Abstract
Inherited disorders of globin synthesis remain desirable targets for hematopoietic stem cell (HSC)-based therapies. Gene transfer using retroviral vectors offers an alternative to allogeneic HSC transplantation by the permanent integration of potentially therapeutic genes into primary autologous HSCs. Although proof of principle has been demonstrated in humans, this approach has been met by formidable obstacles, and large-animal models have become increasingly important for the preclinical development of gene addition strategies. Here we report lentiviral gene transfer of the human β-globin gene under the control of the globin promoter and large fragments of the globin locus control region (LCR) in the nonhuman primate. Using an HIV-1, vesicular stomatitis virus glycoprotein G (VSV-G)-pseudotyped vector, modified to overcome a species-specific restriction to HIV-1, gene transfer to colony-forming units (CFU) derived from mobilized peripheral blood (PB) rhesus CD34+ cells was 84.4 ± 2.33%. Erythroid cells derived from transduced rhesus CD34+ cells expressed human β-globin at high levels as assessed by flow cytometry with a human β-globin-specific antibody. Two rhesus macaques (RQ3586 and RQ3583) were transplanted with mobilized PB CD34+ cells transduced with our modified HIV vector at a multiplicity of infection of 80. High gene transfer rates to CFUs were achieved in vitro (RQ3586, 87.5%; RQ3583, 83.3%), with efficient human β-globin expression among erythroid progeny generated in vitro. Early posttransplantation, gene transfer rates of 5% or higher were detectable and confirmed by genomic Southern blotting, with equivalent-level human β-globin expression detected by flow cytometry. Long-term gene marking levels among mononuclear cells and granulocytes assessed by quantitative polymerase chain reaction gradually decreased to about 0.001% at 2 years, likely due to additional HIV-1 restrictive elements in the rhesus macaque. No evidence of clonal hematopoiesis has occurred in our animals in up to 2 years. Current efforts are aimed at developing a lentiviral vector capable of efficiently transducing both human and rhesus HSCs to allow preclinical modeling of globin gene transfer.
Introduction
The β-thalassemias and sickle cell disease are severe congenital anemias that are caused by mutations that alter production of the β-chain of hemoglobin. Reduced or absent β-chain synthesis characterizing the β-thalassemias leads to the intracellular precipitation of excess α-globin chains, causing ineffective erythropoiesis (Pootrakul et al., 2000; Weatherall, 2001). The production of an abnormal β chain that is prone to polymerization on deoxygenation leads to impaired deformability and sickling of red blood cells, causing the repeated vasoocclusive crises that characterize sickle cell disease. Although supportive treatment has considerably improved the prognosis of the thalassemias and hemoglobinopathies, patients still suffer significant morbidity and early mortality (Cunningham et al., 2004). Allogeneic hematopoietic stem cell (HSC) transplantation remains the only curative treatment; however, this therapeutic option is limited because of high cost, relative necessity for a matched sibling donor, and the risk of graft rejection and graft-versus-host disease (GVHD) (Boulad et al., 1998). Therefore, gene transfer of a functional β-globin gene to autologous HSCs remains an attractive strategy.
To revert the phenotype, the therapeutic gene must be permanently inserted into HSCs and expressed at high levels among erythroid progeny and for the life of the individual. For successful application of gene transfer techniques for the hemoglobin disorders, not only must the entire β-globin gene itself be delivered, but also its control elements are necessary for tissue-restricted β-globin gene expression (Grosveld et al., 1987). Incorporation of small elements derived from the globin locus control region (LCR) into vectors carrying the β-globin gene proved insufficient to achieve efficient expression and genetic stability (Kalberer et al., 2000; Persons and Nienhuis, 2000), and larger LCR elements seemed necessary. The incorporation of larger LCR elements into oncoretroviral vectors proved particularly problematic, leading to vector instability and considerable genomic rearrangements. Lentiviral vector systems possess the advantage of relative genomic stability and packaging capacity (Kumar et al., 2001). Incorporation of large LCR elements into viral vectors became feasible with the development of these lentiviral vector systems. By developing a lentiviral vector (TNS9) based on HIV-1 encoding the human β-globin gene, from which a cryptic polyadenylation site within intron 2 was deleted, flanked by an extended promoter sequence and the β-globin 3′ proximal enhancer as well as large LCR elements (3.2 kb) spanning HS2, 3, and 4, efficient gene transfer and therapeutic human β-globin gene expression in murine model was demonstrated, allowing for the first time regulated human β-globin expression sufficient to revert the phenotype in a murine model of β-thalassemia (May et al., 2000). Long-term follow-up in Hbbth-3/+ mice (Yang et al., 1995), the most severe viable model of disease, demonstrated persistent amelioration of anemia and normalization of hematopoiesis (May et al., 2002). Confirmation in a number of murine models of both sickle cell disease (SCD) and thalassemia followed (Pawliuk et al., 2001; Levasseur et al., 2003; Persons et al., 2003a).
Although encouraging, murine models of HSC gene transfer poorly predict results in humans and large-animal models have become increasingly important as preclinical tools for human translation. The development of such large-animal models has been hampered by the reduced efficiency with which HIV-based vectors transduce rhesus HSCs compared with human HSCs (Hanawa et al., 2004). Although efficient transduction of rhesus HSCs can be achieved with simian immunodeficiency virus (SIV)-based lentiviral vectors (Hanawa et al., 2004), experience with these vectors is considerably less than that with HIV-1-based vectors. The ideal vector system should perform equally well in both nonhuman primates and humans. To address this dilemma, modification of HIV-1-based vectors has been attempted to improve the efficiency of transduction to rhesus hematopoietic stem cells. The low efficiency of HIV-1-based vectors in the rhesus setting has been ascribed in part to the enrichment of tripartite motif-5α (TRIM5α), an inhibitory protein for HIV-1 infection (Stremlau et al., 2004). This antiviral activity appears to be species specific and was shown to block infection by a broad range of retroviruses. Another cellular factor, cyclophilin A (CyPA), is also important in the process of HIV infection, presenting a postentry restriction in the rhesus (Rits et al., 2007). These antiviral factors limit the use of Old World monkeys for long-term safety studies of the HIV-1-based vector. The exchange of the CyPA-binding region in the capsid of an HIV-1-based lentiviral vector with that of macrophage-tropic HIV-1 Ba-L resulted in a vector that was resistant to the inhibitory effect and efficiently transduced simian cells (Kootstra et al., 2003). This chimeric Gag lentivirus also transduced primary baboon CD34+ hematopoietic progenitor cells efficiently, resulting in transduced cell numbers comparable to that of human CD34+ cells. Here we describe our development of a modified HIV-1-based TNS9 vector and report the results of preclinical testing in the nonhuman primate in vivo.
Materials and Methods
Vector construction and production
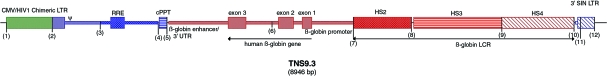
The lentiviral vector used (previously described in May et al., 2000) was modified (TNS9.3; Fig. 1) to include addition of a central polypurine tract (cPPT) element and a 3′ long terminal repeat (LTR) self-inactivating (SIN) deletion. The vector was further modified to allow efficient transduction of simian cells by the substitution of the cyclophilin A-binding domain of the HIV capsid with that of the macrophage-tropic strain as previously described (Kootstra et al., 2003). Viral stocks were generated by triple transfection of the recombinant vectors (TNS9.3 or pHR′eGFP), pCMVR8.9 (Zufferey et al., 1997), and pMD.G (Dull et al., 1998). The pseudotyped virions were concentrated by ultracentrifugation and titrated in NIH3T3 cells as described (May et al., 2000).
FIG. 1.
TNS9.3 vector map. Color images available online at www.liebertonline.com/hum.
Collection of peripheral blood HSCs from humans and rhesus macaques
Human peripheral blood (PB) CD34+ cells were obtained from healthy adult volunteers as previously described (Kang et al., 2002a) through an institutional review board-approved protocol. Briefly, HSCs were mobilized after 5 days of treatment with 10-μg/kg recombinant human granulocyte colony-stimulating factor (rhuG-CSF) (Amgen, Thousand Oaks, CA). On day 5 of mobilization, PB mononuclear cells were collected by one 15-liter leukapheresis, and CD34-positive selection was performed with the Isolex 300i automated immunomagnetic system (Baxter, Deerfield, IL).
The rhesus experiments were approved by the animal care and use committees at the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK, National Institutes of Health [NIH], Bethesda, MD) and the National Heart, Lung, and Blood Institute (NHLBI, NIH). Two female rhesus macaques (Macaca mulatta), RQ 3586 and RQ3583, both weighing 4 to 5 kg, were housed and handled according to the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Research, 1996). The animals were mobilized with a combination of rhuG-CSF at 10 μg/kg (Amgen) and recombinant human stem cell factor (rhuSCF) at 200 μg/kg (Amgen) given as daily subcutaneous injections for 5 days. Mobilized PB cells were collected by leukapheresis on day 5 as previously described (Donahue et al., 1996). Mononuclear cells were isolated by density-gradient centrifugation over lymphocyte separation medium (ICN Biomedicals/Cappel, Aurora, OH). CD34+ cell enrichment was performed with 12.8 immunoglobulin M anti-CD34 biotinylated antibody and MACS (magnetically activated cell sorting) streptavidin microbeads (Miltenyi Biotec, Auburn, CA). Cells were eluted twice from the purification column, and the purity of the product was assessed with a phycoerythrin (PE)-conjugated mouse anti-human CD34 antibody (clone 563; BD Biosciences, San Jose, CA).
Vectors and transduction procedures
CD34-enriched cells were cultured as previously described (Tisdale et al., 1998; Wu et al., 2000) at a starting concentration of 1 × 105 to 2 × 105 cells/ml in filtered vector supernatant, supplemented with rhuSCF (100 ng/ml), recombinant human Flt-3 ligand (rhuFlt-3L, 100 ng/ml), and recombinant human thrombopoietin (rhuTPO, 100 ng/ml), in flasks previously coated with the CH-296 fragment of fibronectin (RetroNectin; Takara Bio, Shiga, Japan) as per the manufacturer's instructions. Every 24 hr, nonadherent cells were collected, spun down, and resuspended in fresh vector supernatant and cytokines and added back to the same fibronectin-coated flask. After 96 hr of total culture, cells were removed from the plates with trypsin and collected.
Colony-forming unit assays
The degree of progenitor enrichment was calculated from CFU assays performed after TNS9.3 transduction. Five hundred or 1000 CD34+ cells were plated. CFU assays were performed with MethoCult H4230 methylcellulose medium (StemCell Technologies, Vancouver, BC, Canada) supplemented with recombinant human erythropoietin (5 U/ml; Amgen), recombinant human granulocyte-macrophage colony-stimulating factor (rhuGM-CSF, 10 ng/ml; Sandoz, East Hanover, NJ), recombinant human interleukin-3 (rhuIL-3, 10 ng/ml; Sandoz), and rhuSCF (100 ng/ml; Amgen) at 37°C in 5% CO2. Between days 10 and 14, colonies of more than 50 cells were counted, and 12 to 24 individual CFUs were plucked from the plates at each time point for polymerase chain reaction (PCR) analysis. Colonies were placed into 50 μl of distilled water and digested with proteinase K (20 μg/ml; Qiagen, Valencia, CA) at 55°C for 1 hr followed by 99°C for 10 min. The resulting DNA-containing cell lysates were analyzed for TNS9.3 by nested PCR. The primer sequences for the TNS9.3 vector for the outer reaction were as follows: forward primer 5′-GGTGGACCATCCTCTAGGTA-3′ and reverse primer 5′-GGCCTTGAGCATCTGGATTC-3′; and the cycle conditions were 94°C for 0.5 min, 55°C for 0.5 min, and 72°C for 1 min for 12 cycles amplified with an Advantage 2 polymerase PCR kit (BD Biosciences). The primer sequences for the inner reaction were as follows: forward primer 5′-CAGCAGACCTCTGATCTCTT-3′ and reverse primer 5′-AGCCTTGACTCCACTCAGTT-3′; and the cycle conditions were 95°C for 0.5 min, 53°C for 0.5 min, and 72°C for 1 min for 36 cycles amplified with Taq DNA polymerase (Qiagen). Products were analyzed on a 1% agarose gel.
Differentiation of human erythroid progenitors in vitro
Rhesus PB CD34+ cells transduced with the modified TNS9.3 vector then underwent erythroid differentiation ex vivo, based on modifications (Wojda et al., 2002) of Fibach's liquid culture protocol (Fibach et al., 1989). Briefly, all the isolated cells were cultured for 14 consecutive days in DMEM containing 30% fetal bovine serum (FBS; Intergen, Purchase, NY), 1% bovine serum albumin (BSA), 10–5 M 2-mercaptoethanol, 10−6 M dexamethasone, transferrin (3 mg/ml), 2 mM glutamine (Biofluids, Rockville, MD), antibiotics (penicillin–streptomycin), 50 ng/ml each of transforming growth factor (TGF)-β and SCF (R&D Systems, Minneapolis, MN), and erythropoietin (4 U/ml; Amgen).
Analysis of red blood cells by permeabilization and intracellular hemoglobin staining by flow cytometry
Red blood cells (RBCs) were permeabilized by placing 5 μl of blood (about 2.5 × 107 RBCs) in a solution containing 1 ml of ice-cold 0.05% glutaraldehyde (Sigma-Aldrich, St. Louis, MO)–phosphate-buffered saline (PBS). After a 10-min incubation, cells were spun down, resuspended in 50% acetone and incubated for 3 min, and then spun for 5 min at 2500 rpm at 4°C. This process of centrifugation, resuspension, and incubation was repeated twice. The permeabilized cells were then resuspended in 0.5 ml of 2% BSA–PBS (Sigma-Aldrich) for staining. Using 5 μl of cells suspended in 85 μl of 2% BSA–PBS, 5 μl of an anti-human β-globin antibody (kindly provided by L. Stanker, U.S. Department of Agriculture, Albany, CA) was added (Stanker et al., 1986). After 30 min of staining on ice, the cells were washed. For secondary staining, the cells were resuspended in 95 μl of 2% BSA–PBS, and 5 μl of fluorescein isothiocyanate (FITC)-conjugated anti-mouse Fab fragment antibody (Sigma-Aldrich) was added to detect the primary antibody and incubated for 30 min on ice. The stained cells were analyzed with a FACSCaliber flow cytometer with CellQuest software (BD Biosciences).
Transduction and transplantation procedures
For both animals, a modified HIV-1-based vesicular stomatitis virus glycoprotein G (VSV-G)-pseudotyped TNS9.3 vector was prepared at concentrations of 7 × 107 infectious particles/ml (IP/ml, 24 ml) and 10.75 × 107 IP/ml (28 ml) and used for transduction. Twenty-four-milliliter volumes of vector supernatant containing 1.68 × 109 and 1.53 × 109 IP total were further concentrated by centrifugation for 90 min at 25,000 rpm (ultracentrifuge, SW28 rotor; Beckman Coulter, Fullerton, CA). Vector was resuspended over 6 hr in 3 ml of medium at 4°C by gentle agitation. Vector was then fully resuspended at the end of the 6 hr by repeat pipetting with an additional 2 ml of medium and was separated into four 1.25-ml aliquots placed on ice at 4°C.
CD34-enriched cells (2 × 107) were cultured at a starting concentration of 5.0 × 105 cells/ml in two 75-cm2 flasks (8.0 ×106 cells in 16 ml per flask) previously coated with the CH-296 fragment of fibronectin (RetroNectin). Cells were initially prestimulated for 36 hr in Iscove's modified Eagle's medium (IMEM; Mediatech, Herndon, VA) supplemented with rhuSCF (100 ng/ml), rhuFlt-3L (100 ng/ml), and TPO (100 ng/ml) (RDI, Flanders, NJ) with 10% FBS (Hyclone, Logan, UT), 4 mM l-glutamine, penicillin (50 mg/ml), and streptomycin (50 mg/ml) at 37°C in 5% CO2. After 36 hr, nonadherent cells were collected and spun down at 1500 rpm in a Sorvall RT7 centrifuge with an RTH-750 rotor (Thermo Fisher Scientific, Waltham, MA). Transductions were performed with 8 ml of freshly thawed viral supernatant combined with 8 ml of freshly prepared medium added to each 75-cm2 flask over two 36-hr exposures. At the end of 72 hr, cultured cells were removed from the plates with trypsin, collected, washed with PBS, and resuspended in IMEM (without serum) containing 1% BSA and fresh cytokines for transport. A small aliquot of cells was plated in methylcellulose with myeloid- and erythroid-specific cytokines (StemCell Technologies). The remaining cells were resuspended with normal saline and transported at ambient temperature just before infusion.
The animals received 500 cGy total body irradiation twice, 48 and 24 hr before infusion. The transduced cells were infused via a central venous catheter over 5 min. Twenty-four hours later, each animal was started on G-CSF intravenously at 5 μg/kg daily to continue until the total white blood cell count reached 6000 cells/μl. Standard supportive care including blood product transfusions and fluid and electrolyte management, and antibiotics were administered as needed. Hematopoietic recovery was monitored by daily complete blood counts (CBCs) (CELL-DYN 3500; Abbott Laboratories, Abbott Park, IL). Electrolyte, renal, and hepatic panels were determined from serum, using the Johnson & Johnson Vitros 250 (Biostad, Sainte Julie, QC, Canada). Reticulocyte counts were determined by flow cytometry as previously described (BD Biosciences).
Quantitative real-time polymerase chain reaction analysis
PB and bone marrow (BM) samples were obtained at various time points after recovery. Granulocytes (Grs) and mononuclear cells (MNCs) were isolated as previously described (Tisdale et al., 1998). Genomic DNA was produced with a Wizard genomic DNA purification kit (Promega, Madison, WI) according to the manufacturer's instructions. The contribution to engraftment by TNS9.3 vector-transduced cells was determined by real-time PCR analysis with TaqMan probes as previously described (Kang et al., 2001, 2002b), using vector-specific primers. Quantitative (Q)-PCR was carried out with the Mx3000P QPCR system (Stratagene, La Jolla, CA), using Brilliant II Q-PCR master mix (Stratagene) according to the manufacturer's instructions. Primers and probes were designed with RealTimeDesign software (Biosearch Technologies, Novato, CA). The primers and probe included 5′-AGCCAGAAGCACCATA-3′ (forward), 5′-ATCTCTTCCTGAATGCTAATC-3′ (reverse), and TaqMan probe 5′-ACATGATAAGGGAGCCAGCAGACC-3′. The results were analyzed with Mx3000P software. Overall levels were determined by normalizing to β-actin. The primers for β-actin were 5′-CATTGTGATGGACTCCGGAGACGG-3′ (forward) and 5′-CATCTCCTGCTCGAAGTCTAGAGC-3′ (reverse).
Genomic Southern blot analysis
For analysis of integrated vector copy number in peripheral blood, genomic DNA was isolated, digested with BamHI, and analyzed by Southern blot as previously described (Sadelain et al., 1995) with a [32P]dCTP-labeled NcoI–BamHI fragment of the human β-globin gene as a probe. To assess integrated vector integrity, Southern blot analysis was performed after NheI digestion, which generates a fragment spanning the entire provirus, using the same human β-globin gene probe.
Mass spectrometry analysis
Peripheral blood from RQ3586 was collected on day 30 posttransplantation. Permeabilized RBCs were stained with a human-specific β-globin antibody as previously described and positive cells were sorted with a FACSAria (BD Biosciences). We sorted four fractions and the cells were pelleted, washed twice and lysed with water, frozen at −20°C for 5 min, and thawed at 37°C for 5 min to collect protein. The fractions were analyzed by mass spectroscopy.
Statistics
Statistical analyses were performed by Student t test, with a p value less than 0.05 deemed significant.
Results
In vitro transduction of human and rhesus CD34+ cells
The vector design of our TNS9.3 has been previously described (Fig. 1) (May et al., 2000). The β-globin transcription unit within the TNS9.3 vector comprises the β-globin promoter, two proximal enhancers, and three large segments encompassing HS2, HS3, and HS4. Other than a small deletion in intron 2, the genomic and coding sequences are unmodified wild-type human sequences. The 3′ long terminal repeat (LTR) comprises a large deletion within the U3 region.
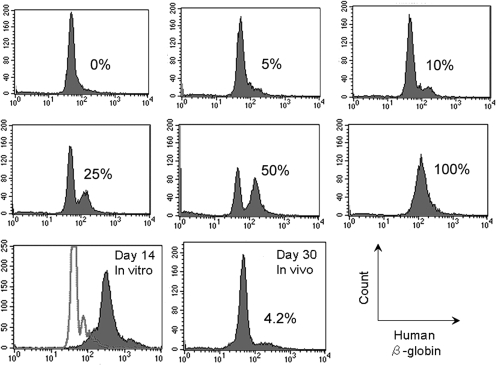
To determine transduction efficiency, both human and rhesus CD34+ progenitor cells were transduced with our modified HIV system. Table 1 shows that a high percentage of human and rhesus CFUs contained TNS9.3 after transduction of CD34+ cells by our modified HIV, with an average transduction efficiency of 97.2% for human and 81.7% for rhesus cells, respectively, at a multiplicity of infection (MOI) of 50. Figure 2 shows a representative flow panel after staining erythroid progeny of transduced rhesus CD34+ progenitor cells with the human β-globin-specific antibody along with controls containing human red blood cells at the indicated input percentage. The majority of the erythroid cells produced in the in vitro erythroid culture system expressed human β-globin (Fig. 2, bottom row, day 14 in vitro).
Table 1.
CFU Assay for Human and Rhesus CD34+ Cells in Vitroa
| |
Experiment 1 |
Experiment 2 |
||
|---|---|---|---|---|
| Human | Rhesus | Human | Rhesus | |
| MOI 50 | 17/18 (94.4%) | 10/12 (83.3%) | 14/14 (100%) | 10/12 (80.0%) |
| MOI 200 | 14/15 (93.3%) | 17/18 (94.4%) | 10/10 (100%) | 20/20 (100%) |
Abbreviations: MOI, multiplicity of infection; TNS9.3, β-globin vector.
The transduction efficiency to human or rhesus CD34+ cells with TNS9.3 at MOI 50 and 200. Tranduction efficiency was estimated by plating colony forming units (CFU) after transduction of human or rhesus CD34+ cells with TNS9.3 gene. After 14 days, individual colonies were picked up and the DNA analyzed by PCR with vector-specific primers. The percentage positive was determined by the fraction of those positive for the housekeeping gene β-actin.
FIG. 2.
Typical flow cytometric analysis of human β-globin expression, using the human-specific H2 antibody. Red blood cells were permeabilized and analyzed for the presence of human β-globin, using the H2 antibody. Concomitant controls of known percentages of human red blood cells mixed with rhesus red blood cells from 0 to 100% were concurrently analyzed and input amounts matched the flow cytometer estimated values. In vitro transduction was estimated after 14 days of erythroid culture (99%, day 14 in vitro) and 30 days after transplantation in animal RQ3586 in vivo (4.2%, day 30 in vivo). The percentages are given in each panel.
Rhesus macaque transplantations
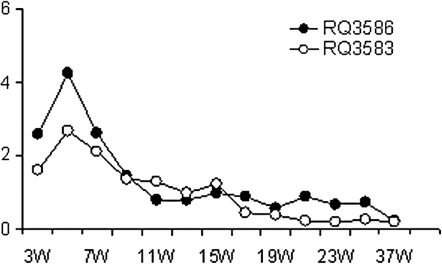
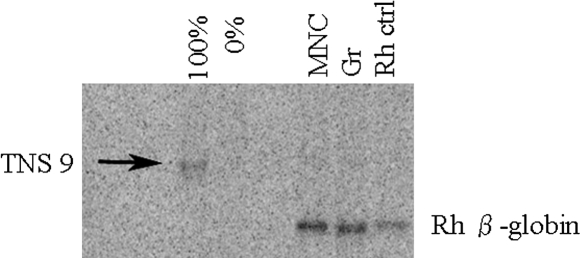
Two rhesus macaques underwent transplantation with our modified TNS9.3 vector-transduced mobilized peripheral blood progenitor cells after irradiation at 500 cGy delivered on days –2 and –1. CD34+ progenitor cells were transduced with our modified TNS9.3 vector, two times at an MOI of 80. Table 2 shows the graft characteristics for both animals. Both animals engrafted rapidly, with neutrophil counts reaching 500 cells/μl by day 12 (RQ3586) and day 10 (RQ3583). A high-level contribution to the early recovery postconditioning by genetically modified cells was demonstrated in RQ3586. Figure 2 demonstrates expression of human β-globin from animal RQ3586. Human β-globin could be detected on day 30 posttransplantation by flow cytometry (Fig. 2, bottom row, day 30 in vivo). To confirm the specificity of our assay for human β-globin production at the protein level, we sorted the β-globin-positive fraction with the H2 human β-globin-specific antibody and assayed for human β-globin by high-performance liquid chromatography analysis, confirming the presence of human β-globin protein by mass spectroscopy. Figure 3 shows the level of human β-globin expression over time posttransplantation in both animals. An initial peak of expression was seen in both animals during recovery, with a gradual fall over time to levels undetectable by flow cytometry. Figure 4 shows genomic Southern blotting for integrated vector on day 30 posttransplantation, with levels of 13% and 32% estimated among MNCs and Grs, respectively.
Table 2.
Graft Characteristicsa
| Animal | Envelope | MOI | Apheresis product | Column yieldb | CD34 purityc | CFU analysisd | Total cell dose infusede |
|---|---|---|---|---|---|---|---|
| RQ3586 | VSV-G | 80 | 3.84 × 109 | 1.62 × 107 | 95.6% | 87.5% | 1.48 × 107 |
| RQ3583 | VSV-G | 80 | 8.84 × 109 | 2.0 × 107 | 98.3% | 83.3% | 2.25 × 107 |
Abbreviations: MOI, multiplicity of infection; TNS9.3, β-globin vector; VSV-G, vesicular stomatitis virus glycoprotein G; CFU, colony-forming units.
Two animals, RQ3586 and RQ3583, underwent transplantation with TNS9.3-transduced cells. The MOI, apheresis product yield in absolute number, the CD34 selection column yield in absolute number, CD34 purity, CFU transduction efficiency, and total cell dose infused are given.
Number of CD34+ cells obtained after selection.
CD34 purity determined by flow cytometry.
CFU transduction efficiency.
Total number of cells infused after transduction.
FIG. 3.
Human β-globin expression over time posttransplantation. Human β-globin expression was analyzed by flow cytometry, using the H2 antibody, and quantitated over 37 weeks posttransplantation in both animals RQ3586 (solid circles) and 3583 (open circles).
FIG. 4.
Genomic Southern blotting from the peripheral blood genomic DNA sample of RQ3586. DNA was extracted from a single copy control (100%), a negative control (0%), peripheral blood MNCs, and Grs from animal 3586 at 30 days posttransplantation, and from peripheral blood MNCs of a control animal, and the DNA was probed for vector-specific sequences (arrow). The percentage positivity estimated, based on the single-copy control, was 13 and 32% for MNCs and Grs, respectively. The lower bands show the endogenous rhesus (Rh) β-globin band.
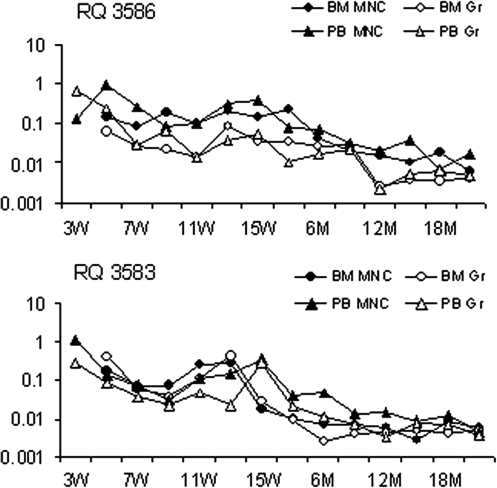
To determine the overall level of engraftment by genetically modified cells long term, real-time PCR for vector-specific sequences was performed over extended follow-up posttransplantation. Figure 5 shows the real-time PCR estimates for the overall level of engraftment by genetically modified cells among MNCs and Grs in both animals. The percentage of cells containing either vector was 1% or higher early on, but gradually decreased and reached stable levels of 0.001–0.01% 1.5 years after transplantation.
FIG. 5.
Quantitation of cells containing integrated TNS9.3 over time posttransplantation. Shown are gene marking levels derived from both animals among BM MNCs, BM Grs, PB MNCs, and PB Grs until 2 years posttransplantation. The data were obtained by real-time PCR using TNS9.3 vector-specific primers and a TaqMan probe. The rate of gene marking was compared with a dilution of a known copy number of integrated TNS9.3 in a background of control rhesus DNA. Top: RQ3586. Bottom: RQ3583.
Discussion
Although remarkable progress in the correction of murine models of β-thalassemia and sickle cell anemia has been reported with the use of lentiviral globin vectors, translation of these results to large animals is essential to address many issues aimed at the maximization of both safety and the likelihood of ultimate clinical success. For human clinical trials to follow, the development of maximally safe vector systems that result in β-globin expression sufficient to correct the phenotype is needed. Our strategy for the development of a model to achieve these goals involves several steps including lentiviral vector optimization for nonhuman primate use, evaluation of stem cell transduction efficiency through in vivo transplantation, maximization of both vector-directed globin gene expression and safety of maximally performing vector systems, and, finally, determination of the minimal degree of host conditioning required for adequate engraftment of genetically modified cells (Tisdale and Sadelain, 2001; Persons and Tisdale, 2004).
Indeed, one of the most difficult issues remains the choice of vector systems. To achieve lifelong clinical efficacy in most disorders of the blood, permanent integration of the vector in hematopoietic stem cells is required. In addition, for disorders of globin synthesis, gene expression must be erythroid specific, high level, and sustained. Lentiviral vectors have inherent advantages in this regard, with promising results obtained in murine models (Rivella and Sadelain, 2002) and verified by several groups (Pawliuk et al., 2001; Levasseur et al., 2003; Persons et al., 2003a,b). In the nonhuman primate model, lentiviral transduction of HSCs with an SIV-based system resulted in moderate levels of in vivo gene transfer and expression in vivo (Hanawa et al., 2004). Although encouraging, we sought to develop vectors based on HIV-1 given the longer duration of use, and the accruing experience with these vectors in human clinical trials (Dropulic and June, 2006). However, when HIV-1-based vectors have been used to transduce rhesus HSCs, poor gene transfer rates were observed owing at least in part to a species-specific block due to a saturable intracellular inhibitor (Besnier et al., 2002; Cowan et al., 2002). Here we developed a modified HIV-1 vector bearing an alternative cyclophilin-binding domain (Kootstra et al., 2003) and demonstrated avoidance of this block in nonhuman primate HSCs in vitro. The modified vector performed well in vitro, with high transduction rates achieved in both human and nonhuman primate CD34+ cells when assessed by the percentage of colonies containing integrated vector by PCR.
To determine the percentage of cells expressing human β-globin, a specific erythroid assay was developed on the basis of small differences between rhesus and human β-globin at the amino acid level. High homology exists between human and rhesus globins, as they are more than 95% identical at both the cDNA and amino acid sequence levels (HomoloGene database at http://www.ncbi.nlm.nih.gov/homologene). The monoclonal antibody (H2, kindly provided by L. Stanker) used in this assay recognizes the human specific β-125 amino acid site where one amino acid change between rhesus and human exists (Stanker et al., 1986). To assay erythroid cells, a modified (Wojda et al., 2002) Fibach two-phase erythroid culture system (Fibach et al., 1989) was developed from which vigorous erythroid output was obtained. This culture system allows the growth of erythroid cells from transduced CD34+ cells that are subsequently permeabilized and assayed with the H2 antibody. Using this technique, we were able to demonstrate by simple flow cytometry an equally high percentage of cells expressing human β-globin after transduction. Although no large-animal models for the thalassemias or the hemoglobinopathies exist, important information regarding the amount of human β-globin expressed per vector copy should ultimately be measurable in this context.
With these tools in place, two rhesus macaques underwent in vivo transplantation with TNS9.3-transduced autologous PB CD34+ cells. In one animal, human β-globin expression at approximately 5% was demonstrated by flow cytometry at early time points. This level of human β-globin expression was confirmed by RNase protection assay (data not shown) and similar levels were estimated at the DNA level by genomic Southern blotting. Furthermore, expression of human β-globin was confirmed by mass spectrometry. Although high human β-globin gene transfer rates in rhesus CD34+ cells and the efficient production of human β-globin could be demonstrated in vitro, the moderate level of human β-globin production in vivo was limited to only a short period posttransplantation, requiring PCR for detection long term. Indeed, PCR optimization for such low levels observed during long-term follow-up may have resulted in an underestimation of the early marking. Factors such as hematopoietic progenitor cell preparation, transduction method, and cytokine combination were all similar to former protocols that our group has already demonstrated elsewhere to be sufficient for sustained, moderate levels of in vivo gene transfer (Kiem et al., 1998, 2004; Wu et al., 2000). In addition, the transduction methods were modeled after those used with SIV in the rhesus macaque (Hanawa et al., 2004). A number of other factors could explain the decline in human transgene expression over time. Others have demonstrated more efficient transduction of human peripheral blood CD34+ cells, using the amphotropic envelope in a nonobese diabetic/severe combined immunodeficient (NOD/SCID) mouse transplant model (Hanawa et al., 2002). We are currently comparing alternative envelopes such as the amphotropic envelope to determine whether this can improve transduction of the more primitive hematopoietic stem cell compartment. However, efficient transduction and engraftment of repopulating cells with VSV-G-pseudotyped lentiviral vectors in the pigtail macaque have been described (Trobridge et al., 2008), and these promising results are likely due to an escape from host restriction by TRIM5α in this model. In addition, equivalent in vivo marking levels were demonstrated when oncoretroviral vectors pseudotyped with either the amphotropic or VSV-G envelope were compared (Shi et al., 2004). HIV-1 vectors encoding marker genes have been more successful in the rhesus model, although a high degree of variation remains present (An et al., 2000, 2001). This discrepancy with our results could relate to the difficulty in achieving high-titer vectors encoding human β-globin along with key regulatory elements. A reduction in absolute HSC numbers could account for the fall in marking derived from the long-term repopulating cell compartment as the gene marking level at 4–6 months or greater posttransplantation represents the “true HSC” marking level (Kim et al., 2000). Unfortunately, our gene marking levels detected by Q-PCR over 6 months posttransplantation were approximately 0.01% in both animals, certainly insufficient to expect clinical benefit in humans, and potentially indicating that our vector system/transduction method has not achieved efficient transduction to LT-HSCs.
The restriction to infection by HIV in rhesus cells was shown to be only partially overcome by modification of the cyclophilin-binding domain (Kahl et al., 2008). Indeed, this modification was insufficient in our hands to achieve high-level engraftment by HSCs transduced with our HIV-1-based vector. The early peak in gene transfer observed thus likely represents a burst in marking derived from successfully transduced committed progenitors but continued restriction of HIV-1 from the HSC compartment. Among the restriction factors identified to date, tripartite motif-5 isoform α (TRIM5α) and apolipoprotein B mRNA-editing catalytic polypeptide 3G (APOBEC3G) have both been shown to restrict HIV-1 infection and in combination may potentially decrease transduction efficiency of HIV-1-based viral vectors (Sakuma et al., 2007). Rhesus TRIM5α binds to the HIV-1 capsid (CA) to initiate E3 ubiquitin ligase-mediated degradation and prevent viral replication (Stremlau et al., 2004), whereas SIV can escape rhesus TRIM5α. Our current work is focused on the development of a lentiviral vector system that can perform with equal efficiency in the human and rhesus nonhuman primate setting. Preliminary results using a newly developed vector system combining components of both HIV with SIV capsid to overcome the restriction demonstrate high-level in vivo marking with a vector encoding green fluorescent protein (GFP) (Uchida et al., 2008).
For human clinical trials to proceed, one must also consider the risk of insertional mutagenesis. Certainly the theoretical risk of insertional oncogenesis has been documented in the nonhuman primate model (Donahue et al., 1992), and unfortunately, more recently in human clinical trials of gene therapy for immunodeficiency diseases (Cavazzana-Calvo et al., 2000; Hacein-Bey-Abina et al., 2003a,b). Although both of our recipient animals maintained normal blood counts during follow-up, the overall low-level contribution by genetically modified cells does not permit a realistic assessment of the risk of insertional mutagenesis with our current vector system and such analysis will have to await further optimization. Indeed, our attempts at cloning lentiviral integrations were met with difficulty owing to the low level of marking and the competing internal bands that are frequent with our construct. The assessment of integration patterns with our lentiviral system will require long-term, high-level engraftment of genetically modified cells in order to assess the safety of our approach. Lentiviral vector have already shown a propensity to insert into transcriptionally active genes (Schroder et al., 2002; Wu et al., 2003; Hematti et al., 2004) and target cells often undergo multiple integration events, particularly at high multiplicities of infection (Woods et al., 2003). Whether vectors that direct erythroid-specific expression of the therapeutic gene will result in an integration site pattern reflecting engraftment patterns that differ from those already described remains of great interest, as vectors with such expression patterns may ultimately prove safer than vectors that constitutively express the therapeutic gene.
In summary, we report transient in vivo β-globin production after lentiviral gene transfer to hematopoietic stem cells in the nonhuman primate. Further optimization should ultimately allow us to comprehensively model HIV-1-based globin gene transfer.
Acknowledgments
This research was supported by the Intramural Research Program of the NIDDK and NHLBI at the NIH and the Leonardo Giambrone Foundation. The authors thank Martha Kirby for cell sorting and D. Eric Anderson for mass spectroscopy. The authors also thank Drs. Isabelle Riviere and Ken Cornetta for helpful discussion on vector production, and Jason Plotkin for assistance with vector construction.
Author Disclosure Statement
There is no significant financial conflict of interest to declare.
References
- An D.S. Wersto R.P. Agricola B.A. Metzger M.E. Lu S. Amado R.G. Chen I.S. Donahue R.E. Marking and gene expression by a lentivirus vector in transplanted human and nonhuman primate CD34+ cells. J. Virol. 2000;74:1286–1295. doi: 10.1128/jvi.74.3.1286-1295.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An D.S. Kung S.K. Bonifacino A. Wersto R.P. Metzger M.E. Agricola B.A. Mao S.H. Chen I.S. Donahue R.E. Lentivirus vector-mediated hematopoietic stem cell gene transfer of common γ-chain cytokine receptor in rhesus macaques. J. Virol. 2001;75:3547–3555. doi: 10.1128/JVI.75.8.3547-3555.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besnier C. Takeuchi Y. Towers G. Restriction of lentivirus in monkeys. Proc. Natl. Acad. Sci. U.S.A. 2002;99:11920–11925. doi: 10.1073/pnas.172384599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulad F. Giardina P. Gillio A. Kernan N. Small T. Brochstein J. Van Syckle K. George D. Szabolcs P. O'Reilly R.J. Bone marrow transplantation for homozygous β-thalassemia: The Memorial Sloan-Kettering Cancer Center experience. Ann. N. Y. Acad. Sci. 1998;850:498–502. doi: 10.1111/j.1749-6632.1998.tb10532.x. [DOI] [PubMed] [Google Scholar]
- Cavazzana-Calvo M. Hacein-Bey S. De Saint Basile G. Gross F. Yvon E. Nusbaum P. Selz F. Hue C. Certain S. Casanova J.L. Bousso P. Deist F.L. Fischer A. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease [see comments] Science. 2000;288:669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- Cowan S. Hatziioannou T. Cunningham T. Muesing M.A. Gottlinger H.G. Bieniasz P.D. Cellular inhibitors with Fv1-like activity restrict human and simian immunodeficiency virus tropism. Proc. Natl. Acad. Sci. U.S.A. 2002;99:11914–11919. doi: 10.1073/pnas.162299499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham M.J. Macklin E.A. Neufeld E.J. Cohen A.R. Complications of β-thalassemia major in North America. Blood. 2004;104:34–39. doi: 10.1182/blood-2003-09-3167. [DOI] [PubMed] [Google Scholar]
- Donahue R.E. Kessler S.W. Bodine D. McDonagh K. Dunbar C. Goodman S. Agricola B. Byrne E. Raffeld M. Moen R. Bacher J. Zsebo K.M. Nienhuis A.W. Helper virus induced T cell lymphoma in nonhuman primates after retroviral mediated gene transfer. J. Exp. Med. 1992;176:1125–1135. doi: 10.1084/jem.176.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue R.E. Kirby M.R. Metzger M.E. Agricola B.A. Sellers S.E. Cullis H.M. Peripheral blood CD34+ cells differ from bone marrow CD34+ cells in Thy-1 expression and cell cycle status in nonhuman primates mobilized or not mobilized with granulocyte colony-stimulating factor and/or stem cell factor. Blood. 1996;87:1644–1653. [PubMed] [Google Scholar]
- Dropulic B. June C.H. Gene-based immunotherapy for human immunodeficiency virus infection and acquired immunodeficiency syndrome. Hum. Gene Ther. 2006;17:577–588. doi: 10.1089/hum.2006.17.577. [DOI] [PubMed] [Google Scholar]
- Dull T. Zufferey R. Kelly M. Mandel R.J. Nguyen M. Trono D. Naldini L. A third-generation lentivirus vector with a conditional packaging system. J. Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fibach E. Manor D. Oppenheim A. Rachmilewitz E.A. Proliferation and maturation of human erythroid progenitors in liquid culture. Blood. 1989;73:100–103. [PubMed] [Google Scholar]
- Grosveld F. Van Assendelft G.B. Greaves D.R. Kollias G. Position-independent, high-level expression of the human β-globin gene in transgenic mice. Cell. 1987;51:975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S. Von Kalle C. Schmidt M. Le Deist F. Wulffraat N. McIntyre E. Radford I. Villeval J.L. Fraser C.C. Cavazzana-Calvo M. Fischer A. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N. Engl. J. Med. 2003a;348:255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S. Von Kalle C. Schmidt M. McCormack M.P. Wulffraat N. Leboulch P. Lim A. Osborne C.S. Pawliuk R. Morillon E. Sorensen R. Forster A. Fraser P. Cohen J.I. De Saint Basile G. Alexander I. Wintergerst U. Frebourg T. Aurias A. Stoppa-Lyonnet D. Romana S. Radford-Weiss I. Gross F. Valensi F. Delabesse E. Macintyre E. Sigaux F. Soulier J. Leiva L.E. Wissler M. Prinz C. Rabbitts T.H. Le Deist F. Fischer A. Cavazzana-Calvo M. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003b;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- Hanawa H. Kelly P.F. Nathwani A.C. Persons D.A. Vandergriff J.A. Hargrove P. Vanin E.F. Nienhuis A.W. Comparison of various envelope proteins for their ability to pseudotype lentiviral vectors and transduce primitive hematopoietic cells from human blood. Mol. Ther. 2002;5:242–251. doi: 10.1006/mthe.2002.0549. [DOI] [PubMed] [Google Scholar]
- Hanawa H. Hematti P. Keyvanfar K. Metzger M.E. Krouse A. Donahue R.E. Kepes S. Gray J. Dunbar C.E. Persons D.A. Nienhuis A.W. Efficient gene transfer into rhesus repopulating hematopoietic stem cells using a simian immunodeficiency virus-based lentiviral vector system. Blood. 2004;103:4062–4069. doi: 10.1182/blood-2004-01-0045. [DOI] [PubMed] [Google Scholar]
- Hematti P. Hong B.K. Ferguson C. Adler R. Hanawa H. Sellers S. Holt I.E. Eckfeldt C.E. Sharma Y. Schmidt M. Von Kalle C. Persons D.A. Billings E.M. Verfaillie C.M. Nienhuis A.W. Wolfsberg T.G. Dunbar C.E. Calmels B. Distinct genomic integration of MLV and SIV vectors in primate hematopoietic stem and progenitor cells. PLoS Biol. 2004;2:e423. doi: 10.1371/journal.pbio.0020423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council. Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, DC: 1996. [Google Scholar]
- Kahl C.A. Cannon P.M. Oldenburg J. Tarantal A.F. Kohn D.B. Tissue-specific restriction of cyclophilin A-independent HIV-1- and SIV-derived lentiviral vectors. Gene Ther. 2008;15:1079–1089. doi: 10.1038/gt.2008.50. [DOI] [PubMed] [Google Scholar]
- Kalberer C.P. Pawliuk R. Imren S. Bachelot T. Takekoshi K.J. Fabry M. Eaves C.J. London I.M. Humphries R.K. Leboulch P. Preselection of retrovirally transduced bone marrow avoids subsequent stem cell gene silencing and age-dependent extinction of expression of human β-globin in engrafted mice [see comments] Proc. Natl. Acad. Sci. U.S.A. 2000;97:5411–5415. doi: 10.1073/pnas.100082597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang E.M. Hanazano Y. Frare P. Vanin E.F. De Witte M. Metzger M. Liu J.M. Tisdale J.F. Persistent low-level engraftment of rhesus peripheral blood progenitor cells transduced with the Fanconi anemia C gene after conditioning with low-dose irradiation. Mol. Ther. 2001;3:911–919. doi: 10.1006/mthe.2001.0337. [DOI] [PubMed] [Google Scholar]
- Kang E.M. Areman E.M. David-Ocampo V. Fitzhugh C. Link M.E. Read E.J. Leitman S.F. Rodgers G.P. Tisdale J.F. Mobilization, collection, and processing of peripheral blood stem cells in individuals with sickle cell trait. Blood. 2002a;99:850–855. doi: 10.1182/blood.v99.3.850. [DOI] [PubMed] [Google Scholar]
- Kang E.M. De Witte M. Malech H. Morgan R.A. Phang S. Carter C. Leitman S.F. Childs R. Barrett A.J. Little R. Tisdale J.F. Nonmyeloablative conditioning followed by transplantation of genetically modified HLA-matched peripheral blood progenitor cells for hematologic malignancies in patients with acquired immunodeficiency syndrome. Blood. 2002b;99:698–701. doi: 10.1182/blood.v99.2.698. [DOI] [PubMed] [Google Scholar]
- Kiem H.P. Andrews R.G. Morris J. Peterson L. Heyward S. Allen J.M. Rasko J.E. Potter J. Miller A.D. Improved gene transfer into baboon marrow repopulating cells using recombinant human fibronectin fragment CH-296 in combination with interleukin-6, stem cell factor, FLT-3 ligand, and megakaryocyte growth and development factor. Blood. 1998;92:1878–1886. [PubMed] [Google Scholar]
- Kiem H.P. Sellers S. Thomasson B. Morris J.C. Tisdale J.F. Horn P.A. Hematti P. Adler R. Kuramoto K. Calmels B. Bonifacino A. Hu J. Von Kalle C. Schmidt M. Sorrentino B. Nienhuis A. Blau C.A. Andrews R.G. Donahue R.E. Dunbar C.E. Long-term clinical and molecular follow-up of large animals receiving retrovirally transduced stem and progenitor cells: No progression to clonal hematopoiesis or leukemia. Mol. Ther. 2004;9:389–395. doi: 10.1016/j.ymthe.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Kim H.J. Tisdale J.F. Wu T. Takatoku M. Sellers S.E. Zickler P. Metzger M.E. Agricola B.A. Malley J.D. Kato I. Donahue R.E. Brown K.E. Dunbar C.E. Many multipotential gene-marked progenitor or stem cell clones contribute to hematopoiesis in nonhuman primates. Blood. 2000;96:1–8. [PubMed] [Google Scholar]
- Kootstra N.A. Munk C. Tonnu N. Landau N.R. Verma I.M. Abrogation of postentry restriction of HIV-1-based lentiviral vector transduction in simian cells. Proc. Natl. Acad. Sci. U.S.A. 2003;100:1298–1303. doi: 10.1073/pnas.0337541100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M. Keller B. Makalou N. Sutton R.E. Systematic determination of the packaging limit of lentiviral vectors. Hum. Gene Ther. 2001;12:1893–1905. doi: 10.1089/104303401753153947. [DOI] [PubMed] [Google Scholar]
- Levasseur D.N. Ryan T.M. Pawlik K.M. Townes T.M. Correction of a mouse model of sickle cell disease: Lentiviral/antisickling β-globin gene transduction of unmobilized, purified hematopoietic stem cells. Blood. 2003;102:4312–4319. doi: 10.1182/blood-2003-04-1251. [DOI] [PubMed] [Google Scholar]
- May C. Rivella S. Callegari J. Heller G. Gaensler K.M. Luzzatto L. Sadelain M. Therapeutic haemoglobin synthesis in β-thalassaemic mice expressing lentivirus-encoded human β-globin. Nature. 2000;406:82–86. doi: 10.1038/35017565. [DOI] [PubMed] [Google Scholar]
- May C. Rivella S. Chadburn A. Sadelain M. Successful treatment of murine β-thalassemia intermedia by transfer of the human β-globin gene. Blood. 2002;99:1902–1908. doi: 10.1182/blood.v99.6.1902. [DOI] [PubMed] [Google Scholar]
- Pawliuk R. Westerman K.A. Fabry M.E. Payen E. Tighe R. Bouhassira E.E. Acharya S.A. Ellis J. London I.M. Eaves C.J. Humphries R.K. Beuzard Y. Nagel R.L. Leboulch P. Correction of sickle cell disease in transgenic mouse models by gene therapy. Science. 2001;294:2368–2371. doi: 10.1126/science.1065806. [DOI] [PubMed] [Google Scholar]
- Persons D.A. Nienhuis A.W. Gene therapy for the hemoglobin disorders: Past, present, and future. Proc. Natl. Acad. Sci. U.S.A. 2000;97:5022–5024. doi: 10.1073/pnas.97.10.5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persons D.A. Tisdale J.F. Gene therapy for the hemoglobin disorders. Semin. Hematol. 2004;41:279–286. doi: 10.1053/j.seminhematol.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Persons D.A. Allay E.R. Sawai N. Hargrove P.W. Brent T.P. Hanawa H. Nienhuis A.W. Sorrentino B.P. Successful treatment of murine β-thalassemia using in vivo selection of genetically modified, drug-resistant hematopoietic stem cells. Blood. 2003a;102:506–513. doi: 10.1182/blood-2003-03-0677. [DOI] [PubMed] [Google Scholar]
- Persons D.A. Hargrove P.W. Allay E.R. Hanawa H. Nienhuis A.W. The degree of phenotypic correction of murine β-thalassemia intermedia following lentiviral-mediated transfer of a human γ-globin gene is influenced by chromosomal position effects and vector copy number. Blood. 2003b;101:2175–2183. doi: 10.1182/blood-2002-07-2211. [DOI] [PubMed] [Google Scholar]
- Pootrakul P. Sirankapracha P. Hemsorach S. Moungsub W. Kumbunlue R. Piangitjagum A. Wasi P. Ma L. Schrier S.L. A correlation of erythrokinetics, ineffective erythropoiesis, and erythroid precursor apoptosis in Thai patients with thalassemia. Blood. 2000;96:2606–2612. [PubMed] [Google Scholar]
- Rits M.A. Van Dort K.A. Munk C. Meijer A.B. Kootstra N.A. Efficient transduction of simian cells by HIV-1-based lentiviral vectors that contain mutations in the capsid protein. Mol. Ther. 2007;15:930–937. doi: 10.1038/mt.sj.6300091. [DOI] [PubMed] [Google Scholar]
- Rivella S. Sadelain M. Therapeutic globin gene delivery using lentiviral vectors. Curr. Opin. Mol. Ther. 2002;4:505–514. [PubMed] [Google Scholar]
- Sadelain M. Wang C.H. Antoniou M. Grosveld F. Mulligan R.C. Generation of a high-titer retroviral vector capable of expressing high levels of the human β-globin gene. Proc. Natl. Acad. Sci. U.S.A. 1995;92:6728–6732. doi: 10.1073/pnas.92.15.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma R. Noser J.A. Ohmine S. Ikeda Y. Inhibition of HIV-1 replication by simian restriction factors, TRIM5α and APOBEC3G. Gene Ther. 2007;14:185–189. doi: 10.1038/sj.gt.3302852. [DOI] [PubMed] [Google Scholar]
- Schroder A.R. Shinn P. Chen H. Berry C. Ecker J.R. Bushman F. HIV-1 integration in the human genome favors active genes and local hotspots. Cell. 2002;110:521–529. doi: 10.1016/s0092-8674(02)00864-4. [DOI] [PubMed] [Google Scholar]
- Shi P.A. De Angioletti M. Donahue R.E. Notaro R. Luzzatto L. Dunbar C.E. In vivo gene marking of rhesus macaque long-term repopulating hematopoietic cells using a VSV-G pseudotyped versus amphotropic oncoretroviral vector. J. Gene Med. 2004;6:367–373. doi: 10.1002/jgm.514. [DOI] [PubMed] [Google Scholar]
- Stanker L.H. Branscomb E. Vanderlaan M. Jensen R.H. Monoclonal antibodies recognizing single amino acid substitutions in hemoglobin. J. Immunol. 1986;136:4174–4180. [PubMed] [Google Scholar]
- Stremlau M. Owens C.M. Perron M.J. Kiessling M. Autissier P. Sodroski J. The cytoplasmic body component TRIM5α restricts HIV-1 infection in Old World monkeys. Nature. 2004;427:848–853. doi: 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
- Tisdale J.F. Sadelain M. Toward gene therapy for disorders of globin synthesis. Semin. Hematol. 2001;38:382–392. doi: 10.1016/s0037-1963(01)90033-2. [DOI] [PubMed] [Google Scholar]
- Tisdale J.F. Hanazono Y. Sellers S.E. Agricola B.A. Metzger M.E. Donahue R.E. Dunbar C.E. Ex vivo expansion of genetically marked rhesus peripheral blood progenitor cells results in diminished long-term repopulating ability. Blood. 1998;92:1131–1141. [PubMed] [Google Scholar]
- Trobridge G.D. Beard B.C. Gooch C. Wohlfahrt M. Olsen P. Fletcher J. Malik P. Kiem H.P. Efficient transduction of pigtailed macaque hematopoietic repopulating cells with HIV-based lentiviral vectors. Blood. 2008;111:5537–5543. doi: 10.1182/blood-2007-09-115022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N. Washington K. Hayakawa J. Hsieh M. Bonifacino A.C. Krouse A.E. Metzger M.E. Donahue R.E. Tisdale J.F. Development of an HIV1-based lentiviral vector able to transduce both human and rhesus blood cells. Blood (ASH Annual Meeting Abstracts) 2008;112:2353. [Google Scholar]
- Weatherall D.J. Phenotype–genotype relationships in monogenic disease: Lessons from the thalassaemias. Nat. Rev. Genet. 2001;2:245–255. doi: 10.1038/35066048. [DOI] [PubMed] [Google Scholar]
- Wojda U. Noel P. Miller J.L. Fetal and adult hemoglobin production during adult erythropoiesis: Coordinate expression correlates with cell proliferation. Blood. 2002;99:3005–3013. [PubMed] [Google Scholar]
- Woods N.B. Muessig A. Schmidt M. Flygare J. Olsson K. Salmon P. Trono D. Von Kalle C. Karlsson S. Lentiviral vector transduction of NOD/SCID repopulating cells results in multiple vector integrations per transduced cell: Risk of insertional mutagenesis. Blood. 2003;101:1284–1289. doi: 10.1182/blood-2002-07-2238. [DOI] [PubMed] [Google Scholar]
- Wu T. Kim H.J. Sellers S. Meade K.E. Agricola B. Metzger M.E. Kato I. Donahue R. Dunbar C. Tisdale J. Prolonged high-level detection of retrovirally marked hematopoietic cells in nonhuman primates after transduction of CD34+ progenitors using clinically feasible methods. Mol. Ther. 2000;1:285–293. doi: 10.1006/mthe.2000.0034. [DOI] [PubMed] [Google Scholar]
- Wu X. Li Y. Crise B. Burgess S.M. Transcription start regions in the human genome are favored targets for MLV integration. Science. 2003;300:1749–1751. doi: 10.1126/science.1083413. [DOI] [PubMed] [Google Scholar]
- Yang B. Kirby S. Lewis J. Detloff P.J. Maeda N. Smithies O. A mouse model for β0-thalassemia. Proc. Natl. Acad. Sci. U.S.A. 1995;92:11608–11612. doi: 10.1073/pnas.92.25.11608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufferey R. Nagy D. Mandel R.J. Naldini L. Trono D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat. Biotechnol. 1997;15:871–875. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]