This study describes the function of RPL4A, a ribosomal protein. It finds a link between ribosomal biogenesis and vacuolar protein sorting and provides insights into the auxin-mediated regulation of vacuolar trafficking in metabolically active tissues.
Abstract
In plants, the mechanisms that regulate the transit of vacuolar soluble proteins containing C-terminal and N-terminal vacuolar sorting determinants (VSDs) to the vacuole are largely unknown. In a screen for Arabidopsis thaliana mutants affected in the trafficking of C-terminal VSD containing proteins, we isolated the ribosomal biogenesis mutant rpl4a characterized by its partial secretion of vacuolar targeted proteins and a plethora of developmental phenotypes derived from its aberrant auxin responses. In this study, we show that ribosomal biogenesis can be directly regulated by auxins and that the exogenous application of auxins to wild-type plants results in vacuolar trafficking defects similar to those observed in rpl4a mutants. We propose that the influence of auxin on ribosomal biogenesis acts as a regulatory mechanism for auxin-mediated developmental processes, and we demonstrate the involvement of this regulatory mechanism in the sorting of vacuolar targeted proteins in Arabidopsis.
INTRODUCTION
A functional vacuole and intact protein trafficking system are necessary for plant cell viability and function. Perturbations of the trafficking machinery often affect vital cellular processes, such as plant hormone responses, cytokinesis, and the development of tissue specificity (Surpin and Raikhel, 2004). In the classical view, many soluble plant vacuolar proteins are sorted away from proteins destined for secretion at the trans-Golgi network, a process that requires the presence of positive sorting signals in the primary sequence of vacuolar proteins (Matsuoka and Nakamura, 1999; Vitale and Raikhel, 1999; Robinson et al., 2005). Two of these sorting signals, an N-terminal propeptide (NTPP) and a C-terminal propeptide (CTPP), are directed to the vacuole by distinct pathways that converge at the prevacuolar compartment (PVC) (Miao et al., 2008). The NTPP pathway is believed to be common to plants and yeast, and several components of the machinery involved in the sorting of NTPP-type cargoes have been characterized (Zheng et al., 1999; Ahmed et al., 2000; Bassham and Raikhel, 2000). The CTPP pathway is believed to be unique to plants, and different genetic approaches have been used to identify components that are specific for that pathway (Sanmartín et al., 2007; Sohn et al., 2007). To isolate new components of the plant-specific CTPP sorting machinery in Arabidopsis thaliana, we used a T-DNA–mutagenized population in the Vac2 background (Vac2 T-DNA). The Vac2 line contains a genetically engineered CLAVATA3 (CLV3) fused to the barley (Hordeum vulgare) lectin C-terminal vacuolar sorting signal (CLV3:CTPPBL) in the clv3-2 mutant background (Figure 1A). Previous studies using genetic crosses and ethyl methanesulfonate mutagenesis of the Vac2 line have been successfully used for the identification of components involved in the specific sorting of CTPP proteins (Sanmartín et al., 2007; Sohn et al., 2007). In this report, the use of the Vac2 T-DNA screen allowed us to identify a previously unknown component of the auxin-mediated vacuolar sorting machinery, the cytosolic ribosomal protein (r-protein) L4/L1 (RPL4A).
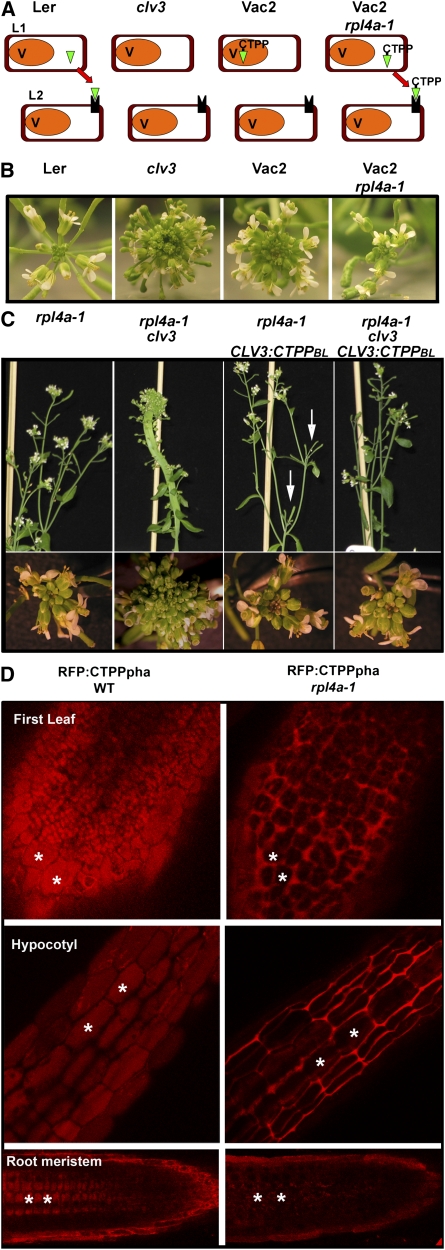
Figure 1.
Identification and Genetic Analysis of rpl4a Using a Visual Screen for Mutants with Altered Trafficking to the Vacuole.
(A) Screening strategy. The Arabidopsis CLV3 protein (green) is synthesized in the shoot apical meristem layers L1 and L2 and secreted to the apoplasm. There, it activates the CLV1/2 LRR kinase receptor (black). Plants lacking CLV3 protein (clv3-2) have uncontrolled growth at the shoot apical meristem. The Vac2 reporter line targets the CLV3:CTPPBL fusion protein to the vacuole (V) in the clv3-2 background. T-DNA plants mutated in components of the vacuolar trafficking machinery shunt CLV3:T7:CTPPBL to the default secretion pathway, thereby complementing the clv3-2 phenotype.
(B) Floral meristems from wild-type Landsberg, clv3-2, Vac2, and the 21-4 (rpl4a-1) mutant isolated in the screen.
(C) Genetic analysis of the clv3-2/CLV3:CTPPBL/rpl4a-1 segregant populations. The top images show representative inflorescences, and the bottom images show representative floral meristems for each genetic background. The rpl4a-1 mutation is unable to bypass genetically the clv3-2 phenotypes (plants rpl4a-1/clv3) unless the CLV3:CTPPBL marker is present (plants rpl4a-1/ CLV3:CTPPBL/clv3). The rpl4a-1/CLV3:CTPPBL plants have terminated floral meristems (arrows) likely due to the secretion of the CLV3:CTPPBL marker to the apoplasm.
(D) The RFP:CTPPpha fluorescent marker is localized in the vacuoles of wild-type plants (left panels, stars), but it is mis-sorted and partially secreted to the apoplast in metabolically active tissues, such as primordial leaves and young hypocotyls, in the 21-4 (rpl4a-1) background (right panels, stars). Notice that the expression level of the RFP:CTPPpha marker decreases in rpl4a-1 root meristems. For each position, the images shown were acquired using the same microscopy settings.
Although protein trafficking defects of ribosomal mutants have not been evaluated, the association of ribosomal mutations with deficient auxin perception and distribution has been widely reported in the literature. The pointed first leaf 1 (pfl1)/r-protein S18 (rps18a), pfl2/rps13b, and the semidominant Arabidopsis minute-like1 (aml1)/rps5a mutants display auxin-related developmental defects, including growth retardation, narrow leaves with reductions in the palisade mesophyll layer, and cotyledon vascular pattern defects (Van Lijsebettens et al., 1994; Ito et al., 2000; Weijers et al., 2001). Mutations in the short valve1 (stv1)/r-protein L24 (rpl24b) result in an apical-basal patterning defect of the gynoecium by influencing the translation of the auxin response factors ARF3 and ARF5 (Nishimura et al., 2005). STV1 together with the proteins RPL28A and RPL5A has been shown to have important roles in specifying leaf adaxial identity (Yao et al., 2008), and RPL5A, RPL10A, and RPL9 have been shown to modulate leaf patterning mechanisms via the ribosome-mediated translational regulation of genes in the HD-ZIPIII-KANADI pathway (Pinon et al., 2008). Finally, mutations in the nucleolar protein PARL1, involved in ribosomal biogenesis, cause similar auxin related developmental defects, suggesting that auxin regulation depends on protein turnover and ribosome biogenesis in areas of growth (Petricka and Nelson, 2007).
In bacteria, the RPL4 protein has been shown to be crucial for the maintenance of ribosomal translational efficiency and fidelity (O'Connor et al., 2004), but aside from structural roles within the ribosome, no other specific function has yet been assigned in plants. In this study, we analyze the implications of the mutations in the Arabidopsis RPL4 family for protein trafficking as well as for hormonal regulation leading to the altered sorting of vacuolar targeted proteins.
The Arabidopsis cytosolic r-protein RPL4 family is composed of two transcriptionally active genes (RPL4A and RPL4D) and two nonexpressed pseudogenes (RPL4B and RPL4C) (Barakat et al., 2001). Our analysis of the two transcriptionally active members of the RPL4 family in Arabidopsis suggests that the RPL4A and RPL4D proteins have equivalent functions and are coexpressed. Mutations in either RPL4 gene cause a similar auxin-related developmental defect, and both proteins are involved in the delivery of vacuolar targeted proteins to the vacuole. Moreover, our results suggest that the sorting defects in rpl4 mutants are due to problems in protein turnover and auxin perception in metabolically active tissues. We propose that ribosomal biogenesis influenced by auxins is a high level regulatory mechanism in metabolically active tissues that regulates the vacuolar delivery of not only CTPP, but also NTPP and recycled proteins.
RESULTS
rpl4a-1 Has Altered Protein Sorting to the Vacuole
rpl4a-1 is a mutant characterized from a T-DNA–mutagenized population in the Arabidopsis Vac2 background (Rojo et al., 2002). The Vac2 line was transformed with the pSKI015 plasmid, and ∼ 8000 lines were divided into 144 independent pools (Koiwa et al., 2006). The pools were screened for complementation of the clv3-2 shoot meristem phenotype (Sanmartín et al., 2007; Sohn et al., 2007; Zouhar et al.,2009) (Figure 1A). By this approach, we identified a mutant designated 21-4 that displayed reduced floral meristem size (Figure 1B). Genetic analyses showed that the 21-4 line harbored one functional T-DNA insertion that caused a recessive mutation in a single nuclear locus (see Supplemental Table 1 online). Thermal asymmetric interlaced PCR was used to determine the T-DNA insertion position and the genomic sequence flanking the T-DNA left border. As a result, a T-DNA insertion located 283 bp downstream of the ATG translation start site in the first exon of the At3g09630 locus was identified. The At3g09630 gene was annotated as the cytosolic L4/L1 protein of the large 60S ribosomal complex subunit (RPL4A). Genetic linkage analysis confirmed that the reduced meristems, the T-DNA insertion in RPL4A, and the developmental phenotypes observed in different rpl4a alleles (Figure 2) were linked within a genetic distance of 0.5 centimorgans (see Supplemental Table 1 online). Upon identification of the mutation in RPL4A, we performed phenotypic and genetic analyses to test whether the mutation acted as a bypass suppressor of clv3-2. The analysis of populations in which rpl4a-1, clv3-2, and CLV3:CTPPBL were segregating indicated that rpl4a-1 mutation was unable to genetically bypass the clv3 phenotypes (plants rpl4a-1/clv3) unless CLV3:CTPPBL was present (plants rpl4a-1/clv3/CLV3:CTPPBL) (Figure 1C). Interestingly, the combination rpl4a-1/CLV3:CTPPBL caused terminated meristems that mimicked the phenotypes observed in CLV3-overexpressing lines (Rojo et al., 2002) (Figure 1C). Based on these results, we concluded that the reduced floral meristem phenotype in 21-4 (rpl4a-1/clv3/CLV3:CTPPBL) was due to the secretion of the CLV3:CTPPBL fusion protein to the apoplasm and that rpl4a-1 was not a bypass mutation of clv3.
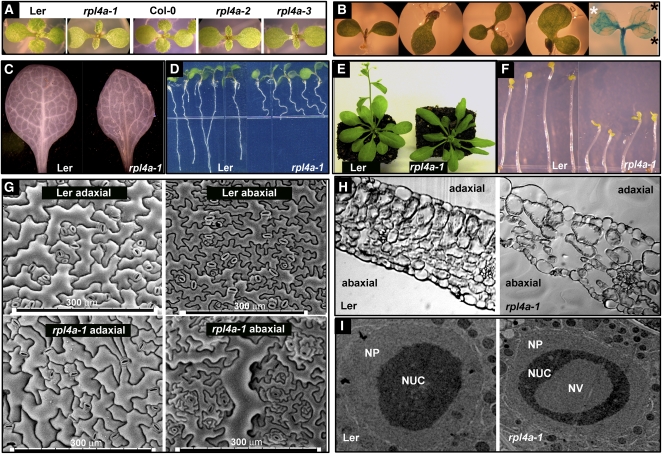
Figure 2.
The rpl4 Family Mutants Display Aberrant Auxin-Related Developmental Defects.
(A) Independent rpl4a alleles display narrow pointed first leaves. The original rpl4a-1 mutant was compared with the wild-type Landsberg, whereas the rpl4a-2 (SALK_130595) and rpl4a-3 (SALK_063782) alleles were compared with the wild-type Col-0.
(B) rpl4a-1 cotyledon structures and auxin maxima localizations. In the far right panel, GUS staining of rpl4a-1 cotyledons expressing the auxin reporter DR5pro:GUS. The right cotyledon shows aberrant auxin maxima localizations (black stars), and the left cotyledon shows the expected apical auxin maxima localization (white star).
(C) to (F) Auxin-related phenotypes in rpl4a-1 include incomplete vascular development (C), reduced root elongation and altered root gravitropic responses (D), delayed transition to reproductive phase (E), and reduced hypocotyl elongation in etiolated seedlings (F).
(G) Scanning electron micrographs of the adaxial and abaxial surfaces of late rosette leaves from 28-d-old plants of the wild type (top) and rpl4a-1 (bottom).
(H) Stained transverse sections of first leaf cells in rpl4a-1 presented many enlarged cells and intercellular spaces in the adaxial palisade region and fewer subepidermal palisade cells than did the wild type.
(I) Transmission electron microscopy of root apical meristem cells shows aberrant nucleolar structures in the rpl4a-1 mutant. NUC, nucleolus; NP, nucleoplasma; NV, nuclear vacuole.
To confirm further that rpl4a-1 causes the secretion of vacuolar targeted proteins at the cellular level, we crossed the rpl4a-1 mutant line with two lines expressing fluorescent vacuolar markers. The first marker contained the vacuolar sorting signal of proricin appended to the N terminus of the monomeric red fluorescent protein RFP1 (NTPPpro:RFP) (Hunter et al., 2007). The second marker contained the vacuolar sorting signal of phaseolin fused to the C terminus of RFP1 (RFP:CTPPpha) (Hunter et al., 2007). Both markers included the signal peptide from sporamine fused to the N-terminal end of RFP1 that caused the reporter to be directed into the default secretion pathway when the vacuolar sorting signals were not recognized (Craddock et al., 2008). Homozygous lines from the F2 generation were obtained, and the localization of the RFP fusion proteins in different tissues and developmental stages using scanning fluorescent confocal microscopy was determined. As shown in Figure 1D, wild-type plants displayed vacuolar localization of the RFP:CTPPpha in all tissues, whereas secretion in the first pair of true leaves and lower hypocotyls and decreased expression and partial secretion in root meristems were observed in the rpl4a-1 line. Similar analyses were performed with the NTPPpro:RFP marker, which presented similar vacuolar localization in wild-type plants, but the secretion of NTPPpro:RFP in different tissues from rpl4a-1 mutants was not as dramatic as in the case of RFP:CTPPpha (see Supplemental Figure 1 online). The differential results obtained with the different vacuolar markers might be partially explained by the lower expression of NTPPpro:RFP in both wild-type and rpl4a backgrounds that, in turn, decreased the marker secretion signal (see Supplemental Figure 1 online). The results obtained from the vacuolar fluorescent markers analyses validate the genetic screen for vacuolar trafficking mutants and suggest that RPL4A is required for the proper sorting of vacuolar-targeted proteins.
rpl4a Displays Aberrant Auxin-Related Developmental Phenotypes
Early in its development, the rpl4a-1 mutant displays very characteristic narrow pointed first leaves (pfl) and retarded growth (Figure 2A). The pfl phenotype in rpl4a-1 mutants resembled the phenotypes of the r-protein knockout mutants rps13b and rps18a (Van Lijsebettens et al., 1994; Ito et al., 2000) and was also similar to the phenotypes observed in RPL23-silenced lines (Degenhardt and Bonham-Smith, 2008). To confirm whether mutations in the RPL4A gene were the cause of the pfl phenotypes, two additional T-DNA alleles in the Columbia-0 (Col-0) genetic background, rpl4a-2 (SALK_130595) and rpl4a-3 (SALK_063782), were isolated. Homozygous rpl4a-2 and rpl4a-3 seedlings exhibited similar developmental phenotypes to those of rpl4a in a Landsberg background (Figure 2A). In early stages of development, it was also observed that rpl4a-1 displayed altered cotyledon architecture associated with aberrant distributions of auxin maxima sites (Figure 2B). The vascular structure in the first leaves of rpl4a-1 deviated from the closed and reticulate venation of controls. rpl4a-1 presented substantially reduced venation, few or no tertiary or quaternary veins, and aberrant anastamosis close to the hypocotyl-petiole junction (Figure 2C). Other physiological and developmental consequences of the rpl4a-1 mutation included reduced root elongation, altered root gravitropic responses, delayed transition to reproductive phase, and decreased hypocotyl elongation in etiolated seedlings (Figures 2D to 2F). Impaired auxin homeostasis may explain these phenotypic traits, which were reminiscent of mutants with altered ribosome biogenesis and auxin responses (for references, see Supplemental Table 2 online). However, the severely altered auxin responses observed in rpl4a-1 did not influence the subcellular localization, polarity, and tissue expression of auxin transporters, such as AUX1, PIN2, and PIN7, in light-incubated rpl4a seedlings (see Supplemental Figure 2 online).
Because rpl4a-1 shared common features with a plethora of previously reported ribosomal mutants, we investigated whether other common phenotypes found in different r-protein mutants were present in rpl4a-1 (see Supplemental Table 2 online). Leaf patterning organization and adaxial-abaxial polarity were disrupted at the cellular level in rpl4a-1. Epidermal cells of the adaxial lamina surface of the first leaf of rpl4a-1 were altered and presented angular lobes as opposed to the jigsaw patterning and smooth lobes in the wild type. Abaxial surfaces in rpl4a-1 were also different and displayed a mosaic pattern of normal abaxial patches mixed with outgrowths and heterogeneous cell sizes (Figure 2G). The stained transverse sections of first leaf cells in rpl4a-1 presented many enlarged cells and intercellular spaces in the adaxial palisade region, and the number of subepidermal palisade cells was fewer than that of the wild type (Figure 2H). Finally, the rpl4a-1 root meristem cells displayed more disorganized nucleolar structures with an abundance of large nucleolar vacuoles (71% of rpl4a nuclei n = 44) compared with controls (10% of Vac2 nuclei n = 39), suggesting that ribosomal biogenesis could be affected in that tissue (Figure 2I). The extensive phenotypic similarities of rpl4a-1 with previously reported r-protein mutants involved in ribosomal biogenesis, together with the aberrant nucleolar structures in rpl4a-1, support the hypothesis that the ribosome per se, and not r-proteins acting independently, regulates auxin-mediated developmental processes.
Arabidopsis Has Heterogeneous RPL4-Containing Ribosomes in Specific Tissues
The RPL4 family in Arabidopsis comprises two nearly identical expressed members, RPL4A and RPL4D (Barakat et al., 2001; Carroll et al., 2008) (Figure 3A). RPL4A is a basic protein (pI = 11.04) of 406 amino acids that shares an overall 95.1% amino acid identity with RPL4D. The T-DNA mutants in the RPL4A (rpl4a-2 and rpl4a-3) and the RPL4D (rpl4d-1 and rpl4d-2) genes in the Col-0 background were isolated from the SALK collection and their mRNA expressions analyzed by RT-PCR. As shown in Figure 3B, the rpl4a-2 mutant (hereafter referred to as rpl4a) showed reduced RPL4A expression, whereas the rpl4a-3, rpl4d-1 (hereafter referred to as rpl4d), and rpl4d-2 mutants showed no discernible transcripts after 35 PCR cycles.
Figure 3.
Genetic Analysis of the F2 Population from the Cross rpl4a × rpl4d.
(A) Exon-intron organization and schematic representation of the locations of the T-DNA insertions in the RPL4A and RPL4D genes. Orange, exon; purple, intron; red, untranslated regions.
(B) RPL4 transcripts detected in 2-week-old seedlings in wild-type (Col-0) and rpl4a and rpl4d mutant alleles using RT-PCR. The positions of the oligonucleotides used for the RT-PCR (F and R) are indicated with arrows in (A).
(C) Genotypes and segregation analysis of the F2 individuals from the cross rpl4a-2 × rpl4d-1 (rpl4a × rpl4d). The possible genotypic F2 combinations derived from the rpl4a × rpl4d cross are represented with green and red bars. Green bars indicate RPL4 genomic copies without T-DNA insertions. Red bars with black triangles indicate RPL4 genomic copies containing T-DNA insertions.
When the rpl4a and rpl4d mutants were grown side by side, they were indistinguishable and displayed similar auxin-related phenotypes (see Supplemental Figure 3 online). The high degree of amino acid identity between RPL4A and RPL4D, together with the similar phenotypes observed in the rpl4a and rpl4d mutants, suggested that both proteins have similar functions. To evaluate further their spatial and temporal equivalence, their respective promoter: β -glucuronidase (GUS) expression patterns were analyzed, and no significant differences between RPL4Apro:GUS and RPL4Dpro:GUS expression patterns in our experimental conditions were observed (Figures 6A to 6F; see Supplemental Figure 4D online). Because a given ribosomal complex contains only one RPL4 family member at a time (Ban et al., 2000), these results suggested that distinct ribosomal populations (RPL4A and RPL4D ribosomal complexes) simultaneously exist in wild-type plants. This conclusion is supported by previous proteomic studies that demonstrated the presence of ribosomal heterogeneity in Arabidopsis (Chang et al., 2005; Giavalisco et al., 2005; Carroll et al., 2008).
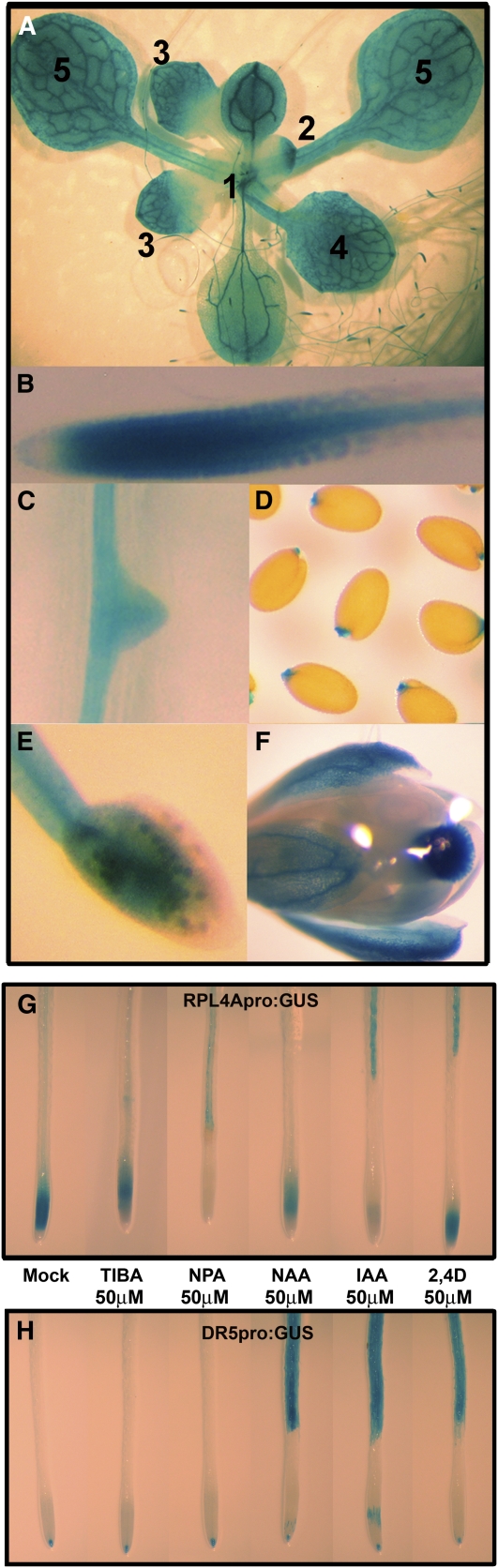
Figure 6.
The RPL4 Expression Correlates with Patterns of Free Auxin Distribution, and It Is Regulated by Auxins.
(A) to (F) GUS activity assay in transgenic plants harboring the RPL4A promoter-GUS fusion.
(A) Whole plant showing GUS staining in leaves at different developmental stages (1 to 5).
(B) Root tip and root vasculature.
(C) Water-embedded seeds.
(D) Lateral root.
(E) Anthers and pollen grains.
(F) Stigma and petals.
(G) GUS activity assay in the presence of auxin transport inhibitors and auxins in RPL4Apro:GUS transgenic plants. TIBA, 2,3,5-triiodobenzoic acid.
(H) The auxin-inducible DR5pro:GUS transgenic lines were used as a control.
The RPL4 Family Requires at Least Two Functional Gene Copies for Plant Viability
We attempted to enhance the phenotypes observed in rpl4 mutants by eliminating both RPL4 proteins or, in case of lethality, to determine the minimum RPL4 gene dosage required for plant viability. For that purpose, we successively eliminated RPL4 gene function by performing reciprocal crosses between the rpl4a and rpl4d mutants. Surprisingly, F1 plants from the rpl4a × rpl4d cross that were heterozygous for both recessive mutations displayed similar morphological defects as the single mutants, with one exception: cleared siliques of either of the single rpl4 mutants were normal, but the double heterozygous plants showed empty spaces in the siliques, indicating embryo lethality of ∼ 30% of expected seeds (see Supplemental Figure 5 online). To confirm this result, phenotypic and genotypic analyses of 144 segregant F2 plants from the rpl4a × rpl4d cross without pfl phenotype and 144 segregant F2 plants with pfl phenotype were completed (Figure 3C). The analysis of the plants without phenotype resulted in 36 wild-type plants and 108 plants with a T-DNA insertion in one of the four RPL4 copies, providing the expected segregation ratio. However, all 144 plants that displayed the pfl phenotype had T-DNA mutations in two RPL4 copies and significant segregation distortions ( χ2 >16.75, P < 0.005) were observed. In this random population, we did not identify plants with insertions in more than two RPL4 copies. This result suggests that two active copies of RPL4, independent of their identities, define the minimum threshold for plant viability.
The rpl4 Mutants Have Altered Ribosomal Functions
In Escherichia coli, multiple defects in translation associated with altered ribosomal protein L4 activity (the homolog of the Arabidopsis RPL4s) have been shown (O'Connor et al., 2004). In Arabidopsis, r-protein mutants, such as stv1/rpl24b (Nishimura et al., 2005), rpl5a, and rpl9 (Yao et al., 2008), and the three piggyback mutants (Pinon et al., 2008) have translational defects due to their aberrant ribosomal function. To test whether the Arabidopsis rpl4 mutations compromised ribosomal function in a similar way to their bacterial counterpart and other Arabidopsis r-proteins, we used an in vivo protoplast transient expression assay. In this assay, various green fluorescent protein (GFP) fusion proteins under the control of the 35S cauliflower mosaic virus promoter were expressed in protoplasts isolated from wild-type and rpl4 seedlings, and the levels of accumulation of different GFP-fused proteins were evaluated temporally using anti-GFP antibodies.
As shown in Figure 4A, the levels of GFP accumulation using a cytosolic 35S:GFP construct were reduced in rpl4a and rpl4d when compared with the wild-type protoplast 6 h after transformation. Those differences were reduced when longer incubation times were analyzed (9 and 12 h), until near-wild-type levels of accumulation were observed after 18 h in rpl4a. In our conditions, rpl4d showed a slightly lower GFP accumulation than rpl4a in all time points likely due to the residual RPL4A expression of the rpl4a mutant allele (Figure 2B).
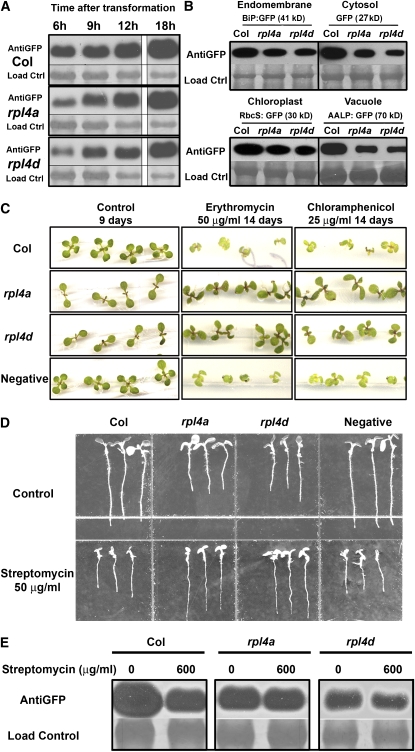
Figure 4.
The rpl4 Mutations Reduce the Rate of Protein Synthesis and Cause Resistance to Specific Antibiotics.
(A) Immunoblots showing the GFP protein accumulation in wild-type and rpl4 backgrounds at different time points. Protoplasts were transformed with a 35S:GFP construct divided in four independent tubes and incubated at 22 ° C in the light for different time periods (6 to18 h). Transformation efficiencies were evaluated, and total proteins were separated by SDS-PAGE and normalized using Coomassie blue staining (load control lanes) prior to the immunoblot analysis.
(B) Immunoblots showing the GFP protein accumulation in wild-type and rpl4 backgrounds 6 h after transformation. The protoplasts were transformed with constructs containing proteins with different organelle specificity fused with GFP and normalized as in (A).
(C) rpl4 mutants shoots are resistant to erythromycin and chloramphenicol. Wild-type and rpl4 seeds were surface sterilized and directly germinated on plates with or without antibiotic. Plates were incubated horizontally in a growth chamber under long-day conditions. An independent kanamycin-resistant line SALK_069239 was used as a negative control to evaluate antibiotic cross-resistance.
(D) rpl4 mutants roots are resistant to streptomycin. The assay was performed as in (C), but the plates were incubated vertically and pictures were taken 7 d after sowing.
(E) Transient expression assay in streptomycin-treated protoplasts. The GFP protein was expressed in protoplasts for 6 h, followed by a treatment with 0 and 600 μ g/mL of antibiotic for 12 additional hours.
Because we determined that the cytosolic GFP levels after 6 h were different in wild-type and rpl4 protoplasts, we tested whether this reduction was a general effect or specific for cytosolic proteins. As shown in Figure 4B, the levels of accumulation of different GFP fusion proteins, which included proteins destined for different compartments such as the ER, cytoplasm, chloroplast, and vacuole, were reduced in both rpl4 mutants compared with the control. These results suggest that independently of their subcellular localization, the rate of protein synthesis was reduced in rpl4 protoplasts; hence, it takes longer to reach the same protein levels in rpl4 mutants relative to the wild type. However, we cannot exclude the possibility that the differential protein accumulation observed after 6 h was due to transcriptional differences or enhanced degradation of newly synthesized polypeptides in rpl4 backgrounds.
Once we determined that rpl4 mutations cause protein synthesis defects, we evaluated whether the altered rpl4 ribosomal function was due to either a structural defect in fully assembled ribosomes or to the lack of ribosomes. For that purpose, we analyzed rpl4 responses against an array of antibiotics with known ribosomal targeting locations in prokaryotes (see Supplemental Table 3 online). The rpl4 mutants did not display increased sensitivity to any tested compounds, including the eukaryotic protein synthesis inhibitor cycloheximide, which binds the eukaryotic ribosomal complexes in a stoichiometic manner (Oleinick, 1977). Moreover, rpl4 mutants displayed mild resistance to chloramphenicol and erythromycin in shoots (Figure 4C) and streptomycin in roots and isolated protoplasts (Figures 4D and 4E). The lack of general resistance or hypersensitivity to antibiotics or cycloheximide in rpl4 suggested that the total number of ribosomes was similar to that in the wild type, and the resistance for specific antibiotics suggested that the protein synthesis problems in the rpl4 background were due to the presence of an aberrant population of ribosomes unable to bind properly to specific antibiotics. Interestingly, the presence of aberrant ribosomes in rpl4 did not trigger an enhanced unfolded protein response as demonstrated by the similar expression of ER resident chaperones at the transcriptional and translational levels (see Supplemental Figure 6 online) and the similar sensitivity to tunicamycin (see Supplemental Table 3 online) of the rpl4 mutants compared with wild-type plants.
The rpl4 Mutants Activate Compensatory Mechanisms at the Protein Level
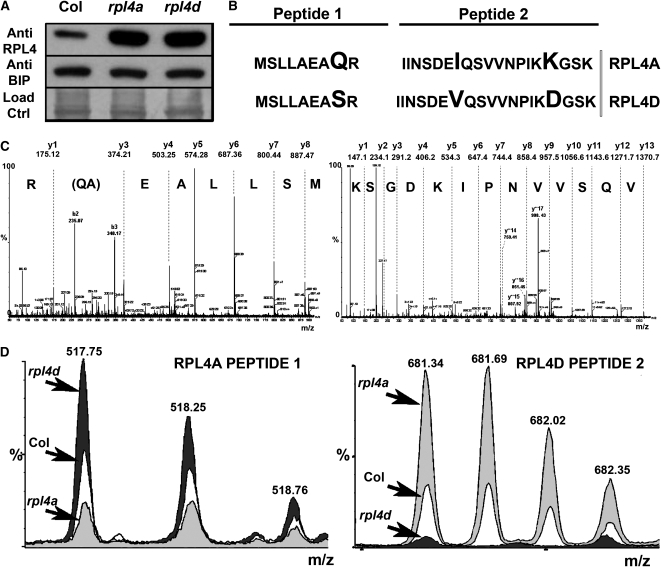
Because the RPL4 family is composed of two coexpressed members, we hypothesized that the general protein accumulation defects in rpl4 might be partially buffered by compensatory mechanisms at the protein level. To test this hypothesis, we checked the levels of total RPL4 protein in both rpl4a and rpl4d single mutant backgrounds compared with the wild type. In this assay, the RPL4 proteins were indistinguishable due to high homology at the amino acid sequence level, so the observed signals using human antiRPL4-specific antibodies accounted for the contributions of both members. As shown in Figure 5A, upregulation of the combined RPL4 proteins was observed in both rpl4a and rpl4d mutants compared with the wild type. Because our mutant lines were knockdowns, it was possible that the lack of one member increased the expression of the other family member to compensate functionally. To test this hypothesis, liquid chromatography/tandem mass spectrometry (LC/MS/MS) analysis was performed using protein extracts from rpl4a and rpl4d 2-week-old seedlings. As shown in Figures 5B and 5C, specific peptides for RPL4A and RPL4D were identified by LC/MS/MS, and the ion chromatograms for the corresponding peptides were quantified. Figure 5D shows the quantification of the RPL4A- and RPL4D-specific peptides, which indicated that the rpl4a mutant expressed more RPL4D protein and vice versa. This compensation at the protein level in rpl4 backgrounds correlated with an increased transcriptional activity of the alternative RPL4 protein and an increased transcriptional activity of the r-protein S6 (see Supplemental Figure 6 online), which is an important regulator of ribosome biogenesis in eukaryotes (Volarevic et al., 2000).
Figure 5.
The rpl4 Mutants Increase the Expression of the Alternative RPL4 Protein.
(A) Immunoblot of RPL4 using human RPL4 polyclonal antibodies. Total protein from 2-week-old seedlings were extracted, separated by SDS-PAGE, and normalized using Coomassie blue staining (load control lanes). Immunoblots with BiP in the same samples were used to confirm equal loading.
(B) Two proteotypic peptides used for the differentiation of the RPL4A and RPL4D proteins. The amino acid differences between RPL4A and RPL4D peptides are indicated with a larger font.
(C) Mass spectra of two proteotypic peptides used in the differentiation of RPL4A and RPL4D proteins.
(D) LC/MS quantitative analysis of RPL4A and RPL4D proteotypic peptides showing the relative abundance of the two proteins in wild-type and rpl4 backgrounds. Light-gray shading, rpl4a mutant; dark-gray shading, rpl4d mutant; no shading, Col.
Although the compensatory mechanisms in planta cannot fully fulfill the requirements of RPL4 protein dosage in specific tissues, this mechanism could explain several observations derived from our study: the relatively mild phenotypes observed in the adult rpl4 plants (Figure 2E), the specificity of the secretion patterns for certain tissues (Figure 1D), and the lack of differences between the wild type and rpl4a when the protein levels of the vacuolar trafficking machinery in planta were analyzed (see Supplemental Figure 6 online).
RPL4A Transcription Is Regulated by Auxins
A model including a general protein accumulation defect in metabolically active tissues is inadequate to explain the specificity of the auxin phenotypes in rpl4a. To link ribosomal function and specific auxin responses, we analyzed whether auxins mediate transcriptional regulation of the RPL4 promoters. Based on the phenotypes observed in Figure 2, we expected that the RPL4 promoter activity would be correlated with patterns of free auxin distribution. To confirm our hypothesis, we characterized the GUS staining patterns of Arabidopsis plants transformed with the RPL4Apro:GUS and RPL4Dpro:GUS reporter gene constructs. As shown in Figure 6A and Supplemental Figures 4A to 4D online, plants transformed with the RPL4Apro:GUS and RPL4Dpro:GUS constructs showed very similar staining distribution that strongly resembled the auxin distribution patterns throughout the Arabidopsis development described by Teale et al. (2006). Briefly, RPL4Apro:GUS expression was strong during early stages of primordia development, and leaf apical dominance was evident in the elongating tip (Figure 6A, primordial leaf 1 and leaf 2). In later stages of leaf development, GUS expression progressed basipetally along the margins (Figure 6A, leaf 3), until its final appearance in the central regions of the lamina (Figure 6A, leaf 4), and in a more diffuse fashion in mature leaf mesophyll cells (Figure 6A, leaf 5). In main and lateral roots, RPL4Apro:GUS expression also correlated with auxin distribution patterns. In the main root, RPL4Apro:GUS staining was more intense toward the root tip and more diffuse in the epidermis (Figure 6B). In lateral roots, the GUS activity started from the stele to the new root tip and then continued through the epidermis (Figure 6C). Finally, GUS staining was also observed in secondary sites of free auxin production, such as the seed endosperm cap, stigma, stamen, and pollen (Figure 6D to 6F). These results suggested that ribosome biogenesis and auxin biosynthesis and transport are tightly linked processes. To confirm further that this linkage exists, we tested whether exogenously applied auxins alter the expression of the RPL4Apro:GUS lines. As shown in Supplemental Figure 4E online, exogenous applications of auxins at physiological levels (10 μ M concentrations for 24 h) caused a general decrease in the RPL4A promoter activity in the root meristem. Although this result suggested that auxins regulate RPL4A expression, no strong conclusions about the relative sensitivity of the RPL4A promoter to different auxins could be reached. To determine whether the different auxin treatments were equivalent, exogenous applications of higher hormonal concentrations (50 μ M concentrations for 4 h) were used. As shown in Figure 6G, the application of the natural auxin indole-3-acetic acid (IAA), as well as the same concentration of the auxin transport inhibitor 1-naphthylphthalamic acid (NPA), completely abolished the RPL4A promoter activity in the root meristem and root elongation zone without modifying the expression patterns in the vasculature of fully developed roots. The same concentrations and incubation times using 1-naphthaleneacetic acid (NAA), 2,4-D, and the auxin transport inhibitor 2,3,5-triiodobenzoic acid caused a moderate decrease in GUS activity in root meristems, suggesting that the promoter response to those treatments was weaker (Figure 6G). As a positive control of induction, we evaluated the effect of the similar treatments using the auxin inducible DR5pro:GUS transgenic line (Ulmasov et al., 1997). As shown in Figure 6H, DR5pro:GUS expression was induced by auxins; however, no differences in induction were observed among the NAA, IAA or 2,4-D treatments. In our conditions, no repression of DR5pro:GUS was observed in the 2,3,5-triiodobenzoic acid and NPA treatments compared with nontreated plants. Based on these results, we concluded that auxins regulate ribosomal biogenesis in root meristems and that the RPL4 promoter is more sensitive to IAA and NPA treatments.
Different Vacuolar Sorting Pathways Are Affected by Auxins and rpl4a
Since IAA and NPA treatments (50 μ M concentrations for 4 h) were able to completely abolish the RPL4A promoter activity in root meristems, we hypothesized that those treatments applied to wild-type plants might mimic the vacuolar sorting defects caused by the rpl4a mutation. As shown in Figures 7A and 7B, the IAA and NPA treatments caused large amounts of secretion of both RFP:CTPPpha and NTPPpro:RFP fluorescent markers restricted to the root meristems and the elongation zone of the root. Since the hormonal treatments were not specific for either RFP:CTPPpha or NTPPpro:RFP markers, we hypothesized that RPL4A acts in a regulatory mechanism that affects vacuolar trafficking generally, not just markers containing C- or N-terminal propeptides. To test that hypothesis, we analyzed the vacuolar transport of the auxin-efflux carrier PIN2, a protein without vacuolar sorting signals that is targeted for vacuolar degradation after darkness treatments (Kleine-Vehn et al., 2008). As shown in Figure 7C, dark-incubated wild-type PIN2:GFP plants displayed fluorescent signals in the plasma membrane, endosomes, and vacuole; however, the combined treatment of darkness and IAA in wild-type plants restricted the PIN2:GFP fluorescent signal to the plasma membrane (Figure 7D). This result is in accordance with previous studies that demonstrated that PIN2 endocytosis, and, therefore, the delivery of the PIN2:GFP marker to the vacuole, was inhibited by treatments with NAA (Paciorek et al., 2005). Once we confirmed that IAA was also able to inhibit the release of the PIN2:GFP marker to the vacuole, we crossed the PIN2:GFP line with rpl4a and analyzed the vacuolar transport of PIN2:GFP in rpl4a backgrounds and isogenic wild-type lines. As shown in Figure 7E, the PIN2:GFP fluorescent signal of dark-treated wild-type plants grown in solid media appeared in plasma membrane, endosomes, and vacuole. However, the PIN2:GFP signal of isogenic rpl4a plants was similar at the plasma membrane but showed a marked decrease in the vacuole (Figure 7F). This result suggested that rpl4a mimics the effect of exogenously applied IAA by partially inhibiting the vacuolar delivery of PIN2:GFP. The partial inhibition of the PIN2:GFP vacuolar delivery in rpl4a correlates with the partial secretion of the RFP:CTPPpha and NTPPpro:RFP markers and suggests that the trafficking of vacuolar targeted proteins is delayed but not totally abolished in rpl4a. Finally, to analyze directly the kinetics of trafficking to the vacuole, we analyzed the vacuolar processing of a marker containing the Barley lectin vacuolar propeptide fused with GFP (GFP:CTPPBL) in transformed wild-type and rpl4a protoplasts. As shown in Figure 7G, 24 h after transformation, a shift in the molecular weight of the GFP:CTPPBL marker was observed in wild-type but not rpl4a protoplasts. This shift is due to the vacuolar processing of the BL propeptide in wild-type plants. Forty-eight hours after transformation, rpl4a protoplasts started to process the marker; however, the ratio of nonprocessed versus processed GFP:CTPPBL was higher in rpl4a than in wild-type protoplasts (Figure 7H). This result confirms that trafficking toward the vacuole was strongly delayed in rpl4a.
Figure 7.
Different Vacuolar Sorting Pathways Are Affected by Auxins and RPL4A.
(A) and (B) Exogenous applications of IAA and NPA cause secretion of vacuolar markers containing C-terminal VSDs (A) and N-terminal VSDs (B). Transgenic plants harboring the RFP:CTPPpha and NTPPpro:RFP markers were incubated in liquid media (4 h) supplemented with DMSO or 50 μ M solutions of IAA or NPA. The RFP fluorescence was analyzed using scanning fluorescent confocal microscopy. All the images shown were acquired using the same settings.
(C) to (F) Vacuolar sorting of the PIN2:GFP marker in the dark.
(C) and (D) PIN2:GFP localization at the plasma membrane and vacuolar compartments in dark-treated (6 h) wild-type epidermal root cells in the absence (C) or presence (D) of IAA. IAA or DMSO was added in darkness 2 h before the end of the 6-h dark treatments.
(E) and (F) PIN2:GFP localization at the plasma membrane and vacuolar compartments in dark-treated (6 h) wild-type (E) or rpl4a (F) root meristems. All the images shown were acquired using the same settings.
(G) Processing of the vacuolar targeted marker GFP:CTPPBL in wild-type and rpl4a protoplasts. Protein extracts from GFP:CTPPBL transformed protoplasts were prepared 24 or 48 h after transformation, and the vacuolar processing was analyzed by immunoblots using anti-GFP antibodies.
DISCUSSION
The results provided by the characterization of the ribosomal rpl4a mutant isolated in this screen demonstrate that the Vac2 T-DNA collection is an excellent tool to describe mechanisms of transport toward the plant vacuole. RPL4A belongs to a totally different group of proteins compared with the ones identified using ethyl methanesulfonate mutagenesis or genetic crosses of the Vac2 line (Sanmartín et al., 2007; Sohn et al., 2007), demonstrating that the Vac2 T-DNA collection can identify unknown components of plant vacuolar trafficking pathways. The described function of RPL4A links vacuolar protein sorting with ribosomal biogenesis and provides new insights into the auxin-mediated regulation of vacuolar trafficking in metabolically active tissues.
The Role of Ribosomal Biogenesis in Auxin-Related Responses
Ribosomes are responsible for protein translation in eukaryotic cells, and their biogenesis is a complex process involving the coordination of many nonribosomal and ribosomal proteins. Previous reports have described individual mutations in r-proteins that cause specific auxin-related phenotypes, and different models have been proposed to explain the specificity of the observed phenotypes. It has been shown that mutations in SHORT VALVE1 (STV1), which encodes an L24 r-protein and causes carpel tissue patterning defects, regulates the translation of the auxin response factors ETTIN and MONOPTEROS via translation of short upstream open reading frames (Nishimura et al., 2005). The defects in the adaxial-abaxial polarity in the ribosomal piggyback mutants have been proposed to be due to defects in the HD-ZIPIII-KANADI pathway in a process regulated by the specific targeting of small RNAs to HD-ZIPIII- KANADI elements (Pinon et al., 2008). Accordingly, a model of the ribosome function in the leaf patterning regulatory network has been proposed by Yao et al. (2008). In this model, the ribosome genetically promotes the HD-ZIPIII–mediated pathway in the adaxial domain of leaves or genetically represses the auxin-responsive genes ARF3/4, KAN, or their downstream genes. However, none of the models directly applied to individual r-proteins properly address a broader question: How does the protein synthesis machinery affect specific auxin responses? To answer this question, it has been proposed that different isoforms and posttranslational modifications of r-proteins, resulting in ribosome heterogeneity, may generate functionally distinct ribosomes with target-transcript specificity (Giavalisco et al., 2005; Carroll et al., 2008; Komili et al., 2007). Indeed, the similar expression patterns of both RPL4 proteins found in this study support that heterogeneous ribosomal populations are present in the same tissues, but also, the similarity of both rpl4 mutant phenotypes suggest that RPL4A and RPL4D-containing ribosomes are equivalent and do not account for the target transcript specificity. In accordance with the equivalence of RPL4 ribosomal types, RPL4 gene dosage, and not the identity of the RPL4 proteins, is the key element for Arabidopsis survival. In our study, 50% RPL4 gene dosage reductions caused similar phenotypes, independent of RPL4 identity, and 75% RPL4 gene dosage reductions caused lethality. Furthermore, the activation of compensatory mechanisms that increased the protein levels of the alternate RPL4 protein in mutant backgrounds suggest that RPL4 ribosomal heterogeneity does not confer auxin specificity, but acts as a backup mechanism to maintain basic cellular functions when RPL4 availability is limited.
To explain the auxin specificity of the r-protein mutant responses, our study uses a global analysis of the phenotypes derived from ribosomal mutations (see Supplemental Table 2 online). Since most of the r-protein mutant phenotypes are similar, we propose that those phenotypes are downstream effects of a failure in a common regulatory mechanism for all r-proteins, the auxin-mediated ribosomal biogenesis. At least four independent lines of evidence were provided to support this model. First, as indicated above, except for variations in the severity of the phenotypes, and rare specific phenotypes that could be associated with extraribosomal functions (Wool, 1996), several independent r-protein mutants described in the literature display very similar auxin-related phenotypes (see Supplemental Table 2 online). Second, parl1, a mutant in a nucleolin that globally affects ribosomal biogenesis (Petricka and Nelson, 2007) displays auxin-related phenotypes similar to those of rpl4 mutants. Third, the transcriptional levels of RPL4, and presumably other coexpressed r-proteins, can be modified by direct application of auxins and auxin transport inhibitors in certain tissues. Finally, our general model predicts that alterations in the auxin-mediated ribosomal biogenesis might regulate auxin-related processes not previously reported, for example, the sorting of vacuolar proteins (see below).
Role of Ribosomal Biogenesis in the Vacuolar Sorting of Proteins
rpl4a was initially isolated in a screen for mutants with altered protein sorting to the vacuole, and we hypothesize that the mis-sorting is caused by an imbalance in the regulation of the auxin-mediated ribosomal biogenesis. To illustrate the role of ribosomal biogenesis in vacuolar trafficking in Arabidopsis, we will use a process known to be regulated by auxins and vacuolar trafficking elements, leaf vascular development. Leaf veins are known to form by differentiation of vascular cells from ground meristem cells in a process regulated by the polar flow of auxins (Gälweiler et al., 1998). In this context, the PVC and vacuoles have recently been reported to function in the regulation of the polarized transport and recycling of the auxin efflux carriers PIN1 and PIN2 (Kleine-Vehn et al., 2008; Laxmi et al., 2008; Shirakawa et al., 2009; Spitzer et al., 2009). Several mutants belonging to the PVC-to-vacuole pathway, including vti11 vti12/+, vps9a, and vam3, have been reported to have defects in protein trafficking and vascular network formation (Shirakawa et al., 2009). VPS9A is the homolog of yeast VPS9, which is involved in vacuolar sorting, the vti11 vti12/+ mutant has defects in the sorting of seed storage protein (Goh et al., 2007, Sanmartín et al., 2007), and vam3 belongs to the vacuolar SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) complex and is reported to be localized to the PVC and the vacuolar membrane (Sato et al., 1997; Sanderfoot et al., 1999, 2001). Since we have shown that the delivery of vacuolar proteins is delayed in rpl4a, it is not surprising that the vascular defects observed in mutants with altered PVC-to-vacuole trafficking resemble the phenotypes observed in many r-protein mutants and particularly rpl4. Interestingly, the explanatory models are highly complementary. From the vacuolar trafficking perspective, the defects in protein trafficking caused by the PVC-to-vacuole mutants modify the distribution of auxin. This is achieved by regulating the localization of auxin carriers (PINs), which in turn, determines where procambium cells are located in the leaf primordium (Shirakawa et al., 2009). In our model (Figure 8), auxin-dependent ribosomal biogenesis affects the sorting of vacuolar targeted proteins, as demonstrated by the delayed processing of the vacuolar targeted protein GFP:CTPPBL, the secretion of the RFP:CTPPpha and NTPPpro:RFP markers, and the delayed processing of PIN2 in the rpl4 backgrounds. Thus, in specific tissues, auxin accumulations cause the transcriptional repression of r-proteins, followed by the translational repression of auxin-responsive factors, such as HD-ZIPIII, KANADI, and ARF3/ARF4, regulated by the r-proteins (Nishimura et al., 2005; Pinon et al., 2008; Yao et al., 2008). As a consequence, downstream elements affecting the sorting of vacuolar targeted proteins are repressed, and the vacuolar delivery of the CTPP:RFPpha, NTPPpro:RFP, NTPPBL:GFP, and PIN2:GFP markers is delayed. The delayed delivery of the CTPP:RFPpha and NTPPpro:RFP markers to the vacuole leads to their partial secretion; meanwhile, the delayed processing of PIN2 likely modifies the auxin polar flow required for the formation of the vascular network. In this way, the delayed vacuolar degradation of PIN2, or other PIN proteins, might explain the vascular defects and other auxin-related developmental phenotypes observed in the rpl4 mutants.
Figure 8.
Model: Ribosomal Biogenesis Affects the Sorting of Vacuolar Proteins in a Process Regulated by Auxins.
Local auxin accumulations cause the transcriptional repression of ribosomal proteins (1), followed by the translational repression of auxin-responsive factors (such as HD-ZIPIII, KANADI, and ARF3/ARF4) (2). r-protein mutations mimic the effect of exogenous auxin application by decreasing the auxin-responsive factor synthesis rate (2). Whether the decreased protein synthesis rate acts as a feedback mechanism that represses auxin biosynthetic genes remain unclear (3). The translational repression of auxin-responsive factors slows down the PVC-to-vacuole delivery of vacuolar sorted markers, such as CTPP:RFPpha, NTPPpro:RFP, NTPPBL:GFP, and PIN2:GFP, either directly or through PVC elements, such as VAM3, VPS9A, or VTI11 (4). The delayed delivery of the CTPP:RFPpha and NTPPpro:RFP markers to the vacuole leads to their partial secretion (5), whereas the delayed processing of PIN2:GFP (6) likely modifies the auxin polar flows and causes the array of auxin-related developmental phenotypes observed in rpl4 mutants (7). Black arrows: normal delivery of CTPP:RFPpha, NTPPpro:RFP, and PIN2 to the vacuole in wild-type Arabidopsis. Dashed arrows: effects of local auxin accumulations or rpl4a backgrounds in vacuolar trafficking.
In conclusion, we speculate that auxin-dependent ribosomal biogenesis is an active part of a previously unknown mechanism regulating the proper delivery of vacuolar targeted proteins (such as PIN2) to the vacuole. Using this mechanism, ribosomal biogenesis might regulate auxin fluxes and influence auxin-related developmental processes. Whether this regulation occurs through general mechanisms dependent on protein and lipid synthesis, for example, of membrane sterols (Pan et al., 2009), or through the inhibition of specific PVC elements, such as VPS9A, VAM3, or VTI11, as suggested by the similarities in the vascular defects when compared with rpl4 mutants is still unclear, and it is the focus of our current research.
METHODS
Plant Materials, Insertion Identification, and Growth Conditions
The Arabidopsis thaliana Vac2 T-DNA insertion lines in the Landsberg background used in the screen were a gift from Ray A. Bressan (Koiwa et al., 2006). The mutant screen was performed as described (Sohn et al., 2007), and the DNA flanking the left border of the inserted T-DNA was isolated by thermal asymmetric interlaced-PCR (Liu et al., 1995; Koiwa et al., 2002). The NTPPpro:RFP and RFP:CTPPpha vacuolar markers (Hunter et al., 2007) were a gift from Lorenzo Frigerio. For histochemical analysis of the RPL4pro:GUS expression, the DR5pro:GUS transgenic line (Ulmasov et al., 1997) was used as a control. The Arabidopsis Col-0 T-DNA insertion mutants SALK_130595 (rpl4a-2), SALK_063782 (rpl4a-3), SALK_029203 (rpl4d-1), and SALK_065625 (rpl4d-2) and the control line SALK_069239 were obtained from the ABRC stock center. The amplified fragments in the 21-4 mutant were sequenced, and genetic cosegregation analysis was performed using an F2 population created by backcrossing 21-4 to Vac2. Primer sets used are shown in Supplemental Table 4 online. For growth assays, surface-sterilized and cold-stratified Arabidopsis seeds were sown onto half-strength Murashige and Skoog phytoagar medium (0.5 × Murashige and Skoog salts, 10 g/L sucrose, and 7 g/L phytoagar, pH 5.7). Phytoagar plates containing sterilized seed were incubated in an environmental chamber that was set for long-day lighting conditions (16 h light/8 h dark) and a temperature of 22 ° C. For antibiotic treatments, the appropriate amounts of filter sterilized antibiotic stock solutions were added to cooled autoclaved growth media. The seeds were directly germinated on the antibiotic-containing plates, and the plates were incubated horizontally for shoot analysis or vertically for root elongation assays for up to 21 d.
RNA Isolation and Transcript Analyses
Total RNA was prepared from 2-week-old Col-0, rpl4a-2, rpl4a-3, rpl4d-1, and rpl4d-2 Arabidopsis plantlets grown in sterile conditions using the RNeasy plant mini kit (Qiagen), and cDNA was synthesized with the Superscript III cDNA synthesis kit (Invitrogen) applying anchored-oligo(dT)18 primer with equalized amounts of template RNA. PCR (30, 35, and 40 cycles) were performed with the cDNAs and ExTaq polymerase (TaKaRa), and products were visualized with ethidium bromide staining. For quantitative PCRs, SYBRGreen Supermix (Bio-Rad) was used in reaction volume of 25 μ L. Reactions were run on an iQ5 thermocycler (Bio-Rad). For relative quantification, ratios were calculated according to the method of Pfaffl (2001). All Ct values were the average of three replicates, and all treatments were subject to three biological replicates. Primer sets used are shown in Supplemental Table 4 online.
RPL4pro:GUS Constructs and Histochemical Analysis
To obtain the RPL4A and RPL4D promoter:GUS-fusions, 1.7 and 1.5 kb of the genomic sequence upstream of the RPL4A and RPL4D translation start sites were cloned using the Gateway recombination cloning technology (Invitrogen) into the binary vector PMDC162 (Curtis and Grossniklaus, 2003) and transformed into Agrobacterium tumefaciens strain GV3101. The primer sets used are shown in Supplemental Table 4 online. Wild-type Col-0 plants were transformed using floral dipping, and primary transformants were isolated using hygromycin as a selective marker. Eight independent homozygous lines were isolated for each construct. For hormonal treatments, the transgenic RPL4Apro:GUS and DR5pro:GUS lines were incubated in liquid media supplemented with 50 μ M solutions of each compound for 4 h, and GUS activity was detected in situ as described (Jefferson et al., 1987).
Microscopy
For scanning electron microscopy of adaxial and abaxial leaf surfaces, fresh leaves were mounted on scanning electron microscopy stubs, flash frozen in liquid air, and imaged in a Hitachi TM-1000 scanning electron microscope at an accelerating voltage of 5 kV. Confocal images of water-mounted seedlings were collected using a Leica TCS SP2/UV fitted with × 20 or × 63 water immersion objectives. A 543-nm line from a He-Ne laser was used to excite RFP, and fluorescence was detected in the 560- to 640-nm range. A 488-nm line from an argon laser was used to excite GFP, and fluorescence was detected in the 500- to 530-nm range. For analysis of leaf vasculature, leaf 5 of 28-d-old plants were fixed in 3% glutaraldehyde, dehydrated through an ethanol series to 100% ethanol, and embedded in JB4 resin (Agar Scientific). Embedded tissue was sectioned at 3 μ m and subsequently stained with 0.02% Toluidine Blue. Images were obtained using a Nikon E800 microscope. For transmission electron microscopy, 5-d-old Vac2 and rpl4a-1 root tips were dissected out, transferred to a B-type aluminum planchette (Technotrade International) containing a 150 mM sucrose solution, and frozen with a HPM 100 high-pressure freezer (Leica). The frozen root tips were freeze-substituted in acetone containing 2% OsO4 at −80 ° C for 4 d. After substitution, the sample temperature was slowly warmed up to −20 ° C over 24 h, from −20 to 4 ° C over 12 h, and from 4 ° C to room temperature over 4 h. The root tips were washed three times with anhydrous acetone and embedded in Epon resin (Ted Pella). After polymerization, samples were sliced into 80-nm sections and stained with uranylacetate solution (2% w/v) and subsequently with lead citrate solution (26 g/L lead nitrate and 35g/L sodium citrate). Electron micrographs were captured with a Hitachi TEM H-7000 operated at 80 kV.
Proteomic Analysis
Total protein extracts purified from 2-week-old Arabidopsis seedlings were separated by SDS-PAGE. The gels were blotted onto nitrocellulose, and immunoblot analysis was performed using human RPL4-specific antibodies (Proteintech Group). The protein amounts were normalized using Coomassie Brilliant Blue staining and AntiBiP antibodies (Rose Biotechnology). Protein bands corresponding to the immunoblot signal were excised and prepared as previously described (Carter et al., 2004). Proteotypic peptides ions for RPL4A and RPL4D were selected based on the criteria described by Baerenfaller et al. (2008) for the inclusion list to perform targeted data-dependent acquisition analysis using a nanoLC/MS/MS system with combination of the nano-Acquity UPLC and Q-TOF Premier (Waters). After peptide sequences were confirmed by the tandem mass spectra, the relative abundance of RPL4A and RPL4D in different backgrounds was evaluated using LC/MS quantitative analysis as previously described (Rojo et al., 2004).
Transient Expression Analysis and in Vivo Translational Assays in Protoplasts
Protoplasts were isolated from 2-week-old seedlings grown on B5 agar media, and the preparations were transformed with 10 μ g of the BiP:GFP (Kim et al., 2001), RbcS:GFP (Lee et al., 2002), AALP:GFP (Sohn et al., 2003), and GFP constructs using a polyethylene glycol–mediated procedure previously described (Jin et al., 2001). Equal transformation efficiencies were ensured measuring the ratio transformed:nontransformed protoplast using confocal microscopy. For the streptomycin-treated protoplasts, the GFP protein was expressed in protoplasts for 6 h, followed by a treatment with 0 and 600 μ g/mL of antibiotic for 12 additional hours. Protein extracts from protoplasts derived from the different assays were prepared at different time points after transformation as described (Sohn et al., 2003). Briefly, protoplast incubation media was collected after centrifugation at 6000g for 5 min. One milligram of BSA was added to the media, which was then precipitated with 10% trichloroacetic acid and followed by centrifugation at 10,000g for 5 min. Precipitated protein aggregates were dissolved in 0.1 M NaOH. Prior to the immunoblot analysis, the protoplast preparations were normalized measuring total protein contents. Immunoblots were developed using a Living Colors GFP Monoclonal antibody (Clontech) and an ECL detection kit (Pierce Biotechnology).
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: RPL4A, locus At3g09630 cDNA, NM_001035586; protein, NP_001030663; RPL4D, locus At5g02870 cDNA NM_001125687; protein, NP_001119159. T-DNA insertion lines SALK_130595 (rpl4a-2), SALK_063782 (rpl4a-3), SALK_029203 (rpl4d-1), SALK_065625 (rpl4d-2), and SALK_069239 (negative control for the antibiotic assays).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. The Secretion of the Vacuolar Markers in rpl4a-1 Depends on Their Expression Levels and Developmental Stage.
Supplemental Figure 2. Cellular Localization of Auxin Transporters in rpl4a-1.
Supplemental Figure 3. The rpl4a and rpl4d Mutants Display Similar Developmental Phenotypes.
Supplemental Figure 4. Histochemical Analysis of RPL4pro:GUS Transgenic Lines.
Supplemental Figure 5. Double Heterozygous rpl4a-rpl4d Plants Have Similar Phenotypes as the Single Mutants.
Supplemental Figure 6. The rpl4a Mutation Does Not Cause Enhanced Unfolded Protein Response or Translational Defects in Vacuolar Trafficking Machinery.
Supplemental Table 1. Genetic Linkage Analysis Indicates That the 21-4 Phenotypes and the Protein Trafficking Defects Are Linked to the Mutation in RPL4A.
Supplemental Table 2. Auxin-Related Phenotypes in Arabidopsis Mutants with Altered Ribosome Biogenesis.
Supplemental Table 3. Specificity of the Antibiotic Response in rpl4 Mutants.
Supplemental Table 4. Primers Used in This Study.
Supplementary Material
Acknowledgments
We thank Glenn Hicks, Marci Surpin, Julia Bailey-Serres, and Patty Springer (University of California, Riverside) for their critical reading of the manuscript. We also thank Mien Van De Ven, April Agee, Latasha Johnson, and Alexander Michkov for technical assistance, L. Frigerio (University of Warwick, UK) for the gift of the NTPPpro:RFP and the RFP:CTPPpha marker lines, and I. Hwang (Pohang University, Korea) for the gift of the BiP:GFP, GFP, RbcS:GFP, and AALP:GFP constructs. We thank the anonymous reviewers for their useful comments and suggestions. This work was funded by Department of Energy, Division of Energy Biosciences, Grant DE-FG03-02ER15295/A000 (to N.V.R.) and by the Spanish Fulbright postdoctoral fellowship MEC-FU-0248-2006 (to A.R.).
References
- Ahmed S., Rojo E., Kovaleva V., Venkataraman S., Dombrowski J., Matsuoka K., Raikhel N. (2000). The plant vacuolar sorting receptor AtELP is involved in transport of NH(2)-terminal propeptide-containing vacuolar proteins in Arabidopsis thaliana. J. Cell Biol. 149: 1335–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baerenfaller K., Grossmann J., Grobei M., Hull R., Hirsch-Hoffmann M., Yalovsky S., Zimmermann P., Grossniklaus U., Gruissem W., Baginsky S. (2008). Genome-scale proteomics reveals Arabidopsis thaliana gene models and proteome dynamics. Science 320: 938–941 [DOI] [PubMed] [Google Scholar]
- Ban N., Nissen P., Hansen J., Moore P., Steitz T. (2000). The complete atomic structure of the large ribosomal subunit at 2.4 A resolution. Science 289: 905–920 [DOI] [PubMed] [Google Scholar]
- Barakat A., Szick-Miranda K., Chang I., Guyot R., Blanc G., Cooke R., Delseny M., Bailey-Serres J. (2001). The organization of cytoplasmic ribosomal protein genes in the Arabidopsis genome. Plant Physiol. 127: 398–415 [PMC free article] [PubMed] [Google Scholar]
- Bassham D., Raikhel N. (2000). Unique features of the plant vacuolar sorting machinery. Curr. Opin. Cell Biol. 12: 491–495 [DOI] [PubMed] [Google Scholar]
- Carroll A., Heazlewood J., Ito J., Millar A. (2008). Analysis of the Arabidopsis cytosolic ribosome proteome provides detailed insights into its components and their post-translational modification. Mol. Cell. Proteomics 7: 347–369 [DOI] [PubMed] [Google Scholar]
- Carter C., Pan S., Zouhar J., Avila E., Girke T., Raikhel N. (2004). The vegetative vacuole proteome of Arabidopsis thaliana reveals predicted and unexpected proteins. Plant Cell 16: 3285–3303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang I., Szick-Miranda K., Pan S., Bailey-Serres J. (2005). Proteomic characterization of evolutionarily conserved and variable proteins of Arabidopsis cytosolic ribosomes. Plant Physiol. 137: 848–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock C., Hunter P., Szakacs E., Hinz G., Robinson D., Frigerio L. (2008). Lack of a vacuolar sorting receptor leads to non-specific missorting of soluble vacuolar proteins in Arabidopsis seeds. Traffic 9: 408–416 [DOI] [PubMed] [Google Scholar]
- Curtis M., Grossniklaus U. (2003). A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol. 133: 462–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt R., Bonham-Smith P. (2008). Arabidopsis ribosomal proteins RPL23aA and RPL23aB are differentially targeted to the nucleolus and are desperately required for normal development. Plant Physiol. 147: 128–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gälweiler L., Guan C., Müller A., Wisman E., Mendgen K., Yephremov A., Palme K. (1998). Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282: 2226–2230 [DOI] [PubMed] [Google Scholar]
- Giavalisco P., Wilson D., Kreitler T., Lehrach H., Klose J., Gobom J., Fucini P. (2005). High heterogeneity within the ribosomal proteins of the Arabidopsis thaliana 80S ribosome. Plant Mol. Biol. 57: 577–591 [DOI] [PubMed] [Google Scholar]
- Goh T., Uchida W., Arakawa S., Ito E., Dainobu T., Ebine K., Takeuchi M., Sato K., Ueda T., Nakano A. (2007). VPS9a, the common activator for two distinct types of Rab5 GTPases, is essential for the development of Arabidopsis thaliana. Plant Cell 19: 3504–3515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter P., Craddock C., Di Benedetto S., Roberts L., Frigerio L. (2007). Fluorescent reporter proteins for the tonoplast and the vacuolar lumen identify a single vacuolar compartment in Arabidopsis cells. Plant Physiol. 145: 1371–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T., Kim G., Shinozaki K. (2000). Disruption of an Arabidopsis cytoplasmic ribosomal protein S13-homologous gene by transposon-mediated mutagenesis causes aberrant growth and development. Plant J. 22: 257–264 [DOI] [PubMed] [Google Scholar]
- Jefferson R., Kavanagh T., Bevan M. (1987). GUS fusions: Beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6: 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J., Kim Y., Kim S., Lee S., Kim D., Cheong G., Hwang I. (2001). A new dynamin-like protein, ADL6, is involved in trafficking from the trans-Golgi network to the central vacuole in Arabidopsis. Plant Cell 13: 1511–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine-Vehn J., Leitner J., Zwiewka M., Sauer M., Abas L., Luschnig C., Friml J. (2008). Differential degradation of PIN2 auxin efflux carrier by retromer-dependent vacuolar targeting. Proc. Natl. Acad. Sci. USA 105: 17812–17817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Eu Y., Yoo C., Kim Y., Pih K., Jin J., Kim S., Stenmark H., Hwang I. (2001). Trafficking of phosphatidylinositol 3-phosphate from the trans-Golgi network to the lumen of the central vacuole in plant cells. Plant Cell 13: 287–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koiwa H., et al. (2002). C-terminal domain phosphatase-like family members (AtCPLs) differentially regulate Arabidopsis thaliana abiotic stress signaling, growth, and development. Proc. Natl. Acad. Sci. USA 99: 10893–10898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koiwa H., Bressan R., Hasegawa P. (2006). Identification of plant stress-responsive determinants in Arabidopsis by large-scale forward genetic screens. J. Exp. Bot. 57: 1119–1128 [DOI] [PubMed] [Google Scholar]
- Komili S., Farny N., Roth F., Silver P. (2007). Functional specificity among ribosomal proteins regulates gene expression. Cell 131: 557–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxmi A., Pan J., Morsy M., Chen R. (2008). Light plays an essential role in intracellular distribution of auxin efflux carrier PIN2 in Arabidopsis thaliana. PLoS One 3: e1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Mitsukawa N., Oosumi T., Whittier R. (1995). Efficient isolation and mapping of Arabidopsis thaliana T-DNA insert junctions by thermal asymmetric interlaced PCR. Plant J. 8: 457–463 [DOI] [PubMed] [Google Scholar]
- Lee M., Min M., Lee Y., Jin J., Shin D., Kim D., Lee K., Hwang I. (2002). ADP-ribosylation factor 1 of Arabidopsis plays a critical role in intracellular trafficking and maintenance of endoplasmic reticulum morphology in Arabidopsis. Plant Physiol. 129: 1507–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka K., Nakamura K. (1999). Large alkyl side-chains of isoleucine and leucine in the NPIRL region constitute the core of the vacuolar sorting determinant of sporamin precursor. Plant Mol. Biol. 41: 825–835 [DOI] [PubMed] [Google Scholar]
- Miao Y., Li K., Li H., Yao X., Jiang L. (2008). The vacuolar transport of aleurain-GFP and 2S albumin-GFP fusions is mediated by the same pre-vacuolar compartments in tobacco BY-2 and Arabidopsis suspension cultured cells. Plant J. 56: 824–839 [DOI] [PubMed] [Google Scholar]
- Nishimura T., Wada T., Yamamoto K., Okada K. (2005). The Arabidopsis STV1 protein, responsible for translation reinitiation, is required for auxin-mediated gynoecium patterning. Plant Cell 17: 2940–2953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor M., Gregory S., Dahlberg A. (2004). Multiple defects in translation associated with altered ribosomal protein L4. Nucleic Acids Res. 32: 5750–5756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleinick N. (1977). Initiation and elongation of protein synthesis in growing cells: Differential inhibition by cycloheximide and emetine. Arch. Biochem. Biophys. 182: 171–180 [DOI] [PubMed] [Google Scholar]
- Paciorek T., Zazímalová E., Ruthardt N., Petrásek J., Stierhof Y., Kleine-Vehn J., Morris D., Emans N., Jürgens G., Geldner N., Friml J. (2005). Auxin inhibits endocytosis and promotes its own efflux from cells. Nature 435: 1251–1256 [DOI] [PubMed] [Google Scholar]
- Pan J., Fujioka S., Peng J., Chen J., Li G., Chen R. (2009). The E3 ubiquitin ligase SCFTIR1/AFB and membrane sterols play key roles in auxin regulation of endocytosis, recycling, and plasma membrane accumulation of the auxin efflux transporter PIN2 in Arabidopsis thaliana. Plant Cell 21: 568–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petricka J., Nelson T. (2007). Arabidopsis nucleolin affects plant development and patterning. Plant Physiol. 144: 173–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl M. (2001). A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinon V., Etchells J., Rossignol P., Collier S., Arroyo J., Martienssen R., Byrne M. (2008). Three PIGGYBACK genes that specifically influence leaf patterning encode ribosomal proteins. Development 135: 1315–1324 [DOI] [PubMed] [Google Scholar]
- Robinson D., Oliviusson P., Hinz G. (2005). Protein sorting to the storage vacuoles of plants: A critical appraisal. Traffic 6: 615–625 [DOI] [PubMed] [Google Scholar]
- Rojo E., Martín R., Carter C., Zouhar J., Pan S., Plotnikova J., Jin H., Paneque M., Sánchez-Serrano J., Baker B., Ausubel F., Raikhel N. (2004). VPEgamma exhibits a caspase-like activity that contributes to defense against pathogens. Curr. Biol. 14: 1897–1906 [DOI] [PubMed] [Google Scholar]
- Rojo E., Sharma V., Kovaleva V., Raikhel N., Fletcher J. (2002). CLV3 is localized to the extracellular space, where it activates the Arabidopsis CLAVATA stem cell signaling pathway. Plant Cell 14: 969–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderfoot A., Kovaleva V., Bassham D., Raikhel N. (2001). Interactions between syntaxins identify at least five SNARE complexes within the Golgi/prevacuolar system of the Arabidopsis cell. Mol. Biol. Cell 12: 3733–3743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderfoot A., Kovaleva V., Zheng H., Raikhel N. (1999). The t-SNARE AtVAM3p resides on the prevacuolar compartment in Arabidopsis root cells. Plant Physiol. 121: 929–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanmartín M., Ordóñez A., Sohn E., Robert S., Sánchez-Serrano J., Surpin M., Raikhel N., Rojo E. (2007). Divergent functions of VTI12 and VTI11 in trafficking to storage and lytic vacuoles in Arabidopsis. Proc. Natl. Acad. Sci. USA 104: 3645–3650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M., Nakamura N., Ohsumi Y., Kouchi H., Kondo M., Hara-Nishimura I., Nishimura M., Wada Y. (1997). The AtVAM3 encodes a syntaxin-related molecule implicated in the vacuolar assembly in Arabidopsis thaliana. J. Biol. Chem. 272: 24530–24535 [DOI] [PubMed] [Google Scholar]
- Shirakawa M., Ueda H., Shimada T., Nishiyama C., Hara-Nishimura I. (2009). Vacuolar SNAREs function in the formation of the leaf vascular network by regulating auxin distribution. Plant Cell Physiol. 50: 1319–1328 [DOI] [PubMed] [Google Scholar]
- Sohn E., Kim E., Zhao M., Kim S., Kim H., Kim Y., Lee Y., Hillmer S., Sohn U., Jiang L., Hwang I. (2003). Rha1, an Arabidopsis Rab5 homolog, plays a critical role in the vacuolar trafficking of soluble cargo proteins. Plant Cell 15: 1057–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn E., Rojas-Pierce M., Pan S., Carter C., Serrano-Mislata A., Madueño F., Rojo E., Surpin M., Raikhel N. (2007). The shoot meristem identity gene TFL1 is involved in flower development and trafficking to the protein storage vacuole. Proc. Natl. Acad. Sci. USA 104: 18801–18806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer C., Reyes F., Buono R., Sliwinski M., Haas T., Otegui M. (2009). The ESCRT-related CHMP1A and B proteins mediate multivesicular body sorting of auxin carriers in Arabidopsis and are required for plant development. Plant Cell 21: 749–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surpin M., Raikhel N. (2004). Traffic jams affect plant development and signal transduction. Nat. Rev. Mol. Cell Biol. 5: 100–109 [DOI] [PubMed] [Google Scholar]
- Teale W., Paponov I., Palme K. (2006). Auxin in action: Signalling, transport and the control of plant growth and development. Nat. Rev. Mol. Cell Biol. 7: 847–859 [DOI] [PubMed] [Google Scholar]
- Ulmasov T., Murfett J., Hagen G., Guilfoyle T. (1997). Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9: 1963–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lijsebettens M., Vanderhaeghen R., De Block M., Bauw G., Villarroel R., Van Montagu M. (1994). An S18 ribosomal protein gene copy at the Arabidopsis PFL locus affects plant development by its specific expression in meristems. EMBO J. 13: 3378–3388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale A., Raikhel N. (1999). What do proteins need to reach different vacuoles?. Trends Plant Sci. 4: 149–155 [DOI] [PubMed] [Google Scholar]
- Volarevic S., Stewart M., Ledermann B., Zilberman F., Terracciano L., Montini E., Grompe M., Kozma S., Thomas G. (2000). Proliferation, but not growth, blocked by conditional deletion of 40S ribosomal protein S6. Science 288: 2045–2047 [DOI] [PubMed] [Google Scholar]
- Weijers D., Franke-van Dijk M., Vencken R., Quint A., Hooykaas P., Offringa R. (2001). An Arabidopsis Minute-like phenotype caused by a semi-dominant mutation in a RIBOSOMAL PROTEIN S5 gene. Development 128: 4289–4299 [DOI] [PubMed] [Google Scholar]
- Wool I. (1996). Extraribosomal functions of ribosomal proteins. Trends Biochem. Sci. 21: 164–165 [PubMed] [Google Scholar]
- Yao Y., Ling Q., Wang H., Huang H. (2008). Ribosomal proteins promote leaf adaxial identity. Development 135: 1325–1334 [DOI] [PubMed] [Google Scholar]
- Zheng H., von Mollard G., Kovaleva V., Stevens T., Raikhel N. (1999). The plant vesicle-associated SNARE AtVTI1a likely mediates vesicle transport from the trans-Golgi network to the prevacuolar compartment. Mol. Biol. Cell 10: 2251–2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouhar J., Rojo E., Bassham D. (2009). AtVPS45 is a positive regulator of the SYP41/SYP61/VTI12 SNARE complex involved in trafficking of vacuolar cargo. Plant Physiol. 149: 1668–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.