Abstract
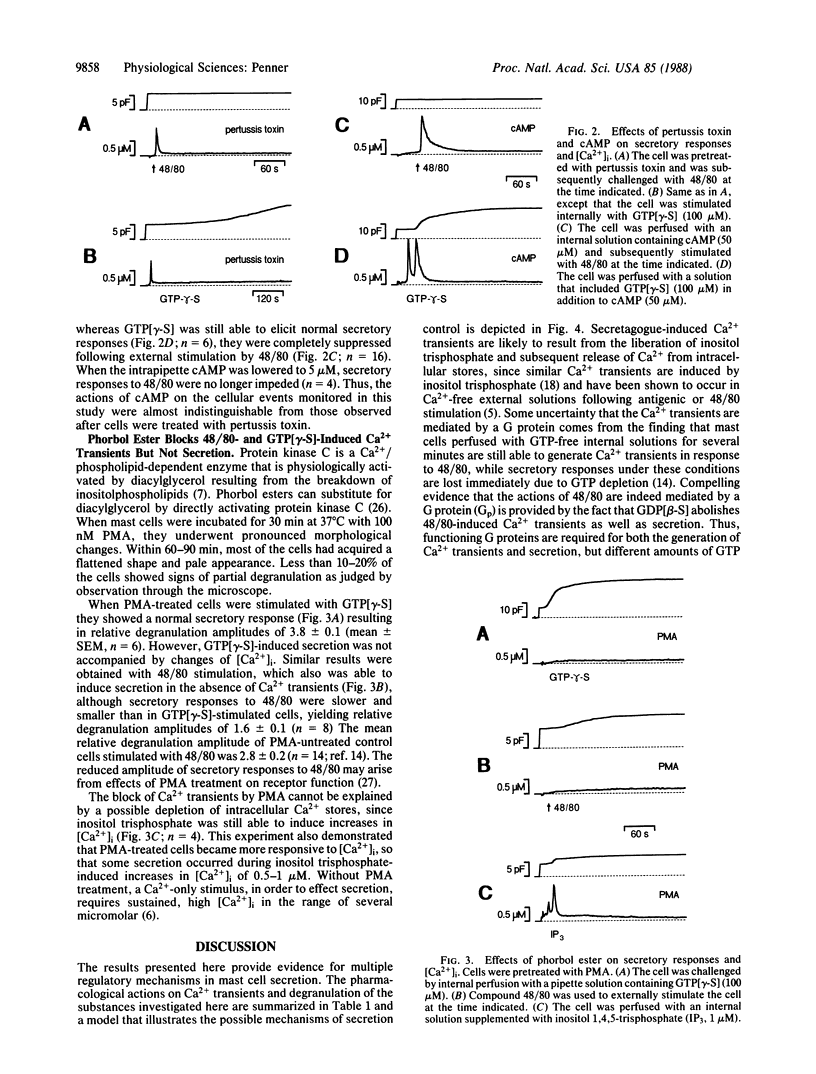
Fura-2 and membrane capacitance measurements were performed to investigate intracellular Ca2+ concentration [( Ca2+]i) and secretory responses of rat peritoneal mast cells following secretagogue stimulation. Compound 48/80 and internally applied guanosine 5'-[gamma-thio]triphosphate (GTP[gamma-S]) induced transient rises in [Ca2+]i and caused membrane capacitance increases as secretion occurred. The 48/80-induced Ca2+ transients and secretory responses were blocked by guanosine 5'-[beta-thio]diphosphate and neomycin, indicating that inositolphospholipid breakdown mediated by guanine nucleotide-binding regulatory protein (G protein) plays an important role in stimulus-secretion coupling. However, pertussis toxin did not block Ca2+ transients induced by 48/80 or GTP[gamma-S], whereas secretory responses were either abolished (48/80) or developed only after a considerable delay (GTP[gamma-S]). Similar effects were obtained by perfusing cells with cAMP: (i) Ca2+ transients following stimulation with 48/80 remained unaffected by cAMP, but secretory responses were abolished; (ii) GTP[gamma-S] induced normal Ca2+ transients and degranulation in the presence of cAMP. Pretreatment of mast cells with phorbol 12-myristate 13-acetate (PMA) abolished 48/80- and GTP[gamma-S]-induced Ca2+ transients (but not inositol trisphosphate-induced Ca2+ transients), whereas secretion still occurred. At the same time, the Ca2+ requirement for secretion was reduced by PMA. These results indicate that secretion in mast cells is under control of an as yet unidentified signaling pathway that involves a G protein. This pathway is distinct from inositolphospholipid turnover and may provide the triggering mechanism for secretion, whereas the inositolphospholipid pathway serves to increase [Ca2+]i and renders the secretory process more sensitive to [Ca2+]i by activating protein kinase C. Persistent activation of protein kinase C through phorbol ester imposes negative feedback control on the inositolphospholipid pathway, whereas cAMP may inhibit the unidentified signaling pathway.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alm E., Bloom G. D. Cyclic nucleotide involvement in histamine release from mast cells--a reevaluation. Life Sci. 1982 Jan 18;30(3):213–218. doi: 10.1016/0024-3205(82)90501-x. [DOI] [PubMed] [Google Scholar]
- Axelrod J., Burch R. M., Jelsema C. L. Receptor-mediated activation of phospholipase A2 via GTP-binding proteins: arachidonic acid and its metabolites as second messengers. Trends Neurosci. 1988 Mar;11(3):117–123. doi: 10.1016/0166-2236(88)90157-9. [DOI] [PubMed] [Google Scholar]
- Barrowman M. M., Cockcroft S., Gomperts B. D. Two roles for guanine nucleotides in the stimulus-secretion sequence of neutrophils. Nature. 1986 Feb 6;319(6053):504–507. doi: 10.1038/319504a0. [DOI] [PubMed] [Google Scholar]
- Beaven M. A., Guthrie D. F., Moore J. P., Smith G. A., Hesketh T. R., Metcalfe J. C. Synergistic signals in the mechanism of antigen-induced exocytosis in 2H3 cells: evidence for an unidentified signal required for histamine release. J Cell Biol. 1987 Sep;105(3):1129–1136. doi: 10.1083/jcb.105.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. Role of guanine nucleotide binding protein in the activation of polyphosphoinositide phosphodiesterase. Nature. 1985 Apr 11;314(6011):534–536. doi: 10.1038/314534a0. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Howell T. W., Gomperts B. D. Two G-proteins act in series to control stimulus-secretion coupling in mast cells: use of neomycin to distinguish between G-proteins controlling polyphosphoinositide phosphodiesterase and exocytosis. J Cell Biol. 1987 Dec;105(6 Pt 1):2745–2750. doi: 10.1083/jcb.105.6.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez J. M., Lindau M., Eckstein F. Intracellular stimulation of mast cells with guanine nucleotides mimic antigenic stimulation. FEBS Lett. 1987 May 25;216(1):89–93. doi: 10.1016/0014-5793(87)80762-7. [DOI] [PubMed] [Google Scholar]
- Fernandez J. M., Neher E., Gomperts B. D. Capacitance measurements reveal stepwise fusion events in degranulating mast cells. 1984 Nov 29-Dec 5Nature. 312(5993):453–455. doi: 10.1038/312453a0. [DOI] [PubMed] [Google Scholar]
- Foreman J. C., Hallett M. B., Mongar J. L. The relationship between histamine secretion and 45calcium uptake by mast cells. J Physiol. 1977 Sep;271(1):193–214. doi: 10.1113/jphysiol.1977.sp011996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Heiman A. S., Crews F. T. Characterization of the effects of phorbol esters on rat mast cell secretion. J Immunol. 1985 Jan;134(1):548–555. [PubMed] [Google Scholar]
- Hepler J. R., Earp H. S., Harden T. K. Long-term phorbol ester treatment down-regulates protein kinase C and sensitizes the phosphoinositide signaling pathway to hormone and growth factor stimulation. Evidence for a role of protein kinase C in agonist-induced desensitization. J Biol Chem. 1988 Jun 5;263(16):7610–7619. [PubMed] [Google Scholar]
- Herrmann E., Jakobs K. H. Stimulation and inhibition of human platelet membrane high-affinity GTPase by neomycin. FEBS Lett. 1988 Feb 29;229(1):49–53. doi: 10.1016/0014-5793(88)80795-6. [DOI] [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K. Activation of mast cells for mediator release through IgE receptors. Prog Allergy. 1984;34:188–235. [PubMed] [Google Scholar]
- Jakobs K. H., Aktories K., Schultz G. Mechanism of pertussis toxin action on the adenylate cyclase system. Inhibition of the turn-on reaction of the inhibitory regulatory site. Eur J Biochem. 1984 Apr 2;140(1):177–181. doi: 10.1111/j.1432-1033.1984.tb08083.x. [DOI] [PubMed] [Google Scholar]
- Katakami Y., Kaibuchi K., Sawamura M., Takai Y., Nishizuka Y. Synergistic action of protein kinase C and calcium for histamine release from rat peritoneal mast cells. Biochem Biophys Res Commun. 1984 Jun 15;121(2):573–578. doi: 10.1016/0006-291x(84)90220-1. [DOI] [PubMed] [Google Scholar]
- Lindau M., Fernandez J. M. IgE-mediated degranulation of mast cells does not require opening of ion channels. Nature. 1986 Jan 9;319(6049):150–153. doi: 10.1038/319150a0. [DOI] [PubMed] [Google Scholar]
- Lindau M., Neher E. Patch-clamp techniques for time-resolved capacitance measurements in single cells. Pflugers Arch. 1988 Feb;411(2):137–146. doi: 10.1007/BF00582306. [DOI] [PubMed] [Google Scholar]
- Lindau M., Nüsse O. Pertussis toxin does not affect the time course of exocytosis in mast cells stimulated by intracellular application of GTP-gamma-S. FEBS Lett. 1987 Oct 5;222(2):317–321. doi: 10.1016/0014-5793(87)80393-9. [DOI] [PubMed] [Google Scholar]
- Metzger H., Alcaraz G., Hohman R., Kinet J. P., Pribluda V., Quarto R. The receptor with high affinity for immunoglobulin E. Annu Rev Immunol. 1986;4:419–470. doi: 10.1146/annurev.iy.04.040186.002223. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Ui M. Simultaneous inhibitions of inositol phospholipid breakdown, arachidonic acid release, and histamine secretion in mast cells by islet-activating protein, pertussis toxin. A possible involvement of the toxin-specific substrate in the Ca2+-mobilizing receptor-mediated biosignaling system. J Biol Chem. 1985 Mar 25;260(6):3584–3593. [PubMed] [Google Scholar]
- Neher E., Almers W. Fast calcium transients in rat peritoneal mast cells are not sufficient to trigger exocytosis. EMBO J. 1986 Jan;5(1):51–53. doi: 10.1002/j.1460-2075.1986.tb04176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. The influence of intracellular calcium concentration on degranulation of dialysed mast cells from rat peritoneum. J Physiol. 1988 Jan;395:193–214. doi: 10.1113/jphysiol.1988.sp016914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Orellana S. A., Solski P. A., Brown J. H. Phorbol ester inhibits phosphoinositide hydrolysis and calcium mobilization in cultured astrocytoma cells. J Biol Chem. 1985 May 10;260(9):5236–5239. [PubMed] [Google Scholar]
- Penner R., Matthews G., Neher E. Regulation of calcium influx by second messengers in rat mast cells. Nature. 1988 Aug 11;334(6182):499–504. doi: 10.1038/334499a0. [DOI] [PubMed] [Google Scholar]
- Penner R., Neher E. Secretory responses of rat peritoneal mast cells to high intracellular calcium. FEBS Lett. 1988 Jan 4;226(2):307–313. doi: 10.1016/0014-5793(88)81445-5. [DOI] [PubMed] [Google Scholar]
- Penner R., Pusch M., Neher E. Washout phenomena in dialyzed mast cells allow discrimination of different steps in stimulus-secretion coupling. Biosci Rep. 1987 Apr;7(4):313–321. doi: 10.1007/BF01121453. [DOI] [PubMed] [Google Scholar]
- Sagi-Eisenberg R., Foreman J. C., Shelly R. Histamine release induced by histone and phorbol ester from rat peritoneal mast cells. Eur J Pharmacol. 1985 Jul 11;113(1):11–17. doi: 10.1016/0014-2999(85)90337-1. [DOI] [PubMed] [Google Scholar]
- Sagi-Eisenberg R., Lieman H., Pecht I. Protein kinase C regulation of the receptor-coupled calcium signal in histamine-secreting rat basophilic leukaemia cells. Nature. 1985 Jan 3;313(5997):59–60. doi: 10.1038/313059a0. [DOI] [PubMed] [Google Scholar]
- Sullivan T. J., Parker C. W. Possible role of arachidonic acid and its metabolites in mediator release from rat mast cells. J Immunol. 1979 Feb;122(2):431–436. [PubMed] [Google Scholar]
- Tasaka K., Mio M., Okamoto M. Intracellular calcium release induced by histamine releasers and its inhibition by some antiallergic drugs. Ann Allergy. 1986 Jun;56(6):464–469. [PubMed] [Google Scholar]
- Watson S. P., Lapetina E. G. 1,2-Diacylglycerol and phorbol ester inhibit agonist-induced formation of inositol phosphates in human platelets: possible implications for negative feedback regulation of inositol phospholipid hydrolysis. Proc Natl Acad Sci U S A. 1985 May;82(9):2623–2626. doi: 10.1073/pnas.82.9.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow C. M., Austen K. F. Role of cyclic nucleotides in the activation-secretion response. Prog Allergy. 1984;34:236–270. [PubMed] [Google Scholar]
- Yamada K., Okano Y., Miura K., Nozawa Y. A major role for phospholipase A2 in antigen-induced arachidonic acid release in rat mast cells. Biochem J. 1987 Oct 1;247(1):95–99. doi: 10.1042/bj2470095. [DOI] [PMC free article] [PubMed] [Google Scholar]


