used in this calculation. A. Comparison of 1760 S. mitis gene products with those annotated in six S. pneumoniae genomes. S. pneumoniae genomes: see text for details. Inner circle: deep red, percentage of features hybridizing with all ten S. mitis strains (S. mitis core); light red, with at least one S. mitis (S. mitis accessory genome); white: no hybridization with any S. mitis (B6 specific); outer circle: proteins present in six S. pneumoniae genomes according to in silico analysis of the annotated gene products, using 70% identity as cut off value and a 60% minimum coverage. Dark blue: genes present in all six S. pneumoniae genomes; light blue: genes present in at least one S. pneumoniae; white: absent in S. pneumoniae. Large numbers indicate the percentage of the 1760 genes represented on the microarray; the number of genes is given in small letters below. B. Homologues of the 109 S. mitis B6 genes not present in the six S. pneumoniae genomes listed in (A). Only >80% identity values were used, and only species with the best hit are listed. The number in brackets below the species name indicates genes exclusively found in this species. S. aga.: S. agalactiae; S. e.: S. equi; S. g.: S. gordonii; S. m.: S. mutans; S. p.: S. pneumoniae; S. py.: S. pyogenes; S. san.: S. sanguinis; S. therm: S. thermophilus; S. u.: Streptococcus uberis; C.d.: Clostridium difficile; R. g.: Ruminococcus gnavus; St.a.: Staphylococcus aureus.
38 gene products (2%) of the S. mitis core had no homologues in S. pneumoniae and thus appear to be S. mitis specific. This set of genes might be of particular interest in respect of the evolution of S. pneumoniae. They include the TCS smi_1072/3 and an adjacent ABC transporter, another two ABC transporters and CBP5 of unknown function, confirming the results obtained with S. pneumoniae R6 in the CGH. Close homologues of the regulatory system and the two adjacent genes exist in other oral streptococci such as S. mutans and S. sanguinis. Curiously, relics of the CBP5 gene are found in the S. pneumoniae strains analyzed here. Recently, a full-length homologue of CBP5 (50% identities in the non-repeat module) was reported in a highly virulent serotype 14 S. pneumoniae [26] and annotated as PcpA. However, only the repeat domain is very similar to that of PcpA whereas the non-repeat module is distinct, representing another example of the versatility of CBPs. In summary, a minority of the S. mitis core genes identified by genomic hybridization distinguishes this species from S. pneumoniae.
All genes required for metabolic pathways in S. pneumoniae as described in TIGR4 [64] are also present in S. mitis B6 with few remarkable exceptions. There are no genes in S. mitis B6 for riboflavin biosynthesis (SP0175–0178) and none for thiamine biosynthesis (SP0717-8 and SP0721-5), both of which are arranged in clusters in S. pneumoniae except for SP0881 thiI which is present in S. mitis B6. Whereas all S. pneumoniae contain these genes, they are absent in most other streptococci except in S. agalactiae, suggesting that they have been imported into an ancient S. pneumoniae. On the other hand, S. mitis B6 contains an intact gene cluster for L-leucine biosynthesis which is highly fragmented in S. pneumoniae, indicating decay in S. pneumoniae.
Comparison with Other Organisms
In order investigate the extent of potential gene transfer events involving other species, a BLAST search of all S. mitis B6 gene products for which no homologues in any of the six S. pneumoniae genomes were found was performed against all other bacterial genomes including many S. pneumoniae genomes not considered in the above analysis. Only identity values >80% over the entire length of the predicted protein were considered. Out of these 338 proteins, 109 fulfilled these criteria (Fig. 6B). 60 were highly related to proteins from at least two streptococcal species, and 24 were found in the other S. pneumoniae genomes. Homologues to S. sanguinis and S. salivarius proteins were predominant and included seven ABC transporters (13 genes). Two proteins related to mercury resistance were S. gordonii homologues. The only genes with no homologues in streptococci were four genes located in the aminoglycoside resistance cluster (see Fig. 2), confirming that oral streptococci represent the main source of DNA for expansion of the accessory genome.
S. mitis B6 and Pneumococcal Virulence Factors
Numerous virulence factors have been described in S. pneumoniae (for reviews, see [65], [66]). Since especially surface proteins are highly variable and thus might escape the detection as homologous in the above analyses, their presence or absence in S. mitis B6 was verified in genomic alignments with S. pneumoniae genomes visualized using the ACT programme in addition to in silico genomic comparisons. Surprisingly, S. mitis B6 contained the majority of virulence factors involved in colonization and adherence, suggesting that they are important for the interaction with host cells also for commensal bacteria. These include the cell surface wall anchor proteins ZmpB, HtrA and NanA and other surface proteins PavA, Enolase and GAPDH, one pair of the recently described histidine-triad proteins, the two hemolysins HlyX and and HlyIII, the CBPs CbpF, LytB, LytC and Pce, the two peptidyl-prolyl isomerases and lipoproteins PpmA and SlrA, oligopeptide transporters AmiA, AliA and AliB, the manganese transporter PsaA, and as mentioned above the repertoire of genes required or phosphoryl-choline decoration of the cell wall. The IgA protease which is absent in S. mitis B6 cannot be regarded as a S. pneumoniae specific component, since IgA activity has been found in over 50% of S. mitis and the presence of an IgA1 genes has been confirmed in this species [12].
Regulatory proteins are also associated with virulence in S. pneumoniae. S. mitis B6 contains all but two of the S. pneumoniae 13 TCS (TCS04/PhoRP and TCS06 are absent). PhoRP has been implicated in phosphate uptake, but there is another phosphate transport system in S. pneumoniae (SP1395–SP1400) which is well conserved in S. mitis B6. The strain-to-strain variation observed in the role of TCS04 in virulence indicates that this might contribute to a modulation in pathogenicity potential but is not required for pneumococcal virulence. In addition, 16 out of 25 listed single regulators associated with virulence in S. pneumoniae [6] are also present in S. mitis B6, and according to theCGH analysis are widespread among S. mitis. Nine of these regulators were detected in all ten S. mitis strains and the other seven in at least four strains. Most of the regulators absent in B6 are located on S. pneumoniae islands coding for PTS systems or ABC transporters and are not part of the pneumococcal core genome such as the rlrA islet regulator SP0461 [67]. No homologues of this cluster nor of the second pilus cluster described recently [68] are found in S. mitis B6.
S. mitis B6 contains also a curious collection of putative virulence genes which are part of the accessory genome of S. pneumoniae: clusters encoding a V-type ATPase, and the Ser-rich LPXTG protein MonX. The genomic hybridizations documented that the monX cluster is present also in other S. mitis. Thus, a repertoire of so-called virulence genes is common to commensal streptococci, probably facilitating and modulating the potential to interact with the host.
S. mitis B6 is lacking the iron uptake system Piu/Pia. However, it contains a siderophore-Fe uptake system tatA/C which belong to the twin-arginine transport (TAT) system [69] also present in several of the S. mitis strains tested on the microarray. A TAT-translocation pathway has been found among streptococci only in the genomes of S. thermophilus and S. sanguinis [70], [71]. In general, TAT excreted proteins are known to be important virulence determinants in Pseudomonas and Yersinia [72], [73]. Interestingly truncated genes of the TAT secretion pathway are found in the genome of S. pneumoniae, indicating that also the loss of potential virulence determinants during the divergent evolution of streptococcal species has occurred.
Only a few components are absent in S. mitis B6 that are crucial for S. pneumoniae pathogenesis: pneumolysin ply, the CBPs pspA, pspC, pcpA, and the hyaluronidase hlyA in addition to the polysaccharide capsule. No S. mitis B6 gene cluster shows signatures related to capsular biosynthesis, also the colony morphology of B6 does not resemble smooth colonies described for encapsulated S. pneumoniae, strongly suggesting that it does not carry a complex polysaccharide capsule. The capsule cluster cps of S. pneumoniae is located between transposase fragments, and is flanked by the conserved genes dexB and aliA. Most strains of the Mitis group were reported to have large inserts up to 30 kb which could be amplified with primers matching dexB and aliA, suggesting the presence of a cps locus [12]. In agreement with these data, all ten S. mitis used in the CGH hybridized with dexB specific oligonucleotides (not shown). In contrast, B6 carries only two genes between dexB and aliA: glf encoding a UDP-galactopyranose mutase which can be found in the cps cluster of a variety of pneumococci, and a non-functional putative aliB-like oligopeptide transporter.
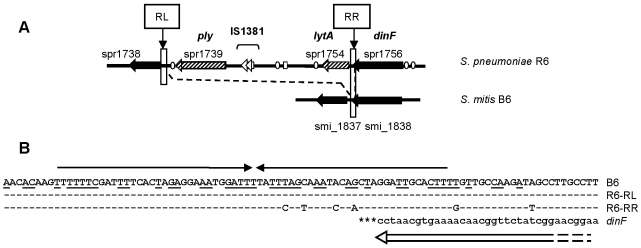
The gene encoding the pneumolysin Ply, a potent cytolysin, is located close to the autolysin lytA on an island which is absent in S. mitis B6. Whereas the LytA gene it is under the control of prophage φB6 in S. mitis B6, it is part of the competence regulon in S. pneumoniae, representing an example that recombination of a partial genetic element can result in its integration into a core regulatory system. The competence regulated induction of lytA mediates the release of Ply, a feature related to the virulence of S. pneumoniae [61], [74]. Close inspection of the ply/lytA region revealed that it is flanked by a 94 bp direct repeat which in itself has an inverted repeat structure (Fig. 7). The repeat is present once in the S. mitis B6 genome and covers the 3′-end of the DinF gene plus downstream sequences. This suggests that these repeats represent the integration site of the ply/lytA region, and the presence of truncated IS elements within that region might be related to this event. In some S. mitis a ply homologue named mitolysin has been described [9]. Occasional isolates of Mitis cluster have been shown to contain both, ply and lytA [12], [75], [76], representing valuable material to elucidate the evolution of these genes in Streptococcus spp. Also, some of the S. mitis strains used in the present study hybridized with ply and/or lytA specific oligonucleotides (Table S2 and S4). Whereas it has been discussed that lytA is a derivative of phages [3], [77], the origin of ply is still not known. The presence of the ply/lytA island, i.e. the combined activity of the autolysin and the cytolysin, has major impact on the clinical manifestation of pneumococcal disease. Pneumolysin has been shown to affect the integrity of brain endothelial cells and thus is important for damaging the blood brain barrier resulting in pneumococcal meningitis [78]. Pneumococcal meningitis is a life threatening disease, but only very few cases of S. mitis meningitis have been reported which occurred primarily after surgical manipulation [79].
Figure 7. Comparison of the ply/lytA island and flanking regions of S. pneumoniae R6 and the dinF region in S. mitis B6.
A: Black: conserved genes in S. mitis B6 and S. pneumoniae; hatched: S. pneumoniae ply and lytA; ovals: BOX elements; small rectangle: RUP elements; RL and RR designate the left and right direct repeats flanking the ply/lytA region (long rectangles). B: Sequences of the left (RL) and right (RR) direct repeat. Arrows above mark the inverted repeat within the direct repeat sequence, and matching nucleotides are underlined; non conserved nucleotides of the S. pneumoniae R6 sequences compared to S. mitis B6 are shown. The DinF gene is indicated below in small letters and as open arrow.
Absent in S. mitis B6 are also the CBPs pspA, pspC, pcpA, and the hyaluronidase hlyA. In order to see whether this is restricted to S. mitis B6, the ten S. mitis strains described above were used for hybridization analysis with S. pneumoniae specific oligonucleotides for these genes. None of the strains hybridized with any of these features (Table S4). The S. pneumoniae hyaluronidase gene hysA (spr0286/SP0314) is located on a large island adjacent to an IS200-like gene including many components involved in sugar metabolism. The entire island is missing in S. mitis B6 in agreement with the finding that S. mitis strains do not express hyaluronidase activity [12]. A role in pathogenesis of HysA has been established, but its precise role in pathogenesis is not yet understood [80]. The three CBPs PspA, PcpA, PspC and the PcpC variant Hic interact with host cells and components of the immune system [66]. PspC is regulated by the adjacent TCS06 [81], and again the entire island is missing in S. mitis B6. The C-terminal CBDs of both, PcpA and PspA, have significant similarity to the CBD of S. mitis B6 Cbp1, suggesting an evolutionary link between these CPBs.
Concluding Remarks
S. mitis B6 represents a striking example for genome modification by the acquisition of genes and gene clusters from other sources. The size of the S. mitis B6 genome with over 2.14 MB is far above the average size of 1.8 Mb of S. mitis genomes as estimated by PFGE [12] and larger S. pneumoniae R6 (2.04 Mb; [82]), suggesting that S. mitis B6 has been unusually successful in gene acquisition. Based on hybridization data on the B6-specific oligonucleotide microarray, the accessory genes constitute over 40% of the B6 genome. The large number of phage related gene clusters, mobile elements including Tn5801, and the presence of all genes involved in competence and transformation described in S. pneumoniae suggests multiple potential routes of gene transfer. Moreover, S. mitis B6 appears to be well equipped with bacteriocins facilitating access to foreign DNA by killing and lysing other bacteria.
Among the special features of B6 is the presence of a large number of the same ISSmi1. None of the ten S. mitis strains hybridized with ISSmi1, and we found only one copy of ISSmi1 in S. mitis NCTC12261, suggesting that expansion of this element has occurred during the evolution of the B6 strain.
We found at least 32 examples of S. mitis B6 homologues as remnants in the genomes of S. pneumoniae R6 and TIGR4. There is little evidence for gene decay in S. mitis B6 (20 truncated genes excluding mobile elements) in comparison to S. pneumoniae R6 and TIGR4 which contain 63 respectively 48 truncated genes. This finding is in agreement with the assumption that S. pneumoniae originated from an ancient S. mitis clone.
S. pneumoniae is particularly rich in sugar-related transport systems. S. pneumoniae TIGR4 contains 21 PTS systems [64], whereas only 10 PTS systems were found in S. mitis B6, five of which are homologues to the S. pneumoniae genes. This confirms that S. pneumoniae is unusually versatile in sugar uptake, probably related to its distinct habitat, the nasopharynx. The combined properties of a comprehensive sugar metabolism, an efficient immunological protection due to the polysaccharide capsule together with the cytolytic activity due to the ply/lytA island could also be related to its capability to survive well and cause damage in the lung and in the middle ear. The general view of the evolution of pathogens is based on the successive import of virulence genes from sources other than the gene pool provided by related commensals [14]. This could be true in case of S. pneumoniae for the hyaluronidase which has not been detected among S. mitis, and the non-repeat modules of the CBPs PcpA, PspC and PspA. On the other hand, loss of genes from the S. mitis core as defined in the present study might also be important for pathogen evolution, even signifying a ‘route of no return’ to a true commensal life style. These features combined with the expansion of sugar uptake and utilizing systems due to the conquest of its specific ecological niche are the recognizable features distinguishing S. pneumoniae from S. mitis.
Materials and Methods
Bacterial Strains
Bacterial strains are listed in Table S1. S. mitis B6 has been described. S. mitis and S. oralis strains used for comparative genomic hybridization have been characterized by MLST analysis [11].
Construction of the Shotgun Libraries
For shotgun sequencing three plasmid libraries with small, medium and large inserts, respectively, have been constructed. The small (1.8–2.2 kb) and medium size (4–5 kb) inserts were generated by ultrasonic treatment. After end repair with T4 polymerase (Roche) 10 µg DNA was loaded on an agarose gel (0.9%) and the appropriate size range was cut from the gel. The extracted DNA was cloned into pUC19 cleaved with SmaI (Roche).
For the large insert plasmid library the bacterial DNA was partially cleaved with the enzyme Sau3AI (Roche). The ends of the fragments were partially filled in and were cloned into the SalI (Roche) cleaved and partially filled low copy vector pMCL210.
Sequencing and Assembly of the Genome
DNA sequencing reactions were set up using Applied Biosystems BigDye Terminator v3.1 Cycle Sequencing Kit. The shotgun clones were sequenced from both sides using an ABI 3730 XL sequencer up to a 6-fold genome coverage. 60% of the data have been generated from small size insert clones, 30% from medium size and 10% from large insert size plasmid clones. The data assembly was performed using the Staden Package software version 4.6 (Roger Staden, Cambridge, UK). Gap closure was performed by combinatorial PCR followed by sequencing of the generated PCR fragments, using results obtained from alignment of the contigs with the S. pneumoniae R6 genome sequence. The sequence includes the MonX gene encoding a highly repetitive 1591 Ser-rich protein. The more precize size of this gene was determined by Southern blot analysis. Chromosomal DNA was digested with restriction endonucleases which cut outside of the repeat regions, and transferred to Hybond N membrane (Amersham) using standard protocols [83]. Two probes unique regions of monX were amplified by PCR using the primers MonX-1f and MonX-1r (GAACACTTCTGCGACAGCAACTGAC and CCGCAGAGTTGACCTTAGTGATAGC; 317 bp) and MonX-2f and MonX-2r (CATCAACTGGATCTGTGTTA and ACCGGTACAATGACCGTTAT; 273 bp). Nonradioactive labelling of the amplicon probe, hybridization to the blots, and signal detection were performed according to the instructions provided by the manufacturer (digoxigenin labelling kit, Boehringer, Mannheim). The size of monX was estimated at 12.600 bp, i.e. over 7.8 kb longer than the sequenced region, corresponding to approximately 4.200 aa.
Bioinformatics Analysis
The finished S. mitis B6 MG1363 sequence was annotated using Glimmer [84], and tRNA genes were identified with tRNAscan-SE [85]. The initial automatic functional annotation was followed by a manual review of the predicted CDSs, and alterations were made on the basis of the presence of potential ribosomal binding sites, predicted transcriptional terminators [86] and sequence alignments; for RNAs, Rfam was used [87]. All ORFs were searched against the nonredundant nucleotide and peptide sequence databases provided by the National Center for Biotechnology Information using BLAST software [88]. A special search for bacteriocins containing a double glycine leader sequence was performed and small ORFs in their vicinity was examined manually to identify putative bacteriocin and immunity protein genes.
Type I signal peptides were predicted using SignalP 3.0 neural networks and hidden Markov model implementation [89]. For the prediction of transmembrane helices in membrane proteins, TMHMM 2.0 [90] and TMpred [91] were used. Sometimes conflicting results are obtained for the prediction of signal peptides and amino-terminal transmembrane helices in proteins containing a single TM-helix. Therefore these proteins were re-analysed manually. Lipoproteins were identified with the stringent motif used by Sutcliffe and Errington [92]. Proteins using a non-classical secretion pathway were predicted using SecretomeP [93] and PSORTb version 2.0.4 [94] for bacterial protein subcellular localization prediction. A SecP score above 0.5 was considered to be significant [93]. From the output of SecretomeP all known ribosomal proteins, DNA-binding proteins (restriction enzymes, integrases, transcriptional regulators) and phage proteins were removed. Type II lipoprotein signal peptides were identified using PROSITE [95] and the searching motif <[MV]-X(0,13)-[RK]-{DERKQ}(6,20)-[LIVMFESTAG]-[LVIAM]-[IVMSTAG]-[AG]-C as defined by Sutcliffe [96]. PECACE domain harbouring, putative cell wall hydrolases were identified using the motif E-[ST]-X-G-X(1,16)-D-X-M-Q-[SA]-[SA]-E-[SG] [97].
Comparative Genome Analysis
For comparative analysis with S. mitis B6, annotated sequences of S. pneumoniae strains R6 [82], TIGR4 [64], ATCC700699 {Croucher, 2009 2435/id}, G54, CGSP14 [26] and U19_6 (http://www.ncbi.nlm.nih.gov/genomes/lproks.cgi) were used. The S. mitis NCTC12261 sequence (http://www.jcvi.org/) was annotated automatically; individual ORFs were investigated manually and compared with S. mitis B6 using the ACT visualization tool [98] and BLAST analyses.
Comparative Genome Hybridizations (CGH)
A 70-mer oligonucleotide microarray was designed based on the initial annotation of S. mitis B6. The oligonucleotides which were synthesized by OPERON (Huntsville, USA) represent 1978 genes, 461 intergenic regions and 171 controls. Oligonucleotides (30 pmol/µl) were spotted on Nexterion HiSens Slides E (SCHOTT Jenaer Glas GmbH) using the SpotArray TM24 Microarray Spotting System (PerkinElmer) with 32 SMP3-Pins (Telechem). S. mitis strains used for CGH have been described [11].
DNA Labelling and Hybridization
Chromosomal DNA was isolated as previously described [48]. 5 µg of heat denatured genomic DNA was used as a template for direct incorporation of alternate fluorescent analogs Cy5- and Cy3-dCTP (Perkin Elmer) by randomly primed polymerization reaction. Ethanol precipitated labeled DNA was resuspended in hybridization buffer (Nexterion Hyb, Formamid 1:1) and denatured twice at 95°C for 5 min. Hybridization was performed following the manufacturers' recommendations using a hybridization temperature of 40°C for 16 h. Labeled chromosomal DNA of S. mitis B6 was used as reference.
Data Processing
Microarrays were scanned on a laser scanner (ScanArray 4000 Microarray Analysis System, PerkinElmer Life Sciences) with a low resolution of 50 µm using ScanArray Express Software, Version 2.1. Photomultiplier Tube (PMT) was adjusted to balance the two fluorescence channels and biochips were scanned with a 10 µm resolution. Replicate spots that had only background values as estimated from the negative controls included on the microarray were discarded. For each experiment, the fluorescence intensity of the test strain was normalized to that obtained for the B6 reference. A histogram was produced for each data set, resulting in positive (+1) and negative (−1) hybridization signals separated by the diagonal; ambiguous spots (0) which were manually adjusted were not considered in the final analysis. The raw data of S. mitis B6 genomic comparison have been deposited in a MIAME compliant database (ArrayExpress accession number E-MEXP-2497).
Accession Numbers
Streptococcus mitis B6 microarray: ArrayDesign B6 ArrayExpress accession A-MEXP-1755. Fully annotated microarray data: ArrayExpress accession number E-MEXP-2497. Streptococcus mitis B6 genome sequence and annotation: EMBL FN568063.
Supporting Information
Repeat sequences of S. mitis LPXTG proteins with coiled-coil domains. The sequences strongly predicted to be involved in coiled-coil domain structure of three LPXTG proteins are underlined; in addition Smi_1306 contains C-terminal repeats of low predicted coiled-coil value. The numbers in brackets designate the position of the first amino acid which is shown in the alignment. Amino acids conserved in the majority of the repeats are shown in grey.
(0.07 MB PDF)
S. mitis strains used for comparative genomic hybridization. Name and references of S. mitis strains used in the study.
(0.04 MB DOC)
Comparative genomic hybridization on a S. mitis B6 specific oligonucleotide microarray using S. mitis DNA. There are 2023 features (70mers) included in the analysis, and evaluated as described in the Materials and Methods section. Hybridization signals are indicated by +1 (positive), -1 (negative), or ambigious (0).
(4.52 MB DOC)
Genomic hybridization on S. mitis B6-specific oligonucleotide microarray data. Only CDS are listed, and mobile elements and phage related gene clusters are not included. Hybridization signals are indicated by +1 (positive, blue), −1 (negative, pink), or ambigious (0). The gene products of six S. pneumoniae finished genomes as indicated above were used for an in silico comparative analysis, using 70% identity as cut off value and a 60% minimum coverage. The presence of the gene products is indicated as (x). S. mitis A: B5; B: Huo8; C: SV5; D: 658; E: Huo1; F: NCTC10712; G: SV10; H: RSA4; I: 697; K: M3; L: S. pneumoniae R6. In silico comparison with S. pneumoniae genomes: I: CGSP14; II: R6; III: TIGR4; IV: U19_6; V: G54; VI: ATCC700699. Using the annotated protein sequences, 60% identity and 70% coverage were defined as presence of the respective gene.
(4.36 MB DOC)
A. Hybridization of S. mitis with oligonucleotides corresponding to S. pneumoniae virulence factors. B. Oligonucleotides. Oligonucleotides specific for S. pneumoniae R6 (spr) or TIGR4 (SP) were used in comparative hybridization using S. mitis DNA. +1: positive signals; negative signals: −1; 0 corresponds to ambiguous signals.
(0.05 MB DOC)
Acknowledgments
We thank Ulrike Schmidt for help in gap closure, Sonja Schröck for technical assistance during DNA sequencing, and Thorsten Kneuper for the electron micrograph of φB6.
Footnotes
Competing Interests: This work has been performed in collaboration with the AGOWA company as part of the BMBF funded sequencing project. There are no issues related to employment, consultancy, patents, products. Therefore, the role of the company for this work does not alter the adherence to all the PLoS ONE policies on sharing data and materials.
Funding: This work was supported by a grant from the Bundesministerium für Bildung und Forschung (BMBF No. PTJ-BIO/0313134) and 0313801 1, the EU (Pneumococcal Resistance Epidemicity and Virulence, An International Study [PREVIS], LSHM-CT-2003-503413 and the Network of Excellence EuroPathoGenomics, LSHB-CT-2005-512061), the AGOWA company, and the Stiftung Rheinland Pfalz für Innovation 15202-386261/580. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bourgault AM, Wilson WR, Washington JA II. Antimicrobial susceptibilities of species of viridans streptococci. J Infect Dis. 1979;140:316–324. doi: 10.1093/infdis/140.3.316. [DOI] [PubMed] [Google Scholar]
- 2.Brandenburg RO, Giuliani ER, Wilson WR, Geraci JE. Infective endocarditis - a 25-year overview of diagnosis and therapy. J Am Coll Cardiol. 1983;1:280–291. doi: 10.1016/s0735-1097(83)80029-1. [DOI] [PubMed] [Google Scholar]
- 3.Van der Meer JTM, van Vianen W, Hu E, van Leeuwen WB, Valkenburg HA, et al. Distribution, antibiotic susceptibility and tolerance of bacterial isolates in culture-positive cases of endocarditis in The Netherlands. Eur J Clin Microbiol Infect Dis. 1991;10:728–734. doi: 10.1007/BF01972497. [DOI] [PubMed] [Google Scholar]
- 4.Henrichsen J. Six newly recognized types of Streptococcus pneumoniae. J Clin Microbiol. 1995;33:2759–2762. doi: 10.1128/jcm.33.10.2759-2762.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park IH, Pritchard DG, Cartee R, Brandao A, Brandileone MC, et al. Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. J Clin Microbiol. 2007;45:1225–1233. doi: 10.1128/JCM.02199-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell TJ. The pathogenesis of streptococcal infections: from tooth decay to meningitis. Nat Rev Microbiol. 2003;1:219–230. doi: 10.1038/nrmicro771. [DOI] [PubMed] [Google Scholar]
- 7.Jedrzejas MJ. Pneumococcal virulence factors: structure and function. Microbiol Mol Biol Rev. 2001;65:187–207. doi: 10.1128/MMBR.65.2.187-207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hakenbeck R, Madhour A, Denapaite D, Brückner R. Versatility of choline metabolism and choline binding proteins in Streptococcus pneumoniae and commensal streptococci. FEMS Microbiol Rev. 2009;33:572–586. doi: 10.1111/j.1574-6976.2009.00172.x. [DOI] [PubMed] [Google Scholar]
- 9.Jefferies J, Nieminen L, Kirkham LA, Johnston C, Smith A, et al. Identification of a secreted cholesterol-dependent cytolysin (mitilysin) from Streptococcus mitis. J Bacteriol. 2007;189:627–632. doi: 10.1128/JB.01092-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Llull D, López R, García E. Characteristic signatures of the lytA gene provide a basis for rapid and reliable diagnosis of Streptococcus pneumoniae. J Bacteriol. 2006;44:1250–1256. doi: 10.1128/JCM.44.4.1250-1256.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chi F, Nolte O, Bergmann C, Ip M, Hakenbeck R. Crossing the barrier: evolution and spread of a major class of mosaic pbp2x in S. pneumoniae, S. mitis and S. oralis. Int J Med Microbiol. 2007;297:503–512. doi: 10.1016/j.ijmm.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Kilian M, Poulsen K, Blomqvist T, Håvarstein LS, Bek-Thomsen M, et al. Evolution of Streptococcus pneumoniae and its close commensal relatives. PLoS ONE. 2008;3:e2683. doi: 10.1371/journal.pone.0002683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bishop CJ, Aanensen DM, Jordan GE, Kilian M, Hanage WP, et al. Assigning strains to bacterial species via the internet. BMC Biol. 2009;7:3. doi: 10.1186/1741-7007-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raskin DM, Seshadri R, Pukatzki SU, Mekalanos JJ. Bacterial genomics and pathogen evolution. Cell. 2006;124:703–714. doi: 10.1016/j.cell.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Hakenbeck R, Balmelle N, Weber B, Gardes C, Keck W, et al. Mosaic genes and mosaic chromosomes: intra- and interspecies variation of Streptococcus pneumoniae. Infect Immun. 2001;69:2477–2486. doi: 10.1128/IAI.69.4.2477-2486.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brückner R, Nuhn M, Reichmann P, Weber B, Hakenbeck R. Mosaic genes and mosaic chromosomes - genomic variation in Streptococcus pneumoniae. Int J Med Microbiol. 2004;294:157–168. doi: 10.1016/j.ijmm.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 17.Dagerhamn J, Blomberg C, Browall S, Sjostrom K, Morfeldt E, et al. Determination of accessory gene patterns predicts the same relatedness among strains of Streptococcus pneumoniae as sequencing of housekeeping genes does and represents a novel approach in molecular epidemiology. J Clin Microbiol. 2008;46:863–868. doi: 10.1128/JCM.01438-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiller NL, Janto B, Hogg JS, Boissy R, Yu S, et al. Comparative genomic analyses of seventeen Streptococcus pneumoniae strains: insights into the pneumococcal supragenome. J Bacteriol. 2007;189:8186–8195. doi: 10.1128/JB.00690-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.König A, Reinert RR, Hakenbeck R. Streptococcus mitis with unusual high level resistance to β-lactam antibiotics. Microb Drug Resist. 1998;4:45–49. doi: 10.1089/mdr.1998.4.45. [DOI] [PubMed] [Google Scholar]
- 20.Hakenbeck R, König A, Kern I, van der Linden M, Keck W, et al. Acquisition of five high-Mr penicillin-binding protein variants during transfer of high-level β-lactam resistance from Streptococcus mitis to Streptococcus pneumoniae. J Bacteriol. 1998;180:1831–1840. doi: 10.1128/jb.180.7.1831-1840.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reichmann P, Hakenbeck R. A XerD recombinase with unusual active site motifs in Streptococcus pneumoniae. J Mol Microbiol Biotechnol. 2001;4:101–110. [PubMed] [Google Scholar]
- 22.Le Bourgeois P, Bugarel M, Campo N, Daveran-Mingot ML, Labonté J, et al. The unconventional Xer recombination machinery of Streptococci/Lactococci. PLoS Genet. 2007;3:e117. doi: 10.1371/journal.pgen.0030117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eisen JA, Heidelberg JF, White O, Salzberg SL. Evidence for symmetric chromosomal inversions around the replication origin in bacteria. Genome Biol. 2000;1:RESEARCH0011. doi: 10.1186/gb-2000-1-6-research0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hendrickson H, Lawrence JG. Selection for chromosome architecture in bacteria. J Mol Evol. 2006;62:615–629. doi: 10.1007/s00239-005-0192-2. [DOI] [PubMed] [Google Scholar]
- 25.Tillier ER, Collins RA. Genome rearrangement by replication-directed translocation. Nat Genet. 2000;26:195–197. doi: 10.1038/79918. [DOI] [PubMed] [Google Scholar]
- 26.Ding F, Tang P, Hsu MH, Cui P, Hu S, et al. Genome evolution driven by host adaptations results in a more virulent and antimicrobial-resistant Streptococcus pneumoniae serotype 14. BMC Genomics. 2009;10:158. doi: 10.1186/1471-2164-10-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuroda M, Ohta T, Uchiyama I, Baba T, Yuzawa H, et al. Whole genome sequencing of methicillin-resistant Staphylococcus aureus. Lancet. 2001;357:1225–1240. doi: 10.1016/s0140-6736(00)04403-2. [DOI] [PubMed] [Google Scholar]
- 28.Burrus V, Pavlovic G, Decaris B, Guedon G. Conjugative transposons: the tip of the iceberg. Mol Microbiol. 2002;46:601–610. doi: 10.1046/j.1365-2958.2002.03191.x. [DOI] [PubMed] [Google Scholar]
- 29.Dai L, Toor N, Olson R, Keeping A, Zimmerly S. Database for mobile group II introns. Nucleic Acids Res. 2003;31:424–426. doi: 10.1093/nar/gkg049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin B, Humbert O, Càmara M, Guenzi E, Walker J, et al. A highly conserved repeated DNA element located in the chromosome of Streptococcus pneumoniae. Nucleic Acids Res. 1992;20:3479–3483. doi: 10.1093/nar/20.13.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oggioni MR, Claverys J-P. Repeated extragenic sequences in prokaryotic genomes: a proposal for the origin and dynamics of the RUP element in Streptococcus pneumoniae. Microbiology. 1999;145:2647–2653. doi: 10.1099/00221287-145-10-2647. [DOI] [PubMed] [Google Scholar]
- 32.Romero P, López R, García E. Characterization of LytA-like N-acetylmuramoyl-L-alanine amidases from two new Streptococcus mitis bacteriophages provides insights into the properties of the major pneumococcal autolysin. J Bacteriol. 2005;186:8229–8239. doi: 10.1128/JB.186.24.8229-8239.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diaz E, Lopez R, Garcia JL. EJ-1, a temperate bacteriophage of Streptococcus pneumoniae with a Myoviridae morphotype. J Bacteriol. 1992;174:5516–5525. doi: 10.1128/jb.174.17.5516-5525.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Black LW. DNA packaging in dsDNA bacteriophages. Annu Rev Microbiol. 1989;43:267–292. doi: 10.1146/annurev.mi.43.100189.001411. [DOI] [PubMed] [Google Scholar]
- 35.Campbell AM. Chromosomal insertion sites for phages and plasmids. J Bacteriol. 1992;174:7495–7499. doi: 10.1128/jb.174.23.7495-7499.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morrison DA, Mortier-Barriere I, Attaiech L, Claverys JP. Identification of the major protein component of the pneumococcal eclipse complex. J Bacteriol. 2007;189:6497–6500. doi: 10.1128/JB.00687-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Croucher NJ, Walker D, Romero P, Lennard N, Paterson GK, et al. The role of conjugative elements in the evolution of the multi-drug resistant pandemic clone Streptococcus pneumoniae Spain23F ST81. J Bacteriol. 2008;191:1480–1489. doi: 10.1128/JB.01343-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loeffler JM, Fischetti VA. Lysogeny of Streptococcus pneumoniae with MM1 phage: improved adherence and other phenotypic changes. Infect Immun. 2006;74:4486–4495. doi: 10.1128/IAI.00020-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baba T, Bae T, Schneewind O, Takeuchi F, Hiramatsu K. Genome sequence of Staphylococcus aureus strain Newman and comparative analysis of staphylococcal genomes: polymorphism and evolution of two major pathogenicity islands. J Bacteriol. 2008;190:300–310. doi: 10.1128/JB.01000-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brussow H, Canchaya C, Hardt WD. Phages and the evolution of bacterial pathogens: from genomic rearrangements to lysogenic conversion. Microbiol Mol Biol Rev. 2004;68:560–602. doi: 10.1128/MMBR.68.3.560-602.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Culebras E, Martinez JL. Aminoglycoside resistance mediated by the bifunctional enzyme 6′-N-aminoglycoside acetyltransferase-2″-O-aminoglycoside phosphotransferase. Front Biosci. 1999;4:D1–D8. doi: 10.2741/culebras. [DOI] [PubMed] [Google Scholar]
- 42.Cerda P, Goni P, Millan L, Rubio C, Gomez-Lus R. Detection of the aminoglycosidestreptothricin resistance gene cluster ant(6)-sat4-aph(3′)-III in commensal viridans group streptococci. Int Microbiol. 2007;10:57–60. [PubMed] [Google Scholar]
- 43.Fatholahzadeh B, Emaneini M, Feizabadi MM, Sedaghat H, Aligholi M, et al. Characterisation of genes encoding aminoglycoside-modifying enzymes among meticillin-resistant Staphylococcus aureus isolated from two hospitals in Tehran, Iran. Int J Antimicrob Agents. 2009;33:264–265. doi: 10.1016/j.ijantimicag.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 44.Yadegar A, Sattari M, Mozafari NA, Goudarzi GR. Prevalence of the genes encoding aminoglycoside-modifying enzymes and methicillin resistance among clinical isolates of Staphylococcus aureus in Tehran, Iran. Microb Drug Resist. 2009;15:109–113. doi: 10.1089/mdr.2009.0897. [DOI] [PubMed] [Google Scholar]
- 45.Ferrandiz MJ, Ardanuy C, Linares J, Garcia-Arenzana JM, Cercenado E, et al. New mutations and horizontal transfer of rpoB among rifampin-resistant Streptococcus pneumoniae from four Spanish hospitals. Antimicrob Agents Chemother. 2005;49:2237–2245. doi: 10.1128/AAC.49.6.2237-2245.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paik J, Kern I, Lurz R, Hakenbeck R. Mutational analysis of the Streptococcus pneumoniae bimodular class A penicillin-binding proteins. J Bacteriol. 1999;181:3852–3856. doi: 10.1128/jb.181.12.3852-3856.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoskins J, Matsushima P, Mullen DL, Tang J, Zhao G, et al. Gene disruption studies of penicillin-binding proteins 1a, 1b and 2a in Streptococcus pneumoniae. J Bacteriol. 1999;181:6552–6555. doi: 10.1128/jb.181.20.6552-6555.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sibold C, Henrichsen J, König A, Martin C, Chalkley L, et al. Mosaic pbpX genes of major clones of penicillin-resistant Streptococcus pneumoniae have evolved from pbpX genes of a penicillin-sensitive Streptococcus oralis. Mol Microbiol. 1994;12:1013–1023. doi: 10.1111/j.1365-2958.1994.tb01089.x. [DOI] [PubMed] [Google Scholar]
- 49.Ton-That H, Marraffini LA, Schneewind O. Protein sorting to the cell wall envelope of Gram-positive bacteria. Biochim Biophys Acta. 2004;1694:269–278. doi: 10.1016/j.bbamcr.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 50.Molina R, González A, Stelter M, Pérez-Dorado I, Kahn R, et al. Crystal structure of CbpF, a bifunctional choline-binding protein and autolysis regulator from Streptococcus pneumoniae. EMBO Rep. 2009;10:246–251. doi: 10.1038/embor.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith AM, Feldman C, Massidda O, McCarthy K, Ndiweni D, et al. Altered PBP 2A and its role in the development of penicillin, cefotaxime, and ceftriaxone resistance in a clinical isolate of Streptococcus pneumoniae. Antimicrob Agents Chemother. 2005;49:2002–2007. doi: 10.1128/AAC.49.5.2002-2007.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Franz CM, van Belkum MJ, Worobo RW, Vederas JC, Stiles ME. Characterization of the genetic locus responsible for production and immunity of carnobacteriocin A: the immunity gene confers cross-protection to enterocin B. Microbiology. 2000;146:621–631. doi: 10.1099/00221287-146-3-621. [DOI] [PubMed] [Google Scholar]
- 53.Takamatsu D, Bensing BA, Cheng H, Jarvis GA, Siboo IR, et al. Binding of the Streptococcus gordonii surface glycoproteins GspB and Hsa to specific carbohydrate structures on platelet membrane glycoprotein Ibalpha. Mol Microbiol. 2005;58:380–392. doi: 10.1111/j.1365-2958.2005.04830.x. [DOI] [PubMed] [Google Scholar]
- 54.Takamatsu D, Bensing BA, Prakobphol A, Fisher SJ, Sullam PM. Binding of the streptococcal surface glycoproteins GspB and Hsa to human salivary proteins. Infect Immun. 2006;74:1933–1940. doi: 10.1128/IAI.74.3.1933-1940.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lupas A, Van DM, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- 56.de Saizieu A, Gardes C, Flint N, Mitchell TJ, Amrein KE, et al. Microarray-based identification of a novel Streptococcus pneumoniae regulon controlled by an autoinduced peptide. J Bacteriol. 2000;182:4696–4703. doi: 10.1128/jb.182.17.4696-4703.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reichmann P, Hakenbeck R. Allelic variation in a peptide-inducible two component system of Streptococcus pneumoniae. FEMS Microbiol Lett. 2000;190:231–236. doi: 10.1111/j.1574-6968.2000.tb09291.x. [DOI] [PubMed] [Google Scholar]
- 58.Lux T, Nuhn M, Hakenbeck R, Reichmann P. Diversity of bacteriocins and activity spectrum in Streptococcus pneumoniae. J Bacteriol. 2007;189:7741–7751. doi: 10.1128/JB.00474-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dawid S, Roche AM, Weiser JN. The blp bacteriocins of Streptococcus pneumoniae mediate intraspecies competition both in vitro and in vivo. Infect Immun. 2007;75:443–451. doi: 10.1128/IAI.01775-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Håvarstein LS, Martin B, Johnsborg O, Granadel C, Claverys JP. New insights into the pneumococcal fracticide: relationship to clumping and identification of a novel immunity factor. Mol Microbiol. 2006;59:1297–1307. doi: 10.1111/j.1365-2958.2005.05021.x. [DOI] [PubMed] [Google Scholar]
- 61.Guiral S, Mitchell TJ, Martin B, Claverys JP. Competence-programmed predation of noncompetent cells in the human pathogen Streptococcus pneumoniae: genetic requirements. Proc Natl Acad Sci U S A. 2005;102:8710–8715. doi: 10.1073/pnas.0500879102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Claverys JP, Martin B, Havarstein LS. Competence-induced fratricide in streptococci. Mol Microbiol. 2007;64:1423–1433. doi: 10.1111/j.1365-2958.2007.05757.x. [DOI] [PubMed] [Google Scholar]
- 63.An FY, Clewell DB. Identification of the cAD1 sex pheromone precursor in Enterococcus faecalis. J Bacteriol. 2002;184:1880–1887. doi: 10.1128/JB.184.7.1880-1887.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tettelin H, Nelson KE, Paulsen IT, Eisen JA, Read TD, et al. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science. 2001;293:498–506. doi: 10.1126/science.1061217. [DOI] [PubMed] [Google Scholar]
- 65.Mitchell TJ, Paterson GK. Genetic regulation of virulence in Streptococcus pneumoniae. In: Hakenbeck R, Chhatwal GS, editors. Molecular Biology of Streptococci. Wymondham, Norfolk UK: Horizon Bioscience; 2007. pp. 205–224. [Google Scholar]
- 66.Bergmann S, Hammerschmidt S. Versatility of pneumococcal surface proteins. Microbiology. 2006;152:295–303. doi: 10.1099/mic.0.28610-0. [DOI] [PubMed] [Google Scholar]
- 67.Aguiar SI, Serrano I, Pinto FR, Melo-Cristino J, Ramirez M. The presence of the pilus locus is a clonal property among pneumococcal invasive isolates. BMC Microbiol. 2008;8:41. doi: 10.1186/1471-2180-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bagnoli F, Moschioni M, Donati C, Dimitrovska V, Ferlenghi I, et al. A second pilus type in Streptococcus pneumoniae is prevalent in emerging serotypes and mediates adhesion to host cells. J Bacteriol. 2008;190:5480–5492. doi: 10.1128/JB.00384-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Berks BC, Palmer T, Sargent F. Protein targeting by the bacterial twin-arginine translocation (Tat) pathway. Curr Opin Microbiol. 2005;8:174–181. doi: 10.1016/j.mib.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 70.Bolotin A, Quinquis B, Renault P, Sorokin A, Ehrlich SD, et al. Complete sequence and comparative genome analysis of the dairy bacterium Streptococcus thermophilus. Nat Biotechnol. 2004;22:1554–1558. doi: 10.1038/nbt1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu P, Alves JM, Kitten T, Brown A, Chen Z, et al. Genome of the opportunistic pathogen Streptococcus sanguinis. J Bacteriol. 2007;189:3166–3175. doi: 10.1128/JB.01808-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Voulhoux R, Ball G, Ize B, Vasil ML, Lazdunski A, et al. Involvement of the twin-arginine translocation system in protein secretion via the type II pathway. EMBO J. 2001;20:6735–6741. doi: 10.1093/emboj/20.23.6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lavander M, Ericsson SK, Broms JE, Forsberg A. The twin arginine translocation system is essential for virulence of Yersinia pseudotuberculosis. Infect Immun. 2006;74:1768–1776. doi: 10.1128/IAI.74.3.1768-1776.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lock RA, Hansman D, Paton JC. Comparative efficacy of autolysin and pneumolysin as immunogens protecting mice against infection by Streptococcus pneumoniae. Microb Pathogen. 1992;12:137–143. doi: 10.1016/0882-4010(92)90116-6. [DOI] [PubMed] [Google Scholar]
- 75.Whatmore AM, Efstratiou A, Pickerill AP, Broughton K, Woodward G, et al. Genetic relationships between clinical isolates of Streptococcus pneumoniae, Streptococcus oralis, and Streptococcus mitis: characterization of “atypical” pneumococci and organisms allied to S. mitis harboring S. pneumoniae virulence factor-encoding genes. Infect Immun. 2000;68:1374–1382. doi: 10.1128/iai.68.3.1374-1382.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kearns AM, Wheeler J, Freeman R, Seiders PR, Perry J, et al. Pneumolysin detection identifies atypical isolates of Streptococcus pneumoniae. J Clin Microbiol. 2000;38:1309–1310. doi: 10.1128/jcm.38.3.1309-1310.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Whatmore AM, Dowson CG. The autolysin-encoding gene (lytA) of Streptococcus pneumoniae displays restricted allelic variation despite localized recombination events with genes of pneumococcal bacteriophage encoding cell wall lytic enzymes. Infect Immun. 1999;67:4551–4556. doi: 10.1128/iai.67.9.4551-4556.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zysk G, Schneider-Wald B, Hwang JH, Bejo L, Kim KS, et al. Pneumolysin is the main inducer of cytotoxicity to brain microvascular endothelial cells caused by Streptococcus pneumoniae. Infect Immun. 2000;69:845–852. doi: 10.1128/IAI.69.2.845-852.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schneeberger PM, Janssen M, Voss A. Alpha-hemolytic streptococci: a major pathogen of iatrogenic meningitis following lumbar puncture. Case reports and a review of the literature. Infection. 1996;24:29–33. doi: 10.1007/BF01780647. [DOI] [PubMed] [Google Scholar]
- 80.Hammerschmidt S. Pneumococcal virulence factors and adhesion proteins targeting the host. In: Hakenbeck R, Chhatwal GS, editors. Molecular Biology of Streptococci. Wymondham, Norfolk UK: Horizon Press; 2007. pp. 141–203. [Google Scholar]
- 81.Standish AJ, Strocher UH, Paton JC. The two-component signal transduction system RR06/HK06 regulates expression of cbpA in Streptococcus pneumoniae. Proc Natl Acad Sci USA. 2005;102:7701–7706. doi: 10.1073/pnas.0409377102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hoskins J, Alborn WE, Jr, Arnold J, Blaszczak LC, Burgett S, et al. Genome of the bacterium Streptococcus pneumoniae strain R6. J Bacteriol. 2001;183:5709–5717. doi: 10.1128/JB.183.19.5709-5717.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sambrook J, Fritsch EF, Maniatis T. Plainview. New York: Cold Spring Harbor Laboratory Press; 1989. Molecular Cloning: A Laboratory Manual. [Google Scholar]
- 84.Delcher AL, Harmon D, Kasif S, White O, Salzberg SL. Improved microbial gene identification with GLIMMER. Nucleic Acids Res. 1999;27:4636–4641. doi: 10.1093/nar/27.23.4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25:955–964. doi: 10.1093/nar/25.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ermolaeva MD, Khalak HG, White O, Smith HO, Salzberg SL. Prediction of Transcription Terminators in Bacterial Genomes. J Mol Biol. 2000;301:27–33. doi: 10.1006/jmbi.2000.3836. [DOI] [PubMed] [Google Scholar]
- 87.Griffiths-Jones S, Moxon S, Marshall M, Khanna A, Eddy SR, et al. Rfam: annotating non-coding RNAs in complete genomes. Nucleic Acids Res. 2005;33:D121–D124. doi: 10.1093/nar/gki081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, et al. Gapped BLAST and PSI-BLAST: a new generationcof protein database search programs. Nucleic Acids Res. 1997;25:3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bendtsen JD, Nielsen H, von HG, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol. 2004;340:783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 90.Krogh A, Larsson B, Von Heijne G, Sonnhammer ELL. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J Mol Biol. 2001;305:657–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 91.Hofman K, Stoffel W. TMbase - A database of membrane spanning proteins segments. Bio Chem Hoppe-Seyler. 1993;347:166. [Google Scholar]
- 92.Sutcliffe IC, Harrington DJ. Putative lipoproteins of Streptococcus agalactiae identified by bioinformatic genome analysis. Antonie van Leeuwenhoek. 2004;85:305–315. doi: 10.1023/B:ANTO.0000020166.29833.9a. [DOI] [PubMed] [Google Scholar]
- 93.Bendtsen JD, Nielsen H, Widdick D, Palmer T, Brunak S. Prediction of twin-arginine signal peptides. BMC Bioinformatics. 2005;6:167. doi: 10.1186/1471-2105-6-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rey S, Acab M, Gardy JL, Laird MR, deFays K, et al. PSORTdb: a protein subcellular localization database for bacteria. Nucleic Acids Res. 2005;33:D164–D168. doi: 10.1093/nar/gki027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gattiker A, Gasteiger E, Bairoch A. ScanProsite: a reference implementation of a PROSITE scanning tool. Appl Bioinformatics. 2002;1:107–108. [PubMed] [Google Scholar]
- 96.Sutcliffe IC, Harrington DJ. Pattern searches for the identification of putative lipoprotein genes in Gram-positive bacterial genomes. Microbiology. 2002;148:2065–2077. doi: 10.1099/00221287-148-7-2065. [DOI] [PubMed] [Google Scholar]
- 97.Pagliero E, Dideberg O, Vernet T, Di Guilmi AM. The PECACE domain: a new family of enzymes with potential peptidoglycan cleavage activity in Gram-positive bacteria. BMC Genomics. 2005;6:19. doi: 10.1186/1471-2164-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rutherford K, Parkhill J, Crook J, Horsnell T, Rice P, et al. Artemis: sequence visualisation and annotation. Bioinformatics. 2000;16:944–945. doi: 10.1093/bioinformatics/16.10.944. [DOI] [PubMed] [Google Scholar]
- 99.Zhou L, Hui FM, Morrison DA. Characterization of IS1167, a new insertion sequence in Streptococcus pneumoniae. Plasmid. 1995;33:127–138. doi: 10.1006/plas.1995.1014. [DOI] [PubMed] [Google Scholar]
- 100.Sánchez-Beato AR, García E, López R, García JL. Identification and characterization of IS1381, a new insertion sequence in Streptococcus pneumoniae. J Bacteriol. 1997;179:2459–2463. doi: 10.1128/jb.179.7.2459-2463.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen C, Tang J, Dong W, Wang C, Feng Y, et al. A glimpse of streptococcal toxic shock syndrome from comparative genomics of S. suis 2 Chinese isolates. PLoS ONE. 2007;2:e315. doi: 10.1371/journal.pone.0000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ajdic D, McShan WM, McLaughlin RE, Savic G, Chang J, et al. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc Natl Acad Sci U S A. 2002;99:14434–14439. doi: 10.1073/pnas.172501299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Krauβ J, Hakenbeck R. A mutation in the D,D-carboxypeptidase penicillin-binding protein 3 of Streptococcus pneumoniae contributes to cefotaxime resistance of the laboratory mutant C604. Antimicrob Agents Chemother. 1997;41:936–942. doi: 10.1128/aac.41.5.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Repeat sequences of S. mitis LPXTG proteins with coiled-coil domains. The sequences strongly predicted to be involved in coiled-coil domain structure of three LPXTG proteins are underlined; in addition Smi_1306 contains C-terminal repeats of low predicted coiled-coil value. The numbers in brackets designate the position of the first amino acid which is shown in the alignment. Amino acids conserved in the majority of the repeats are shown in grey.
(0.07 MB PDF)
S. mitis strains used for comparative genomic hybridization. Name and references of S. mitis strains used in the study.
(0.04 MB DOC)
Comparative genomic hybridization on a S. mitis B6 specific oligonucleotide microarray using S. mitis DNA. There are 2023 features (70mers) included in the analysis, and evaluated as described in the Materials and Methods section. Hybridization signals are indicated by +1 (positive), -1 (negative), or ambigious (0).
(4.52 MB DOC)
Genomic hybridization on S. mitis B6-specific oligonucleotide microarray data. Only CDS are listed, and mobile elements and phage related gene clusters are not included. Hybridization signals are indicated by +1 (positive, blue), −1 (negative, pink), or ambigious (0). The gene products of six S. pneumoniae finished genomes as indicated above were used for an in silico comparative analysis, using 70% identity as cut off value and a 60% minimum coverage. The presence of the gene products is indicated as (x). S. mitis A: B5; B: Huo8; C: SV5; D: 658; E: Huo1; F: NCTC10712; G: SV10; H: RSA4; I: 697; K: M3; L: S. pneumoniae R6. In silico comparison with S. pneumoniae genomes: I: CGSP14; II: R6; III: TIGR4; IV: U19_6; V: G54; VI: ATCC700699. Using the annotated protein sequences, 60% identity and 70% coverage were defined as presence of the respective gene.
(4.36 MB DOC)
A. Hybridization of S. mitis with oligonucleotides corresponding to S. pneumoniae virulence factors. B. Oligonucleotides. Oligonucleotides specific for S. pneumoniae R6 (spr) or TIGR4 (SP) were used in comparative hybridization using S. mitis DNA. +1: positive signals; negative signals: −1; 0 corresponds to ambiguous signals.
(0.05 MB DOC)