Abstract
Human lens proteins (HLP) become chemically modified by kynurenines and advanced glycation end products (AGEs) during aging and cataractogenesis. We investigated the effects of kynurenines on AGE synthesis in HLP. We found that incubation with 5 mM ribose or 5 mM ascorbate produced significant quantities of pentosidine, and this was further enhanced in the presence of two different kynurenines (200–500 µM): N-formylkynurenine (Nfk) and kynurenine (Kyn). Another related compound, 3-hydroxykynurenine (3OH-Kyn), had disparate effects; low concentrations (10–200 µM) promoted pentosidine synthesis, but high concentrations (200–500 µM) inhibited it. 3OH-Kyn showed similar effects on pentosidine synthesis from Amadori-enriched HLP or ribated lysine. Chelex-100 treatment of phosphate buffer reduced pentosidine synthesis from Amadori-enriched HLP by ~90%, but it did not inhibit the stimulating effect of 3OH-Kyn and EDTA. 3OH-Kyn (100–500 µM) spontaneously produced copious amounts of H2O2 (10–25 µM), but externally added H2O2 had only a mild stimulating effect on pentosidine but had no effect on Nε-carboxymethyl lysine (CML) synthesis in HLP from ribose and ascorbate. Further, human lens epithelial cells incubated with ribose and 3OH-Kyn showed higher intracellular pentosidine than cells incubated with ribose alone. CML synthesis from glycating agents was inhibited 30 to 50% by 3OH-Kyn at concentrations of 100–500 µM. Argpyrimidine synthesis from 5 mM methylglyoxal was slightly inhibited by all kynurenines at concentrations of 100–500 µM. These results suggest that AGE synthesis in HLP is modulated by kynurenines, and such effects indicate a mode of interplay between kynurenines and carbohydrates important for AGE formation during lens aging and cataract formation.
Keywords: Kynurenines, Glycation, Lens proteins, Cataract
1. Introduction
The glycation of proteins via the Maillard reaction involves multi-step, spontaneous reactions between carbonyl groups of sugars and free amino groups in proteins. In addition to sugars, carbonyls such as methylglyoxal (MGO), glyoxal and ascorbic acid oxidation products can also initiate this process. Upon reaction of sugars with protein amino groups, a Schiff base is formed, which is then converted to a more stable Amadori product. A cascade of rearrangements and further reactions converts this intermediate into structurally divergent products known collectively as advanced glycation end products (AGEs) [1, 2].
The lens of the human eye consists of highly packed fiber cells that contain abundant proteins and lack organelles. Lens proteins have minimal turnover, and as a result, accumulate post-translational modifications throughout their lifespans [3]. The Maillard reaction is well recognized as a prominent mechanism for post-translational modification of lens proteins, producing AGE modifications. Several AGEs have been detected in the human lens, including 6-{2-[(4-amino-4-carboxybutyl)amino]-3H-imidazo[4,5-b]pyridin-4-ium-4-yl}norleucine (pentosidine) [4], N5-(5-hydroxy-4,6-dimethyl-2-pyrimidin-2-yl)ornithine (argpyrimidine) [5], hydroimidazolones [6], Nε-carboxymethyl lysine (CML) [7], 1,4-bis(5-amino-5-carboxypentyl)-6-hydroxy-1H-pyrrolo[3,2-b]pyridinium (vesperlysine A) [8], 2-Ammonio-6-(3-oxidopyridinium-1-yl)hexanoate (OP-lysine) [9] and 1-(5-amino-5-carboxypentyl)-4-(5-amino-5-carboxypentyl-amino)-3-hydroxy-2, 3-dihydropyridinium (K2P) [10]. Since AGE formation can result in protein conformational changes and crosslinking, and because these changes accumulate in lens proteins, this mechanism is thought to contribute to lens aging and cataract formation.
The precursors of pentosidine and CML are hexoses, pentoses and ascorbic acid [4, 11]. Pentosidine formation from ascorbic acid and glucose depends on molecular oxygen [11]. Whether synthesis from ribose has a similar requirement is not clear, but one study has shown only partial inhibition of pentosidine synthesis under conditions of oxygen depletion [12]. CML formation from sugars occurs by metal ion-dependent cleavage of the Schiff’s base and Amadori product, and by autoxidation of sugars before they react with proteins [13].
Kynurenines are diffusible components of the lens that absorb UVA and UVB radiation and thus are believed to protect the retina from light damage [14]. They are produced by the oxidation of tryptophan [15]. The enzymatic pathway, known as the kynurenine pathway, is initiated by indoleamine 2,3-dioxygenase (IDO), which oxidizes tryptophan to N-formylkynurenine (Nfk). Nfk is subsequently hydrolyzed to kynurenine (Kyn) by kynurenine formidase [14]. Kyn is then hydroxylated by kynurenine 3-hydroxylase to 3-hydroxykynurenine (3OH-Kyn). The reported concentrations of Kyn and 3OH-Kyn in the human lens are 5–30 nmol/g and 2–15 nmol/g, respectively [16]. Human lens kynurenines also exist as O-β-D-glucosides, of which 3-hydroxykynurenine O-β-D-glucoside (3OH-KynG) and 4-(2-amino-3-hydroxyphenyl)-4-oxabutanoic acid O-β-D-glucoside are the most abundant forms [17].
Kynurenines are unstable under physiological conditions; they undergo deamination with a half life of approximately seven days [18]. The deaminated products form α,β-ketoalkenes that can react with lens proteins and modify specific amino acids [19–21]. Consequently, such modifications are thought to contribute to age and cataract-associated changes in human lens proteins [17, 20, 22]. In recent studies, we demonstrated that overexpression of IDO in the mouse lens causes kynurenine-mediated modification of lens proteins, lens epithelial cell cycle arrest, apoptosis of fiber cells, and cataracts, further underlining the importance of kynurenines in lens protein modification [23, 24]. It is also known that 3OH-Kyn can spontaneously generate hydrogen peroxide in a transition metal ion-dependent manner [25]. Incubation of bovine α-crystallins with low concentrations of 3OH-Kyn causes protein crosslinking and oxidation of methionine and tryptophan residues [26], indicating that the protein damage likely results from generation of reactive oxygen species. These observations suggest overlapping mechanisms in kynurenine- and AGE-mediated protein modifications in the lens. Therefore, the present study was designed to investigate the possible effects of kynurenines on AGE formation in lens proteins. We chose three AGE markers (pentosidine, CML and argpyrimidine) to determine the effects of kynurenines on ribose-, ascorbate- and MGO-initiated Maillard reactions.
2. Materials and methods
2.1. Materials
Nα-acetyl arginine, Nα-acetyl lysine, methylglyoxal (40%), D-ribose, ascorbic acid sodium salt, Chelex-100, Kyn and 3OH-Kyn were from Sigma Chemical Co. (St. Louis, MO). Nfk was synthesized as previously described [27]. Nα-BOC lysine was from Bachem Americas, Inc. (Torrance, CA). The CML monoclonal antibody was a kind gift from Jes Thorn Clausen (Novo Nordisk, Germany).
2.2. Incubation of human lens proteins with kynurenines and glycating agents
a) Continuous glycation
Proteins were extracted from pooled normal human lenses obtained from donors of ages 20–50 years. Each lens was homogenized in 1 ml of PBS and centrifuged at 20,000 g for 30 min at 4°C. The supernatant, which contained water-soluble proteins (HLP) was dialyzed against 2 L of PBS for 24 hrs. HLP samples (1 mg/ml) were incubated for 7 days at 37°C in 0.1 M sodium phosphate buffer (pH 7.4) with one of the following glycating agents: 5 mM ribose, 5 mM ascorbate or 5 mM MGO in the presence or absence of 0–500 µM kynurenines. All reaction mixtures were sterile-filtered with 0.2-µm syringe filters (Millipore Corp, MA) before incubation with the glycating agents. In another experiment HLP was similarly incubated with 3OH-Kyn in the absence of oxygen (incubation was done in glass ampules after bubbling argon for 10 min and sealing the ampules). BSA at 1 mg/ml was similarly incubated with 5 mM ribose in the presence of 0–500 µM 3OH-Kyn. After incubation, samples were dialyzed against 2 L of PBS for 48 hrs with a change after 24 hrs.
b) Interrupted glycation
HLP (1 mg/ml) was incubated with 5 M ribose for 24 hrs at 37 °C in 0.4 M sodium phosphate buffer (pH 7.4) and dialyzed for 48 hrs against 0.05 M sodium phosphate buffer (pH 7.4). It has been reported that incubation of proteins with 5 M ribose for this period of time enriches them with Amadori product [28]. After dialysis, the proteins were incubated with or without one of the following: 1 mM Nα-acetyl arginine, 1 mM Nα-acetyl arginine + 10–50 µM 3OH-Kyn, 1 mM Nα-acetyl arginine + 10–50 µM 3OH-Kyn + 1 mM EDTA, 10–50 µM 3OH-Kyn, or 1 mM EDTA for 7 days at 37 °C in 0.1 M sodium phosphate buffer (pH 7.4). The protein was then dialyzed against PBS for 24 hrs. The dialyzed protein was acid hydrolyzed and analyzed for pentosidine (see below). An identical set of incubations was done in Chelex-100 treated sodium phosphate buffer (pH 7.4). These samples were dialyzed as above, except that the buffer was also Chelex-100 treated.
2.3. Incubation of ribated-Nα-BOC-lysine
Ribated-Nα-BOC-lysine (referred to as ribated lysine henceforth) was prepared following the method of Grandhee and Monnier [4]. Briefly, Nα-BOC-lysine was mixed with 0.04 M ribose in 100 ml methanol and refluxed for 45 min. Methanol was removed under vacuum, and the resulting gummy material was resuspended in 0.2 M pyridine formate buffer (pH 3.5) and loaded onto a Dowex 50×4 cation exchange column (in pyridine form) using the same buffer as the eluant. The column eluate was collected in 4.0 ml fractions, and the presence of ribated lysine was assessed by TLC using silica gel plates with ninhydrin as the detection reagent. Fractions with ribated lysine were pooled and lyophilized. Then, the ribated lysine in 0.1 M sodium phosphate buffer, pH 7.4 was mixed with 1 mM Nα-acetyl arginine and incubated in the presence or absence of the following: 0–300 µM 3OH-Kyn or 0–300 µM 3OH-Kyn + 1 mM EDTA for 3 days at 37 °C. Samples were hydrolyzed with 6 N HCl at 110 °C for 16 to 20 hrs, dried in a Speed Vac concentrating system and subjected to pentosidine analysis as described below.
2.4. Measurement of pentosidine and argpyrimidine
Protein samples (1 mg/ml) were subjected to acid hydrolysis as described above. Dried samples were reconstituted in 250 µl of water and filtered through 0.45-µm centrifugal filters. Pentosidine and argpyrimidine were measured by HPLC using a reversed-phase C18 column as described previously [29]. The amino acid content of the acid hydrolysates was estimated by the ninhydrin assay [30]. Pentosidine and argpyrimidine concentrations in the protein samples were calculated based on the standard curves generated using synthetic compounds and were expressed as pmoles/µmol of amino acid.
2.5. Measurement of CML
CML was measured by a direct ELISA. Microplate wells were coated with protein (1 µg/well) diluted in 50 mM carbonate buffer (pH 9.6) and incubated overnight at 4°C. The wells were washed three times with PBS containing 0.05 % Tween-20 (PBS-T) and blocked with 5% non-fat dry milk (NFDM) for 2 h at room temp. After washing three times with PBS-T, the plate was incubated for 1 h at 37°C with the anti-CML monoclonal antibody diluted 1:5,000 in 5% NFDM (Bio-Rad) prepared in PBS-T. After washing three times with PBS-T, the plate was incubated for 1 h at 37°C with goat anti-mouse IgG conjugated with HRP (Promega Corp., Madison, WI) that was diluted in PBS-T 1:5,000. After three washings with PBS-T, the reaction was developed by the addition of 100 µl of 3,3’,5,5’-tetramethylbenzidine substrate (Sigma Aldrich) to each well. The enzyme reaction was stopped by the addition of 50 µl of 2 N H2SO4. Absorption at 450 nm was measured in a microplate reader.
2.6. Formation of hydrogen peroxide by 3OH-Kyn
HLP samples (1 mg/ml) were incubated with 5 mM ribose, ascorbate or MGO in 0.1 M sodium phosphate buffer (pH 7.4) in the presence of 0–500 µM 3OH-Kyn. After incubation at 37°C for 7 days, 50-µl aliquots were withdrawn, and hydrogen peroxide was measured with an Amplex Red Hydrogen Peroxide/Peroxidase Assay Kit (Invitrogen-Molecular Probes, Eugene, OR). HLP incubated with 0–500 µM 3OH-Kyn alone served as controls.
2.7. Effect of 3OH-Kyn on intracellular glycation in HLE B-3 cells
HLE B-3 cells (passage number between 5 and 8) were cultured in minimum essential medium (MEM) with 20% fetal calf serum (FCS), 50 µg/ml gentamycin and 2 mM glutamine. Cells at 50% confluence were weaned into FCS-free MEM containing 0–100 µM 3OH-Kyn and 20 mM ribose and cultured for 4 days. Cells were harvested, washed three times with PBS, hydrolyzed with 6 N HCl and subjected to pentosidine analysis (as above).
3. Results
3.1. Kynurenines modulate AGE synthesis
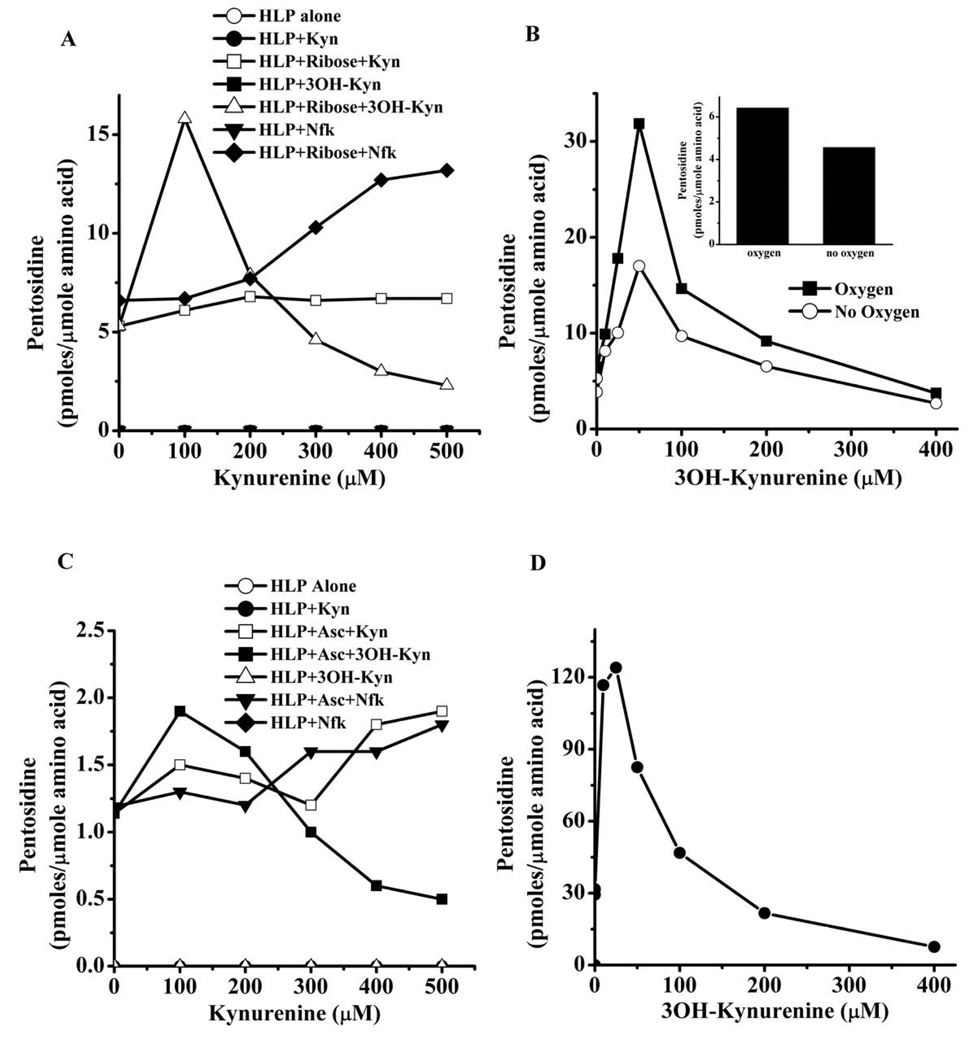
We used ribose in our incubation because it is one of the best precursor sugars for CML and pentosidine synthesis. HLP incubated with ribose in the presence of Nfk and Kyn promoted pentosidine formation at concentrations higher than 200 µM (Fig. 1A). After 7 days of incubation, 500 µM Nfk increased pentosidine synthesis in HLP nearly 2-fold over that incubated with ribose alone. Five hundred µM Kyn had a weaker effect, with an increase in pentosidine from 5.3 to 6.7 pmoles/µmol amino acids. In contrast, 3OH-Kyn at concentrations greater than 200 µM inhibited pentosidine formation, and it was almost completely blocked by 500 µM 3OH-Kyn. Conversely, lower concentrations of 3OH-Kyn (< 200 µM) promoted pentosidine formation from ribose. Pentosidine levels increased with the increase in 3OH-Kyn concentration from 0–50 µM, with the highest stimulating effect occurring with 50 µM 3OH-Kyn (Fig. 1B). At this 3OH-Kyn concentration, the pentosidine level was more than 7-fold higher than in the absence of 3OH-Kyn. Any further increase in the concentration of 3OH-Kyn reduced its promotional effect. It is possible, that at high concentrations 3OH-Kyn might have catalyzed degradation of pentosidine.
Fig. 1. Kynurenines modulate pentosidine synthesis.
HLP was incubated with 5 mM ribose (A) or 5 mM ascorbate (C) in the presence of 0–500 µM kynurenines for one week at pH 7.4 and 37 °C. Samples were exhaustively dialyzed to remove free reactants, and proteins were hydrolyzed with 6 N HCl and analyzed by fluorescence (335/385 nm)-reverse-phase HPLC. The effects of low concentrations of 3OH-Kyn (<100 µM) and absence of oxygen on ribose-mediated pentosidine are shown in B. Effect of 3OH-Kyn on ribose-mediated pentosidine synthesis in BSA is shown in D. For quantification of pentosidine in the samples, a standard curve was generated using synthetic pentosidine. Each data point is the average of two independent experiments. Asc=ascorbate.
3OH-Kyn undergoes autoxidation under aerobic conditions. To determine if such autoxidation played a role in its effect on pentosidine synthesis, we incubated HLP with ribose and 3OH-Kyn in the absence of oxygen for 7 days at 37 °C. We found that in the absence of oxygen 3OH-Kyn had similar effects on pentosidine synthesis as in the presence of oxygen, although the pentosidine levels were 30–50% lower (Fig. IB). This decrease was not due to oxidation of 3OH-Kyn, because in the absence of oxygen and 3OH-Kyn there was ~40% decrease in pentosidine from ribose (Fig. 1B inset).
It was possible that intrinsic UV filters present in human lens proteins could have played a role in the observed effects. To verify this possibility, we incubated bovine serum albumin (1mg/ml) with 5 mM ribose and 0–400 µM 3OH-Kyn for 7 days. 3OH-Kyn at low concentrations promoted and at high concentrations inhibited pentosidine synthesis (Fig. 1D). These results are similar to the results from HLP incubations, ruling out a role for intrinsic UV filters in effects seen with HLP.
Pentosidine synthesis from ascorbate was 5 times lower when compared to ribose (Fig. 1C). However, the effect of kynurenines on pentosidine formation was similar. At low concentrations (<200 µM), both Nfk and Kyn slightly increased the formation of pentosidine; at 100 µM, the increase was ~9 and 17% with Nfk and Kyn, respectively. However, the increase with Nfk was more apparent at higher concentrations; at 500 µM concentration there was a ~2.5-fold higher level of pentosidine than in control. 3OH-Kyn, at concentrations up to 200 µM, increased pentosidine synthesis, but at concentrations above 200 µM, pentosidine synthesis was inhibited. The highest stimulatory effect on pentosidine synthesis was seen with 50 µM 3OH-Kyn, which increased the pentosidine level nearly 80% higher than the control reaction without 3OH-Kyn.
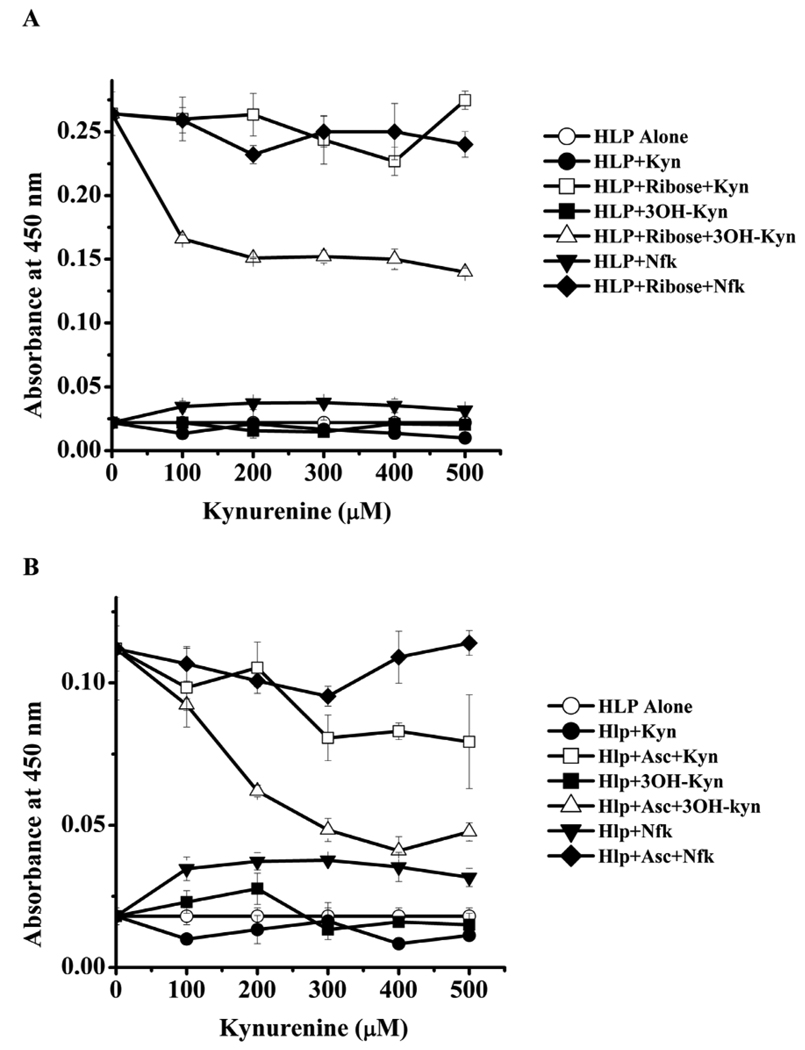
We then investigated the effect of kynurenines on CML formation from ribose and ascorbate. CML formation from ribose was unaffected by Nfk and Kyn, but 3OH-Kyn inhibited formation (Fig. 2A). Inhibition of ~40% was seen with 100 µM 3OH-Kyn, and it increased to ~52% with 500 µM 3OH-Kyn. CML levels from ascorbate were about 40% lower when compared to those with ribose (Fig. 2B). Kyn inhibited CML formation from ascorbate at concentrations >300 µM. At 500 µM, there was nearly a 25% inhibition. The effect by 3OH-Kyn was more robust; at 200 µM there was nearly 50% inhibition which increased to 60% at 400 µM.
Fig. 2. Kynurenines inhibit CML formation.
HLP was incubated with 5 mM of either ribose (A) or ascorbate (B) in the presence of 0–500 µM kynurenines as described in Fig. 1. Samples were extensively dialyzed and assayed by a direct ELISA. Each point is mean ±SD of three assays. Asc=ascorbate.
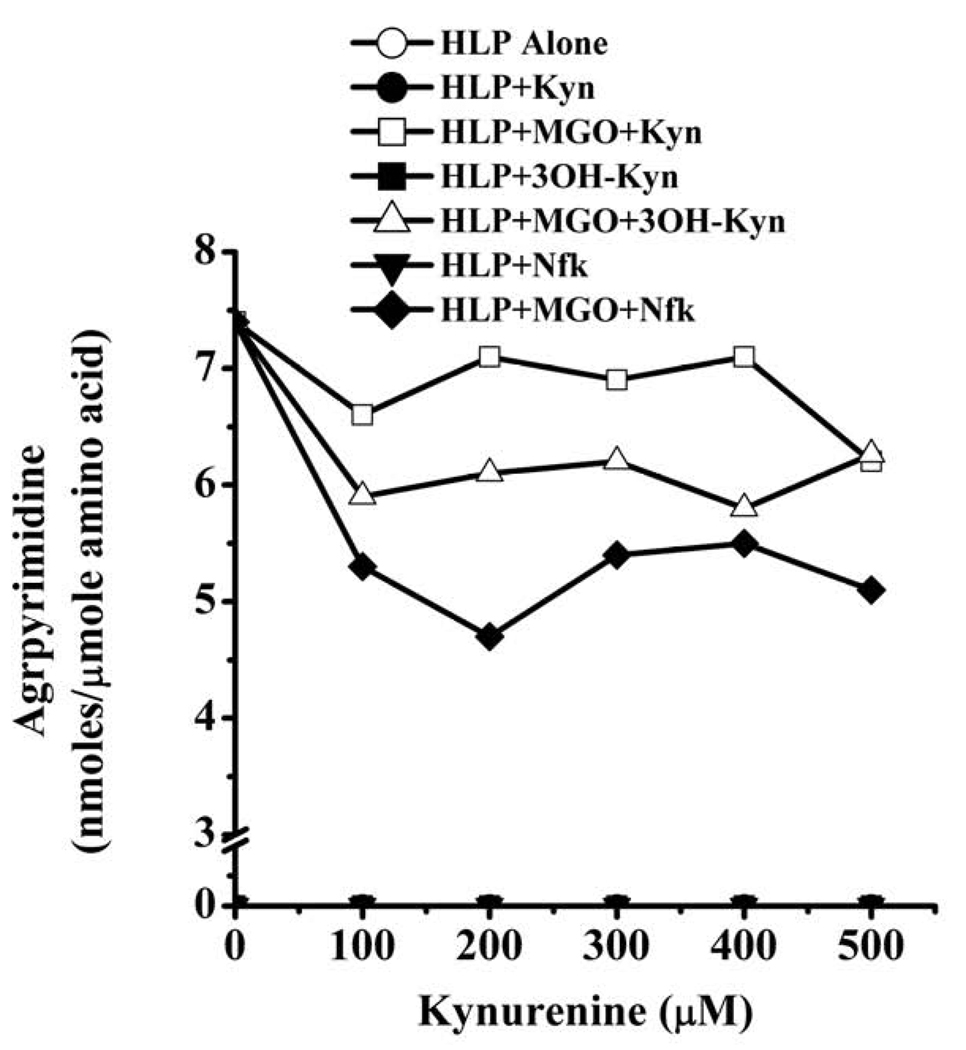
All kynurenines had inhibitory effects on argpyrimidine formation (Fig. 3). Inhibition by Nfk was slightly better than the other two kynurenines. At 500 µM, the inhibition by Nfk, Kyn and 3OH-Kyn was about 22, 22 and 38%, respectively. These data imply that kynurenines have negative effects on MGO-mediated Maillard reactions.
Fig. 3. Effect of kynurenines on argpyrimidine formation.
HLP was incubated with 5 mM MGO in the presence of 0–500 µM kynurenines for one week at pH 7.4 and 37 °C. Samples were extensively dialyzed to remove free reactants, and the proteins were hydrolyzed with 6 N HCl and analyzed by fluorescence (335/385 nm) reverse-phase HPLC. For quantification of argpyrimidine in samples, a standard curve was generated using a preparation of synthetic argpyrimidine.
3.2. Effect of 3OH-Kyn on pentosidine synthesis from Amadori-HLP
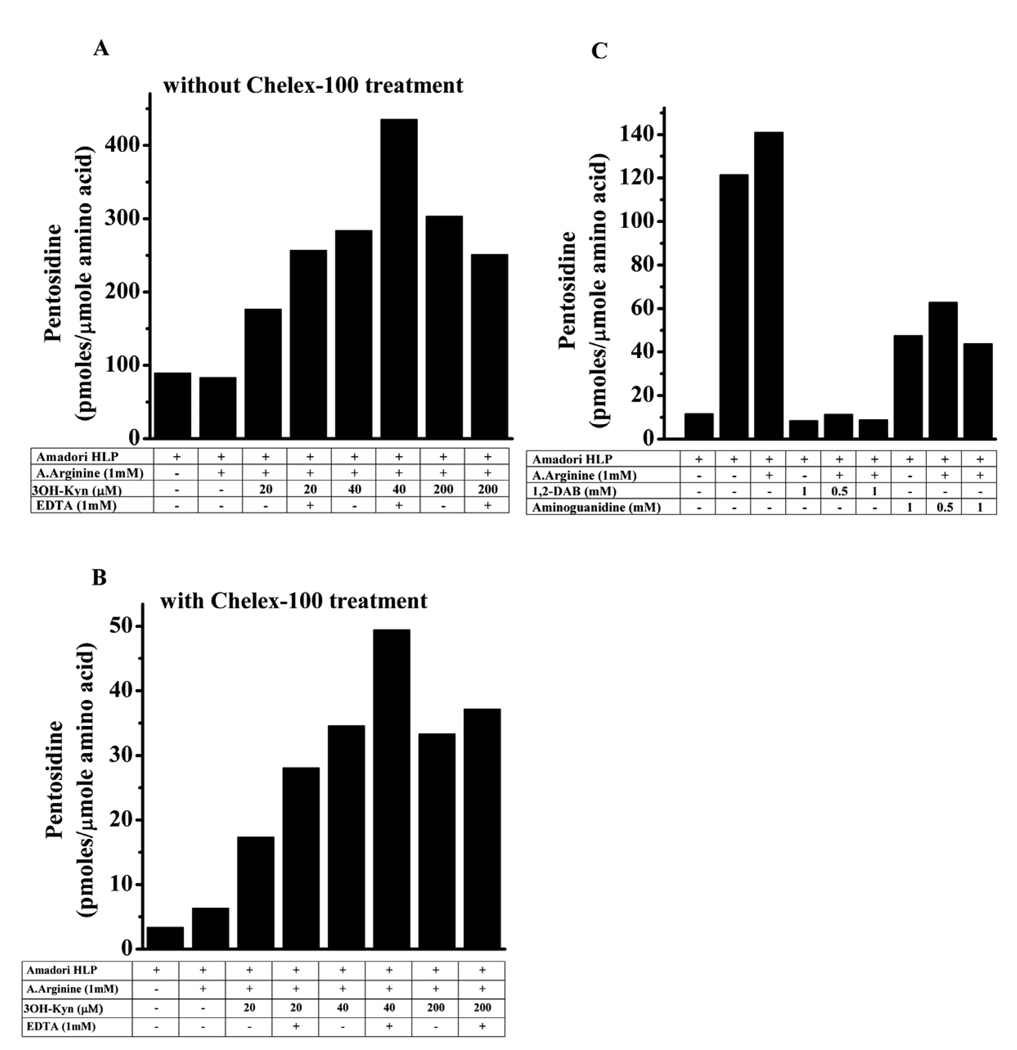
The effect of 3OH-Kyn on pentosidine synthesis was further investigated. To determine whether the disparate effects of 3OH-Kyn on pentosidine synthesis are occurring pre- or post-Amadori reaction, we incubated Amadori-enriched HLP (Amadori-HLP) with Nα-acetyl arginine and various concentrations of 3OH-Kyn (0–200 µM) for 3 days. A similar experiment was also done in parallel with Chelex-100 treated buffer to determine if metal ions played a role. The pentosidine level was 98 pmoles/µmole amino acid in the Amadori-HLP (Fig. 4A). Surprisingly, the addition of Nα-acetyl arginine did not increase pentosidine levels, suggesting that pentosidine synthesis occurs mostly in an intramolecular manner in proteins (i.e., involving lysine and arginine residues of the same protein molecule). Inclusion of 20 and 40 µM 3OH-Kyn in the incubation mixture resulted in a 2- and 3-fold increase in pentosidine concentration, respectively. Further increases in the concentration of 3OH-Kyn (200 µM) did not increase pentosidine concentrations beyond what was observed with 40 µM 3OH-Kyn. The addition of 1 mM EDTA along with 3OH-Kyn further increased pentosidine concentrations at all concentrations of 3OH-Kyn.
Fig. 4. Effect of dicarbonyl trapping and metal ion chelation on pentosidine synthesis from Amadori-HLP.
HLP was incubated for 24 hrs with 5 M ribose in Chelex-100 treated or untreated 0.4 M sodium phosphate buffer, pH 7.4. The incubated samples were dialyzed extensively against 0.05 M sodium phosphate buffer, pH 7.4. (A) Effect of incubation in Chelex-100 untreated and (B) Chelex-100 treated buffer. Samples were incubated with indicated reagents for 3 days. (C) Effect of dicarbonyl trapping agents on pentosidine synthesis. The samples were dialyzed and processed for pentosidine estimation as described in Fig. 1. A.Arginine=Nα-acetyl arginine, DAB=1,2-diaminobenzene.
3.3. Effect of Chelex-100 treatment of the buffer on pentosidine formation
Chelex-100 treatment of the reaction buffer drastically reduced pentosidine formation in Amadori-HLP; the levels were ~10 times lower when compared to those seen without Chelex-100 treatment (Fig. 4B). However, the effect of 3OH-Kyn was very similar with or without Chelex-100 treatment. Surprisingly, the addition of 1 mM EDTA to Chelex-100 treated buffer also increased the pentosidine concentration in these samples. Taken together, these results suggest that pentosidine formation in HLP principally occurs intramolecularly, and 3OH-Kyn and EDTA enhance pentosidine synthesis in post-Amadori reactions.
Addition of 500 or 1000 µM 1,2-diaminobenzene, a powerful dicarbonyl trapping agent [31], almost completely inhibited pentosidine formation from Amadori-HLP (Fig. 4C). Similar observations, albeit with lower inhibition (~60%), were made with aminoguanidine (another dicarbonyl trapping agent) [32], suggesting the involvement of dideoxyosone intermediates in pentosidine synthesis.
3.4. Effect of 3OH-Kyn on pentosidine synthesis from ribated lysine
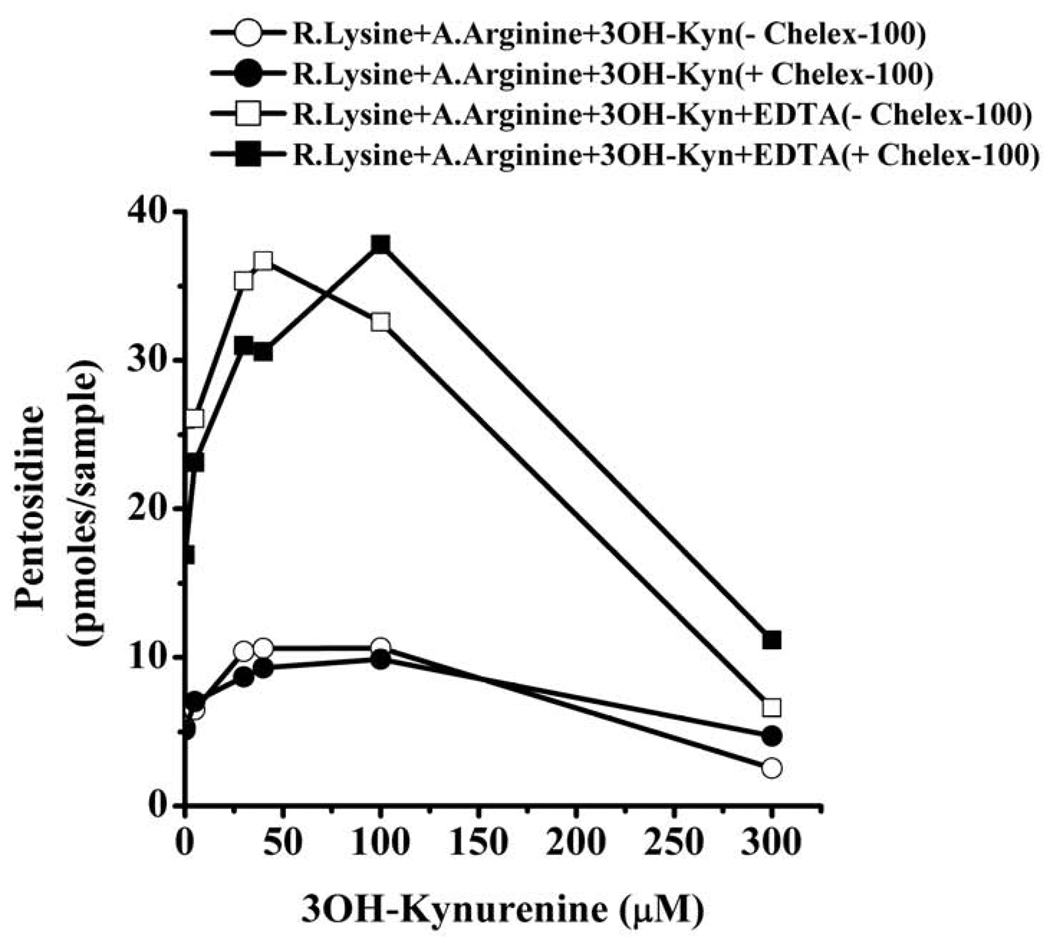
Next, we investigated the effects of 3OH-Kyn and EDTA on pentosidine synthesis from ribated lysine. This is a much simpler system due to the lack of interference from other reactive groups present in proteins. As expected, incubation of ribated lysine with Nα-acetyl arginine led to pentosidine formation (Fig. 5). The pentosidine level was 5.2 pmoles/25 µl of reaction mixture without 3OH-Kyn. However, upon addition of 5–40 µM 3OH-Kyn, the pentosidine level linearly increased; at 40 µM 3OH-Kyn, the pentosidine concentration was approximately 2-fold higher (10 pmoles) than the control (without 3OH-Kyn). At a higher concentration of 3OH-Kyn (300 µM), the pentosidine level declined to 3.2 pmoles/25 µl of reaction mixture. The effects of 3OH-Kyn were unaltered when incubations were carried out with Chelex-100 treated buffer.
Fig. 5. 3OH-Kyn modulates pentosidine synthesis from ribated lysine.
Ribated lysine was incubated with Nα-acetyl arginine in the presence or absence of 3OH-Kyn for 3 days in 0.1 M sodium phosphate buffer, pH 7.4 and 37 °C. The incubation buffer was either pre-treated with Chelex-100 or untreated. The reaction mixture was then subjected to acid hydrolysis, and 25 µl samples were analyzed by HPLC for pentosidine. R.Lysine=ribated lysine, A.Arginine=Nα-acetyl arginine.
Addition of EDTA along with 3OH-Kyn produced dramatic increases in pentosidine concentrations. The pentosidine levels increased from 5.2 pmoles in the control to 36 pmoles with EDTA + 40 µM 3OH-Kyn, but levels decreased to 8 pmoles at 300 µM 3OH-Kyn. When incubations were carried out with Chelex-100 treated buffer, the effect of 3OH-Kyn and EDTA were similar, but the peak pentosidine synthesis (37 pmoles) occurred with 100 µM 3OH-Kyn as opposed to 40 µM 3OH-Kyn (in the absence of Chelex-100 treatment). These results are consistent with the data in Fig. 1, where pentosidine synthesis was enhanced at low concentrations but inhibited by high concentrations of 3OH-Kyn. Addition of 1 mM EDTA along with 5 µM 3OH-Kyn resulted in a 5-fold increase in pentosidine concentration. This further increased to 7-fold with 40 µM 3OH-Kyn. However, at 300 µM of 3OH-Kyn and 1 mM EDTA, the pentosidine level dropped to the level observed in the absence of EDTA. The effect of 3OH-Kyn and EDTA on pentosidine synthesis from ribated lysine was independent of metal ions, as incubation with Chelex-100 treated buffer did not significantly change the synthesis pattern seen in the absence of Chelex-100 treatment.
3.5. Generation of H2O2 by 3OH-Kyn
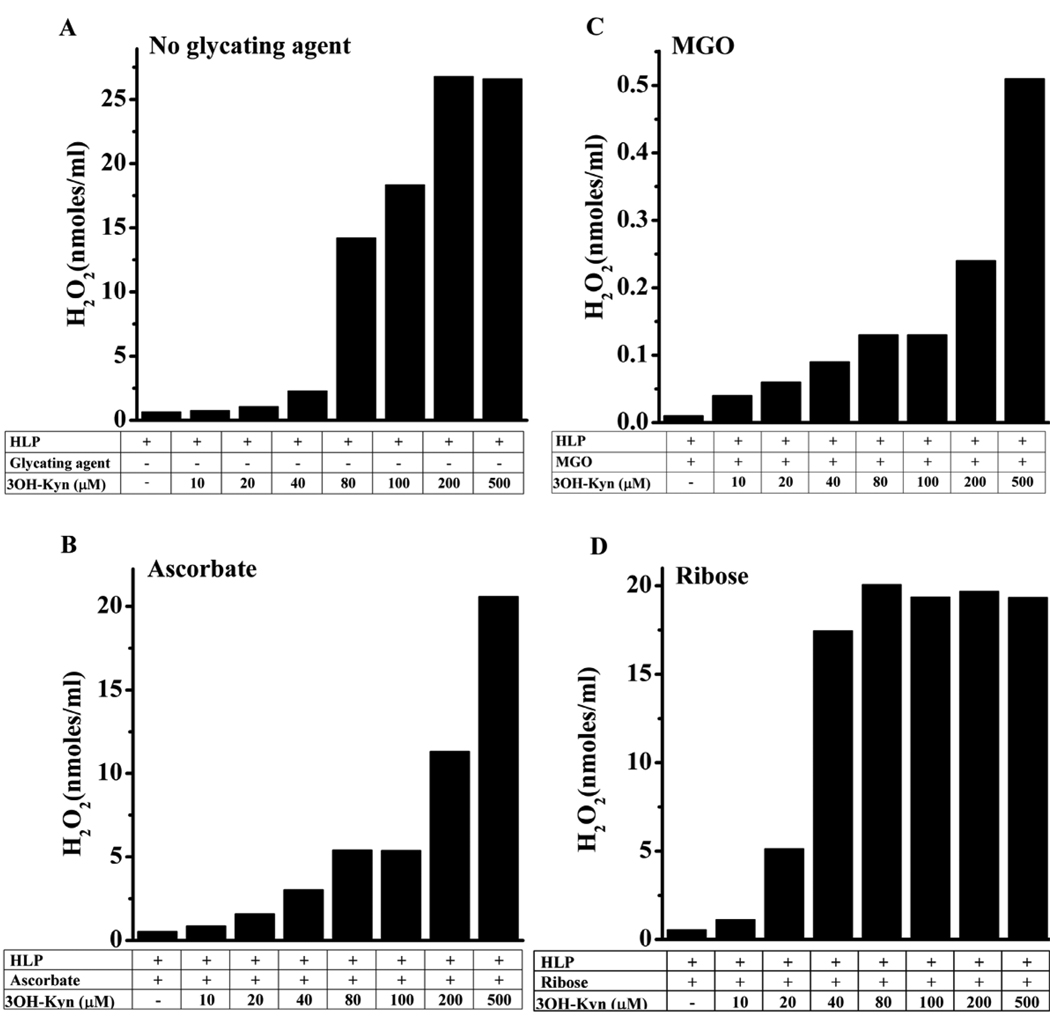
Since 3OH-Kyn binds transition metal ions, and in the presence of O2 generates H2O2 [25], we reasoned that this property could be a factor in its effect on pentosidine synthesis. First, we assayed H2O2 production by 3OH-Kyn in the presence of HLP. After one week of incubation with HLP, there was very little H2O2 detected in reactions containing 10–40 µM 3OH-Kyn, but significant amounts of H2O2 were observed with 80–500 µM 3OH-Kyn (Fig. 6A). The H2O2 concentration was 27 nmoles/ml with 200 µM 3OH-Kyn, as compared to 1 nmole/ml with 10 µM 3OH-Kyn. Next, we checked H2O2 production in the presence of glycating agents. In samples containing ascorbate or MGO, the H2O2 concentration increased with increases in 3OH-Kyn concentration; while 20 nmoles/ml H2O2 was observed with ascorbate and 500 µM 3OH-Kyn, 40 times lower (0.5 nmoles) levels were observed with MGO and 500 µM 3OH-Kyn (Fig. 6B and C). Notably, the values with MGO were approximately 150-fold lower than those observed in the absence of the glycating agent. With ribose, the highest concentration of H2O2 (~15 nmoles/ml) was observed with 80 µM 3OH-Kyn (Fig. 6D). However, further increases of 3OH-Kyn (up to 500 µM) did not increase H2O2 production. These data show that 3OH-Kyn can produce significant quantities of H2O2 under the conditions used for glycation in this study, and the production of H2O2 is muted in the presence of glycating agents, especially MGO.
Fig. 6. H2O2 production by 3OH-Kyn.
HLP was incubated with various concentrations of 3OH-Kyn alone (A) 3OH-Kyn + ascorbate (B) 3OH-Kyn + MGO (C) or 3OH-Kyn + ribose (D) at pH 7.4 and 37 °C for 7 days. H2O2 estimation in the samples was performed using the Amplex Red H2O2 Assay Kit as described in the Methods.
3.6. Effect of H2O2 on AGE synthesis
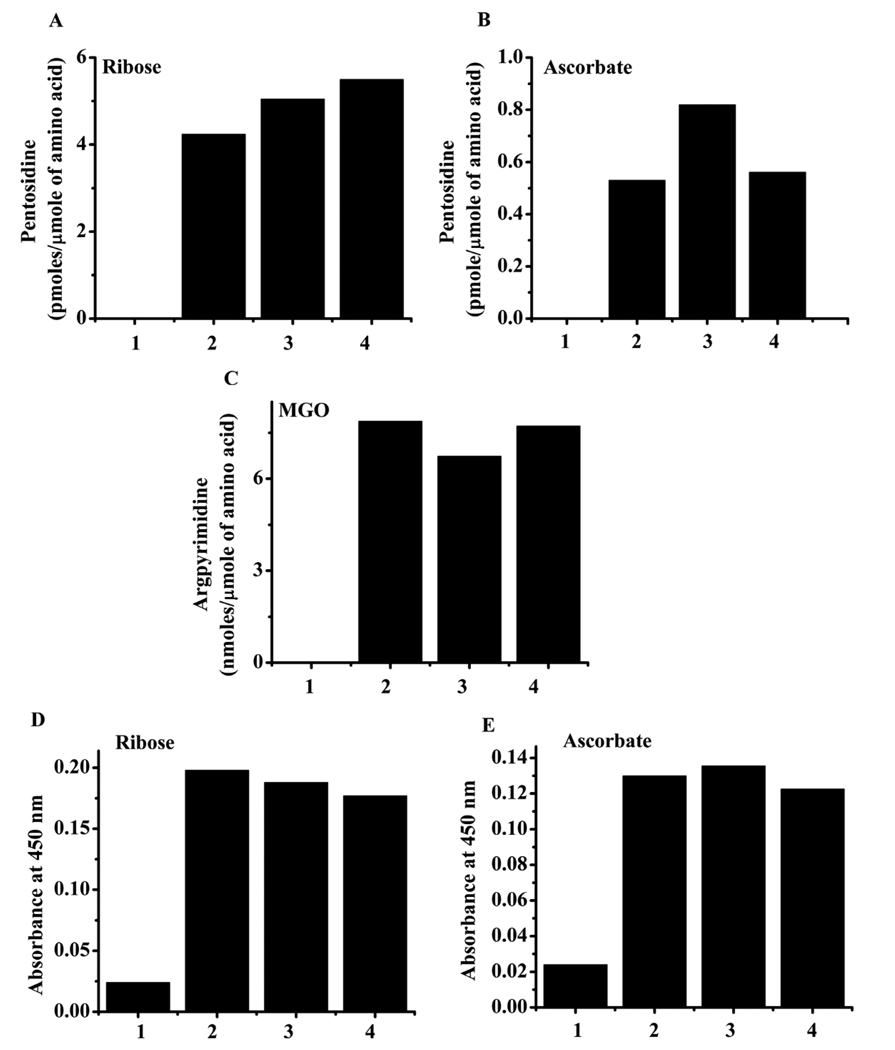
Since H2O2 production was robust in our reactions, we sought to determine the effect of H2O2 on AGE synthesis in HLP. The addition of 50 and 500 µM H2O2 to HLP + ribose or ascorbate samples slightly increased (20 to 25%) the yield of pentosidine, although with 500 µM H2O2, the pentosidine level was slightly lower than at 100 µM. (Fig. 7A and B). However, at this concentration, H2O2 had no effect on argpyrimidine synthesis from MGO (Fig. 7C) or CML formation from ribose and ascorbate (Fig. 7D and E). These data indicate that even though 3OH-Kyn produces significant quantities of H2O2, its effect on AGE synthesis is minimal.
Fig. 7. Effect of H2O2 on AGE formation.
HLP was incubated with ribose (A and D), ascorbate (B and E) or MGO (C) in the presence of 0, 50 or 500 µM H2O2 for 7 days at pH 7.4 and 37 °C. The incubated samples were dialyzed against PBS and either: 1) hydrolyzed with acid and analyzed for pentosidine or argpyrimidine by HPLC as in Fig. 1 and Fig. 3; or 2) analyzed for CML by ELISA as in Fig. 2. Effect on pentosidine formation from ribose (A) and ascorbate (B), the effect on argpyrimidine formation from MGO (C) and effect on CML formation from ribose (D) and ascorbate (E) are shown. In each figure, 1 = HLP alone, 2 = HLP + glycating agent, 3 = HLP + glycating agent + 50 µM H2O2 and 4 = HLP + glycating agent + 500 µM H2O2.
3.7. Effect of 3OH-Kyn on intracellular pentosidine formation
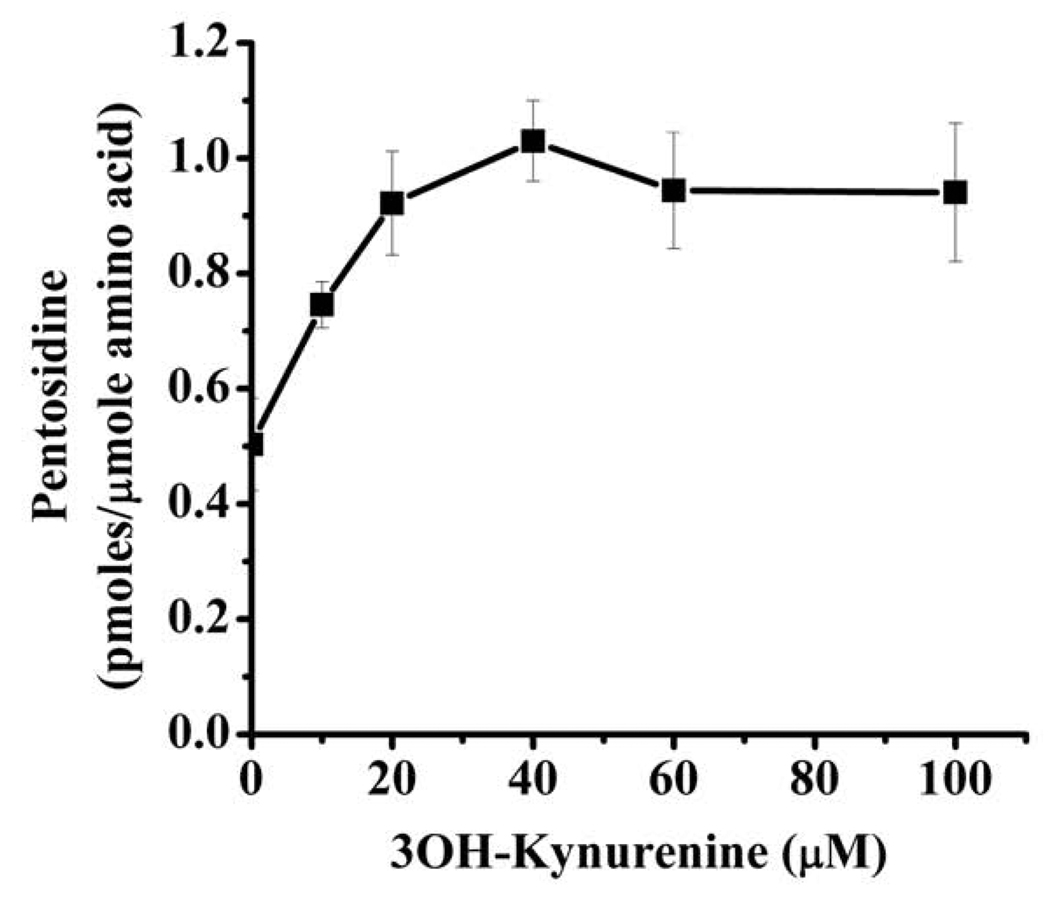
Next, we investigated if 3OH-Kyn exhibits similar effects on pentosidine synthesis in HLE-B3 cells. Incubation with 20 mM ribose for 4 days resulted in the formation of 0.5 pmoles/µmole amino acid of pentosidine in intracellular proteins (Fig. 8). This was further increased by the addition of 10–100 µM 3OH-Kyn. The increase was 2-fold with 40 µM 3OH-Kyn, but further increases in 3OH-Kyn (>40 µM) showed no change in pentosidine synthesis.
Fig. 8. 3OH-Kyn modulates pentosidine synthesis in human lens epithelial cells.
HLE-B3 cells were cultured in the presence or absence of 20 mM ribose and 0–100 µM 3OH-Kyn for 4 days in the absence of serum in the medium. Cells were thoroughly washed with PBS, hydrolyzed with acid and analyzed for pentosidine as described in Fig. 1. Each point is the mean ±SD from three independent experiments.
4. Discussion
The purpose of our study was to determine if kynurenines influence AGE formation in the human lens. There is reason to believe that these compounds contribute to deleterious changes in lens proteins that occur during aging and cataract formation. Kynurenines are formed from oxidation of tryptophan, primarily by the action of IDO. This enzyme is present mainly in the epithelial cells of the lens (15). An elegant series of studies by Truscott and colleagues demonstrated kynurenine-modified amino acids in lens proteins [17, 20, 26]. We enlarged upon their findings by using specific monoclonal antibodies to identify kynurenine-modified proteins in human cataractous lenses [22, 33].
Like kynurenine-mediated protein modification, glycation of lens proteins is also a posttranslational modification process. Here we chose to work with three markers of glycation: pentosidine, CML and argpyrimidine. While pentosidine is formed from sugars, CML is formed from sugars and dicarbonyls. Conversely, argpyrimidine is produced exclusively from MGO. Thus, these AGEs represent three different, albeit related, pathways of glycation. Pentosidine, CML and argpyrimidine concentrations increase with lens aging, and they accumulate to relatively high concentrations in cataractous lenses [5, 7, 30]. Several studies have documented that reactive oxygen species are a catalyst for the formation of AGEs. It has been shown that H2O2 generated during glycation (or added externally) enhances the formation of pentosidine from sugars [34], and hydroxyl radicals promote CML synthesis from sugars [35]. Kynurenines bind and reduce transitional metal ions and in the process produce H2O2 [25]. H2O2 thus formed can be converted to hydroxyl radicals by metal ion-dependent Fenton reactions. These observations, together with possible competition of kynurenines with glycating agents for reaction with lens proteins, prompted us to undertake a systematic study to determine the effects of kynurenines on glycation.
The principal findings in this study are: 1) 3OH-Kyn exerts a stimulating effect at low concentrations and an inhibitory effect at high concentrations on pentosidine synthesis from ascorbate and ribose; 2) Low concentrations of 3OH-Kyn enhance pentosidine synthesis from ribated lysine and Amadori-enriched HLP and in HLE B-3 cells; 3) Chelex-100 treatment reduces pentosidine formation from ribose, but the enhancement effect of 3OH-Kyn occurs even in Chelex-100 treated buffer; 4) in combination with 3OH-Kyn, EDTA promotes pentosidine synthesis from ribated lysine and Amadori-HLP, and this effect is independent of metal ions; and 5) CML synthesis from ribose and ascorbate, and argpyrimidine synthesis from MGO, are inhibited by kynurenines.
All three kynurenines (Nfk, Kyn and 3OH-Kyn) inhibited CML formation from ascorbate, but they had negligible effect on pentosidine synthesis from ribose and ascorbate at low concentrations (<200 µM). However, at higher concentrations (>200 µM) they promoted pentosidine synthesis from ascorbate and ribose (only Nfk) (Fig. 1). It is possible that Nfk and Kyn might have degraded during incubation and the degradation products could have promoted pentosidine synthesis similar to 3OH-Kyn. Further work is needed to address this possibility.
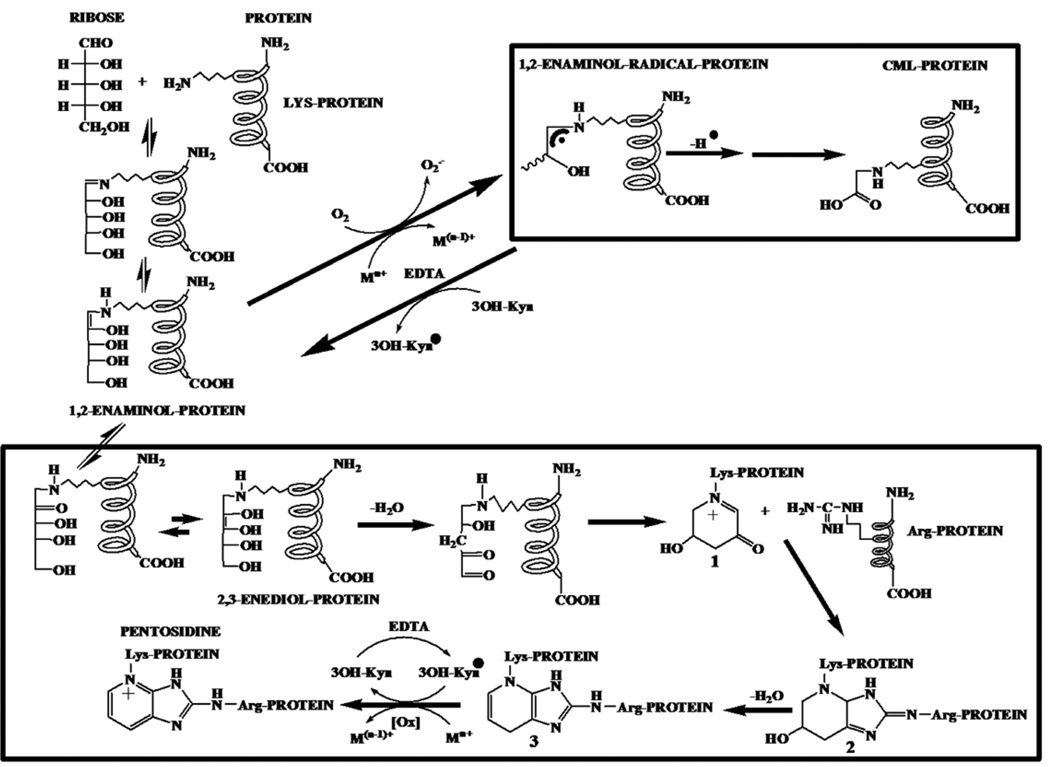
We can speculate on how 3OH-Kyn and EDTA promote pentosidine synthesis from ribose. It is likely that 3OH-Kyn prevents metal-catalyzed oxidation of the 1,2-enaminol intermediate to the 1,2-enaminol radical, which is a precursor for CML. During this process, 3OH-Kyn is oxidized to a 3OH-Kyn radical. This inhibition of CML by 3OH-Kyn favors pentosidine synthesis by channeling the Amadori product through conversion to 2,3-enediol and dideoxyosone intermediates (Scheme I). The 3OH-Kyn radical oxidizes the penultimate intermediate 3 to pentosidine during this process. Obviously, 3OH-Kyn has an oxidation potential in between the CML precursor and the pentosidine precursor, and thus could transfer electrons between these species. As a result, oxidation to pentosidine proceeds to the disadvantage of oxidation to CML. The robust inhibitory effect on pentosidine synthesis from Amadori-HLP by 1,2-diaminobenzene, which may bind to dideoxyosones in proteins, supports this view. At high concentration (>200 µM), 3OH-Kyn may compete with ribose or ascorbate for reaction with lysine residues in proteins and inhibit pentosidine formation. Indeed, 3OH-Kyn has previously been shown to react with lysine residues in proteins [22]. As for the promotional effect of EDTA on pentosidine synthesis from Amadori-HLP, it is possible that EDTA recycles the 3OH-Kyn radical and consequently catalyzes oxidation of intermediate 3 to pentosidine. The inhibition of pentosidine synthesis from Amadori-HLP in Chelex-100 treated buffer (Fig. 4) is possibly due to the inhibition of metal-catalyzed oxidation of intermediate 3 to pentosidine. Thus, oxidation of intermediate 3 to pentosidine is likely both metal ion-dependent and independent.
Scheme I.
These observations are somewhat similar to those reported for anti-oxidants and pyridoxamine derivatives, which were also found to enhance pentosidine formation in post-Amadori reactions [36]. These compounds resemble 3OH-Kyn in that they have a phenolic group that can act as both a metal chelator and a free radical trap. The fact that the other two kynurenines (Nfk and Kyn) did not elicit such a response suggests that the α-aminophenol nature of 3OH-Kyn was largely responsible for the observed effects.
Additionally, H2O2 did not have much of an effect on pentosidine or CML synthesis from either ribose or ascorbate. Since 3OH-Kyn produces large quantities of H2O2, we reasoned that it would affect pentosidine synthesis. In fact, a previous study has shown the promotional effect of H2O2 on pentosidine synthesis [37]. However, our results are in contrast to this report. It has been shown that CML synthesis is enhanced by hydroxyl radicals [35]. The findings in the present study that CML synthesis from Amadori-HLP is unaffected by H2O2 suggest that hydroxyl radicals mediate cleavage of sugars to products such as glyoxal prior to Amadori product formation, and this is possibly a mechanism by which H2O2 promotes CML synthesis, as previously proposed [13].
The relevance of our finding to changes in human lenses during aging can be appreciated by the fact that both kynurenines and glycating agents are present in the lens. Among kynurenines, 3OH-Kyn accounts for a large portion of protein free kynurenines. Its concentration is approximately 15 nmol/g in young lenses and 2 nmol/g in aged lenses [16]. These concentrations translate into 2–15 µM in normal aging lens. Although the ribose concentration in the human lens is not known, we can appreciate the relevance of work in the context of ascorbate concentration. In the adult lens, ascorbate is present at concentrations of 1–2 mM [38, 39]. Thus, the ratio of ascorbate:3OH-Kyn in the lens is 100:1 (young) to 750:1 (aged) (by considering the mean ascorbate concentration to be 1.5 mM, however, in aged lenses ascorbate concentration is expected to be lower, but data on absolute amounts are not available). In our experimental conditions, a 50% increase in pentosidine synthesis was already seen with an ascorbate:3OH-Kyn ratio of 500:1, and this rose to 125% when the ratio was 125:1. These ratios are within the range present in human lenses, and thus it is conceivable that the stimulatory effect of 3OH-Kyn on pentosidine synthesis can occur in vivo in aging human lenses. Moreover, it is possible that protein-bound 3OH-Kyn in the human lens [20] could have effects similar to the protein-free 3OH-Kyn used in the present study. If this does occur, it would further enhance AGE formation from ascorbate in the human lens. The lens environment could favor these reactions, as it contains high levels of protein (300–500 mg/ml)[40] and the proteins have negligible turnover rate. Further, it should be pointed out that pentosidine is one of the many AGEs that can be generated from ascorbate; there are reports of other AGEs that are derived from ascorbate, such as, vesperlysine A [8], K2P and OP-lysine [9, 10]. Whether their synthesis is also affected by kynurenines remains to be investigated.
The relevance of the effect of 3OH-Kyn on pentosidine synthesis in human lens aging was further investigated using HLE-B3 cells. 3OH-Kyn, at concentrations up to 40 µM, enhanced ribose-mediated pentosidine synthesis in intracellular proteins. However, at higher concentrations, it did not further increase pentosidine synthesis. These results indicate three possibilities: 1) 3OH-Kyn uptake by HLE-B3 cells is regulated, and reaches saturation at ~40 µM 3OH-Kyn; 2) although unlikely, 3OH-Kyn concentration in cells might be regulated by enzymes that catalyze its degradation; or 3) 3OH-Kyn in cells may be converted to 3OH-KynG, which is not an aminophenol and therefore cannot have the effects of 3OH-Kyn on pentosidine synthesis. Nevertheless, this finding is analogous to that in our previous study which showed that kynurenine uptake by mouse lens epithelial cells is regulated [24]. Together, these results demonstrate that lens epithelial cells may have regulatory mechanisms to limit kynurenine accumulation and prevent intracellular protein damage.
Several factors could dictate the interaction of kynurenines with Maillard reaction intermediates. For instance, GSH is present in the lens in relatively large concentrations. In the adult human lens, the GSH concentration is between 4–6 mM [41]. Kynurenines exist as GSH adducts in the lens [42, 43], and such an association with GSH may limit reaction with Maillard intermediates. Furthermore, with age (especially after 50 years), free kynurenines decrease in the lens, possibly as a result of their reaction with lens proteins [17]. This may also affect their reaction with Maillard reaction intermediates. The reaction of proteins with kynurenines is partially reversible with GSH [17, 43], allowing kynurenines to exist in the free form. In addition, GSH levels decrease during aging and cataract formation [44], which may favor reaction of kynurenines with Maillard intermediates. Another important factor that may affect is the oxygen tension in the lens. The human lens contains low levels of oxygen [45]. This could limit pentosidine formation, as molecular oxygen is required for its synthesis. Yet another factor is the light. Lens receives UV light (UVA and UVB) through the cornea. It is known that kynurenines are photosensitizers and they can oxidize ascobate [46].Whether such oxidation occurs in low oxygen environment is not known, but it is likely. Lastly, it is possible that the age-associated loss in kynurenines could be due to their reaction with Maillard intermediates.
Our findings imply that the synthesis of AGEs in the lens depends, at least in part, on the concentrations and types of kynurenines. Our observations thus have important implications for aging and possibly cataractogenesis in the human lens.
Acknowledgements
This study was supported from NIH grants R01EY-016219 and R01EY-09912 (RHN), P30EY-11373 (Visual Sciences Research Center of CWRU), Carl F. Asseff, M.D. Professorship to RHN, Research to Prevent Blindness (RPB), NY and Ohio Lions Eye Research Foundation.
Abbreviations
- HLP
human lens proteins
- Kyn
kynurenine
- Nfk
N-formyl kynurenine
- 3OH-Kyn
3OH-kynurenine
- MGO
methylglyoxal
- AGEs
advanced glycation end products
- CML
Nε-carboxymethyl lysine
- IDO
indoleamine 2, 3-dioxygenase
- BSA
bovine serum albumin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thorpe SR, Baynes JW. Maillard reaction products in tissue proteins: new products and new perspectives. Amino Acids. 2003;25:275–281. doi: 10.1007/s00726-003-0017-9. [DOI] [PubMed] [Google Scholar]
- 2.Monnier VM, Sell DR. Prevention and repair of protein damage by the Maillard reaction in vivo. Rejuvenation Res. 2006;9:264–273. doi: 10.1089/rej.2006.9.264. [DOI] [PubMed] [Google Scholar]
- 3.Bloemendal H, de Jong W, Jaenicke R, Lubsen NH, Slingsby C, Tardieu A. Ageing and vision: structure, stability and function of lens crystallins. Prog. Biophys. Mol. Biol. 2004;86:407–485. doi: 10.1016/j.pbiomolbio.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Grandhee SK, Monnier VM. Mechanism of formation of the Maillard protein cross-link pentosidine. Glucose, fructose, and ascorbate as pentosidine precursors. J. Biol. Chem. 1991;266:11649–11653. [PubMed] [Google Scholar]
- 5.Padayatti PS, Ng AS, Uchida K, Glomb MA, Nagaraj RH. Argpyrimidine, a blue fluorophore in human lens proteins: high levels in brunescent cataractous lenses. Invest. Ophthalmol. Vis. Sci. 2001;42:1299–1304. [PubMed] [Google Scholar]
- 6.Ahmed N, Thornalley PJ, Dawczynski J, Franke S, Strobel J, Stein G, Haik GM. Methylglyoxal-derived hydroimidazolone advanced glycation end-products of human lens proteins. Invest. Ophthalmol. Vis. Sci. 2003;44:5287–5292. doi: 10.1167/iovs.03-0573. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed MU, Thorpe SR, Baynes JW. Identification of N epsilon-carboxymethyllysine as a degradation product of fructoselysine in glycated protein. J. Biol. Chem. 1986;261:4889–4894. [PubMed] [Google Scholar]
- 8.Tessier F, Obrenovich M, Monnier VM. Structure and mechanism of formation of human lens fluorophore LM-1. Relationship to vesperlysine A and the advanced Maillard reaction in aging, diabetes, and cataractogenesis. J. Biol. Chem. 1999;274:20796–20804. doi: 10.1074/jbc.274.30.20796. [DOI] [PubMed] [Google Scholar]
- 9.Argirov OK, Lin B, Ortwerth BJ. 2-ammonio-6-(3-oxidopyridinium-1-yl)hexanoate (OP-lysine) is a newly identified advanced glycation end product in cataractous and aged human lenses. J. Biol. Chem. 2004;279:6487–6495. doi: 10.1074/jbc.M309090200. [DOI] [PubMed] [Google Scholar]
- 10.Cheng R, Feng Q, Argirov OK, Ortwerth BJ. Structure elucidation of a novel yellow chromophore from human lens protein. J. Biol. Chem. 2004;279:45441–45449. doi: 10.1074/jbc.M405664200. [DOI] [PubMed] [Google Scholar]
- 11.Litchfield JE, Thorpe SR, Baynes JW. Oxygen is not required for the browning and crosslinking of protein by pentoses: relevance to Maillard reactions in vivo. Int J Biochem Cell Biol. 1999;31:1297–1305. doi: 10.1016/s1357-2725(99)00091-6. [DOI] [PubMed] [Google Scholar]
- 12.Chellan P, Nagaraj RH. Early glycation products produce pentosidine cross-links on native proteins. novel mechanism of pentosidine formation and propagation of glycation. J Biol Chem. 2001;276:3895–3903. doi: 10.1074/jbc.M008626200. [DOI] [PubMed] [Google Scholar]
- 13.Glomb MA, Monnier VM. Mechanism of protein modification by glyoxal and glycolaldehyde, reactive intermediates of the Maillard reaction. J. Biol. Chem. 1995;270:10017–10026. doi: 10.1074/jbc.270.17.10017. [DOI] [PubMed] [Google Scholar]
- 14.Wood AM, Truscott RJ. UV filters in human lenses: tryptophan catabolism. Exp. Eye Res. 1993;56:317–325. doi: 10.1006/exer.1993.1041. [DOI] [PubMed] [Google Scholar]
- 15.Takikawa O, Littlejohn TK, Truscott RJ. Indoleamine 2,3-dioxygenase in the human lens, the first enzyme in the synthesis of UV filters. Exp. Eye Res. 2001;72:271–277. doi: 10.1006/exer.2000.0951. [DOI] [PubMed] [Google Scholar]
- 16.Bova LM, Sweeney MH, Jamie JF, Truscott RJ. Major changes in human ocular UV protection with age. Invest. Ophthalmol. Vis. Sci. 2001;42:200–205. [PubMed] [Google Scholar]
- 17.Korlimbinis A, Aquilina JA, Truscott RJ. Protein-bound UV filters in normal human lenses: the concentration of bound UV filters equals that of free UV filters in the center of older lenses. Invest. Ophthalmol. Vis. Sci. 2007;48:1718–1723. doi: 10.1167/iovs.06-1134. [DOI] [PubMed] [Google Scholar]
- 18.Taylor LM, Andrew Aquilina J, Jamie JF, Truscott RJ. UV filter instability: consequences for the human lens. Exp. Eye Res. 2002;75:165–175. doi: 10.1006/exer.2002.2012. [DOI] [PubMed] [Google Scholar]
- 19.Aquilina JA, Truscott RJ. Kynurenine binds to the peptide binding region of the chaperone alphaB-crystallin. Biochem. Biophys. Res. Commun. 2001;285:1107–1113. doi: 10.1006/bbrc.2001.5288. [DOI] [PubMed] [Google Scholar]
- 20.Korlimbinis A, Truscott RJ. Identification of 3-hydroxykynurenine bound to proteins in the human lens. A possible role in age-related nuclear cataract. Biochemistry (Mosc) 2006;45:1950–1960. doi: 10.1021/bi051744y. [DOI] [PubMed] [Google Scholar]
- 21.Hood BD, Garner B, Truscott RJ. Human lens coloration and aging. Evidence for crystallin modification by the major ultraviolet filter, 3-hydroxy-kynurenine O-beta-D-glucoside. J. Biol. Chem. 1999;274:32547–32550. doi: 10.1074/jbc.274.46.32547. [DOI] [PubMed] [Google Scholar]
- 22.Staniszewska MM, Nagaraj RH. 3-hydroxykynurenine-mediated modification of human lens proteins: structure determination of a major modification using a monoclonal antibody. J. Biol. Chem. 2005;280:22154–22164. doi: 10.1074/jbc.M501419200. [DOI] [PubMed] [Google Scholar]
- 23.Mailankot M, Staniszewska MM, Butler H, Caprara MH, Howell S, Wang B, Doller C, Reneker LW, Nagaraj RH. Indoleamine 2,3-dioxygenase overexpression causes kynurenine-modification of proteins, fiber cell apoptosis and cataract formation in the mouse lens. Lab. Invest. 2009;89:498–512. doi: 10.1038/labinvest.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mailankot M, Smith D, Howell S, Wang B, Jacobberger JW, Stefan T, Nagaraj RH. Cell cycle arrest by kynurenine in lens epithelial cells. Invest. Ophthalmol. Vis. Sci. 2008;49:5466–5475. doi: 10.1167/iovs.08-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldstein LE, Leopold MC, Huang X, Atwood CS, Saunders AJ, Hartshorn M, Lim JT, Faget KY, Muffat JA, Scarpa RC, Chylack LT, Jr, Bowden EF, Tanzi RE, Bush AI. 3-Hydroxykynurenine and 3-hydroxyanthranilic acid generate hydrogen peroxide and promote alpha-crystallin cross-linking by metal ion reduction. Biochemistry (Mosc) 2000;39:7266–7275. doi: 10.1021/bi992997s. [DOI] [PubMed] [Google Scholar]
- 26.Korlimbinis A, Hains PG, Truscott RJ, Truscott J, Aquilina JA. 3-Hydroxykynurenine oxidizes alpha-crystallin: potential role in cataractogenesis. Biochemistry (Mosc) 2006;45:1852–1860. doi: 10.1021/bi051737+. [DOI] [PubMed] [Google Scholar]
- 27.Simat TJ, Steinhart H. Oxidation of Free Tryptophan and Tryptophan Residues in Peptides and Proteins. J. Agric. Food Chem. 1998;46:490–498. doi: 10.1021/jf970818c. [DOI] [PubMed] [Google Scholar]
- 28.Khalifah RG, Todd P, Booth AA, Yang SX, Mott JD, Hudson BG. Kinetics of nonenzymatic glycation of ribonuclease A leading to advanced glycation end products. Paradoxical inhibition by ribose leads to facile isolation of protein intermediate for rapid post-Amadori studies. Biochemistry (Mosc) 1996;35:4645–4654. doi: 10.1021/bi9525942. [DOI] [PubMed] [Google Scholar]
- 29.Puttaiah S, Biswas A, Staniszewska M, Nagaraj RH. Methylglyoxal inhibits glycation-mediated loss in chaperone function and synthesis of pentosidine in alpha-crystallin. Exp. Eye Res. 2007;84:914–921. doi: 10.1016/j.exer.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 30.Nagaraj RH, Sell DR, Prabhakaram M, Ortwerth BJ, Monnier VM. High correlation between pentosidine protein cross-links and pigmentation implicates ascorbate oxidation in human lens senescence and cataractogenesis. Proc. Natl. Acad. Sci. U. S. A. 1991;88:10257–10261. doi: 10.1073/pnas.88.22.10257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puttaiah S, Zhang Y, Pilch HA, Pfahler C, Oya-Ito T, Sayre LM, Nagaraj RH. Detection of dideoxyosone intermediates of glycation using a monoclonal antibody: characterization of major epitope structures. Arch. Biochem. Biophys. 2006;446:186–196. doi: 10.1016/j.abb.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 32.Brownlee M. Pharmacological modulation of the advanced glycosylation reaction. Prog. Clin. Biol. Res. 1989;304:235–248. [PubMed] [Google Scholar]
- 33.Staniszewska M, Nagaraj RH. Detection of kynurenine modifications in proteins using a monoclonal antibody. J. Immunol. Methods. 2007;324:63–73. doi: 10.1016/j.jim.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Monnier VM, Glomb M, Elgawish A, Sell DR. The mechanism of collagen crosslinking in diabetes: a puzzle nearing resolution. Diabetes. 1996;45 Suppl 3:S67–S72. doi: 10.2337/diab.45.3.s67. [DOI] [PubMed] [Google Scholar]
- 35.Nagai R, Ikeda K, Higashi T, Sano H, Jinnouchi Y, Araki T, Horiuchi S. Hydroxyl radical mediates N epsilon-(carboxymethyl)lysine formation from Amadori product. Biochem. Biophys. Res. Commun. 1997;234:167–172. doi: 10.1006/bbrc.1997.6608. [DOI] [PubMed] [Google Scholar]
- 36.Culbertson SM, Vassilenko EI, Morrison LD, Ingold KU. Paradoxical impact of antioxidants on post-Amadori glycoxidation: Counterintuitive increase in the yields of pentosidine and Nepsilon-carboxymethyllysine using a novel multifunctional pyridoxamine derivative. J. Biol. Chem. 2003;278:38384–38394. doi: 10.1074/jbc.M305099200. [DOI] [PubMed] [Google Scholar]
- 37.Elgawish A, Glomb M, Friedlander M, Monnier VM. Involvement of hydrogen peroxide in collagen cross-linking by high glucose in vitro and in vivo. J. Biol. Chem. 1996;271:12964–12971. doi: 10.1074/jbc.271.22.12964. [DOI] [PubMed] [Google Scholar]
- 38.Taylor A, Jacques PF, Nadler D, Morrow F, Sulsky SI, Shepard D. Relationship in humans between ascorbic acid consumption and levels of total and reduced ascorbic acid in lens, aqueous humor, and plasma. Curr. Eye Res. 1991;10:751–759. doi: 10.3109/02713689109013869. [DOI] [PubMed] [Google Scholar]
- 39.Varma SD, Richards RD. Ascorbic acid and the eye lens. Ophthalmic Res. 1988;20:164–173. doi: 10.1159/000266579. [DOI] [PubMed] [Google Scholar]
- 40.Obalinsky TR. Nova Sciences Publishers, Inc; 2006. [Google Scholar]
- 41.Lou MF. Thiol regulation in the lens. J. Ocul. Pharmacol. Ther. 2000;16:137–148. doi: 10.1089/jop.2000.16.137. [DOI] [PubMed] [Google Scholar]
- 42.Taylor LM, Andrew Aquilina J, Jamie JF, Truscott RJ. Glutathione and NADH, but not ascorbate, protect lens proteins from modification by UV filters. Exp. Eye Res. 2002;74:503–511. doi: 10.1006/exer.2001.1165. [DOI] [PubMed] [Google Scholar]
- 43.Garner B, Vazquez S, Griffith R, Lindner RA, Carver JA, Truscott RJ. Identification of glutathionyl-3-hydroxykynurenine glucoside as a novel fluorophore associated with aging of the human lens. J. Biol. Chem. 1999;274:20847–20854. doi: 10.1074/jbc.274.30.20847. [DOI] [PubMed] [Google Scholar]
- 44.Giblin FJ. Glutathione: a vital lens antioxidant. J. Ocul. Pharmacol. Ther. 2000;16:121–135. doi: 10.1089/jop.2000.16.121. [DOI] [PubMed] [Google Scholar]
- 45.McNulty R, Wang H, Mathias RT, Ortwerth BJ, Truscott RJ, Bassnett S. Regulation of tissue oxygen levels in the mammalian lens. J Physiol. 2004;559:883–898. doi: 10.1113/jphysiol.2004.068619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ortwerth BJ, Bhattacharyya J, Shipova E. Tryptophan metabolites from young human lenses and the photooxidation of ascorbic acid by UVA light. Invest. Ophthalmol. Vis. Sci. 2009;50:3311–3319. doi: 10.1167/iovs.08-2927. [DOI] [PubMed] [Google Scholar]