Abstract
Purpose
To determine the effects of corneal epithelial membrane-type 1 matrix metalloproteinase (MT1-MMP) on vascular endothelial migration and proliferation.
Methods
We generated immortalized wild-type, MT1-MMP knockout and MT1-MMP knockin corneal epithelial cells. Calf pulmonary arterial endothelial (CPAE) cell proliferation and Boyden chamber migration were assayed.
Results
Conditioned media from MT1-MMP epithelial knockout cells significantly increased CPAE proliferation BrdU incorporation, and CPAE migration as compared with wild-type epithelial cells. Conditioned media from knockin cells reversed the increase in CPAE proliferation, BrdU incorporation and CPAE migration. Knockin cells transfected with mutant MT1-MMP (E240A) did not abrogate the reversal effect.
Conclusions
Corneal epithelial MT1-MMP is anti-angiogenic. This anti-angiogenic activity does not require the catalytic domain.
Introduction
The broad substrate specificity of Matrix metalloproteinases (MMPs) against extracellular matrix (ECM) molecules makes MMPs important in normal tissue remodeling and in disease states.1-3 During angiogenesis, membrane-type 1 MMP (MT1-MMP or MMP-14) is expressed at the surface of invading vascular endothelial cells.4 MT1-MMP has been shown to possess pro-angiogenic properties, partly mediated by MMP-2 activation and regulated by the tissue inhibitor of MMP, TIMP-2. 5 Implantation of fibroblast growth factor-2 (FGF-2) pellets in the corneas of MT1-MMP knockout (KO) mice fails to promote angiogenesis,6 indicating the importance of MT1-MMP for this process.
The mechanisms by which corneal epithelial cells contribute to corneal avascularity have not been fully elucidated.7 Corneal avascularity requires a balance between several angiogenic (VEGF, bFGF) and anti-angiogenic (endostatin, thrombospondin-1) factors.7, 8, 9 The corneal epithelium expresses soluble forms of VEGF receptor-1 and -3, which act as endogenous VEGF traps.10, 11 MMPs expressed in the corneal epithelium generate the anti-angiogenic factors, angiostatin and endostatin, through their proteolytic activity on plasminogen and collagen XVIII, respectively.12, 13, 14 We have shown that MT1-MMP is expressed in corneal epithelial cells, primarily in the basal epithelium, in unwounded corneas, and after keratectomy wounds in vivo.15 In contrast to its pro-angiogenic role in vascular endothelial cells and stromal fibroblasts, we report here that MT1-MMP has an anti-angiogenic role in corneal epithelial cells, which may be a contributing factor to the anti-angiogenic role of epithelial cells in the cornea.
Materials and Methods
Immortalized cell lines
MT1-MMP KO mouse corneas were obtained from Dr. Zhongjun Zhou (Department of Medical Biochemistry and Biophysics, Karolinska Institute, Stockholm, Sweden). Immortalized WT and KO mouse corneal epithelial cell lines were generated and established after infection with the large T antigen-encoding retroviral expressing vector (pZIPTEX). Cells were grown in supplemented hormone epithelial medium (SHEM).
Characterization of corneal epithelial cells
Immortalized corneal epithelial cells from WT and MT1-MMP KO mice were subcloned to generate corneal epithelial cell lines. Cell lines were characterized immunohistochemically using anti-keratin AE1/AE3 and anti-α smooth muscle actin (αSMA) antibodies.
Cultured epithelial cells were fixed for 15 min in 1 ml cold (−20°C) methanol and rinsed three times in phosphate-buffered saline (PBS). Fixed cells were incubated in a blocking buffer of 1% bovine serum albumin (Sigma-Aldrich, St. Louis, MO) and 0.2% Triton X-100 (Sigma-Aldrich) in PBS for 30 min at room temperature to block nonspecific binding. Cells were then incubated with the following primary antibodies for 1 hr at room temperature: monoclonal mouse anti-keratin (AE1/AE3) antibody (ICN Biomedicals, Costa Mesa, CA; diluted 1:100 in 1% BSA-PBS) and monoclonal mouse anti-αSMA antibody (ICN Biomedicals; diluted 1:200 in 1% BSA-PBS). The fixed cultured cells were then incubated with secondary antibody (fluorescein-conjugated donkey anti-mouse IgG, Jackson ImmunoResearch Laboratories, West Grove, PA; diluted 1:200 in 1% BSA-PBS) for 1 hr at room temperature. As a negative control, the primary antibody was omitted. Coverslips were washed three times in PBS and mounted with mounting medium containing propidium iodide (PI; Vector Laboratories, Burlingame, CA) to permit visualization of nuclei. Cells were viewed under an epifluorescence microscope (Eclipse E 800; Nikon, Tokyo, Japan).
Cell cultures
Calf pulmonary arterial endothelial (CPAE) cells were obtained from VEC Technologies (Rensselaer, NY). Cells were routinely grown in MCDB-131 complete medium (VEC Technologies, Inc.). WT and MT1-MMP KO corneal epithelial cells, as well as stable transfected cell lines, were grown in Dulbecco's modified Eagle's medium (DMEM; Cellgro by Mediatech Inc., Herndon, VA) supplemented with 10% heat-inactivated FCS (Sigma), 100 U/ml penicillin, 100 μg/ml streptomycin, and 250 ng/ml amphotericin B (Cellgro), in 5% CO2 at 37°C. All experiments were initiated with cells in the log phase of growth. Cells were approximately 80% confluent upon completion of the experiment. DMEM containing 0.5% FCS was used for starvation.
Western blots were performed on lysed cells. Cells were washed three times with PBS and incubated in fresh serum-free medium for at least 24 hrs. The serum-free conditioned medium was then collected, followed by cell lyses.
Plasmid construction and cloning into retroviral vector
Using primers and mouse MT1-MMP cDNA as a template, we generated MT1-MMP cDNA fragments. Genes encoding the full-length mouse WT and an enzymatically-inactive mutant of MT1-MMP (E240A) were cloned into plasmid pFB. An internal ribosome entry site (IRES) and enhanced green fluorescent protein (EGFP) were linked to this construction. All constructs were confirmed by DNA sequencing. For the MT1-MMP-E240A, site-directed mutagenesis was performed on MT1-MMPs cDNA and the codon GAG coding for Glutamic acid was mutated to the GCG codon for Alanine (E240A; Stratagene's-QuikChange-II-Site-Directed-Mutagenesis-Kit).
A replication-defective retroviral gene transfer system was used to create MT1-MMP knock-in corneal cell lines. In order to generate infectious virus particles that carry the gene of interest, HEK-293T cells were co-transfected (Effectene Transfection Reagent; Qiagen, Valencia, CA) with expression vectors encoding the viral internal structural proteins, reverse transcriptase, integrase (pVPack-Gag-Pol vector), viral envelope protein (pVPack-Eco vector) and the expression plasmid (pFB, pFB MT1-MMP, or pFB MT1-MMP-E240A) for the production of the replication defective retrovirus. The procedure was performed in accordance with the manufacturer's instructions (Stratagene, La Jolla, CA).
Transfection for generation and amplification of recombinant virus in 293T cells
Recombinant viruses were generated by cotransfection of 293T cells with recombinant EGFP-expressing pFB vector-coding MT1-MMP and its mutant forms and E240A. An empty vector was also transfected. 293T cells were used to generate high-titer virus stocks by amplifying viruses. Effectene transfection reagent (Qiagen, Valencia, CA) was used to achieve high transfection efficiency. Briefly, 2 μl of DNA, 1 μg of virus envelope and 1 μg of gag-pol were mixed with 16 μl of Enhancer and 300 μl of buffer for efficient DNA condensation. The mixture was vortexed and incubated for 5 min. Then, 60 μl of Effectene transfection reagent was added. The medium from the second and third days after transfection was collected and used for infection of cell lines. This medium contained the highest concentration of virus.
Expression of MT1-MMP introduced into WT or MT1-MMP KO epithelial cells by retroviral infection
Corneal epithelial cell lines from WT or MT1-MMP KO mice were stably transfected with either MT1-MMP, an empty vector, or the MT1-MMP mutant (E240A). Cells were sorted using fluorescence (EGFP; Table 1). A high-titer recombinant virus-containing medium, amplified in 293T cells, was used to infect epithelial cell lines. Cells transfected with an empty vector and non-transfected cells were used as controls.
Table 1.
MT1-MMP-overexpressing, Knockout, and Knockin Mouse Corneal Epithelial Cell Lines
| I. Wild Type Epithelial Cell Lines with MT1-MMP Over-expression | |
| WT Epi / MT1-MMP: | WT corneal epithelial cells transfected with MT1-MMP |
| WT Epi / MT1-MMP-E240A: | WT corneal epithelial cells transfected with mutant MT1-MMP-E240A |
| II. MT1-MMP Epithelial Knockout Cell Line | |
| KO Epi: | MT1-MMP KO corneal epithelial cells (non-transfected) |
| III. MT1-MMP Knockin Epithelial Cell Lines | |
| KI Epi / MT1-MMP: | MT1-MMP KO corneal epithelial cells transfected with MT1-MMP |
| KI Epi / MT1-MMP-E240A: | MT1-MMP KO corneal epithelial cells transfected with mutant MT1-MMP-E240A |
MT1-MMP: membrane type-1 metalloproteinase; WT: wild type; KO: knockout, KI: knockin.
WT and MT1-MMP epithelial cell lines were seeded in cell culture plates until they reached 80% confluence. To enhance the infection process, 4 μg/μl polybrene was added to the solution. Cells were incubated at 37°C for 1-3 days. Cells were monitored daily for the presence of EGFP expression using an epi-fluorescence microscope.
Flow cytometry and cell sorting
Cells were sorted based on the expression of EGFP. Single cell suspensions were prepared and washed with cold PBS, trypsinized, and centrifuged at 1,000 rpm for 5 min. Suspensions were fixed in PBS containing 1% paraformaldehyde. Flow cytometry analysis was performed using a Coulter EPICS XL-MCL flow cytometer (Coulter Electronics Inc., Miami, FL). Stained cells were sorted using a Coulter ELITE cell sorter to yield two cell populations: EGFP-positive and -negative cells.
Viable cells were gated based on their forward and side scatter characteristics. Gates were set to sort positive and negative cell populations. The percentage of EGFP-positive cells was determined. The EGFP-positive population was cultured and sorted again when needed. The stability of the expression of EGFP was monitored by flow cytometric analysis. The expression of MT1-MMP was examined by western blotting analysis.
Collection of cell lysates and conditioned media
EGFP-positive cells were sorted and plated in a 75 cm2 flask at a concentration of 10,000 cells/ml in DMEM supplemented with 10% heat-inactivated FCS at 37°C in 5% CO2. When they reached confluence, cells were kept in 5ml serum-free medium for 24 hrs, followed by collection of cell lysates and conditioned media. After incubation with serum-free medium, the conditioned media was clarified, filtered through a 0.45-μm Millipore filter (Millipore, Bedford, MA), aliquoted, and stored at -80°C. Fresh serum-free medium was used as a negative control. Medium was concentrated to one-one hundredth of its original volume (50 μl/5 ml). To minimize variability that can occur as a result of adsorption to the surface of the concentration device, we used a speed vacuum concentrator (Savant Instruments, Farmingdale, NY) rather than a centrifugal filter device to concentrate the media.
Cultured cells were washed in cold phosphate-buffered saline, scraped in 200μl (per 75 cm2 flask) extraction buffer containing 150 mM NaCl, 50 mM Tris-HCl, pH 7.5, 1% Nonidet P-40, 0.25% deoxycholate, 0.1% SDS, and incubated on ice for 20 min. Cells were then homogenized for 60 s on ice using a portable, motorized tissue grinder (Pellet Pestle Motor, Kontes, Vineland, NJ). Homogenates were centrifuged at 14,000 × g for 15 min at 4°C. The supernatant was collected and stored at -80°C until further analysis.
Western blotting
Cell lysates and conditioned media were analyzed by western blotting. Supernatants (10 μl) from cell lysates or concentrated conditioned media were recovered and subjected to western blot analysis with anti-MT1-MMP antibodies. The anti-MT1MMP antibodies were generated and characterized in our laboratory. 16 17 The antibody recognizes the N-terminal of MT1-MMP, and detects MT1-MMP as a 63 kDa band on western Blot analysis. Band densities from western blot were quantified by using Scion Image software (version 4.0.3, Scion Corp., Frederick, MD).
CPAE cell proliferation
CPAE cells were harvested with 0.05% trypsin-EDTA solution (Cellgro) and counted under a phase-contrast microscope using a hemocytometer. These cells were seeded in a 96-well plate (15,000 cells/well). In each well, 100 μl DMEM containing 0.5% FCS was added and cells were then starved for 24 hr. After the starvation period, the medium was replaced by supernatant obtained from all cell lines. The supernatant had been previously filtered and stored at -80°C. Equal amount of protein (500 ng) from the conditioned media were added to the MCDB-131 media.
Forty-eight hours later, a colored formazan product assay was used to determine the number of living CPAE cells. In this assay, 20 μl/well MTS tetrazolium solution (Promega Corp., Madison, WI) was used. CPAE cells converted the solution into a water-soluble formazan that absorbs light at 490 nm, which was measured by a microplate reader (EAR 400AT; SLT Labinstruments, Austria) or using a Tecan microplate reader (Tecan. Switzerland).
BrdU incorporation proliferation assay
Incorporation of the thymidine analogue, 5-bromo-2′-deoxy-uridine (BrdU), into DNA served as an index of DNA synthesis and cellular proliferation. BrdU incorporation was assayed using a commercially available chemiluminescent ELISA kit (Roche Germany) according to the manufacturer's instructions. Briefly, CPAE cells were plated in MCDB-131 complete medium on 0.1% gelatin-coated 96-well plates (6,000 cells/well). After incubation for 24 hrs, cells were starved for 6 hrs in serum-free medium. The cells were then incubated for 24 hrs with concentrated conditioned medium containing 500 ng of protein. After that, the cells were incubated with 10 ng/ml of BrdU for 6 hrs, washed with fresh medium, and fixed with 100 ul of pre-cooled ethanol-HCl. Incorporated BrdU was visualized by incubating cells with anti-BrdU-POD for 60 min, rinsing them four times with a washing solution, and adding a chemiluminescent reagent. The relative luminescence units were read using a luminometer (Tecan, Switzerland).
CPAE cell migration
Migration assays were conducted in a modified 10-well Boyden chamber (Neuro Probe, Inc., Gaithersburg, MD). Cells migrated through a membrane toward a chemoattractant represented by a culture medium conditioned by all cell lines. Polyvinylpyrrolidone-free polycarbonate filters (8-μm pore size) (Neuro Probe) were coated with fibronectin (10 ng/μl; BD Biosciences, Bedford, MA). Cell lines were preincubated for 24 hr in starvation medium (with 0.5% FCS) to allow for collection of the conditioned medium to be used as a chemoattractant. The bottom-plate wells were filled with 400 μl supernatant from the cell lines. Non-migrated cells on the upper surface of the filter were wiped off after the seeding of 2 × 105 CPAE cells/well onto the filters in the upper chamber.
For qualitative analysis, the filter was rinsed in PBS, incubated with 100 μM/ml Cell Tracker Green CMFDA (5-chloromethylfluorescein diacetate; Molecular Probes, Inc., Eugene, OR), and diluted in DMEM for 15 min at 37°C. The filter was then incubated in DMEM for 30 min and fixed with 3.7% formaldehyde. The cells that actively migrated to the under surface of the filter were analyzed by using an epi-fluorescence microscope. Photographs were taken using RT Color and SPOT software (Diagnostic Instruments, Inc., Sterling Heights, MI). Control experiments were performed using 0.5% FCS in DMEM as a negative control. The value of the control medium containing 0.5% FCS was set to 100%.
Data analysis
Statistical analyses were performed using SPSS software (version 15.0). For two group comparisons student's T-test was performed. Analysis of Variance (ANOVA) was performed to compare the optical density between the different conditioned media. Post-Hoc analysis in conjunction with the ANOVA was performed for pair-wise comparisons. A P-value < 0.05 was considered statistically significant.
Results
Characterization of Immortalized Corneal Epithelial Cells
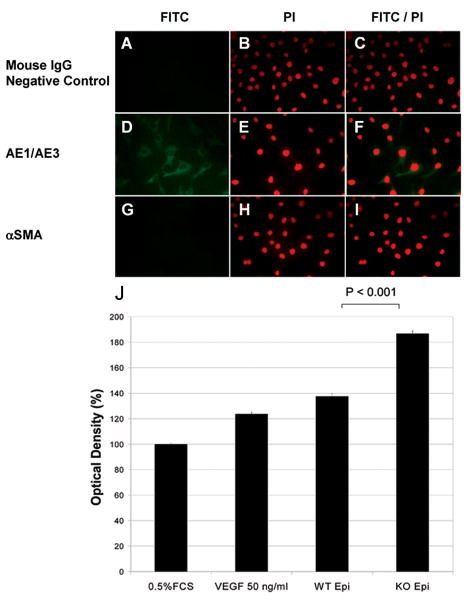
Immortalized corneal cells from WT mice were subcloned to generate corneal epithelial cell lines. The epithelial cells were isolated and characterized by immunohistochemical labeling for positive staining with anti-keratin AE1/AE3, but not with anti-α smooth muscle actin antibodies (Fig. 1 A-I).
Figure 1.
A-I: Immunohistochemical characterization of a corneal epithelial cell line using anti-keratin AE1/AE3 and anti-α smooth muscle actin antibodies.
Immortalized corneal cells from wild-type (WT) mice were subcloned to generate corneal epithelial cells. In double-staining experiments (C, F, I), conditioned epithelial cells were immunohistochemically characterized using anti-keratin AE1/AE3 and anti-α smooth muscle actin (αSMA) antibodies. Nuclei were stained with propidium iodide (PI; B, E, H). The corneal epithelial cells in the culture stained with AE1/AE3 antibodies (D, E, F), but not αSMA antibodies (G, H, I). There was no immunostaining in the negative controls, in which mouse serum was substituted for the primary antibody (A, B, C). J: Calf pulmonary arterial endothelial (CPAE) proliferation assay using WT and membrane-type 1 matrix metalloproteinase knockout (MT1-MMP KO) epithelial cells. CPAE cells were seeded in a 96-well plate (15,000 cells/well). DMEM (100 μl) containing 0.5% FCS was added to each well. Cells were then starved for 24 hours. Starvation medium was replaced by the supernatant obtained from cultured WT or MT1-MMP KO corneal epithelial cells. Control experiments were performed using 0.5% FCS in DMEM as a negative control. Medium containing 50 ng/mL VEGF was also used for comparison (positive control). Forty-eight hours later, the number of living CPAE cells in each well was calculated. Proliferation assays revealed a statistically significant difference between WT and MT1-MMP KO epithelial cell lines (P < 0.0001).
CPAE Cell Proliferation in Media of Corneal Epithelial Cells
CPAE cells were seeded in 96-well plates, starved for 24 hr, and subsequently incubated in media from cultured epithelial cells. Forty-eight hours later, the colored formazan product assay was used to calculate the number of living CPAE cells. Normalized data were compared using a two-tailed t-test. Figure 1J shows the percentage of activity with respect to 0.5% fetal calf serum (FCS) control (set at 100%). There was a significant increase in CPAE cell proliferation in conditioned media from the MT1-MMP KO corneal epithelial cells (mean ± SEM, 186.68% ± 1.29%) when compared with the conditioned media from the WT epithelial cells (137.45% ± 1.81%, P < 0.001).
Overexpression of MT1-MMP in WT Corneal Epithelial Cells
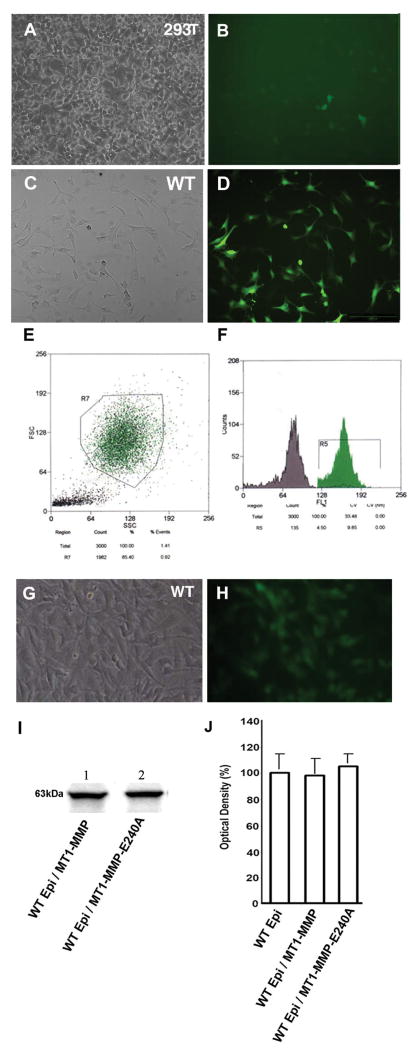
Recombinant viruses were generated by cotransfection of 293T cells with plasmids encoding gag-pol and env, with a pFB vector containing MT1-MMP to produce cell surface forms of MT1-MMP (Fig. 2A, B). 293T cells were used to generate high-titer virus stocks by amplifying viruses. High-titer recombinant virus-containing medium, amplified in 293T cells, was used to infect WT epithelial cell lines. WT epithelial cells were infected with a retrovirus containing MT1-MMP (Fig. 2C, D). Cells were sorted by flow cytometry based on EGFP expression (Fig. 2E, F), which served as a marker for MT1-MMP expression. The levels of EGFP were observed in the cytoplasm of sorted epithelial cells (Fig. 2G, H).
Figure 2.
Using primers, membrane-type 1 matrix metalloproteinase (MT1-MMP) cDNA fragments of mouse MT1-MMP were generated and subcloned and EGFP-containing constructs were isolated. Recombinant viruses were generated by cotransfection of 293T cells with an EGFP-expressing recombinant pFB vector coding MT1-MMP (A, B). High-titer recombinant virus-containing medium amplified in 293T cells was used to infect a wild-type (WT) epithelial cell line (C). Infection efficiency was determined by observing EGFP expression (D). The WT epithelial cells transfected with MT1-MMP were sorted based on EGFP expression by flow cytometry (E, F). Stably-transfected epithelial cell lines were generated (G) and confirmed by fluorescence microscopy (H). The stability of MT1-MMP expression was confirmed in WT epithelial cell lysates by western blot analysis (I, lane 1). Similar experiments were performed in which 293T cells were cotransfected with plasmids of gag-pol, env and pFB vectors containing MT1-MMP-E240A. After cotransfection, WT epithelial cells were transfected and sorted by flow cytometry. Stably transfected cell lines of MT1-MMP-E240A were generated and confirmed by western blot analysis (Fig 2I lane 2). Calf pulmonary arterial endothelial (CPAE) proliferation assays were performed using conditioned media from WT and MT1-MMP overexpressing epithelial cells (transfected with WT MT1-MMP and MT1-MMP-E240A; J). The CPAE proliferation data of WT epithelial cells was set as 100%. Conditioned media from transfection of WT epithelial cell lines with MT1-MMP or its mutant E240A did not result in significant alteration of CPAE proliferation.
Similar experiments were performed using the MT1-MMP mutant. The cDNA fragments encoding the MT1-MMP mutant were subcloned separately into pFB plasmids. Recombinant viruses were generated by cotransfection of 293T cells with plasmids encoding gag-pol and env with a pFB vector containing MT1-MMP-E240A. The expression of WT and mutant MT1-MMP in the transfected (overexpressing) WT epithelial cells was confirmed by western blot analysis (Fig. 2I).
CPAE cell proliferation was assayed in conditioned media from the wild type and MT1-MMP overexpressing cells. Cell proliferation was assayed in vitro. CPAE proliferation was not altered significantly in the conditioned media of WT epithelial cell lines transfected with either MT1-MMP or its mutant form as compared to that of non-transfected WT epithelial cells (Fig. 2J). This finding is consistent with our previous report of MT1-MMP expression in the corneal epithelium during wound healing.15 Overexpression of the WT or mutated form of MT1-MMP in WT corneal epithelial cells did not significantly augment the anti-angiogenic effect of endogenous MT1-MMP.
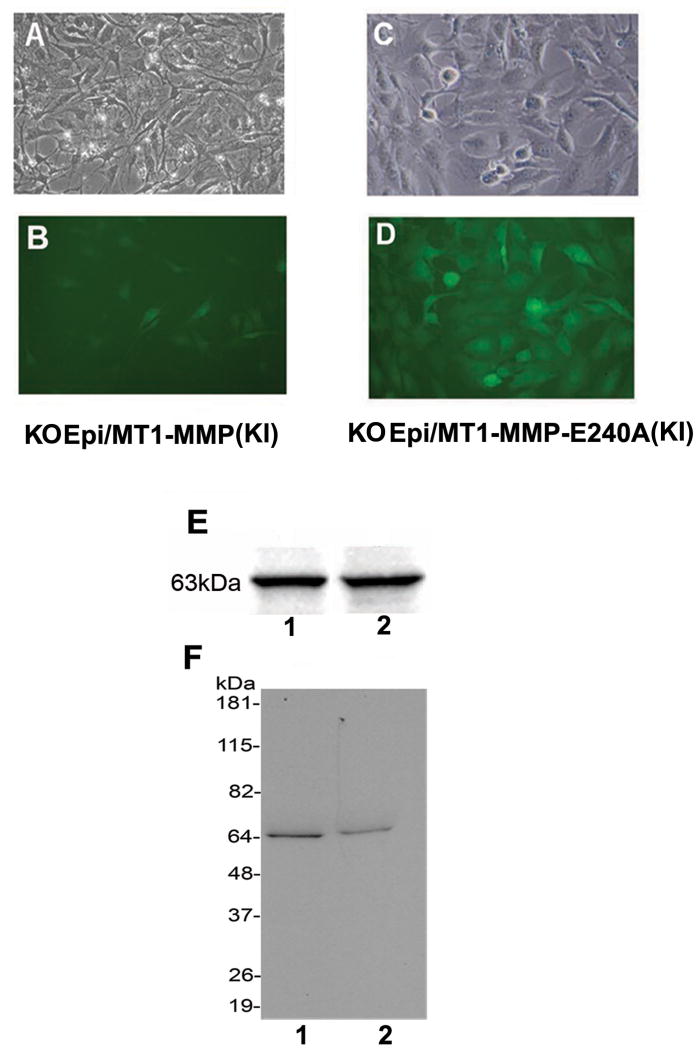
Corneal Epithelial Knockin Cell Lines
MT1-MMP KO epithelial cells were transfected with WT and mutant MT1-MMP. High-titer recombinant virus-containing medium amplified in 293T cells was used to infect MT1-MMP KO epithelial cell lines with MT1-MMP or MT1-MMP-E240A. Each transfected cell line (KO Epi/MT1-MMP (KI) or KO Epi/MT1-MMP-E240A (KI)) was sorted by flow cytometry based on EGFP expression. Stably-transfected epithelial cell lines were generated and confirmed by fluorescence microscopy: KO Epi/MT1-MMP (KI) (Fig. 3A, B) and KO Epi/MT1-MMP-E240A (KI) (Fig. 3C, D). The stability of expression of MT1-MMP (Fig. 3E, lane 1) and MT1-MMP-E240A (Fig. 3E, lane 2) in MT1-MMP KO epithelial cells was confirmed by analyzing cell lysates by western blotting. MT1-MMP levels in conditioned media were determined (Fig. 3F; lanes 1 and 2, respectively).
Figure 3.
A-F: Corneal epithelial knockin (KI) cell lines. Recombinant viruses were generated by cotransfection of 293T cells with EGFP-expressing recombinant pFB vectors coding either MT1-MMP or MT1-MMP-E240A. High-titer recombinant viruses containing medium amplified in 293T cells were used to infect MT1-MMP KO epithelial cell lines with MT1-MMP or MT1-MMP-E240A. Each transfected cell line (KO Epi/MT1-MMP (KI) or KO Epi/MT1-MMP-E240A (KI)) was sorted based on EGFP expression using flow cytometry. Stably-transfected epithelial cell lines were generated and confirmed by fluorescence microscopy: KO Epi/MT1-MMP (KI) (Fig. 3A, B) and KO Epi/MT1-MMP-E240A (KI) (Fig. 3C, D). E: Expression of MT1-MMP (E, lane 1) and MT1-MMP-E240A (E, lane 2) in MT1-MMP KO epithelial cell lysates was determined by western blot. F: Expression of MT1-MMP (F, lane 1) and MT1-MMP-E240A (F, lane 2) was also assayed by western blotting of conditioned media.
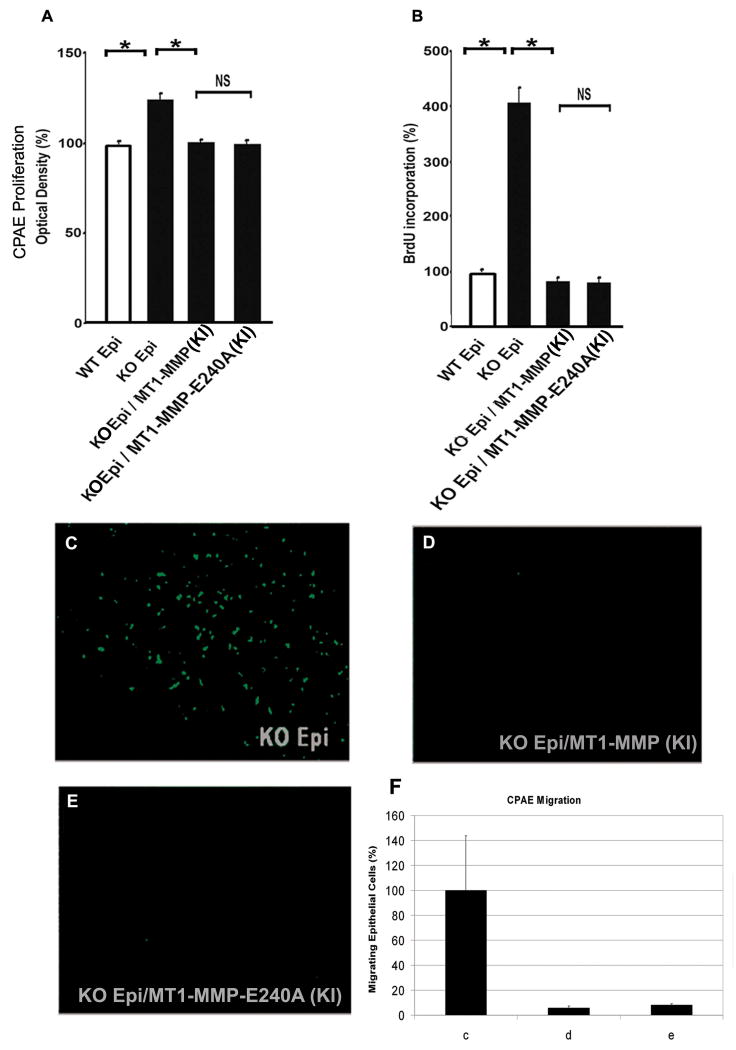
CPAE Proliferation and Migration of conditioned media of MT1-MMP KI Epithelial Cells
CPAE proliferation was assayed in the conditioned media of WT, KO, and KI corneal epithelial cells. CPAE proliferation in the conditioned media of WT epithelial cells was set as 100%. Conditioned media from MT1-MMP KO epithelium significantly increased CPAE proliferation (124.63% ± 2.79%) as compared to WT epithelium (100% ± 1.43%). CPAE proliferation in conditioned media from the KO Epi/MT1-MMP (KI) (100.51% ±1.41%) and KO Epi / MT1-MMP-E240A (KI) (98.76% ± 2.40%) was not statistically significantly different from that in conditioned media of WT epithelial cells (Fig. 4A).
Figure 4. CPAE Proliferation and Migration.
A: Calf pulmonary arterial endothelial (CPAE) proliferation assays using conditioned media from wild-type (WT) and MT1-MMP knockout (KO) epithelial cells after stable transfection of WT MT1-MMP and MT1-MMP-E240A. Conditioned media from MT1-MMP KO epithelium significantly increased CPAE proliferation as compared to WT epithelium. Conditioned media from MT1-MMP KO cell transfected with either WT or mutant MT1-MMP-E240A did not increase CPAE proliferation. B: Exposure to conditioned media from MT1-MMP KO epithelial cells was associated with greater BrdU incorporation in vascular endothelial cells than exposure to conditioned media from MT1-MMP KO epithelial cells transfected with MT1-MMP WT or MT1-MMP-E240A. C-F: CPAE migration assays using conditioned media from KO MT1-MMP epithelial cells after stable transfection of WT MT1-MMP and MT1-MMP-E240A. To assess the effects of MT1-MMP on migration, CPAE cells migrating through the membrane were observed using the Boyden chamber method. Conditioned medium (440 μl/well) from each cell line was used as a chemoattractant in the lower chamber, while 250,000 CPAE cells/well were seeded in the upper chamber. CPAE cell migration was observed in the supernatant from the MT1-MMP KO cell line (C). When the MT1-MMP KO cell line was knocked-in with MT1-MMP (D) or MT1-MMP-E240A (E), CPAE migration was not observed. Experiments were done in triplicates. Number of cells (measured by Image-J) is graphically represented in F normalized to 100% for the KO epithelial cells (c: KO epithelium; d: KO epithelium/MT1-MMP (KI); e: KO epithelium/MT1-MMP-E240A (KI)).
Analysis of DNA synthesis revealed that exposure to conditioned media from KO Epi was associated with significantly greater BrdU incorporation in CPAE cells than exposure to conditioned media from KO Epi/MT1-MMP (KI) or KO Epi / MT1-MMP-E240A (KI) cells (Fig. 4B).
A Boyden chamber migration assay was used to evaluate CPAE cell migration through the 8-μm membrane pores. CPAE cell migration was assayed in conditioned media from the MT1-MMP KO epithelial cells (Fig. 4C). CPAE cell proliferation in conditioned media of the MT1-MMP KI cell lines (Figs. 4D, E) was inhibited.
Discussion
In the present work, we compared the angiogenic potential of wild-type MT1-MMP with its mutant counterparts that were expressed in MT1-MMP knockout corneal epithelial cells. CPAE proliferation and migration was significantly enhanced when MT1-MMP was knocked out in corneal epithelial cells suggesting that MT1-MMP contributes to anti-angiogenesis. In these cells, when WT MT1-MMP or mutated catalytically-inactive MT1-MMP (MT1-MMP-E240A) was overexpressed, CPAE proliferation and migration reverted to levels similar to WT MT1-MMP. Taken together, these data suggest that MT1-MMP contributes to anti-angiogenic potential in cultured corneal epithelial cells and that this effect is independent of its catalytic activity. Overall, our results imply that MT1-MMP inhibits angiogenesis in cultured corneal epithelial cells independently of its enzymatic activity.
Corneal epithelial cells express MT1-MMP during wound healing, and only the basal epithelial cell layer express MT1-MMP in unwounded corneas. Therefore in the experimental design in this manuscript we knocked out the MT1-MMP and re-expressed it in the epithelial cells.
Although the corneal epithelium is avascular in vivo, cultured corneal epithelial cells have angiogenic potential. Cultured corneal epithelial cells induce vascular endothelial cell invasion, migration, and tube formation.18 Kanayama et al, used ELISA to identify candidate factors that have angiogenesis-induction capabilities in cultured corneal epithelial cells. Angiogenic factors such as fibroblast growth factor-2 (FGF-2), vascular endothelial growth factor (VEGF), angiopoietin-1 and transforming growth factor β1 were present in the conditioned medium. Sekiyama et al used immunohistochemical staining to show that anti-angiogenic factors [Thrombospondin-1 (TSP-1), pigment epithelium derived factor (PEDF), endostatin, angiostatin] as well as VEGF and FGF-2 are expressed in cultured corneal epithelial cells. Several recent studies have shown that conditioned media modulates vascular endothelial cell proliferation, migration, and/or tube formation. Pollina et al. have demonstrated that the balance between the angiogenesis inducers and inhibitors secreted in the microenvironment controls the rate of new blood vessel formation.19 MT1-MMP appears to be a hitherto unrecognized anti-angiogenic factor expressed in cultured corneal epithelial cells. We have previously shown that MT1-MMP is localized to the corneal basal epithelium and stromal keratocytes in unwounded corneas.15 Under physiological conditions, epithelial-derived MT1-MMP may inhibit angiogenesis and contribute to the maintenance of corneal avascularity.
MT1-MMP contains several domain motifs, including a signal peptide, a prodomain, a catalytic domain, a hinge region, a hemopexin domain, a transmembrane domain, and a cytoplasmic domain.20 One physiological function of MT1-MMP is to cleave and activate proMMP2, and several of the MT1-MMP domains differentially regulate this function. 21 For example, the cytoplasmic tail of MT1-MMP may negatively regulate pro-MMP2 activation by mediating internalization of this proenzyme from the cell surface.22 On the other hand, MT1-MMP-mediated surface activation of proMMP2 has recently been shown to require homophilic complex formation, which is dependent on either the hemopexin domain or cytoplasmic tail. 23-25 Our data show that the anti-angiogenic potential of MT1-MMP in cultured corneal epithelial cells is independent of its catalytic activity. Biological effects that are independent of catalytic activity have been reported for other molecules as well. For example, cathepsin D overexpressed by cancer cells can enhance apoptosis-dependent chemo-sensitivity independently of its catalytic activity. 26
The mechanisms by which MT1-MMP alters the corneal angiogenic privilege remain elusive. Our experiments show that when transmembrane and cytoplasmic domain mutated MT1-MMP (MT1-MMP-ΔTC; data not shown) was overexpressed in MT1-MMP knockout cultured corneal epithelial cells, BrdU incorporation levels were significantly higher then when WT MT1-MMP or mutated catalytically-inactive MT1-MMP (MT1-MMP-E240A) were overexpressed. This suggests that the transmembrane and cytoplasmic domain of MT1-MMP may be involved in the anti-angiogenic role of MT1-MMP in the corneal epithelium.
Various studies show that MT1-MMP levels are modulated by angiogenesis-regulating cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), epidermal growth factor (EGF), and bFGF.7, 16, 27, 28 MT1-MMP expression may also be regulated by the cytoskeleton and stress fibers, as MT1-MMP is upregulated in fibroblasts grown in relaxed collagen lattices. In accordance with the ability of angiogenesis regulators to modulate MT1-MMP expression, MT1-MMP as well as other MMPs have been shown to participate in angiogenic processes.29, 30 The role of MMPs in the regulation of angiogenesis is often ambiguous, as the same molecule often has both pro-angiogenic or anti-angiogenic effects.31 The dual function of MMPs during angiogenesis can be explained by the ability of MMPs to generate anti-angiogenic fragments from precursors, which lack angiogenic properties, as well as the ability of MMPs to degrade the ECM, allowing MMP-bearing endothelial cells to invade the tissue 32 or release matrix-bound cytokines or growth factors.32-34
Specifically, in the context of cornea, MT1-MMP is pro-angiogenic in the corneal stroma via several mechanisms: breakdown of corneal ECM, activation of MMP-2, breakdown of anti-angiogenic factors, upregulation of VEGF and synergistic effect with bFGF (in angiogenesis models). The importance of our finding that epithelial MT1-MMP is anti-angiogenic is that the overall corneal in vivo pro-angiogenic effect is despite the anti-angiogenic effect of epithelial MT1MMP (Table 2). Another possible explanation for the anti-angiogenic potential of epithelial MT1-MMP is the possible generation of Neostatin-14 (MT1-MMP proteolytic fragment of collagen XVIII) by epithelial MT1MMP, which is a potent anti-angiogenic factor. One limitation of this study is that we do not provide an in vivo correlate to our findings. The main reason for not performing in vivo corneal NV experiments to confirm our findings is that the extent of NV in murine models is dependent upon the balance between anti-angiogenic and angiogenic factors in epithelium as well as in stroma, therefore we may not be able to distinguish the contribution of corneal epithelium specifically. Additional studies will be required to understand the molecular mechanisms underlying the ability of corneal epithelial MT1-MMP to inhibit vascular endothelial cell proliferation and migration (e.g. levels of angiostatin or endostatin). Understanding how corneal epithelial MT1-MMP regulates anti-angiogenic or pro-angiogenic factors involved in corneal neovascularization may aid the development of therapeutic interventions aimed at treating angiogenesis-related corneal disorders.
Table 2. Pro- and Anti-Angiogenic Effects of MT1-MMP in the Cornea.
| Pro-angiogenic |
|---|
| Break down of corneal extra-cellular matrix7 |
| Corneal stroma extra-cellular matrix breakdown leading to release of matrix-bound cytokines or growth factors (example: bFGF)7 |
| Activation of MMP-27 |
| Breakdown of anti-angiogenic extra-cellular matrix macromolecules (example, Decorin)28 |
| Upregulation of VEGF16,27 |
| Synergistic effect with bFGF27 |
| Anti-angiogenic |
| Generation of Neostatin- 14 (MT1-MMP proteolytic fragment of collagen XVIII)12,13 |
| Breakdown of angiogenic Factors (FGF-2, VEGF, angiopoietin-1, transforming growth factor β1)7 |
Acknowledgments
NIH EY10101 (D.T.A.), EY001792 (D.T.A.), EY14048 (J.H.C.), and an unrestricted departmental Grant from Research to Prevent Blindness (New York, NY).
Abbreviations
- MMP
matrix metalloproteinases
- TIMPs
tissue inhibitors of metalloproteinases
Footnotes
Commercial Relationships: None
References
- 1.Werb Z. ECM and cell surface proteolysis: regulating cellular ecology. Cell. 1997;91:439–442. doi: 10.1016/s0092-8674(00)80429-8. [DOI] [PubMed] [Google Scholar]
- 2.Sato H, Takino T, Okada Y, et al. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994;370:61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- 3.Coussens LM, Jacks T. Genetic and cellular mechanisms of oncogenesis. Curr Opin Genet Dev. 2008;18:1–2. doi: 10.1016/j.gde.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Rooprai HK, Van Meter T, Rucklidge GJ, et al. Comparative analysis of matrix metalloproteinases by immunocytochemistry, immunohistochemistry and zymography in human primary brain tumours. Int J Oncol. 1998;13:1153–1157. doi: 10.3892/ijo.13.6.1153. [DOI] [PubMed] [Google Scholar]
- 5.Williamson RA, Hutton M, Vogt G, et al. Tyrosine 36 plays a critical role in the interaction of the AB loop of tissue inhibitor of metalloproteinases-2 with matrix metalloproteinase-14. J Biol Chem. 2001;276:32966–32970. doi: 10.1074/jbc.M101843200. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Z, Apte SS, Soininen R, et al. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc Natl Acad Sci U S A. 2000;97:4052–4057. doi: 10.1073/pnas.060037197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azar DT. Corneal angiogenic privilege: angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2006;104:264–302. [PMC free article] [PubMed] [Google Scholar]
- 8.Maharaj AS, D'Amore PA. Roles for VEGF in the adult. Microvasc Res. 2007;74:100–113. doi: 10.1016/j.mvr.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Amore PA. Vascular endothelial cell growth factor-a: not just for endothelial cells anymore. Am J Pathol. 2007;171:14–18. doi: 10.2353/ajpath.2007.070385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ambati BK, Nozaki M, Singh N, et al. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443:993–997. doi: 10.1038/nature05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cursiefen C, Chen L, Saint-Geniez M, et al. Nonvascular VEGF receptor 3 expression by corneal epithelium maintains avascularity and vision. Proc Natl Acad Sci U S A. 2006;103:11405–11410. doi: 10.1073/pnas.0506112103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gabison E, Chang JH, Hernandez-Quintela E, et al. Anti-angiogenic role of angiostatin during corneal wound healing. Exp Eye Res. 2004;78:579–589. doi: 10.1016/j.exer.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Chang JH, Javier JA, Chang GY, et al. Functional characterization of neostatins, the MMP-derived, enzymatic cleavage products of type XVIII collagen. FEBS Lett. 2005;579:3601–3606. doi: 10.1016/j.febslet.2005.05.043. [DOI] [PubMed] [Google Scholar]
- 14.Kure T, Chang JH, Kato T, et al. Corneal neovascularization after excimer keratectomy wounds in matrilysin-deficient mice. Invest Ophthalmol Vis Sci. 2003;44:137–144. doi: 10.1167/iovs.01-1058. [DOI] [PubMed] [Google Scholar]
- 15.Ye HQ, Maeda M, Yu FS, et al. Differential expression of MT1-MMP (MMP-14) and collagenase III (MMP-13) genes in normal and wounded rat corneas. Invest Ophthalmol Vis Sci. 2000;41:2894–2899. [PubMed] [Google Scholar]
- 16.Azar DT, Casanova FH, Mimura T, et al. Effect of MT1-MMP deficiency and overexpression in corneal keratocytes on vascular endothelial cell migration and proliferation. Curr Eye Res. 2008;33:954–962. doi: 10.1080/02713680802461106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kojima T, Chang JH, Azar DT. Proangiogenic role of ephrinB1/EphB1 in basic fibroblast growth factor-induced corneal angiogenesis. Am J Pathol. 2007;170:764–773. doi: 10.2353/ajpath.2007.060487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanayama S, Nishida K, Yamato M, et al. Analysis of angiogenesis induced by cultured corneal and oral mucosal epithelial cell sheets in vitro. Exp Eye Res. 2007;85:772–781. doi: 10.1016/j.exer.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Pollina EA, Legesse-Miller A, Haley EM, et al. Regulating the angiogenic balance in tissues. Cell Cycle. 2008;7:2056–2070. doi: 10.4161/cc.7.13.6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seiki M. The cell surface: the stage for matrix metalloproteinase regulation of migration. Curr Opin Cell Biol. 2002;14:624–632. doi: 10.1016/s0955-0674(02)00363-0. [DOI] [PubMed] [Google Scholar]
- 21.Jiang A, Pei D. Distinct roles of catalytic and pexin-like domains in membrane-type matrix metalloproteinase (MMP)-mediated pro-MMP-2 activation and collagenolysis. J Biol Chem. 2003;278:38765–38771. doi: 10.1074/jbc.M306618200. [DOI] [PubMed] [Google Scholar]
- 22.Jiang A, Lehti K, Wang X, et al. Regulation of membrane-type matrix metalloproteinase 1 activity by dynamin-mediated endocytosis. Proc Natl Acad Sci U S A. 2001;98:13693–13698. doi: 10.1073/pnas.241293698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Itoh Y, Ito N, Nagase H, et al. The second dimer interface of MT1-MMP, the transmembrane domain, is essential for ProMMP-2 activation on the cell surface. J Biol Chem. 2008;283:13053–13062. doi: 10.1074/jbc.M709327200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lehti K, Lohi J, Juntunen MM, et al. Oligomerization through hemopexin and cytoplasmic domains regulates the activity and turnover of membrane-type 1 matrix metalloproteinase. J Biol Chem. 2002;277:8440–8448. doi: 10.1074/jbc.M109128200. [DOI] [PubMed] [Google Scholar]
- 25.Rozanov DV, Deryugina EI, Ratnikov BI, et al. Mutation analysis of membrane type-1 matrix metalloproteinase (MT1-MMP). The role of the cytoplasmic tail Cys(574), the active site Glu(240), and furin cleavage motifs in oligomerization, processing, and self-proteolysis of MT1-MMP expressed in breast carcinoma cells. J Biol Chem. 2001;276:25705–25714. doi: 10.1074/jbc.M007921200. [DOI] [PubMed] [Google Scholar]
- 26.Beaujouin M, Liaudet-Coopman E. Cathepsin D overexpressed by cancer cells can enhance apoptosis-dependent chemo-sensitivity independently of its catalytic activity. Adv Exp Med Biol. 2008;617:453–461. doi: 10.1007/978-0-387-69080-3_44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Onguchi T, Han KY, Chang JH, et al. Membrane type-1 matrix metalloproteinase potentiates basic fibroblast growth factor-induced corneal neovascularization. Am J Pathol. 2009;174:1564–1571. doi: 10.2353/ajpath.2009.080452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mimura T, Onguchi T, Chang JH, et al. MT1-MMP-mediated cleavage of decorin in corneal angiogenesis. J Vasc Res. 2009 doi: 10.1159/000226222. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghajar CM, George SC, Putnam AJ. Matrix metalloproteinase control of capillary morphogenesis. Crit Rev Eukaryot Gene Expr. 2008;18:251–278. doi: 10.1615/critreveukargeneexpr.v18.i3.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Langlois S, Nyalendo C, Di Tomasso G, et al. Membrane-type 1 matrix metalloproteinase stimulates cell migration through epidermal growth factor receptor transactivation. Mol Cancer Res. 2007;5:569–583. doi: 10.1158/1541-7786.MCR-06-0267. [DOI] [PubMed] [Google Scholar]
- 31.Raza SL, Cornelius LA. Matrix metalloproteinases: pro- and anti-angiogenic activities. J Investig Dermatol Symp Proc. 2000;5:47–54. doi: 10.1046/j.1087-0024.2000.00004.x. [DOI] [PubMed] [Google Scholar]
- 32.Lamoreaux WJ, Fitzgerald ME, Reiner A, et al. Vascular endothelial growth factor increases release of gelatinase A and decreases release of tissue inhibitor of metalloproteinases by microvascular endothelial cells in vitro. Microvasc Res. 1998;55:29–42. doi: 10.1006/mvre.1997.2056. [DOI] [PubMed] [Google Scholar]
- 33.Reed MJ, Koike T, Sadoun E, et al. Inhibition of TIMP1 enhances angiogenesis in vivo and cell migration in vitro. Microvasc Res. 2003;65:9–17. doi: 10.1016/s0026-2862(02)00026-2. [DOI] [PubMed] [Google Scholar]
- 34.Gallery ED, Campbell S, Arkell J, et al. Preeclamptic decidual microvascular endothelial cells express lower levels of matrix metalloproteinase-1 than normals. Microvasc Res. 1999;57:340–346. doi: 10.1006/mvre.1998.2142. [DOI] [PubMed] [Google Scholar]