Abstract
Purpose
To determine double-strand-break (DSB) yields produced by decay of minor-groove-bound 123I-labeled Hoechst 33342 (123IEH) in supercoiled (SC) and linear (L) forms of pUC19 DNA, to compare strand-break efficiency of 123IEH with that of 125IEH, and to examine the role of DNA topology in DSB induction by these Auger electron emitters.
Materials and methods
Tritium-labeled SC and L pUC19 DNA were incubated with 123IEH (0–10.9 MBq) at 4°C. After 123I had completely decayed (10 days), samples were analyzed on agarose gel, and single-strand-break (SSB) and DSB yields were measured.
Results
Each 123I decay in SC DNA produces a DSB yield of 0.18 ± 0.01. On the basis of DSB yields for 125IEH (0.52 ± 0.02 for SC and 1.62 ± 0.07 for L, reported previously) and dosimetric expectations, a DSB yield of ~0.5 (3 × 0.18) per 123I decay is expected for L DNA. However, no DSB are observed for the L form, even after ~2 × 1011 decays of 123I per μg DNA, whereas a similar number of 125I decays produces DSB in ~40% of L DNA.
Conclusion
123IEH-induced DSB yield for SC but not L DNA is consistent with the dosimetric expectations for Auger electron emitters. These studies highlight the role of DNA topology in DSB production by Auger emitters and underscore the failure of current theoretical dosimetric methods per se to predict the magnitude of DSB.
Keywords: DNA strand break, DNA topology, Auger electron, 123I, 125I
Introduction
Many radionuclides exhibit the Auger effect, a phenomenon which is characterized by the emission of a cascade of low-energy electrons (Auger 1925). The decay of such radioactive atoms is accompanied by the creation of a primary vacancy in the inner shell consequent to electron capture and/or internal conversion and leads to the emission of X-ray photons and monoenergetic Auger or Coster-Kronig electrons with energies ranging from a few eV to ~1 keV (Cole 1969, Pomplun et al. 1987, Kassis 2004). Auger electrons travel a short distance of 2 to 500 nm; therefore, they behave like high linear energy transfer (LET) radiation (~2 to ~25 keV/μm) and must be in close proximity to DNA, to cause cytotoxicity (Sastry and Rao 1984, Charlton et al. 1987, Kassis 2004).
Among the Auger electron emitters, Iodine-125 and iodine-123 have attracted considerable attention in the development of cancer therapy (Bloomer and Adelstein 1977, Kassis et al. 1998, Kassis 2004, Kassis et al. 2004). The similar size of an iodine atom and a –CH3 group has permitted the in vivo incorporation of radioiodine – as 5-[125I/123I]iodo-2′-deoxyuridine (125IUdR, 123IUdR) in the place of thymidine – into the nuclear DNA during cell division, thus facilitating the proximity of the decaying radioactive iodine atom to the DNA strand and inducing cell kill (Hofer and Hughes 1971, Feinendegen 1975, Chan et al. 1976, 1977, Makrigiorgos et al. 1989). Iodine-125-labeled DNA intercalators (Martin 1977, Kassis et al. 1989), 125I-labeled benzimidazole minor-groove binders such Hoechst 33342 and 33258 (Martin and Holmes 1983, Kassis et al. 1999a, 1999b, 2000), 123I-labeled steroid hormones (DeSombre et al. 1992, DeSombre et al. 2000), 125I-internalizing antibodies and 125I-deoxycytidine in homopyrimidine triplex-forming oligonucleotides (Panyutin et al. 2000, 2001) have also been used in positioning radioiodine decay proximal to the DNA in the nucleus.
Historically, radioiodine has been useful in the diagnosis and treatment of thyroid-related diseases (Rawson et al. 1951). Recently, 123I, which emits a 159 keV γ-photon, has emerged as an alternative for thyroid imaging by single photon emission computed tomography (SPECT) (Reynolds and Robbins 1997, Yaakob et al. 1999). Unlike 131I, the decay of 123I does not emit β-particles and, therefore, delivers less radiation to the surrounding normal tissue. However, when 123I is proximal to nuclear DNA, the cytotoxicity of Auger electron cascade could be a nuisance in 123I imaging. Hence, a clear understanding of the mechanisms underlying 123I induced DNA damage and cell death is necessary in developing reliable radiation safety tools to protect normal cells from radiation risk during these diagnostic procedures.
We have been interested in exploring the biophysical mechanisms underlying DNA damage induced by Auger emitters, particularly 125I and 123I. We had hypothesized that DNA compaction favors the formation of >1 DSB by •OH-mediated indirect mechanisms when DNA-incorporated 125I decays (Walicka et al. 1998). Consequently, a higher DSB was expected for the supercoiled (SC) form of naked plasmid DNA due to its compacted state than for its torsionally relaxed, non-supercoiled counterpart, the linear (L) form. However, our recent study (Balagurumoorthy et al. 2008) using the minor-groove binder 125I-labeled m-iodo-p-ethoxyHoechst 33342 (125IEH) showed that supercoiling of naked plasmid DNA significantly reduces the magnitude of 125I-induced DSB yield and affects the mechanism (direct versus indirect) of DSB production. A 3-fold higher DSB yield is observed for torisonally relaxed L DNA (~1.6 per decay of 125I) than for SC DNA (~0.5 per 125I decay), and both direct and indirect mechanisms produce DSB in the L form compared with only the direct mechanism in the SC form. In here, we compared 123IEH-induced DSB yields for SC and L forms of pUC19 plasmid DNA and have analyzed the differences in the magnitude and mechanism of Auger-electron-induced DSB yield on DNA topology observed recently for 125I.
Materials and methods
Synthesis of 123I-/125I-labeled m-iodo-p-ethoxyHoechst 33342 (123IEH/125IEH)
The radioiodinated (123I/125I) analogs of the DNA minor-groove-binding drug m-iodo-p-ethoxyHoechst 33342 (123IEH/125IEH) were synthesized from its trimethylstannyl derivative (Harapanhalli et al. 1996, Kassis et al. 1999a). In essence, carrier-free, dried Na123I powder (370 MBq) in 0.1 M NaOH, purchased from MDS Nordion (Ottawa, Canada) was neutralized with 0.1 M HCl, and the pH of the solution was adjusted to ~7. To a vial coated with iodogen (5 μg), trimethylstannylHoechst 33342 (1 μl, 3μg/μl in dimethyl sulfoxide (DMSO), 0.1X phosphate buffered saline (PBS) (2 μl, pH 7.4), and Na123I (11 μl, 185 MBq, ~8,800 TBq/mmole) were added, and the mixture was vortexed for 2 min at room temperature. The radioiodination was followed by analyzing 0.5-μl aliquots of reaction mixture on HPLC (Waters, Milford MA) with a reverse phase Zorbax SB C18 column (9.4 × 250 mm) (Harapanhalli et al. 1996, Kassis et al. 1999a). The radioiodinated product was identified using the retention time of nonradioactive (127I) iodoHoechst 33342 run under identical conditions. Ultra-violet (UV) absorption (Waters 486 detector) and γ-ray emission (gamma-ram, IN/US Systems) were used to detect non-radioiodinated and radioiodinated products, respectively. Fractions containing 123I-labeled Hoechst 33342 were collected, dried, and redissolved in water. The radiochemical yield was 85% and the radiochemical purity >98%. Since the retention times of Na123I, 123IEH, and Hoechst 33342, are distinct, the specific activity of the 123I-labeled derivative is ~8,800 TBq/mmole. 125IEH was synthesized in a similar fashion using Na125I (Perkin Elmer Life and Analytical Sciences, Waltham MA, USA).
Preparation of 3H-pUC19 plasmid DNA
pUC19 plasmid DNA (30 ng, New England Biolabs, Incorporated, Beverly MA, USA) was transformed into Escherichia coli DH5α competent cells (Invitrogen Incorporated, Gibco BRL, CA). Plasmid DNA was isolated from bacterial cultures grown for 16 h at 37°C in the presence of 3H-thymidine (3H-TdR, 370 KBq/ml) and ampicillin (50 μg/ml) (Balagurumoorthy et al. 2006). The medium was centrifuged at 6000 rpm in a GSA rotor, and the plasmid (3H-pUC19) was isolated from the bacterial cell pellet using the Qiagen Plasmid Maxi kit. DNA was precipitated with ethanol and redissolved in PBS, (pH 7.4). DNA concentration was determined spectrophotometrically from absorbance at 260 nm. Agarose gel electrophoresis indicated that >99% of the DNA is in the SC state.
Linearization of 3H-pUC19 plasmid DNA
Supercoiled 3H-pUC19 plasmid DNA (100 μg in 134 μl 1X PBS, pH 7.4) was digested with EcoRI (1,000 units, 50 μl) in EcoRI buffer (1×, 500 μl, New England Biolabs Inc, Beverly MA, USA) for 16 h at 37°C. Linear (L) DNA formation in the reaction mixture was assessed on 1% agarose gel, another 100 units of EcoRI was added, and the incubation was continued for 12 h longer at 37°C. Subsequent agarose gel analysis indicated 100% linearization and no residual SC DNA left undigested. The reaction mixture was extracted twice with equal volumes of phenol equilibrated with Tris buffer (pH >7.5) and once with chloroform:isoamyl alcohol (24:1, v/v). Linear 3H-pUC19 DNA was precipitated with ethanol, and the dried DNA pellet was redissolved in PBS (1 ×, 200 μl, pH 7.4). DNA concentration, measured spectrophotometrically from absorbance at 260 nm, was 0.375 μg/μl; the total yield was ~75 μg.
Incubation of supercoiled and linear forms of 3H-pUC19 plasmid DNA with m-[123I]iodo-p-ethoxyHoechst 33342 (123IEH)
The pUC19 plasmid DNA concentration used in the 123IEH–DNA (SC or L) incubations is 0.053 μM (1.8 μg, or 1.06 pmoles, in 20 μl), which is the same as that used in our previous studies with 125IEH. The highest dose 10.9 MBq corresponds to 1.2 pmoles of 123IEH. Individual incubations of DNA with increasing amounts of 123IEH (in the range of 0–10.9 MBq) in PBS at 4°C were continued for two weeks. Then aliquots (6 μl) were removed from the incubation mixtures, combined with 3 μl loading-dye-glycerol mixture and 3 μl PBS, and loaded onto 1% agarose gel in Tris-acetate-EDTA (TAE) buffer (0.5X) containing ethidium bromide (0.5 μg/ml). We estimate that the random pipetting error in loading the samples on the agarose gel is 3–7% of the total volume of the sample (12 μl). The gels were run at 200 volts (~7 volts/cm) and photographed on a transilluminator (long wave) attached to a charge coupled device (CCD) camera. For SC DNA–123IEH incubations, DNA bands corresponding to SC, N (nicked-circular), and L forms were excised; for L DNA–123IEH incubations, bands corresponding to intact L DNA were excised. The gel pieces were dissolved in scintillation cocktail (OPTI-FLOR) and 3H content was determined by scintillation counting.
Calculation of 123IEH-induced double-strand breaks in supercoiled and linear forms of 3H-pUC19 plasmid DNA
The fraction of SC DNA remaining intact at each time point is plotted as a function of number of 123IEH decays/ml. The number of 123I disintegrations required to reduce the total number of DNA molecules initially present to 37% is defined by D0 = [(log10 37) − log10100]/X, where X is the slope obtained from the linear regression of the semilogarithmic plot. Since at zero decay, the fraction of intact DNA equals unity, the regression line is forced through 1. Statistically this is permissible because, in the absence of forcing through one, the y intercept is not significantly different. The strand-break calculations are based on the assumption that the binding of 123IEH to DNA and, hence, the strand breaks, follow Poisson distribution.
The mean number of double (XDSB)-strand breaks per SC DNA molecule are calculated from the experimentally observed fractions of L DNA formed after exposure to a given number of 123I disintegrations, using the following relationship (Cowan et al. 1987):
where FL is the fraction of L DNA formed as a result of exposure to accumulated 123IEH decays (Kassis et al. 1999a, 1999b).
For the L form, the rate of formation of DSB must be equal to the rate of L DNA disappearance, as DSB are the only cause of reduction in intact L DNA band intensity. The mean number of DSB (XDSB) per L DNA molecule are determined from the experimentally observed fractions of fragmented L DNA formed and intact L DNA remaining after exposure to a given number of disintegrations:
where Fi is the fraction of intact L DNA remaining at any given time (ratio of 3H dpm in intact L DNA remaining after 123IEH-exposure to that in unirradiated control L DNA band), and Ff is the fraction of fragmented DNA occurring as a result of DSB due to 123I decays (1 − Fi).
The rate of formation of DSB per DNA molecule per decay of 123I is obtained by plotting XDSB as a function of the number of accumulated 123I decays per ml. We have carried out duplicate experiments and plotted them together in such a way as to nullify random errors associated with pipetting, loading, and recovery of samples. The standard errors obtained for the slopes of the linear regressions reflect such random pipetting, loading, and recovery errors. The straight lines are forced through zero, as at zero decay there would be neither single- nor double-strand breaks. The slopes of these linear regressions reflect DSB yield expressed as number of DSB generated in one DNA molecule per decay of 123I in one ml, and the reciprocal of the slopes represents D0, the number of decays per ml required to form one DSB in one DNA molecule. The slope, when multiplied by the total number of plasmid DNA molecules per ml, gives the yield of DSB per decay of 123I:
where [DNA]=3.06 × 1013 molecules/ml.
Results and discussion
Detection of 123IEH-induced double strand breaks in supercoiled 3H-pUC19 plasmid DNA
Supercoiled 3H-pUC19 DNA was incubated with 123IEH for two weeks to allow complete decay of all the 123I atoms present, and the DNA was analyzed on 1% agarose gels (Figure 1). Under the experimental conditions in these studies, the number of 123I atoms per plasmid DNA molecule is 0.3 for the lowest added dose (2.8 MBq) and 1.2 for the highest dose (10.9 MBq) of 123IEH. The gels show that SC DNA does not undergo strand breakage in the absence of 123IEH during the course of incubation (Figure 1, Lane 1). However, there is a gradual decrease in fluorescence intensity of the SC DNA bands with increases in 123IEH doses (Figure 1, lanes 2–6). The disappearance of SC DNA is accompanied by a concomitant appearance of N and L DNA indicating that the decay of 123IEH causes both SSB and DSB in SC pUC19 plasmid DNA.
Figure 1.
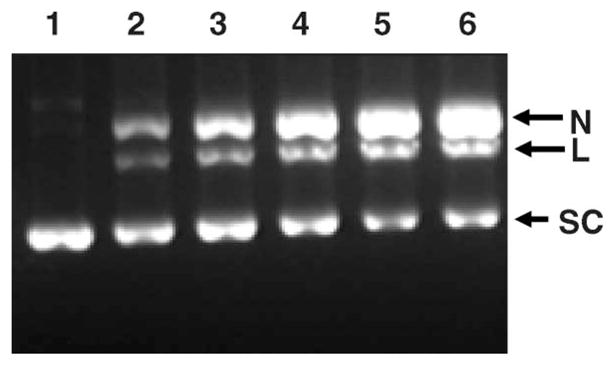
Agarose gel analysis of SC 3H-pUC19 plasmid DNA incubated with 123IEH at 4°C in PBS (pH 7.4): lane 1, control (no 123IEH); lane 2 9.7 × 1012 decays/ml; lane 3, 13.7 × 1012 decays/ml; lane 4, 23.5 × 1012 decays/ml; lane 5, 34.9 × 1012 decays/ml; and lane 6, 37.6 × 1012 decays/ml. Gels (containing ethidium bromide) were visualized using ultraviolet (320 nm) transillumination.
Quantitative analysis of DNA strand breaks in supercoiled 3H-pUC19 DNA
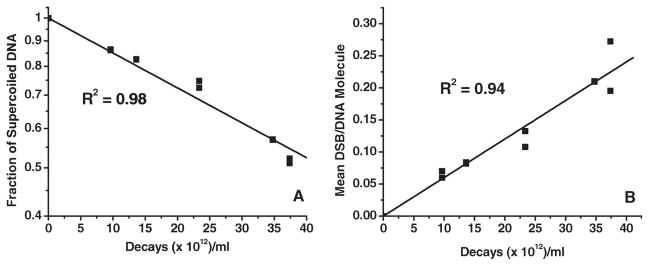
The bands corresponding to SC, N and L forms of DNA in each lane in the agarose gel shown in Figure 1 were excised and the fraction of the DNA in each of these topological forms was determined by assaying the associated tritium content. Analysis of the fractions of SC, L and N present at various doses of 123I reveals that: (i) At the lowest dose when the number of 123I atoms decayed per plasmid molecule is 0.3, ~15% of the SC DNA disappear, producing ~6% L and ~9% N forms. A similar trend is observed even at the highest dose at which the number of 123I atoms decayed per DNA molecule is ~1.2. At this 123IEH:DNA ratio, ~50% of SC molecules disappear producing ~20% L and ~30% N forms through DSB and SSB, respectively.
Double-strand break yields are calculated using the method of Cowan et al. (1987). Figure 2A shows the disappearance of SC DNA as a function of 123IEH decays. The D0 for 123IEH-decay-induced disappearance of SC DNA, calculated from the slope of the linear regression (Figure 2A) is (6.15 ± 0.19) × 1013 decays/ml, which is ~7 times higher than the D0 for 125IEH-induced disappearance of SC DNA (Balagurumoorthy et al. 2006).
Figure 2.
Quantitative analysis of data from agarose gel electrophoresis assessing disappearance of SC 3H-pUC19 plasmid DNA (A) and appearance of L DNA (B), indicator of DSB formation, as function of accumulated 123I decays.
The rate of formation of L DNA following DSB in SC DNA as a function of 123IEH decays is shown in Figure 2B. The D0 of (16.64 ± 0.67) × 1013 decays/ml for the formation of the L form is ~3-fold higher than that induced by 125IEH decays (Balagurumoorthy et al. 2006). Accordingly, the DSB yield for 123IEH (0.18 ± 0.01) is 3-fold lower than that generated by 125IEH decays (Table I). This 0.18 DSB/123I decay yield is ~3 times lower than that reported by Lobachevsky and Martin (2005) when SC DNA from a different plasmid (pBR322) was incubated with another Hoechst analog, 123I-iodoHoechst 33258 (123IMH). These authors also reported the ratio of DSB yields induced by the two Auger electron emitters 123I and 125I as 0.77 (123IMH: 0.62 DSB/decay; 125IMH: 0.82 DSB/decay), a value that differs from the ratio of 0.33 obtained when the DSB yields after 123IEH (current studies) and 125IEH (Balagurumoorthy et al. 2006) are compared. These differences may be attributed to the subtle dissimilarities in the plasmid DNA model (pUC19 vs. pBR322), the two carrier Hoechst ligands used (IEH vs. IMH), each with its binding specificity and affinity, and/or variation in experimental conditions. However, our finding that 125IEH is ~3 times more efficient than 123IEH in inducing DNA DSB in naked SC plasmid DNA (in comparison to the 1.3 value reported for IMH) is in line with our previous experimental studies in which 125I was ~ 2.2 times more efficient than 123I in causing DNA DSB in mammalian DNA (Makrigiorgos et al. 1992), a value that is also similar to the ratios obtained when the energy deposited in small spheres (2–50 nm radius) around decaying 125I and 123I atoms is estimated using semiempirical Monte Carlo calculations (Makrigiorgos et al. 1989).
Table I.
DSB yields in pUC19 SC and L plasmid DNA after decay of 123I bound to minor groove (123IEH): Comparison with 125IEH.
Data previously published (Balagurumoorthy et al. 2008). Standard errors are obtained from the slope of the linear regressions used to calculate the DSB yields.
123IEH-induced double-strand-breaks in linear DNA
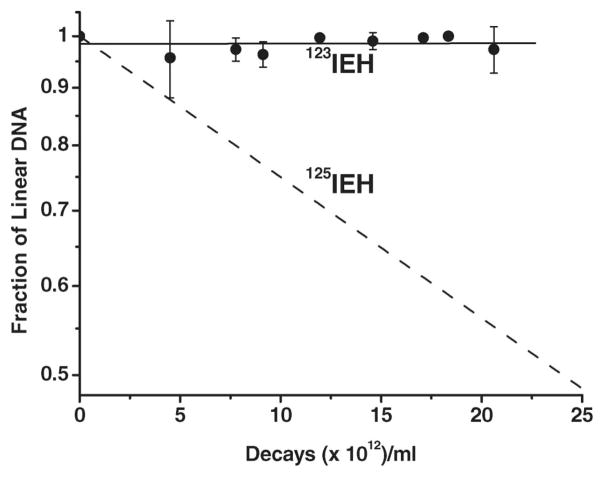
We have recently examined the effect of DNA topology on the mechanism and magnitude of DSB produced in plasmid pUC19 DNA by 125IEH (Balagurumoorthy et al. 2008) and γ rays (unpublished results). The data indicate that DSB yield is influenced by DNA topology for both types of radiation. For example, the DSB yield per DNA molecule following γ-ray irradiation of L DNA ([30.9 ± 2.17] × 10−4/Gy) is ~2.4-fold higher that obtained with SC DNA ([13.0 ± 0.51] × 10−4/Gy). Similarly, 125IEH-induced DSB yield in the L form (1.62 ± 0.07) is 3-fold higher than that in the SC form (0.52 ± 0.02). These results lead us to conclude that DNA topology affects the DSB yield following γ-irradiation or 125I decay in close proximity to DNA.
In analogy with these latter findings, we expected each decay of 123IEH in L DNA to generate a 2- to 3-fold higher DSB yield (0.4–0.6 DSB/decay) than that obtained for SC DNA (0.18 ± 0.01). However, when L DNA samples were incubated with the same 123IEH concentration and run on 1% agarose gel, we could not detect any reduction in the fluorescence intensity of the intact L DNA band due to DSB (Figure 3, lanes 1–5), except for the faint smear seen in the lanes (insignificant compared to the total amount of DNA present in the lanes). These observations indicate that, within the radioactive concentrations used, the decay of 123IEH is either unable to or minimally able to induce DSB. This conclusion is supported by our data (3H-TdR counts in excised fluorescent L DNA band) showing that the amount of intact L DNA present in the gels is not reduced following exposure to 123IEH. Consequently, when the fractions of L DNA remaining post irradiation are plotted as a function of 123IEH decays/ml (Figure 4), a very shallow slope (6.2 × 10−5) is obtained (essentially, there is no quantifiable decrease in the amount of L DNA). For comparison, the rapid rate at which DNA disappears following exposure of L DNA to 125IEH decays (under similar experimental conditions) is shown as a dotted line (Balagurumoorthy et al. 2008). Note that ~50% of the L DNA molecules exposed to ~25 × 1012 decays per ml 125IEH have disappeared but that for a similar number of 123IEH decays, ~100% of the L DNA is intact. Thus, unlike 125IEH which induces DSB in both SC and L DNA, 123IEH induces DSB only in the SC form.
Figure 3.
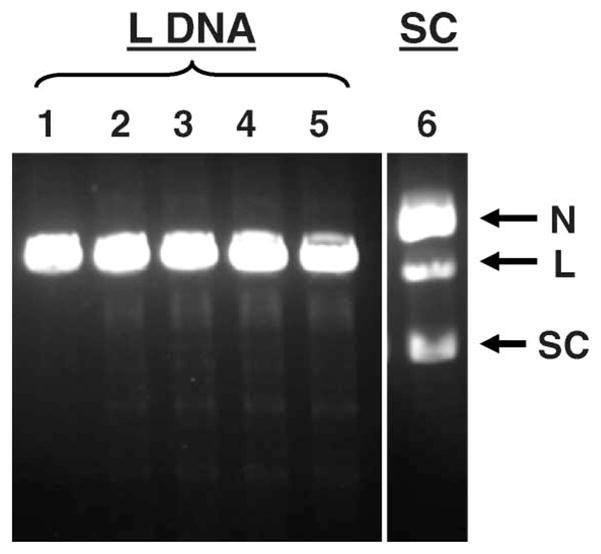
Agarose gel analysis of L form of 3H-pUC19 plasmid DNA incubated with 123IEH at 4°C in PBS (pH 7.4): lane 1, control (no 123IEH); lane 2, 4.6 × 1012 decays/ml; lane 3, 8.1 × 1012 decays/ml; lane 4, 11.5 × 1012 decays/ml; and lane 5, 21.9 × 1012 decays/ml, lane 6, SC DNA exposed at the highest dose (21.9 × 1012 decays/ml) showing DSB formation for comparison.
Figure 4.
Quantitative analysis of data obtained from agarose gel electrophoresis indicating disappearance of L 3H-pUC19 plasmid DNA after exposure to 123IEH (●). Error bars are the standard deviation of the mean for three independent experiments. Dotted line shows rate of disappearance of L DNA exposed to 125IEH decays (Balagurumoorthy et al. 2008).
To confirm these observations, the disappearance of L DNA following exposure to the same number of 123IEH and 125IEH decays (~2 × 1011) was compared. The results (Figure 5) show that the fluorescence intensity of intact L DNA band after 125I decays is clearly diminished when compared with the DNA control band whereas nearly 100% of L DNA remains intact after being irradiated with the same number of 123IEH decays (very similar to the unirradiated pUC19 L DNA controls). When the amount of L DNA in each band was quantified, the data demonstrate that exposure to 2 × 1011 125IEH decays leads to the disappearance of ~50% of L DNA (due to DSB) whereas > 95% of 123I-exposed DNA is still present as L DNA.
Figure 5.
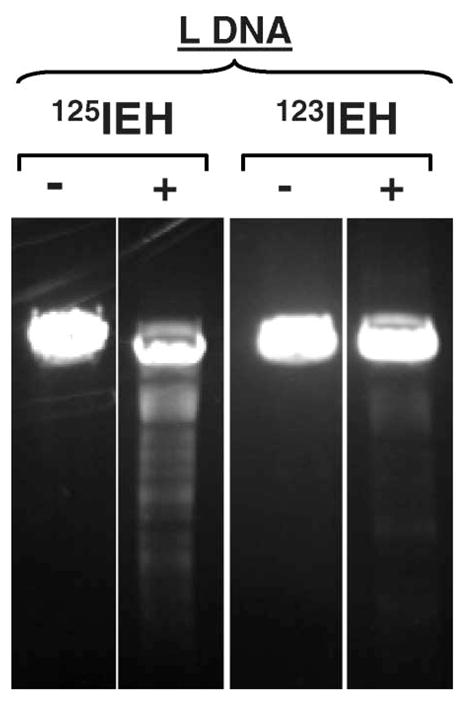
Comparison of pUC19 L DNA exposed to the same number (~2 × 1011) of 125IEH and 123IEH decays per μg DNA: (−) and (+) indicate absence and presence of radioiodinated ligand, respectively.
We have no ready explanation for the failure of 123I decay to produce DSB in L DNA. 123I differs from 125I in several ways: The latter decays in two steps –electron capture (EC) and internal conversion (IC); each step produces an average of ~10 low-energy electrons. Iodine-123 decays by EC alone, producing ~10 electrons only. Consequently, 125I builds up twice the positive charge as that of 123I and the dissipation of the potential energy associated with the higher positive charge of the residual 125I atom and its neutralization may, in principle, also act concomitantly and be responsible for the differences in the observed effects. In addition the decay constant for 123II is considerably greater than that for 125I, i.e., 123I has a much shorter half-life.
We can only speculate on some of the possible reasons underlying this unexpected finding (Table II): (1) The DSB yield following the decay of the two iodine isotopes is the same for the common EC step – 0.2 in SC DNA and 0.0 in L DNA; (2) the differences in total DSB yield are mainly due to the IC step (125I decay) − 0.3 and 1.6 DSB respectively for SC and L. Without IC decay, there are no subsequent DSB produced by 123I.
Table II.
Comparison of DSB yields among different plasmid DNA forms: Dependence of DNA topology.
| DSB per decay |
|||||||
|---|---|---|---|---|---|---|---|
| 1st step: EC | 2nd step: IC | Highly compacted Highly curved | Terminal nucleotide(s) | Experimental | Assigned* | ||
| SC | 125I | Yes | – | Yes | No | 0.5 | 0.2 |
| – | Yes | Yes | Yes | 0.3 | |||
| 123I | Yes | – | Yes | No | 0.2 | 0.2 | |
| – | No | NA | NA | NA | |||
| L | 125I | Yes | – | No | Yes | 1.6 | 0.0 |
| – | Yes | No | Yes | 1.6 | |||
| 123I | Yes | – | No | Yes | 0.0 | 0.0 | |
| No | NA | NA | NA | ||||
Segregation calculated assuming the same DSB yields for both radionuclides following EC.
What mechanisms might prevent the EC step from producing DSB in L DNA? Obviously, the topology of the L DNA molecules, which differs from that of SC DNA, may play an important role, e.g., the greater stokes radius of the L form, the prominent curvatures within the compacted SC DNA molecules vs. the relaxed elongated linear structure of L DNA molecules. For example, the curvature could increase the DSB yield in SC DNA consequent to the positioning of some bases that are hundreds/thousands of angstroms away from the minor groove-bound decaying 123IEH but within the range of emitted electrons and/or radicals formed along their tracks. Another possibility is electron tunneling/migration within double stranded DNA molecules, a phenomenon that leads to the induction of DNA lesions over long distances (Bixon et al. 1999, Nunez et al. 1999, Giese 2002, 2006). One can imagine that migration of emitted low energy Auger electrons within the plasmid DNA is more likely to damage SC DNA (with electrons being trapped within the circular structure and a higher probability of interacting with an atom within the DNA molecule and forming a break) than L DNA (with particles escaping from the termini of L DNA and thereby reducing the opportunity to interact with atoms within the DNA molecule and rupture it). Alternatively, to the extent that charge neutralization may also contribute to DSB production (Charlton et al. 1987, Kassis et al. 1987, Lobachevsky and Martin 2000), a greater charge may be necessary to fracture linear DNA than the supercoiled form. Clearly, further experimentation will be necessary to gain insights into the rationale behind this surprising phenomenon.
Conclusions
Comparison of DSB produced by 123IEH and 125IEH following their decay within the minor groove of plasmid SC DNA indicates that the DSB yield for these two Auger emitters is consistent with dosimetric expectations: 123IEH is ~3 times less efficient than 125IEH in inducing DSB, and this relative efficacy is in reasonable agreement with studies in mammalian cells using 123IUdR and 125IUdR. That 123IEH decay does not induce DSB in the relaxed L form whereas 125IEH decay leads to a 3-fold increase in DSB yield (compared with the yield in SC DNA) highlights the important and unpredictable role of DNA topology (and other factors) in DSB production by Auger emitters. This observation underscores the failure of current dosimetric methods to predict the magnitude of DSB. The current work suggests the need for developing more comprehensive models that include DNA structure and topology for examining the biophysical mechanisms underlying DSB produced by Auger emitters.
Acknowledgments
This work was supported in part by NIH 5 R01 CA15523 (Amin I. Kassis) and NIH 5 T32 CA009078 (Pichumani Balagurumoorthy).
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Publisher's Disclaimer: Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
References
- Auger P. Sur les rayons b secondaires produits dans un gaz par des rayons X. Comptes Rendues Hebdomadaires des Seances de l’Academie des Sciences. 1925;180:65–68. [Google Scholar]
- Balagurumoorthy P, Chen K, Adelstein SJ, Kassis AI. Auger electron-induced double-strand breaks depend on DNA topology. Radiation Research. 2008;170:70–82. doi: 10.1667/RR1072.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balagurumoorthy P, Chen K, Bash RC, Adelstein SJ, Kassis AI. Mechanisms underlying production of double-strand breaks in plasmid DNA after decay of 125I-Hoechst. Radiation Research. 2006;166:333–344. doi: 10.1667/RR3591.1. [DOI] [PubMed] [Google Scholar]
- Bixon M, Giese B, Wessely S, Langenbacher T, Michel-Beyerle ME, Jortner J. Long-range charge hopping in DNA. Proceedings of the National Academy of Sciences of the USA. 1999;96:11713–11716. doi: 10.1073/pnas.96.21.11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomer WD, Adelstein SJ. 5-125I-iododeoxyuridine as prototype for radionuclide therapy with Auger emitters. Nature. 1977;265:620–621. doi: 10.1038/265620a0. [DOI] [PubMed] [Google Scholar]
- Chan PC, Lisco E, Lisco H, Adelstein SJ. The radiotoxicity of iodine-125 in mammalian cells. II. A comparative study on cell survival and cytogenetic responses to 125IUdR, 131IUdR, and 3HTdR. Radiation Research. 1976;67:332–343. [PubMed] [Google Scholar]
- Chan PC, Lisco E, Lisco H, Adelstein SJ. Cell survival and cytogenetic responses to 125I-UdR in cultured mammalian cells. Current Topics in Radiation Research Quarterly. 1977;12:426–435. [PubMed] [Google Scholar]
- Charlton DE, Pomplun E, Booz J. Some consequences of the Auger effect: Fluorescence yield, charge potential, and energy imparted. Radiation Research. 1987;111:553–564. [PubMed] [Google Scholar]
- Cole A. Absorption of 20-eV to 50,000-eV electron beams in air and plastic. Radiation Research. 1969;38:7–33. [PubMed] [Google Scholar]
- Cowan R, Collis CM, Grigg GW. Breakage of double-stranded DNA due to single-stranded nicking. Journal of Theoretical Biology. 1987;127:229–245. doi: 10.1016/s0022-5193(87)80133-9. [DOI] [PubMed] [Google Scholar]
- DeSombre ER, Hughes A, Hanson RN, Kearney T. Therapy of estrogen receptor-positive micrometastases in the peritoneal cavity with Auger electron-emitting estrogens: theoretical and practical considerations. Acta Oncologica. 2000;39:659–666. doi: 10.1080/028418600750063695. [DOI] [PubMed] [Google Scholar]
- DeSombre ER, Hughes A, Shafii B, Púy L, Kuivanen PC, Hanson RN, Harper PV. Estrogen receptor-directed radiotoxicity with Auger electron-emitting nuclides: E-17a-[123I] iodovinyl-11b-methoxyestradiol and CHO-ER cells. In: Howell RW, Narra VR, Sastry KSR, Rao DV, editors. Biophysical aspects of Auger processes; American Association of Physicists in Medicine Symposium Series No. 8; Woodbury, NY: American Institute of Physics; 1992. pp. 352–371. [Google Scholar]
- Feinendegen LE. Biological damage from the Auger effect, possible benefits. Radiation and Environmental Biophysics. 1975;12:85–99. doi: 10.1007/BF01328970. [DOI] [PubMed] [Google Scholar]
- Giese B. Long-distance electron transfer through DNA. Annual Review of Biochemistry. 2002;71:51–70. doi: 10.1146/annurev.biochem.71.083101.134037. [DOI] [PubMed] [Google Scholar]
- Giese B. Electron transfer through DNA and peptides. Bioorganic and Medicinal Chemistry. 2006;14:6139–6143. doi: 10.1016/j.bmc.2006.05.067. [DOI] [PubMed] [Google Scholar]
- Harapanhalli RS, McLaughlin LW, Howell RW, Rao DV, Adelstein SJ, Kassis AI. [125I/127I]iodoHoechst 33342: synthesis, DNA binding, and biodistribution. Journal of Medicinal Chemistry. 1996;39:4804–4809. doi: 10.1021/jm9602672. [DOI] [PubMed] [Google Scholar]
- Hofer KG, Hughes WL. Radiotoxicity of intranuclear tritium, 125iodine and 131iodine. Radiation Research. 1971;47:94–109. [PubMed] [Google Scholar]
- Kassis AI. The amazing world of Auger electrons. International Journal of Radiation Biology. 2004;80:789–803. doi: 10.1080/09553000400017663. [DOI] [PubMed] [Google Scholar]
- Kassis AI, Fayad F, Kinsey BM, Sastry KSR, Adelstein SJ. Radiotoxicity of a 125I-labeled DNA intercalator in mammalian cells. Radiation Research. 1989;118:283–294. [PubMed] [Google Scholar]
- Kassis AI, Harapanhalli RS, Adelstein SJ. Comparison of strand breaks in plasmid DNA after positional changes of Auger electron-emitting iodine-125. Radiation Research. 1999a;151:167–176. [PubMed] [Google Scholar]
- Kassis AI, Harapanhalli RS, Adelstein SJ. Strand breaks in plasmid DNA after positional changes of Auger electron-emitting iodine-125: Direct compared to indirect effects. Radiation Research. 1999b;152:530–538. [PubMed] [Google Scholar]
- Kassis AI, Kirichian AM, Wang K, Safaie Semnani E, Adelstein SJ. Therapeutic potential of 5-[125I]iodo-2′-deoxyuridine and methotrexate in the treatment of advanced neoplastic meningitis. International Journal of Radiation Biology. 2004;80:941–946. doi: 10.1080/09553000400017671. [DOI] [PubMed] [Google Scholar]
- Kassis AI, Sastry KSR, Adelstein SJ. Kinetics of uptake, retention, and radiotoxicity of 125IUdR in mammalian cells: Implications of localized energy deposition by Auger processes. Radiation Research. 1987;109:78–89. [PubMed] [Google Scholar]
- Kassis AI, Walicka MA, Adelstein SJ. Double-strand break yield following 125I decay: Effects of DNA conformation. Acta Oncologica. 2000;39:721–726. doi: 10.1080/028418600750063785. [DOI] [PubMed] [Google Scholar]
- Kassis AI, Wen PY, Van den Abbeele AD, Baranowska-Kortylewicz J, Makrigiorgos GM, Metz KR, Matalka KZ, Cook CU, Sahu SK, Black PM, Adelstein SJ. 5-[125I]iodo-2′-deoxyuridine in the radiotherapy of brain tumors in rats. Journal of Nuclear Medicine. 1998;39:1148–1154. [PubMed] [Google Scholar]
- Lobachevsky PN, Martin RF. Iodine-125 decay in a synthetic oligodeoxynucleotide. II. The role of Auger electron irradiation compared to charge neutralization in DNA breakage. Radiation Research. 2000;153:271–278. doi: 10.1667/0033-7587(2000)153[0271:idiaso]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Lobachevsky PN, Martin RF. DNA breakage by decay of Auger electron emitters: experiments with 123I-iodo-Hoechst 33258 and plasmid DNA. Radiation Research. 2005;164:766–773. doi: 10.1667/rr3469.1. [DOI] [PubMed] [Google Scholar]
- Makrigiorgos GM, Berman RM, Baranowska-Kortylewicz J, Bump E, Humm JL, Adelstein SJ, Kassis AI. DNA damage produced in V79 cells by DNA-incorporated iodine-123: A comparison with iodine-125. Radiation Research. 1992;129:309–314. [PubMed] [Google Scholar]
- Makrigiorgos GM, Kassis AI, Baranowska-Kortylewicz J, McElvany KD, Welch MJ, Sastry KSR, Adelstein SJ. Radiotoxicity of 5-[123I]iodo-2′-deoxyuridine in V79 cells: a comparison with 5-[125I]iodo-2′-deoxyuridine. Radiation Research. 1989;118:532–544. [PubMed] [Google Scholar]
- Martin RF. Induction of double-stranded breaks in DNA by binding with a 125I-labelled acridine. International Journal of Radiation Biology. 1977;32:491–497. doi: 10.1080/09553007714551261. [DOI] [PubMed] [Google Scholar]
- Martin RF, Holmes N. Use of a 125I-labelled DNA ligand to probe DNA structure. Nature. 1983;302:452–454. doi: 10.1038/302452a0. [DOI] [PubMed] [Google Scholar]
- Nunez ME, Hall DB, Barton JK. Long-range oxidative damage to DNA: Effects of distance and sequence. Chemistry and Biology. 1999;6:85–97. doi: 10.1016/S1074-5521(99)80005-2. [DOI] [PubMed] [Google Scholar]
- Panyutin IG, Winters TA, Feinendegen LE, Neumann RD. Development of DNA-based radiopharmaceuticals carrying Auger-electron emitters for anti-gene radiotherapy. Quarterly Journal of Nuclear Medicine. 2000;44:256–267. [PubMed] [Google Scholar]
- Panyutin IV, Luu AN, Panyutin IG, Neumann RD. Strand breaks in whole plasmid DNA produced by the decay of 125I in a triplex-forming oligonucleotide. Radiation Research. 2001;156:158–166. doi: 10.1667/0033-7587(2001)156[0158:sbiwpd]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Pomplun E, Booz J, Charlton DE. A Monte Carlo simulation of Auger cascades. Radiation Research. 1987;111:533–552. [PubMed] [Google Scholar]
- Rawson RW, Rall JE, Peacock W. Limitations and indications in the treatment of cancer of the thyroid with radioactive iodine. Journal of Clinical Endocrinology and Metabolism. 1951;11:1128–1142. doi: 10.1210/jcem-11-10-1128. [DOI] [PubMed] [Google Scholar]
- Reynolds JC, Robbins J. The changing role of radioiodine in the management of differentiated thyroid cancer. Seminars in Nuclear Medicine. 1997;27:152–164. doi: 10.1016/s0001-2998(97)80045-1. [DOI] [PubMed] [Google Scholar]
- Sastry KSR, Rao DV. Dosimetry of low energy electrons. In: Rao DV, Chandra R, Graham MC, editors. Physics of nuclear medicine: Recent advances. Woodbury, NY: American Institute of Physics; 1984. pp. 169–208. [Google Scholar]
- Walicka MA, Adelstein SJ, Kassis AI. Indirect mechanisms contribute to biological effects produced by decay of DNA-incorporated iodine-125 in mammalian cells in vitro: Clonogenic survival. Radiation Research. 1998;149:142–146. [PubMed] [Google Scholar]
- Yaakob W, Gordon L, Spicer KM, Nitke SJ. The usefulness of iodine-123 whole-body scans in evaluating thyroid carcinoma and metastases. Journal of Nuclear Medicine Technology. 1999;27:279–281. [PubMed] [Google Scholar]