Abstract
The main issue regarding the approach to the patient with uninvestigated dyspepsia are whether the symptoms are the result of important clinical illness which then determines the appropriate management strategy for treatment of the symptoms. A initial trial of empiric anti-secretory drugs is recommended for those without H. pylori infection and no alarm symptoms whereas H. pylori eradication is recommended for those with an active H. pylori infection. Treatment expectations for H. pylori infections should theoretically be similar to other common infectious diseases. In most regions clarithromycin resistance has undermined traditional triple therapy such that it is no longer a suitable choice as an empiric therapy. Four drug therapies such as sequential, concomitant, and bismuth-quadruple therapy are generally still acceptable choices as empiric therapies. Post eradication testing is highly recommended to provides early identification of otherwise unrecognized increasing antimicrobial resistance. However, despite the ability to successfully cure H. pylori infections, a symptomatic response can be expected in only a minority of those with dyspepsia not associated with ulcers (so called non-ulcer dyspepsia). Overall, from the patients stand point, symptomatic relief is often difficult to achieve and physicians must relay on reassurance along with empiric and individualized care.
Keywords: Dyspepsia, Helicobacter pylori, diagnosis, non-ulcer dyspepsia, gastric ulcer, H. pylori therapy
Case vignette
Scenario 1
A 57 year old Korean-American man presented with a 6 month history of daily epigastric discomfort relieved by eating. He had not experienced this problem previously. There were no aggravating factors. He was otherwise healthy, had no other gastrointestinal complaints, and had not lost weight. He did not smoke and was a social drinker. He took no drugs. He had been born in Korea and had come to the United States at age 6 as an adoptee. The family history was unknown. Physical examination and basic laboratory tests (complete blood count, basic metabolic panel, and urinalysis) were normal. The stool guaiac was negative.
Scenario 2
same patient but 25 years old
The clinical problem
Dyspepsia (bad digestion) is a common (ie, 15 to 40% of the population) and perplexing global problem with a broad differential. Initially, patients are characterized as having “uninvestigated” dyspepsia which simply means that the patient has not undergone specific diagnostic investigations most especially upper gastrointestinal endoscopy. After an appropriate evaluation the patient would be recharacterized as either having dyspepsia associated with a specific disease (eg, peptic ulcer disease), condition (eg, NSAID use), or as functional dyspepsia.
Dyspepsia like gastritis is a term has had variable use by both clinicians and patients. Some order was introduced by the ROME meetings on functional gastrointestinal disorders which have since 1988 grappled with bringing order to a variety of common gastrointestinal symptom complexes (1;2). The ROME II criteria defined functional dyspepsia as pain or discomfort centered in the upper abdomen without a definite structural or biochemical explanation. More recent iterations have separated patients with substernal discomfort and typical heartburn from those with dyspepsia. The most recent ROME III criteria reclassified functional dyspepsia with two new symptom entities: epigastric pain syndrome and meal-related symptoms termed postprandial pain syndrome (3;4). These working definitions are expected to continue to evolve as new etiological conditions are identified allowing symptom based definitions to be separated based on specific etiologies.
Strategies and Evidence
The diagnostic and management strategies for patients with dyspepsia are based first on the degree of concern regarding the presence of a serious disease and second on cost effectiveness. The 4 common approaches include: prompt endoscopy, an empiric trial of antisecretory drugs, test for H. pylori and treat those who test positive, and test for H. pylori and endoscope those who test positive. These approaches have been compared in randomized controlled trials (5-12).
The most common important clinical diagnoses presenting as dyspepsia are gastric-esophageal malignancies and peptic ulcer disease. Because of its better accuracy and the ability to obtain biopsies, upper gastrointestinal endoscopy has generally replaced barium radiographic studies as the diagnostic test of choice. The choice of a particular strategy depends on the pretest probability of finding an serious condition (defined as one in which a definite diagnose might favorably influence outcome). There is now general agreement that prompt endoscopy is the preferred strategy for those over a predefined age (typically 50 or 55) and those with “alarm symptoms” (Table 1) (13-19). The difference in recommended age cut-off is related to the fact that the prevalence of gastric carcinoma varies greatly among population (eg, 45 years in areas where gastric cancer is common and 55 in areas where it is not). Overall, the positive predictive value of alarm symptoms is poor; most will have normal upper endoscopies, and the few malignancies that are found are typically advanced (20;21). For those who do not qualify for prompt endoscopy, the decision to try empiric antisecretory therapy or test for H. pylori depends on the prevalence of H. pylori in the population. Generally empiric antisecretory therapy is recommended when the prevalence of H. pylori is 10% or less (13). In most regions, the prevalence of H. pylori is above this threshold and test and treat is the most cost effective strategy (22).
Table 1.
Alarm symptoms
| Age over 55 with new onset symptoms |
| Family history of gastric cancer |
| Unintended weight loss |
| Gastrointestinal bleeding |
| Progressive dysphagia |
| Odynophagia |
| Unexplained iron deficiency anemia |
| Persistent vomiting |
| Palpable mass or lymphadenopathy |
| Jaundice |
Scenario 1
This patient meets the criteria for early endoscopy based on age and recent onset.
Scenario 2
No alarm features are present; the prevalence of H. pylori is likely greater than 10% making the test and treat strategy like the most cost effective.
Diagnosis
Scenario 1
The patient underwent upper gastrointestinal endoscopy which showed a 1.5 cm benign appearing gastric ulcer 1 cm proximal to the angularis incisura. Biopsies of the ulcer edge and base and from the normal appearing mucosa of the antrum and corpus were taken according to the recommendations of the updated Sydney system. “Salvage” cytology was also performed. (23) and was negative for malignancy showing “Mucosecreting epithelia with regressive artifacts and leucocytes”). Histological interpretation was: “Antral biopsy samples shows mild intestinal metaplasia (atrophy +--) coexisting with active and follicular inflammation. The biopsy sample from the transitional zone features hyperplastic epithelial changes, and scattered intestinal metaplasia (+--) (vial 1). Both oxyntic samples (vial 2) show superficial inflammatory infiltrate with no atrophic changes. Biopsy samples obtained from the ulcer edge/base (vial 3) consist of connective tissue, partially covered by columnar hyperplastic epithelia including goblet cells; necrotic debris are also present. In both antral and corpus biopsy samples, bacteria with morphology consistent with H. pylori are present (++-)”.
Interpretation of the gastric histology
The histology shows a H. pylori-associated gastritis with atrophic gastritis of the antrum, non-atrophic corpus gastritis, coexisting with morphological features indicative of non-malignant active gastric ulcer. The availability of targeted antral and corpus specimens allowed the pathologist to provide an estimate of gastric cancer risk using the newly introduced OLGA staging system (Figure 1) (24). Using only the traditional histology report the clinician might be concerned about the finding of intestinal metaplasia, overestimate the cancer risk, and be unsure about what follow-up might be indicated. This case was assessed as OLGA-Stage II which signifies a low cancer risk.
Figure 1.
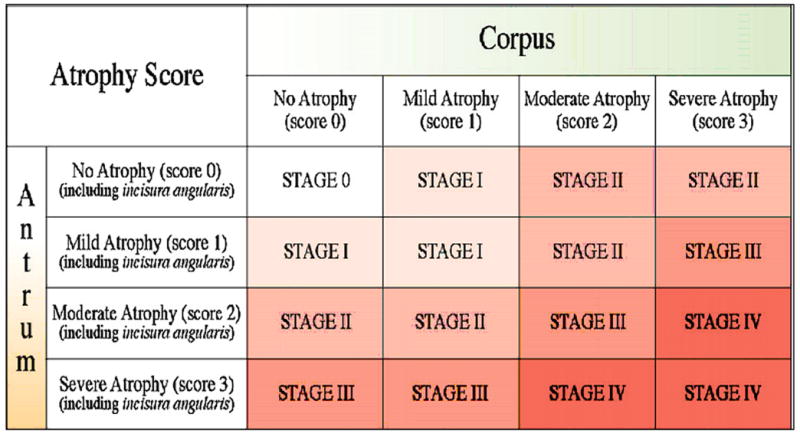
OLGA staging system for risk of gastric cancer
Scenario 2
The choices for non-invasive testing for H. pylori include IgG serology (IgM and IgA anti-H. pylori antibody tests are unreliable), stool antigen or urea breath testing. Serologic testing is no longer recommended in part because of lower sensitivity and the presence of serum anti-H. pylori antibodies can not distinguish between present and past infection. Both stool antigen testing and the urea breath test detect the presence of active infection and have excellent sensitivity and specificity. Stool antigen tests using monoclonal antibodies are preferred over those using polyclonal antibodies. One caveat is that the use of antibiotics, bismuth, or proton pump inhibitors will reduce the bacterial load in the stomach and may result in false negative results. This is a problem both with non-invasive tests (urea breath test and stool antigen test) and with invasive tests such as histology, rapid urease testing, and culture. It is therefore recommended that when possible testing not be done within one, preferably 2 weeks of the use of any of these drugs. When there is a high pretest probability for H. pylori infection (ie, presence of an ulcer) and the tests are negative, the test should be repeated after discontinuing these drugs. H2-receptor antagonists do not effect the H. pylori bacterial load and thus one can temporarily switch from PPI to an H2RA for patients who require continuous antisecretory drug therapy.
The patient in Scenario 2 received a urea breath test which was positive. In most instances endoscopy would not be done in a young person without alarm systems because even if an ulcer were present, the ulcer disease would be cured along with the H. pylori infection.
Management
Scenario 1
The issues that need to be addressed include a strategy for obtaining relief of symptoms, healing of the ulcer, choice of anti-H. pylori therapy, deciding on a strategy for confirmation of cure of the infection, and deciding whether endoscopic confirmation of healing of the ulcer was warranted.
The issues for Scenario 2 would be choice of anti-H. pylori therapy, deciding on a strategy for confirmation of cure of the infection (Figure 2).
Figure 2.
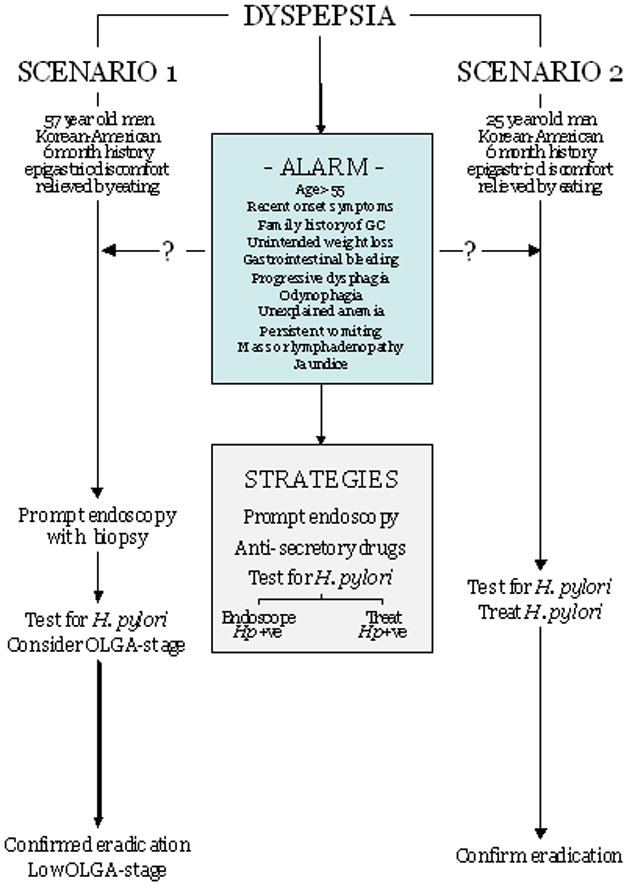
Schema for evaluation of the patient
Choice of anti-H. pylori therapy
Both scenario 1 and 2 require choosing an appropriate anti-H. pylori therapy. This decision has become increasingly difficult because, with few exceptions, worldwide empiric use of traditional (legacy) triple therapy with a PPI, clarithromycin and amoxicillin no longer provides an acceptable cure rate (ie, cure rates now typically below 80%) (25-33;33-37). This decline in effectiveness is primarily related to the increasing prevalence of clarithromycin resistance. The decline in effectiveness has often been unrecognized by practicing clinicians in part because, in contrast to other common infectious diseases, regional and local data concerning antibiotic resistance patterns is usually unavailable. In addition, post treatment testing for cure is often not routinely done such that clinicians were not alerted to the increasing tide of failures (37-39). Recent expert consensus statements and guidelines have also failed to effectively deal with this issue. They generally have acknowledged that legacy triple therapy should not be used empirically in regions where resistance is common, but fail to note with few exceptions (eg, Northern Eruope) high level resistance is now the rule and not the exception. It is ironic that when increasing resistance results in reduced cure rates for common bacterial infections (eg, Streptococcus pneumoniae), previously recommended therapies are rapidly abandoned for therapies that provide high cure rates. In contrast, with H. pylori infections, steadily falling cure rates have until recently been accepted and even recommended as first line. The H. pylori literature is also replete with examples of comparative trials in region where clarithromycin resistance has made triple therapy ineffective allowing investigators to “prove” that a new therapy A is superior to one with proven unacceptable low cure rates (38). Hopefully, H. pylori like other infectious diseases will begin to stress cure rates, only highly successful therapies will be compared, patients will no longer be randomized to known inferior regimens, and meta-analyses will no longer recommend as equivalent therapies that provide less than acceptable cure rates (ie, if both are bad, can one be better) (32-34;37;38;40).
High cure rates for H. pylori infections currently consists of multidrug therapies and include an antisecretory drug. Therapy tailored to antimicrobial susceptibility is preferred for all infectious diseases but is not yet widely available (41). Four drug combinations currently provide the best results although studies evaluating dose, duration, formulation, etc. have generally not been done such that the therapy is still more an art than a science (39). The currently best 4 drug combinations are shown in Table 2 and consist of two general combinations A) a PPI, amoxicillin, clarithromycin, metronidazole/tinidazole given either sequentially or concomitantly, or B) a PPI, a bismuth, tetracycline HCl, and metronidazole/tinidazole (37;42). Metronidazole resistance, in contrast to clarithromycin resistance, can be partially over come by increasing the dosage and duration of therapy. Both commercially available bismuth-containing convenience packs available in the United states (Helidac® and Pylera®) contain suboptimal doses of metronidazole (250 mg q.i.d. for Helidac® and 375 t.i.d. for Pylera® and in areas where metronidazole resistance is likely to be prevalent (ie, women, Hispanics, individuals from developing countries), it may be prudent to add an additional prescription to bring the dose up to 500 mg t.i.d. In addition, Pylera® contains a suboptimal dose of tetracycline (375 mg t.i.d.) instead of the recommended 500 mg q.i.d.
Table 2.
Four-drug therapies for H. pylori in the era of prevalent clarithromycin resistance
|
Confirmation of cure
As there is no universally effective empiric therapy, post eradication testing is recommended (eg, with a urea breath test or stool antigen test) to both confirm cure and to serve as an early warning of the rise in antibiotic resistance locally (37). Post eradication testing should be delayed a minimum of 4 weeks after the end of therapy to allow regrowth of any remaining bacteria. The caveats regarding avoiding use of antibiotics, bismuth and PPIs mentioned above also applies.
Confirmation of healing of the gastric ulcer
Until recently, it was standard of care to follow-up all gastric ulcers to confirm healing and more importantly to reconfirm that the ulcer was not actually a gastric cancer. This strategy was based on the knowledge that 1 to 5% of benign appearing ulcers are actually gastric cancers (43;44). As a general rule, gastric ulcers should be followed to complete healing confirmed endoscopically. Confirmation of cure of H. pylori can be done histologically at the same time. Endoscopic follow-up of duodenal ulcers is unnecessary.
Guidelines
Five guidelines and recommendations for dyspepsia have been published including those from North America, Europe and Asia (13-19) as well as a recent excellent review of the various guidelines (45). The agree on the major points. Differences primarily relate to the initial steps for those without alarm symptoms and these differences generally relate to the differences audiences (general physicians vs. gastroenterologists). There is also a well thought out discussion of dyspepsia associated with non-steroidal anti-inflammatory drug therapy with a useful algorithm (46).
Conclusions and Recommendations
The main issues regarding the approach to a patient with uninvestigated dyspepsia are whether the symptoms are the result of important clinical illness and what is the appropriate management strategy for treatment of the symptoms. A initial trial of empiric anti-secretory drugs is recommended for those without H. pylori infection and no alarm symptoms whereas H. pylori eradication is recommended for those with an active H. pylori infection. H. pylori infection is etiologically associated with clinical outcomes such as duodenal ulcer disease, gastric ulcer disease, and atrophic gastritis. Atrophic gastritis in turn may lead to iron and/or vitamin B12 deficiency, gastric adenocarcinoma and/or primary B-cell gastric lymphoma.(47-50). To Eradication is associated with healing of the gastritis prevention or cure of peptic ulcers. If done before the onset of atrophy will likely prevent gastric cancer. Thus, it is recommended for all H. pylori infections.
The treatment of H. pylori infection is being rethought to bring the expectations into line with other common treatable infectious diseases. It is now recognized that recommendations regarding the continued use of triple therapy are generally not useful for clinicians in that they lack specific details about the global problem of clarithromycin resistance and it deleterious effects on cure rates. In general, recent “consensus” groups and Society guidelines have recommended legacy triple therapy with caveat that if there is a high prevalence of resistance alternate therapy should be used. The general high prevalence of resistance supports the recommendation currently all patients should considered to have clarithromycin resistant H. pylori infections and legacy triple therapies should be avoided as an empiric choice (37-39). Hopefully, the next generation of recommendations will focus on empiric therapies that reliably provide high cure rates (Grade A or B results on the scale of A = ≥9% to F ≤80%)(35). The gold standard therapy should be “the one that works locally”. Recent consensus statements and guidelines agree regarding the importance of diagnostic testing both pre and post therapy (51;52). Post eradication confirmation of cure is especially because it can provide early identification of otherwise unrecognized increasing antimicrobial resistance. However, despite the ability to successfully cure H. pylori infections, a symptomatic response can be expected in only a minority of those with dyspepsia not associated with ulcers (so called non-ulcer dyspepsia) (53). Overall, from the patients stand point, symptomatic relief is often difficult to achieve and physicians must relay on reassurance along with empiric and individualized care. For many patients, therapy consists of reassurance along with empiric and individualized.
Acknowledgments
This material is based upon work supported in part by the Office of Research and Development Medical Research Service Department of Veterans Affairs. Dr. Graham is supported in part by Public Health Service grant DK56338 which funds the Texas Medical Center Digestive Diseases Center and R01 CA116845. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the VA or NIH. In the last 3 years, Dr. Graham has received small amounts of grant support and/or free drugs or urea breath tests from Meretek and BioHit for investigator initiated and completely investigator controlled research. Dr. Graham is a consultant for Novartis in relation to vaccine development for treatment or prevention of H. pylori infection. Dr. Graham is a also a paid consultant for Otsuka Pharmaceuticals and until July 2007 was a member of the Board of Directors of Meretek, Diagnostics, the manufacturer of the 13C-urea breath test. Until October 2009, Dr. Graham received royalties on the Baylor College of Medicine patent covering materials related to 13C-urea breath test.
References
- 1.Drossman DA. The functional gastrointestinal disorders and the Rome II process. Gut. 1999;45(Suppl 2):II1–II5. doi: 10.1136/gut.45.2008.ii1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson WG. The road to Rome. Gut. 1999;45(Suppl 2):II80. doi: 10.1136/gut.45.2008.ii80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clouse RE, Mayer EA, Aziz Q, et al. In: ROME III The functional gastrointestinal disorders. 3. Drossman DA, Corazziari E, Delvaux M, Spiller RC, Talley NJ, Thompson WG, et al., editors. Allen Press; Lawrence, KS: 2006. pp. 557–594. [Google Scholar]
- 4.Drossman DA. Rome III: the new criteria. Chin J Dig Dis. 2006;7:181–185. doi: 10.1111/j.1443-9573.2006.00265.x. [DOI] [PubMed] [Google Scholar]
- 5.Delaney BC, Wilson S, Roalfe A, et al. Randomised controlled trial of Helicobacter pylori testing and endoscopy for dyspepsia in primary care. BMJ. 2001;322:898–901. doi: 10.1136/bmj.322.7291.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delaney BC, Wilson S, Roalfe A, et al. Cost effectiveness of initial endoscopy for dyspepsia in patients over age 50 years: a randomised controlled trial in primary care. Lancet. 2000;356:1965–1969. doi: 10.1016/s0140-6736(00)03308-0. [DOI] [PubMed] [Google Scholar]
- 7.Lewin van den Broek NT, Numans ME, Smout AJ, et al. A randomised controlled trial of four management strategies for dyspepsia: relationships between symptom subgroups and strategy outcome. Br J Gen Pract. 2001;51:619–624. [PMC free article] [PubMed] [Google Scholar]
- 8.Laheij RJ, Severens JL, van de Lisdonk EH, et al. Randomized controlled trial of omeprazole or endoscopy in patients with persistent dyspepsia: a cost-effectiveness analysis. Aliment Pharmacol Ther. 1998;12(12):1249–1256. doi: 10.1046/j.1365-2036.1998.00423.x. [DOI] [PubMed] [Google Scholar]
- 9.McColl KE, Murray LS, Gillen D, et al. Randomised trial of endoscopy with testing for Helicobacter pylori compared with non-invasive H pylori testing alone in the management of dyspepsia. BMJ. 2002;324:999–1002. doi: 10.1136/bmj.324.7344.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arents NL, Thijs JC, van Zwet AA, et al. Approach to treatment of dyspepsia in primary care: a randomized trial comparing “test-and-treat” with prompt endoscopy. Arch Intern Med. 2003;163:1606–1612. doi: 10.1001/archinte.163.13.1606. [DOI] [PubMed] [Google Scholar]
- 11.Heaney A, Collins JS, Watson RG, et al. A prospective randomised trial of a “test and treat” policy versus endoscopy based management in young Helicobacter pylori positive patients with ulcer-like dyspepsia, referred to a hospital clinic. Gut. 1999;45:186–190. doi: 10.1136/gut.45.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manes G, Menchise A, de Nucci C, et al. Empirical prescribing for dyspepsia: randomised controlled trial of test and treat versus omeprazole treatment. BMJ. 2003;326:1118. doi: 10.1136/bmj.326.7399.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Talley NJ. American Gastroenterological Association medical position statement: evaluation of dyspepsia. Gastroenterology. 2005;129:1753–1755. doi: 10.1053/j.gastro.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 14.Talley NJ, Vakil NB, Moayyedi P. American gastroenterological association technical review on the evaluation of dyspepsia. Gastroenterology. 2005;129:1756–1780. doi: 10.1053/j.gastro.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 15.Talley NJ, Lam SK, Goh KL, et al. Management guidelines for uninvestigated and functional dyspepsia in the Asia-Pacific region: First Asian Pacific Working Party on Functional Dyspepsia. J Gastroenterol Hepatol. 1998;13:335–353. doi: 10.1111/j.1440-1746.1998.tb00644.x. [DOI] [PubMed] [Google Scholar]
- 16.Talley NJ, Vakil N. Guidelines for the management of dyspepsia. Am J Gastroenterol. 2005;100:2324–2337. doi: 10.1111/j.1572-0241.2005.00225.x. [DOI] [PubMed] [Google Scholar]
- 17.Veldhuyzen van Zanten SJ, Flook N, et al. An evidence-based approach to the management of uninvestigated dyspepsia in the era of Helicobacter pylori. Canadian Dyspepsia Working Group. CMAJ. 2000;162(12 Suppl):S3–23. [PMC free article] [PubMed] [Google Scholar]
- 18.National Institute for Clinical Excellence. Dyspepsia: Managing dyspepsia in adults in primary care. 2004 www.nice.org.uk/pdf/CG-017fullguideline.pdf.
- 19.Scottish Intercollegiate Guidelines Network. Dyspepsia: A national clinical guideline. 2003 www.sign.ac.uk/pdf/sign68.pdf.
- 20.Adang RP, Vismans JF, Talmon JL, et al. Appropriateness of indications for diagnostic upper gastrointestinal endoscopy: association with relevant endoscopic disease. Gastrointest Endosc. 1995;42:390–397. doi: 10.1016/s0016-5107(95)70037-4. [DOI] [PubMed] [Google Scholar]
- 21.Vakil N, Moayyedi P, Fennerty MB, et al. Limited value of alarm features in the diagnosis of upper gastrointestinal malignancy: systematic review and meta-analysis. Gastroenterology. 2006;131:390–401. doi: 10.1053/j.gastro.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 22.Ford AC, Qume M, Moayyedi P, et al. Helicobacter pylori “test and treat” or endoscopy for managing dyspepsia: an individual patient data meta-analysis. Gastroenterology. 2005;128(7):1838–1844. doi: 10.1053/j.gastro.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Green LK, Zachariah S, Graham DY. The use of gastric salvage cytology in the diagnosis of malignancy: a review of 731 cases. Diagn Cytopathol. 1990;6:1–4. doi: 10.1002/dc.2840060102. [DOI] [PubMed] [Google Scholar]
- 24.Rugge M, Correa P, Di Mario F, et al. OLGA staging for gastritis: a tutorial. Dig Liver Dis. 2008;40:650–658. doi: 10.1016/j.dld.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 25.Yamaoka Y, Graham DY, Lu H. Should triple therapy for Helicobacter pylori infection be abandoned as no longer effective? US Gastroenterology. 2008;4:65–67. [Google Scholar]
- 26.Huang AH, Sheu BS, Yang HB, et al. Impact of Helicobacter pylori antimicrobial resistance on the outcome of 1-week lansoprazole-based triple therapy. J Formos Med Assoc. 2000;99:704–709. [PubMed] [Google Scholar]
- 27.Zanten SJ, Bradette M, Farley A, et al. The DU-MACH study: eradication of Helicobacter pylori and ulcer healing in patients with acute duodenal ulcer using omeprazole based triple therapy. Aliment Pharmacol Ther. 1999;13:289–295. doi: 10.1046/j.1365-2036.1999.00471.x. [DOI] [PubMed] [Google Scholar]
- 28.Sheu BS, Wu JJ, Lo CY, et al. Impact of supplement with Lactobacillus- and Bifidobacterium-containing yogurt on triple therapy for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2002;16:1669–1675. doi: 10.1046/j.1365-2036.2002.01335.x. [DOI] [PubMed] [Google Scholar]
- 29.Bazzoli F, Pozzato P, Rokkas T. Helicobacter pylori: the challenge in therapy. Helicobacter. 2002;7(Suppl 1):43–49. doi: 10.1046/j.1523-5378.7.s1.7.x. [DOI] [PubMed] [Google Scholar]
- 30.Georgopoulos SD, Ladas SD, Karatapanis S, et al. Effectiveness of two quadruple, tetracycline-or clarithromycin-containing, second-line, Helicobacter pylori eradication therapies. Aliment Pharmacol Ther. 2002;16:569–575. doi: 10.1046/j.1365-2036.2002.01220.x. [DOI] [PubMed] [Google Scholar]
- 31.Peitz U, Sulliga M, Wolle K, et al. High rate of post-therapeutic resistance after failure of macrolide-nitroimidazole triple therapy to cure Helicobacter pylori infection: impact of two second-line therapies in a randomized study. Aliment Pharmacol Ther. 2002;16:315–324. doi: 10.1046/j.1365-2036.2002.01173.x. [DOI] [PubMed] [Google Scholar]
- 32.Vakil N, Lanza F, Schwartz H, et al. Seven-day therapy for Helicobacter pylori in the United States. Aliment Pharmacol Ther. 2004;20:99–107. doi: 10.1111/j.1365-2036.2004.02029.x. [DOI] [PubMed] [Google Scholar]
- 33.Fuccio L, Minardi ME, Zagari RM, et al. Meta-analysis: duration of first-line proton-pump inhibitor based triple therapy for Helicobacter pylori eradication. Ann Intern Med. 2007;147:553–562. doi: 10.7326/0003-4819-147-8-200710160-00008. [DOI] [PubMed] [Google Scholar]
- 34.Zagari RM, Bianchi-Porro G, Fiocca R, et al. Comparison of 1 and 2 weeks of omeprazole, amoxicillin and clarithromycin treatment for Helicobacter pylori eradication: the HYPER Study. Gut. 2007;56:475–479. doi: 10.1136/gut.2006.102269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graham DY, Lu H, Yamaoka Y. A report card to grade Helicobacter pylori therapy. Helicobacter. 2007;12:275–278. doi: 10.1111/j.1523-5378.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 36.Fischbach L, Evans EL. Meta-analysis: the effect of antibiotic resistance status on the efficacy of triple and quadruple first-line therapies for Helicobacter pylori. Aliment Pharmacol Ther. 2007;26:343–357. doi: 10.1111/j.1365-2036.2007.03386.x. [DOI] [PubMed] [Google Scholar]
- 37.Graham DY, Shiotani A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat Clin Pract Gastroenterol Hepatol. 2008;5:331. doi: 10.1038/ncpgasthep1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Graham DY. Efficient Identification and Evaluation of Effective Helicobacter pylori Therapies. Clin Gastroenterol Hepatol. 2009;7:145–148. doi: 10.1016/j.cgh.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graham DY, Lu H, Yamaoka Y. Therapy for Helicobacter pylori infection can be improved : sequential therapy and beyond. Drugs. 2008;68:725–736. doi: 10.2165/00003495-200868060-00001. [DOI] [PubMed] [Google Scholar]
- 40.Graham DY, Yamaoka Y. One- or two-week triple therapy for Helicobacter pylori: questions of efficacy and inclusion of a dual therapy treatment arm. Gut. 2007;56:1021–1023. doi: 10.1136/gut.2006.118109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Furuta T, Shirai N, Kodaira M, et al. Pharmacogenomics-based tailored versus standard therapeutic regimen for eradication of H. pylori Clin Pharmacol Ther. 2007;81:521–528. doi: 10.1038/sj.clpt.6100043. [DOI] [PubMed] [Google Scholar]
- 42.Essa AS, Kramer JR, Graham DY, et al. Meta-analysis: four-drug, three-antibiotic, non-bismuth-containing “concomitant therapy” versus triple therapy for Helicobacter pylori eradication. Helicobacter. 2009;14:109–118. doi: 10.1111/j.1523-5378.2009.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pruitt RE, Truss CD. Endoscopy, gastric ulcer, and gastric cancer. Follow-up endoscopy for all gastric ulcers? Dig Dis Sci. 1993;38:284–288. doi: 10.1007/BF01307545. [DOI] [PubMed] [Google Scholar]
- 44.Podolsky I, Storms PR, Richardson CT, et al. Gastric adenocarcinoma masquerading endoscopically as benign gastric ulcer. A five-year experience. Dig Dis Sci. 1988;33:1057–1063. doi: 10.1007/BF01535778. [DOI] [PubMed] [Google Scholar]
- 45.Ford AC, Moayyedi P. Current guidelines for dyspepsia management. Dig Dis. 2008;26:225–230. doi: 10.1159/000121351. [DOI] [PubMed] [Google Scholar]
- 46.Gupta S, McQuaid K. Management of nonsteroidal, anti-inflammatory, drug-associated dyspepsia. Gastroenterology. 2005;129:1711–1719. doi: 10.1053/j.gastro.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 47.Cardenas VM, Mulla ZD, Ortiz M, et al. Iron deficiency and Helicobacter pylori infection in the United States. Am J Epidemiol. 2006;163:127–134. doi: 10.1093/aje/kwj018. [DOI] [PubMed] [Google Scholar]
- 48.Dholakia KR, Dharmarajan TS, Yadav D, et al. Vitamin B12 deficiency and gastric histopathology in older patients. World J Gastroenterol. 2005;11:7078–7083. doi: 10.3748/wjg.v11.i45.7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DuBois S, Kearney DJ. Iron-deficiency anemia and Helicobacter pylori infection: a review of the evidence. Am J Gastroenterol. 2005;100:453–459. doi: 10.1111/j.1572-0241.2005.30252.x. [DOI] [PubMed] [Google Scholar]
- 50.Hershko C, Lahad A, Kereth D. Gastropathic sideropenia. Best Pract Res Clin Haematol. 2005;18:363–380. doi: 10.1016/j.beha.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 51.Chey WD, Wong BC. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808–1825. doi: 10.1111/j.1572-0241.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- 52.Malfertheiner P, Megraud F, O’Morain C, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772–781. doi: 10.1136/gut.2006.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moayyedi P, Soo S, Deeks J, et al. Eradication of Helicobacter pylori for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2006:CD002096. doi: 10.1002/14651858.CD002096.pub4. [DOI] [PubMed] [Google Scholar]


