Abstract
Of all the intracellular organelles, secretory granules contain by far the highest calcium concentration; secretory granules of typical neuroendocrine chromaffin cells contain ∼40 mM Ca2+ and occupy ∼20% cell volume, accounting for >60% of total cellular calcium. They also contain the majority of cellular inositol 1,4,5-trisphosphate receptors (IP3Rs) in addition to the presence of >2 mM of chromogranins A and B that function as high-capacity, low-affinity Ca2+ storage proteins. Chromogranins A and B also interact with the IP3Rs and activate the IP3R/Ca2+ channels. In experiments with both neuroendocrine PC12 and nonneuroendocrine NIH3T3 cells, in which the number of secretory granules present was changed by either suppression or induction of secretory granule formation, secretory granules were demonstrated to account for >70% of the IP3-induced Ca2+ releases in the cytoplasm. Moreover, the IP3 sensitivity of secretory granule IP3R/Ca2+ channels is at least ∼6- to 7-fold more sensitive than those of the endoplasmic reticulum, thus enabling secretory granules to release Ca2+ ahead of the endoplasmic reticulum. Further, there is a direct correlation between the number of secretory granules and the IP3 sensitivity of cytoplasmic IP3R/Ca2+ channels and the increased ratio of IP3-induced cytoplasmic Ca2+ release, highlighting the importance of secretory granules in the IP3-dependent Ca2+ signaling. Given that secretory granules are present in all secretory cells, these results presage critical roles of secretory granules in the control of cytoplasmic Ca2+ concentrations in other secretory cells.—Yoo, S. H. Secretory granules in inositol 1,4,5-trisphosphate-dependent Ca2+ signaling in the cytoplasm of neuroendocrine cells.
Keywords: chromogranin A, chromogranin B, IP3 receptor/Ca2+ channel, chromaffin cell
Several cytoplasmic organelles, including the endoplasmic reticulum (ER), secretory granules, mitochondria (1,2,3,4), lysosomes, and endosomes (5,6,7) have been shown to function as intracellular Ca2+ stores. Yet studies on the intracellular Ca2+ stores in the past have often centered around the ER (8,9,10), presumably because of the first identification of the inositol 1,4,5-trisphosphate receptor (IP3R) in the ER (11) and the importance of the ER in the cell. The possibility of secretory granules serving as an intracellular Ca2+ store was first raised in chromaffin cells when chromaffin granules rapidly released Ca2+ in response to IP3 (12), and then in pancreatic acinar cells (13). IP3 releases substantial amounts of Ca2+ rapidly from secretory granules, which is followed by immediate sequestration of the released Ca2+ into secretory granules, both in the absence of exogenous energy supply (12,13,14). The IP3-induced release and sequestration of granular Ca2+ can be repeated several times without diminution of the magnitude and speed of Ca2+ release and sequestration (Fig. 1). The IP3-mediated Ca2+ release from secretory granules has now been demonstrated in many types of secretory cells (15,16,17,18,19,20), and the acidic intragranular milieu (pH 5.5) is considered to be responsible for the energy-independent Ca2+ sequestration (20). In a recent review (7), participation of secretory granules, along with the ER and other intracellular organelles, in the control of intracellular Ca2+ concentrations in exocrine cells is discussed. Hence, in light of increasing evidence demonstrating the major role of secretory granules in the control of IP3-dependent Ca2+ mobilization in the cytoplasm of neuroendocrine cells, the molecular basis that gives rise to the primary role of secretory granules in IP3-dependent Ca2+ signaling in the cytoplasm is reviewed here.
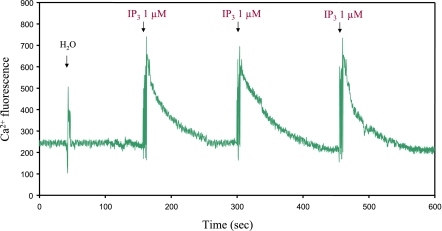
Figure 1.
IP3-induced Ca2+ release from secretory granules of chromaffin cells. IP3-induced Ca2+ release from isolated secretory granules of bovine chromaffin cells was measured using fluorescence microscopy after adding 1 μl IP3 (1 μM final) aliquots to 200 μl of secretory granule solution (1 mg protein/ml) in the presence of 15 μM Calcium Green-1 (111). To perform IP3-induced Ca2+ release experiments, secretory granules that had been prepared as described previously (12) were resuspended in buffer I (10 mM HEPES/KOH, pH 7.2; 140 mM KCl; 0.25 M sucrose). Final Ca2+ concentration of the secretory granule solution was adjusted to 0.3 μM by adding appropriate amounts of EGTA. Sample chamber containing Calcium Green-1 was placed on the stage of a Zeiss Axiovert S 100 microscope (Carl Zeiss, Jena, Germany), and 1 μl IP3 (1 μM final) aliquots were added. IP3-induced Ca2+ release was analyzed by excitation of Calcium Green-1 at 495 nm with a Lambda LS xenon arc lamp and Lambda 10-2 optical filter changer (Sutter Instrument, Novato, CA, USA). The emission fluorescence signals at 530 nm were collected using a bandpass filter of D510/40 nm (Chroma Technology Corp., Rockingham, VT, USA) and Hamamatsu C4742-95 digital CCD camera (Hamamatsu Photonics, Hamamatsu, Japan). Approximately 4 images/s were acquired; imaging continued with successive addition of IP3. Changes of fluorescence were calculated by MetaFluor software (Meta Imaging 6.1; Universal Imaging Corporation, West Chester, PA, USA).
Ca2+ CONCENTRATIONS IN SECRETORY GRANULES
Secretory granules of secretory cells are known to contain the most intracellular calcium in the cytoplasm, containing 15–40 mM (7, 21,22,23). However, most of the intragranular calcium stays bound to chromogranins (24), and secretory granules maintain free Ca2+ concentrations of only 28–100 μM (13, 14, 19, 20, 24, 25) although 1.4 μM (26) and 300 μM (18) are also reported. Despite the seemingly low free-Ca2+ concentrations inside secretory granules, IP3 triggers a rapid Ca2+ release from secretory granules (12,13,14, 17,18,19,20, 27). In addition to 40 mM calcium in secretory granules (21, 22), there are on average ∼15,000–30,000 secretory granules in each adrenal chromaffin cell (28,29,30,31,32), occupying ∼20% of the total cell volume (28,29,30) and accounting for >60% of total cellular calcium of these cells (21) (Table 1). On the other hand, the Ca2+ concentrations of the ER are known to be ∼3 mM (10, 33), and the ER of bovine adrenal chromaffin cells occupies ∼14% of the cell volume (28), which indicates that ∼5% of total cellular calcium of chromaffin cells is stored in the ER (Table 1). The unusually high calcium-storing capability of secretory granules is attributed to the presence of high concentrations of Ca2+-storage proteins chromogranins A and B (CGA and CGB), and secretogranin II (SgII) in secretory granules (Table 2) (24, 34,35,36).
TABLE 1.
Calcium and IP3R concentrations in the ER and secretory granules of bovine chromaffin cells
| Location | Cell volume (%) | Calcium concentration (mM) | Total calcium (% of cell) | IP3R1
|
IP3R2
|
IP3R3
|
|||
|---|---|---|---|---|---|---|---|---|---|
| Relative concentration | Total IP3R1 (% of cell) | Relative concentration | Total IP3R2 (% of cell) | Relative concentration | Total IP3R3 (% of cell) | ||||
| Endoplasmic reticulum | 14.2 | 3 | 5 | 1.20 | 15.2 | 1.55 | 19.8 | 0.74 | 15.9 |
| Secretory granule | 20.5 | 40 | 60 | 3.77 | 69.3 | 3.48 | 64.3 | 1.88 | 58.1 |
Percentages of cell volume are from ref. 28. Calcium concentrations for the ER and secretory granules are from refs. 10 and 21, 22, respectively. Percentage of ER calcium is calculated assuming that 60% of total cell calcium is stored in secretory granules (21). Relative concentration values are obtained assuming the relative concentration of each IP3R isoform of the nucleus as 1 (41).
TABLE 2.
Ca2+-binding properties of chromogranins/secretogranin II and their respective concentrations in the ER and secretory granules of bovine chromaffin cells
| Protein | Ca2+ binding (mol/mol protein) | Kd (mM) | Concentration (μM)
|
|
|---|---|---|---|---|
| Endoplasmic reticulum | Secretory granule | |||
| Chromogranin A | 32–55 | 2.7–4.0 | >140 | >1800 |
| Chromogranin B | 50–93 | 1.5–3.1 | >120 | >200 |
| Secretogranin II | 30–61 | 2.2–3.0 | >10 | >30 |
IP3R/Ca2+ CHANNELS IN SECRETORY GRANULES
As the first IP3-induced Ca2+ release from secretory granules was demonstrated with chromaffin granules (12), so was the first direct evidence for the existence of the IP3Rs in the membranes of secretory granules obtained from chromaffin granules (37). In the same year, the insulin-containing secretory granules of pancreatic β cells were reported to contain type 3 IP3R (IP3R3) (38), but this report was questioned by another group (39) who raised the possibility of cross-reactivity of the IP3R3 antibody, which had been used in previous studies with insulin molecules that are present inside the insulin-containing granules of pancreatic β cells. Yet, subsequent studies confirmed not only the presence of IP3Rs (17) but also of all three types (types 1, 2, and 3) of IP3Rs in the secretory granules of pancreatic β cells (16). It appears that depending on the epitope specificity of the antibodies used, the presence of the IP3Rs in secretory granules has occasionally not been detected. On the other hand, in a study using vesicle membrane protein synaptobrevin-aequorin chimera that is expressed in a β-cell line (MIN6 cells) as a reporter, IP3 was shown not to lower the inside Ca2+ concentrations of secretory granules ([Ca2+]SG) in permeabilized MIN6 cells, whereas the ryanodine receptor activator caffeine caused a dramatic fall in [Ca2+]SG of intact MIN6 cells (25), leading the researchers to conclude that only the ryanodine receptors, not the IP3Rs, localize in β-cell granules. Given that the IP3 effect was tested with permeabilized cells while the caffeine effect was with intact cells, it is likely that either permeabilization destroyed the IP3Rs, and the signal receiving machinery on secretory granules or synaptobrevin-aequorin chimera was not positioned in the right structure along with other membrane components in the granules to respond to IP3.
In addition to the IP3Rs in secretory granules of chromaffin cells, pancreatic β and acinar cells, secretory granules of airway goblet cells (14) and mast cells (15, 20) are also known to contain the IP3Rs. Utilizing the thin optical sectioning method and measuring the changes in Ca2+ concentrations in both secretory granules and the surrounding cytoplasm simultaneously, Verdugo and colleagues (14) showed that the IP3-induced Ca2+ releases from secretory granules dictate the Ca2+ concentrations in the neighboring cytoplasm of goblet cells. Again taking advantage of the mast cell granules of mutant beige mice, which can reach up to 2–3 μm in diameter, both the IP3-induced Ca2+ release from secretory granules and the IP3-dependent intracellular Ca2+ oscillator role of secretory granules were unequivocally demonstrated (15, 20), thus establishing the presence of the IP3Rs in secretory granules of airway goblet and mast cells.
The amounts of IP3R isoforms that exist in each cellular organelle vary depending on the type of cells (40), yet the majority of cellular IP3Rs in chromaffin cells is localized in secretory granules (41). Assuming the relative concentration of each IP3R isoform in the nucleus as 1 and taking the cell volume of each organelle of bovine adrenal chromaffin cells (28) into consideration, the relative concentration and the total amount of each IP3R isoform in each organelle can be estimated (Table 1). As summarized in Table 1, secretory granules contain ∼58–69% of total cellular IP3Rs, with relative concentrations of 1.8–3.7, while the ER contains ∼15–20% of total cellular IP3Rs, with relative concentrations of 0.7–1.5 (41). This indicates that the total amounts and relative concentrations of the IP3Rs in secretory granules are higher than those of the ER by 3- to 4-fold and 2- to 3-fold, respectively (41, 42).
CHROMOGRANINS IN SECRETORY GRANULES
The major proteins of secretory granules are chromogranins A and B (43,44,45,46); thus, they are called marker proteins of secretory granules (47). Bovine secretory granules contain >1.8 mM CGA and >0.2 mM CGB (43, 44), while the ER contains >0.14 mM CGA and >0.12 mM CGB (Table 2). A third major member of the granin family proteins, secretogranin II, is also found in secretory granules, although its concentration is significantly lower than that of chromogranins (43, 44) (Table 2). Chromogranins A and B are multifunctional proteins (see below) that are present primarily in secretory cells though they are also found in cells such as cardiomyocytes (48) that are not traditionally considered secretory cells.
Granulogenesis
Chromogranins A and B participate directly in secretory granule biogenesis of secretory cells (49,50,51). Studies that had been done with both neuroendocrine and nonneuroendocrine cells have demonstrated the granulogenic roles of chromogranins. Expression of the granins in nonneuroendocrine cells, such as NIH3T3 or COS cells that do not normally contain secretory granules, induce formation of secretory granules in these cells, whereas suppression of granin expression in neuroendocrine cells substantially reduce the number of secretory granules formed in these cells (49,50,51). Nonetheless, it was initially reported that only CGA, not CGB, is capable of functioning as the granulogenic factor (51), which proved not to be the case in later studies (49, 50). The granulogenic ability of CGB is, in fact, shown to be ∼60% greater than that of CGA in certain conditions (50), and this appeared to reflect the markedly stronger ability of CGB than CGA to interact with other proteins in secretory granules (52, 53). Analogous to the granulogenic roles of chromogranins A and B, secretogranins have also been shown to participate in secretory granule formation (49, 54).
Ca2+ storage, and catecholamine and ATP binding
The high Ca2+ concentrations in secretory granules are due to the presence of large amounts of acidic protein chromogranins that have a high-capacity, low-affinity Ca2+-binding property (Table 2). Chromogranin A binds 32–55 mol of Ca2+/mol, with dissociation constants (Kd) of 2.7–4 mM (35), whereas chromogranin B binds 50–93 mol of Ca2+/mol, with Kd of 1.5–3.1 mM (34, 36). As a result, most (>99.9%) of the intragranular calcium stays bound to chromogranins, and only a very small amount of Ca2+ stays in free state, ranging from ∼28 to 100 μM (13, 14, 19, 20, 24, 25).
In light of the presence of >2 mM chromogranins in secretory granules, the high-capacity Ca2+ binding property of chromogranins would be sufficient to store ∼40 mM Ca2+ inside secretory granules. Moreover, the low affinity of chromogranins for Ca2+ would facilitate ready exchange of bound and free Ca2+ within secretory granules, so that intragranular calcium could actively participate in the Ca2+ flow through the IP3R/Ca2+ channels of secretory granules. Analogous to the high-capacity, low-affinity Ca2+-binding property of chromogranins A and B, secretogranin II also binds 30–61 mol of Ca2+/mol with Kd of 2.2–3.0 mM (Table 2), implying active roles of SgII in the storage of Ca2+ in secretory granules.
Chromogranins are also known to bind catecholamines (55) and ATP (56) that exist in secretory granules at ∼600 mM and ∼150 mM, respectively (22, 57). Chromogranin A is reported to bind 32 mol of norepinephrine/mol, with a Kd of 2.1 mM (55). However, considering the intragranular concentrations of chromogranins and catecholamines, the binding of 32 mol of norepinephrine/mol of CGA is estimated to account for only ∼5.3% of norepinephrine present inside secretory granules (55). This result suggests that chromogranins are partly responsible for the accumulation of catecholamines in secretory granules. In line with this finding, the intragranular content, as well as the amount of catecholamines released in chromaffin cells from CGA-knockout mice, were shown to be reduced ∼30% (58), suggesting the role of chromogranin A in the storage of catecholamines. Chromogranin A also interacts with ATP through the adenine base of the nucleotide (56), yet its binding stoichiometry or affinity is not known. In view of the coexistence of catecholamines, ATP, the granins and divalent cations in high concentrations and the tendency of these molecules to interact with each other, it is likely that the intragranular residents form complexes, as has been proposed previously (59).
IP3R/Ca2+ channel modulation
The IP3Rs bind chromogranins A and B directly at the intragranular pH 5.5 (60), and the bound chromogranins activate the IP3R/Ca2+ channels to an open-ready state (61), increasing the open probability of the channels 8- to 16-fold and the mean open time 9- to 42-fold (62, 63). In contrast to CGA that fails to interact with the IP3Rs at a near physiological pH 7.5, CGB still binds the IP3Rs even at pH 7.5 (60) and increases the open probability of the Ca2+ channels 8-fold and the mean open time 24-fold (62, 63), suggesting critical roles chromogranins play in both storage and flux of the intragranular calcium through the IP3R/Ca2+ channels (64). Further, the IP3Rs undergo conformational changes on IP3 binding (65), which will, in turn, lead to conformational changes of coupled chromogranins (60, 64). Given that the Ca2+-binding affinity and capacity of chromogranins differ depending on the conformation (34,35,36), the IP3-induced conformational changes of chromogranins will lead to changes in the Ca2+-binding affinity and capacity of the coupled chromogranins, thereby enabling rapid exchanges of bound and free Ca2+ inside the granules. This rapid exchange of bound and free Ca2+ may serve as a basis for secretory granules to be able to repeatedly release and sequester Ca2+ on multiple IP3 challenges.
Moreover, at pH 5.5, chromogranin A not only self-associates to form a CGA homotetramer (66, 67) but also interacts with chromogranin B to form a CGA2CGB2 heterotetramer (68). Given that the IP3Rs form a homotetrameric or heterotetrameric complex to function as a Ca2+ channel (40), formation of a homotetrameric or heterotetrameric chromogranin complex in secretory granules implies coupling of tetrameric chromogranins with and activation to an open-ready state of the tetrameric IP3R/Ca2+ channels (64). Nonetheless, this will not be the case in the ER, where the pH is maintained at ∼7.4 (69,70,71); chromogranin A fails to bind the IP3Rs at a near physiological pH (60), although it interacts with chromogranin B to form a CGA-CGB heterodimer at pH 7.5 (68). In contrast, chromogranin B binds the IP3Rs and activates the IP3R/Ca2+ channels to an open-ready state even at a near-physiological pH 7.5 (63), thereby suggesting additional Ca2+ channel modulatory roles of CGB in organelles other than secretory granules. Accordingly, chromogranin B has indeed been shown to play essential roles in the activity of IP3R/Ca2+ channels of the ER in neuronally differentiated PC12 cells (72) and neurons (73), thus controlling the IP3-dependent Ca2+ release from the ER and the intracellular Ca2+ concentrations. It is also shown in cardiomyocytes that CGB controls the IP3-mediated Ca2+ release in these cells, which is closely linked to ventricular cardiac hypertrophy (48).
As a source of bioactive peptides
In addition, chromogranins further exert important physiological functions even after leaving secretory granules, i.e., after exocytosis. Although intragranular chromogranins A and B remain bound to the membranes of secretory granule at the intragranular pH 5.5 through their binding to the integral membrane protein IP3Rs (60), this state will change during exocytosis when they are exposed to the pH of the extracellular fluid; chromogranin A dissociates completely from the IP3Rs, while chromogranin B dissociates partially at that pH (60). Therefore, large amounts of chromogranins will dissociate from the granules during exocytosis and be released into the bloodstream. The secreted chromogranins serve as the source of many bioactive peptides, including catestatin, vasostatin, pancreastatin, and secretoneurin, that circulate in the bloodstream (74,75,76,77,78,79,80,81,82). Among a variety of functions that are attributed to these peptides, catestatin and vasostatin are known to control the release of catecholamines (74, 75) and blood vessel dilation (76, 77), respectively, whereas pancreastatin and secretoneurin are known to reduce glucose metabolism (80) and to potentiate angiogenesis (78, 79), respectively.
SECRETORY GRANULES ARE RESPONSIBLE FOR THE MAJORITY OF IP3-INDUCED Ca2+ RELEASES IN THE CYTOPLASM
In view of the presence of the majority of cellular calcium, IP3R/Ca2+ channels, and chromogranins in secretory granules, which is necessary for an organelle to function as the major IP3-sensitive intracellular Ca2+ store, it would be natural for secretory granules to play the most significant role in the IP3-dependent intracellular Ca2+ control mechanism. Whether secretory granules play the major role in the cytoplasmic IP3-dependent Ca2+ control mechanism could be discerned by comparing the magnitudes of the IP3-mediated Ca2+ releases between the cells that contain different numbers of secretory granules (27, 83).
Although both chromogranins A and B play granulogenic roles (49,50,51), CGB can induce ∼60% more secretory granule formation than CGA (50). Hence, by taking advantage of the different granulogenic effects of CGA and CGB, the number of secretory granules produced in nonneuroendocrine cells can be controlled. Moreover, the granulogenic role of chromogranins can also be used in controlling the number of secretory granules formed in neuroendocrine cells; the number of secretory granules formed in neuroendocrine cells decreases by suppressing the intrinsic chromogranin expression in these cells by treating the cells with siRNA-chromogranin. Yet the extent of reduction in the number of secretory granules formed differs markedly depending on whether CGA or CGB expression is suppressed (50). Reflecting the stronger granulogenic role of CGB, suppression of CGB expression is far more effective in reducing the secretory granule formation that the CGB suppression in PC12 cells decreases the number of secretory granules formed ∼90% more than can be achieved with the CGA suppression (50). Thus utilizing this property of chromogranins, the number of secretory granules formed in both neuroendocrine and nonneuroendocrine cells can be controlled, thereby making it possible to examine the specific role of secretory granules in the IP3-dependent Ca2+ releases in the cell.
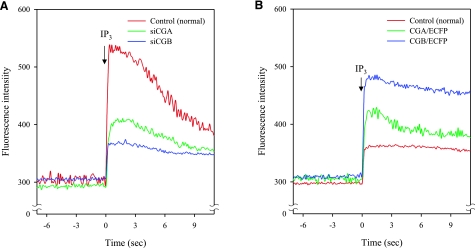
When the formation of secretory granules in PC12 cells that normally contain chromogranins and intrinsic secretory granules was suppressed by suppressing the intrinsic CGA expression, the magnitude of IP3-induced Ca2+ release in the cytoplasm was reduced to less than a half that of control cells (Fig. 2A), but when the CGB expression was suppressed, the magnitude of IP3-induced Ca2+ release was reduced to approximately one-quarter that of control cells (Fig. 2A). Given that only the number of secretory granules formed is reduced in the cytoplasm, these results indicate that secretory granules are responsible for the majority of IP3-mediated cellular Ca2+ release in the cytoplasm of neuroendocrine PC12 cells (27). On the other hand, induction of secretory granule formation by expressing chromogranins in nonneuroendocrine NIH3T3 cells that normally do not contain any secretory granules markedly increased the magnitude of IP3-induced Ca2+ release of these cells (27, 83). When secretory granule formation was induced by expressing CGA in NIH3T3 cells, the magnitude of IP3-induced Ca2+ release in the cytoplasm of NIH3T3 cells increased ∼2.7-fold over the control (Fig. 2B). But when the number of newly formed secretory granules was further increased by expressing CGB in NIH3T3 cells, the magnitude of IP3-induced Ca2+ release in the cytoplasm of NIH3T3 cells increased ∼3.7-fold over the control (Fig. 2B), further underscoring the major role of secretory granules in IP3-dependent Ca2+ mobilization. Consistent with the results shown for the PC12 cells, these results demonstrate that secretory granules are the major IP3-sensitive intracellular Ca2+ store, accounting for >70% of the cytoplasmic Ca2+ released by IP3.
Figure 2.
Microinjection of IP3 and Ca2+ mobilization in the cytoplasm of PC12 and NIH3T3 cells. A) IP3-induced Ca2+ releases in the cytoplasm of control (normal), siCGA-treated (siCGA), and siCGB-treated (siCGB) PC12 cells were measured after microinjection of 10 nM IP3 into the cytoplasm of the cell. B) IP3-induced Ca2+ releases in the cytoplasm of control (normal), CGA-transfected (CGA/ECFP), and CGB-transfected (CGB/ECFP) NIH3T3 cells were measured after microinjection of 10 nM IP3 into the cytoplasm of the cell. Figure is modified from Figs. 2 and 7 of ref. 83.
Although it is possible that the effect shown by CGB expression is a combined effect of secretory granules and the CGB-containing ER, the effect exerted by the CGB-containing ER will be very minimal compared to that shown by secretory granules because of the large difference in the total calcium stored and the IP3R concentrations that exist in each organelle, and the significantly lower IP3 sensitivity of the IP3R/Ca2+ channels of the ER. In addition, the weaker binding of CGB to the IP3Rs (84,85,86,87) and the lower Ca2+ binding capacity of CGB (36) at the pH of the ER compared to those shown at the intragranular pH could also contribute to the minimal effect. That the effect shown by chromogranin expression or suppression is very closely tied to the number of secretory granules formed or reduced further strengthens this assessment.
SECRETORY GRANULE IP3R/Ca2+ CHANNELS ARE MORE SENSITIVE TO IP3 THAN THE ER IP3R/Ca2+ CHANNELS
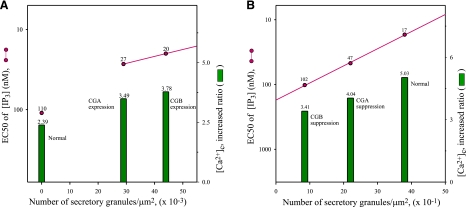
By examining the IP3 sensitivity of the IP3R/Ca2+ channels in the cytoplasm of cells in which the formation of secretory granules is either induced or suppressed without disturbing the ER, it is possible to estimate the respective IP3 sensitivity of the IP3R/Ca2+ channels of the ER and secretory granules. The IP3 sensitivity of the IP3R/Ca2+ channels, which is normally expressed in EC50 values of IP3 concentration, will predict which IP3R/Ca2+ channels open first in response to IP3. In this regard, the EC50 value of IP3 concentration of the cytoplasmic IP3R/Ca2+ channels in nonneuroendocrine NIH3T3 cells that do not have secretory granules is shown to be 110 nM (88), but in the NIH3T3 cells that contain newly formed secretory granules as a result of chromogranin A expression, the EC50 value changes to 27 nM (Fig. 3A), increasing the IP3 sensitivity of the cytoplasmic IP3R/Ca2+ channels ∼4-fold (88). Further, expression of CGB in the same cells changes the EC50 of cytoplasmic IP3R/Ca2+ channels to 20 nM (Fig. 3A), increasing the IP3 sensitivity of the cytoplasmic IP3R/Ca2+ channels of the NIH3T3 cells ∼6-fold (88). Here, the effect of CGB expression is significantly greater than CGA. In view of the fact that induction of secretory granule formation by CGB expression increased the IP3 sensitivity of the NIH3T3 cells ∼6-fold compared to the same NIH3T3 cells without secretory granules, it is apparent that the IP3R/Ca2+ channels of secretory granules are at least 6-fold more sensitive to IP3 than those of the ER.
Figure 3.
Interrelation between number of secretory granules and EC50 of cytoplasmic IP3Rs and magnitude of IP3-induced Ca2+ release. Interrelation between number of secretory granules per square micrometer of cell area on one hand and EC50 value of IP3 concentrations for cytoplasmic IP3Rs and increased ratio of IP3-induced Ca2+ release in cytoplasm (in fold increase over resting state) after stimulation with IP3-producing ATP on the other is shown for both nonneuroendocrine NIH3T3 and neuroendocrine PC12 cells. A) Control (normal), CGA-expressed, and CGB-expressed NIH3T3 cells. B) Control (normal), CGA-suppressed, and CGB-suppressed PC12 cells. Numbers of secretory granules per square micrometer of cell area are from Table 1 of ref. 50, EC50 values are from Table 1 of ref. 88, and the IP3-induced [Ca2+]c increased ratio (fold increases) are from Figs. 1 and 6 of ref. 83.
In contrast, when the formation of intrinsic secretory granules in PC12 cells is suppressed by inhibiting the CGA expression, the EC50 of IP3 concentration of the cytoplasmic IP3R/Ca2+ channels changes from a normal value of 17 to 47 nM (Fig. 3B), decreasing the IP3 sensitivity of the PC12 cells that contain the reduced number of secretory granules ∼3-fold (88). However, inhibition of the CGB expression in the same cells changes the EC50 from 17 to 102 nM (Fig. 3B), decreasing the IP3 sensitivity of the cytoplasmic IP3R/Ca2+ channels of the PC12 cells that contain the reduced number of secretory granules ∼6-fold (88). Again, the effect that results from CGB suppression is markedly greater than that of CGA suppression (50). These results indicate that secretory granules can release Ca2+ in response to less than one-sixth the IP3 concentration that is required to release Ca2+ from the ER. Considering that a mere reduction of the number of secretory granules, not a complete absence, made the cytoplasmic IP3R/Ca2+ channels of PC12 cells ∼6-fold less sensitive in the presence of the identical ER, it is clear that the IP3R/Ca2+ channels of secretory granules are at least 6- to 7-fold more sensitive to IP3 than those of the ER. This, in turn, predicts that secretory granules of secretory cells will release Ca2+ in response to IP3, whose concentrations are too low for the ER of both secretory and nonsecretory cells to even sense the presence of IP3, not to mention of releasing Ca2+.
DIRECT CORRELATION BETWEEN THE NUMBER OF SECRETORY GRANULES AND THE EC50 OF CYTOPLASMIC IP3R/Ca2+ CHANNELS AND THE INCREASED RATIO OF IP3-INDUCED Ca2+ RELEASE
As discussed above, the IP3-mediated Ca2+ release properties of the cells undergo profound changes depending on the number of secretory granules in the cells, underscoring the major role of secretory granules in the IP3-dependent intracellular Ca2+ control. Not only is the IP3 sensitivity of the cytoplasmic IP3R/Ca2+ channels of secretory cells 6- to 7-fold higher than those of nonsecretory cells (Fig. 3A, B) but also the magnitude of IP3-induced Ca2+ release of secretory cells is severalfold higher than that of nonsecretory cells (27, 83). This result indicates that the cells that contain secretory granules release far greater amounts of Ca2+ in response to IP3 than the cells without granules or with reduced number of secretory granules (27, 50). Therefore, it appears that the IP3 sensitivity of the IP3R/Ca2+ channels and the magnitude of IP3-induced Ca2+ release of secretory cells reflect the number of secretory granules present in the cells, as these parameters either increase or decrease, depending on the induction or suppression of secretory granule formation in the cells. In line with the critical roles of secretory granules in the control of IP3-dependent cytoplasmic Ca2+ concentrations, the IP3sensitivity of the cytoplasmic IP3R/Ca2+ channels of bovine chromaffin cells that contain far more secretory granules than PC12 cells was ∼4-fold higher than that of PC12 cells, and the magnitude of IP3-induced Ca2+ release in chromaffin cells was also significantly higher than that in PC12 cells (unpublished results).
The relevant relationship becomes apparent by expressing 3 parameters in the published literature, i.e., the number of secretory granules/unit area, the EC50 values of IP3 concentration of the cytoplasmic IP3Rs, and the increased ratio of IP3-induced cytoplasmic Ca2+ release ([Ca2+]c) over the resting state, in one figure (Fig. 3). Figure 3A shows the relationship obtained from both the normal and chromogranin-transfected NIH3T3 cells: when the secretory granule formation was induced from 0 to 0.0287 and 0.0445 granules/μm2 of cell area (50), the increased ratio in IP3-induced Ca2+ release over the resting state increased from 2.39 to 3.49 and 3.78, respectively (83). Moreover, in accordance with the increase in the number of secretory granules formed, the IP3 sensitivity also increased (Fig. 3A). Taken together, in the NIH3T3 cells that contain the induced secretory granules, the IP3 sensitivity of the cytoplasmic IP3Rs and the increased ratio of the IP3-induced cytoplasmic Ca2+ release show a linear relationship with the increase in the number of secretory granules in the cell (Fig. 3A), although in normal NIH3T3 cells without secretory granules, the linear relationship appears to deviate slightly. The slight deviation is not unexpected, considering that the CGA-induced secretory granules contain only CGA, while the CGB-induced secretory granules contain only CGB in the newly formed granules, features that could easily influence both the Ca2+ storage and the IP3-dependent Ca2+ release properties of secretory granules.
Further, the results obtained from PC12 cells that contain intrinsic secretory granules reveal a clearer relationship (Fig. 3B): when the number of secretory granules was reduced from 3.8 to 2.2 and 0.86 granules/μm2 of cell area (50), the increased ratio in IP3-induced Ca2+ release over the resting state decreased from 5.03 to 4.04 and 3.41, respectively (83). Again, in accordance with the number of secretory granules reduced, the IP3 sensitivity also decreased (Fig. 3B) (88). Remarkably, both the IP3 sensitivity of the cytoplasmic IP3Rs and the increased ratio of the IP3-induced cytoplasmic Ca2+ release of the PC12 cells demonstrate a direct correlation with the number of secretory granules present in the cell (Fig. 3B). From these results, it is apparent that both the IP3 sensitivity of the cytoplasmic IP3R/Ca2+ channels and the increased ratio of IP3-induced Ca2+ release in the cell that contain secretory granules, whether intrinsic or induced, are directly related to the number of secretory granules in the cell; the more the secretory granules in the cell, the higher the sensitivity of the IP3R/Ca2+ channels and the increased ratio of IP3-induced Ca2+ release of the cell (27, 50).
PHYSIOLOGICAL SIGNIFICANCE
It is of crucial physiological importance that secretory cells that secrete the secretory granule contents in response to a sudden increase of intracellular Ca2+ concentration, which can be initiated by IP3-mediated Ca2+ release, are equipped with molecular machinery that responds to IP3 with a significantly higher sensitivity than nonsecretory cells (88). In this regard, the high sensitivity of IP3R/Ca2+ channels and the high magnitude of IP3-induced Ca2+ release in the cytoplasm of neuroendocrine cells are in line with the physiological needs of secretory cells to quickly produce the secretory cargos and release at the site and precisely at the moment they are needed.
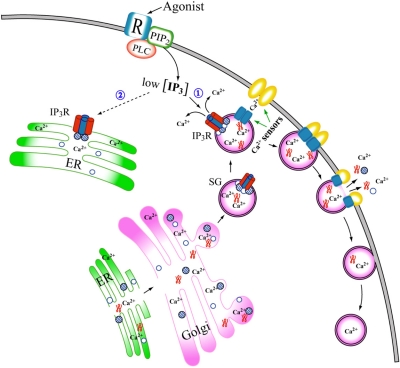
The roles of secretory granules and their key components chromogranins play in the physiology of neuroendocrine cells are shown in a schematic drawing (Fig. 4). As shown in Fig. 4, both chromogranins A and B are present in the ER and secretory granules, but given that only chromogranin B binds to the IP3Rs at the pH of the ER (60), it would be chromogranin B, not chromogranin A, that couple to the IP3R/Ca2+ channels in the ER and modulate the Ca2+ channel activity. However, in secretory granules, both CGA homotetramers and CGA2CGB2 heterotetramers will be present (66,67,68), but CGA2CGB2 heterotetramers will preferentially couple to the tetrameric IP3R/Ca2+ channels due to the markedly stronger affinity of CGB for the IP3Rs (84,85,86,87). The affinity of CGB for the IP3R at pH 5.5 is several orders of magnitude stronger than that of CGA at the same pH and substantially stronger than that of CGB for the IP3R at pH 7.5 (84,85,86,87).
Figure 4.
Secretory granule formation and IP3-induced Ca2+ mobilization in cytoplasm of neuroendocrine cells. Process of secretory granule biogenesis is shown from the ER via the Golgi. Tetrameric IP3R/Ca2+ channels are shown as red and blue columns, and chromogranins A and B are shown as open and hatched circles, respectively. Note that only chromogranin B can couple to tetrameric IP3Rs in the ER, whereas both chromogranins A and B, which form a CGA2CGB2 heterotetrameric complex, couple to tetrameric IP3Rs in secretory granules. Other contents of secretory granules are shown as twisted red structures. Generation of IP3 at the plasma membrane as a result of agonist stimulation is the first step in IP3-dependent Ca2+ release from intracellular Ca2+ stores in cytoplasm. Depending on amount of IP3 produced and sensitivity of IP3R/Ca2+ channels to IP3, each intracellular Ca2+ store will respond differently to IP3. The significantly higher sensitivity of secretory granule IP3R/Ca2+ channels would enable secretory granules to release Ca2+ in response to low IP3 concentrations (1) that are not high enough for the ER IP3R/Ca2+ channels to sense the presence of IP3 (2), thereby initiating the exocytotic processes. Ca2+ sensor molecules on the membranes of secretory granules and the plasma membranes, which participate in the Ca2+-dependent fusion process, are shown in blue and yellow, respectively. Exocytosed secretory granule contents will enter the bloodstream as mixtures of both intact and cleaved molecules.
As a result of the coupling of CGB to the IP3R/Ca2+ channels at pH 5.5, both the channel open probability and the mean open time of the IP3R/Ca2+ channels are ∼2-fold higher than those that result from the same coupling at pH 7.5 (63). The interaction of CGA or CGB with the IP3Rs changes the conformation of the IP3Rs (61, 63), and the stronger interaction of CGB with the IP3Rs at pH 5.5 than at pH 7.5 changes the conformation of the IP3Rs to a greater extent (63). It appears that chromogranin coupling to the IP3Rs changes the conformation of the IP3R/Ca2+ channels to a more ordered structure that can respond better to IP3 (61,62,63), apparently explaining the higher sensitivity of secretory granule IP3R/Ca2+ channels than the ER Ca2+ channels. From a mechanistic point, it is highly likely that the tightly coupled CGB maintains the IP3R/Ca2+ channels in a high-tension state that is open-ready in case IP3 arrives in the cytoplasmic side of the channels. This high-tension, open-ready state of the IP3R/Ca2+ channels may not exist in the ER and is likely to differentiate the IP3R/Ca2+ channels of secretory granules from those of the ER. Hence, the initial Ca2+ released from secretory granules would be able to serve as the trigger signal for the ensuing additional Ca2+ releases, influx of extracellular Ca2+, and the secretory processes.
Fitting with the presence of the majority of cellular calcium and IP3R/Ca2+ channels in secretory granules, Ca2+ release from secretory granules was shown to be essential for insulin secretion (89, 90) and sufficient to initiate exocytosis in chromaffin, pancreatic acinar, and β cells (13, 16, 17, 91,92,93,94,95). For example, application of acetylcholine, which is known to produce intracellular IP3, to the basal surface of pancreatic acinar cells in the absence of external Ca2+ invoked the initial IP3-induced Ca2+ release in the granule-rich apical zone and led to exocytosis (94). Although the initial IP3-induced Ca2+ release in the granular zone of pancreatic acinar cells (13, 94, 95) has not always been ascribed to the secretory granules that are exclusively clustered in the granular zone, it appears plain that the IP3-induced Ca2+ release from secretory granules in the absence of external Ca2+ is sufficient to cause exocytosis. In a more recent study, application of IP3-producing ATP to pancreatic β cells in the absence of external Ca2+ caused release of Ca2+ from secretory granules of these cells, and this IP3-induced Ca2+ release was shown to initiate exocytosis in pancreatic β cells (17). Therefore, it seems natural to think that the IP3-induced Ca2+ release from secretory granules in the absence of external Ca2+ will not only be sufficient but also be the first signal to initiate exocytosis of secretory cells.
Yet, in exocrine pancreatic acinar cells, the results have not been consistent. Despite the early report of IP3-induced Ca2+ release from secretory granules of exocrine pancreatic acinar cells (13), secretory granules of the same cells that had been purified by Percoll gradient failed to release Ca2+ in response to IP3 in another study (96), although it is possible that residual Percoll, which is detrimental to IP3-mediated Ca2+ release, disenabled the release process. In the same year, in a study using immunofluorescence microscopy with very low magnification, the IP3Rs were reported not to localize in zymogen granules of the apical (granular) zone of acinar cells (97). Nonetheless, in a more recent and thorough study, it was further shown that secretory granules of exocrine pancreatic acinar cells release Ca2+ in response not only to IP3 but also to cyclic ADP-ribose or nicotinic acid adenine dinucleotide phosphate (18).
Because one of the primary functions of secretory cells is to store and secrete molecules essential for cellular communication, and the trigger signal for secretion is Ca2+ (7,8,9, 33), the presence of most of cellular Ca2+ and IP3R/Ca2+ channels along with the highest concentrations of chromogranins in secretory granules highlights the extraordinary efficiency of secretory cells to deal with their complex physiological needs. Moreover, secretory granules also contain Ca2+ sensor proteins, such as synaptotagmin and syntaxin (98,99,100), which are known to participate in the Ca2+-initiated membrane fusion and exocytotic processes (101,102,103). These membrane proteins will initiate the fusion process and pave the way for secretion of intragranular contents to the extracellular space and on to the bloodstream (Fig. 4). Once released, both intact chromogranins and chromogranin-derived peptides circulate in the bloodstream and serve as the source of a number of bioactive peptides (74,75,76,77,78,79,80). The concentration of chromogranin-derived proteins in the blood is a very good indicator of secretory activity of secretory cells in the body; hence, precise measurements of these proteins in the blood samples have been widely used in assessing a number of clinical symptoms that include tumors of secretory cell origin and blood pressure (104,105,106,107,108).
PERSPECTIVES
Traditionally, secretory granules have simply been regarded as organelles that store and transport secretory contents, and the unique and extraordinary roles exerted by secretory granules in the physiology of neuroendocrine cells have mostly not been duly appreciated. Secretory granules and the associated molecules are not stationary entities in the cytoplasm; rather, they are constantly on the move and are in the center of dynamic key cellular activities of secretory cells. Secretory granules and the associated molecules actively participate in the IP3-dependent Ca2+ signaling in neuroendocrine cells, and the roles described above point out the hitherto underappreciated roles of these. The functions uncovered thus far strongly hint at a number of additional roles of major importance by this fascinating organelle and its companions.
The fate of secretory granules and the associated molecules is intimately tied together from the beginning of their birth in the cell to their final destination at the secretion site as transport vehicles and secreted products. Only at the secretion site will the secretory cargos separate from the granules and venture into a new environment (Fig. 4). Even at this point, the amounts and types of secreted molecules will differ from one secretion site to another and from one granule to another, depending on the nature of the interaction between the granule membranes and the granule contents. Therefore, the importance of future studies on secretory granules and the associated molecules goes far beyond the neuroendocrine cells and could extend to other types of secretory cells, covering a variety of cellular activities, such as secretory granule biogenesis, protein trafficking, control of intracellular Ca2+ concentration, membrane fusion, exocytosis, production of biopeptides, and control of hormone secretion.
On a new front, recent studies indicate the presence and operation of an IP3-dependent Ca2+ store inside the nucleus (109, 110), which is independent of Ca2+ stores of cytoplasmic or nuclear envelope origin, and chromogranin B is shown to be a key component in the system (27, 88). Chromogranin B is present in the IP3-sensitive nucleoplasmic Ca2+ store vesicles (109, 110), and the nuclear chromogranin B is presumed to play key roles in the IP3-dependent Ca2+ control mechanism through its specific interaction with the IP3R/Ca2+ channels. Moreover, secretogranin II is also shown recently to be present in the nucleus, localizing in the IP3-sensitive nucleoplasmic Ca2+ store vesicles along with CGB and the IP3Rs (36). This implies that secretogranin II may also assume an important role in the control of nuclear calcium in conjunction with other Ca2+ storage proteins and the Ca2+ channels. Hence, further studies on the function of the granins will be pivotal in obtaining a complete picture of the IP3-dependent Ca2+ signaling mechanisms of neuroendocrine cells encompassing both the cytoplasm and the nucleus.
Acknowledgments
The author thanks past collaborators and former and present members of the author’s laboratory who have contributed to the development of the topic. Research covered in this review was funded by the U.S. National Institutes of Health in its early stage and by the Creative Research Initiative program of the Republic of Korea in its later stage.
References
- Rizzuto R, Bernardi P, Pozzan T. Mitochondria as all-round players of the calcium game. J Physiol. 2000;529:37–47. doi: 10.1111/j.1469-7793.2000.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordas G, Renken C, Varnai P, Walter L, Weaver D, Buttle K F, Balla T, Mannella C A, Hajnoczky G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederkehr A, Wollheim C B. Impact of mitochondrial calcium on the coupling of metabolism to insulin secretion in the pancreatic beta-cell. Cell Calcium. 2008;44:64–76. doi: 10.1016/j.ceca.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Duchen M R, Verkhratsky A, Muallem S. Mitochondria and calcium in health and disease. Cell Calcium. 2008;44:1–5. doi: 10.1016/j.ceca.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Churchill G C, Okada Y, Thomas J M, Genazzani A A, Patel S, Galione A. NAADP mobilizes Ca2+ from reserve granules, lysosome-related organelles, in sea urchin eggs. Cell. 2002;111:703–708. doi: 10.1016/s0092-8674(02)01082-6. [DOI] [PubMed] [Google Scholar]
- Menteyne A, Burdakov A, Charpentier G, Petersen O H, Cancela J M. Generation of specific Ca2+ signals from Ca2+ stores and endocytosis by differential coupling to messengers. Curr Biol. 2006;16:1931–1937. doi: 10.1016/j.cub.2006.07.070. [DOI] [PubMed] [Google Scholar]
- Petersen O H, Tepikin A V. Polarized calcium signaling in exocrine gland cells. Annu Rev Physiol. 2008;70:273–299. doi: 10.1146/annurev.physiol.70.113006.100618. [DOI] [PubMed] [Google Scholar]
- Berridge M J, Bootman M D, Roderick H L. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- Clapham D E. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Meldolesi J, Pozzan T. The endoplasmic reticulum Ca2+ store: a view from the lumen. Trends Biochem Sci. 1998;23:10–14. doi: 10.1016/s0968-0004(97)01143-2. [DOI] [PubMed] [Google Scholar]
- Spat A, Bradford P G, McKinney J S, Rubin R P, Putney J W., Jr A saturable receptor for 32P-inositol-1,4,5-triphosphate in hepatocytes and neutrophils. Nature. 1986;319:514–516. doi: 10.1038/319514a0. [DOI] [PubMed] [Google Scholar]
- Yoo S H, Albanesi J P. Inositol 1,4,5-trisphosphate-triggered Ca2+ release from bovine adrenal medullary secretory vesicles. J Biol Chem. 1990;265:13446–13448. [PubMed] [Google Scholar]
- Gerasimenko O V, Gerasimenko J V, Belan P V, Petersen O H. Inositol trisphosphate and cyclic ADP-ribose-mediated release of Ca2+ from single isolated pancreatic zymogen granules. Cell. 1996;84:473–480. doi: 10.1016/s0092-8674(00)81292-1. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Chin W C, Verdugo P. Role of Ca2+/K+ ion exchange in intracellular storage and release of Ca2+ Nature. 1998;395:908–912. doi: 10.1038/27686. [DOI] [PubMed] [Google Scholar]
- Quesada I, Chin W C, Steed J, Campos-Bedolla P, Verdugo P. Mouse mast cell secretory granules can function as intracellular ionic oscillators. Biophys J. 2001;80:2133–2139. doi: 10.1016/S0006-3495(01)76186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava M, Atwater I, Glasman M, Leighton X, Goping G, Caohuy H, Miller G, Pichel J, Westphal H, Mears D, Rojas E, Pollard H B. Defects in inositol 1,4,5-trisphosphate receptor expression, Ca2+ signaling, and insulin secretion in the anx7+/− knockout mouse. Proc Natl Acad Sci U S A. 1999;96:13783–13788. doi: 10.1073/pnas.96.24.13783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Zhang M, Zhou W, Wu Z, Ding J, Chen L, Xu T. Extracellular ATP stimulates exocytosis via localized Ca release from acidic stores in rat pancreatic beta cells. Traffic. 2006;7:429–439. doi: 10.1111/j.1600-0854.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- Gerasimenko J V, Sherwood M, Tepikin A V, Petersen O H, Gerasimenko O V. NAADP, cADPR and IP3 all release Ca2+ from the endoplasmic reticulum and an acidic store in the secretory granule area. J Cell Sci. 2006;119:226–238. doi: 10.1242/jcs.02721. [DOI] [PubMed] [Google Scholar]
- Santodomingo J, Vay L, Camacho M, Hernandez-SanMiguel E, Fonteriz R I, Lobaton C D, Montero M, Moreno A, Alvarez J. Calcium dynamics in bovine adrenal medulla chromaffin cell secretory granules. Eur J Neurosci. 2008;28:1265–1274. doi: 10.1111/j.1460-9568.2008.06440.x. [DOI] [PubMed] [Google Scholar]
- Quesada I, Chin W C, Verdugo P. ATP-independent luminal oscillations and release of Ca2+ and H+ from mast cell secretory granules: implications for signal transduction. Biophys J. 2003;85:963–970. doi: 10.1016/S0006-3495(03)74535-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigh J R, Parris R, Phillips J H. Free concentrations of sodium, potassium, and calcium in chromaffin granules. Biochem J. 1989;259:485–491. doi: 10.1042/bj2590485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H, Westhead E. The molecular organization of adrenal chromaffin granules. Neuroscience. 1980;5:1803–1823. doi: 10.1016/0306-4522(80)90031-7. [DOI] [PubMed] [Google Scholar]
- Hutton J C. The insulin secretory granule. Diabetologia. 1989;32:271–281. doi: 10.1007/BF00265542. [DOI] [PubMed] [Google Scholar]
- Bulenda D, Gratzl M. Matrix free Ca2+ in isolated chromaffin vesicles. Biochemistry. 1985;24:7760–7765. doi: 10.1021/bi00347a039. [DOI] [PubMed] [Google Scholar]
- Mitchell K J, Pinton P, Varadi A, Tacchetti C, Ainscow E K, Pozzan T, Rizzuto R, Rutter G A. Dense core secretory vesicles revealed as a dynamic Ca2+ store in neuroendocrine cells with a vesicle-associated membrane protein aequorin chimaera. J Cell Biol. 2001;155:41–51. doi: 10.1083/jcb.200103145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahapatra N R, Mahata M, Hazra P P, McDonough P M, O'Connor D T, Mahata S K. A dynamic pool of calcium in catecholamine storage vesicles. Exploration in living cells by a novel vesicle-targeted chromogranin A-aequorin chimeric photoprotein. J Biol Chem. 2004;279:51107–51121. doi: 10.1074/jbc.M408742200. [DOI] [PubMed] [Google Scholar]
- Huh Y H, Jeon S H, Yoo J A, Park S Y, Yoo S H. Effects of chromogranin expression on inositol 1,4,5-trisphosphate-induced intracellular Ca2+ mobilization. Biochemistry. 2005;44:6122–6132. doi: 10.1021/bi048070w. [DOI] [PubMed] [Google Scholar]
- Huh Y H, Bahk S J, Ghee J Y, Yoo S H. Subcellular distribution of chromogranins A and B in bovine adrenal chromaffin cells. FEBS Lett. 2005;579:5145–5151. doi: 10.1016/j.febslet.2005.08.025. [DOI] [PubMed] [Google Scholar]
- Plattner H, Artalejo A R, Neher E. Ultrastructural organization of bovine chromaffin cell cortex-analysis by cryofixation and morphometry of aspects pertinent to exocytosis. J Cell Biol. 1997;139:1709–1717. doi: 10.1083/jcb.139.7.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne R D. Control of exocytosis in adrenal chromaffin cells. Biochim Biophys Acta. 1991;1071:174–202. doi: 10.1016/0304-4157(91)90024-q. [DOI] [PubMed] [Google Scholar]
- Nordmann J J. Combined stereological and biochemical analysis of storage and release of catecholamines in the adrenal medulla of the rat. J Neurochem. 1984;42:434–437. doi: 10.1111/j.1471-4159.1984.tb02696.x. [DOI] [PubMed] [Google Scholar]
- Vitale M L, Seward E P, Trifaro J M. Chromaffin cell cortical actin network dynamics control the size of the release-ready vesicle pool and the initial rate of exocytosis. Neuron. 1995;14:353–363. doi: 10.1016/0896-6273(95)90291-0. [DOI] [PubMed] [Google Scholar]
- Garcia A G, Garcia-De-Diego A M, Gandia L, Borges R, Garcia-Sancho J. Calcium signaling and exocytosis in adrenal chromaffin cells. Physiol Rev. 2006;86:1093–1131. doi: 10.1152/physrev.00039.2005. [DOI] [PubMed] [Google Scholar]
- Yoo S H, Oh Y S, Kang M K, Huh Y H, So S H, Park H S, Park H Y. Localization of three types of the inositol 1,4,5-trisphosphate receptor/Ca2+ channel in the secretory granules and coupling with the Ca2+ storage proteins chromogranins A and B. J Biol Chem. 2001;276:45806–45812. doi: 10.1074/jbc.M107532200. [DOI] [PubMed] [Google Scholar]
- Yoo S H, Albanesi J P. High capacity, low affinity Ca2+ binding of chromogranin A. Relationship between the pH-induced conformational change and Ca2+ binding property. J Biol Chem. 1991;266:7740–7745. [PubMed] [Google Scholar]
- Yoo S H, Chu S Y, Kim K D, Huh Y H. Presence of secretogranin II and high-capacity, low-affinity Ca2+ storage role in nucleoplasmic Ca2+ store vesicles. Biochemistry. 2007;46:14663–14671. doi: 10.1021/bi701339m. [DOI] [PubMed] [Google Scholar]
- Yoo S H. pH-dependent interaction of chromogranin A with integral membrane proteins of secretory vesicle including 260-kDa protein reactive to inositol 1,4,5-triphosphate receptor antibody. J Biol Chem. 1994;269:12001–12006. [PubMed] [Google Scholar]
- Blondel O, Moody M M, Depaoli A M, Sharp A H, Ross C A, Swift H, Bell G I. Localization of inositol trisphosphate receptor subtype 3 to insulin and somatostatin secretory granules and regulation of expression in islets and insulinoma cells. Proc Natl Acad Sci U S A. 1994;91:7777–7781. doi: 10.1073/pnas.91.16.7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravazzola M, Halban P A, Orci L. Inositol 1,4,5-trisphosphate receptor subtype 3 in pancreatic islet cell secretory granules revisited. Proc Natl Acad Sci U S A. 1996;93:2745–2748. doi: 10.1073/pnas.93.7.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foskett J K, White C, Cheung K H, Mak D O. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev. 2007;87:593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh Y H, Yoo J A, Bahk S J, Yoo S H. Distribution profile of inositol 1,4,5-trisphosphate receptor isoforms in adrenal chromaffin cells. FEBS Lett. 2005;579:2597–2603. doi: 10.1016/j.febslet.2005.03.076. [DOI] [PubMed] [Google Scholar]
- Huh Y H, Yoo S H. Presence of the inositol 1,4,5-triphosphate receptor isoforms in the nucleoplasm. FEBS Lett. 2003;555:411–418. doi: 10.1016/s0014-5793(03)01273-0. [DOI] [PubMed] [Google Scholar]
- Helle K B. The chromogranins. Historical perspectives. Adv Exp Med Biol. 2000;482:3–20. doi: 10.1007/0-306-46837-9_1. [DOI] [PubMed] [Google Scholar]
- Winkler H, Fischer-Colbrie R. The chromogranins A and B: the first 25 years and future perspectives. Neuroscience. 1992;49:497–528. doi: 10.1016/0306-4522(92)90222-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taupenot L, Harper K L, O'Connor D T. The chromogranin-secretogranin family. N Engl J Med. 2003;348:1134–1149. doi: 10.1056/NEJMra021405. [DOI] [PubMed] [Google Scholar]
- Montero-Hadjadje M, Vaingankar S, Elias S, Tostivint H, Mahata S K, Anouar Y. Chromogranins A and B and secretogranin II: evolutionary and functional aspects. Acta Physiol (Oxf) 2008;192:309–324. doi: 10.1111/j.1748-1716.2007.01806.x. [DOI] [PubMed] [Google Scholar]
- Huttner W B, Gerdes H H, Rosa P. The granin (chromogranin/secretogranin) family. Trends Biochem Sci. 1991;16:27–30. doi: 10.1016/0968-0004(91)90012-k. [DOI] [PubMed] [Google Scholar]
- Heidrich F M, Zhang K, Estrada M, Huang Y, Giordano F J, Ehrlich B E. Chromogranin B regulates calcium signaling, nuclear factor-κB activity, and brain natriuretic peptide production in cardiomyocytes. Circ Res. 2008;102:1230–1238. doi: 10.1161/CIRCRESAHA.107.166033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuret N, Stettler H, Renold A, Rutishauser J, Spiess M. Expression of regulated secretory proteins is sufficient to generate granule-like structures in constitutively secreting cells. J Biol Chem. 2004;279:20242–20249. doi: 10.1074/jbc.M310613200. [DOI] [PubMed] [Google Scholar]
- Huh Y H, Jeon S H, Yoo S H. Chromogranin B-induced secretory granule biogenesis: comparison with the similar role of chromogranin A. J Biol Chem. 2003;278:40581–40589. doi: 10.1074/jbc.M304942200. [DOI] [PubMed] [Google Scholar]
- Kim T, Tao-Cheng J H, Eiden L E, Loh Y P. Chromogranin A, an “on/off” switch controlling dense-core secretory granule biogenesis. Cell. 2001;106:499–509. doi: 10.1016/s0092-8674(01)00459-7. [DOI] [PubMed] [Google Scholar]
- Yoo S H. Purification and pH-dependent secretory vesicle membrane binding of chromogranin B. Biochemistry. 1995;34:8680–8686. doi: 10.1021/bi00027a017. [DOI] [PubMed] [Google Scholar]
- Yoo S H. pH-dependent association of chromogranin A with secretory vesicle membrane and a putative membrane binding region of chromogranin A. Biochemistry. 1993;32:8213–8219. doi: 10.1021/bi00083a023. [DOI] [PubMed] [Google Scholar]
- Prasad P, Yanagihara A A, Small-Howard A L, Turner H, Stokes A J. Secretogranin III directs secretory vesicle biogenesis in mast cells in a manner dependent upon interaction with chromogranin A. J Immunol. 2008;181:5024–5034. doi: 10.4049/jimmunol.181.7.5024. [DOI] [PubMed] [Google Scholar]
- Videen J S, Mezger M S, Chang Y M, O'Connor D T. Calcium and catecholamine interactions with adrenal chromogranins. Comparison of driving forces in binding and aggregation. J Biol Chem. 1992;267:3066–3073. [PubMed] [Google Scholar]
- Yoo S H, Albanesi J P, Jameson D M. Fluorescence studies of nucleotide interactions with bovine adrenal chromogranin A. Biochim Biophys Acta. 1990;1040:66–70. doi: 10.1016/0167-4838(90)90146-7. [DOI] [PubMed] [Google Scholar]
- Phillips J H. Dynamic aspects of chromaffin granule structure. Neuroscience. 1982;7:1595–1609. doi: 10.1016/0306-4522(82)90017-3. [DOI] [PubMed] [Google Scholar]
- Montesinos M S, Machado J D, Camacho M, Diaz J, Morales Y G, de la Varez R D, Carmona E, Castaneyra A, Viveros O H, O'Connor D T, Mahata S K, Borges R. The crucial role of chromogranins in storage and exocytosis revealed using chromaffin cells from chromogranin A null mouse. J Neurosci. 2008;28:3350–3358. doi: 10.1523/JNEUROSCI.5292-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels A J, Williams R J, Wright P E. The character of the stored molecules in chromaffin granules of the adrenal medulla: a nuclear magnetic resonance study. Neuroscience. 1978;3:573–585. doi: 10.1016/0306-4522(78)90022-2. [DOI] [PubMed] [Google Scholar]
- Yoo S H, So S H, Kweon H S, Lee J S, Kang M K, Jeon C J. Coupling of the inositol 1,4,5-trisphosphate receptor and chromogranins A and B in secretory granules. J Biol Chem. 2000;275:12553–12559. doi: 10.1074/jbc.275.17.12553. [DOI] [PubMed] [Google Scholar]
- Yoo S H, Jeon C J. Inositol 1,4,5-trisphosphate receptor/Ca2+ channel modulatory role of chromogranin A, a Ca2+ storage protein of secretory granules. J Biol Chem. 2000;275:15067–15073. doi: 10.1074/jbc.M909391199. [DOI] [PubMed] [Google Scholar]
- Thrower E C, Park H Y, So S H, Yoo S H, Ehrlich B E. Activation of the inositol 1,4,5-trisphosphate receptor by the calcium storage protein chromogranin A. J Biol Chem. 2002;277:15801–15806. doi: 10.1074/jbc.M110139200. [DOI] [PubMed] [Google Scholar]
- Thrower E C, Choe C U, So S H, Jeon S H, Ehrlich B E, Yoo S H. A functional interaction between chromogranin B and the inositol 1,4,5-trisphosphate receptor/Ca2+ channel. J Biol Chem. 2003;278:49699–49706. doi: 10.1074/jbc.M309307200. [DOI] [PubMed] [Google Scholar]
- Yoo S H. Coupling of the IP3 receptor/Ca2+ channel with Ca2+ storage proteins chromogranins A and B in secretory granules. Trends Neurosci. 2000;23:424–428. doi: 10.1016/s0166-2236(00)01621-0. [DOI] [PubMed] [Google Scholar]
- Mignery G A, Sudhof T C. The ligand binding site and transduction mechanism in the inositol-1,4,5-triphosphate receptor. EMBO J. 1990;9:3893–3898. doi: 10.1002/j.1460-2075.1990.tb07609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S H, Lewis M S. Effects of pH and Ca2+ on monomer-dimer and monomer-tetramer equilibria of chromogranin A. J Biol Chem. 1992;267:11236–11241. [PubMed] [Google Scholar]
- Thiele C, Huttner W B. The disulfide-bonded loop of chromogranins, which is essential for sorting to secretory granules, mediates homodimerization. J Biol Chem. 1998;273:1223–1231. doi: 10.1074/jbc.273.2.1223. [DOI] [PubMed] [Google Scholar]
- Yoo S H, Lewis M S. Effects of pH and Ca2+ on heterodimer and heterotetramer formation by chromogranin A and chromogranin B. J Biol Chem. 1996;271:17041–17046. doi: 10.1074/jbc.271.29.17041. [DOI] [PubMed] [Google Scholar]
- Anderson R G, Pathak R K. Vesicles and cisternae in the trans Golgi apparatus of human fibroblasts are acidic compartments. Cell. 1985;40:635–643. doi: 10.1016/0092-8674(85)90212-0. [DOI] [PubMed] [Google Scholar]
- Kim J H, Johannes L, Goud B, Antony C, Lingwood C A, Daneman R, Grinstein S. Noninvasive measurement of the pH of the endoplasmic reticulum at rest and during calcium release. Proc Natl Acad Sci U S A. 1998;95:2997–3002. doi: 10.1073/pnas.95.6.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L, Ravazzola M, Amherdt M, Madsen O, Perrelet A, Vassalli J D, Anderson R G. Conversion of proinsulin to insulin occurs coordinately with acidification of maturing secretory vesicles. J Cell Biol. 1986;103:2273–2281. doi: 10.1083/jcb.103.6.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe C U, Harrison K D, Grant W, Ehrlich B E. Functional coupling of chromogranin with the inositol 1,4,5-trisphosphate receptor shapes calcium signaling. J Biol Chem. 2004;279:35551–35556. doi: 10.1074/jbc.M311261200. [DOI] [PubMed] [Google Scholar]
- Jacob S N, Choe C U, Uhlen P, DeGray B, Yeckel M F, Ehrlich B E. Signaling microdomains regulate inositol 1,4,5-trisphosphate-mediated intracellular calcium transients in cultured neurons. J Neurosci. 2005;25:2853–2864. doi: 10.1523/JNEUROSCI.4313-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahata S K, O'Connor D T, Mahata M, Yoo S H, Taupenot L, Wu H, Gill B M, Parmer R J. Novel autocrine feedback control of catecholamine release. A discrete chromogranin a fragment is a noncompetitive nicotinic cholinergic antagonist. J Clin Invest. 1997;100:1623–1633. doi: 10.1172/JCI119686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahata S K, Mahata M, Wen G, Wong W B, Mahapatra N R, Hamilton B A, O'Connor D T. The catecholamine release-inhibitory “catestatin” fragment of chromogranin a: naturally occurring human variants with different potencies for multiple chromaffin cell nicotinic cholinergic responses. Mol Pharmacol. 2004;66:1180–1191. doi: 10.1124/mol.104.002139. [DOI] [PubMed] [Google Scholar]
- Aardal S, Helle K B. The vasoinhibitory activity of bovine chromogranin A fragment (vasostatin) and its independence of extracellular calcium in isolated segments of human blood vessels. Regul Pept. 1992;41:9–18. doi: 10.1016/0167-0115(92)90509-s. [DOI] [PubMed] [Google Scholar]
- Tota B, Mazza R, Angelone T, Nullans G, Metz-Boutigue M H, Aunis D, Helle K B. Peptides from the N-terminal domain of chromogranin A (vasostatins) exert negative inotropic effects in the isolated frog heart. Regul Pept. 2003;114:123–130. doi: 10.1016/s0167-0115(03)00112-5. [DOI] [PubMed] [Google Scholar]
- Egger M, Schgoer W, Beer A G, Jeschke J, Leierer J, Theurl M, Frauscher S, Tepper O M, Niederwanger A, Ritsch A, Kearney M, Wanschitz J, Gurtner G C, Fischer-Colbrie R, Weiss G, Piza-Katzer H, Losordo D W, Patsch J R, Schratzberger P, Kirchmair R. Hypoxia up-regulates the angiogenic cytokine secretoneurin via an HIF-1α- and basic FGF-dependent pathway in muscle cells. FASEB J. 2007;21:2906–2917. doi: 10.1096/fj.06-7440com. [DOI] [PubMed] [Google Scholar]
- Fischer-Colbrie R, Kirchmair R, Kahler C M, Wiedermann C J, Saria A. Secretoneurin: a new player in angiogenesis and chemotaxis linking nerves, blood vessels and the immune system. Curr Protein Pept Sci. 2005;6:373–385. doi: 10.2174/1389203054546334. [DOI] [PubMed] [Google Scholar]
- Zhang K, Rao F, Wen G, Salem R M, Vaingankar S, Mahata M, Mahapatra N R, Lillie E O, Cadman P E, Friese R S, Hamilton B A, Hook V Y, Mahata S K, Taupenot L, O'Connor D T. Catecholamine storage vesicles and the metabolic syndrome: The role of the chromogranin A fragment pancreastatin. Diabetes Obes Metab. 2006;8:621–633. doi: 10.1111/j.1463-1326.2006.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchmair R, Hogue-Angeletti R, Gutierrez J, Fischer-Colbrie R, Winkler H. Secretoneurin–a neuropeptide generated in brain, adrenal medulla and other endocrine tissues by proteolytic processing of secretogranin II (chromogranin C) Neuroscience. 1993;53:359–365. doi: 10.1016/0306-4522(93)90200-y. [DOI] [PubMed] [Google Scholar]
- Tatemoto K, Efendic S, Mutt V, Makk G, Feistner G J, Barchas J D. Pancreastatin, a novel pancreatic peptide that inhibits insulin secretion. Nature. 1986;324:476–478. doi: 10.1038/324476a0. [DOI] [PubMed] [Google Scholar]
- Huh Y H, Chu S Y, Park S Y, Huh S K, Yoo S H. Role of nuclear chromogranin B in inositol 1,4,5-trisphosphate-mediated nuclear Ca2+ mobilization. Biochemistry. 2006;45:1212–1226. doi: 10.1021/bi051594r. [DOI] [PubMed] [Google Scholar]
- Yoo S H, Lewis M S. Interaction of chromogranin B and the near N-terminal region of chromogranin B with an intraluminal loop peptide of the inositol 1,4, 5-trisphosphate receptor. J Biol Chem. 2000;275:30293–30300. doi: 10.1074/jbc.M001204200. [DOI] [PubMed] [Google Scholar]
- Yoo S H, Lewis M S. pH-dependent interaction of an intraluminal loop of inositol 1,4,5-trisphosphate receptor with chromogranin A. FEBS Lett. 1994;341:28–32. doi: 10.1016/0014-5793(94)80234-3. [DOI] [PubMed] [Google Scholar]
- Yoo S H, Lewis M S. Interaction between an intraluminal loop peptide of the inositol 1,4,5-trisphosphate receptor and the near N-terminal peptide of chromogranin A. FEBS Lett. 1998;427:55–58. doi: 10.1016/s0014-5793(98)00393-7. [DOI] [PubMed] [Google Scholar]
- Yoo S H, Lewis M S. Thermodynamic study of the pH-dependent interaction of chromogranin A with an intraluminal loop peptide of the inositol 1,4,5-trisphosphate receptor. Biochemistry. 1995;34:632–638. doi: 10.1021/bi00002a030. [DOI] [PubMed] [Google Scholar]
- Huh Y H, Kim K D, Yoo S H. Comparison of and chromogranin effect on inositol 1,4,5-trisphosphate sensitivity of cytoplasmic and nucleoplasmic inositol 1,4,5-trisphosphate receptor/Ca2+ channels. Biochemistry. 2007;46:14032–14043. doi: 10.1021/bi701364p. [DOI] [PubMed] [Google Scholar]
- Mitchell K J, Lai F A, Rutter G A. Ryanodine receptor type I and nicotinic acid adenine dinucleotide phosphate receptors mediate Ca2+ release from insulin-containing vesicles in living pancreatic beta-cells (MIN6) J Biol Chem. 2003;278:11057–11064. doi: 10.1074/jbc.M210257200. [DOI] [PubMed] [Google Scholar]
- Scheenen W J, Wollheim C B, Pozzan T, Fasolato C. Ca2+ depletion from granules inhibits exocytosis. A study with insulin-secreting cells. J Biol Chem. 1998;273:19002–19008. doi: 10.1074/jbc.273.30.19002. [DOI] [PubMed] [Google Scholar]
- Mundorf M L, Troyer K P, Hochstetler S E, Near J A, Wightman R M. Vesicular Ca2+ participates in the catalysis of exocytosis. J Biol Chem. 2000;275:9136–9142. doi: 10.1074/jbc.275.13.9136. [DOI] [PubMed] [Google Scholar]
- Camacho M, Machado J D, Alvarez J, Borges R. Intravesicular calcium release mediates the motion and exocytosis of secretory organelles: a study with adrenal chromaffin cells. J Biol Chem. 2008;283:22383–22389. doi: 10.1074/jbc.M800552200. [DOI] [PubMed] [Google Scholar]
- Haynes C L, Buhler L A, Wightman R M. Vesicular Ca2+-induced secretion promoted by intracellular pH-gradient disruption. Biophys Chem. 2006;123:20–24. doi: 10.1016/j.bpc.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama Y, Inooka G, Li Y X, Miyashita Y, Kasai H. Agonist-induced localized Ca2+ spikes directly triggering exocytotic secretion in exocrine pancreas. EMBO J. 1993;12:3017–3022. doi: 10.1002/j.1460-2075.1993.tb05970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama Y, Petersen O H. Delay in granular fusion evoked by repetitive cytosolic Ca2+ spikes in mouse pancreatic acinar cells. Cell Calcium. 1994;16:419–430. doi: 10.1016/0143-4160(94)90035-3. [DOI] [PubMed] [Google Scholar]
- Yule D I, Ernst S A, Ohnishi H, Wojcikiewicz R J. Evidence that zymogen granules are not a physiologically relevant calcium pool. Defining the distribution of inositol 1,4,5-trisphosphate receptors in pancreatic acinar cells. J Biol Chem. 1997;272:9093–9098. doi: 10.1074/jbc.272.14.9093. [DOI] [PubMed] [Google Scholar]
- Lee M G, Xu X, Zeng W, Diaz J, Wojcikiewicz R J, Kuo T H, Wuytack F, Racymaekers L, Muallem S. Polarized expression of Ca2+ channels in pancreatic and salivary gland cells. Correlation with initiation and propagation of [Ca2+]i waves. J Biol Chem. 1997;272:15765–15770. doi: 10.1074/jbc.272.25.15765. [DOI] [PubMed] [Google Scholar]
- Yoo S H, You S H, Huh Y H. Presence of syntaxin 1A in secretory granules of chromaffin cells and interaction with chromogranins A and B. FEBS Lett. 2005;579:222–228. doi: 10.1016/j.febslet.2004.11.079. [DOI] [PubMed] [Google Scholar]
- Saegusa C, Fukuda M, Mikoshiba K. Synaptotagmin V is targeted to dense-core vesicles that undergo calcium-dependent exocytosis in PC12 cells. J Biol Chem. 2002;277:24499–24505. doi: 10.1074/jbc.M202767200. [DOI] [PubMed] [Google Scholar]
- Tagaya M, Toyonaga S, Takahashi M, Yamamoto A, Fujiwara T, Akagawa K, Moriyama Y, Mizushima S. Syntaxin 1 (HPC-1) is associated with chromaffin granules. J Biol Chem. 1995;270:15930–15933. doi: 10.1074/jbc.270.27.15930. [DOI] [PubMed] [Google Scholar]
- Gerber S H, Rah J C, Min S W, Liu X, de W H, Dulubova I, Meyer A C, Rizo J, Arancillo M, Hammer R E, Verhage M, Rosenmund C, Sudhof T C. Conformational switch of syntaxin-1 controls synaptic vesicle fusion. Science. 2008;321:1507–1510. doi: 10.1126/science.1163174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraudo C G, Eng W S, Melia T J, Rothman J E. A clamping mechanism involved in SNARE-dependent exocytosis. Science. 2006;313:676–680. doi: 10.1126/science.1129450. [DOI] [PubMed] [Google Scholar]
- Martens S, Kozlov M M, McMahon H T. How synaptotagmin promotes membrane fusion. Science. 2007;316:1205–1208. doi: 10.1126/science.1142614. [DOI] [PubMed] [Google Scholar]
- Gregorc V, Spreafico A, Floriani I, Colombo B, Ludovini V, Pistola L, Bellezza G, Vigano M G, Villa E, Corti A. Prognostic value of circulating chromogranin A and soluble tumor necrosis factor receptors in advanced nonsmall cell lung cancer. Cancer. 2007;110:845–853. doi: 10.1002/cncr.22856. [DOI] [PubMed] [Google Scholar]
- O'Connor D T, Zhu G, Rao F, Taupenot L, Fung M M, Das M, Mahata S K, Mahata M, Wang L, Zhang K, Greenwood T A, Shih P A, Cockburn M G, Ziegler M G, Stridsberg M, Martin N G, Whitfield J B. Heritability and genome-wide linkage in US and Australian twins identify novel genomic regions controlling chromogranin a: implications for secretion and blood pressure. Circulation. 2008;118:247–257. doi: 10.1161/CIRCULATIONAHA.107.709105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stridsberg M, Eriksson B, Janson E T. Measurements of secretogranins II, III, V and proconvertases 1/3 and 2 in plasma from patients with neuroendocrine tumours. Regul Pept. 2008;148:95–98. doi: 10.1016/j.regpep.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Metz D C, Jensen R T. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology. 2008;135:1469–1492. doi: 10.1053/j.gastro.2008.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrott-Fischer A, Bitsche M, Humpel C, Walcher C, Maier H, Jellinger K, Rabl W, Glueckert R, Marksteiner J. Chromogranin peptides in amyotrophic lateral sclerosis. Regul Pept. 2009;152:13–21. doi: 10.1016/j.regpep.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Huh Y H, Huh S K, Chu S Y, Kweon H S, Yoo S H. Presence of a putative vesicular inositol 1,4,5-trisphosphate-sensitive nucleoplasmic Ca2+ store. Biochemistry. 2006;45:1362–1373. doi: 10.1021/bi051837f. [DOI] [PubMed] [Google Scholar]
- Yoo S H, Nam S W, Huh S K, Park S Y, Huh Y H. Presence of a nucleoplasmic complex composed of the inositol 1,4,5-trisphosphate receptor/Ca2+ channel, chromogranin B, and phospholipids. Biochemistry. 2005;44:9246–9254. doi: 10.1021/bi047427t. [DOI] [PubMed] [Google Scholar]
- Gerasimenko O V, Gerasimenko J V, Tepikin A V, Petersen O H. ATP-dependent accumulation and inositol trisphosphate- or cyclic ADP-ribose-mediated release of Ca2+ from the nuclear envelope. Cell. 1995;80:439–444. doi: 10.1016/0092-8674(95)90494-8. [DOI] [PubMed] [Google Scholar]