Abstract
The emergence of highly aggressive subtypes of human cutaneous squamous cell carcinoma (SCC) often reflects increased autocrine/paracrine TGF-β synthesis and epidermal growth factor receptor (EGFR) amplification. Cooperative TGF-β/EGFR signaling promotes cell migration and induces expression of both proteases and protease inhibitors that regulate stromal remodeling resulting in the acquisition of an invasive phenotype. In one physiologically relevant model of human cutaneous SCC progression, TGF-β1+EGF stimulation increases the production of several matrix metalloproteinases (MMPs), among the most prominent of which is MMP-10—an MMP known to be elevated in SCC in situ. Activation of stromal plasminogen appears to be critical in triggering downstream MMP activity. Paradoxically, PAI-1, the major physiological inhibitor of plasmin generation, is also upregulated under these conditions and is an early event in progression of incipient epidermal SCC. One testable hypothesis proposes that TGF-β1+EGF-dependent MMP-10 elevation directs focalized matrix remodeling events that promote epithelial cell plasticity and tissue invasion. Increased PAI-1 expression serves to temporally and spatially modulate plasmin-initiated pericellular proteolysis, further facilitating epithelial invasive potential. Defining the complex signaling and transcriptional mechanisms that maintain this delicate balance is critical to developing targeted therapeutics for the treatment of human cutaneous malignancies.
1. Epithelial Skin Cancer Initiation
Nonmelanoma skin cancers (NMSCs) (i.e., basal cell, squamous cell, and Merkel cell carcinomas) are the most common human malignancies [1, 2]. In North America alone, >50% of all neoplasms arise in the skin [3] and the incidence of NMSC in Australia for the year 2002 was more than five times the incidence of all other cancers combined [4]. Relative to other cutaneous tumors, advanced squamous cell carcinoma (SCC) is aggressive, resistant to localized therapy with significant associated mortality and increasing in frequency [5].
The emergence of epithelial skin tumors appears causally linked to ultraviolet (UV) radiation exposure. Specific UV-B “signature” base changes (C→T or CC→TT), particularly in codons 177 (basal cell carcinoma) and 278 (SCC) in the tumor suppressor p53 gene [6–8], likely occur early in epidermal carcinogenesis. Indeed, UV-associated p53 mutations are prevalent in solar radiation-induced actinic keratosis; 10% of these lesions progress to SCC and 60% of all SCC arise within actinic keratoses [9–11]. Transition of a normal keratinocyte to an initiated pre- or early malignant phenotype, in fact, often involves loss- or gain-of-function mutations in p53, with characteristic karyotypic changes including gains in chromosomes 7, 9, 18 (early on) and 3q, 8q, 9q, and 11q in later stages of tumor progression, ras gene mutation/activation/amplified expression (10–30% incidence), and inactivation of cell cycle inhibitors [7, 12–15]. While epidermal cancers associated with mutant ras expression may be cell type-dependent [16], molecular events that accompany the development of lesional subsets in both premalignant cutaneous lesions (actinic keratosis) and SCC [10, 11, 17] are similar. p53 gain-of-function versus loss-of-function mutations, moreover, may actually influence different stages in cutaneous SCC progression with gain-of-function changes associated with acceleration to SCC in the context of an oncogenic ras gene [14, 18]. At least one p53-activating gain-of-function mutation (p53R172H) results in increased skin tumor formation/progression and metastatic spread [18].
2. Epithelial Cell Plasticity and Tumor Progression
The accumulated genetic/epigenetic changes accompanying evolution of aggressive subtypes of cutaneous SCC are intertwined in a complex signaling landscape emanating from both tumor cells and stromal-derived elements (e.g., hepatocyte growth factor (HGF); epidermal growth factor (EGF); platelet-derived growth factor (PDGF); transforming growth factor-β (TGF-β)) [19–24]. TGF-β1 is a particularly robust initiator of epithelial “plasticity” (usually referred to as epithelial-to-mesenchymal transition or EMT), a likely facilitator of tumor invasion and metastasis (see, e.g., [22, 24]). The EMT “phenotome” however depends on physiologic context (i.e., embryogenesis, fibrosis/wound healing, tumor progression), the involved cell type, and the actual initiating stimulus [24].
Elevated expression of transforming growth factor-β1 (TGF-β1) in the tumor microenvironment appears causally linked to creation of highly aggressive metastatic variants [19–23]. Acquired resistance to TGF-β1-mediated growth suppression, moreover, is frequently accompanied by mutation, allelic loss, or misregulation of elements within the TGF-β1 signaling network (e.g., TGF-βRI, TGF-βRII, SMAD2, SMAD4, the coreceptors endoglin, and betaglycan) (see, e.g., [25]). Such signaling defects, particularly in later stage tumors, are often coupled to constitutive epidermal growth factor receptor (EGFR) activation as a result of receptor amplification and/or autocrine ligand release [26–30]. The associated reprogramming of gene expression initiates and perpetuates TGF-β1-induced phenotypic plasticity [21, 31–37].
Recent data mining of the actual repertoire of plasticity genes (i.e., the EMT transcriptome) has significantly enhanced our understanding of the biology of human cutaneous tumor progression while also providing a comparative map of expressed/repressed genes in actinic keratosis and SCC versus normal skin [38, 39]. Although the spectrum of likely candidate genes identified in different studies varies, plasminogen activator inhibitor type-1 (PAI-1; SERPINE1), the major physiologic regulator of the pericellular plasmin-generating cascade, has consistently emerged as a prominent member of the subset of TGF-β1-induced, EMT-associated genes in transformed human keratinocytes [34, 40]. PAI-1 is significantly increased in epithelial cells undergoing a mesenchymal-like conversion following activation of the E-cadherin transcriptional repressors, EMT-inducers, Snail, Slug, or E47 indicating that expression of this serine protease inhibitor is a general characteristic of the plastic phenotype [41]. Use of a novel, physiologically-relevant (i.e., p53 mutant, Ha-ras-expressing), dual growth factor (TGF-β1+EGF)-stimulated model of EMT in transformed human keratinocytes (HaCaT II-4 cells) (Figure 1) and microarray profiling defined PAI-1 as the most highly upregulated transcript of the early gene set (Figure 2; Table 1). The acquisition of a spindle-like, actively motile, behavior in this system was preceded by a decrease in E-cadherin immunoreactivity, the induction of vimentin and α-smooth muscle actin (Figure 1), and a genetic signature typical of an aggressive epithelial cell type (Table 1). Ingenuity Pathway analyses of many of these genes (Figure 2; Table 1) indicate that several (e.g., MMPs, uPA, uPAR, SERPINE1) are direct targets of TGF-β1, as well as NF-κB, highlighting complex associations among EMT, the tumor microenvironment, and the attendant inflammatory response. Importantly, such clustergrams illustrate the highly coordinate and interdependent nature of the defined pericellular proteolytic cascades involved in focalized stromal degradation and tumor invasion (see, e.g., [34, 35, 38–41]).
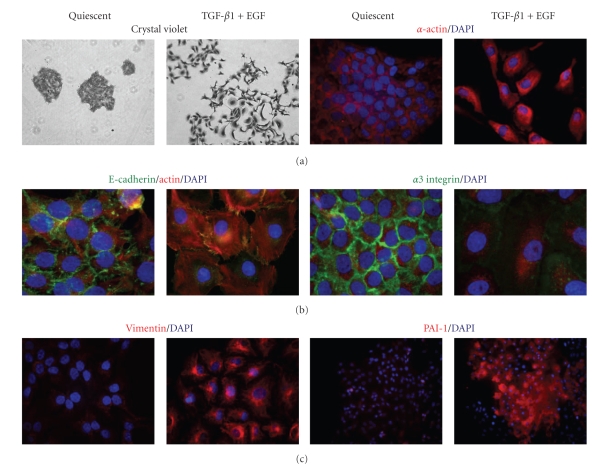
Figure 1.
Combination stimulation with TGF-β1+EGF induces a plastic response in HaCaT II-4 cells. A model system was devised in which small colonies of HaCaT II-4 cells, seeded on tissue culture plastic, were serum-starved followed by addition of TGF-β1 (1 ng/mL) + EGF (10 ng/mL). The induced acquisition of a spindle-shaped, highly migratory phenotype, resulted in marked colony dispersal within 24–48 hours. Cell scattering was accompanied by the loss of E-cadherin (green) and α3 integrin (red) immunostaining at cell-cell junctions, and the gain of several mesenchymal markers, such as α-smooth muscle actin and vimentin with construction of a well-developed vimentin filament network. Induced PAI-1 expression (within 6 hours) was a prominent and early feature of growth factor-stimulated EMT.
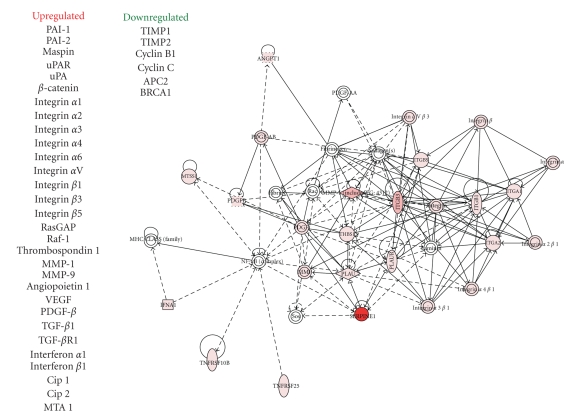
Figure 2.
Microarray transcript profiling and pathway analysis of TGF-β1+EGF-impacted genes in HaCaT II-4 keratinocytes. Focused microarrays of dual growth factor-stimulated HaCaT II-4 cells revealed the increased expression of mRNAs encoding proteins involved in angiogenesis, stromal invasion, and control of pericellular proteolysis. PAI-1 transcripts were the most highly upregulated (>168-fold), induced early (within 6 hours) of stimulation and prior to the onset of colony dispersal. Ingenuity Pathway clustergram mapping describes potential functional interactions among the complement of induced genes. Pathway analyses of many of these genes (see also Table 1) indicate that several including uPA, uPAR, SERPINE1, and the MMPs are TGF-β1 targets and encode key elements in the integrative proteolytic cascades that regulate focalized stromal degradation and tumor invasion.
Table 1.
Transcript levels for select Cancer Pathway genes.
| Gene name | Symbol | Quiescent versus TGF-β1+EGF |
|---|---|---|
| Angiopoietin 1 | ANGPT1 | 3.01 |
| Breast cancer 1, early onset | BRCA1 | −3.18 |
| Cyclin-dependent kinase 2 | CDK2 | 2.46 |
| Cyclin-dependent kinase inhibitor 1A (p21, Cip1) | CDKN1A | 7.41 |
| Interferon α1 | IFNα1 | 5.66 |
| Interferon β1, fibroblast | IFNβ1 | 6.87 |
| Integrin α1 | ITGα1 | 5.66 |
| Integrin α2 | ITGα2 | 18.25 |
| Integrin β1 | ITGβ1 | 11.71 |
| Integrin β3 | ITGβ3 | 59.30 |
| Integrin β5 | ITGβ5 | 5.82 |
| Matrix metallopeptidase 1 | MMP1 | 59.30 |
| Matrix metallopeptidase 9 | MMP9 | 55.33 |
| Metastasis associated 1 | MTA1 | 2.19 |
| Metastasis associated 1 family, member 2 | MTA2 | 1.82 |
| Metastasis suppressor 1 | MTSS1 | 5.58 |
| Platelet-derived growth factor β polypeptide | PDGFB | 9.51 |
| Plasminogen activator, urokinase | PLAU | 2.64 |
| Plasminogen activator, urokinase receptor | PLAUR | 8.00 |
| Serpin peptidase inhibitor, clade E (plasminogen activator inhibitor-1) | SERPINE1 | 168.90 |
| Transforming growth factor β1 | TGFβ1 | 5.54 |
| Transforming growth factor β receptor 1 | TGF-βR1 | 3.46 |
| Thrombospondin 1 | THBS1 | 9.25 |
| Tumor necrosis factor receptor superfamily, member 10b | TNFRSF10B | 2.16 |
| Tumor necrosis factor receptor superfamily, member 25 | TNFRSF25 | 3.53 |
| Vascular endothelial growth factor A | VEGFA | 23.26 |
Elevated PAI-1 tumor levels signal a poor prognosis and reduced disease-free survival in patients with various malignancies including breast, lung, ovarian, and oral SCC [42–46]. Current data suggest a model in which this SERPIN maintains an angiogenic “scaffold,” stabilizes nascent capillary vessel structure, and facilitates tumor cell stromal invasion through precise control of the peritumor proteolytic microenvironment [42, 47, 48]. Indeed, recent targeting of PAI-1 expression in endothelial cells and exogenous introduction of stable PAI-1 variants confirmed that PAI-1 is critical to nascent vessel stabilization and preservation of collagen matrix integrity [35, 49, 50]. In vivo studies, moreover, clearly implicate PAI-1 as an important, perhaps stage-dependent, determinant in cutaneous tumor invasion and the associated angiogenic response [47, 48, 51, 52] (Figure 3). PAI-1 likely “titrates” the extent and locale of collagen matrix remodeling, facilitating tumor invasion into the stroma while maintaining an angiogenic network by inhibiting capillary regression. Molecular knockdown “rescue” strategies, in fact, confirmed PAI-1 to be a positive regulator of keratinocyte migration and an inhibitor of plasminogen-induced anoikis [53]. PAI-1 upregulation is an early event in the progression of incipient epidermal SCC, often localizing to tumor cells and cancer-associated myofibroblasts at the invasive front [54–56] and, more importantly, is a marker with significant prognostic value [43–46]. Identification of PAI-1 (Figure 4(a)) in SCC-proximal α-SMA-positive stromal myofibroblasts (Figure 4(b)), furthermore, implies a more global involvement as a matricellular modulator of invasive potential [55–57] consistent with the increasing appreciation of the role of tumor stromal fibroblasts in cancer progression [58].
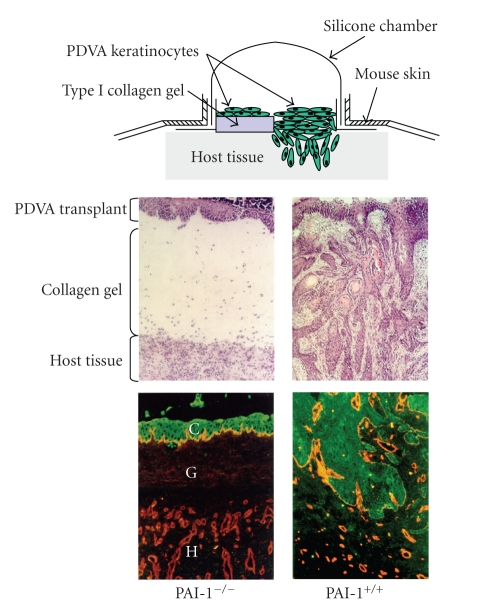
Figure 3.
Cutaneous carcinoma invasion and tumor angiogenesis are suppressed in PAI-1−/− mice. Malignant murine (PDVA) keratinocytes, cultured on a collagen gel in a silicone implantation chamber (top schematic), were transplanted onto PAI-1−/− and wild-type PAI-1+/+ mice. Tumor implantation in PAI-1−/− hosts resulted in a dramatic impairment of stromal invasion and failure to develop a supporting angiogenic network unlike the robust responses evident in wild-type animals. Tissue sections were stained with hematoxylin/eosin (two upper panels) or immunostained for keratin (green; to identify transplanted carcinoma cells) and type IV collagen (red; to delineate capillary vessel basement membrane (two lower panels)).
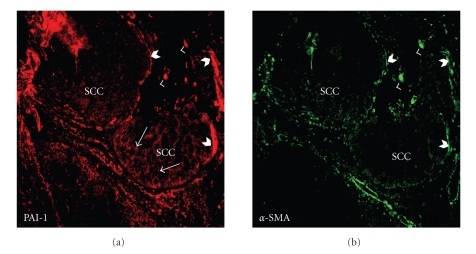
Figure 4.
In situ distribution of PAI-1 in an early invasive human cutaneous squamous cell carcinoma. Sections were dually stained for PAI-1 (red; (a)) and α-smooth muscle actin (α-SMA, green; (b)). PAI-1 was evident in the SCC epithelium at the invasive front (arrows). Prominent PAI-1-expression also colocalized to α-SMA-positive cells, a marker for myofibroblasts, at the carcinoma periphery. Barbed arrowheads indicate PAI-1/α-SMA at the tumor perimeter while arrowheads depict PAI-1/α-SMA in stromal cells.
2.1. Stromal Remodeling Accompanies the Acquisition of Epithelial Plasticity
Costimulation of human cutaneous SCC (HaCaT II-4) cells with TGF-β1+EGF promotes a plastic transition typical of late-stage tumor progression [35, 61] (Figure 1). This conversion to a more aggressive phenotype appears to be due, in part, to deregulated growth factor signaling and the transcriptional reprogramming that supports stromal remodeling events [62–69]. Plasmin generation, in particular, accompanies cooperative TGF-β1/EGFR signaling during the evolution of keratinocyte cell plasticity and is a critical event in the downstream activation of a complex and highly interdependent uPA-plasmin-matrix metalloproteinase (MMP) cascade [35, 70–80]. uPA, uPAR, and MMP expression levels are, in fact, significantly upregulated in HaCaT II-4 cells following stimulation with TGF-β1+EGF (e.g., Figure 2). The combination of TGF-β1+EGF, therefore, augments both matrix deposition, through TGF-β1-dependent upregulation of fibronectin, laminin, proteoglycans, tenascin, thrombospondin and PAI-1 production, and focal degradation by dependent increases in MMPs-1, -2, -3, -9, -10, -11, -13, and -21 [35, 66–71, 81–83].
TGF-β1- and/or EGF-stimulated synthesis of the generally epithelial-restricted MMP-10 (stromelysin-2) [72, 73], which targets a broad spectrum of matrix components including collagens types III, IV, and V, gelatin, elastin, fibronectin, proteoglycans and laminin, as well as proMMPs-1, -7, -8, -9, and -13 [74] is particularly significant. SCC of the head and neck, esophagus, oral cavity, and skin expresses elevated levels of MMP-10 [75–78]. While not detectable in intact skin, during cutaneous wound healing MMP-10 is expressed by keratinocytes that comprise the migrating tongue [79], where its activity appears to be important in stromal remodeling during cutaneous wound healing [79]. Despite an inability to cleave collagen type-I, a major dermal component, MMP-10, promotes plasmin-dependent collagenolysis by TGF-β1+EGF-stimulated HaCaT II-4 cells in a 3-dimensional system [35]. MMP-10, in fact, “superactivates” collagenase 1 (MMP-1), increasing MMP-1-dependent activity >10-fold compared to its activation by plasmin alone [72] creating a significant proteolytic axis within the cutaneous environment.
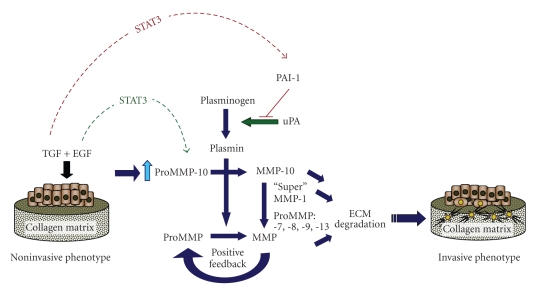
Several MMPs, including MMP-10, are synergistically increased following costimulation of intestinal epithelial cells with TGF-β1+EGF [80]. In HaCaT II-4 keratinocytes, dual stimulation with TGF-β1+EGF induces MMP-10 expression while dramatically enhancing PAI-1 production and stromal invasion [35]. Since type-1 collagen degradation is essential for dermal remodeling, cutaneous tumor invasion may well be considerably dependent on MMP-10 activity. Indeed, MMP-10 upregulation, concomitant with increased STAT3 phosphorylation, accompanies the development of invasive behavior in breast cancer [81]. Similarly, EGF-dependent MMP-10 expression in bladder tumor cells is associated with changes in STAT3 signaling [82]. While the link between STAT3 activation and MMP-10 expression in cutaneous tumor progression remains to be determined, STAT3 over-expression/activation parallels invasive traits in cutaneous SCC [83, 84] suggesting that STAT3 may temporally regulate expression of proteolytically active components in the stromal microenvironment. Our studies indicate, moreover, that PAI-1 regulates MMP-10-dependent collagenolysis in TGF-β1+EGF-stimulated HaCaT II-4 keratinocytes [35]. Collectively, the current data suggest a model (Figure 5) in which MMP-10 induction in response to coincubation with TGF-β1+EGF activates MMPs-1, -7, -8, -9, and -13 stimulating plasmin-dependent matrix proteolysis. A corresponding upregulation of PAI-1 provides a sensitive focalized mechanism for titering the extent and duration of extracellular matrix degradation consequently sustaining a stromal scaffold necessary for tissue invasion. STAT3 in this context may promote this phenotype by regulating growth factor-dependent expression of critical remodeling factors such as MMP-10 and PAI-1 (Figure 5).
Figure 5.
Proposed mechanistic context for TGF-β1+EGF-enhanced plasmin-dependent collagen matrix remodeling and its contribution to development of an invasive phenotype. Dual growth factor-stimulated HaCaT II-4 keratinocytes cultured on a three-dimensional collagen gel upregulate critical stromal remodeling factors that both disrupt and preserve matrix integrity. In the presence of active plasmin, increased MMP-10 promotes MMP activation and creates a proteolytic axis that accelerates collagen degradation through “superactivation” of MMP-1. STAT3 phosphorylation may serve as a temporal switch in this process, through its ability to both promote EGF-stimulated proMMP-10 expression and antagonize induction of TGF-β1 target genes (i.e., PAI-1, fibronectin) [59]. The synergistic upregulation of PAI-1 in response to TGF-β1+EGF may subsequently shift this proteolytic balance, enabling PAI-1 to “titrate” the extent and locale of collagen matrix remodeling to facilitate tumor cell stromal invasion. Indeed, PAI-1 induction is an early event in this phenotypic transition and required for the motile response since PAI-1 knockdown (with siRNA constructs) effectively inhibited TGF-β1+EGF-initiated colony scattering [60].
2.2. TGF-β1/EGFR Pathway Integration in PAI-1 Expression Control
In several common carcinoma types, including cutaneous SCC, the combination of TGF-β1+EGF effectively initiates and maintains the dramatic morphological restructuring and genomic responses characteristic of the plastic phenotype [35, 61, 80]. In particular tumor models, the addition of EGF serves to activate the ras →raf →MEK→ERK cascade as a collateral stimulus to TGF-βR-dependent signaling. Clearly, cooperative, albeit complex, interactions between TGF-β1- and EGFR-activated pathways involving EGFR/pp60c-src, p21ras and mitogen-activated extracellular kinase (MEK) [85] and the MAP kinases ERK/p38 appear mechanistically linked to epithelial tumor cell plasticity, at least in HaCaT II-4 cells [28, 30, 86–88]. The nonreceptor tyrosine kinase pp60c-src is, in fact, a critical intermediate in a TGF-β1-initiated transduction cascade leading to MEK involvement, PAI-1 transcription, and downstream phenotypic responses [28, 85, 86, 88]. TGF-β1 complements EGF-mediated signaling to the MAPK/AKT pathways to effect EMT consistent with the requirement for oncogenic ras in TGF-β1-induced EMT [89, 90]. Disruption of TGF-β1-stimulated ERK1/2 phosphorylation and PAI-1 transcription by src family kinase inhibitors, as well as blockade of EGFR signaling with AG1478, suggests that pp60c-src, perhaps through phosphorylation of the Y845 src-kinase EGFR target residue, regulates MEK-ERK-dependent PAI-1 expression [28, 85, 91] (Figure 6). While the actual mechanism underlying TGF-β1-associated pp60c-src kinase/EGFR stimulation remains to be determined, TGF-β1-dependent release of EGFR ligands (e.g., HB-EGF, amphiregulin and/or TGF-α) via MMP- or ADAM-dependent processes is one likely possibility for at least some cell types [60, 92]. Alternatively, the TGF-β1-stimulated formation of integrin/FAK/p130cas/EGFR complexes may initiate ligand-independent EGFR activation and pp60c-src recruitment [91, 93, 94]. Indeed, in HaCaT cells, TGF-β1 transactivates the EGFR in a complex manner requiring src kinase signaling and production of reactive oxygen species but may not involve the shedding of EGFR ligands [30, 95]. The effective blockade of TGF-β1-stimulated ERK1/2 phosphorylation and PAI-1 transcription by src kinase-targeting pharmacologic agents, as well as the EGFR inhibitor AG1478, and the requirement for MEK-ERK signaling for the full inductive effect of TGF-β1, suggests that pp60c-src, perhaps through phosphorylation of the Y845 src-specific EGFR substrate residue regulates the MEK-ERK-dependent PAI-1 expression transduction cascade [28, 30, 51, 85, 86]. While specific mechanisms remain to be clarified, it is apparent that cooperative EGFR signaling is an essential aspect of TGF-β1-stimulated PAI-1 expression which provides novel insights as to the impact of TGF-β1 in late-stage human cutaneous tumor progression.
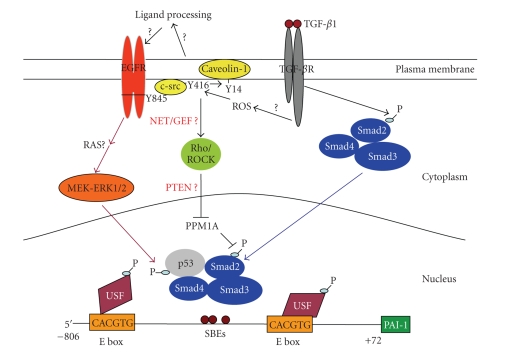
Figure 6.
A model for TGF-β1 induced PAI-1 transcription. Emerging studies suggest that transcriptional outputs from both SMAD2/3 as well as non-SMAD (e.g., EGFR-MEK/ERK) pathways are absolutely critical for TGF-β1-mediated PAI-1 induction. Activated Src kinases (e.g., c-Src), downstream of TGF-β1 receptor, function as an upstream regulator of EGFR transactivation (by Y845 phosphorylation). c-Src also modulates Caveolin-1Y14 phosphorylation, and likely stimulates Rho/ROCK-dependent maintenance of SMAD2/3 transcriptional activity (by suppressing nuclear levels or activity of the SMAD2/3 phosphatase PPM1A). ERK1/2 (downstream of EGFR activation), or p38 kinases, may phosphorylate p53 and the bHLH-LZ upstream stimulatory factor proteins 1/2 (USF1/2) in response to TGF-β1. Indeed, SMAD2/3 appears to cooperate with p53 and USF family transcription factors for maximal TGF-β1-directed PAI-1 gene expression.
2.3. PAI-1 Transcription: Links to p53
Members of the p53 family are critical elements in a subset of TGF-β1 transcriptional responses due, at least in part, to the ability of MAP kinase-phosphorylated p53 to bind SMAD2 [96–100]. DNase I footprinting/methylation interference and oligonucleotide mobility shift analyses confirmed, moreover, that p53 binds to a recognition motif in the PAI-1 promoter resulting in both p53 sequence-driven reporter gene transcription and induced expression of the endogenous PAI-1 gene [101]. Two p53 half-sites (AcACATGCCT, cAGCAAGTCC) [Profile Hidden Markov Model score= 82; 89] likely regulate p53-dependent PAI-1 gene activation [102]. p53-mediated PAI-1 expression control, moreover, is likely to involve nontranscriptional mechanisms as well since p53 binds to a 70 nt sequence on the PAI-1 mRNA 3′ UTR resulting in increased mRNA stabilization [103].
p53 is also required for maximal PAI-1 expression in response to TGF-β1 since induced transcription is significantly attenuated in p53 siRNA knockdown cells [98] as well as in p53−/− mouse fibroblasts (Samarakoon and Higgins, unpublished data). This is consistent with the observation that p53-deficient lung tumor cells synthesize little or no PAI-1 while vector-engineered introduction of wild-type p53 rescues both basal and inducible PAI-1 expressions [103]. The recent analysis of the upstream region of the PAI-1 gene provides some insight as to the possible mechanisms underlying p53-dependent PAI-1 gene control. The PAI-1 promoter PE2 region hexanucleotide E box (CACGTG), a site juxtaposed to 3 SMAD-binding elements, is occupied by upstream stimulatory factor (USF) in response to TGF-β1 stimulation [85, 104]. Phasing analysis revealed that certain bHLH-LZ members of the MYC family (including USF) orient DNA bending toward the minor groove [105] which could potentially promote interactions between p53, bound to its downstream half-site motif, with SMAD2 tethered to the upstream PE2 region SMAD-binding elements, thus, providing a molecular basis for SMAD2/p53 complex formation and subsequent transcriptional activation of the PAI-1 gene (Figure 6).
2.4. Implications on Cell Growth Control
p53 mutations occur in 40–60% of all skin cancers [9, 106] suggesting that direct p53 transcriptional targets (such as the PAI-1 gene) may be activated, repressed, or dysregulated as a consequence of p53 mutation with associated loss or gain of function. Indeed, p53 is a major element in PAI-1 induction in response to TGF-β1 [98] and may be critical particularly in the setting of increased autocrine TGF-β1 expression during cutaneous SCC progression. The role of PAI-1 in subsequent tumor progression, however, may be more complex than previously appreciated. Ectopic expression of wild-type PAI-1 in breast cancer cells or in p53-deficient murine and human fibroblasts, in fact, initiates a senescence-like growth arrest [92, 107] while RNAi-mediated PAI-1 knockdown (PAI-1KD) or PAI-1 genetic deficiency (PAI-1−/− genotype) results in escape from replicative senescence in primary mouse and human fibroblasts [107]. Proliferation of PAI-1−/− endothelial cells, and PAI-1KD fibroblasts appears due, at least in part, to sustained activation of the PI3K-AKT-GSK3β pathway, increased AKTSer473 phosphorylation, nuclear retention of cyclin D1 [107, 108] and, perhaps, increased inactivation of the tumor suppressor PTEN [108]. Importantly, PAI-1−/− mouse embryo fibroblasts (MEFs), PAI-1KD HaCaT keratinocytes, and PAI-1KD MEFs are resistant to TGF-β1-initiated growth inhibition although PAI-1 deficiency does not interfere with canonical TGF-β1 signaling such as SMAD phosphorylation or p21CIP1 and p15INK4B induction [109].
Collectively, these data suggest a multifunctional relationship between PAI-1 expression and tumor progression. Elevated PAI-1 levels may inhibit (at least transiently) tumor cell proliferation while stimulating migration and stromal invasion by providing a sensitive focalized mechanism for titering the extent and duration of extracellular matrix degradation, sustaining a stromal scaffold necessary for tissue invasion. This carefully orchestrated process may also serve to promote tumor cell survival by preventing anoikis during the precarious process of cell detachment and readhesion to a new, likely foreign, tissue microenvironment. Importantly, these findings underscore the potential diversity of new molecular targets that can be exploited for therapeutic benefit. Refining the current understanding of PAI-1 gene regulation, and relevant signaling pathways, may lead to the discovery of critical regulatory factors that ultimately prove important in stage-specific treatment of human cutaneous malignancies.
Acknowledgment
This work is supported by NIH Grant GM57242.
Abbreviations
- SCC:
Squamous cell carcinoma
- EGF:
Epidermal growth factor
- EGFR:
Epidermal growth factor receptor
- TGF-β1:
Transforming growth factor-β1
- MMP:
Matrix metalloproteinase
- NMSC:
Nonmelanoma skin cancer
- UV:
Ultraviolet
- TGF-βR:
TGF-β receptor
- EMT:
Epithelial-to-mesenchymal transition
- PAI-1:
Plasminogen activator inhibitor type-1
- SERPINE1:
Serine protease inhibitor, clade E, member 1
- uPA:
Urokinase plasminogen activator
- uPAR:
Urokinase plasminogen activator receptor
- STAT3:
Signal transducer and activators of transcription protein 3
- SMAD:
Sma/Mad homologues
- ERK:
Extracellular signal-regulated kinases
- MEK:
Mitogen-activated protein kinase/ERK kinase
- FAK:
Focal adhesion kinase
- MEFs:
Mouse embryo fibroblasts.
References
- 1.Eide MJ, Weinstock MA. Epidemiology of skin cancer. In: Rigel DS, Friedman RJ, Dzubow LM, Reintgen DS, Bystryn J-C, Marks R, editors. Cancer of the Skin. Philadelphia, Pa, USA: Elsevier; 2005. pp. 47–60. [Google Scholar]
- 2.Lang PG, Maize JC. Basal cell carcinoma. In: Rigel DS, Friedman RJ, Dzubow LM, Reintgen DS, Bystryn J-C, Marks R, editors. Cancer of the Skin. Philadelphia, Pa, USA: Elsevier; 2005. pp. 101–132. [Google Scholar]
- 3.Dlugosz A, Merlino G, Yuspa SH. Progress in cutaneous cancer research. Journal of Investigative Dermatology Symposium Proceedings. 2002;7(1):17–26. doi: 10.1046/j.1523-1747.2002.19631.x. [DOI] [PubMed] [Google Scholar]
- 4.Staples MP, Elwood M, Burton RC, Williams JL, Marks R, Giles GG. Non-melanoma skin cancer in Australia: the 2002 national survey and trends since 1985. Medical Journal of Australia. 2006;184(1):6–10. doi: 10.5694/j.1326-5377.2006.tb00086.x. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen TH, Yoon J. Squamous cell carcinoma. In: Rigel DS, Friedman RJ, Dzubow LM, Reintgen DS, Bystryn J-C, Marks R, editors. Cancer of the Skin. Philadelphia, Pa, USA: Elsevier; 2005. pp. 133–150. [Google Scholar]
- 6.Boukamp P. UV-induced skin cancer: similarities—variations. Journal of the German Society of Dermatology. 2005;3(7):493–503. doi: 10.1111/j.1610-0387.2005.05037.x. [DOI] [PubMed] [Google Scholar]
- 7.Boukamp P, Peter W, Pascheberg U, et al. Step-wise progression in human skin carcinogenesis in vitro involves mutational inactivation of p53, ras(H) oncogene activation and additional chromosome loss. Oncogene. 1995;11(5):961–969. [PubMed] [Google Scholar]
- 8.Giglia-Mari G, Sarasin A. TP53 mutations in human skin cancers. Human Mutation. 2003;21(3):217–228. doi: 10.1002/humu.10179. [DOI] [PubMed] [Google Scholar]
- 9.Dans M, Fakharzadeh SS. Genetic basis of skin cancer. In: Rigel DS, Friedman RJ, Dzubow LM, Reintgen DS, Bystryn J-C, Marks R, editors. Cancer of the Skin. Philadelphia, Pa, USA: Elsevier; 2005. pp. 15–27. [Google Scholar]
- 10.Smoller BR. Squamous cell carcinoma: from precursor lesions to high-risk variants. Modern Pathology. 2006;19(supplement 2):S88–S92. doi: 10.1038/modpathol.3800509. [DOI] [PubMed] [Google Scholar]
- 11.Tsai KY, Tsao H. The genetics of skin cancer. American Journal of Medical Genetics. 2004;131C(1):82–92. doi: 10.1002/ajmg.c.30037. [DOI] [PubMed] [Google Scholar]
- 12.Boukamp P. Non-melanoma skin cancer: what drives tumor development and progression? Carcinogenesis. 2005;26(10):1657–1667. doi: 10.1093/carcin/bgi123. [DOI] [PubMed] [Google Scholar]
- 13.Yuspa SH. The pathogenesis of squamous cell cancer: lessons learned from studies of skin carcinogenesis. Journal of Dermatological Science. 1998;17(1):1–7. doi: 10.1016/s0923-1811(97)00071-6. [DOI] [PubMed] [Google Scholar]
- 14.Frame S, Crombie R, Liddell J, et al. Epithelial carcinogenesis in the mouse: correlating the genetics and the biology. Philosophical Transactions of the Royal Society B. 1998;353(1370):839–845. doi: 10.1098/rstb.1998.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsantoulis PK, Kastrinakis NG, Tourvas AD, Laskaris G, Gorgoulis VG. Advances in the biology of oral cancer. Oral Oncology. 2007;43(6):523–534. doi: 10.1016/j.oraloncology.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Brown K, Strathdee D, Bryson S, Lambie W, Balmain A. The malignant capacity of skin tumours induced by expression of a mutant H-ras transgene depends on the cell type targeted. Current Biology. 1998;8(9):516–524. doi: 10.1016/s0960-9822(98)70203-9. [DOI] [PubMed] [Google Scholar]
- 17.Akhurst RJ, Balmain A. Genetic events and the role of TGFβ in epithelial tumour progression. Journal of Pathology. 1999;187(1):82–90. doi: 10.1002/(SICI)1096-9896(199901)187:1<82::AID-PATH248>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 18.Caulin C, Nguyen T, Lang GA, et al. An inducible mouse model for skin cancer reveals distinct roles for gain- and loss-of-function p53 mutations. Journal of Clinical Investigation. 2007;117(7):1893–1901. doi: 10.1172/JCI31721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui W, Fowlis DJ, Bryson S, et al. TGFβ1 inhibits the formation of benign skin tumors, but enhances progression to invasive spindle carcinomas in transgenic mice. Cell. 1996;86(4):531–542. doi: 10.1016/s0092-8674(00)80127-0. [DOI] [PubMed] [Google Scholar]
- 20.Portella G, Cumming SA, Liddell J, et al. Transforming growth factor β is essential for spindle cell conversion of mouse skin carcinoma in vivo: implications for tumor invasion. Cell Growth and Differentiation. 1998;9(5):393–404. [PubMed] [Google Scholar]
- 21.Derynck R, Akhurst RJ, Balmain A. TGF-β signaling in tumor suppression and cancer progression. Nature Genetics. 2001;29(2):117–129. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- 22.Moreno-Bueno G, Portillo F, Cano A. Transcriptional regulation of cell polarity in EMT and cancer. Oncogene. 2008;27(55):6958–6969. doi: 10.1038/onc.2008.346. [DOI] [PubMed] [Google Scholar]
- 23.Han G, Lu S-L, Li AG, et al. Distinct mechanisms of TGF-β1-mediated epithelial-to-mesenchymal transition and metastasis during skin carcinogenesis. Journal of Clinical Investigation. 2005;115(7):1714–1723. doi: 10.1172/JCI24399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. Journal of Clinical Investigation. 2009;119(6):1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bierie B, Moses HL. Tumour microenvironment: TGFβ: the molecular Jekyll and Hyde of cancer. Nature Reviews Cancer. 2006;6(7):506–520. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- 26.Moghal N, Sternberg PW. Multiple positive and negative regulators of signaling by the EGF-receptor. Current Opinion in Cell Biology. 1999;11(2):190–196. doi: 10.1016/s0955-0674(99)80025-8. [DOI] [PubMed] [Google Scholar]
- 27.Rho O, Beltran LM, Gimenez-Conti IB, DiGiovanni J. Altered expression of the epidermal growth factor receptor and transforming growth factor-α during multistage skin carcinogenesis in SENCAR mice. Molecular Carcinogenesis. 1994;11(1):19–28. doi: 10.1002/mc.2940110105. [DOI] [PubMed] [Google Scholar]
- 28.Samarakoon R, Higgins CE, Higgins SP, Higgins PJ. TGF-β1-induced expression of the poor prognosis SERPINE1/PAI-1 gene requires EGFR signaling: a new target for anti-EGFR therapy. Journal of Oncology. 2009;2009:6 pages. doi: 10.1155/2009/342391. Article ID 342391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murillo MM, del Castillo G, Sánchez A, Fernández M, Fabregat I. Involvement of EGF receptor and c-Src in the survival signals induced by TGF-β1 in hepatocytes. Oncogene. 2005;24(28):4580–4587. doi: 10.1038/sj.onc.1208664. [DOI] [PubMed] [Google Scholar]
- 30.Joo C-K, Kim H-S, Park J-Y, Seomun Y, Son M-J, Kim J-T. Ligand release-independent transactivation of epidermal growth factor receptor by transforming growth factor-β involves multiple signaling pathways. Oncogene. 2008;27(5):614–628. doi: 10.1038/sj.onc.1210649. [DOI] [PubMed] [Google Scholar]
- 31.O-Charoenrat P, Rhys-Evans P, Modjtahedi H, Court W, Box G, Eccles S. Overexpression of epidermal growth factor receptor in human head and neck squamous carcinoma cell lines correlates with matrix metalloproteinase-9 expression and in vitro invasion. International Journal of Cancer. 2000;89(2):307–317. doi: 10.1002/(sici)1097-0215(20000501)86:3<307::aid-ijc2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 32.O-charoenrat P, Rhys-Evans PH, Archer DJ, Eccles SA. C-erbB receptors in squamous cell carcinomas of the head and neck: clinical significance and correlation with matrix metalloproteinases and vascular endothelial growth factors. Oral Oncology. 2002;38(1):73–80. doi: 10.1016/s1368-8375(01)00029-x. [DOI] [PubMed] [Google Scholar]
- 33.Zavadil J, Böttinger EP. TGF-β and epithelial-to-mesenchymal transitions. Oncogene. 2005;24(37):5764–5774. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- 34.Zavadil J, Bitzer M, Liang D, et al. Genetic programs of epithelial cell plasticity directed by transforming growth factor-β . Proceedings of the National Academy of Sciences of the United States of America. 2001;98(12):6686–6691. doi: 10.1073/pnas.111614398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilkins-Port CE, Ye Q, Mazurkiewicz JE, Higgins PJ. TGF-β1 + EGF-initiated invasive potential in transformed human keratinocytes is coupled to a plasmin/MMP-10/MMP-1-dependent collagen remodeling axis: role for PAI-1. Cancer Research. 2009;69(9):4081–4091. doi: 10.1158/0008-5472.CAN-09-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mani SA, Guo W, Liao M-J, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Developmental Cell. 2008;14(6):818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 38.Nindl I, Dang C, Forschner T, et al. Identification of differentially expressed genes in cutaneous squamous cell carcinoma by microarray expression profiling. Molecular Cancer. 2006;5:1–17. doi: 10.1186/1476-4598-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dang C, Gottschling M, Manning K, et al. Identification of dysregulated genes in cutaneous squamous cell carcinoma. Oncology Reports. 2006;16(3):513–519. [PubMed] [Google Scholar]
- 40.Akiyoshi S, Ishii M, Nemoto N, Kawabata M, Aburatani H, Miyazono K. Targets of transcriptional regulation by transforming growth factor-β: expression profile analysis using oligonucleotide arrays. Japanese Journal of Cancer Research. 2001;92(3):257–268. doi: 10.1111/j.1349-7006.2001.tb01090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moreno-Bueno G, Cubillo E, Sarrió D, et al. Genetic profiling of epithelial cells expressing E-cadherin repressors reveals a distinct role for Snail, Slug, and E47 factors in epithelial- mesenchymal transition. Cancer Research. 2006;66(19):9543–9556. doi: 10.1158/0008-5472.CAN-06-0479. [DOI] [PubMed] [Google Scholar]
- 42.Andreasen PA, Kjøller L, Christensen L, Duffy MJ. The urokinase-type plasminogen activator system in cancer metastasis: a review. International Journal of Cancer. 1997;72(1):1–22. doi: 10.1002/(sici)1097-0215(19970703)72:1<1::aid-ijc1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 43.Hundsdorfer B, Zeilhofer HF, Bock KP, Dettmar P, Schmitt M, Horch HH. The prognostic importance of urinase type plasminogen activators (uPA) and plasminogen activator inhibitors (PAI-1) in the primary resection of oral squamous cell carcinoma. Mund-, Kiefer- und Gesichtschirurgie. 2004;8(3):173–179. doi: 10.1007/s10006-003-0520-x. [DOI] [PubMed] [Google Scholar]
- 44.Annecke K, Schmitt M, Euler U, et al. uPA and PAI-1 in breast cancer: review of their clinical utility and current validation in the prospective NNBC-3 trial. Advances in Clinical Chemistry. 2008;45:31–45. doi: 10.1016/s0065-2423(07)00002-9. [DOI] [PubMed] [Google Scholar]
- 45.Harbeck N, Schmitt M, Paepke S, Allgayer H, Kates RE. Tumor-associated proteolytic factors uPA and PAI-1: critical appraisal of their clinical relevance in breast cancer and their integration into decision-support algorithms. Critical Reviews in Clinical Laboratory Sciences. 2007;44(2):179–201. doi: 10.1080/10408360601040970. [DOI] [PubMed] [Google Scholar]
- 46.Vairaktaris E, Yapijakis C, Serefoglou Z, et al. Plasminogen activator inhibitor-1 polymorphism is associated with increased risk for oral cancer. Oral Oncology. 2006;42(9):888–892. doi: 10.1016/j.oraloncology.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 47.Bajou K, Masson V, Gerard RD, et al. The plasminogen activator inhibitor PAI-1 controls in vivo tumor vascularization by interaction with proteases, not vitronectin: implications for antiangiogenic strategies. Journal of Cell Biology. 2001;152(4):777–784. doi: 10.1083/jcb.152.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bajou K, Noel A, Gerard RD, et al. Absence of host plasminogen activator inhibitor 1 prevents cancer invasion and vascularization. Nature Medicine. 1998;4(8):923–928. doi: 10.1038/nm0898-923. [DOI] [PubMed] [Google Scholar]
- 49.Davis GE, Pintar Allen KA, Salazar R, Maxwell SA. Matrix metalloproteinase-1 and -9 activation by plasmin regulates a novel endothelial cell-mediated mechanism of collagen gel contraction and capillary tube regression in three-dimensional collagen matrices. Journal of Cell Science. 2001;114(5):917–930. doi: 10.1242/jcs.114.5.917. [DOI] [PubMed] [Google Scholar]
- 50.Higgins SP, Samarakoon R, Higgins CE, Freytag J, Wilkins-Port C, Higgins PJ. TGF-β1-induced expression of the anti-apoptotic PAI-1 protein requires EGFR signaling. Cell Communication Insights. 2009;2:1–11. doi: 10.4137/cci.s2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maillard C, Jost M, Rømer MU, et al. Host plasminogen activator inhibitor-1 promotes human skin carcinoma progression in a stage-dependent manner. Neoplasia. 2005;7(1):57–66. doi: 10.1593/neo.04406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bacharach E, Itin A, Keshet E. Apposition-dependent induction of plasminogen activator inhibitor type 1 expression: a mechanism for balancing pericellular proteolysis during angiogenesis. Blood. 1998;92(3):939–945. [PubMed] [Google Scholar]
- 53.Providence KM, Higgins SP, Mullen A, Battista A, Higgins CE, Higgins PJ. SERPINE1 (PAI-1) is deposited into keratinocyte migration “trails” and required for optimal monolayer wound repair. Archives of Dermatological Research. 2008;300(6):303–310. doi: 10.1007/s00403-008-0845-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilkins-Port CE, Higgins CE, Freytag J, Higgins SP, Carlson JA, Higgins PJ. PAI-1 is a critical upstream regulator of the TGF-β1/EGF-induced invasive phenotype in mutant p53 human cutaneous squamous cell carcinoma. Journal of Biomedicine and Biotechnology. 2007;2007:1–8. doi: 10.1155/2007/85208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Illemann M, Hansen U, Nielsen HJ, et al. Leading-edge myofibroblasts in human colon cancer express plasminogen activator inhibitor-1. American Journal of Clinical Pathology. 2004;122(2):256–265. doi: 10.1309/F32X-WQ20-T568-H8VP. [DOI] [PubMed] [Google Scholar]
- 56.Offersen BV, Nielsen BS, Høyer-Hansen G, et al. The myofibroblast is the predominant plasminogen activator inhibitor-1-expressing cell type in human breast carcinomas. American Journal of Pathology. 2003;163(5):1887–1899. doi: 10.1016/S0002-9440(10)63547-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maquerlot F, Galiacy S, Malo M, et al. Dual role for plasminogen activator inhibitor type 1 as soluble and as matricellular regulator of epithelial alveolar cell wound healing. American Journal of Pathology. 2006;169(5):1624–1632. doi: 10.2353/ajpath.2006.051053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parrinello S, Coppe J-P, Krtolica A, Campisi J. Stromal-epithelial interactions in aging and cancer: senescent fibroblasts alter epithelial cell differentiation. Journal of Cell Science. 2005;118(3):485–496. doi: 10.1242/jcs.01635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Giannopoulou M, Iszkula SC, Dai C, et al. Distinctive role of Stat3 and Erk-1/2 activation in mediating interferon-γ inhibition of TGF-β1 action. American Journal of Physiology. 2006;290(5):F1234–F1240. doi: 10.1152/ajprenal.00388.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Freytag J, Wilkins-Port CE, Higgins CE, Higgins SP, Samarakoon R, Higgins PJ. PAI-1 mediates the TGF-β1+EGF-induced “scatter”-response in transformed human keratinocytes. doi: 10.1038/jid.2010.106. Journal of Investigative Dermatology. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davies M, Robinson M, Smith E, Huntley S, Prime S, Paterson I. Induction of an epithelial to mesenchymal transition in human immortal and malignant keratinocytes by TGF-β1 involves MAPK, Smad and AP-1 signalling pathways. Journal of Cellular Biochemistry. 2005;95(5):918–931. doi: 10.1002/jcb.20458. [DOI] [PubMed] [Google Scholar]
- 62.Chin D, Boyle GM, Parsons PG, Coman WB. What is transforming growth factor-beta (TGF-β)? British Journal of Plastic Surgery. 2004;57(3):215–221. doi: 10.1016/j.bjps.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 63.Streuli CH, Schmidhauser C, Kobrin M, Bissell MJ, Derynck R. Extracellular matrix regulates expression of the TGF-β1 gene. Journal of Cell Biology. 1993;120(1):253–260. doi: 10.1083/jcb.120.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vollberg TM, Sr., George MD, Jetten AM. Induction of extracellular matrix gene expression in normal human keratinocytes by transforming growth factor β is altered by cellular differentiation. Experimental Cell Research. 1991;193(1):93–100. doi: 10.1016/0014-4827(91)90542-3. [DOI] [PubMed] [Google Scholar]
- 65.Wikner NE, Elder JT, Persichitte KA, Mink P, Clark RAF. Transforming growth factor-β modulates plasminogen activator activity and plasminogen activator inhibitor type-1 expression in human keratinocytes in vitro. Journal of Investigative Dermatology. 1990;95(5):607–613. doi: 10.1111/1523-1747.ep12505603. [DOI] [PubMed] [Google Scholar]
- 66.Ahokas K, Lohi J, Illman SA, et al. Matrix metalloproteinase-21 is expressed epithelially during development and in cancer and is up-regulated by transforming growth factor-β1 in keratinocytes. Laboratory Investigation. 2003;83(12):1887–1899. doi: 10.1097/01.lab.0000106721.86126.39. [DOI] [PubMed] [Google Scholar]
- 67.Johansson N, Westermarck J, Leppä S, et al. Collagenase 3 (matrix metalloproteinase 13) gene expression by HaCaT keratinocytes is enhanced by tumor necrosis factor α and transforming growth factor β1. Cell Growth and Differentiation. 1997;8(2):243–250. [PubMed] [Google Scholar]
- 68.Kim ES, Kim MS, Moon A. TGF-beta-induced upregulation of MMP-2 and MMP-9 depends on p38 MAPK, but not ERK signaling in MCF10A human breast epithelial cells. International Journal of Oncology. 2004;25(5):1375–1382. [PubMed] [Google Scholar]
- 69.Kim E-S, Kim M-S, Moon A. Transforming growth factor (TGF)-β in conjunction with H-ras activation promotes malignant progression of MCF10A breast epithelial cells. Cytokine. 2005;29(2):84–91. doi: 10.1016/j.cyto.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 70.Kim H-S, Shang T, Chen Z, Pflugfelder SC, Li D-Q. TGF-β1 stimulates production of gelatinase (MMP-9), collagenases (MMP-1, -13) and stromelysins (MMP-3, -10, -11) by human corneal epithelial cells. Experimental Eye Research. 2004;79(2):263–274. doi: 10.1016/j.exer.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 71.Munshi HG, Wu YI, Mukhopadhyay S, et al. Differential regulation of membrane type 1-matrix metalloproteinase activity by ERK 1/2- and p38 MAPK-modulated tissue inhibitor of metalloproteinases 2 expression controls transforming growth factor-β1-induced pericellular collagenolysis. Journal of Biological Chemistry. 2004;279(37):39042–39050. doi: 10.1074/jbc.M404958200. [DOI] [PubMed] [Google Scholar]
- 72.Windsor LJ, Grenett H, Birkedal-Hansen B, Bodden MK, Engler JA, Birkedal-Hansen H. Cell type-specific regulation of SL-1 and SL-2 genes. Induction of the SL- 2 gene but not the SL-1 gene by human keratinocytes in response to cytokines and phorbolesters. Journal of Biological Chemistry. 1993;268(23):17341–17347. [PubMed] [Google Scholar]
- 73.Madlener M, Mauch C, Conca W, Brauchle M, Parks WC, Werner S. Regulation of the expression of stromelysin-2 by growth factors in keratinocytes: implications for normal and impaired wound healing. Biochemical Journal. 1996;320(2):659–664. doi: 10.1042/bj3200659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T. Regulation of matrix metalloproteinases. An overview. Molecular and Cellular Biochemistry. 2003;253(1-2):269–285. doi: 10.1023/a:1026028303196. [DOI] [PubMed] [Google Scholar]
- 75.O-charoenrat P, Rhys-Evans PH, Eccles SA. Expression of matrix metalloproteinases and their inhibitors correlates with invasion and metastasis in squamous cell carcinoma of the head and neck. Archives of Otolaryngology. 2001;127(7):813–820. [PubMed] [Google Scholar]
- 76.Impola U, Uitto VJ, Hietanen J, et al. Differential expression of matrilysin-I (MMP-7), 92 kD gelatinase (MMP-9), and metalloelastase (MMP-12) in oral verrucous and squamous cell cancer. Journal of Pathology. 2004;202(1):14–22. doi: 10.1002/path.1479. [DOI] [PubMed] [Google Scholar]
- 77.Kerkelä E, Ala-aho R, Jeskanen L, et al. Differential patterns of stromelysin-2 (MMP-10) and MT1-MMP (MMP-14) expression in epithelial skin cancers. British Journal of Cancer. 2001;84(5):659–669. doi: 10.1054/bjoc.2000.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mathew R, Khanna R, Kumar R, Mathur M, Shukla NK, Ralhan R. Stromelysin-2 overexpression in human esophageal squamous cell carcinoma: potential clinical implications. Cancer Detection and Prevention. 2002;26(3):222–228. doi: 10.1016/s0361-090x(02)00035-1. [DOI] [PubMed] [Google Scholar]
- 79.Krampert M, Bloch W, Sasaki T, et al. Activities of the matrix metalloproteinase stromelysin-2 (MMP-10) in matrix degradation and keratinocyte organization in wounded skin. Molecular Biology of the Cell. 2004;15(12):5242–5254. doi: 10.1091/mbc.E04-02-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Uttamsingh S, Bao X, Nguyen KT, et al. Synergistic effect between EGF and TGF-β1 in inducing oncogenic properties of intestinal epithelial cells. Oncogene. 2008;27(18):2626–2634. doi: 10.1038/sj.onc.1210915. [DOI] [PubMed] [Google Scholar]
- 81.Hsieh F-C, Cheng G, Lin J. Evaluation of potential Stat3-regulated genes in human breast cancer. Biochemical and Biophysical Research Communications. 2005;335(2):292–299. doi: 10.1016/j.bbrc.2005.07.075. [DOI] [PubMed] [Google Scholar]
- 82.Itoh M, Murata T, Suzuki T, et al. Requirement of STAT3 activation for maximal collagenase-1 (MMP-1) induction by epidermal growth factor and malignant characteristics in T24 bladder cancer cells. Oncogene. 2006;25(8):1195–1204. doi: 10.1038/sj.onc.1209149. [DOI] [PubMed] [Google Scholar]
- 83.Sumita N, Bito T, Nakajima K, Nishigori C. Stat3 activation is required for cell proliferation and tumorigenesis but not for cell viability in cutaneous squamous cell carcinoma cell lines. Experimental Dermatology. 2006;15(4):291–299. doi: 10.1111/j.0906-6705.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- 84.Suiqing C, Min Z, Lirong C. Overexpression of phosphorylated-STAT3 correlated with the invasion and metastasis of cutaneous squamous cell carcinoma. Journal of Dermatology. 2005;32(5):354–360. doi: 10.1111/j.1346-8138.2005.tb00906.x. [DOI] [PubMed] [Google Scholar]
- 85.Kutz SM, Higgins CE, Samarakoon R, et al. TGF-β1-induced PAI-1 expression is E box/USF-dependent and requires EGFR signaling. Experimental Cell Research. 2006;312(7):1093–1105. doi: 10.1016/j.yexcr.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 86.Samarakoon R, Higgins CE, Higgins SP, Kutz SM, Higgins PJ. Plasminogen activator inhibitor type-1 gene expression and induced migration in TGF-β1-stimulated smooth muscle cells is p p60c−src/MEK-dependent. Journal of Cellular Physiology. 2005;204(1):236–246. doi: 10.1002/jcp.20279. [DOI] [PubMed] [Google Scholar]
- 87.Hu PP-C, Shen X, Huang D, Liu Y, Counter C, Wang X-F. The MEK pathway is required for stimulation of p21(WAF1/CIP1) by transforming growth factor-β . Journal of Biological Chemistry. 1999;274(50):35381–35387. doi: 10.1074/jbc.274.50.35381. [DOI] [PubMed] [Google Scholar]
- 88.Sato M, Kawai-Kowase K, Sato H, et al. c-Src and hydrogen peroxide mediate transforming growth factor-β1-induced smooth muscle cell-gene expression in 10T1/2 cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25(2):341–347. doi: 10.1161/01.ATV.0000152608.29351.8f. [DOI] [PubMed] [Google Scholar]
- 89.Oft M, Peli J, Rudaz C, Schwarz H, Beug H, Reichmann E. TGF-β1 and Ha-Ras collaborate in modulating the phenotypic plasticity and invasiveness of epithelial tumor cells. Genes and Development. 1996;10(19):2462–2477. doi: 10.1101/gad.10.19.2462. [DOI] [PubMed] [Google Scholar]
- 90.Oft M, Akhurst RJ, Balmain A. Metastasis is driven by sequential elevation of H-ras and Smad2 levels. Nature Cell Biology. 2002;4(7):487–494. doi: 10.1038/ncb807. [DOI] [PubMed] [Google Scholar]
- 91.Biscardi JS, Maa MC, Tice DA, Cox ME, Leu TH, Parsons SJ. c-Src-mediated phosphorylation of the epidermal growth factor receptor on tyr 845 and tyr 1101 is associated with modulation of receptor function. Journal of Biological Chemistry. 1999;274:8335–8343. doi: 10.1074/jbc.274.12.8335. [DOI] [PubMed] [Google Scholar]
- 92.Beaulieu LM, Whitley BR, Wiesner TF, et al. Breast cancer and metabolic syndrome linked through the plasminogen activator inhibitor-1 cycle. BioEssays. 2007;29(10):1029–1038. doi: 10.1002/bies.20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moro L, Dolce L, Cabodi S, et al. Integrin-induced epidermal growth factor (EGF) receptor activation requires c-Src and p130Cas and leads to phosphorylation of specific EGF receptor tyrosines. Journal of Biological Chemistry. 2002;277(11):9405–9414. doi: 10.1074/jbc.M109101200. [DOI] [PubMed] [Google Scholar]
- 94.Miranti CK, Brugge JS. Sensing the environment: a historical perspective on integrin signal transduction. Nature Cell Biology. 2002;4(4):E83–E90. doi: 10.1038/ncb0402-e83. [DOI] [PubMed] [Google Scholar]
- 95.Kim J-T, Joo C-K. Involvement of cell-cell interactions in the rapid stimulation of Cas tyrosine phosphorylation and Src kinase activity by transforming growth factor-β1. Journal of Biological Chemistry. 2002;277(35):31938–31948. doi: 10.1074/jbc.M201178200. [DOI] [PubMed] [Google Scholar]
- 96.Khwaja FW, Svoboda P, Reed M, Pohl J, Pyrzynska B, Van Meir EG. Proteomic identification of the wt-p53-regulated tumor cell secretome. Oncogene. 2006;25(58):7650–7661. doi: 10.1038/sj.onc.1209969. [DOI] [PubMed] [Google Scholar]
- 97.Piccolo S. p53 regulation orchestrates the TGF-β response. Cell. 2008;133(5):767–769. doi: 10.1016/j.cell.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 98.Cordenonsi M, Dupont S, Maretto S, Insinga A, Imbriano C, Piccolo S. Links between tumor suppressors: p53 is required for TGF-β gene responses by cooperating with Smads. Cell. 2003;113(3):301–314. doi: 10.1016/s0092-8674(03)00308-8. [DOI] [PubMed] [Google Scholar]
- 99.Cordenonsi M, Montagner M, Adorno M, et al. Integration of TGF-β and Ras/MAPK signaling through p53 phosphorylation. Science. 2007;315(5813):840–843. doi: 10.1126/science.1135961. [DOI] [PubMed] [Google Scholar]
- 100.Dupont S, Zacchigna L, Adorno M, et al. Convergence of p53 and TGFβ signaling networks. Cancer Letters. 2004;213(2):129–138. doi: 10.1016/j.canlet.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 101.Kunz C, Pebler S, Otte J, von der Ahe D. Differential regulation of plasminogen activator and inhibitor gene transcription by the tumor suppressor p53. Nucleic Acids Research. 1995;23(18):3710–3717. doi: 10.1093/nar/23.18.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Riley T, Sontag E, Chen P, Levine A. Transcriptional control of human p53-regulated genes. Nature Reviews Molecular Cell Biology. 2008;9(5):402–412. doi: 10.1038/nrm2395. [DOI] [PubMed] [Google Scholar]
- 103.Shetty S, Shetty P, Idell S, Velusamy T, Bhandary YP, Shetty RS. Regulation of plasminogen activator inhibitor-1 expression by tumor suppressor protein p53. Journal of Biological Chemistry. 2008;283(28):19570–19580. doi: 10.1074/jbc.M710268200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Allen RR, Qi L, Higgins PJ. Upstream stimulatory factor regulates E box-dependent PAI-1 transcription in human epidermal keratinocytes. Journal of Cellular Physiology. 2005;203(1):156–165. doi: 10.1002/jcp.20211. [DOI] [PubMed] [Google Scholar]
- 105.Fisher DE, Parent LA, Sharp PA. Myc/Max and other helix-loop-helix/leucine zipper proteins bend DNA toward the minor groove. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(24):11779–11783. doi: 10.1073/pnas.89.24.11779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Scheinfeld N, DeLeo V. Etiological factors in skin cancers: environmental and biological. In: Rigel DS, Friedman RJ, Dzubow LM, Reintgen DS, Bystryn J-C, Marks R, editors. Cancer of the Skin. Philadelphia, Pa, USA: Elsevier; 2005. pp. 61–70. [Google Scholar]
- 107.Kortlever RM, Higgins PJ, Bernards R. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nature Cell Biology. 2006;8(8):878–884. doi: 10.1038/ncb1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Balsara RD, Castellino FJ, Ploplis VA. A novel function of plasminogen activator inhibitor-1 in modulation of the AKT pathway in wild-type and plasminogen activator inhibitor-1-deficient endothelial cells. Journal of Biological Chemistry. 2006;281(32):22527–22536. doi: 10.1074/jbc.M512819200. [DOI] [PubMed] [Google Scholar]
- 109.Kortlever RM, Nijwening JH, Bernards R. Transforming growth factor-β requires its target plasminogen activator inhibitor-1 for cytostatic activity. Journal of Biological Chemistry. 2008;283(36):24308–24313. doi: 10.1074/jbc.M803341200. [DOI] [PMC free article] [PubMed] [Google Scholar]