Abstract
An important discovery of recent years has been that lifestyle and environmental factors affect cancer initiation, promotion and progression, suggesting that many malignancies are preventable. Epidemiological studies strongly suggest that excessive adiposity, decreased physical activity, and unhealthy diets are key players in the pathogenesis and prognosis of many common cancers. In addition, calorie restriction (CR), without malnutrition, has been shown to be broadly effective in cancer-prevention in laboratory strains of rodents. Adult-onset moderate CR also reduces cancer incidence by 50% in monkeys. Whether the anti-tumorigenic effects of CR will apply to humans is unknown, but CR results in a consistent reduction in circulating levels of growth factors, anabolic hormones, inflammatory cytokines and oxidative stress markers associated with various malignancies. Here, we discuss the link between nutritional interventions and cancer prevention with focus on the mechanisms that may be responsible for these effects in simple systems and mammals with a view to developing chemoprevention agents.
INTRODUCTION
Cancer is a complex multistage disease associated with accumulation of multiple DNA mutations that cause a deregulation of cell proliferation and differentiation, loss of normal tissue organization, and eventually tissue invasion and dislocation to distant sites (1). DNA damage, which occurs continuously in both the dividing and non-dividing cells of the human body, and can increase after exposure to exogenous genotoxic carcinogens (e.g. radiations, chemicals, tobacco smoke, viruses, aflatoxin and other food derived carcinogens), can be prevented or repaired by endogenous protective small molecules and enzymes (2–4). However, these detoxification and repair systems may fail, especially in environments that promote cell proliferation and inhibit cell apoptosis, but also in non-dividing cells in which the opportunity for repair may be limited (5, 6). Accumulation of multiple DNA mutations in critical genes (i.e. oncogenes or tumor suppressor genes) of particular cells, if not properly controlled through induction of senescence or apoptosis, can lead to uncontrolled cell proliferation and progressive transformation of normal human cells into highly malignant tumor cells (5–7). Moreover, recent data suggest that the surrounding microenvironment and cell-to-cell interactions between cancer cells and their neighbour stromal and inflammatory cells play a central role in driving tumor cell proliferation, tissue invasion and metastasis, that ultimately are responsible for ~90% of human cancer deaths (8, 9).
Metabolic, hormonal and growth factor alterations associated with increased food consumption, decreased physical activity, and excessive adiposity, affect the regulation and expression of genes involved in DNA repair, cell proliferation and differentiation or apoptosis, allowing cells to accumulate damage and mutations, survive, proliferate and under permissive conditions, undergo malignant transformation (10, 11). These detrimental effects can be potentiated by exposure to non-genotoxic carcinogens (e.g. ethanol, saccharin, 1,4-dichlorobenzene, 17beta-estradiol, arsenic and beryllium) that induce cell damage, by inflammation, increased oxidative stress and secretion of anabolic hormones, immunosuppression, and activation of signal-transduction pathways that result in genomic instability, loss of proliferation control, and resistance to apoptosis (12).
Calorie restriction (CR) without malnutrition is the most potent and reproducible physiological intervention for increasing lifespan and protecting against cancer in mammals (13, 14). CR reduces the levels of a number of anabolic hormones, growth factors and inflammatory cytokines, reduces oxidative stress and cell proliferation, enhances autophagy and several DNA repair processes (13, 14). Hence, understanding the metabolic and molecular mechanisms responsible for the CR-mediated cancer preventive effect has the potential to lead to drugs and therapies for broad-spectrum prevention and treatment of cancer. Here, we discuss nutritional interventions that have been shown to prevent cancer and to ameliorate cancer prognosis. We also describe the genetic pathways and mechanisms that appear to be crucial for the effects of CR and discuss the evidence for potentially protective but also detrimental effects in humans.
NUTRITION AND CANCER
Epidemiological data on geographical and chronological variations in cancer incidence have shown that environmental factors have profound effects in the initiation, promotion and progression of some of the most common cancers in Western countries (15, 16). In fact, studies of populations migrating from a low- to a high-risk area for cancer have shown major changes in rates of several common cancers. For example, the incidence of stomach cancer decreased and the rates of breast, colon and prostate cancer increased within a single generation after migration of Japanese people from Japan to Hawaii (15). Moreover, the recent shift from traditional dietary patterns to Western diet patterns in many developed and developing countries has produced a striking increase in the most common cancers (e.g. colon, breast and prostate cancer) within populations previously considered to have a low prevalence rate (15, 16). For example, in the last 4 decades in Japan the age-standardized incidence rate of colon cancer has increased 9.4 times for males and 4.7 times for females, in conjunction with a significant increase in height and body weight (17). These studies, together with the investigation of lifestyle factors and behaviors, have led to the conclusion that the majority of cancer deaths in many developed countries can be attributed to factors such as unhealthy diets, tobacco, alcoholism, infections and occupational exposures.
In particular, data from several epidemiological studies support the theory that diet plays an important role in the initiation, promotion and progression of many common cancers in Western countries (18). Despite frequent reports of specific vitamins, micronutrients, nutrients, or foods having a favorable or harmful effect on cancer risk, few of these have a recognized cause-effect link to cancer (i.e. fried, broiled or roasted red meat, aflatoxin-contaminated food, preserved salty foods, excessive alcohol consumption) (19, 20). These results are often based on data obtained from cell culture or animals studies, and not from randomized clinical trial in humans (19). To date, there is consensus that excessive adiposity due to overconsumption of energy-dense foods and a sedentary lifestyle increases the risk of developing cancer (10, 21). Nonetheless, data from several epidemiological studies suggest that cancer risk can also be reduced by an overall dietary pattern that favors a high intake of plant foods (e.g. vegetables, beans, fruits, and whole grains) rich in a wide range of phytochemicals, and a limited consumption of animal fat, meat and dairy products (18, 21, 22). Greater consumption of a wide variety of vegetables, beans and fruits has been associated with a lower risk of developing colon, lung, oral, esophageal, and stomach cancer (22). Evidence that high intake of vegetables and fruits reduce the risk of developing breast and prostate cancer is less strong (22).
Excessive adiposity and cancer risk
Several epidemiological studies have consistently shown associations between adiposity and increased risk of cancers of the endometrium, breast (postmenopausal), colon, esophagus (adenocarcinoma), kidney (renal-cell), pancreas, gallbladder and liver (10, 23). No association is seen between adiposity and the risk of developing lung and prostate cancer, whereas premenopausal breast cancer is inversely correlated with body mass index (10, 21).
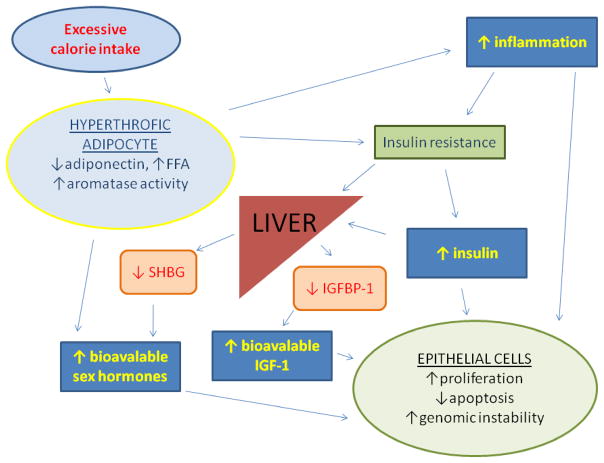
Excessive adiposity due to chronic energy imbalance is associated with increased oxidative stress, insulin resistance, inflammation, and changes in hormone and growth factor concentrations that play a key role in the pathogenesis of many cancers (Figure 1) (10, 21, 23, 24). Persistent positive energy balance promotes hypertrophy of adipose tissue, reduced adiponectin production, insulin resistance, and compensatory hyperinsulinemia (25). Adiposity generally shows a direct linear relationship with serum insulin concentrations (26). Chronically elevated insulin concentrations have been associated with cancers of the breast, colon, pancreas, endometrium (27–30). A number of mechanisms likely account for the tumorigenic effects of insulin, which exerts direct powerful mitogenic effects through the insulin receptor expressed on many cell types, especially in pre-neoplastic cells (31). In addition, hyperinsulinemia inhibits the hepatic production of sex hormone-binding globulin and therefore increases the circulating concentrations of bioavailable sex hormones (32). Hyperinsulinemia also stimulates the ovarian production of androgens (33). Finally, insulin increases the biological activity of IGF-1, in part by reducing synthesis and secretion of IGFBP-1 (34). Adipose tissue is considered to be one of the major sources of extra-glandular estrogen, produced by aromatization of androgen precursors (35). Estrogens, androgens and IGF-1 are strong mitogens for cells, and stimulate the development and growth of several tumors (31, 35, 36). For example, women who are overweight, especially those with visceral obesity, which is associated with increased risk of breast cancer in postmenopausal women, frequently have insulin resistance, hyperinsulinemia, low plasma levels of sex hormone-binding globulin, and high total and free sex hormone levels (37). Finally, fat cells produce and secrete molecules such as IGF-1, IL-6, leptin and type VI collagen that promote cell survival and tumor growth (25, 38, 39).
Fig. 1. Effects of excessive calorie intake and adiposity on hormones and growth-factor production and cell proliferation.
Excessive calorie intake and a sedentary lifestyle promote hypertrophy of adipose tissue, reduce adiponectin production, and increase circulating free fatty acids (FFA) and inflammation, leading to insulin resistance and compensatory hyperinsulinemia. Increased serum insulin concentration causes a reduction in hepatic synthesis of insulin-like growth factor binding protein 1 (IGFBP1) and steroid hormone binding globulin (SHBG), that leads to increased bioavailability of insulin growth factor 1 (IGF-1) and sex hormones. Adipose tissue is also a major source of extra-glandular estrogens. Chronically elevated circulating levels of insulin, IGF-1, sex hormones and inflammatory cytokines promote cellular proliferation, genomic instability, and inhibit apoptosis in many cell types.
Recent data indicate that long-term cancer mortality after weight loss induced by gastric bypass surgery is significantly reduced (40, 41). Exercise- and/or CR-induced weight loss improves several metabolic and hormonal alterations associated with excessive adiposity in overweight and obese subjects (13, 42). Weight loss induced by a negative energy balance is associated with decreased fat cell size, a reduction in visceral, hepatic and skeletal muscle fat, improved adipokine profile and insulin sensitivity, and reduced circulating insulin levels (25, 43–45). Reduced fat mass is also associated with a reduction in circulating estrogens levels due to reduction in aromatase activity (23, 33, 46). The reduction in insulin levels is associated with: (1) increased SHBG and reduced free estrogens and testosterone, (2) increased IGFBP-1 and reduced free IGF-1 (10, 21, 23, 32, 34, 37, 42). Weight loss is also associated with a reduction in inflammatory cytokines and prostaglandins, and in several markers of oxidative stress and DNA damage (47–50). Finally, a 13 months weight loss intervention has recently been reported to be associated with increased telomere length in the rectal tissue biopsies obtained from a small group of obese patients (51), suggesting that CR may contribute to the prevention of telomere shortening, which may also be important in modulating the pathogenesis of cancer and biology of aging (52).
Calorie restriction and cancer
In 1909, Moreschi published the first scientific paper to report that CR inhibits the growth of tumors transplanted into mice (53). Subsequently, data have shown that CR, defined as a reduction in calorie intake below usual ad libitum intake without malnutrition, inhibits spontaneous, chemically-induced and radiation-induced tumors in several animal models of cancer (54–60). More recently, adult-onset moderate 30% CR has been shown to reduce cancer morbidity and mortality also in non-human primates. The incidence of cancer (mainly gastrointestinal adenocarcinoma) was reduced by 50% in the CR monkeys as compared to that in controls (61). The age when CR is started, the severity of CR, and the strain/genetic background of the animals determine the magnitude of cancer prevention or delay (54–60, 62). In rodents, 15%–53% reduction in calorie intake below usual ad libitum intake caused a proportionate linear 20%–62% reduction in tumor incidence (63). Nonetheless, the effects of CR on cancer are not homogeneous. Some cancers show a greater response to CR than others, and a small portion of tumors is resistant to the effects of CR (54, 56, 57, 60, 62).
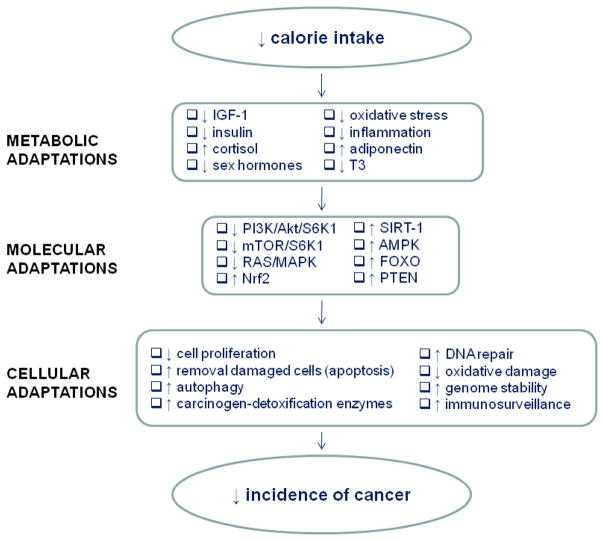
The mechanisms responsible for CR-mediated beneficial effects on cancer observed in rodents, and now also in monkeys, which will be discussed in more detail in the following sections, are thought to involve the metabolic adaptations to CR itself (Figure 2), including: 1) decreased production of growth factors and anabolic hormones (64–67); 2) decreased reactive oxygen species production and modulation of the endogenous antioxidant systems, which decrease oxidative stress and free radical-induced DNA damage (68–70); 3) decreased plasma concentrations of inflammatory cytokines and an increase in circulating corticosteroids, ghrelin and adiponectin that results in a reduction in inflammation (71–75), 4) protection against aging-associated deterioration in immunosurveillance (76–77). In addition, CR simultaneously affects multiple processes that are involved in the pathogenesis of cancer, including enhanced DNA repair processes, increased removal of damaged cells through apoptosis, enhanced autophagy and protection from the damaging effects of a range of damaging agents (e.g., toxic and genotoxic compounds) (78–81). Many of the effects of CR are likely mediated by regulating gene expression including the up-regulation of tumor-suppressor genes, of genes promoting DNA and cellular repair, protein turnover, stress resistance, and anti-oxidant genes, and the down-regulation of pro-inflammatory genes and modulation of energy metabolism pathways (82, 83). Whether CR with adequate nutrition will reduce cancer incidence in humans is unknown, but data from studies of long-term CR in humans suggest that the metabolic and physiological responses to CR are similar to those in rodents and monkeys (13, 84–87).
Fig. 2. Mechanisms for cancer prevention by calorie restriction.
CR causes several key metabolic/hormonal adaptations, that alter the expression of several genes and signaling pathways (up-regulation of certain genes/signaling pathways and down-regulation of others as indicated by the arrows), which produce major cellular adaptations (e.g. a reduction in cell proliferation, increased removal of damaged organelles or cells via autophagy or apoptosis, up-regulation of DNA repair systems and genomic stability) that result in a reduced cancer incidence (see the text). T3 = triiodothyronine; PI3K = phosphatidylinositol-3 kinase; AKT = kinase AKT, also known as protein kinase B; S6K1 = ribosomal S6 protein kinase 1; mTOR = mammalian target of rapamycin; MAPK = mitogen-activated protein kinase; NRF2 = transcription factors NF-E2-related factor 2; SIRT-1 = silent mating type information regulation 2 homolog 1; AMPK = adenosine monophosphate (AMP)–activated protein kinase; FOXO = Forkhead transcription factors; PTEN = phosphatase and tensin homolog.
Endocrine regulation of cancer by insulin-like signals
Insulin-like growth factor 1 (IGF-1), a growth factor produced primarily from the liver, acts synergistically with other anabolic hormones (e.g. insulin, sex steroids), and in relation to calorie and protein availability, to regulate energy metabolism, cell proliferation, cell differentiation, body size and longevity (31, 35, 36). In addition, IGF-1 exerts a potent mitogenic effect on a variety of cancer cells, by increasing proliferation rate and inhibiting apoptosis (31, 88).
Long-term calorie restriction, but not endurance exercise, decreases serum IGF-1 concentration by approximately 30–40% in rodents, and this CR-mediated reduction in IGF-1 levels is believed to play a key role in protecting against cancer and slowing aging (11, 64, 89–90). The powerful CR-mediated protective effect against carcinogenesis in rodents is reversed by infusing growth hormone or IGF-1 (91–93), further underlining the critical role of these growth factors in the pathogenesis of cancer. In addition, growth hormone receptor (GHR) knockout mice, that have low serum IGF-1 concentrations, exhibit a ~50% reduction in tumor burden and incidence of lethal cancers, in agreement with results in growth hormone (GH) deficient Snell dwarf, in which cancer is either reduced or postponed (94, 95). A lower mutation rate in middle-aged GH deficient Ames dwarf mice was observed in kidney, liver and intestine, providing some mechanistic explanation for the delay in neoplastic diseases (96). In contrast, GH overexpressing mice have very high concentrations of IGF-1, increased body size up to 100%, increased incidence and early on-set of tumors, and a marked reduction in lifespan compared to their normal siblings (97). Nonetheless, down-regulation of IGF-I signalling may account for only part of the effects of CR, GH and GHR deficiencies in protecting against cancer. The CR-mediated increase in corticosterone, for example, may also play an important role in preventing cancer, as adrenalectomy reverses the cancer-protective effects of CR, and glucocorticoid supplementation restores inhibition (98).
The role of IGF-1 in the pathogenesis of some human cancers is supported by epidemiologic studies, which have found that high serum concentrations of IGF-I are associated with increased risk of several common cancers, including those of the prostate, breast, and colon (99). An elevated incidence of tumors is observed also in acromegalic patients, who have elevated IGF-I levels (100). Nutrition is one of the major regulators of circulating IGF-1 levels. Fasting in humans markedly reduces serum IGF-1 concentration into the range observed for GH-deficient patients (101), but long-term severe CR does not reduce circulating IGF-1 levels in middle-aged healthy men and women if protein intake is high (102, 103). In contrast, strict vegetarians consuming a moderately restricted protein diet (~0.75 g of protein/kg body weight/day) display significantly lower serum concentrations of total and free IGF-1 (102). Moreover, reducing protein intake in individuals practicing severe CR with high protein intake (~1.65 g of protein/kg body weight/day) results in a 25% reduction in serum IGF-1 (from 194 ng/ml to 152 ng/ml), suggesting that protein intake is a key determinant of circulating IGF-1 levels in humans (102). These findings are in agreement with a series of data from epidemiological studies that show a positive association between protein intake and serum IGF-I concentration in both men and women eating typical Western diets (104, 105). In women the association between protein intake and circulating IGF-1 concentrations was stronger than the relationship between calorie intake and IGF-I concentration (104). In men animal and vegetable protein consumption was associated with an increase in serum IGF-1 concentrations, whereas calorie intake was associated with an increase in serum IGF-I concentration only in lean men with a BMI <25 kg/m2 (105). These data might help to explain, at least in part, the link between the consumption of high animal protein Western diets and the increased incidence of two adiposity-independent common cancers, such as prostate and premenopausal breast cancer (15, 16, 99, 106, 107). It is important to note that the recommended daily allowance for protein intake in healthy adults is 0.83 g/kg of body weight/day (108), whereas at least 50% of the US males are eating ≥ 1.34 g of protein/kg of body weight/day, which is 40% or more protein than the RDA recommended intakes, and therefore associated with a positive nitrogen balance (108, 109). More studies are necessary to understand the metabolic and clinical implications of a chronic positive nitrogen balance on serum IGF-1 and IGFBPs concentrations, and on cancer biology, especially in sedentary adults with a positive family history for cancer.
The hypothesis that reduction of GH/IGF-I signaling can modulate/prevent cancer in humans can also be tested by following individuals that have deficiencies in the GH/IGF-I axis. Preliminary data from a unique population of GHR deficient patients (Laron dwarfs), who are deficient in IGF-1, suggest a protective effect against tumorigenesis (110). However, this report is indicative but no conclusive since the mean age of the Laron dwarfs was much lower than the mean age of the controls. Although animal studies suggest that GH and IGF-1 play an important role in modulating cancer, to determine whether CR and/or GH and/or IGF-I deficiencies indeed reduce cancer risk in human we must await properly controlled studies of CR and Laron dwarfs or similar populations. Nevertheless, even if GH and/or IGF-1 deficiencies may decrease cancer risk, it is well known that low levels of IGF-1 are associated with increased obesity and heart disease in people who are not restricting calorie intake (111). On the other hand, severe CR (when protein intake is normal) causes a reduction in IGF-I without leading to obesity, and while concomitantly reducing many of the markers of heart diseases (84). Not surprisingly, GH and IGF-I deficient dwarf mice become obese at middle age and live approximately 40% longer (112), but when these mice are calorie restricted, they no longer become obese and can live up to 100% longer than wild type and ad lib fed mice (113).
MOLECULAR TARGETS OF CALORIE RESTRICTION
Because CR extends the life span of all the major model organisms used to study aging, the nutrient-response pathways that regulate aging and age-dependent genomic instability in these organisms can provide insights on the mechanisms linking CR and the reduction in cancer. Although worms and flies are excellent model organisms to study aging, their mostly non-dividing cellular network and rare tumor phenotypes limit their value in studies of the mechanisms of age-dependent tumorigenesis. By contrast, the simple S. cerevisiae can provide evidence for the fundamental molecular mechanisms of age-dependent genomic instability whereas mice can be studied to determine whether similar mechanisms apply to mammals. Here, we review the major pathways and mechanisms believed to connect CR and genomic instability and/or cancer.
Pathways that mediate CR-dependent longevity extension regulate genomic instability and cancer-like phenotypes in S. cerevisiae
S. cerevisiae has the advantages of being perhaps the simplest and the best characterized model system to study aging. As observed in mammals (114), mutations, which increase with age in S. cerevisiae by as much as 10-fold, are reduced by CR (115–117). The deletion of either the TOR/SCH9, the yeast homologues of mammalian target of rapamycin (mTOR), and S6 kinase (S6K) and/or AKT genes, respectively, and the down-regulation of the Ras/adenylyl cyclase (AC)/protein kinase A (PKA) pathway postpone this age-dependent increase in spontaneous mutations (118–120). Notably, the down-regulation of the Tor/Sch9 and the Ras/AC/PKA pathways are required to extend life span in yeast by a mechanism that involves the up-regulation of stress resistance transcription factors described below.
Analogously to the accumulation of somatic mutations playing key roles in cancer development in mammals (114), cancer-like mutant cells that “regrow” under conditions that block the growth of normal cells, are generated within aging S. cerevisiae populations (116, 117). Such regrowth can be studied in S. cerevisiae liquid cultures by monitoring the takeover of the culture by the mutated sub-population that utilizes the limited nutrients available to grow while normal cells are aging and dying (116). The frequency of this cancer-like regrowth phenotype is greatly reduced in CR yeast and yeast with mutations in the Tor/Sch9 and Ras/AC/PKA pathways (117). The reduced frequency of age-dependent mutations in CR cells and cells lacking TOR1/SCH9 (118–120) suggests that oncogene homologs control age-dependent regrowth in part by regulating the generation of the one or multiple mutations necessary for growth under unfavorable conditions. The effect of Tor/Sch9 and Ras/AC/PKA on DNA mutations is due in part to regulation of antioxidant defenses and the generation of oxidants. In fact, the generation of oxidants is elevated and resistance to oxidants and life span are decreased in S. cerevisiae expressing an oncogene-like constitutively active RAS2val19 (121, 122) or overexpressing SCH9. Furthermore, CR cells and cells lacking RAS2 or TOR1/SCH9 are highly protected against oxidative damage (119, 120, 121, 123) and age-dependent regrowth frequency doubles in cells lacking cytosolic superoxide dismutase but is reduced in cells overexpressing antioxidant enzymes (116, 119, 121). Supporting this model is the very high frequency of nuclear mutations in aging yeast lacking mitochondrial superoxide dismutase (SOD)-2 or SOD-1 (115, 116), with cells lacking SOD1 being among the genetic manipulations causing the highest rate of DNA mutations. The down-regulation of superoxide generation and increase in antioxidant defenses in Ras/AC/PKA and/or Tor/Sch9 deficient cells is mediated by serine threonine kinase Rim15 and transcription factors Msn2/4 and Gis1, which regulate a number of stress resistance genes including the mitochondrial superoxide dismutase SOD2 (119, 122). CR is likely to regulate pro- and anti-oxidant systems by similar mechanisms since life span extension caused by CR is largely reversed in cells lacking these 3 stress resistance transcription factors (i.e. Msn2/4 and Gis1) (124).
In addition to the oxidative stress systems, the Tor/Sch9 and Ras/AC/PKA pathways regulate the expression of a number of DNA repair genes (125). Among them is the REV1 gene, which functions in the error-prone translation repair system together with the polymerase Polzeta. It is the Rev1/Polzeta system that is responsible for a major portion of the age-dependent DNA mutations (119). The evidence suggests that the Tor/Sch9 pathway promotes superoxide generation and reduces the anti-oxidant protection by down-regulating Msn2/4 and Gis1. These changes result in increased oxidative damage to the DNA but also in increased expression of the REV1 gene, which, together, promote age-dependent point mutations (119). The activation of the error prone Rev1/Polzeta appears to generate point mutations as part of a process required to prevent the potentially more detrimental gross chromosomal rearrangements that occur when replication is stalled. Whether Rev1/Polzeta is involved in the CR-dependent effect on DNA protection is unknown.
Molecular pathways that mediate the anti-cancer effects of calorie restriction
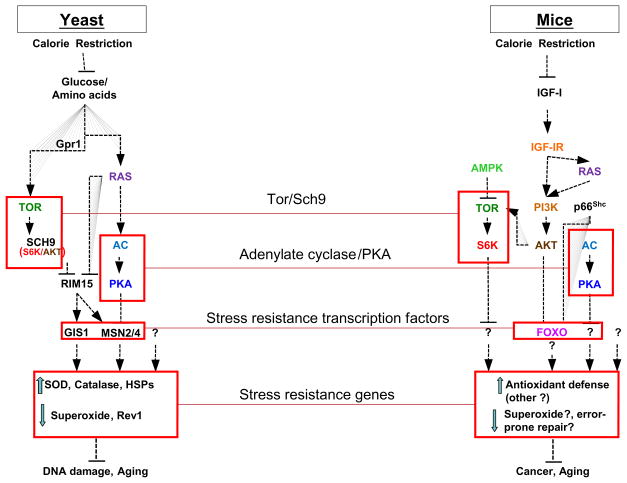
Studies in yeast, worms, and flies indicate that the insulin and IGF-I pathways may be important for aging and cancer in mammals (Figure 3). In mice, mutations in the Prop-1 or Pit-1 genes, which cause severe deficiencies in GH and IGF-I, extend life span by 25 to 65% and cause dwarfism (126, 127). These deficiencies appear to mediate the effects of Prop-1 and Pit-1 mutations on longevity, since the mice that cannot release GH in response to growth hormone releasing hormone also live longer (128). In fact, dwarf mice with high plasma GH, but a 90% lower IGF-I (GHR/BP null mice) and heterozygous female IGF-I receptor knockout mice live longer than the wild type controls (129, 130).
Fig. 3. Pro-aging and pro-cancer pathways in yeast and mice.
Similar pathways, including Ras, Tor, S6 kinase (S6K), adenylate cyclase (AC), and PKA, have been shown to promote aging in both yeast and mice. In yeast, CR causes the down-regulation of these proteins, which promote DNA mutations by reducing the activity of stress resistance transcription factors including Msn2/4 and Gis1 and subsequently increasing the level of superoxide and the activity of error-prone polymerases (Rev1, etc). In yeast, this DNA damage-promoting mode can occur largely independently of cell division. In mice, orthologues of yeast Tor, S6K, AC, and PKA promote aging but are also components of some of the most common oncogenic pathways. CR reduces IGF-I and consequently can reduce the activity of protein functioning downstream of IGF-IR including Tor and S6K. Activation of Tor and S6K but also of AC and PKA may promote DNA damage and cancer in part by promoting cell growth and inhibiting apoptosis in damaged cells and in part by promoting aging and genomic instability independently of the rate of cell growth. These pathways may also contribute to cancer and metastases by affecting inflammation and the cellular environment of the malignant and pre-cancerous cells. The mechanisms connecting IGF-I signaling pathways and cancer in mammals are poorly understood but may involve mechanisms similar to those identified in yeast (147).
Reduced insulin/IGF-1 signaling in mice not only increases life span, but also delays or reduces aging-related pathology, most notably spontaneous tumors (94, 131) in agreement with the association between IGF-1 level and cancer incidence. Notably, IGF-I and age-dependent cancer are both decreased in CR mice (14, 54–60). The mechanism behind the increased survival and reduced cancer incidence associated with CR and low IGF-1 signals may be partly explained by the increased resistance to oxidative and other types of damage demonstrated in yeast, nematode and flies with defects in insulin/IGF-1-like pathways (132). In fact, fibroblasts from long-lived, adult Ames or Snell dwarf mice as well as growth hormone receptor knockout mice are resistant, in vitro, to a variety of toxic agents, such as hydrogen peroxide and ultraviolet light (133) and hepatocytes from Ames dwarf mice more readily undergo apoptosis than wild-type cells, when experiencing an oxidative challenge (134) and IGF-I reverses the beneficial effects of calorie restriction against a bladder carcinogen (93). Activation of the transcription factors NF-E2-related factor 2 (Nrf2), which increases the transcription and activity of a variety of antioxidative and carcinogen-detoxification enzymes, may also be important in mediating the anti-cancer effects, but not the insulin sensitizing and anti-aging effects of CR. The anti-tumorigenesis effects of CR are significantly impaired in Nrf2 deficient mice exposed to carcinogens (135). In agreement with these results, Mn-superoxide dismutase (MnSOD) heterozygous knockout mice, that have reduced MnSOD activity, have increased levels of oxidative damage to DNA in all tissues, a 100% increase in cancer incidence, but average and maximal lifespan is not affected (136). These data suggest that the relationship between oxidative stress, cancer and aging is complex, and may also indicate that the conditions that increase mutations and cancer are not necessarily accelerating aging.
In addition to reducing growth and enhancing apoptotic pathways, CR and reduced IGF-I may contribute to cancer by also reducing genomic instability, possibly via a Ras- or phosphatidylinositol-3 kinase (PI3K)/Akt/Tor/S6kinase-dependent mechanisms. For example, the degree of activation of PI3K in cancer cells plays an important role in regulating cell proliferation, tumor growth, and sensitivity to CR. Tumors that are CR-resistant originate from cancer cells with constitutive activation of the PI3K pathway, which in culture proliferate in the absence of IGF-1 and insulin (137). Replacement of an activated mutant allele of PI3K with a wild-type PI3K allele in these cancer cells restored CR-sensitivity in vitro and in vivo, underlining the importance of the insulin/IGF-1 signalling pathway in mediating the anti-cancer effects of CR. The expression of the tumor suppressor phosphatase and tensin homologue (PTEN), an inhibitor of PI3K activity, in the CR-resistant cancer cells was also sufficient to restore CR-sensitivity in vitro and in vivo in tumor xenografts animal models (137). Several downstream effectors of PI3K/AKT [e.g. forkhead transcription factors (FOXO), mTOR, adenosine monophosphate (AMP)–activated protein kinase (AMPK), S6K, silent mating type information regulation 2 homolog 1 (SIRT1)] might be responsible for the anti-tumorigenic effects of CR (138–142). Interestingly, although the nuclear transcription factor p53, a tumor suppressor that regulates cell cycle, apoptosis and cell senescence, down-regulates the expression of the IGF-1 receptor and the insulin/IGF-1 pathway in post-mitotic fully differentiated cells (143), the anti-tumorigenic effects of CR are similar in p53 overexpressing and p53 knockout mice (144). A better characterization of the molecular signaling pathways by which CR mediates its cancer inhibitory activity in some, but not all cancers, is essential to design new drugs and interventions that prevent tumor initiation or block tumor promotion and progression.
Conclusions and future directions
The probability of developing cancer is remarkably high in the US, with approximately 44% of the men and ~37% of the women will develop cancer during their lifetime (145). Although genetic inheritance influences the risk of cancer (146), most of the variation in cancer risk across populations and among individuals is due to lifestyle and environmental factors. Data from experimental and epidemiological studies indicate that excessive adiposity due to excessive energy intake and minimal physical activity increase the risk of developing cancer. In contrast, calorie restriction without malnutrition, and possibly protein restriction, prevent cancer. More studies are needed to elucidate the molecular mechanisms underlying the beneficial effects of CR and other interventions (e.g. fasting, protein restriction, phytochemicals) in preventing cancer by avoiding accumulation of DNA damage or by potentiating the regression of preneoplastic lesions. More research is also needed to understand how we can block cancer before it becomes invasive or metastic, since metastases are the cause of 90% of human cancer deaths (9). Cancer is not a disease limited to a number of proliferating mutated cells, but a complex process that also involves interactions with the neighboring not-mutated mesenchymal and inflammatory cells that are also affected by aging and/or cancer risk factors (8, 9). CR (and other interventions) by reducing the activity of pro-aging pathways, reducing growth and inflammation in the pre-cancerous and normal neighbor cells, and increasing apoptosis in damaged cells may reduce oncogene mutations frequency but also modulate the growth and invasiveness potentials of the mutated cancerous cells. Hence, understanding the role of CR and of other dietary manipulations in cancer, and identifying the metabolic and molecular mechanisms responsible for the CR-dependent cancer preventive effect has the potential to lead to drugs and therapies for broad-spectrum prevention and treatment of cancer.
Acknowledgments
Funding/Support: Supported by Grant Number UL1 RR024992 from the National Center for Research Resources (a component of the National Institutes of Health and NIH Roadmap for Medical Research), by Istituto Superiore di Sanità/National Institutes of Health Collaboration Program Grant, grants from Ministero della Salute, a grant from the Longer Life Foundation (an RGA/Washington University Partnership), a grant from the Bakewell Foundation, and a donation from the Scott and Annie Appleby Charitable Trust.
Role of the Sponsor: The funding agency had no role in the analysis or interpretation of the data or in the decision to submit the report for publication.
Footnotes
Financial Disclosures: The authors had no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Valter D Longo, Email: vlongo@usc.edu.
Luigi Fontana, Email: lfontana@dom.wustl.edu.
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Ames BN, Gold LS. Endogenous mutagens and the causes of aging and cancer. Mutat Res. 1991;250(1–2):3–16. doi: 10.1016/0027-5107(91)90157-j. [DOI] [PubMed] [Google Scholar]
- 3.Luch A. Nature and nurture - lessons from chemical carcinogenesis. Nat Rev Cancer. 2005;5(2):113–25. doi: 10.1038/nrc1546. [DOI] [PubMed] [Google Scholar]
- 4.Sancar A, Lindsey-Boltz LA, Unsal-Kaçmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- 5.Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396(6712):643–649. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- 6.Bishop JM, Weinberg RA. Molecular oncology. New York: Scientific American Inc; 1996. [Google Scholar]
- 7.Sharpless NE, DePinho RA. Cell. 1. Vol. 110. 2002. p53: good cop/bad cop; pp. 9–12. [DOI] [PubMed] [Google Scholar]
- 8.Kinzler KW, Vogelstein B. Landscaping the cancer terrain. Science. 1998;280(5366):1036–7. doi: 10.1126/science.280.5366.1036. [DOI] [PubMed] [Google Scholar]
- 9.Fidler IJ. The pathogenesis of cancer metastasis: The “seed and soil” hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 10.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 11.Hursting SD, Slaga TJ, Fischer SM, DiGiovanni J, Phang JM. Mechanism-based cancer prevention approaches: targets, examples, and the use of transgenic mice. J Natl Cancer Inst. 1999;91(3):215–25. doi: 10.1093/jnci/91.3.215. [DOI] [PubMed] [Google Scholar]
- 12.Hernández LG, van Steeg H, Luijten M, van Benthem J. Mechanisms of non-genotoxic carcinogens and importance of a weight of evidence approach. Mutat Res. 2009 doi: 10.1016/j.mrrev.2009.07.002. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Fontana L, Klein S. Aging, adiposity, and calorie restriction. JAMA. 2007;297(9):986–94. doi: 10.1001/jama.297.9.986. [DOI] [PubMed] [Google Scholar]
- 14.Hursting SD, Lavigne JA, Berrigan D, Perkins SN, Barrett JC. Calorie restriction, aging, and cancer prevention: mechanisms of action and applicability to humans. Annu Rev Med. 2003;54:131–52. doi: 10.1146/annurev.med.54.101601.152156. [DOI] [PubMed] [Google Scholar]
- 15.Kolonel LN, Altshuler D, Henderson BE. The multiethnic cohort study: exploring genes, lifestyle and cancer risk. Nat Rev Cancer. 2004;4(7):519–27. doi: 10.1038/nrc1389. [DOI] [PubMed] [Google Scholar]
- 16.Baade PD, Youlden DR, Krnjacki LJ. International epidemiology of prostate cancer: geographical distribution and secular trends. Mol Nutr Food Res. 2009;53(2):171–84. doi: 10.1002/mnfr.200700511. [DOI] [PubMed] [Google Scholar]
- 17.Minami Y, Nishino Y, Tsubono Y, Tsuji I, Hisamichi S. Increase of colon and rectal cancer incidence rates in Japan: trends in incidence rates in Miyagi Prefecture. J Epidemiol. 2006;16:240–8. doi: 10.2188/jea.16.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kushi LH, Byers T, Doyle C, Bandera EV, McCullough M, McTiernan A, Gansler T, Andrews KS, Thun MJ American Cancer Society 2006 Nutrition and Physical Activity Guidelines Advisory Committee. American Cancer Society Guidelines on Nutrition and Physical Activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2006;56(5):254–81. doi: 10.3322/canjclin.56.5.254. [DOI] [PubMed] [Google Scholar]
- 19.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3(10):768–80. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 20.Sugimura T. Nutrition and dietary carcinogens. Carcinogenesis. 2000;21(3):387–95. doi: 10.1093/carcin/21.3.387. [DOI] [PubMed] [Google Scholar]
- 21.World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, and Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: AICR; 2007. [Google Scholar]
- 22.Fruits and Vegetables. Vol. 8. Lyon, France: International Agency for Research on Cancer, World Health Organization; 2003. [Google Scholar]
- 23.Kaaks R, Lukanova A. Effects of weight control and physical activity in cancer prevention: role of endogenous hormone metabolism. Ann N Y Acad Sci. 2002;963:268–81. doi: 10.1111/j.1749-6632.2002.tb04118.x. [DOI] [PubMed] [Google Scholar]
- 24.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–56. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 26.Nagulesparan M, Savage PJ, Unger RH, Bennett PH. A simplified method using somatostatin to assess in vivo insulin resistance over a range of obesity. Diabetes. 1979;28(11):980–3. doi: 10.2337/diab.28.11.980. [DOI] [PubMed] [Google Scholar]
- 27.Gunter MJ, Hoover DR, Yu H, Wassertheil-Smoller S, Rohan TE, Manson JE, Li J, Ho GY, Xue X, Anderson GL, Kaplan RC, Harris TG, Howard BV, Wylie-Rosett J, Burk RD, Strickler HD. Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2009;101(1):48–60. doi: 10.1093/jnci/djn415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giovannucci E. Insulin and colon cancer. Cancer Causes Control. 1995;6:164–179. doi: 10.1007/BF00052777. [DOI] [PubMed] [Google Scholar]
- 29.Kaaks R. Nutrition, hormones, and breast cancer: is insulin the missing link? Cancer Causes Control. 1996;7:605–625. doi: 10.1007/BF00051703. [DOI] [PubMed] [Google Scholar]
- 30.Weiderpass E, Partanen T, Kaaks R, Vainio H, Porta M, Kauppinen T, Ojajärvi A, Boffetta P, Malats N. Occurrence, trends and environment etiology of pancreatic cancer. Scand J Work Environ Health. 1998;24(3):165–174. doi: 10.5271/sjweh.295. [DOI] [PubMed] [Google Scholar]
- 31.Prisco M, Romano G, Peruzzi F, Valentinis B, Baserga R. Insulin and IGF-I receptors signaling in protection from apoptosis. Horm Metab Res. 1999;31(2–3):80–9. doi: 10.1055/s-2007-978703. [DOI] [PubMed] [Google Scholar]
- 32.Pugeat M, Crave JC, Elmidani M, Nicolas MH, Garoscio-Cholet M, Lejeune H, Déchaud H, Tourniaire J. Pathophysiology of sex hormone binding globulin (SHBG): relation to insulin. J Steroid Biochem Mol Biol. 1991;40(4–6):841–849. doi: 10.1016/0960-0760(91)90310-2. [DOI] [PubMed] [Google Scholar]
- 33.Poretsky L, Kalin MF. The gonadotropic function of insulin. Endocr Rev. 1987;8:132–141. doi: 10.1210/edrv-8-2-132. [DOI] [PubMed] [Google Scholar]
- 34.Powell DR, Suwanichkul A, Cubbage ML, DePaolis LA, Snuggs MB, Lee PD. Insulin inhibits transcription of the human gene for insulin-like growth factor-binding protein-1. J Biol Chem. 1991;266:18868–76. [PubMed] [Google Scholar]
- 35.Flötotto T, Djahansouzi S, Gläser M, Hanstein B, Niederacher D, Brumm C, Beckmann MW. Hormones and hormone antagonists: mechanisms of action in carcinogenesis of endometrial and breast cancer. Horm Metab Res. 2001;33:451–457. doi: 10.1055/s-2001-16936. [DOI] [PubMed] [Google Scholar]
- 36.Yu H, Shu XO, Li BD, Dai Q, Gao YT, Jin F, Zheng W. Joint effect of insulin-like growth factors and sex steroids on breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2003;12:1067–73. [PubMed] [Google Scholar]
- 37.Berrino F, Bellati C, Secreto G, Camerini E, Pala V, Panico S, Allegro G, Kaaks R. Reducing bioavailable sex hormones through a comprehensive change in diet: the diet and androgens (DIANA) randomized trial. Cancer Epidemiol Biomarkers Prev. 2001;10(1):25–33. [PubMed] [Google Scholar]
- 38.Somasundar P, McFadden DW, Hileman SM, Vona-Davis L. Leptin is a growth factor in cancer. J Surg Res. 2004;116(2):337–49. doi: 10.1016/j.jss.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Iyengar P, Espina V, Williams TW, Lin Y, Berry D, Jelicks LA, Lee H, Temple K, Graves R, Pollard J, Chopra N, Russell RG, Sasisekharan R, Trock BJ, Lippman M, Calvert VS, Petricoin EF, 3rd, Liotta L, Dadachova E, Pestell RG, Lisanti MP, Bonaldo P, Scherer PE. Adipocyte-derived collagen VI affects early mammary tumor progression in vivo, demonstrating a critical interaction in the tumor/stroma microenvironment. J Clin Invest. 2005;115(5):1163–76. doi: 10.1172/JCI23424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, LaMonte MJ, Stroup AM, Hunt SC. Long-Term Mortality after Gastric Bypass Surgery. N Engl J Med. 2007;357:753–61. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 41.Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, Bengtsson C, Dahlgren S, Gummesson A, Jacobson P, Karlsson J, Lindroos AK, Lonroth H, Naslund I, Olbers T, Stenlof K, Torgerson J, Agren G, Carlsson LM Swedish Obese Subjects Study. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8):741–52. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 42.Pi-Sunyer FX. A review of the long-term studies evaluating the efficacy of weight loss in ameliorating disorders associated with obesity. Clin Therapeu. 1996;18:1006–1035. doi: 10.1016/s0149-2918(96)80057-9. [DOI] [PubMed] [Google Scholar]
- 43.Racette SB, Weiss EP, Villareal DT, et al. The Washington University School of Medicine CALERIE Group. One Year of Caloric Restriction in Humans: Feasibility and Effects on Body Composition and Abdominal Adipose Tissue. J Gerontol A Biol Sci Med Sci. 2006;61:943–950. doi: 10.1093/gerona/61.9.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weiss EP, Racette SB, Villareal DT, et al. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr. 2006;84(5):1033–42. doi: 10.1093/ajcn/84.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Larson-Meyer DE, Heilbronn LK, Redman LM, Newcomer BR, Frisard MI, Anton S, Smith SR, Alfonso A, Ravussin E. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care. 2006;29(6):1337–44. doi: 10.2337/dc05-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Villareal DT, Fontana L, Weiss EP, Racette SB, Steger-May K, Schechtman KB, Klein S, Holloszy JO. Bone mineral density response to caloric restriction-induced weight loss or exercise-induced weight loss: A randomized controlled trial. Arch Intern Med. 2006;166(22):2502–10. doi: 10.1001/archinte.166.22.2502. [DOI] [PubMed] [Google Scholar]
- 47.Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, Giugliano D. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 2003;289(14):1799–804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 48.Fontana L, Villareal DT, Weiss EP, Racette SB, Steger-May K, Klein S, Holloszy JO the Washington University School of Medicine CALERIE Group. Calorie restriction or exercise: effects on coronary heart disease risk factors. A randomized, controlled trial. Am J Physiol Endocrinol Metab. 2007;293(1):E197–202. doi: 10.1152/ajpendo.00102.2007. [DOI] [PubMed] [Google Scholar]
- 49.Hofer T, Fontana L, Weiss EP, Anton S, Malayappan B, Holloszy JO, Leeuwenburgh C. One year of caloric restriction and exercise in humans and the effects on DNA and RNA oxidation levels in white blood cells and urine. Rejuvenation Research. 2008;11(4):793–799. doi: 10.1089/rej.2008.0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heilbronn LK, de Jonge L, Frisard MI, et al. Pennington CALERIE Team. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295:1539–48. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Callaghan NJ, Clifton PM, Noakes M, Fenech M. Weight loss in obese men is associated with increased telomere length and decreased a basic sites in rectal mucosa. Rejuvenation Res. 2009;12(3):169–76. doi: 10.1089/rej.2008.0819. [DOI] [PubMed] [Google Scholar]
- 52.Rudolph KL, Chang S, Lee HW, Blasco M, Gottlieb GJ, Greider C, DePinho RA. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell. 1999;96(5):701–12. doi: 10.1016/s0092-8674(00)80580-2. [DOI] [PubMed] [Google Scholar]
- 53.Moreschi C. Beziehungen zwischen ernährung und tumorwachstum. Z Immunitätsforsch Orig. 1909;2:651–675. [Google Scholar]
- 54.Tannenbaum A, Silverstone H. The influence of the degree of caloric restriction on the formation of skin tumors and hepatomas in mice. Cancer Res. 1949;9(12):724–7. [PubMed] [Google Scholar]
- 55.Cheney KE, Liu RK, Smith GS, Leung RE, Mickey MR, Walford RL. Survival and disease patterns in C57BL/6J mice subjected to undernutrition. Exp Gerontol. 1980;15(4):237–58. doi: 10.1016/0531-5565(80)90029-7. [DOI] [PubMed] [Google Scholar]
- 56.Cheney KE, Liu RK, Smith GS, Meredith PJ, Mickey MR, Walford RL. The effect of dietary restriction of varying duration on survival, tumor patterns, immune function, and body temperature in B10C3F1 female mice. J Gerontol. 1983;38(4):420–30. doi: 10.1093/geronj/38.4.420. [DOI] [PubMed] [Google Scholar]
- 57.Weindruch R, Walford RL. The retardation of aging and disease by dietary restriction. Springfield, IL: Charles C Thomas Publisher; 1988. [Google Scholar]
- 58.Shimokawa I, Higami Y, Yu BP, Masoro EJ, Ikeda T. Influence of dietary components on occurrence of and mortality due to neoplasms in male F344 rats. Aging (Milano) 1996;8(4):254–62. doi: 10.1007/BF03339576. [DOI] [PubMed] [Google Scholar]
- 59.Klurfeld DM, Welch CB, Davis MJ, Kritchevsky D. Determination of degree of energy restriction necessary to reduce DMBA-induced mammary tumorigenesis in rats during the promotion phase. J Nutr. 1989;119(2):286–91. doi: 10.1093/jn/119.2.286. [DOI] [PubMed] [Google Scholar]
- 60.Thompson HJ, Zhu Z, Jiang W. Dietary energy restriction in breast cancer prevention. J Mammary Gland Biol Neoplasia. 2003;8(1):133–42. doi: 10.1023/a:1025743607445. [DOI] [PubMed] [Google Scholar]
- 61.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325(5937):201–4. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pugh TD, Oberley TD, Weindruch R. Dietary intervention at middle age: caloric restriction but not dehydroepiandrosterone sulfate increases lifespan and lifetime cancer incidence in mice. Cancer Res. 1999;59(7):1642–8. [PubMed] [Google Scholar]
- 63.Albanes D. Total calories, body weight, and tumor incidence in mice. Cancer Res. 1987;47(8):1987–92. [PubMed] [Google Scholar]
- 64.Sonntag WE, et al. Pleiotropic effects of growth hormone and insulin-like growth factor (IGF)-1 on biological aging: inferences from moderate caloric-restricted animals. J Gerontol A Biol Sci Med Sci. 1999;54:B521–38. doi: 10.1093/gerona/54.12.b521. [DOI] [PubMed] [Google Scholar]
- 65.Kalant N, Stewart J, Kaplan R. Effect of diet restriction on glucose metabolism and insulin responsiveness in aging rats. Mech Ageing Dev. 1988;46(1–3):89–104. doi: 10.1016/0047-6374(88)90117-0. [DOI] [PubMed] [Google Scholar]
- 66.Kemnitz JW, Roecker EB, Weindruch R, Elson DF, Baum ST, Bergmann RN. Dietary restriction increases insulin sensitivity and lowers blood glucose in rhesus monkeys. Am J Physiol. 1994;266:E540–E547. doi: 10.1152/ajpendo.1994.266.4.E540. [DOI] [PubMed] [Google Scholar]
- 67.Merry BJ, Holehan AM. Serum profiles of LH, FSH, testosterone and 5 alpha-DHT from 21 to 1000 days of age in ad libitum fed and dietary restricted rats. Exp Gerontol. 1981;16(6):431–44. doi: 10.1016/0531-5565(81)90025-5. [DOI] [PubMed] [Google Scholar]
- 68.Sohal RS, Weindruch R. Oxidative stress, caloric restriction and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sohal RS, Agarwal S, Candas M, Forster MJ, Lal H. Effect of age and caloric restriction on DNA oxidative damage in different tissues of C57BL/6 mice. Mech Ageing Dev. 1994;76(2–3):215–24. doi: 10.1016/0047-6374(94)91595-4. [DOI] [PubMed] [Google Scholar]
- 70.Youngman LD, Park JY, Ames BN. Protein oxidation associated with aging is reduced by dietary restriction of protein or calories. Proc Natl Acad Sci U S A. 1992;89(19):9112–6. doi: 10.1073/pnas.89.19.9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Matsuzaki J, Kuwamura M, Yamaji R, Inui H, Nakano Y. Inflammatory responses to lipopolysaccharide are suppressed in 40% energy-restricted mice. J Nutr. 2001;131:2139–2144. doi: 10.1093/jn/131.8.2139. [DOI] [PubMed] [Google Scholar]
- 72.Ershler WB, Sun WH, Binkley N, et al. Interleukin-6 and aging: blood levels and mononuclear cell production increase with advancing age and in vitro production is modifiable by dietary restriction. Lymphokine Cytokine Res. 1993;12:225–30. [PubMed] [Google Scholar]
- 73.Han ES, Evans TR, Shu JH, Lee S, Nelson JF. Food restriction enhances endogenous and corticotropin-induced plasma elevations of free but not total corticosterone throughout life in rats. J Gerontol A Biol Sci Med Sci. 2001;56:B391–7. doi: 10.1093/gerona/56.9.b391. [DOI] [PubMed] [Google Scholar]
- 74.Yang H, Youm YH, Nakata C, Dixit VD. Chronic caloric restriction induces forestomach hypertrophy with enhanced ghrelin levels during aging. Peptides. 2007;28(10):1931–6. doi: 10.1016/j.peptides.2007.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grossmann ME, Nkhata KJ, Mizuno NK, Ray A, Cleary MP. Effects of adiponectin on breast cancer cell growth and signaling. Br J Cancer. 2008;98(2):370–9. doi: 10.1038/sj.bjc.6604166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Spaulding CC, Walford RL, Effros RB. The accumulation of non-replicative, non-functional, senescent T cells with age is avoided in calorically restricted mice by an enhancement of T cell apoptosis. Mech Ageing Dev. 1997;93(1–3):25–33. doi: 10.1016/s0047-6374(96)01808-8. [DOI] [PubMed] [Google Scholar]
- 77.Messaoudi I, Warner J, Fischer M, Park B, Hill B, Mattison J, Lane MA, Roth GS, Ingram DK, Picker LJ, Douek DC, Mori M, Nikolich-Zugich J. Delay of T cell senescence by caloric restriction in aged long-lived nonhuman primates. Proc Natl Acad Sci U S A. 2006;103(51):19448–53. doi: 10.1073/pnas.0606661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weraarchakul N, Strong R, Wood WG, Richardson A. Effect of aging and dietary restriction on DNA repair. Exp Cell Res. 1989;181:197–204. doi: 10.1016/0014-4827(89)90193-6. [DOI] [PubMed] [Google Scholar]
- 79.Wachsman JT. The beneficial effects of dietary restriction: reduced oxidative damage and enhanced apoptosis. Mutat Res. 1996;350(1):25–34. doi: 10.1016/0027-5107(95)00087-9. [DOI] [PubMed] [Google Scholar]
- 80.Leeuwenburgh C, Wagner P, Holloszy JO, Sohal RS, Heinecke JW. Caloric restriction attenuates dityrosine cross-linking of cardiac and skeletal muscle proteins in aging mice. Arch Biochem Biophys. 1997;346:74–80. doi: 10.1006/abbi.1997.0297. [DOI] [PubMed] [Google Scholar]
- 81.Cuervo AM, Bergamini E, Brunk UT, Dröge W, Ffrench M, Terman A. Autophagy and aging: the importance of maintaining “clean” cells. Autophagy. 2005;1(3):131–40. doi: 10.4161/auto.1.3.2017. [DOI] [PubMed] [Google Scholar]
- 82.Park SK, Prolla TA. Gene expression profiling studies of aging in cardiac and skeletal muscles. Cardiovasc Res. 2005;66:205–12. doi: 10.1016/j.cardiores.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 83.Dhahbi JM, Kim HJ, Mote PL, Beaver RJ, Spindler SR. Temporal linkage between the phenotypic and genomic responses to caloric restriction. Proc Natl Acad Sci U S A. 2004;101:5524–9. doi: 10.1073/pnas.0305300101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fontana L, Meyer TE, Klein S, Holloszy JO. Long-Term Calorie Restriction Is Highly Effective In Reducing The Risk For Atherosclerosis In Humans. Procedings of the National Academy of Science USA. 2004;101(17):6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Meyer TE, Kovács SJ, Ehsani AA, Klein S, Holloszy JO, Fontana L. Long-term Caloric Restriction Ameliorates the Decline in Diastolic Function in Humans. Journal of American Collage Cardiology. 2006;47(2):398–402. doi: 10.1016/j.jacc.2005.08.069. [DOI] [PubMed] [Google Scholar]
- 86.Fontana L, Klein S, Holloszy JO, Premachandra BN. Effect of Long-term Calorie Restriction with Adequate Protein and Micronutrients on Thyroid Hormones. Journal of Clinical Endocrinology & Metabolism. 2006;91:3232–3235. doi: 10.1210/jc.2006-0328. [DOI] [PubMed] [Google Scholar]
- 87.Fontana L, Klein S, Holloszy JO. Effects of long-term calorie restriction and endurance exercise on glucose tolerance, insulin action and adipokine production. Age. 2009 doi: 10.1007/s11357-009-9118-z. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ramsey MM, Ingram RL, Cashion AB, Ng AH, Cline JM, Parlow AF, Sonntag WE. Growth hormone-deficient dwarf animals are resistant to dimethylbenzanthracine (DMBA)-induced mammary carcinogenesis. Endocrinology. 2002;143(10):4139–42. doi: 10.1210/en.2002-220717. [DOI] [PubMed] [Google Scholar]
- 89.Colbert LH, Westerlind KC, Perkins SN, Haines DC, Berrigan D, Donehower LA, Fuchs-Young R, Hursting SD. Exercise effects on tumorigenesis in a p53-deficient mouse model of breast cancer. Med Sci Sports Exerc. 2009;41(8):1597–605. doi: 10.1249/MSS.0b013e31819f1f05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Colbert LH, Mai V, Perkins SN, Berrigan D, Lavigne JA, Wimbrow HH, Alvord WG, Haines DC, Srinivas P, Hursting SD. Exercise and intestinal polyp development in APCMin mice. Med Sci Sports Exerc. 2003;35(10):1662–9. doi: 10.1249/01.MSS.0000089349.54813.41. [DOI] [PubMed] [Google Scholar]
- 91.Tomas FM, Chandler CS, Coyle P, Bourgeois CS, Burgoyne JL, Rofe AM. Effects of insulin and insulin-like growth factors on protein and energy metabolism in tumour-bearing rats. Biochem J. 1994;301 (Pt 3):769–75. doi: 10.1042/bj3010769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hursting SD, Switzer BR, French JE, Kari FW. The growth hormone: insulin-like growth factor 1 axis is a mediator of diet restriction-induced inhibition of mononuclear cell leukemia in Fischer rats. Cancer Res. 1993;53(12):2750–7. [PubMed] [Google Scholar]
- 93.Dunn SE, et al. Dietary restriction reduces insulin-like growth factor I levels, which modulates apoptosis, cell proliferation, and tumor progression in p53-deficient mice. Cancer Res. 1997;57:4667–72. [PubMed] [Google Scholar]
- 94.Ikeno Y, Hubbard GB, Lee S, Cortez LA, Lew CM, Webb CR, Berryman DE, List EO, Kopchick JJ, Bartke A. Reduced incidence and delayed occurrence of fatal neoplastic diseases in growth hormone receptor/binding protein knockout mice. J Gerontol A Biol Sci Med Sci. 2009;64(5):522–9. doi: 10.1093/gerona/glp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vergara M, Smith-Wheelock M, Harper JM, Sigler R, Miller RA. Hormone-treated snell dwarf mice regain fertility but remain long lived and disease resistant. J Gerontol A Biol Sci Med Sci. 2004;59(12):1244–50. doi: 10.1093/gerona/59.12.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Garcia AM, Busuttil RA, Calder RB, Dollé ME, Diaz V, McMahan CA, Bartke A, Nelson J, Reddick R, Vijg J. Effect of Ames dwarfism and caloric restriction on spontaneous DNA mutation frequency in different mouse tissues. Mech Ageing Dev. 2008;129(9):528–33. doi: 10.1016/j.mad.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bartke A, Chandrashekar V, Bailey B, Zaczek D, Turyn D. Consequences of growth hormone (GH) overexpression and GH resistance. Neuropeptides. 2002;36(2–3):201–8. doi: 10.1054/npep.2002.0889. [DOI] [PubMed] [Google Scholar]
- 98.Stewart JW, Koehler K, Jackson W, Hawley J, Wang W, Au A, Myers R, Birt DF. Prevention of mouse skin tumor promotion by dietary energy restriction requires an intact adrenal gland and glucocorticoid supplementation restores inhibition. Carcinogenesis. 2005;26(6):1077–84. doi: 10.1093/carcin/bgi051. [DOI] [PubMed] [Google Scholar]
- 99.Renehan AG, Zwahlen M, Minder C, O’Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363(9418):1346–53. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 100.Ron E, Gridley G, Hrubec Z, Page W, Arora S, Fraumeni JF., Jr Acromegaly and gastrointestinal cancer. Cancer. 1991;68(8):1673–7. doi: 10.1002/1097-0142(19911015)68:8<1673::aid-cncr2820680802>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 101.Thissen JP, Ketelslegers JM, Underwood LE. Nutritional regulation of the insulin-like growth factors. Endocr Rev. 1994;15(1):80–101. doi: 10.1210/edrv-15-1-80. [DOI] [PubMed] [Google Scholar]
- 102.Fontana L, Weiss EP, Villareal DT, Klein S, Holloszy JO. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell. 2008;7(5):681–7. doi: 10.1111/j.1474-9726.2008.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Redman LM, Veldhuis JD, Rood J, Smith SR, Williamson D, Ravussin E for the Pennington CALERIE Team. The effect of caloric restriction interventions on growth hormone secretion in non-obese men and women. Aging Cell. 2009 doi: 10.1111/j.1474-9726.2009.00530.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Holmes MD, Pollak MN, Willett WC, Hankinson SE. Dietary correlates of plasma insulin-like growth factor I and insulin-like growth factor binding protein 3 concentrations. Cancer Epidemiol Biomarkers Prev. 2002;11(9):852–61. [PubMed] [Google Scholar]
- 105.Giovannucci E, Pollak M, Liu Y, Platz EA, Majeed N, Rimm EB, Willett WC. Nutritional predictors of insulin-like growth factor I and their relationships to cancer in men. Cancer Epidemiol Biomarkers Prev. 2003 Feb;12(2):84–9. [PubMed] [Google Scholar]
- 106.Linos E, Willett WC, Cho E, Colditz G, Frazier LA. Red meat consumption during adolescence among premenopausal women and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(8):2146–51. doi: 10.1158/1055-9965.EPI-08-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Michaud DS, Augustsson K, Rimm EB, Stampfer MJ, Willet WC, Giovannucci E. A prospective study on intake of animal products and risk of prostate cancer. Cancer Causes Control. 2001;12(6):557–67. doi: 10.1023/a:1011256201044. [DOI] [PubMed] [Google Scholar]
- 108.Rand WM, Pellett PL, Young VR. Meta-analysis of nitrogen balance studies for estimating protein requirements in healthy adults. Am J Clin Nutr. 2003;77:109–27. doi: 10.1093/ajcn/77.1.109. [DOI] [PubMed] [Google Scholar]
- 109.Moshfegh A, Goldman J, Cleveland L. What we eat in America, NHANES 2001–2002: Usual nutrient intakes from food compared to Dietary Reference Intakes. [Accessed Sep 20, 2009];2005 Available at: http://www.ars.usda.gov/SP2UserFiles/Place/12355000/pdf/usualintaketables2001-02.pdf.
- 110.Shevah O, Laron Z. Patients with congenital deficiency of IGF-I seem protected from the development of malignancies: a preliminary report. Growth Horm IGF Res. 2007;17(1):54–7. doi: 10.1016/j.ghir.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 111.Laughlin GA, Barrett-Connor E, Criqui MH, Kritz-Silverstein D. The prospective association of serum insulin-like growth factor I (IGF-I) and IGF-binding protein-1 levels with all cause and cardiovascular disease mortality in older adults: the Rancho Bernardo Study. J Clin Endocrinol Metab. 2004;89(1):114–20. doi: 10.1210/jc.2003-030967. [DOI] [PubMed] [Google Scholar]
- 112.Berryman DE, List EO, Coschigano KT, Behar K, Kim JK, Kopchick JJ. Comparing adiposity profiles in three mouse models with altered GH signaling. Growth Horm IGF Res. 2004;14(4):309–18. doi: 10.1016/j.ghir.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 113.Bartke A, Bonkowski M, Masternak M. THow diet interacts with longevity genes. Hormones (Athens) 2008;7(1):17–23. doi: 10.14310/horm.2002.1111033. [DOI] [PubMed] [Google Scholar]
- 114.Busuttil RA, Dollé M, Campisi J, Vijga J. Genomic instability, aging, and cellular senescence. Ann N Y Acad Sci. 2004;1019:245–55. doi: 10.1196/annals.1297.041. [DOI] [PubMed] [Google Scholar]
- 115.Longo VD, Liou LL, Valentine JS, Gralla EB. Mitochondrial superoxide decreases yeast survival in stationary phase. Arch Biochem Biophys. 1999;365(1):131–42. doi: 10.1006/abbi.1999.1158. [DOI] [PubMed] [Google Scholar]
- 116.Fabrizio P, Battistella L, Vardavas R, Gattazzo C, Liou LL, Diaspro A, Dossen JW, Gralla EB, Longo VD. Superoxide is a mediator of an altruistic aging program in Saccharomyces cerevisiae. J Cell Biol. 2004;166(7):1055–67. doi: 10.1083/jcb.200404002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Madia F, Gattazzo C, Fabrizio P, Longo VD. A simple model system for age-dependent DNA damage and cancer. Mech Ageing Dev. 2007;128(1):45–9. doi: 10.1016/j.mad.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fabrizio P, Gattazzo C, Battistella L, Wei M, Cheng C, McGrew K, Longo VD. Sir2 blocks extreme life-span extension. Cell. 2005;123(4):655–67. doi: 10.1016/j.cell.2005.08.042. [DOI] [PubMed] [Google Scholar]
- 119.Madia F, Wei M, Yuan V, Hu J, Gattazzo C, Pham P, Goodman MF, Longo VD. Oncogene homologue Sch9 promotes age-dependent mutations by a superoxide and Rev1/Polzeta-dependent mechanism. J Cell Biol. 2009;186(4):509–23. doi: 10.1083/jcb.200906011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Madia F, Gattazzo C, Wei M, Fabrizio P, Burhans WC, Weinberger M, Galbani A, Smith JR, Nguyen C, Huey S, Comai L, Longo VD. Longevity mutation in SCH9 prevents recombination errors and premature genomic instability in a Werner/Bloom model system. J Cell Biol. 2008;180(1):67–81. doi: 10.1083/jcb.200707154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fabrizio P, Liou LL, Moy VN, Diaspro A, Valentine JS, Gralla EB, Longo VD. SOD2 functions downstream of Sch9 to extend longevity in yeast. Genetics. 2003;163(1):35–46. doi: 10.1093/genetics/163.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hlavatá L, Aguilaniu H, Pichová A, Nyström T. The oncogenic RAS2(val19) mutation locks respiration, independently of PKA, in a mode prone to generate ROS. EMBO J. 2003;22(13):3337–45. doi: 10.1093/emboj/cdg314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD. Regulation of longevity and stress resistance by Sch9 in yeast. Science. 2001;292(5515):288–90. doi: 10.1126/science.1059497. [DOI] [PubMed] [Google Scholar]
- 124.Wei M, Fabrizio P, Hu J, Ge H, Cheng C, Li L, Longo VD. Life span extension by calorie restriction depends on Rim15 and transcription factors downstream of Ras/PKA, Tor, and Sch9. PLoS Genet. 2008;4(1):e13. doi: 10.1371/journal.pgen.0040013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wei M, Fabrizio P, Madia F, Hu J, Ge H, Li LM, Longo VD. Tor1/Sch9-regulated carbon source substitution is as effective as calorie restriction in life span extension. PLoS Genet. 2009;5(5):e1000467. doi: 10.1371/journal.pgen.1000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384(6604):33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- 127.Flurkey K, Papaconstantinou J, Harrison DE. The Snell dwarf mutation Pit1(dw) can increase life span in mice. Mech Ageing Dev. 2002;123(2–3):121–30. doi: 10.1016/s0047-6374(01)00339-6. [DOI] [PubMed] [Google Scholar]
- 128.Flurkey K, Papaconstantinou J, Miller RA, Harrison DE. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc Natl Acad Sci U S A. 2001;98(12):6736–41. doi: 10.1073/pnas.111158898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Holzenberger M, Dupont J, Ducos B, Leneuve P, Géloën A, Even PC, Cervera P, Le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421(6919):182–7. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 130.Coschigano KT, Clemmons D, Bellush LL, Kopchick JJ. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology. 2000;141(7):2608–13. doi: 10.1210/endo.141.7.7586. [DOI] [PubMed] [Google Scholar]
- 131.Ikeno Y, Bronson RT, Hubbard GB, Lee S, Bartke A. Delayed occurrence of fatal neoplastic diseases in ames dwarf mice: correlation to extended longevity. J Gerontol A Biol Sci Med Sci. 2003;58(4):291–6. doi: 10.1093/gerona/58.4.b291. [DOI] [PubMed] [Google Scholar]
- 132.Longo VD, Finch CE. Evolutionary medicine: from dwarf model systems to healthy centenarians? Science. 2003;299(5611):1342–6. doi: 10.1126/science.1077991. [DOI] [PubMed] [Google Scholar]
- 133.Salmon AB, Murakami S, Bartke A, Kopchick J, Yasumura K, Miller RA. Fibroblast cell lines from young adult mice of long-lived mutant strains are resistant to multiple forms of stress. Am J Physiol Endocrinol Metab. 2005;289(1):E23–9. doi: 10.1152/ajpendo.00575.2004. [DOI] [PubMed] [Google Scholar]
- 134.Kennedy MA, Rakoczy SG, Brown-Borg HM. Long-living Ames dwarf mouse hepatocytes readily undergo apoptosis. Exp Gerontol. 2003;38(9):997–1008. doi: 10.1016/s0531-5565(03)00164-5. [DOI] [PubMed] [Google Scholar]
- 135.Pearson KJ, Lewis KN, Price NL, Chang JW, Perez E, Cascajo MV, Tamashiro KL, Poosala S, Csiszar A, Ungvari Z, Kensler TW, Yamamoto M, Egan JM, Longo DL, Ingram DK, Navas P, de Cabo R. Nrf2 mediates cancer protection but not prolongevity induced by caloric restriction. Proc Natl Acad Sci U S A. 2008;105(7):2325–30. doi: 10.1073/pnas.0712162105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Van Remmen H, Ikeno Y, Hamilton M, Pahlavani M, Wolf N, Thorpe SR, Alderson NL, Baynes JW, Epstein CJ, Huang TT, Nelson J, Strong R, Richardson A. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol Genomics. 2003;16(1):29–37. doi: 10.1152/physiolgenomics.00122.2003. [DOI] [PubMed] [Google Scholar]
- 137.Kalaany NY, Sabatini DM. Tumours with PI3K activation are resistant to dietary restriction. Nature. 2009;458(7239):725–31. doi: 10.1038/nature07782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jiang W, Zhu Z, Thompson HJ. Dietary energy restriction modulates the activity of AMP-activated protein kinase, Akt, and mammalian target of rapamycin in mammary carcinomas, mammary gland, and liver. Cancer Res. 2008;68(13):5492–9. doi: 10.1158/0008-5472.CAN-07-6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kopelovich L, Fay JR, Sigman CC, Crowell JA. The mammalian target of rapamycin pathway as a potential target for cancer chemoprevention. Cancer Epidemiol Biomarkers Prev. 2007;16(7):1330–40. doi: 10.1158/1055-9965.EPI-07-0045. [DOI] [PubMed] [Google Scholar]
- 140.Moore T, Beltran L, Carbajal S, Strom S, Traag J, Hursting SD, DiGiovanni J. Dietary energy balance modulates signaling through the Akt/mammalian target of rapamycin pathways in multiple epithelial tissues. Cancer Prev Res (Phila Pa) 2008;1(1):65–76. doi: 10.1158/1940-6207.CAPR-08-0022. [DOI] [PubMed] [Google Scholar]
- 141.Luo Z, Saha AK, Xiang X, Ruderman NB. AMPK, the metabolic syndrome and cancer. Trends Pharmacol Sci. 2005;26(2):69–76. doi: 10.1016/j.tips.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 142.Firestein R, Blander G, Michan S, Oberdoerffer P, Ogino S, Campbell J, Bhimavarapu A, Luikenhuis S, de Cabo R, Fuchs C, Hahn WC, Guarente LP, Sinclair DA. The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth. PLoS One. 2008;3(4):e2020. doi: 10.1371/journal.pone.0002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Scrable H, Medrano S, Ungewitter E. Running on empty: how p53 controls INS/IGF signaling and affects life span. Exp Gerontol. 2009;44(1–2):93–100. doi: 10.1016/j.exger.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hursting SD, Perkins SN, Brown CC, Haines DC, Phang JM. Calorie restriction induces a p53-independent delay of spontaneous carcinogenesis in p53-deficient and wild-type mice. Cancer Res. 1997;57(14):2843–6. [PubMed] [Google Scholar]
- 145.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 146.Lichtenstein P, Holm NV, Verkasalo PK, et al. Environmental and heritable factors in the causation of cancer—analyses of cohorts of twins from Sweden, Denmark and Finland. N EnglJ Med. 2000;343:78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 147.Longo VD, Lieber MR, Vijg J. Turning anti-ageing genes against cancer. Nat Rev Mol Cell Biol. 2008 Nov;9(11):903–10. doi: 10.1038/nrm2526. [DOI] [PubMed] [Google Scholar]