Abstract
Obesity-related disorders are associated with the development of ischemic heart disease. Adiponectin is a circulating adipose-derived cytokine that is downregulated in obese individuals and after myocardial infarction. Here, we examine the role of adiponectin in myocardial remodeling in response to acute injury. Ischemia-reperfusion in adiponectin-deficient (APN-KO) mice resulted in increased myocardial infarct size, myocardial apoptosis and tumor necrosis factor (TNF)-α expression compared with wild-type mice. Administration of adiponectin diminished infarct size, apoptosis and TNF-α production in both APN-KO and wild-type mice. In cultured cardiac cells, adiponectin inhibited apoptosis and TNF-α production. Dominant negative AMP-activated protein kinase (AMPK) reversed the inhibitory effects of adiponectin on apoptosis but had no effect on the suppressive effect of adiponectin on TNF-α production. Adiponectin induced cyclooxygenase (COX)-2–dependent synthesis of prostaglandin E2 in cardiac cells, and COX-2 inhibition reversed the inhibitory effects of adiponectin on TNF-α production and infarct size. These data suggest that adiponectin protects the heart from ischemia-reperfusion injury through both AMPK- and COX-2–dependent mechanisms.
Ischemic heart disease including myocardial infarction is the major cause of death in industrial countries1,2. Obesity-linked disorders are thought to be involved in the severity and outcome of ischemic heart disease3,4, but the link between obesity and the development of heart disease is poorly understood at the molecular level. Adiponectin, also referred to as ACRP30, AdipoQ and gelatin-binding protein-28 (refs. 5-7), is an adipocyte-specific cytokine. Circulating adiponectin levels are diminished in obese individuals8 and are inversely correlated with cardiovascular risk factors including hyperlipidemia, blood pressure and C-reactive protein (CRP) levels9,10. It has also been shown that hypoadiponectinemia is an independent risk factor for developing type 2 diabetes11, hypertension12 and coronary artery disease13. Adiponectin-knockout (APN-KO) mice have diet-induced insulin resistance14, impaired angiogenic responses to ischemia15 and excessive cardiac remodeling after pressure overload16. Conversely, overexpression of adiponectin promotes insulin sensitivity and angiogenesis, and inhibits cardiac hypertrophy. These data suggest that adiponectin functions as a mediator of obesity-linked cardiovascular and metabolic disorders, and that it may have utility for the treatment of a number of chronic diseases including type 2 diabetes, peripheral artery disease and hypertrophic cardiomyopathy.
Recently, it was shown that high plasma adiponectin levels are associated with a lower risk of myocardial infarction independent of CRP levels and glycemic status17. Furthermore, it is recognized that adiponectin levels rapidly decline after acute myocardial infarction18. Here, we investigate whether adiponectin confers resistance to acute ischemic injury in the heart. We tested the effects of adiponectin on myocardial infarct size, apoptotic activity and inflammation with loss- and gain-of-function genetic manipulations. Our observations indicate that adiponectin is cardioprotective in the context of ischemia-reperfusion injury through both AMPK-dependent antiapoptotic actions and cyclooxygenase (COX)-2–dependent anti-inflammatory actions on cardiac cells.
RESULTS
Increased myocardial infarct size in APN-KO mice
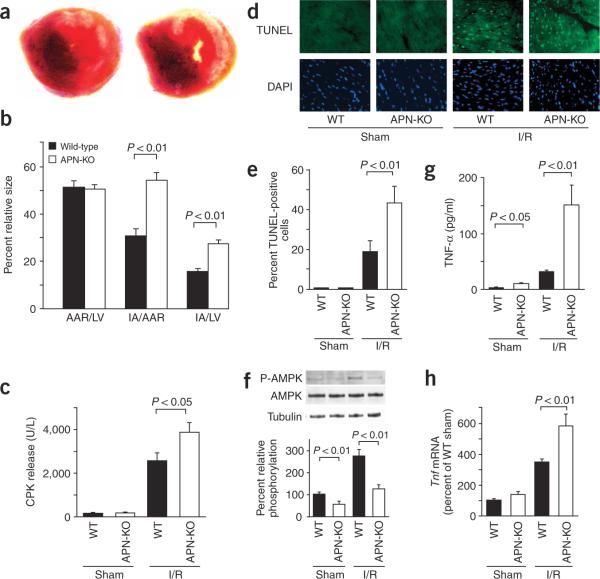
We subjected wild-type and APN-KO mice to 30 min of left anterior descending (LAD) vessel ligation followed by 48 h of reperfusion. All mice survived the surgical induction of ischemia and reperfusion. Body weight and blood pressure did not differ between wild-type and APN-KO mice (data not shown). Representative photographs of myocardial tissues after staining with Evans blue dye to delineate area at risk (AAR) and 2,3,5-triphenyltetrazolium chloride to delineate infarct area in wild-type and APN-KO mice are shown in Figure 1a. The ratio of AAR to left ventricular area was the same in APN-KO and wild-type mice (Fig. 1b). But the ratios of infarct area to AAR and infarct area to left ventricular area were increased 78% and 76%, respectively, in APN-KO mice compared with those of wild-type mice. Serum creatine phosphokinase (CPK) level, an index of myocyte injury, was also significantly higher in APN-KO mice compared with wild-type mice after ischemia and 6 h of reperfusion (Fig. 1c).
Figure 1.
Increased myocardial infarction, myocardial apoptosis and TNF-α expression in APN-KO mice subjected to ischemia-reperfusion injury. (a) Representative pictures of myocardial tissues from wild-type (left) and APN-KO (right) mice at 48 h after ischemia-reperfusion. The nonischemic area is indicated by blue, the AAR by red and the infarct area by white. (b) Quantification of infarct size in wild-type (n = 7) and APN-KO (n = 7) mice. AAR/LV, ratio of AAR to left ventricular area; IA/AAR, ratio of infarct area to AAR; IA/LV, ratio of infarct area to left ventricular area. (c) Release of CPK from wild-type (n = 4) and APN-KO mice (n = 4) after sham operation or ischemia-reperfusion. (d) Representative photographs of TUNEL-stained heart sections from wild-type and APN-KO mice at 48 h after sham operation or ischemia-reperfusion. Apoptotic nuclei were identified by TUNEL staining (green) and total nuclei by DAPI counterstaining (blue). (e) Quantitative analysis of apoptotic nuclei from wild-type (n = 5) and APN-KO mice (n = 5) hearts after sham operation or ischemia-reperfusion. TUNEL-positive nuclei are expressed as a percentage of the total number of nuclei. (f) Phosphorylation of AMPK in heart tissues from wild-type and APN-KO mice at 48 h after sham operation or ischemia-reperfusion. (n = 4 hearts per group). (g) Serum levels of TNF-α, determined by ELISA, in wild-type (n = 5) and APN-KO (n = 5) mice at 48 h after sham operation or ischemia-reperfusion.
(h) Myocardial levels of Tnf transcript in wild-type (n = 5) and APN-KO (n = 5) mice. Tnf mRNA in myocardium of wild-type and APN-KO mice were quantified by RT-PCR. Results are presented as mean ± s.d. WT, wild-type; I/R, ischemia-reperfusion.
Increased myocardial apoptosis in APN-KO mice
To investigate the extent of apoptosis in the AAR regions, we performed TUNEL staining on the different experimental groups. Representative photographs of TUNEL-positive nuclei in the heart are shown in Figure 1d. Quantitative analysis showed a significantly higher proportion of TUNEL-positive cells in the myocardium of APN-KO mice compared with wild-type mice after ischemia-reperfusion injury, whereas little or no TUNEL-positive cells could be detected in the hearts of wild-type or APN-KO mice after sham operation (Fig. 1e).
Because AMP-activated protein kinase (AMPK) is reported to protect myocytes from ischemia-reperfusion injury19, we assessed the phosphorylation status of AMPK at threonine residue 172 in heart tissue by western blotting (Fig. 1f). Ischemia-reperfusion increased phosphorylation of AMPK in wild-type hearts, but this induction was markedly attenuated in the APN-KO hearts. Basal levels of AMPK phosphorylation were also reduced in the sham-operated hearts of APN-KO compared to wild-type mice. Adiponectin-activated AMPK signaling in endothelial cells is proangiogenic20, but no difference in capillary density was detected between wild-type and APN-KO 2 d after injury (Supplementary Fig. 1 online).
Elevated production of TNF-α in APN-KO mice
Because inflammation contributes to myocardial injury after ischemia and reperfusion21, we assessed serum and cardiac levels of TNF-α in each experimental group (Fig. 1g,h). Ischemia-reperfusion led to an increase in serum TNF-α in wild-type mice, consistent with previous reports21, but the magnitude of this induction was greater in APN-KO than in wild-type mice (Fig. 1g). Basal serum TNF-α levels in sham-operated mice were mildly elevated in APN-KO compared with wild-type mice. We quantified Tnf mRNA (which encodes TNF-α) in heart by real-time PCR. Cardiac Tnf mRNA was elevated by ischemia-reperfusion injury to a greater degree in APN-KO mice than in wild-type mice (Fig. 1h). We also measured serum levels of interleukin (IL)-1β and IL-6 in each experimental group. Both cytokines were upregulated after ischemia-reperfusion but, in contrast to TNF-α, levels of serum IL-1β and IL-6 did not differ between APN-KO and wild-type mice (Supplementary Fig. 2 online).
Adiponectin supplementation is cardiac protective
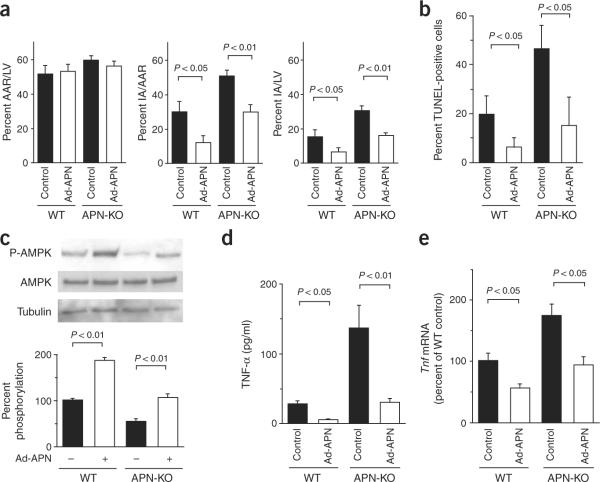
To test whether increased expression of adiponectin could minimize infarct area, we pretreated wild-type and APN-KO mice with adenoviral vectors expressing either adiponectin (Ad-APN) or β-galactosidase (Ad-βgal) as a control. When mice were killed, circulating adiponectin levels were 11.1 ± 1.8 μg/ml in wild-type control, 25.2 ± 4.5 μg/ml in wild-type Ad-APN–treated, <0.05 μg/ml in APN-KO control and 14.8 ± 8.1 μg/ml in APN-KO Ad-APN–treated mice. Both wild-type and APN-KO mice treated with Ad-APN showed a significant decrease in infarct area after ischemia-reperfusion compared with mice receiving the control vector (Fig. 2a). These data indicate that adiponectin replacement can rescue the increase in infarct size seen in APN-KO mice and that overexpression of adiponectin can protect against myocardial injury after ischemia-reperfusion in wild-type mice.
Figure 2.
Adenovirus-mediated expression of adiponectin diminishes infarct size, apoptosis and TNF-α production after ischemia-reperfusion in wild-type (WT) and APN-KO mice. Ad-APN (2 × 108 plaque-forming units total) was delivered intravenously through the jugular vein 3 d before ischemia-reperfusion injury. (a) Quantification of infarct size in wild-type (n = 4) and APN-KO (n = 4) treated with Ad-APN or Ad-βgal (control) at 48 h after surgery. AAR/LV, ratio of AAR to left ventricular area; IA/AAR, ratio of infarct area to AAR; IA/LV, ratio of infarct area to left ventricular area. (b) Quantitative analysis of apoptotic nuclei from wild-type (n = 4) and APN-KO (n = 4) mice hearts treated with Ad-APN or Ad-βgal after ischemia-reperfusion. TUNEL-positive nuclei were counted in several randomly selected fields and expressed as a percentage of the total number of nuclei. (c) Phosphorylation of AMPK in heart tissues of wild-type and APN-KO mice treated with Ad-APN or Ad-βgal at 48 h after sham operation or ischemia-reperfusion. AMPK phosphorylation (P-AMPK) and total AMPK in myocardium was analyzed by western blotting. Phosphorylation levels of AMPK were quantified and expressed relative to untreated WT. Immunoblots were normalized to total loaded protein (n = 4 hearts per experimental group). (d) Serum levels of TNF-α, in wild-type (n = 4) and APN-KO (n = 4) mice treated with Ad-APN or Ad-βgal at 48 h after ischemia-reperfusion. (e) Myocardial levels of Tnf transcript in wild-type (n = 4) and APN-KO (n = 4) mice treated with Ad-APN or Ad-βgal at 48 h after ischemia-reperfusion. Results are presented as mean ± s.d.
To examine whether an increase in adiponectin levels has antiapoptotic actions in vivo, we assessed the viability of myocardial cells by TUNEL assay in tissue sections after ischemia-reperfusion in both wild-type and APN-KO mice treated with Ad-APN or Ad-βgal. Ad-APN treatment decreased the frequency of TUNEL-positive cells in both the wild-type and APN-KO mice (Fig. 2b). The reduction in myocyte apoptosis by adiponectin was associated with increases in the regulatory phosphorylation of AMPK at Thr172 in wild-type and APN-KO mice (Fig. 2c).
To test whether increased expression of adiponectin could decrease production of TNF-α after infarction, we determined serum levels of TNF-α and myocardial levels of Tnf mRNA after ischemia-reperfusion in wild-type and APN-KO mice treated with Ad-APN or Ad-βgal. Ad-APN treatment markedly decreased serum TNF-α after infarction in both wild-type and APN-KO mice (Fig. 2d). Ad-APN treatment also decreased Tnf mRNA in the hearts of both wild-type and APN-KO mice (Fig. 2e).
Adiponectin inhibits apoptosis through AMPK
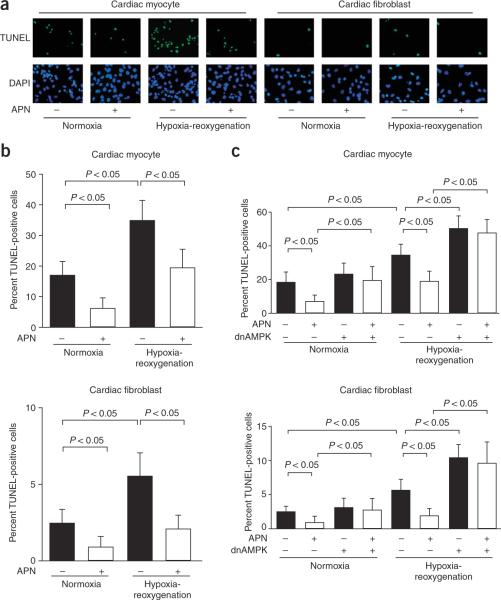
To analyze the antiapoptotic actions of adiponectin at a cellular level, primary cultures of neonatal rat ventricular myocytes or fibroblasts were deprived of serum under conditions of normoxia or hypoxia-reoxygenation in the presence or absence of recombinant adiponectin. We examined TUNEL-positive cells after 48 h of serum deprivation or after 12 h of hypoxia followed by 24 h of reoxygenation under conditions of serum deprivation (Fig. 3a). Pretreatment with adiponectin diminished the frequency of TUNEL-positive cells under normoxic conditions by 69% in cardiomyocytes and by 45% in cardiac fibroblasts (Fig. 3b). Hypoxia-reoxygenation increased the frequency of TUNEL-positive cardiomyocytes and cardiac fibroblasts. Pretreatment with adiponectin suppressed the frequency of TUNEL-positive cells under conditions of hypoxia-reoxygenation by 47% in cardiomyocytes and by 62% in cardiac fibroblasts. To test whether AMPK signaling was involved in the antiapoptotic actions of adiponectin, we pretreated cultured cardiac cells with an adenoviral vector expressing a dominant negative mutant of AMPK (Ad-dnAMPK). Transduction with Ad-dnAMPK effectively suppressed adiponectin-induced phosphorylation of acetyl-CoA carboxylase, a downstream target of AMPK, in both cardiac myocytes and fibroblasts (data not shown), and reversed the inhibitory effects of adiponectin on apoptosis under conditions of serum deprivation and hypoxia-reoxygenation in both cell types (Fig. 3c). In contrast, treatment with the COX-2 inhibitor NS398 had no effect on the protective actions of adiponectin on apoptosis of cardiac myocytes or fibroblasts (Supplementary Fig. 3 online).
Figure 3.
AMPK-dependent inhibition of cardiac myocyte and fibroblast apoptosis by adiponectin. Rat neonatal myocytes and fibroblasts were treated with adiponectin (30 μg/ml) or vehicle in serum-free media for 48 h under normoxic conditions or 12 h under hypoxic conditions followed by 24 h of reoxygenation. (a) Representative photomicrographs of TUNEL-positive cardiac myocytes and fibroblasts. Apoptotic nuclei were identified by TUNEL staining (green) and total nuclei by DAPI counterstaining (blue). (b) Quantitative analysis of TUNEL-positive cells under conditions of normoxia or hypoxia-reoxygenation with adiponectin (APN) or vehicle. TUNEL-positive nuclei were counted in several randomly selected fields and expressed as a percentage of the total number of nuclei. (c) Effect of Ad-dnAMPK on adiponectin inhibition of myocyte and fibroblast apoptosis under conditions of normoxia or hypoxia-reoxygenation. Cells were transduced with Ad-dnAMPK or Ad-βgal (control) for 24 h and then treated with adiponectin or vehicle under conditions of normoxia or hypoxia-reoxygenation. Results are presented as mean ± s.d. (n = 3).
Adiponectin inhibits TNF-α production through COX-2
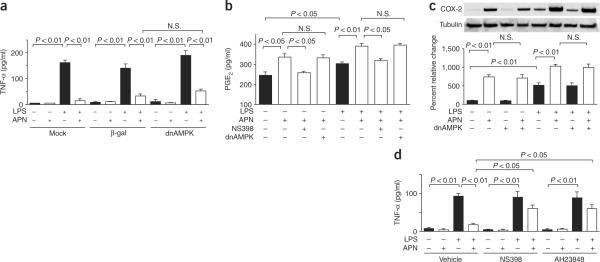
Cardiac cells produce TNF-α when stimulated with lipopolysaccharide (LPS)22. To test the effects of adiponectin on production of TNF-α, we subjected cultured neonatal cardiac myocytes and fibroblasts to stimulation with LPS for 6 h in the presence or absence of adiponectin. We determined the accumulation of TNF-α in the culture medium by ELISA. Exposure to LPS increased the secretion of TNF-α by 42-fold in cardiomyocytes and 15-fold in cardiac fibroblasts, and pretreatment with adiponectin markedly inhibited LPS-induced production of TNF-α in both cell types (Fig. 4a and Supplementary Fig. 4 online). In contrast to the effects on cell viability, transduction with Ad-dnAMPK had little or no effect on LPS-induced production of TNF-α in either cell type.
Figure 4.
Adiponectin suppresses LPS-induced secretion of TNF-α from neonatal myocytes through a COX-2–dependent pathway. (a) Effect of adiponectin (APN) on LPS-induced production of TNF-α. TNF-α levels in media were determined by ELISA. Mock-transduced cells were compared with cells that were transduced with Ad-dnAMPK or Ad-βgal for 24 h, and then treated with adiponectin or vehicle, and stimulated with or without LPS. (b) Adiponectin stimulates secretion of PGE2 from cardiac myocytes. Pretreatment with the COX-2 inhibitor NS398 blocked adiponectin-stimulated production of PGE2 in the presence or absence of LPS. Transduction with Ad-dnAMPK did not affect adiponectin-induced production of PGE2. Transduction with Ad-βgal had no effect on production of PGE2. (c) Effect of adiponectin on expression of COX-2 in myocytes. Transduction with dominant negative AMPK did not affect induction of COX-2 in cultured cardiac myocytes. (d) Contribution of the COX-2–PGE2 pathway to adiponectin inhibition of LPS-induced production of TNF-α from myocytes. Cells were pretreated with a EP4-selective antagonist, AH23848, NS398 or vehicle, treated with adiponectin or vehicle and stimulated with or without LPS. Results are presented as mean ± s.d. (n = 3–5). N.S., not statistically significant.
Prostaglandin E2 (PGE2) inhibits LPS-induced production of TNF-α in monocytic cells23,24. Therefore, we assessed whether adiponectin regulates the synthesis of PGE2 in cardiac cells. Adiponectin stimulated the production of PGE2 in both myocytes and fibroblasts (Fig. 4b and Supplementary Fig. 4 online). Adiponectin-stimulated production of PGE2 was inhibited by the selective COX-2 inhibitor NS398 but not by transduction with Ad-dnAMPK. LPS increased production of PGE2 in cardiac neonatal myocytes and fibroblasts, consistent with observations in other cell types25, and adiponectin further augmented the production of PGE2 under these conditions in a COX-2–dependent, AMPK-independent manner (Fig. 4b and Supplementary Fig. 4 online). In separate experiments, exogenous PGE2 at a concentration of 350 pg/ml inhibited LPS-induced TNF-α production by 91% ± 4% in myocyte cultures (data not shown), indicating that functionally relevant levels of PGE2 are produced in response to adiponectin stimulation in vitro. Adiponectin also increased the expression of COX-2, the rate-limiting step for PGE2 synthesis, in both basal and LPS-stimulated myocytes and fibroblasts (Fig. 4c and Supplementary Fig. 4 online). This induction was not suppressed by transduction with Ad-dnAMPK in either cell type. Finally, we also examined the effects of adiponectin on this regulatory system in cultures of rat cardiac myocytes prepared from adult left ventricle. Adiponectin inhibited LPS-induced production of TNF-α, enhanced production of PGE2 in the presence or absence of LPS and increased basal and LPS-stimulated expression of COX-2 (Supplementary Fig. 5 online).
The cardioprotective actions of PGE2 are mediated, at least in part, by the EP4 receptor that is highly expressed in heart26. Thus, to test whether EP4 participates in the inhibitory effect of adiponectin on secretion of TNF-α, we treated neonatal cardiac myocytes or fibroblasts with the EP4 receptor–selective antagonist AH23848 and then assessed LPS-induced production of TNF-α. AH23848 reversed the inhibitory actions of adiponectin on LPS-induced secretion of TNF-α from both myocytes and fibroblasts (Fig. 4d and Supplementary Fig. 4 online). The COX-2 inhibitor NS398 also blocked the suppressive effect of adiponectin on LPS-induced secretion of TNF-α from myocytes and fibroblasts. Collectively, these data suggest that adiponectin suppresses LPS-induced secretion of TNF-α through a COX-2–PGE2–EP4–dependent pathway that is independent of AMPK signaling.
COX-2 contributes to the protective actions of adiponectin
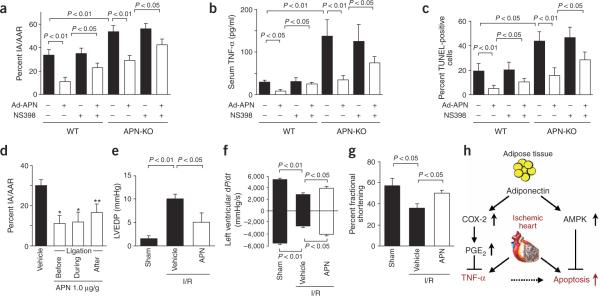
To examine the role of COX-2 signaling in the cardioprotective action of adiponectin in vivo, we administered daily intraperitoneal injections of the COX-2 inhibitor NS398 (5 mg/kg) from 3 d before surgery until mice were killed. Although NS398 did not affect infarct size in Ad-βgal–treated wild-type and APN-KO mice, it abrogated the infarct-sparing actions of exogenous adiponectin by 53% in wild-type and 48% in APN-KO mice (Fig. 5a). Consistent with the results from LPS-stimulated cardiac myocyte and fibroblast cultures (Fig. 4d and Supplementary Fig. 4 online), NS398 significantly reversed the suppressive effect of adiponectin on serum levels of TNF-α after infarction in both wild-type and APN-KO mice (Fig. 5b). Treatment with NS398 also abrogated the adiponectin-induced decrease in TUNEL-positive cells in wild-type and APN-KO mice (Fig. 5c). NS398 did not affect apoptosis or serum levels of TNF-α in Ad-βgal–treated wild-type or APN-KO mice. These data indicate that COX-2–dependent signaling contributes to the protective action of adiponectin in myocardial ischemia-reperfusion injury through the suppression of inflammatory cytokines and improved cell survival.
Figure 5.
Inhibition of COX-2 partially prevents the protective actions of adiponectin on myocardial infarct size after ischemia-reperfusion injury in wild-type (WT) and APN-KO mice. (a) The COX-2 inhibitor NS398 was injected intraperitoneally from 3 d before ischemia-reperfusion injury until mice were killed, and Ad-APN or Ad-βgal was injected into the jugular vein 3 d before surgery. Infarct size in the heart tissue was quantified (n = 5 mice per experimental group). IA/AAR, ratio of infarct area to AAR. (b) NS398 reversed the suppressive effect of adiponectin on serum levels of TNF-α, after infarction in both wild-type (WT) and APN-KO mice. (c) NS398 increased the frequencies of TUNEL-positive cells after ischemia-reperfusion in Ad-APN-treated wild-type and APN-KO mice. (d) Administration of recombinant adiponectin minimized the effects of ischemia-reperfusion on infarct size and heart function. Quantification of infarct size in wild-type mice treated with recombinant adiponectin before, during and after ischemic injury (n = 5 per group). *P < 0.01, **P < 0.05 versus vehicle. (e) Effect of recombinant adiponectin on left ventricular end-diastolic pressure (LVEDP) in wild-type mice at 24 h after sham operation or ischemia-reperfusion. Recombinant adiponectin (1.0 μg/g) or vehicle was injected in wild-type mice before ischemia-reperfusion (n = 5). (f) Left ventricular dP/dt in wild-type mice (n = 5) treated with adiponectin (1.0 μg/g) or vehicle at 24 h after sham operation or ischemia-reperfusion. (g) Left ventricular fractional shortening assessed by echocardiography in wild-type (n = 4) treated with recombinant adiponectin (1.0 μg/g) at 7 d after ischemia-reperfusion. (h) Adiponectin protects the myocardium from cardiac injury in response to ischemia by protecting cardiac cells from apoptosis through activation of AMPK signaling and by the suppression of cardiac production of TNF-α by the activation of the COX-2–PGE2 pathway.
Recombinant adiponectin protein minimizes infarct size
To test whether administration of adiponectin could minimize infarct area before or after ischemia-reperfusion, we administered recombinant adiponectin (1.0 μg/g) to wild-type mice either 30 min before the induction of ischemia, during ischemia or 15 min after reperfusion. The administration of adiponectin at any of these time points led to a reduction in infarct size relative to control mice (Fig. 5d). To examine the effect of adiponectin on hemodynamic properties, we measured left ventricular end-diastolic pressure (LVEDP) and the derivative of left ventricular pressure (dP/dt) using a micromanometer-tipped catheter at 24 h after ischemia-reperfusion surgery in wild-type mice that had received 1.0 μg/g adiponectin or vehicle 15 min before ischemia. Whereas control mice showed a marked elevation in LVEDP, the increase in LVEDP diminished in the adiponectin-treated animals (Fig. 5e). Furthermore, pretreatment with adiponectin increased dP/dtmax and decreased dP/dtmin at 24 h after ischemia-reperfusion (Fig. 5f). Finally, to test whether adiponectin affects echocardiographic parameters, we measured left ventricular fractional shortening by echocardiography on day 7 after operation in wild-type mice (Fig. 5g). Treatment with adiponectin before ischemia-reperfusion led to a statistically significant increase in fractional shortening, indicative of improved myocardial remodeling.
Discussion
Our data provide evidence that adiponectin confers resistance to acute myocardial damage. Adiponectin-deficient mice showed increased infarct size after ischemia-reperfusion, whereas exogenous adiponectin reduced infarct size in both adiponectin-deficient and wild-type mice. It has been shown in men that plasma adiponectin is a marker of risk for myocardial infarction17. The current study suggests that this reduction in adiponectin level is a causal factor that contributes to the severity of infarction. Adiponectin protects the heart from injury in response to ischemia-reperfusion through at least two mechanisms: improvements in viability of myocardial cells and suppression of cardiac production of TNF-α (Fig. 5h). The antiapoptotic action of adiponectin is likely to be mediated by the direct activation of AMPK signaling within cardiac myocytes. Consistent with this hypothesis, ex vivo experiments have shown that the expression of dominant negative AMPK in the heart from a cardiac myocyte–specific promoter leads to increased apoptosis and cardiac dysfunction after ischemia-reperfusion injury19. Furthermore, here we show that the level of activated AMPK was reduced in the hearts of APN-KO mice. Transduction with dominant negative AMPK abrogated the antiapoptotic activities of adiponectin in cardiac myocytes in response to serum deprivation and hypoxia-reoxygenation in vitro. Fibroblasts in the heart also participate in remodeling after ischemia27, and here we show that adiponectin also protected cardiac fibroblasts from apoptosis through an AMPK-dependent mechanism. Adiponectin stimulation inhibits apoptosis of endothelial cells through an AMPK-dependent mechanism20,28, and this feature could also contribute to the protective action of adiponectin. But we detected no significant difference in capillary density between wild-type and APNKO mice 2 d after ischemia-reperfusion injury, suggesting that the effect of adiponectin on infarct size is not mediated by the angiogenic properties of this cytokine at this early time point. Finally, administration of adiponectin reduced CPK release into the circulation, indicative of diminished myocardial necrosis. This effect may be mediated by the ability of adiponectin to increase AMPK-induced glucose transport29.
The mechanisms by which adiponectin suppress inflammatory reactions are poorly understood. Here we show that adiponectin functions to suppress myocardial production of TNF-α in vitro and in vivo. The increased production of proinflammatory cytokines is an important component of postischemic myocardial injury30,31. Studies have shown that TNF-α–deficient mice have decreased myocardial damage in response to ischemia-reperfusion injury and that treatment with TNF-α–specific antibody limits the damage caused by acute myocardial injury in rat hearts ex vivo32,33. Clinically, the reduction of plasma adiponectin levels after acute myocardial infarction is negatively correlated with plasma CRP levels18, suggesting that hypoadiponectinemia is associated with an increased inflammatory response to acute myocardial ischemia. We found that adiponectin deficiency resulted in markedly higher TNF-α levels in the serum and heart tissue after ischemia-reperfusion injury, whereas elevated adiponectin expression reduced serum and myocardial TNF-α levels in both APN-KO and wild-type mice. Adiponectin also suppressed LPS-induced production of TNF-α in cultured cardiac myocytes and fibroblasts. This anti-inflammatory action was independent of AMPK signaling.
Our results indicate that the inhibitory action of adiponectin on myocardial production of TNF-α results from an activation of the COX-2–PGE2–EP4 pathway. COX-2 has important protective roles in the regulation of myocardial damage after ischemia-reperfusion injury34,35, and it could function to limit oxidative damage in the heart36. Furthermore, the COX-2 metabolite PGE2 protects hearts from ischemia-reperfusion injury, an effect that is mediated by the EP3 and EP4 receptor subtypes26,37. We found that adiponectin increased expression of COX-2 and release of PGE2 from myocytes and fibroblasts in an AMPK-independent manner. COX-2 also produces PGI2, which is reported to have cardioprotective effects against ischemia-reperfusion injury38.
The protective action of adiponectin overexpression on myocardial infarct size was inhibited when we administered a COX-2 inhibitor to either wild-type or APN-KO mice. Abrogation of adiponectin-protective actions by inhibition of COX-2 was associated with increases in apoptosis and production of TNF-α. In contrast, in vitro studies showed that the antiapoptotic actions of adiponectin were not reversed by inhibition of COX-2. These data suggest a complex interplay between COX-2–dependent production of TNF-α and cell-death pathways in the heart that are not reflected by experiments with cultured cardiac cells (Fig. 5h). In this regard, production of TNF-α after ischemia-reperfusion injury has been shown to have a major role in apoptosis and myocardial damage39,40. Presumably, the proapoptotic actions of COX-2 inhibition are only observed in vivo because cardiac levels of TNF-α are higher than those produced in the cell-culture experiments or because a large portion of the apoptosis observed in vivo is the consequence of inflammatory cell infiltration in response to this cytokine.
Notably, inhibition of COX-2 had no detectable effect on infarct size in untreated mice. These data are consistent with reports showing that although COX-2 is required for late preconditioning, treatment with COX-2 inhibitors does not result in a statistically significant increase in infarct size34,41,42. The inability of COX-2 inhibitors to influence infarct size in untreated mice could result from the decline in adiponectin levels that occurs in response to acute myocardial injury. In our studies, adiponectin levels declined 32% after ischemia-reperfusion injury (data not shown), which is comparable to the 29% decrease found in men 72 h after a myocardial infarction18. Recent clinical trials indicate that treatment with selective COX-2 inhibitors results in an increased risk for myocardial infarction and other serious cardiovascular events43,44. The adverse effects of COX-2 inhibitors on cardiovascular events are thought to result from a perturbation of vascular homeostasis due to reduced synthesis of prostacyclin45. Our data suggest that inhibition of COX-2 could also contribute to the severity of myocardial infarction by interfering with the protective actions of adiponectin on cardiac myocytes.
Previous studies have shown that adiponectin might have potential utility for the treatment of a variety of chronic diseases including obesity46, insulin resistance47,48 and hypertrophic cardiomyopathy16. But adiponectin is an abundant serum protein and its long-term administration could be problematic. Here, it is shown that adiponectin can limit the damage from an acute myocardial infarction, suggesting that the short-term administration of this factor may have practical clinical utility.
METHODS
Materials
We purchased phosphorylated AMPK (Thr172) and pan-α-AMPK from Cell Signaling Technology. We purchased COX-2–specific antibody and NS398 from Cayman Chemical Co; tubulin-specific antibody from Oncogene; phosphorylated ACC (Ser79), ACC and c-Myc–specific tag antibody from Upstate Biotechnology. We purchased LPS of E. coli 0127 and AH23848 from Sigma Chemical Co. We prepared recombinant mouse adiponectin in E. coli as described previously20. Adenovirus vectors containing the gene for β-galactosidase (Ad-βgal), full-length mouse adiponectin (Ad-APN) and dominant negative AMPKα2 (Ad-dnAMPK) were described previously14,49.
Mouse model of myocardial ischemia-reperfusion
Studies using APN-KO and wild-type mice in a C57BL/6 background were approved by the Institutional Animal Care and Use Committee at Boston University14. We anesthetized 10–12-week-old mice with sodium pentobarbital. We cannulated the trachea with a polyethylene tube connected to a respirator with a tidal volume of 0.6 ml (110 breaths/min). We performed left thoracotomy between the fourth and fifth ribs. We removed the pericardial tissue and visualized the LAD artery under a microscope and ligated it with 8-0 silk suture using a snare occluder. We subjected mice to 30 min of LAD ligation followed by 48 h of reperfusion. In some experiments, we injected 2 × 108 plaque-forming units of Ad-APN or Ad-βgal into the jugular vein of mice 3 d before the ischemia-reperfusion injury. We determined mouse adiponectin levels by ELISA kit (Otsuka Pharmaceutical Co. Ltd.)16. In some experiments, we intraperitoneally injected the COX-2 inhibitor NS398 (5 mg/kg/d) or vehicle (dimethylsulfoxide) into the abdomen of the APN-KO and wild-type mice from 3 d before ischemia-reperfusion injury and until the mice were killed. In other experiments, we injected recombinant adiponectin or PBS vehicle into the jugular vein of mice at 30 min before LAD ligation, after 15 min of LAD ligation or 15 min after reperfusion. We performed hemodynamic measurements after 24 h of reperfusion using a 1.4F catheter tip micromanometer (ARIA, Millar Instruments) inserted through the right carotid artery into the left ventricular cavity. We analyzed the first derivative of left ventricular pressure (dP/dt) using Power Lab SP Software (Ad Instruments). We performed echocardiography with an Acuson Sequoia C-256 machine using a 15-MHz probe. We calculated fractional shortening as (LVEDD − LVESD)/LVEDD × 100 and expressed the result as a percentage. LVEDD is left ventricular end diastolic dimension and LVESD is left ventricular end systolic dimension.
Determination of area at risk and infarct size
We reoccluded the LAD artery, and injected 1 ml of 1.0% Evans blue (Sigma Chemical Co.) through the jugular vein to delineate the nonischemic tissue. We then excised the heart, washed it with PBS and cut it into four transverse slices. We stained slices for 5 min at 23 °C with 1.0 ml of 1.5% 2,3,5-triphenyltetrazolium chloride (Sigma Chemical Co.) to determine infarct area. We weighed sections and photographed them under a microscope. We determined left ventricular area, AAR and infarct area by computerized planimetry using Image J software. We expressed infarct area as a percentage of the AAR and left ventricular area.
Analysis of myocardial injury and apoptosis
We assessed an index of myocyte injury by determining the release of CPK (Catachem Inc.). We collected blood from tail veins at 6 h after operation. We qualitatively analyzed myocardial apoptosis by TUNEL staining as previously described50. We examined five randomly chosen microscopic fields from four different sections in each tissue block for each mouse specimen.
Measurement of TNF-α
We quantified plasma TNF-α with the use of ELISA kits (R&D Systems). We quantified Tnf mRNA in myocardium by real-time PCR. We prepared total RNA using a Qiagen kit. We produced cDNA using ThermoScript RT-PCR Systems (Invitrogen). We performed PCR on iCycler iQ Real-Time PCR Detection System (BIO-RAD) using SYBR Green 1 as a double-standard DNA-specific dye (Applied Biosystems)9. We used the following primers: 5′-CATCTTCTCAAAATTCGAGTGACAA-3′, and 5′-TGGGAGTAGACAAGGTACAACCC-3′ for Tnf and 5′-TCACCACCATGGAGAAGGC-3′ and 5′-GCTAAGCAGTTGGTGGTGCA-3′ for Gapdh.
Cell culture and adiponectin treatment
We prepared primary cultures of neonatal and adult rat ventricular myocytes as described previously. We cultured neonatal myocytes in DMEM containing 7% FCS16. We cultured adult myocytes in ACTT medium. We cultured neonatal rat ventricular nonmyocytes, which are predominantly fibroblasts, in DMEM containing 1% FCS and passaged them with trypsin-EDTA. We examined cell number and TUNEL-positive cells after serum deprivation for 48 h under normoxic conditions or 12 h of hypoxia (<1% O2 and 5% CO2, 37 °C) followed by 24 h of reoxygenation (21% O2 and 5% CO2, 37 °C) in the presence or absence of recombinant adiponectin (30 μg/ml). We generated hypoxia by using a GasPak Plus system (Becton Dickinson). We pretreated myocyte cultures for 18 h in the presence or absence of bacterially produced adiponectin20 (30 μg/ml) and subjected them to LPS stimulation (1 μg/ml) for 6 h. In other experiments, we preincubated the cells with AH23848 (100 μM), NS398 (2 μM) or vehicle for 30 min before adiponectin treatment. We infected cells with Ad-βgal and Ad-dnAMPK at a multiplicity of infection of 50 for 24 h before treatments. We measured levels of TNF-α, IL-1β and IL-6 using ELISA (R&D Systems). We also determined PGE2 concentrations by ELISA (Cayman Chemical Co.).
Western blot analysis
We homogenized tissue samples obtained at 48 h after surgery and separated proteins (30 μg) with denaturing SDS 10% polyacryl-amide gels. After transfer to membranes, we performed immunoblot analysis with the indicated antibodies at a 1:1,000 dilution. This was followed by incubation with secondary antibody conjugated with horseradish peroxidase at a 1:5,000 dilution. We used the ECL-PLUS Western Blotting Detection kit (Amersham Pharmacia Biotech) for detection. We normalized the relative changes to the tubulin signal and expressed them as percent relative to control.
Statistical analysis
Data are presented as mean ± s.d. We performed statistical analysis using Scheffe F test or analysis of variance to determine group differences. A value of P < 0.05 was accepted as statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by US National Institutes of Health (NIH) grants HL66957, HL77774, AR40197 and AG15052 (to K.W.); NIH Cardiovascular Scientist Training Grant HL07224 (to D.R.P.); and Grant-in-Aid for Scientific Research on Priority Areas (to S.K. and T.F.). R.S. was supported by grants from the American Heart Association Postdoctoral Fellowship Award, Northeast Affiliate and the Uehara Memorial Foundation. N.O. was supported by a Department of Medicine Pilot Project Grant from Boston University. We gratefully acknowledge the technical assistance of S. Tanaka and A. Bialik.
Footnotes
Note: Supplementary information is available on the Nature Medicine website.
COMPETING INTERESTS STATEMENT The authors declare that they have no competing financial interests.
References
- 1.Eisenberg MS, Mengert TJ. Cardiac resuscitation. N. Engl. J. Med. 2001;344:1304–1313. doi: 10.1056/NEJM200104263441707. [DOI] [PubMed] [Google Scholar]
- 2.Rogers WJ, et al. Temporal trends in the treatment of over 1.5 million patients with myocardial infarction in the US from 1990 through 1999: the National Registry of Myocardial Infarction 1, 2 and 3. J. Am. Coll. Cardiol. 2000;36:2056–2063. doi: 10.1016/s0735-1097(00)00996-7. [DOI] [PubMed] [Google Scholar]
- 3.Wolk R, Berger P, Lennon RJ, Brilakis ES, Somers VK. Body mass index: a risk factor for unstable angina and myocardial infarction in patients with angiographically confirmed coronary artery disease. Circulation. 2003;108:2206–2211. doi: 10.1161/01.CIR.0000095270.85646.E8. [DOI] [PubMed] [Google Scholar]
- 4.Orlander PR, et al. The relation of diabetes to the severity of acute myocardial infarction and post-myocardial infarction survival in Mexican-Americans and non-Hispanic whites. The Corpus Christi Heart Project. Diabetes. 1994;43:897–902. doi: 10.2337/diab.43.7.897. [DOI] [PubMed] [Google Scholar]
- 5.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 6.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J. Biol. Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 7.Maeda K, et al. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1) Biochem. Biophys. Res. Commun. 1996;221:286–289. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- 8.Arita Y, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 9.Ouchi N, et al. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation. 2003;107:671–674. doi: 10.1161/01.cir.0000055188.83694.b3. [DOI] [PubMed] [Google Scholar]
- 10.Ouchi N, Kihara S, Funahashi T, Matsuzawa Y, Walsh K. Obesity, adiponectin and vascular inflammatory disease. Curr. Opin. Lipidol. 2003;14:561–566. doi: 10.1097/00041433-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Hotta K, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler. Thromb. Vasc. Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 12.Iwashima Y, et al. Hypoadiponectinemia is an independent risk factor for hypertension. Hypertension. 2004;43:1318–1323. doi: 10.1161/01.HYP.0000129281.03801.4b. [DOI] [PubMed] [Google Scholar]
- 13.Kumada M, et al. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler. Thromb. Vasc. Biol. 2003;23:85–89. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 14.Maeda N, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat. Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 15.Shibata R, et al. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of amp-activated protein kinase signaling. J. Biol. Chem. 2004;279:28670–28674. doi: 10.1074/jbc.M402558200. [DOI] [PubMed] [Google Scholar]
- 16.Shibata R, et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat. Med. 2004;10:1384–1389. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pischon T, et al. Plasma adiponectin levels and risk of myocardial infarction in men. J. Am. Med. Assoc. 2004;291:1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 18.Kojima S, et al. The variation of plasma concentrations of a novel, adipocyte derived protein, adiponectin, in patients with acute myocardial infarction. Heart. 2003;89:667. doi: 10.1136/heart.89.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russell RR, III, et al. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J. Clin. Invest. 2004;114:495–503. doi: 10.1172/JCI19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ouchi N, et al. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J. Biol. Chem. 2004;279:1304–1309. doi: 10.1074/jbc.M310389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc. Res. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 22.Wagner DR, et al. Adenosine inhibits lipopolysaccharide-induced cardiac expression of tumor necrosis factor-alpha. Circ. Res. 1998;82:47–56. doi: 10.1161/01.res.82.1.47. [DOI] [PubMed] [Google Scholar]
- 23.Meja KK, Barnes PJ, Giembycz MA. Characterization of the prostanoid receptor(s) on human blood monocytes at which prostaglandin E2 inhibits lipopolysaccharide-induced tumour necrosis factor-alpha generation. Br. J. Pharmacol. 1997;122:149–157. doi: 10.1038/sj.bjp.0701360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pruimboom WM, et al. Interactions between cytokines and eicosanoids: a study using human peritoneal macrophages. Immunol. Lett. 1994;41:255–260. doi: 10.1016/0165-2478(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 25.Eliopoulos AG, Dumitru CD, Wang CC, Cho J, Tsichlis PN. Induction of COX-2 by LPS in macrophages is regulated by Tpl2-dependent CREB activation signals. EMBO J. 2002;21:4831–4840. doi: 10.1093/emboj/cdf478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao CY, et al. Prostaglandin E2 protects the heart from ischemia-reperfusion injury via its receptor subtype EP4. Circulation. 2004;109:2462–2468. doi: 10.1161/01.CIR.0000128046.54681.97. [DOI] [PubMed] [Google Scholar]
- 27.Parsa CJ, et al. Cardioprotective effects of erythropoietin in the reperfused ischemic heart: a potential role for cardiac fibroblasts. J. Biol. Chem. 2004;279:20655–20662. doi: 10.1074/jbc.M314099200. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi H, et al. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ. Res. 2004;94:e27–e31. doi: 10.1161/01.RES.0000119921.86460.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russell RR, III, Bergeron R, Shulman GI, Young LH. Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. Am.J.Physiol. 1999;277:H643–H649. doi: 10.1152/ajpheart.1999.277.2.H643. [DOI] [PubMed] [Google Scholar]
- 30.Frangogiannis NG, et al. Resident cardiac mast cells degranulate and release preformed TNF-alpha, initiating the cytokine cascade in experimental canine myocardial ischemia/reperfusion. Circulation. 1998;98:699–710. doi: 10.1161/01.cir.98.7.699. [DOI] [PubMed] [Google Scholar]
- 31.Kupatt C, et al. Tumor necrosis factor-alpha contributes to ischemia- and reperfusion-induced endothelial activation in isolated hearts. Circ. Res. 1999;84:392–400. doi: 10.1161/01.res.84.4.392. [DOI] [PubMed] [Google Scholar]
- 32.Maekawa N, et al. Improved myocardial ischemia/reperfusion injury in mice lacking tumor necrosis factor-alpha. J. Am. Coll. Cardiol. 2002;39:1229–1235. doi: 10.1016/s0735-1097(02)01738-2. [DOI] [PubMed] [Google Scholar]
- 33.Gurevitch J, et al. Anti-tumor necrosis factor-alpha improves myocardial recovery after ischemia and reperfusion. J. Am. Coll. Cardiol. 1997;30:1554–1561. doi: 10.1016/s0735-1097(97)00328-8. [DOI] [PubMed] [Google Scholar]
- 34.Bolli R, et al. Discovery of a new function of cyclooxygenase (COX)-2: COX-2 is a cardioprotective protein that alleviates ischemia/reperfusion injury and mediates the late phase of preconditioning. Cardiovasc. Res. 2002;55:506–519. doi: 10.1016/s0008-6363(02)00414-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Camitta MG, et al. Cyclooxygenase-1 and -2 knockout mice demonstrate increased cardiac ischemia/reperfusion injury but are protected by acute preconditioning. Circulation. 2001;104:2453–2458. doi: 10.1161/hc4401.098429. [DOI] [PubMed] [Google Scholar]
- 36.Adderley SR, Fitzgerald DJ. Oxidative damage of cardiomyocytes is limited by extracellular regulated kinases 1/2-mediated induction of cyclooxygenase-2. J. Biol. Chem. 1999;274:5038–5046. doi: 10.1074/jbc.274.8.5038. [DOI] [PubMed] [Google Scholar]
- 37.Hohlfeld T, Meyer-Kirchrath J, Vogel YC, Schror K. Reduction of infarct size by selective stimulation of prostaglandin EP(3)receptors in the reperfused ischemic pig heart. J. Mol. Cell. Cardiol. 2000;32:285–296. doi: 10.1006/jmcc.1999.1072. [DOI] [PubMed] [Google Scholar]
- 38.Xiao CY, et al. Roles of prostaglandin I(2) and thromboxane A(2) in cardiac ischemia-reperfusion injury: a study using mice lacking their respective receptors. Circulation. 2001;104:2210–2215. doi: 10.1161/hc4301.098058. [DOI] [PubMed] [Google Scholar]
- 39.Bryant D, et al. Cardiac failure in transgenic mice with myocardial expression of tumor necrosis factor-alpha. Circulation. 1998;97:1375–1381. doi: 10.1161/01.cir.97.14.1375. [DOI] [PubMed] [Google Scholar]
- 40.Sugano M, et al. Local delivery of soluble TNF-alpha receptor 1 gene reduces infarct size following ischemia/reperfusion injury in rats. Mol. Cell. Biochem. 2004;266:127–132. doi: 10.1023/b:mcbi.0000049149.03964.c9. [DOI] [PubMed] [Google Scholar]
- 41.Li Q, et al. Gene therapy with inducible nitric oxide synthase protects against myocardial infarction via a cyclooxygenase-2-dependent mechanism. Circ. Res. 2003;92:741–748. doi: 10.1161/01.RES.0000065441.72685.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arnaud C, Joyeux-Faure M, Godin-Ribuot D, Ribuot C. COX-2: an in vivo evidence of its participation in heat stress-induced myocardial preconditioning. Cardiovasc. Res. 2003;58:582–588. doi: 10.1016/s0008-6363(03)00295-5. [DOI] [PubMed] [Google Scholar]
- 43.Solomon SD, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N. Engl. J. Med. 2005;352:1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 44.Bresalier RS, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N. Engl. J. Med. 2005;352:1092–1102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- 45.Fitzgerald GA. Coxibs and cardiovascular disease. N. Engl. J. Med. 2004;351:1709–1711. doi: 10.1056/NEJMp048288. [DOI] [PubMed] [Google Scholar]
- 46.Fruebis J, et al. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc. Natl. Acad. Sci. USA. 2001;98:2005–2010. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat. Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 48.Yamauchi T, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 49.Nagata D, Mogi M, Walsh K. AMP-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J. Biol. Chem. 2003;278:31000–31006. doi: 10.1074/jbc.M300643200. [DOI] [PubMed] [Google Scholar]
- 50.Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation. 2000;101:660–667. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.