Abstract
Maternal separation during early life is an established chronic behavioral model of early life stress in rats. It is known that perinatal adverse environments increase activity of the renin-angiotensin system, specifically angiotensin II (ang II), in adulthood. The aim of this study was to investigate whether the effects of early life stress augments the sensitivity of the ang II pathway. Using Wistar Kyoto rats, the maternal separation (MS) protocol was performed separating approximately half of the male pups from their mother 3 hs/day from day 2–14 of life. Pups remaining with the mother at all times were utilized as controls. Maternal separation did not influence the plasma basal parameters such blood glucose, insulin, ang II, ang 1–7 and PRA. Furthermore, body weight, blood pressure and heart rate were similar in MS and control rats. The acute pressor response to ang II was not different in anesthetized MS and control rats. However, the chronic infusion of ang II (65 ng/day, s.c.) elicited an exaggerated hypertensive response in MS compared to control rats (p<0.05). Surprisingly, HR was dramatically increased during the second week of ang II infusion in MS compared with control rats (p<0.05). This enhanced ang II sensitivity was accompanied by a greater vascular inflammatory response in MS vs. control rats. Chronic ang II-infusion increased vascular wall structure in both groups similarly. These data indicate that early life stress sensitizes rats to an increased hemodynamic and inflammatory response during ang II-induced hypertension.
Keywords: maternal separation, ang II-induced hypertension, vascular inflammation
Introduction
Traditionally, cardiovascular and metabolic disorders are thought to result from lifestyle risk factors acting upon a certain genetic background. More recently, investigators have described a phenomenon whereby adverse stimuli affect the fetal environment altering the development of organs or tissues increasing the susceptibility to disease later in life, known as “early” or “fetal” origins of adult disease (1–3). In humans, adverse experiences in early childhood create and/or interact with genetic-based vulnerabilities which are correlated with a greater incidence of endocrine, cardiovascular and metabolic disease in adulthood (4–6). In animal models, it is well accepted that environmental factors during development influence the risk for multiple forms of chronic adult diseases such as depression, diabetes, and heart disease (7–9).
The renin-angiotensin system (RAS) is an important regulator of arterial pressure and body fluid balance through both the systemic and the intra-renal actions of angiotensin II (ang II) (10). Excessive activation of the RAS also stimulates the sympathetic nervous system as well as mediating vascular hypertrophy and pro-inflammatory pathways. These ang II-dependent mechanistic pathways are known to contribute towards cardiovascular dysfunction, especially hypertension, in adults. Animal models of fetal programming, such as maternal low protein diet or early gestational exposure to glucocorticoid, lead to alterations in RAS activity and adult onset of hypertension and renal dysfunction (11,12).
Maternal separation is a widely used animal model of chronic behavioral stress during early life (13,14). Variations in maternal care during early life are considered a stressful experience associated with alterations in the endocrine response (15,16). Male rat pups separated from their mothers daily for a prolonged period (180 min) show, as adults, abnormal anxiety-related behavior and exaggerated endocrine responses to an acute stress; whereas females display normal or minimally-exaggerated responses (14,17,18). In a study of over 17,000 adults, early life stress or adverse childhood events is reported to be highly correlative with ischemic heart disease in adulthood more so than the traditional risk factors of smoking, obesity, and hypertension (4). Despite these dramatic observations, very little research has been conducted to determine whether early life behavioral stress leads to cardiovascular dysfunction and/or activation of the RAS system. We hypothesized that early life stress may activate the RAS signaling pathway leading to cardiovascular dysfunction in adults. This study was designed to 1) investigate whether the rat model of early life stress, maternal separation, influences basal parameters of cardiovascular function such as blood pressure, heart rate, vascular morphology and vascular inflammation in male rats; and, 2) elucidate whether maternal separation sensitizes male rats to ang II-induced cardiovascular dysfunction by monitoring the same parameters.
Methods
Early life stress: maternal separation protocol
Close to delivery, pregnant Wistar Kyoto rats (WKY) were carefully observed to determine the exact day of birth (postnatal day 0). Approximately half of the male pups from each litter were removed from the mother’s cage and their tails were snipped and cauterized with silver nitrate for identification as “maternally separated” (MS). From postnatal days 2 to 14 of life, MS pups were separated from their mothers and littermates, at the same time of day by transferring the pups to a clean cage in an incubator (30±1° C) for 3 hours. The control group (C) consisted of counterparts that remained with their mother and were non-handled. Routinely, each breeding cycle includes 5 breeding pairs at one time. When the 5 female rats deliver their pups, it generated a total number of 18–24 male pups. We subjected approximately half of these pups to maternal separation, thus, we consistently obtained one group of control rats (n=9–12) along with a group of separated rats (n=9–12). Subsequent treatment groups are divided among breeding pairs as well as across breeding cycles. Therefore, we included rats from at least 3 different litters in each experimental group. Weaning was performed at postnatal day 28. Body weights were determined every 2 days on post natal days 2–14 and weekly until 12 weeks old.
Plasma assays
Plasma glucose, insulin, plasma renin activity (PRA), ang II, and ang (1–7) peptide concentrations were assessed in baseline conditions. Additional methods are provided in the online Data Supplement (http://hyper.ahajournals.org).
Whole animal pressor responses
Rats were anesthetized with thiobutabarbital (inactin; 65 mg/kg, i.p.) and the right femoral artery and vein were isolated and cannulated with PE-50 for monitoring MAP and drug infusion, respectively. Rats were allowed to equilibrate for 40 min and ang II (Phoenix Pharmaceutical Inc., Burlingame, CA) was given as an i.v. bolus (0.04, 0.08, 0.16 and 0.32 μg/kg) in randomized order. After a 30-minute recovery period, animals were given chlorisondamine (chlor, 5 mg/kg, i.v.). MAP and HR were allowed to return to baseline between each dose. All measurements were recorded using a computer-based data acquisition system (PowerLab, ADInstruments, Inc., CO).
Osmotic mini-pump and telemetry transmitter implantation
Rats were implanted with telemetry transmitters at 9–10 weeks old (Data Sciences, Inc., St. Paul, MN) as previously described (19). Minipumpscontaining ang II (65 ng/min infusion rate) were implanted according to the manufacturer’s instructions. See details in http://hyper.ahajournals.org.
Aortic tissue morphology and immunohistochemical analysis
Aortic rings were collected in 10% phosphate-buffered formalin, embedded in paraffin, and sectioned at a thickness of 4 μm onto Superfrost plus slides. CD68 antibody was used for staining monocytes/macrophages and anti-CD3 antibody for T-cell staining (20). The number of CD68 and CD3-positive cells was counted in a blinded manner. Sections were stained with hematoxylin and eosin (Surgipath Medical Industries, Leica, IL). Aorta thickness, external and internal diameter and wall area were determined by software analysis (Meta Morph). See details in http://hyper.ahajournals.org.
Statistical analysis
Telemetry data are presented as the mean value of 12 or 24 hours and 95% confidence interval. For statistical analysis, 72 values for every 12 h (1 value at each 10 min) for each rat were computed. Comparisons of changes in MAP, HR and locomotor activity, basal parameters and aortic tissue morphology or inflammatory cell counts were made by unpaired Student’s t-test (Prism 5.01, GraphPad Software, 2007). A value of p < 0.05 was considered statistically significant.
Results
Body weight and basal parameters in adult rats
We did not observe differences in body weight (BW) between MS and C rats during the maternal separation period (days 2 to 14 postnatal) (figure S1, inset) or up to 3 months old (figure S1 and table 1). MAP and HR were not different between adult MS and C rats (table 1). Maternal separation did not produce any significant change in plasma insulin and blood glucose values between MS and C rats (table 1). In addition, maternal separation did not modify the levels of plasma ang II, plasma ang 1–7 and plasma renin activity (PRA) in MS rats compared with C rats (table 1).
Table 1.
Basal parameters in 12 week old male rats. There are no significant differences between C and MS rats in body weight, MAP (mean arterial pressure), HR (heart rate), glycemia, plasma insulin, plasma ang II, plasma ang 1–7, or plasma renin activity (PRA).
| Basal Parameter | C | MS |
|---|---|---|
| Body Weight, g | 292.0 ± 13.4 (n=12) | 271.9 ± 15.8 (n=14) |
| MAP, mmHg | 110 ± 1 (n=8) | 108 ± 1 (n=11) |
| HR, bpm | 355 ± 9 (n=8) | 366 ± 7 (n=11) |
| Glycemia, mg/dl | 93.8 ± 2.9 (n=6 | 88.3 ± 2.0 (n=6) |
| Insulin, ng/ml | 2.25 ± 0.36 (n=6) | 1.70 ± 0.27 (n=7) |
| Ang II, pg/ml | 53.1 ± 4.1 (n=10) | 50.5 ± 3.4 (n=10) |
| Ang 1–7, pg/ml | 29.6 ± 4.0 (n=10) | 21.7 ± 3.7 (n=10) |
| PRA, ng AngI/ml/h | 18.1 ± 2.6 (n=6) | 20.7 ± 3.5 (n=7) |
Acute ang II administration in anesthetized rats
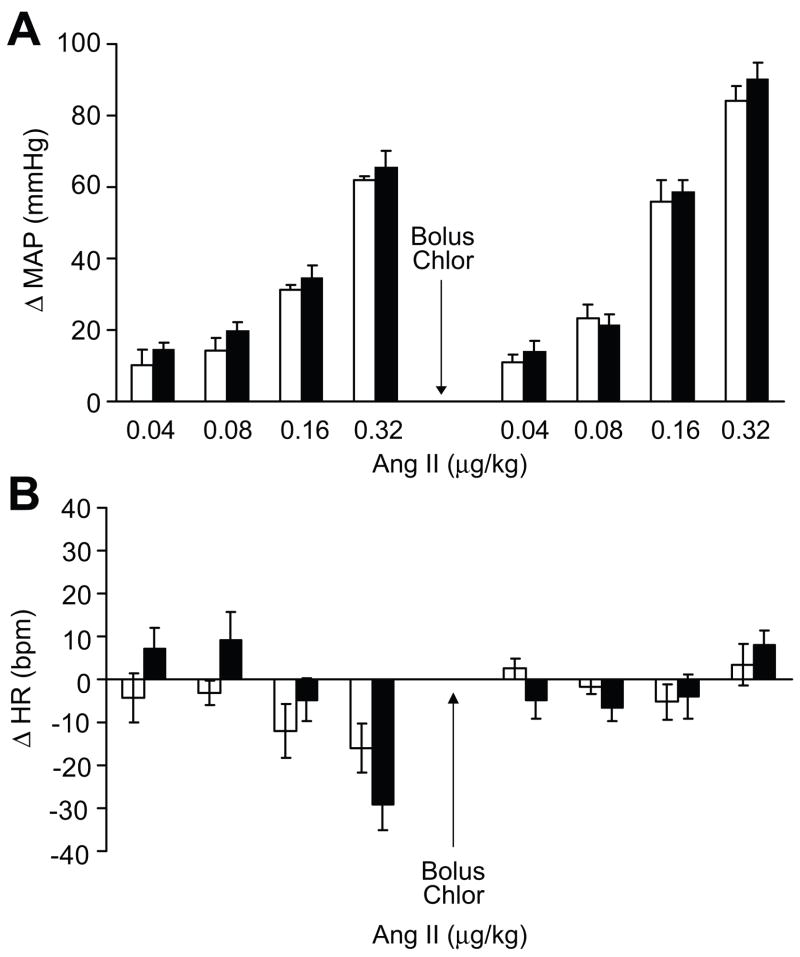
The blood pressure (MAP) and heart rate (HR) response to acute i.v. bolus of ang II in anesthetized rats was not different in MS and C rats (table S1A and S1B, respectively). Figure 1A represents the change in MAP (ΔMAP) in response to the ang II bolus. Increments of change in HR (ΔHR) were not different in MS and C rats in response to the ang II bolus as well (figure 1B). Chlorisondamine administration (5 mg/kg) abolished the HR responses to ang II as expected (table S1B; figure 1B).
Figure 1.
Change in blood pressure (MAP) and heart rate (HR) in anesthetized rats in response to i.v. bolus of ang II with increasing doses in MS (open bars) and C (black bars) rats, n=5.
Responses to chronic ang II infusion
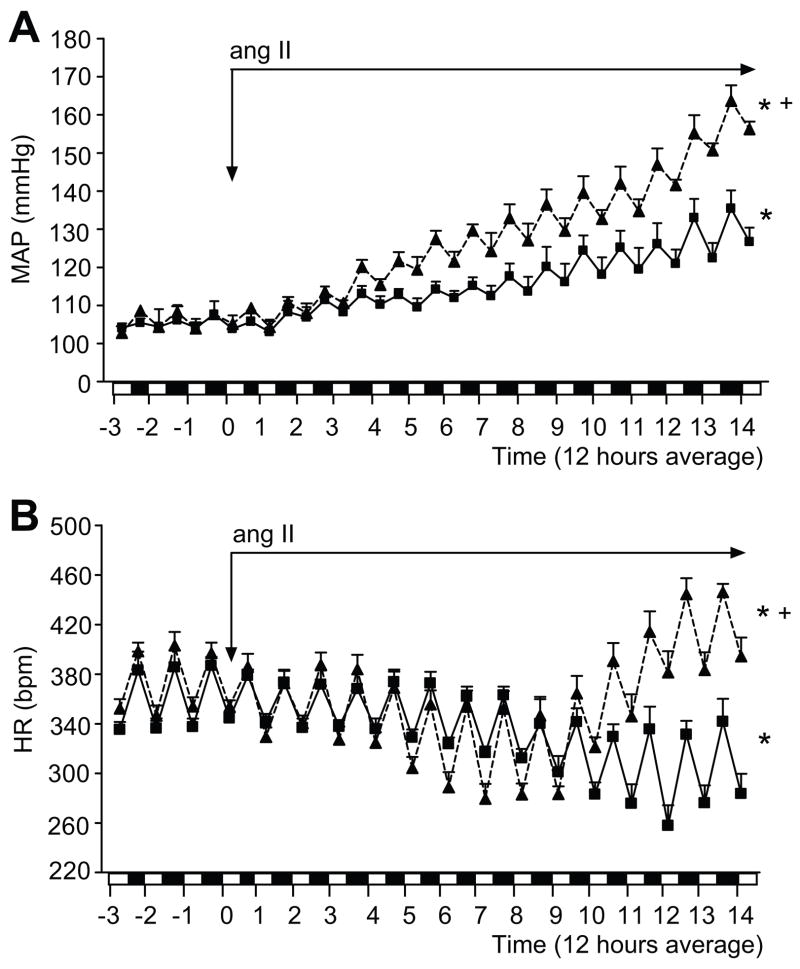
In parallel, we tested the blood pressure, heart rate and locomotor activity changes in response to a chronic infusion of ang II in telemetry implanted rats. MAP (24 hour average) in MS (100.9±3.0 mmHg) and C rats (104.9±3.2 mmHg) was similar prior to infusion of ang II (table 1; figure 2A). After 3 days of ang II infusion, MAP increased significantly in MS rats compared to C rats (117.1±2.1 vs.111.2±1.7 mmHg, p<0.05, respectively). This enhanced blood pressure response in MS rats continued during the second week of ang II infusion (figure 2A).
Figure 2.
MAP (panel 2A) and HR (panel 2B) in non-infused (baseline; prior to ang II infusion) and ang II-infused telemetry implanted MS (▲, dashed line) (n=7) and C rats (■, solid line) (n=8). Data is expressed as 12 hour average. On the x-axis: white bar represents 6:00 a.m. to 6:00 p.m. period; black bar represents 6:00 p.m. to 6:00 a.m. period. * p <0.05 vs. baseline; + p <0.05 vs. C.
As a normal baroreflex response to an increase in BP, HR decreased significantly during the first week of ang II infusion in C rats (figure 2B; 371.2±7.8 bpm baseline vs. 334.8±7.0 bpm at 7 days, p<0.05). Furthermore, HR was maintained at a reduced level compared with the baseline (320.0±4.5 bpm at 14 days, p<0.05) during the second week of ang II infusion in C rats. MS rats have a similar HR at baseline (figure 2B; 373.0±4.5 bpm) when compared to C rats. Also, similar to C rats, after one week of ang II infusion HR decreased significantly (373.2±4.5 bpm at baseline vs. 321.4±8.1 bpm at 7 days, p<0.05; figure 2B) in MS rats. However, after 8 days of ang II infusion, the HR in MS rats increased dramatically over the course of the last 4 days of ang II infusion (403.0±9.0 bpm at 14 days, p<0.05; figure 2B). The circadian rhythm of the MAP and HR was similar in both groups at baseline and during the ang II infusion (figure 2A and 2B).
Locomotor activity was similar between C and MS rats at baseline conditions (figure S2). Interestingly, locomotor activity in MS rats infused with ang II was significantly higher compared to C rats infused with ang II (3.1±0.3 vs. 2.3±0.2, respectively, p<0.05) during the active cycle (6:00 p.m to 6:00 a.m.), but not during the inactive cycle (6:00 a.m to 6:00 p.m.; figure S2). However, the activity increase did not correlate with the progressive augmentation in blood pressure or the changes in HR observed with chronic ang II infusion in MS rats (figures 2 and S2).
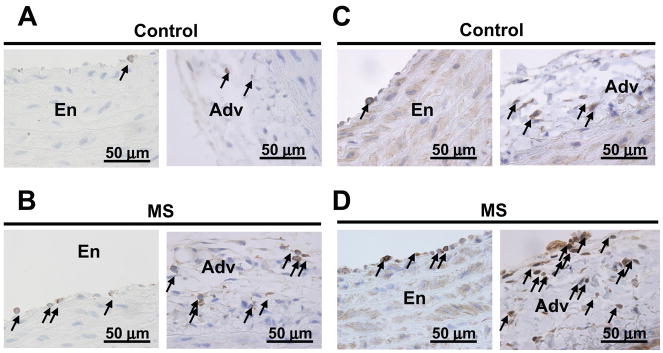
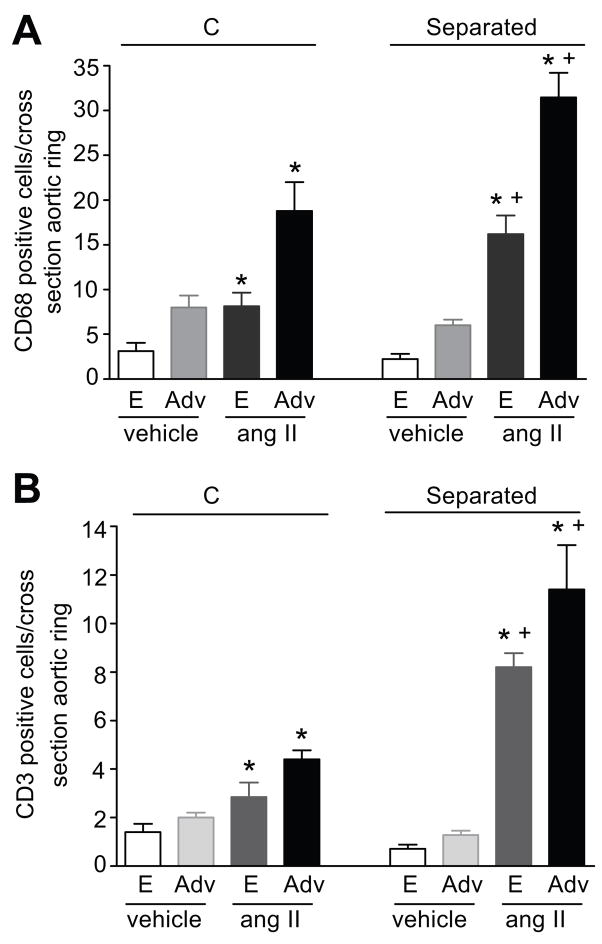
Our study of aortic morphology showed that the aortic thickness, external diameter, wall area and cell density were not different between MS and C rats at baseline (table S2). Ang II infusion significantly increased aortic wall thickness, external diameter and wall area in both MS and C rats, similarly (table S2). Immunolocalization of inflammatory cells in the aortas from MS and C rats demonstrated that monocytes/macrophages (CD68 marker) and T cells (CD3 marker) were found associated with the endothelium and the peri-vascular adventitia at baseline conditions and after chronic ang II infusion (figure 3). No immunostaining for CD68 or CD3 positive cells was detected in the smooth muscle layer (data not shown). At baseline, the numbers of CD68 (figure 4A) and CD3 (figure 4B) positive cells were similar in the aortas from MS and C rats. Chronic ang II-infusion results in a significant increase in the number of CD68 and CD3 positive cells in both the endothelium and peri-vascular adventitia of aortas from MS rats and C rats (figure 4). Furthermore, the increase in inflammatory cells in aortas from MS rats was significantly higher than in aortas from C rats (figure 4).
Figure 3.
Localization of inflammatory cells in aortic tissue from ang II-infused rats. Panels 3A and 3B: representative micrographs of CD68 positive cells in ang II-infused control, C (3A) and maternally separated, MS (3B) rats, respectively. Panels 3C and 3D: representative micrographs of CD3 positive cells in ang II-infused control, C (3C) and maternally separated, MS (3D) rats, respectively. Arrows point to CD68 or CD3 positive cells in the endothelium (En) or adventitia (Adv).
Figure 4.
Quantitation of inflammatory cells in the thoracic aorta from vehicle and ang II-infused rats. Panel A: CD68 positive cells in ang II-infused C (n=5) and MS (n=9) rats; vehicle-infused C (n=4) and MS (n=4) rats. Panel B: CD3 positive cells in ang II-infused C (n=6) and MS (n=5) rats; vehicle-infused C (n=4) and MS (n=4) rats. E: Endothelium, Adv: Adventitia. * p <0.05 vs. vehicle. + p <0.05 vs. C.
Discussion
Foremost, our findings demonstrate that early life stress, specifically maternal separation, augments the responses to activation of the ang II pathway in adult male rats. Our findings show that maternal separation does not induce hypertension as observed in other models of fetal programming; however, the consequent increased ang II sensitivity may contribute to the pathogenesis of hypertension and cardiovascular disease. Specifically, maternal separation during the postnatal period sensitizes rats to ang II-dependent hypertension, increased heart rate, and vascular inflammation. Taken together, these data support a role for early life stress in the genesis of a risk factor mediating enhanced cardiovascular reactivity to ang II.
Interestingly, rats display a stress hyporesponsive period to stress during neonatal life as evidenced by a markedly attenuated response of the hypothalamus-pituitary-adrenocortical axis to environmental stressors (21,22). It has been shown that imposition of a stressor during the hyporesponsive period can promote changes in adult behavior and neuroendocrine function (17,18). Coincidently, the maternal separation protocol was conducted during this stress hyporesponsive period. We sought to elucidate the cardiovascular phenotype of adult rats that were exposed to maternal separation under baseline conditions and in response to exogenous ang II. Our results have led us to hypothesize that early life stress elicits a sensitization of ang II signaling in male rats. The sensitization of ang II signaling may involve modulation of neuroendocrine mechanisms and/or represent a change in postnatal organ development modulating the susceptibility to cardiovascular disease. Typically, chronic infusion of an acutely sub-pressor dose of ang II increases blood pressure after several days (23,24) through mechanisms involving vascular dysfunction, a dysfunctional pressure/natriuresis relationship, and/or elevated sympathetic activity. At this point, it is not clear whether the mechanism of ang II-induced hypertension is similar in the MS and C rats. Additional research is needed to further elucidate the mechanisms related to early life stress induced sensitization of ang II signaling.
Very few studies have explored the effects of maternal separation on cardiovascular parameters and/or function. Sanders et al (25) examined the effects of early life stress on cardiovascular function in borderline hypertensive rats (BHR). They also report that baseline MAP and HR is similar between control and maternal separation rats, nevertheless MS rats subjected to restraint stress demonstrate an increased HR response, although they reported no significant differences in blood pressure responses. These data, in conjunction with our study, would indicate that early life stress does not elicit an obvious cardiovascular pathological phenotype in adulthood unless provoked by exogenous stimuli.
Interestingly, the HR responses to chronic ang II infusion in the MS rats showed a dramatic and rather unexpected biphasic response. The MS rats appeared to maintain a normal baroreflex response during the first week of ang II infusion, yet during the second week developed tachycardia. Our finding may suggest that chronic ang II sensitization in the MS rats provoke actions in the forebrain and/or the heart that increase sympathetic nerve activity to override the reflex bradycardia (26). Acute handling (3 min/day) of neonatal rats was found to alter autonomic controls of HR in pre-weanling SHR and WKY rats (27,28). Alternatively, the biphasic HR response may be the result of secondary actions. Thus, a further exploration of the effects of maternal separation of the autonomic neural development controlling blood pressure may address the mechanisms triggering the increased heart rate.
Ang II-induced hypertension was accompanied by an increase in inflammatory cell numbers, identified by CD68 and CD3 immunostaining, in the aortic endothelium and perivascular adventitia that was more pronounced in MS rats. Interestingly, aortic morphological analysis and inflammatory cell numbers were similar in both MS and C rats at baseline conditions. Ang II-dependent aortic hypertrophy was similar in both MS and C rats suggesting that early life stress may mediate a specific alteration in the immune response. Vanbesien-Mailliot (29) has shown that prenatal stress produces alterations in immune function. Effects of perinatal stress enhancing the immune response have been documented in pre-term lambs (30), neonate rhesus monkeys (31), and young adult rats (32). The mechanism of the enhanced immune response in these models is undefined. Furthermore, it is unclear whether the increase in aortic inflammatory cells is due to the increased blood pressure in the MS rats, or that the MS rats elicit a differential activation of inflammatory cells, which may play a role in the exaggerated ang II sensitivity and resulting elevation in blood pressure. Guzik et al have demonstrated that T cell activation is critical for the full realization of ang II-dependent hypertension (33). Future experiments are planned to examine whether inflammatory cells, specifically T cells, are involved in the ang II-dependent sensitivity induced by early life stress.
The acute response to an intravenous bolus of ang II in anesthetized rats did not demonstrate an exaggerated blood pressure or heart rate response in the MS rats. This result is contrary to the chronic actions of ang II with conscious telemetry measurements of blood pressure and heart rate. Thus, it is important to consider the effect of sympathetic control under anesthesia and the sensitivity of the response to ang II in the intact animal. Further analysis of acute responses to ang II in conscious animals as well as isolated vascular preparations and/or intact organs may reveal an exaggerated difference in the MS rats (34). Alternatively, chronic ang II-infusion may invoke further mechanisms, such as activation of inflammatory cells or alterations in renal function that unmask the effects of early life stress.
Rat models of fetal programming that predict a subsequent cardiovascular pathological phenotype involve modulation of the maternal environment during fetal development or in the neonate (35–37). Increased expression of ang II type 1 and type 2 receptor, angiotensinogen, plasma renin activity and angiotensin-converting enzyme have been reported in the adult brain and kidney from animals exposed to dexamethasone during development (38–40). Various models of developmental plasticity have observed changes in RAS components when a hypertensive phenotype co-exists (39,41,42). Thus, differential expression and/or function of the RAS pathway may be a target of fetal programming models.
In summary, these findings demonstrate that a model of early life stress, maternal separation, does not induce hypertension in adult WKY rats as observed in other models of fetal programming. Yet, maternal separation renders the adult rats susceptible to activation of ang II-dependent hypertension, increased heart rate, and vascular inflammation, which may contribute to the pathogenesis of cardiovascular disease. Overall, our findings support a role for early life stress in the genesis of a risk factor mediating enhanced cardiovascular reactivity to ang II and provide justification for further investigation.
Perspectives
Chronic stressors are known to sensitize humans to risk factors for cardiovascular disease. This study utilized a model of chronic stress during early life and demonstrating a sensitization to ang II-dependent responses. A complete understanding of the mechanisms involved in the ang II-dependent chronic stress responses may disclose future therapeutic approaches and preventative measures for minimizing the risk of hypertension.
Supplementary Material
Acknowledgments
We gratefully acknowledge the participation of Dr Bridget Brosnihan for the determinations of plasma angiotensin II and angiotensin 1–7.
This study was performed with outstanding technical support from Hiram Ocasio, Janet Hobbs, Carolyn Rhoden and Amy Dukes.
Sources of Funding
NIH P01 HL69999; AHA Postdoctoral Fellowship to ASL
Footnotes
Disclosures
none
References
- 1.Alexander BT. Fetal programming of hypertension. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1–R10. doi: 10.1152/ajpregu.00417.2005. [DOI] [PubMed] [Google Scholar]
- 2.McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005;85:571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 3.Zandi-Nejad K, Luyckx VA, Brenner BM. Adult hypertension and kidney disease: the role of fetal programming. Hypertension. 2006;47:502–508. doi: 10.1161/01.HYP.0000198544.09909.1a. [DOI] [PubMed] [Google Scholar]
- 4.Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, Anda RF. Insights into causal pathways for ischemic heart disease: adverse childhood experiences study. Circulation. 2004;110:1761–1766. doi: 10.1161/01.CIR.0000143074.54995.7F. [DOI] [PubMed] [Google Scholar]
- 5.Kelishadi R, Mirghaffari N, Poursafa P, Gidding SS. Lifestyle and environmental factors associated with inflammation, oxidative stress and insulin resistance in children. Atherosclerosis. 2009;203:311–319. doi: 10.1016/j.atherosclerosis.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 6.Tyrka AR, Wier L, Price LH, Ross N, Anderson GM, Wilkinson CW, Carpenter LL. Childhood parental loss and adult hypothalamic-pituitary-adrenal function. Biol Psychiatry. 2008;63:1147–1154. doi: 10.1016/j.biopsych.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holness MJ, Langdown ML, Sugden MC. Early-life programming of susceptibility to dysregulation of glucose metabolism and the development of Type 2 diabetes mellitus. Biochem J. 2000;349:657–665. doi: 10.1042/bj3490657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Igosheva N, Klimova O, Anishchenko T, Glover V. Prenatal stress alters cardiovascular responses in adult rats. J Physiol. 2004;557:273–285. doi: 10.1113/jphysiol.2003.056911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EH, Piersma AH, Ozanne SE, Twinn DF, Remacle C, Rowlerson A, Poston L, Taylor PD. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension. 2008;51:383–392. doi: 10.1161/HYPERTENSIONAHA.107.101477. [DOI] [PubMed] [Google Scholar]
- 10.Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev. 2000;52:639–672. [PubMed] [Google Scholar]
- 11.Langely-Evans SC, Welham SJ, Jackson AA. Fetal exposure to a maternal protein diet impairs nephrogenesis and promotes hypertension in the rat life. Life Sci. 1999;64:965–974. doi: 10.1016/s0024-3205(99)00022-3. [DOI] [PubMed] [Google Scholar]
- 12.Roghair RD, Lamb FS, Bedell KA, Smith OM, Scholz TD, Segar JL. Late-gestation betamethasone enhances coronary artery responsiveness to angiotensin II in fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2004;286:R80–88. doi: 10.1152/ajpregu.00421.2003. [DOI] [PubMed] [Google Scholar]
- 13.Lehmann J, Stohr T, Feldon J. Long-term effects of prenatal stress experiences and postnatal maternal separation on emotionality and attentional processes. Behav Brain Res. 2000;107:133–144. doi: 10.1016/s0166-4328(99)00122-9. [DOI] [PubMed] [Google Scholar]
- 14.Neumann ID, Wigger A, Krömer S, Frank E, Landgraf R, Bosch OJ. Differential effects of periodic maternal separation on adult stress coping in a rat model of extremes in trait anxiety. Neuroscience. 2005;132:867–877. doi: 10.1016/j.neuroscience.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 15.Caldji C, Diorio J, Meaney MJ. Variations in maternal care in infancy regulate the development of stress reactivity. Biol Psychiatry. 2000;48:1164–1174. doi: 10.1016/s0006-3223(00)01084-2. [DOI] [PubMed] [Google Scholar]
- 16.Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- 17.Lippmann M, Bress A, Nemeroff CB, Plotsky PM, Monteggia LM. Long-term behavioural and molecular alterations associated with maternal separation in rats. Eur J Neurosci. 2007;5:3091–3098. doi: 10.1111/j.1460-9568.2007.05522.x. [DOI] [PubMed] [Google Scholar]
- 18.Plotsky PM, Meaney MJ. Early postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 19.D’Angelo G, Pollock JS, Pollock DM. In vivo evidence for endothelin-1-mediated attenuation of alpha1-adrenergic stimulation. Am J Physiol Heart Circ Physiol. 2006;290:H1251–1258. doi: 10.1152/ajpheart.00203.2005. [DOI] [PubMed] [Google Scholar]
- 20.Sasser JM, Sullivan JC, Hobbs JL, Yamamoto T, Pollock DM, Carmines PK, Pollock JS. Endothelin A receptor blockade reduces diabetic renal injury via an anti-inflammatory mechanism. J Am Soc Nephrol. 2007;18:143–154. doi: 10.1681/ASN.2006030208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology. 2005;30:939–946. doi: 10.1016/j.psyneuen.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 22.Okimoto DK, Blaus A, Schmidt MV, Gordon MK, Dent GW, Levine S. Differential expression of c-fos and tyrosine hydroxylase mRNA in the adrenal gland of the infant rat: evidence for an adrenal hyporesponsive period. Endocrinology. 2002;143:1717–1725. doi: 10.1210/endo.143.5.8819. [DOI] [PubMed] [Google Scholar]
- 23.Ortiz MC, Manriquez MC, Romero JC, Juncos LA. Antioxidants block angiotensin II-induced increases in blood pressure and endothelin. Hypertension. 2001;38:655–659. doi: 10.1161/01.hyp.38.3.655. [DOI] [PubMed] [Google Scholar]
- 24.Szentiványi M, Jr, Zou AP, Mattson DL, Soares P, Moreno C, Roman RJ, Cowley AW., Jr Renal medullary nitric oxide deficit of Dahl S rats enhances hypertensive actions of angiotensin II. Am J Physiol Regul Integr Comp Physiol. 2002;283:R266–272. doi: 10.1152/ajpregu.00461.2001. [DOI] [PubMed] [Google Scholar]
- 25.Sanders BJ, Anticevic A. Maternal separation enhances neuronal activation and cardiovascular responses to acute stress in borderline hypertensive rats. Behav Brain Res. 2007;183:25–30. doi: 10.1016/j.bbr.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porter JP, Phillips A, Rich J, Wright D. Effect of chronic stress on the cardiac baroreflex in the post-weanling rat. Life Sci. 2004;75:1595–1607. doi: 10.1016/j.lfs.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 27.Tucker DC, Bhatnagar RK, Johnson AK. Genetic and environmental influences on developing autonomic control of heart rate. Am J Physiol. 1984;246:R578–586. doi: 10.1152/ajpregu.1984.246.4.R578. [DOI] [PubMed] [Google Scholar]
- 28.Tucker DC, Johnson AK. Influence of neonatal handling on blood pressure, locomotor activity, and preweanling heart rate in spontaneously hypertensive and Wistar Kyoto rats. Dev Psychobiol. 1984;17:587–600. doi: 10.1002/dev.420170603. [DOI] [PubMed] [Google Scholar]
- 29.Vanbesien-Mailliot CC, Wolowczuk I, Mairesse J, Viltart O, Delacre M, Khalife J, Chartier-Harlin MC, Maccari S. Prenatal stress has pro-inflammatory consequences on the immune system in adult rats. Psychoneuroendocrinology. 2007;32:114–124. doi: 10.1016/j.psyneuen.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Kallapur SG, Kramer BW, Moss TJ, Newnham JP, Jobe AH, Ikegami M, Bachurski CJ. Maternal glucocorticoids increase endotoxin-induced lung inflammation in preterm lambs. Am J Physiol Lung Cell Mol Physiol. 2003;284:633–642. doi: 10.1152/ajplung.00344.2002. [DOI] [PubMed] [Google Scholar]
- 31.Coe CL, Kramer M, Kirschbaum C, Netter P, Fuchs E. Prenatal stress diminishes the cytokine response of leukocytes to endotoxin stimulation in juvenile rhesus monkeys. J Clin Endocrinol Metab. 2002;87:675–681. doi: 10.1210/jcem.87.2.8233. [DOI] [PubMed] [Google Scholar]
- 32.Klein SL, Rager DR. Prenatal stress alters immune function in the offspring of rats. Dev Psychobiol. 1995;28:321–336. doi: 10.1002/dev.420280603. [DOI] [PubMed] [Google Scholar]
- 33.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:449–2456. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loria A, Kang K, Pollock D, Pollock J. Maternal Separation Enhances Angiotensin II-induced Aortic Contraction Via Reduced NO Signaling. Hypertension. 2008;52:e74. Abstract. [Google Scholar]
- 35.Bertram C, Trowern AR, Copin N, Jackson AA, Whorwood CB. The maternal diet during pregnancy programs altered expression of the glucocorticoid receptor and type 2 11b-hydroxysteroid dehydrogenase: potential molecular mechanisms underlying the programming of hypertension in utero. Endocrinology. 2001;142:2841–2853. doi: 10.1210/endo.142.7.8238. [DOI] [PubMed] [Google Scholar]
- 36.Guron G, Friberg P. An intact renin-angiotensin system is a prerequisite for normal renal development. J Hypertens. 2000;18:123–137. doi: 10.1097/00004872-200018020-00001. [DOI] [PubMed] [Google Scholar]
- 37.O’Regan D, Kenyon CJ, Seckl JR, Holmes MC. Glucocorticoid exposure in late gestation in the rat permanently programs gender-specific differences in adult cardiovascular and metabolic physiology. Am J Physiol Endocrinol Metab. 2004;287:E863–870. doi: 10.1152/ajpendo.00137.2004. [DOI] [PubMed] [Google Scholar]
- 38.Manning J, Vehaskari VM. Postnatal modulation of prenatally programmed hypertension by dietary Na and ACE inhibition. Am J Physiol Regul Integr Physiol. 2004;288:80–84. doi: 10.1152/ajpregu.00309.2004. [DOI] [PubMed] [Google Scholar]
- 39.Moritz KM, Johnson K, Douglas-Denton R, Wintour EM, Dodic M. Maternal glucocorticoid treatment programs alterations in the renin-angiotensin system of the ovine fetal kidney. Endocrinology. 2002;43:4455–4463. doi: 10.1210/en.2002-220534. [DOI] [PubMed] [Google Scholar]
- 40.Shaltout HA, Figueroa JP, Rose JC, Diz DI, Chappell MC. Alterations in circulatory and renal angiotensin-converting enzyme and angiotensin-converting enzyme 2 in fetal programmed hypertension. Hypertension. 2009;53:404–408. doi: 10.1161/HYPERTENSIONAHA.108.124339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bogdarina I, Welham S, King PJ, Burns SP, Clark AJ. Epigenetic modification of the renin-angiotensin system in the fetal programming of hypertension. Circ Res. 2007;100:520–526. doi: 10.1161/01.RES.0000258855.60637.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Contreras RJ. Differences in perinatal NaCl exposure alters blood pressure levels of adult rats. Am J Physiol. 1989;256:79–77. doi: 10.1152/ajpregu.1989.256.1.R70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.