Abstract
Objective
To explore general practitioners' perceptions of their role in implementing genetic technology.
Design
Grounded theory interview study.
Setting
Primary care.
Subjects
Purposive sample of 30 general practitioners with a further theoretical sample of 14.
Results
Inconsistencies were identified between policy makers' and general practitioners' definitions of general practitioners' role in implementing the new genetics. General practitioners emphasised the need to build on current practice, whereas policy makers focused on transforming practice to include the new specialised roles and skills. Two core themes were identified: genetics in a generalist context, which included appropriate generalist intervention, the ethical dilemmas implicit in the “therapeutic gap,” the familial-hereditary distinction in primary care, and the implications for generalist identity, including the potential marginalisation of generalism.
Conclusion
New technologies such as genetics that require implementation in general practice should be integrated within existing generalist frameworks.
Key messages
Tensions exist between the role of general practitioners in implementing genetic advances identified by policy makers and that identified by general practitioners themselves
General practitioners' ability to integrate patient experiences with genetic and other biomedical knowledge is a key generalist skill
New genetic technology should be integrated into existing generalist frameworks
General practitioners identified ethical dilemmas associated with the therapeutic gap
Introduction
Policy makers, clinical geneticists, and more recently the Royal College of General Practitioners advise that some level of genetic services should be offered within primary care.1–8 Specifically, they recommend that most patients' inquiries about genetic susceptibility to common diseases—for example, breast and colon cancer—should be managed by general practitioners. This reflects concern about meeting a predicted rise in public demand for genetic counselling. General practitioners will necessarily have a role since the ratio of consultant geneticists to general practitioners in the United Kingdom is roughly 500:1.8 However, this role has been defined by policy makers and experts with little research about practitioners' perspectives. This study investigates general practitioners' perceptions of the effect that genetic advances will have on their practice.
Participants and methods
We used grounded theory9–11 to guide sampling, data collection, and data analysis. Thirty general practitioners who had attended a Genetics for GPs course were invited to participate. Five declined because of time constraints and one because of illness; the 24 who agreed formed a purposive sample of informed general practitioners (table 1).12 The course lasted five half days, was delivered by geneticists, and covered basic genetics, recent advances in cancer genetics (including genes for breast and ovarian cancer), genetic screening (including the use of family history as a screening tool), and clinical genetics in practice. SK recruited the general practitioners by telephone over two weeks after the end of the course.
Table 1.
Key characteristics of purposive sample of general practitioners (n=24)
| No of general practitioners | |
|---|---|
| Male | 16 |
| Female | 8 |
| Full time principals | 20 |
| Part time principals | 4 |
| Singlehanded | 1 |
| Possession of MRCGP | 20 |
| Qualified overseas | 1 |
In keeping with grounded theory, a further theoretical sample (selection guided by the emerging analysis)11,12 of 14 general practitioners was interviewed (table 2). The specific aims were to extend and challenge existing data and to test the integrity and credibility of the developing analysis.
Table 2.
People included in theoretical sample and what was discussed with them
| Theoretical sample (n=14) | Specific issues explored* |
|---|---|
| GP principal and professor of general practice | Interpretation of hereditary and familial disease; role of GPs; generalism/specialism |
| Lead GP who advises on health policy | Role of GPs; generalism |
| GP principal with a specific interest in genetics | Role of GPs; hereditary and familial disease; generalism |
| Member of GP research network | Role of GPs; hereditary and familial disease |
| 1 GP teacher and 1 tutor | Ethical information needs; holism; generalism |
| 6 GP principals from local practices (with no specific interest in genetics) | Impact of genetics on current practice; role of GPs; hereditary and familial disease; generalism/specialism |
| Consultant in cancer genetics | Heriditary and familial disease; generalism |
| Professor of genetics | Heriditary and familial disease; generalism |
Only those issues relevant to this paper are listed.
The final analysis was presented to a group of 20 general practitioners who had no specific interest in genetics. They comprised doctors from academic departments, service practices, and general practice teachers and trainers. They judged our analysis to be consistent with their perceptions and experiences of genetics, which indicated a degree of external validity for our findings.13,14
Data collection
SK conducted face to face semistructured interviews at participants' surgeries.15,16 To refine questions and interview technique an interview guide (developed from observations during the course by MG) was piloted on six local general practitioners; these data were not included in the final analysis. Questions addressed the following broad areas: opinions and beliefs about general practitioners' role in providing genetic counselling and risk assessment; knowledge of advances in genetics; current skills they could immediately use; current clinical situations in which they advised on genetic risk; attitudes to extending their services to provide genetic counselling for common conditions; and biographical and demographic data. Responses moved from predictable statements of professional and practice policy to more critical reflection as general practitioners were encouraged to focus on a specific personal or professional experience. In order to seek respondent validation SK regularly summarised and fed back his interpretation to general practitioners during the interview.13 All interviews were audiotaped and transcribed verbatim. Respondents were offered a further opportunity to review the transcriptions as well as the final analysis. However, all declined because of time constraints.
Data analysis
Constant comparison analysis was used to interpret the data.9 To maximise theoretical sensitivity10 we both coordinated the development of the conceptual framework. The first step, open coding, was achieved by deconstructing each interview sentence by sentence to identify key categories and concepts. These were compared across scripts and with established concepts in the literature. Data collection and analyses were iterative, with new data used to assess the integrity of the conceptual framework. The concepts identified were reintegrated into themes which provide the structure for the results.
Results
The analysis is presented as two core themes: genetics in the generalist context and implications for the generalist identity. Each theme comprises a series of concepts, supported by extracts from interviews drawn from the larger data set.
Genetics in the generalist context
Rarity
All respondents highlighted the paucity of training in genetics:
I qualified in 1959, my knowledge about genetics is rusty, we hardly got any training in medical school about genetics, and I don't think I ever saw a geneticist until I went on this course.
I qualified in 1985 and we got a few lectures on genetics but they didn't go much beyond Mendel.
This lack of training was not, however, considered problematic because of the perceived rarity of genetic conditions in general practices:
These diseases are all, even cystic fibrosis, very rare, because genetic diseases are actually few and far between from our perspective.
In this context genetic advances were seen as having little relevance for practice:
Genetic conditions are not our bread and butter; the new genetics has little impact on my day to day clinical work.
Ethical dilemmas associated with therapeutic gap
Surprisingly, given the prominence of genetics, all respondents thought that genetic advances would have little effect on their management of common diseases. However, they arrived at this position for different reasons. Participants who were aware of the ongoing genetic redefinition of common diseases (such as BRCA1 and BRCA2 conferring susceptibility to breast and ovarian cancer) were reluctant to alert patients to genetic risk in the absence of effective screening technologies and therapies to reduce risk or prevent disease—in other words they identified the therapeutic gap as an ethical dilemma:
The problem with these diseases [breast and ovarian cancer] is what can you do about it if you've got the gene? Is there any point in giving young patients a death sentence? You must be able to alter the natural history of the disease and if at the moment we can't do that effectively then I can't see myself raising the issue.
By raising the issue of genetic risk, general practitioners thought they would simply create another at risk group likely to seek additional advice from practice nurses and practitioners:
I don't raise the issue of genetic risk for common diseases unless I think there is something positive that can be done, which there isn't, and anyhow even if I did it would only make them and their family anxious and so possibly more work for us.
They recognised too the potential tensions patients and professionals face in managing behavioural and lifestyle changes in the context of genetic determinism:
It will be very difficult for patients to balance the finality of carrying genes for heart disease or cancer and then receive advice to give up smoking, take exercise, and increase fruit and vegetable intake. How do genes and lifestyle interact? Will adopting a healthy lifestyle change their genes, their genetic risk? These are the things I need to know.
Appropriate generalist intervention
It was in the context of established genetic diseases that general practitioners saw a clear role for themselves, using family histories collected in specific circumstances (preconceptual or antenatal advice or child health surveillance clinics). Thus doctors who did not identify a genetic component to common cancers envisaged the genetic advances affecting established genetic diseases such as cystic fibrosis:
Most of these advances will be for improving our treatment of diseases like cystic fibrosis or screening for Down's syndrome.
Common diseases were described as multifactorial in cause, but general practitioners spoke only in terms of lifestyle, nutrition, and environmental toxins—not genes:
For cancers I usually try to make people aware of what lifestyle changes they can make. I don't so much talk figures but say cut down on smoking, eat more fruit and vegetables, eat less saturated fat and take exercise—things like that, healthy advice.
The existence of multiple cases in a family was interpreted as exposure to common carcinogens rather than shared genes. These general practitioners collected family history to gain insight into the possible psychological and social impact the disease had on family members:
For a woman with a strong family history of breast cancer I would ask how old her mother was when she got the breast cancer, I would talk to them about the fact it was common and I would want to know this in order to understand if they knew what the cancer could do to them, it was not to find out if they were at risk of cancer.
Familial and hereditary distinction
These findings led us to explore how general practitioners differentiated between the terms familial and hereditary disease in the context of common diseases. Familial was consistently used to describe conditions shared by family members who were not genetically related such as husband and wife:
I would take ‘familial’ to mean conditions that crop up in the family in relatives who aren't related—for example, I see depression, alcoholism, and obesity affecting husbands and wives.
Hereditary, on the other hand was consistently understood to imply shared genes. This distinction was not made by the consultant cancer geneticist or the professor of genetics (in the theoretical sample), who used the terms synonymously to signify shared genes. However, informed general practitioners used hereditary breast cancer to distinguish women where genes exert a powerful effect; familial breast cancer was taken to mean exposure to shared environmental carcinogens. In making this distinction, general practitioners are beginning to recognise that common diseases may be genetic or have a genetic component. This is crucial if they are to use family history to assess genetic risk. As the director of the Genetics Interest Group has pointed out: “Patients do not go to the doctor saying,‘I think I have a genetic disorder.’ It's a matter of getting a light to come on in the GP's head saying, ‘I wonder if this is genetic?’”1
Implications of genetics for their generalist identity
Managing change
All respondents perceived the new genetics as another in a series of changes imposed on general practice. This view was reinforced by policy makers and experts characterising the new genetics as a series of additional tasks requiring new knowledge and skills, rather than an extension of current practice.1–6
Genetics is an expanding field, it's not that long ago when it was just a lab subject; it's only now that it might be useful to us. As far as general practice is concerned, it's going to mean even more change for us.
The traditional role of GPs is doing what GPs do and that is defined in our service agreement. GPs haven't traditionally looked after people with chronic renal failure and they haven't traditionally done warfarin clinics and certainly haven't done genetic counselling.
Marginalisation of generalism?
Respondents from both samples highlighted how the debate surrounding genetics related to the broader discussion of the core values of general practice, which is fuelled by continuing contractual change.17–19 These general practitioners resisted taking on genetic risk calculation for common diseases because it challenged their perception of themselves as autonomous providers of personal, holistic, and generalist medical care:
Because genetics has long term implications for the family, primary care is inevitably called in. For example, my role in genetic counselling would be supportive, dealing with the implications for the family and the children. I don't want to calculate genetic risk.
Additionally, concern was expressed that increasing specialisation would threaten their traditional and core skills:
GPs are sick to death of being asked to do traditional secondary care as primary care. Where do we get the time to see our normal patients and do what GPs traditionally do?
My strength lies in knowing ... in which direction to send the patients. I am not an expert in genetic counselling or calculating risks but I can help explain things to patients once they've seen the geneticists, and I can help them through the social and psychological effects.
This reinforces arguments that general practitioners are being asked to take on more and more, with the consequent marginalisation of the real substance of their work, specifically patient advocacy and centredness.17–20 Only one general practitioner (interviewed as part of the theoretical sample because of previous experience of community screening for cystic fibrosis) welcomed the new genetics as an opportunity to extend specialist skills and link with clinical geneticists but recognised this would entail a reduced commitment to routine clinical work.
Discussion
We selected methods that were designed to maximise both internal and external validity. Decisions on sampling, data collection, and data analysis were made within the framework of grounded theory. We selected informants who were “information rich”11,12 in the area of interest and so maximised the potential for identifying pertinent issues. We collected data by qualitative interviews because of their flexibility and their ability to access respondents' definitions and interpretations and to penetrate public accounts.21,22 Responses that were consistent with and contradictory to those of the initial sample were deliberately sought and explored during the theoretical sampling phase and by presenting the analysis to a broader range of general practitioners.
Policy implications
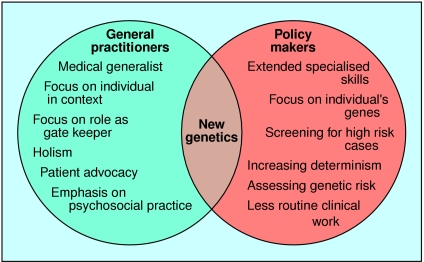
Our data suggest that many general practitioners do not believe that genetic testing for susceptibility to common disorders is likely to become a routine part of their practice in the near future. We have sought the opinions of a range of general practitioners, and our results highlight tensions between how policy makers and general practitioners view the role of general practitioners in genetics (figure).
Genetic technology has often been presented in terms of “revolution”1 and “radical change,”6 with implementation requiring generalists to perform more specialised tasks. In adopting this approach, policy makers and experts have either overlooked or not recognised the value of general practitioners' existing generalist skills and knowledge in implementing genetic advances. General practitioners draw on a wide range of theoretical disciplines23 to explore the effect of factors such as poverty, unemployment, and social isolation on the experience of illness and disease. In integrating information from different disciplines, the generalist is uniquely placed to mediate between biological and holistic models of health. This is a key generalist skill because of the potential of genetics to undermine the consideration of social, psychological, economic, and political causes of ill health.24
In this study informed general practitioners identified the “therapeutic gap” as a reason for not raising the issue of genetic risk in the context of common diseases. The potential of genetics to cause harm has not been fully addressed by policy makers. They are wrong to assume that education, training, and decision support systems will ensure that general practitioners are willing to implement the new genetics. Resistance to implementing new genetic knowledge is more than defensive fence building25; it reflects a commitment to holism that is sustained by current generalist training and practice in Britain23,26 and which may be diminished by further specialisation.17–23
Figure.
Roles and skills identified by general practitioners and policy makers1–9 in describing general practitioners' role in implementing genetic advances
Acknowledgments
We thank all the interviewees and transcribers.
Footnotes
Funding: SK was funded by a Southern and Western NHS Research and Development research studentship grant.
Competing interests: None declared.
References
- 1.Lenaghan J. Brave new NHS? The impact of the new genetics on the health service. London: Institute for Public Policy Research; 1998. [Google Scholar]
- 2.Kinmonth A-L, Reinhard J, Bobrow M, Pauker S. Implications for genetic services in Britain and the United States. BMJ. 1998;316:767–770. doi: 10.1136/bmj.316.7133.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Austoker J. Cancer prevention in primary care. BMJ. 1994;309:517–520. doi: 10.1136/bmj.309.6953.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris R. Genetics in a reformed health service. J R Coll Phys. 1992;26:437–441. [PMC free article] [PubMed] [Google Scholar]
- 5.Calman K. GPs need to keep abreast of genetics. BMJ. 1995;310:1142. [Google Scholar]
- 6.Royal College of General Practitioners. Genetics in primary care. A report from the faculty genetics group. London: RCGP; 1998. (Occasional paper 77.) [Google Scholar]
- 7.Hayflick SJ, Eiff PM. Role of primary care providers in the delivery of genetic services. Community Genet. 1998;1:18–22. doi: 10.1159/000016131. [DOI] [PubMed] [Google Scholar]
- 8.Sweeney B. Genetic advances: great promise tempered with concern. J R Coll Gen Pract. 1997;44:544. [PMC free article] [PubMed] [Google Scholar]
- 9.Glaser B, Strauss A. The discovery of grounded theory: strategies for qualitative research. New York: Aldine; 1967. [Google Scholar]
- 10.Glaser B. Advances in the methodology of grounded theory: theoretical sensitivity. Mill Valley: Sociology Press; 1978. [Google Scholar]
- 11.Strauss A, Corbin J. Basics of qualitative research, grounded theory procedures. London: Sage; 1990. [Google Scholar]
- 12.Patton MQ. Qualitative evaluation and research methods. 2nd ed. London: Sage; 1990. [Google Scholar]
- 13.Kirk J, Miller M. Reliability and validity in qualitative research. London: Sage; 1986. [Google Scholar]
- 14.Mays N, Pope C. Rigour and qualitative research. BMJ. 1995;311:109–112. doi: 10.1136/bmj.311.6997.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCraken G. The long interview: qualitative research methods series 13. London: Sage; 1998. [Google Scholar]
- 16.Britten N. Qualitative interviews in medical research. BMJ. 1995;311:251–253. doi: 10.1136/bmj.311.6999.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oelsen NDL. Sustaining general practice. BMJ. 1996;312:525–526. doi: 10.1136/bmj.312.7030.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pringle M, Heath I. Primary care opportunities and threats. BMJ. 1997;314:595–599. doi: 10.1136/bmj.314.7080.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heath I. The mystery of general practice. London: John Fry Trust Fellowship; 1995. [Google Scholar]
- 20.Fugelli P, Heath I. The nature of general practice. BMJ. 1996;312:456–457. doi: 10.1136/bmj.312.7029.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chard J, Liliford R, Gardinder D. Looking beyond the next patient: sociology and modern health care. Lancet. 1999;353:486–489. doi: 10.1016/S0140-6736(98)07304-8. [DOI] [PubMed] [Google Scholar]
- 22.Murphy E, Dingwall R, Greatbatch D, Parker S, Watson P. Qualitative research methods in health technology assessment; a review of literature. Health Technology Assessment 1998;2(16). [PubMed]
- 23.Royal College of General Practitioners. The nature of general medical practice. Report from general practice. London: RCGP; 1996. [Google Scholar]
- 24.Neighbour R. The inner consultation: how to develop an effective and intuitive consultation style. London: Kluwer Academic; 1992. [Google Scholar]
- 25.Horton R. Evidence and primary care. Lancet. 1999;353:609–610. doi: 10.1016/S0140-6736(99)00056-2. [DOI] [PubMed] [Google Scholar]
- 26.Clarke AJ. Population screening for genetic susceptibility to disease. BMJ. 1995;311:35–38. doi: 10.1136/bmj.311.6996.35. [DOI] [PMC free article] [PubMed] [Google Scholar]