Abstract
Magnetic susceptibilities of both reduced and oxidized ferredoxin from Clostridium pasteurianum were obtained in solution. Whereas the reduced form exhibits a Curie law behavior, the magnetic susceptibility of oxidized ferredoxin in fact increases with temperature and suggests extensive antiferromagnetic exchange coupling between the component iron atoms. Contact-shifted resonances are observed for both forms of ferredoxin that are attributed to the β-CH2 protons of the eight cysteine residues. A model based on these results is presented.
Full text
PDF
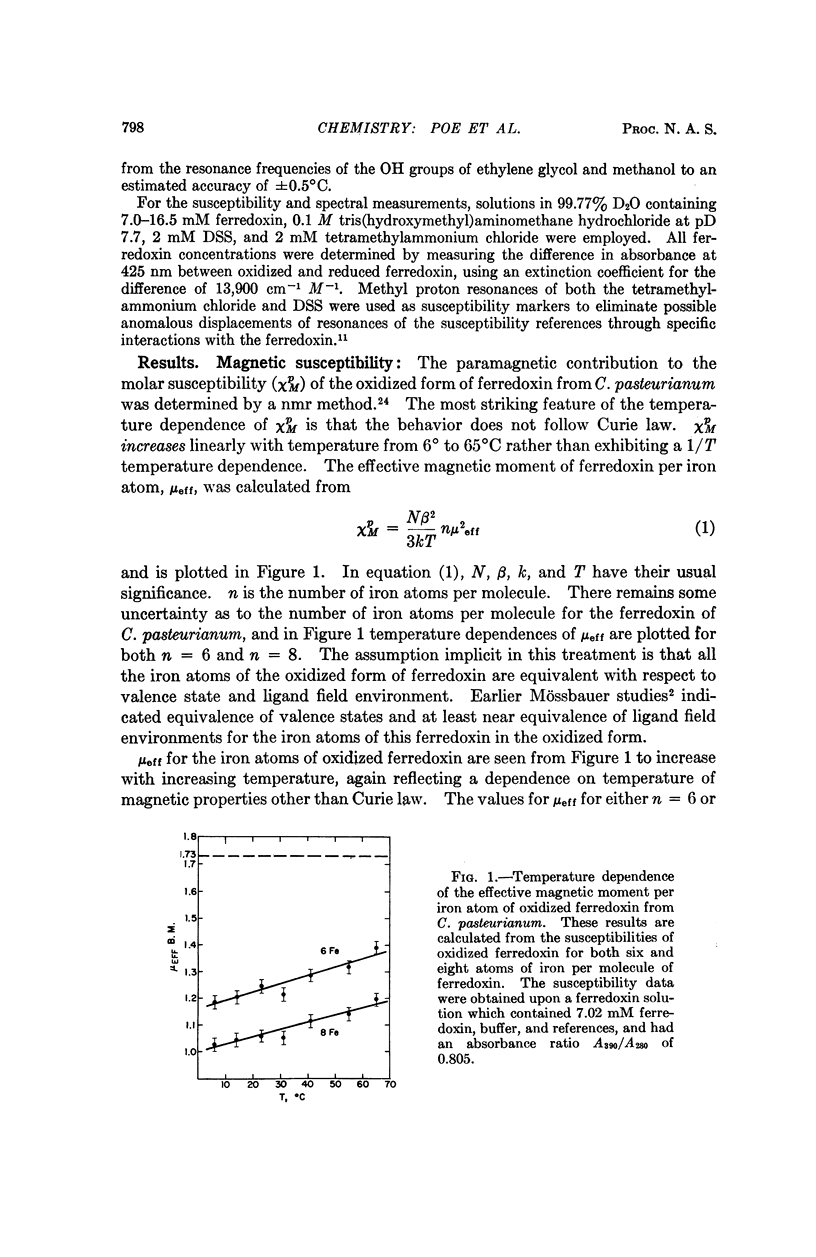

Selected References
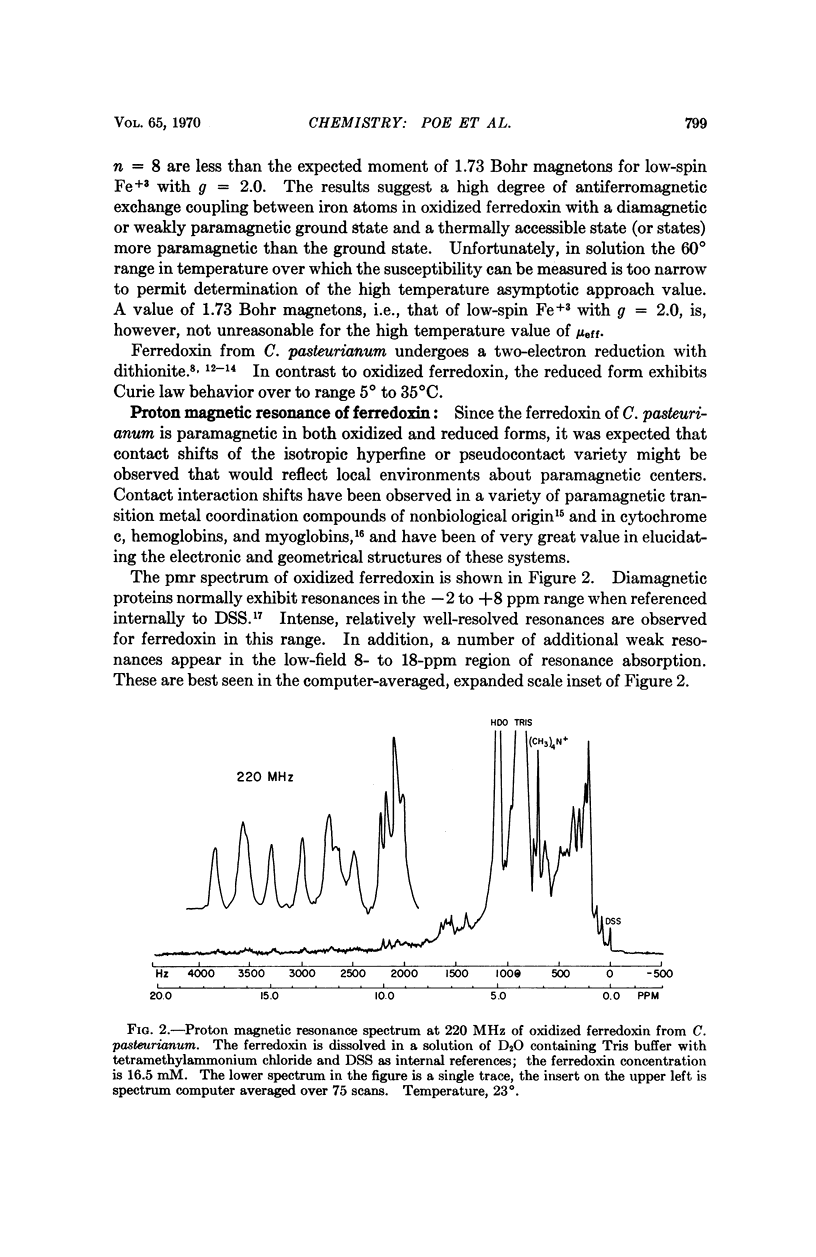
These references are in PubMed. This may not be the complete list of references from this article.
- BLOMSTROM D. C., KNIGHT E., Jr, PHILLIPS W. D., WEIHER J. F. THE NATURE OF IRON IN FERREDOXIN. Proc Natl Acad Sci U S A. 1964 Jun;51:1085–1092. doi: 10.1073/pnas.51.6.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer E., Eckstein H., Hagenmaier H., Josef D., Koch J., Krauss P., Röder A., Schretzmann P. Untersuchungen zur Struktur der Ferredoxine. Eur J Biochem. 1969 Mar;8(1):33–49. doi: 10.1111/j.1432-1033.1969.tb00492.x. [DOI] [PubMed] [Google Scholar]
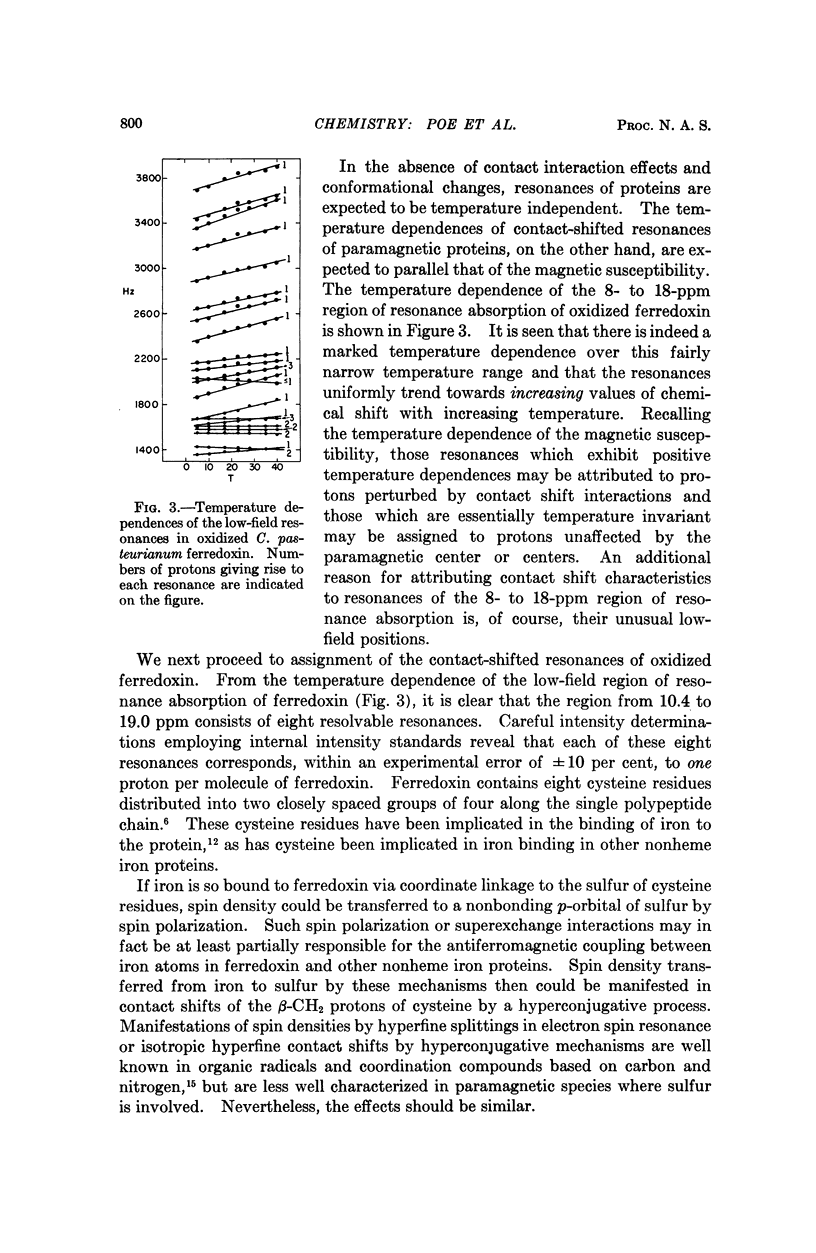
- Eisenstein K. K., Wang J. H. Conversion of light to chemical free energy. I. Porphyrin-sensitized photoreduction of ferredoxin by glutathione. J Biol Chem. 1969 Apr 10;244(7):1720–1728. [PubMed] [Google Scholar]
- Epstein M., Navon G. DSS as an activator of carboxypeptidase A. Biochem Biophys Res Commun. 1969 Jul 7;36(1):126–130. doi: 10.1016/0006-291x(69)90658-5. [DOI] [PubMed] [Google Scholar]
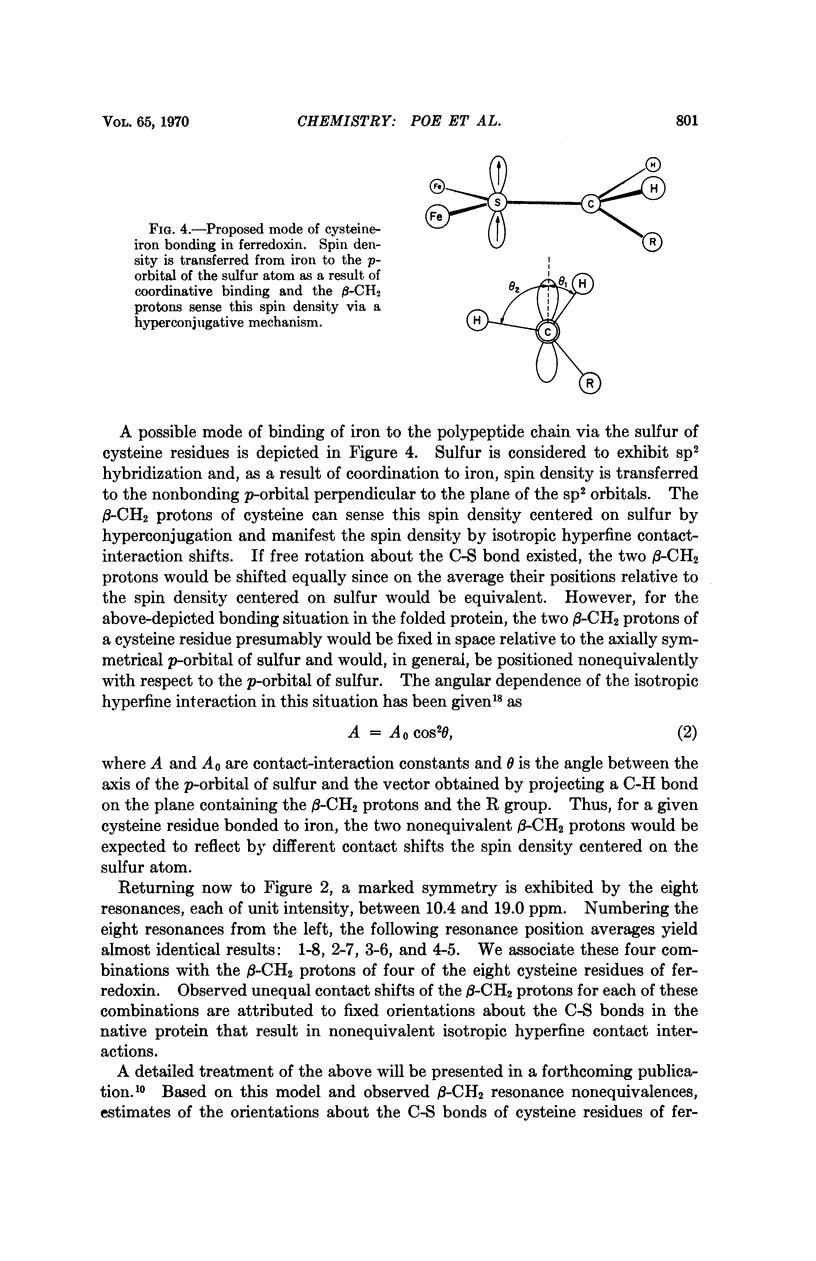
- Kurland R. J., Davis D. G., Ho C. Paramagnetic proton nuclear magnetic resonance shifts of metmyoglobin, methemoglobin, and hemin derivatives. J Am Chem Soc. 1968 May 8;90(10):2700–2701. doi: 10.1021/ja01012a048. [DOI] [PubMed] [Google Scholar]
- LOVENBERG W., BUCHANAN B. B., RABINOWITZ J. C. STUDIES ON THE CHEMICAL NATURE OF CLOSTRIDIAL FERREDOXIN. J Biol Chem. 1963 Dec;238:3899–3913. [PubMed] [Google Scholar]
- MORTENSON L. E., VALENTINE R. C., CARNAHAN J. E. An electron transport factor from Clostridium pasteurianum. Biochem Biophys Res Commun. 1962 Jun 4;7:448–452. doi: 10.1016/0006-291x(62)90333-9. [DOI] [PubMed] [Google Scholar]
- Malkin R., Rabinowitz J. C. Additional observations on the chemistry of clostridial ferredoxin. Biochemistry. 1966 Apr;5(4):1262–1268. doi: 10.1021/bi00868a020. [DOI] [PubMed] [Google Scholar]
- Mayhew S. G., Petering D., Palmer G., Foust G. P. Spectrophotometric titration of ferredoxins and Chromatium high potential iron protein with sodium dithionite. J Biol Chem. 1969 Jun 10;244(11):2830–2834. [PubMed] [Google Scholar]
- McDonald C. C., Phillips W. D. Manifestations of the tertiary structures of proteins in high-frequency nuclear magnetic resonance. J Am Chem Soc. 1967 Nov 22;89(24):6332–6341. doi: 10.1021/ja01000a061. [DOI] [PubMed] [Google Scholar]
- McDonald C. C., Phillips W. D. Proton magnetic resonance spectra of proteins in random-coil configurations. J Am Chem Soc. 1969 Mar 12;91(6):1513–1521. doi: 10.1021/ja01034a039. [DOI] [PubMed] [Google Scholar]
- Orme-Johnson W. H., Beinert H. Heterogeneity of paramagnetic species in two iron-sulfur proteins: Clostridium pasteurianum ferredoxin and milk xanthine oxidase. Biochem Biophys Res Commun. 1969 Aug 7;36(3):337–344. doi: 10.1016/0006-291x(69)90569-5. [DOI] [PubMed] [Google Scholar]
- Palmer G., Sands R. H., Mortenson L. E. Electron paramagnetic resonance studies on the ferredoxin from Clostridium pasteurianum. Biochem Biophys Res Commun. 1966 May 25;23(4):357–362. doi: 10.1016/0006-291x(66)90733-9. [DOI] [PubMed] [Google Scholar]
- SIEKER L. C., JENSEN L. H. AN X-RAY INVESTIGATION OF THE STRUCTURE OF A BACTERIAL FERREDOXIN. Biochem Biophys Res Commun. 1965 Jun 18;20:33–35. doi: 10.1016/0006-291x(65)90945-9. [DOI] [PubMed] [Google Scholar]
- Shulman R. G., Ogawa S., Wüthrich K., Yamane T., Peisach J., Blumberg W. E. The absence of "heme-heme" interactions in hemoglobin. Science. 1969 Jul 18;165(3890):251–257. doi: 10.1126/science.165.3890.251. [DOI] [PubMed] [Google Scholar]
- Sobel B. E., Lovenberg W. Characteristics of Clostridium pasteurianum ferredoxin in oxidation-reduction reactions. Biochemistry. 1966 Jan;5(1):6–13. doi: 10.1021/bi00865a002. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Nakashima T., Benson A., Mower H., Tasunobu K. T. The amino acid sequence of Clostridium pasteurianum ferredoxin. Biochemistry. 1966 May;5(5):1666–1681. doi: 10.1021/bi00869a032. [DOI] [PubMed] [Google Scholar]
- Wüthrich K., Shulman R. G., Peisach J. High-resolution proton magnetic resonance spectra of sperm whale cyanometmyoglobin. Proc Natl Acad Sci U S A. 1968 Jun;60(2):373–380. doi: 10.1073/pnas.60.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]


