Abstract
Although the role of the classic retinoic acid (RA)-induced genomic pathway in cancer cell differentiation is well recognized, the underlying mechanisms remain to be dissected. Retinoic acid receptor α (RARα) is a transcription factor activated by RA, and its serine 77 (RARαS77) is the main residue phosphorylated by the cyclin-dependent kinase (CDK)-activating kinase (CAK) complex. We report here that in both human myeloid leukemia and mouse embryonic teratocarcinoma stem cells, either RA-suppressed CAK phosphorylation of RARα or mutation of RARαS77 to alanine (RARαS77A) coordinates CAK-dependent G1 arrest with cancer cell differentiation by transactivating RA-target genes. Both hypophosphorylated RARα and RARαS77A reduce binding to retinoic acid-responsive elements (RARE) in the promoters of RA-target genes while stimulating gene transcription. The enhanced transactivation and reduced RARα-chromatin interaction are accompanied by RARα dissociation from the transcriptional repressor N-CoR and are association with the coactivator NCoA-3. Such effects of decreased CAK phosphorylation of RARαS77 on mediating RA-dependent transcriptional control of cancer cell differentiation are examined correspondingly in both RA-resistant myeloid leukemia and embryonic teratocarcinoma stem RARα−/− cells. These studies demonstrate, for the first time, that RA couples G1 arrest to transcriptional control of cancer cell differentiation by suppressing CAK phosphorylation of RARα to release transcriptional repression.—Wang, A., Alimova, I. N., Luo, P. Jong, A., Triche, T. J., Wu, L. Loss of CAK phosphorylation of RARα mediates transcriptional control of retinoid-induced cancer cell differentiation.
Keywords: decreased CAK activity by RA, cell cycle G1 exit, gene transcription, transcriptional repression
The regulatory effects of RA on cell differentiation are elicited through both classic and nonclassic pathways. Two types of receptors, the retinoic acid receptors (RARs) and the retinoid X receptors (RXRs), mediate RA signaling at the genomic level (so-called classic genomic pathway) by binding to RARE in the promoters of target genes to activate gene transcription (1,2,3). Besides this well-known classic mode of genomic action, RA can also exert rapid nongenomic effects independently of RAR/RXR-mediated gene transcription (4), leading to regulation of cell differentiation or apoptosis (5, 6). Although the role of the classic RA-induced genomic pathway in mediating cancer cell differentiation (7,8,9,10) is well recognized (11, 12), the underlying mechanisms of RAR/RXR-mediated transcriptional control remain largely unknown.
Comprehensive studies have shown that the transcriptional activation function (AF) domains of the retinoid receptors, AF-1 and AF-2, possess a conserved structure that allows synergistic transactivation of RA-target genes (12, 13). RARαS77 in the AF-1 domain is the main residue phosphorylated by CAK (14). Expression of S74A/S77A mutants in mouse F9 embryonic teratocarcinoma stem cells (F9 cells) induces primitive and parietal endoderm-like cell differentiation (7, 8), demonstrating that inhibition of RARαAF-1 phosphorylation can mediate cancer cell differentiation. Indeed, either RA-suppressed CAK phosphorylation of RARα or expression of the RARαS77A mutant inhibits cancer cell proliferation (15), mediates cancer cell differentiation (10, 16, 17), and enhances normal granulocytic development (18). However, the mechanisms by which decreased CAK phosphorylation of RARαS77 mediates RA-dependent transactivation are unknown.
The CAK complex consists of CDK7 (19), cyclin H (20), and assembly factor ménage à trois 1 (MAT1) (21). The changes in MAT1 levels determine CAK’s substrate-specificity (21, 22), and RA-induced ubiquitination-degradation of MAT1 leads to suppression of CAK phosphorylation of RARα (10, 17, 23). CAK exists in cells as free CAK or as part of the general transcription factor IIH (TFIIH) complex (24). CAK mediates cell cycle progression by phosphorylation-activation of CDKs (24, 25), whereas loss of CAK phosphorylation of retinoblastoma tumor suppressor protein (pRb) leads to cell cycle G1 exit (26). Furthermore, TFIIH-containing CAK mediates transcription initiation (27,28,29) by phosphorylating the largest subunit of RNA polymerase II (RNA Pol II) (27, 29). These studies suggest that CAK cross-regulates cell cycle and gene transcription, although the underlying mechanisms remain to be dissected.
Recent studies have demonstrated that RARα interacts with CAK (10, 14, 23) and binds to TFIIH (14). Either free CAK or TFIIH-containing CAK phosphorylates RARα (10, 14, 17) on RARαS77 (14). Since CAK regulates G1 exit (10, 16, 26) whereas RARα mediates transcription of RA-target genes (12), we reasoned that RA-induced changes in CAK phosphorylation of RARα may be involved in coordinating cell cycle exit with gene transcription to induce cancer cell differentiation. We tested this hypothesis by using well-established RA-RARα signaling models, including the human myeloid leukemia cell lines HL60 and HL60R (9, 10, 30), as well as F9 and F9 RARα−/− cells (7, 8, 31).
MATERIALS AND METHODS
Cell culture
F9 cells (American Type Culture Collection, Manassas, VA, USA) and F9 RARα−/− cells (kindly provided by Dr. Lorraine J. Gudas, Cornell University, Ithaca, NY, USA) were grown as monolayers on gelatinized surfaces in Dulbecco’s modified Eagle medium (Invitrogen, Carlsbad, CA, USA), supplemented with 10% fetal bovine serum (FBS) as described previously (32). HL60 and HL60R cells were cultured in RPMI 1640 medium plus 10% FBS as described previously (10). All-trans retinoic acid (ATRA; Sigma, St. Louis, MO, USA) was dissolved in ethanol. ATRA (1 μM) or vehicle (0.1% ethanol) was used in the experiments.
Lentiviral plasmid construction, virion production, titration, and transduction
Human wild-type RARα and phosphorylation-defective RARαS77A (S77A) cDNAs were, respectively, cloned into pCCL-c-MNDU3c-X2-PGK-EGFP lentiviral vectors as described previously (18). By using our established techniques (18), virions were prepared 48 h after transfection of 293FT cells. Virion titers were determined as described previously (18). Cells were transduced with lentiviral pCCL-S77A, pCCL-RARα, and pCCL as described previously (18) at a multiplicity of infection (MOI) of 10 using modified manufacturer’s methods (Invitrogen).
Luciferase assays
The βRARE-TK-Luc (firefly) plasmid harboring the luciferase reporter gene with the RARE of the RARβ promoter was kindly provided by Dr. J. Song (University of California, Los Angeles, CA, USA; ref. 33). pRL-TK-Luc (Renilla) plasmid (for normalization of luciferase activity) and a dual-luciferase reporter assay kit were purchased from Promega (Madison, WI, USA). Triplicate wells of cells were transiently cotransfected with βRARE-TK-Luc and pRL-TK-Luc vectors at 100:1 ratio using Lipofectamine Plus (Life Technologies, Gaithersburg, MD, USA). The luciferase activity was measured with a luminometer (Tecan Genios; Tecan, San Jose, CA, USA). The firefly luciferase activities were normalized based on Renilla activity and protein concentration (33).
Proliferation and cell cycle analyses
Cell replication and cell cycle analysis were performed as described previously (10, 18).
Plasmid construction and induction of glutathione S-transferase fusion proteins
The cDNAs of RARα, S77A, and RXRα were cloned into the pGEX-2T plasmid (Amersham Pharmacia, Piscataway, NJ, USA) as described previously (17). Induction and purification of glutathione S-transferase (GST)-RARα, S77A, and RXRα fusion proteins were performed as described previously (17, 26).
Electrophoretic mobility shift assay (EMSA)
The cell nuclear extracts were prepared with NE-PER Nuclear and Cytoplasmic Extraction reagents (Pierce Biotechnology, Rockford, IL, USA). EMSA was performed with the LightShift Chemiluminescent EMSA kit (Pierce Biotechnology) as described by the manufacturer. Biotin end-labeled oligonucleotides (Operon, Huntsville, AL, USA) containing DNA binding motifs were RARE sequences of human RARβ2 promoter (hβRARE) 5′-GGGTAGGGTTCACCGAAAGTTCACTCG-3′ (34, 35), and human E-box sequences 5′-TTCCCAGCACAGCCCCATGTGAGAGCTCCCTGGCTC-3′ (accession no. NW001838432) containing a CANNTG core (36) for nonspecific control. For supershift experiments, rabbit polyclonal anti-RARα or RXRα antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were incubated with GST-RARα, GST-S77A, GST-RXRα, or nuclear protein extracts before addition of the labeled oligonucleotides.
Chromatin immunoprecipitation (ChIP)
ChIP assays were performed by following the manufacturer’s recommendations (Upstate Biotechnology, Lake Placid, NY, USA). Each 1 × 107 cells were cross-linked with formaldehyde, and chromatins were sonicated to an average length of 200–500 bp. Following precleaning, 1% of each chromatin supernatant was used as input loading control, whereas the remaining chromatin supernatants were incubated with rabbit polyclonal RARα antibodies (Santa Cruz Biotechnology) or preimmune IgG (PI) for immunoprecipitation (IP). Protein-chromatin complexes were eluted and followed by reverse-cross-link to recover free DNA. Purified DNA was analyzed by polymerase chain reaction (PCR) analysis with a HotStarTaq Master Mix Kit (Qiagen, Hilden, Germany) using specific primers (Supplemental Table 1).
IP, Western blotting (WB), and immunofluorescence analysis
IP and WB were performed as described previously (10, 26). Rabbit polyclonal anti-phosphorylation serine antibodies were from Invitrogen. Antibodies against the C-terminal RARα (C-20) were from Santa Cruz Biotechnology. Immunofluorescence analysis was performed as described previously (18). Mouse monoclonal antibodies against stage-specific embryonic antigen-1 (SSEA-1) and all other antibodies were from Santa Cruz Biotechnology.
Kinase assay
In vitro kinase assays were performed in the presence of [γ32P]ATP or unlabeled ATP as described (17, 26).
Reverse transcription-PCR (RT-PCR) analysis
RT-PCR was performed as described previously (18). RNA quantity was normalized by determining the transcripts of human β-actin or mouse 36B4 that is not responsive to retinoid stimuli (8).
Statistical analysis
Whenever appropriate, we subjected our results to statistical testing, using ANOVA or Student’s unpaired 2-tailed t test.
Supplemental data
The online Supplemental Data section includes Supplemental Fig. 1 and Supplemental Table 1.
RESULTS
RA links cell cycle G1 arrest to differentiation in cancer cells by suppressing CAK phosphorylation of RARα
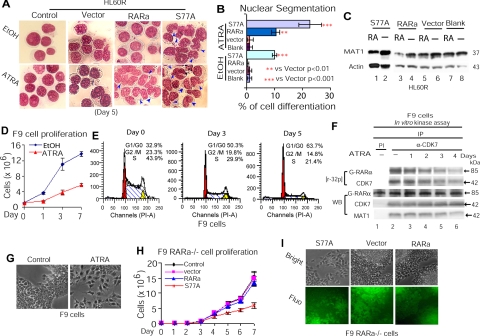
In contrast to RA-sensitive HL60 cells (10, 17, 23), HL60R cells harboring a C-terminal truncation-mutation RARα (37) are RA-resistant (10, 37). To test that decreased CAK phosphorylation of RARα due to RA-induced MAT1 degradation indeed induces HL60 cell differentiation (10, 17, 23), we transduced HL60R cells with lentivirus-RARαS77A (Supplemental Fig. 1A, B), a phosphorylation-defective mutant of RARα (14) blocking CAK phosphorylation of RARαS77 (Supplemental Fig. 1C). The results showed that HL60R-S77A cells underwent granulocytic differentiation in the absence of RA, whereas such morphological differentiation was induced in HL60R-RARα cells only in the presence of RA (Fig. 1A, B). In contrast to MAT1 degradation in HL60R cells expressing S77A or RARα in the presence of RA (Fig. 1C, lanes 1, 3 vs. 2, 4–8), S77A expression in HL60R cells did not induce MAT1 degradation (Fig. 1C, lanes 1 vs. 2). These results support the notion that MAT1 degradation is an upstream event induced by RA to suppress CAK phosphorylation of RARα (10, 23) and that loss of CAK phosphorylation of RARαS77 mediates leukemia cell differentiation.
Figure 1.
ATRA links cell cycle G1 arrest to differentiation in cancer cells by suppressing CAK phosphorylation of RARα. A) Granulocytic differentiation of HL60R cells expressing S77A or RARα. EtOH, ethanol; S77A; RARαS77A. B) Quantification of granulocytic differentiation of HL60R cells in panel A. C) WB analysis of MAT1 levels in transduced HL60R cells. D) Proliferation analysis of F9 cells by cell count. E) F9 cell cycle profile in the presence of ATRA. F) In vitro kinase assay of CAK activity in phosphorylation of GST-RARα. CAK complexes were immunoprecipitated from F9 cells treated with or without ATRA. Autoradiography blot was reimmunoblotted with different antibodies as indicated. G) F9 cell differentiation into primitive endoderm-like cells after ATRA treatment for 4 d. H) Proliferation analysis of transduced F9 RARα−/− cells by cell count. I) Primitive endodermal differentiation of F9 RARα−/− cells expressing S77A.
We next investigated whether RA-induced F9 cell differentiation (7) involves CAK-dependent G1 arrest, as we observed before in HL60 cells (10, 17). The results show that in F9 cells treated with RA, proliferation inhibition (Fig. 1D) was associated with cell cycle G1 arrest (Fig. 1E). Cellular CAK activity in phosphorylation of GST-RARα was significantly inhibited after 48 h of RA treatment (Fig. 1F), as evidenced by a progressive decrease in levels of [32P]RARα phosphorylation (Fig. 1F, lanes 4–6; densitometer results not shown). Loss of RARα phosphorylation corresponded with decreased CAK activity, as shown by a decline in autophosphorylated [32P]CDK7. IP with PI resulted in a negative precipitation of cellular CDK7 or MAT1 (Fig. 1F, lane 1). WB analysis showed that while CDK7 levels remained unchanged, decreased levels of MAT1 (densitometer results not shown) corresponded to decreased CAK activities, as reflected by decreased [32P]CDK7 autophosphorylation. Both G1 arrest and decreased CAK phosphorylation of RARα were associated with F9 cell morphological differentiation characterized by a flat triangular morphology (Fig. 1G), as described previously (7, 38). These data indicate that in F9 cells, RA links CAK-dependent G1 arrest to differentiation by suppressing CAK phosphorylation of RARα.
To demonstrate further that loss of CAK phosphorylation of RARα induces F9 cell differentiation, we transduced F9 RARα−/− cells with lentivirus-RARαS77A (Supplemental Fig. 1D). We found that proliferation inhibition (Fig. 1H) induced by S77A expression (Supplemental Fig. 1E) was not associated with significant G1 arrest (Supplemental Fig. 1F) compared to F9 cells treated with RA (Fig. 1E), supporting the notion that RARα hypophosphorylation is a downstream event of decreased CAK activities, which simultaneously induce G1 arrest and suppress RARα phosphorylation (Fig. 1E, F). Furthermore, F9 RARα−/− cells expressing S77A appeared to undergo a primitive endoderm-like cell differentiation (Fig. 1I), similar to the RA-induced morphological differentiation of F9 cells (Fig. 1G). These studies demonstrate that in F9 cells, loss of CAK phosphorylation of RARαS77 links CAK-dependent G1 exit to differentiation.
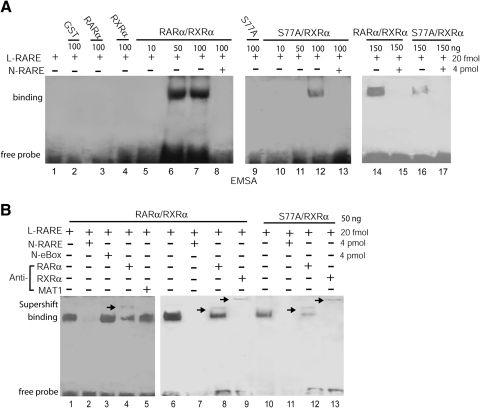
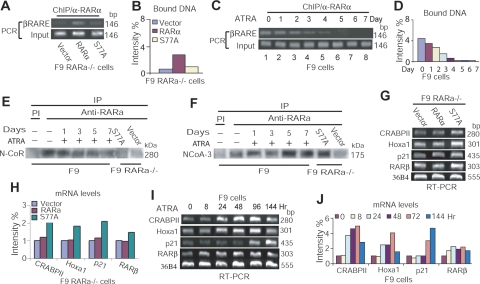
Hypophosphorylated RARα diminishes binding to RARE of the RARβ2 promoter
RARβ is the best-characterized RA-responsive gene, while RARα binds to RARE of RARβ promoter (34, 39). To investigate whether decreased RARαS77 phosphorylation induces cancer cell differentiation (Fig. 1) through transactivating RA-target genes, we first tested whether hypophosphorylated RARαS77 interacts with RARE of human RARβ (hβRARE) promoter. By using the EMSA assay, we incubated biotin end-labeled hβRARE with GST-RARα, GST-RXRα, GST-S77A, GST-RARα/RXRα, or GST-S77A/RXRα. The results showed that heterodimers RARα/RXRα and S77A/RXRα bind to hβRARE in a dose-dependent manner (Fig. 2A, lanes 5–7, 10–12). RARα/RXRα-hβRARE interactions were abolished by an excess of 200-fold unlabeled hβRARE (Fig. 2A, lanes 8, 13, 15, 17). Interestingly, S77A/RXRα showed less binding intensity to hβRARE than RARα/RXRα (Fig. 2A, lanes 14 vs. 16). While anti-MAT1 antibodies did not recognize RARα/RXRα-DNA complexes (Fig. 2B, lanes 5 vs. 4), the slowly migrating supershift complexes were retained by anti-RARα (Fig. 2B, lanes 8, 12) or anti-RXRα antibodies in parallel (Fig. 2B, lanes 9, 13). In contrast to unlabeled hβRARE, the eBox sequence failed to block RARα/RXRα-hβRARE interactions (Fig. 2B, lanes 2 vs. 3). Together, the results above show that 1) only RARα/RXRα or RARαS77A/RXRα heterodimers, but no RARα/RARα or RXRα/RXRα homodimers, bound to RARE (Fig. 2A, lanes 6, 7, 12, 14, 16 vs. 3, 4); and 2) the supershift assays confirmed that by using anti-RARα and anti-RXRα antibodies in parallel to immunoprecipitate protein-DNA complexes, only heterodimer RARα/RXRα or RARαS77A/RXRα bond to RARE (Fig. 2B, lanes 8, 9 and 12, 13); and 3) The RARα/RXRα heterodimers showed stronger protein-DNA binding than RARαS77A/RXRα heterodimers (Fig. 2B, lanes 6 vs. 10). Hence, these studies suggest that although hypophosphorylated RARα can form a complex with RXRα, such a heterodimer’s protein-DNA binding intensity is decreased due to loss of RARα phosphorylation by CAK.
Figure 2.
Hypophosphorylated RARα diminishes binding to RARE of the RARβ2 promoter. A) Dose-dependent analysis of heterodimer GST-RARα/RXRα or GST-S77A/RXRα binding to hβRARE. L-RARE, biotin end-labeled hβRARE; N-RARE, nonlabeled hβRARE. B) EMSA supershift analyses of the specific binding of GST-RARα/RXRα or GST-S77A/RXRα to hβRARE. N-eBox, nonlabeled eBox sequences.
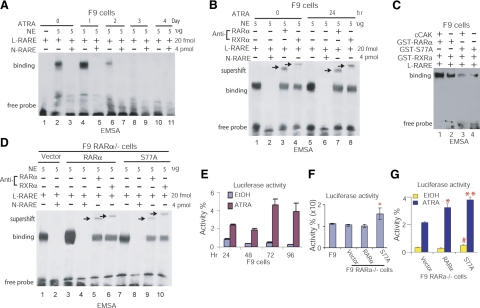
Hypophosphorylated RARα enhances transcriptional activity while diminishing binding to RARE
Because recombinant RARαS77A diminishes binding to hβRARE (Fig. 2), we examined whether RA-induced RARα hypophosphorylation in cells has a similar effect on RARα-hβRARE interaction. After treating F9 cells with RA at different time points, we extracted the nuclear proteins and incubated them with biotin end-labeled hβRARE to allow for the formation of protein-DNA complexes. We found that extended RA treatment progressively decreased binding of nuclear proteins to hβRARE, and that protein-hβRARE binding was abolished after 72 h of RA treatment (Fig. 3A, lanes 2, 4 vs. 6, 8, 10). The specific binding of cellular RARα/RXRα heterodimer to hβRARE was determined by supershift assay using anti-RARα or anti-RXRα antibodies in parallel (Fig. 3B, lanes 3, 4, 7, 8). Because RA inhibits CAK phosphorylation of RARα (Fig. 1F) and decreases RARα/RXRα binding to hβRARE (Fig. 3A, B), these results indicate that RA-induced RARα hypophosphorylation diminishes binding of RARα/RXRα heterodimer to hβRARE, similar to decreased binding of recombinant RARαS77A/RXRα heterodimer to hβRARE (Fig. 2).
Figure 3.
Hypophosphorylated RARα enhances transcriptional activity while diminishing binding to RARE. A) EMSA analysis of RA-induced interaction between nuclear protein and hβRARE. NE, nuclear extracts isolated from F9 cells. B) EMSA supershift assay of specific binding of nuclear RARα/RXRα to hβRARE. C) EMSA analysis of the effect of CAK phosphorylation of RARα on RARα-hβRARE interaction. cCAK, cellular CAK immunoprecipitated from F9 cells. D) EMSA and EMSA supershift analyses of specific interactions between hβRARE and cellular S77A or RARα expressed in F9 RARα−/− cells. E) Luciferase reporter assay of transcriptional activities in F9 cells treated with ATRA. F) S77A expressed in F9 RARα−/− cells enhances luciferase activity in the absence of ATRA. *P < 0.005 vs. other groups. G) S77A expressed in F9 RARα−/− cells enhances luciferase activity in the presence or absence of ATRA. *P < 0.003, **P < 0.001 vs. vector; #P < 0.02 vs. RARα and vector.
To confirm the effect of CAK phosphorylation of RARα on RARα/RXRα-hβRARE interaction, both phosphorylated and nonphosphorylated RAR/RXR complexes were used in EMSA analysis. After immunoprecipitating cellular CAK complexes from F9 cells using anti-CDK7 antibodies, CAK phosphorylation of GST-RARα or GST-S77A was performed in an in vitro kinase reaction as described (17) in the presence of unlabeled ATP. The reaction mixtures were then subjected to EMSA analysis in the presence of GST-RXRα and hβRARE. We found that GST-RARα/RXRα had higher binding intensity toward hβRARE than did GST-S77A/RXRα incubated either with or without CAK (Fig. 3C, lanes 1 vs. 3, 4), supporting the notion that the decreased binding intensity of RARα/RXRα heterodimer to hβRARE resulted from loss of CAK phosphorylation of RARα, as represented by RARαS77A that prevented phosphorylation by CAK (Supplemental Fig. 1C). To further test that cellular hypophosphorylated RARα diminishes interaction of RARα/RXRα heterodimer with hβRARE, we transduced F9 RARα−/− cells with lentivirus-RARαS77A or RARα. We found that F9 RARα−/− cells sufficiently express both transduced RARα and RARαS77A proteins (Supplemental Fig. 1E). Nuclear proteins extracted from these cells were incubated with hβRARE and then immunoprecipitated with anti-RARα or anti-RXRα antibodies in parallel. The results showed that both RARα/RXRα-hβRARE and S77A/RXRα-hβRARE complexes were supershifted (Fig. 3D, lanes 5, 6, 9, 10). S77A/RXRα heterodimers isolated from F9 RARα−/− cells expressing S77A reduced binding to hβRARE comparing to RARα/RXRα heterodimers isolated from F9 RARα−/− cells expressing RARα (Fig. 3D, lanes 3 vs. 7). Vector-transduced F9 RARα−/− cells showed protein-DNA interaction (Fig. 3D, lane 1), which likely resulted from other transcription factors having cross-interaction with hβRARE. This explanation was confirmed by competition analysis, in which such interaction was abolished by addition of nonlabeled hβRARE (Fig. 3D, lanes 1 vs. 2). Together, these data with the results in Figs. 1 and 3A–D, demonstrate that RARαS77A is effective in forming heterodimer with RXRα and that either RA stimulus or loss of RARα phosphorylation decreases interaction of RARα/RXRα heterodimer with hβRARE.
We further investigated whether the diminished binding of hypophosphorylated RARα to βRARE (Figs. 2 and 3A–D) alters transcription by using luciferase reporter assay. We transiently cotransfected βRARE-TK-Luc and pRL-TK-Luc plasmids into F9 cells as well as F9 RARα−/− cells expressing S77A, RARα, or vector for 24 h. To induce RA-dependent RARα hypophosphorylation, we treated F9 cells with RA for up to 96 h post-transfection. We found that RA constitutively induced transactivation of βRARE-TK-Luc, as shown by increased luciferase activity (Fig. 3E). In the absence of RA, S77A expression in F9 RARα−/− cells significantly stimulated transactivation, compared to F9 cells or F9 RARα−/− cells expressing RARα or vector (Fig. 3F). RA also enhanced S77A-mediated transactivation in F9 RARα−/− cells (Fig. 3G). These results indicate that hypophosphorylated RARα stimulates transcription (Fig. 3E–G) while diminishing binding to the RARE of target gene promoters (Figs. 2 and 3A–D).
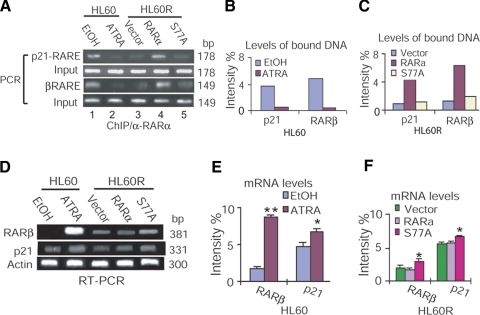
Diminished binding of hypophospharylated RARα to RARE leads to transactivation of RA-target genes in RA-sensitive and RA-resistant cells
According to the current model of gene regulation by retinoids (12), unliganded and DNA-bound retinoid receptors repress transcription. Ligand-induced conformational changes in the receptors may decompact such repressive chromatin to induce transactivation. By using the ChIP assay, we tested whether loss of CAK phosphorylation of RARα releases transcriptional repression to induce transactivation. We overexpressed RARαS77A in HL60R cells resistant to both RA-induced RARα hypophosphorylation (10) and granulocytic differentiation (Fig. 1A, B). The cross-linked protein-chromatin complexes were sonicated and extracted from HL60R cells expressing RARα or S77A, as well as from HL60 cells treated with RA. The soluble chromatins were then immunoprecipitated using anti-RARα antibodies. DNA bound by RARα was examined by PCR with different primers flanking the RARE region of the human RARβ or CDK inhibitor p21CIP1 (p21) promoter (Supplemental Table 1). Whereas ChIP by PI precipitation showed negative binding (data not shown), hypophosphorylated RARα reduced binding to RARE of human p21 (hp21RARE) or hβRARE in either HL60R cells expressing S77A or HL60 cells treated with RA (Fig. 4A–C). We also simultaneously examined whether such decreased RARαS77A interaction with RARE enhances RA-target gene expression in HL60 and HL60R cells. RT-PCR analysis showed that transcriptional expression of RARβ and p21 were up-regulated in HL60R cells transduced with S77A, similar to HL60 cells treated with RA (Fig. 4D–F). These results demonstrate that the reduced binding of hypophosphorylated RARα to RARE leads to transactivation of RA-target genes.
Figure 4.
Diminished binding of hypophospharylated RARα to RARE leads to transactivation of RA-target genes in RA-sensitive and RA-resistant cells. A) ChIP analysis of S77A-hp21RARE or S77A-hβRARE interaction in HL60 and HL60R cells. C-terminal anti-RARα antibodies that did not recognize the endogenous mutant RARα in HL60R cells were used for ChIP assay. α-RARα, c-terminal anti-RARα antibodies. B, C) Densitometry quantification of RARα-bound DNA in HL60 cells (B) and HL60R cells (C) in panel A. Data represent 3 independent experiments with similar results, normalized against levels of input DNA. D) RT-PCR analysis of RARβ and p21 expression in HL60 and HL60R cells. E, F) Densitometry quantification of mRNA levels in HL60 cells (E; *P<0.005, **P=0.000) and HL60R cells (F; *P<0.05 vs. vector or RARα) in panel D. Data represent 3 independent experiments with similar results, normalized against actin expression.
Reduced RARα-RARE interaction and enhanced transactivation are accompanied by RARα dissociation from corepressor and association with coactivator
To directly test the role of hypophosphorylated RARα in mediating chromatin interaction and gene transcription, we performed ChIP assay by using F9 RARα−/− cells expressing lentivirus-S77A or -RARα. The amount of RARE of mouse RARβ (mβRARE) bound by transduced S77A or RARα in these cells was amplified with PCR primers that flank the mβRARE region (see Supplemental Table 1). We found that S77A expressed in F9 RARα−/− cells had less binding intensity toward mβRARE comparing to RARα expressed in F9 RARα−/− cells (Fig. 5A, B). ChIP by PI precipitation showed negative binding (data not shown). Similarly, the binding intensity of RARα-mβRARE in F9 cells was decreased ∼40% after 48 h of RA stimulation and then diminished to ∼88% after 96 h of RA stimulation (Fig. 5C, D). Hence, the data derived from RARα-chromatin interactions in those different F9 RARα−/− cellular models further demonstrate that hypophosphorylated RARα decreases binding to the RARE of target genes.
Figure 5.
Reduced RARα-RARE interaction and enhanced transactivation are accompanied by RARα dissociation from corepressor and association with coactivator. A) ChIP analysis of RARα-mβRARE interaction in transduced F9 RARα−/− cells. B) Densitometry quantification of RARα-bound DNA in panel A. Data represent 3 independent experiments with similar results, normalized against levels of input DNA. C) ChIP analysis of RARα-mβRARE interaction in F9 cells treated with ATRA. D) Densitometry quantification of RARα-bound DNA in panel C. Data represent 3 independent experiments with similar results, normalized against levels of input DNA. E) IP analysis of RARα/N-CoR interaction. F) IP analysis of RARαNCoA-3 interaction. G) RT-PCR analysis of gene expression in transduced F9 RARα−/− cells. H) Densitometry quantification of mRNA levels in panel G. Data represent 3 independent experiments with similar results, normalized against 36B4 levels. I) RT-PCR analysis of RA-target gene expression in F9 cells. J) Densitometry quantification of mRNA levels in panel I. Data represent 3 independent experiments with similar results, normalized against 36B4 expression.
It is suggested that ligand-dependent transcriptional regulation by retinoid receptors requires their dissociation from and association with various cofactors functioning as either corepressors or coactivators (12). We therefore investigated whether reduced RARα-chromatin interaction and enhanced transactivation in F9 RARα−/− cells expressing S77A (Figs. 3 and 5A–D) are accompanied by such dissociation/association of RARα from the corepressor N-CoR (40) or with the coactivator NCoA-3 (41). We extracted nuclear proteins from those F9 RARα−/− cells transduced with S77A or from F9 cells treated with RA. IP analysis determined that either RA treatment or S77A expression decreased RARα interaction with N-CoR (Fig. 5E) but increased association with NCoA-3 (Fig. 5F). We did not detect an interaction between RARα and SMRTe (42) using our IP systems (data not shown). These results suggest that in the absence of RA, hyperphosphorylated RARα binds to the RARE of target genes in association with corepressors and thus represses transcription. However, a conformational change in RARα due to loss of RARαS77 phosphorylation reduces RARα binding to RARE, leading to RARα dissociation from corepressor N-CoR and association with coactivator NCoA-3 to induce transcription.
We then examined in parallel whether decreased RARα-chromatin interaction and increased RARα-NCoA-3 association (Fig. 5A–F) lead to gene transcription in F9 RARα−/− cells expressing S77A and in F9 cells treated with RA. We found that in the absence of RA, S77A up-regulated transcriptional expression of RA-target genes, including RARβ, Hoxa-1, p21, and CRABPII (Fig. 5G, H). Such S77A-effect was similar to RA-induced gene transcription in F9 cells (Fig. 5I, J). These findings demonstrate that the reduced binding of hypophosphorylated RARα to RARE leads to transactivation of RA-target genes. Moreover, although RARαS77A significantly transactivated RA-target genes in the absence of ligand, RA induced a higher level of gene transcription than did S77A in HL60R cells (Fig. 4E vs. F) or F9 RARα−/− cells (Fig. 5H vs. J), indicating that RA stimulation might induce additional signaling events to cooperate with the effect of RARα hypophosphorylation on gene transcription.
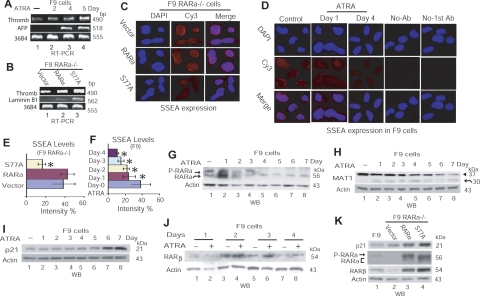
Loss of CAK phosphorylation of RARα leads to cancer cell differentiation
We further evaluated the effects of decreased CAK phosphorylation of RARα on embryonic carcinoma stem differentiation, using F9 cells treated with RA or F9 RARα−/− cells expressing S77A. RT-PCR analyses showed that RA treatment or S77A expression induced expression of differentiation marker-genes, including thrombomodulin, α-fetoprotein (AFP), and laminin B1 (Fig. 6A, B), which are related to primitive endodermal differentiation, parietal endodermal differentiation, or visceral endodermal differentiation, respectively (38, 43, 44). Immunofluorescence assay showed that the expression of SSEA-1, a marker of stem cells and embryonic carcinoma cells that is lost on differentiation (45, 46), was indeed decreased in F9 RARα−/− cells expressing S77A (Fig. 6C, 6E) and in F9 cells treated with RA (Fig. 6D, 6F). Such increased expression of differentiation marker-genes and decreased SSEA-1 levels (Fig. 6A–F) corresponded to morphological differentiation of F9 cells treated with RA and of F9 RARα−/− cells expressing S77A (Fig. 1G, I). Furthermore, RA stimulation led to a shift from RARα hyperphosphorylation in proliferating cells to RARα hypophosphorylation in differentiating cells (Fig. 6G). Simultaneously, MAT1, which sustains CAK phosphorylation of RARα (10, 23), was degraded (Fig. 6H), while the differentiation regulators p21 and RARβ were up-regulated (Fig. 6I, J). Similarly, in F9 RARα−/− cells expressing S77A, RARα hypophosphorylation, reflected by a faster migration of S77A comparing to RARα (Fig. 6K, lanes 3 vs. 4), was associated with increased protein levels of RARβ and p21 (Fig. 6K). These data demonstrate that loss of CAK phosphorylation of RARα is crucial to retinoid-induced cancer cell differentiation.
Figure 6.
Loss of RARαS77 phosphorylation leads to cancer cell differentiation. A, B) RT-PCR analysis of differentiation marker-gene expression in F9 cells (A) or transduced F9 RARα−/− cells (B). Thromb, thrombomodulin. C, D) Immunofluorescence detection of SSEA-1 expression in transduced F9 RARα−/− cells (C) or F9 cells treated with ATRA (D). E, F) Densitometry quantification of SSEA-1 levels in F9 RARα−/− cells in panel C (E; *P=0.000 vs. vector or RARα) and in F9 cells in panel D (F; *P=0.000 vs. control). Panel F presents expression levels of SSEA-1 at d 2 and 3; corresponding images are not shown in panel D. Values are means ± sd from 3 independent experiments. G–K) WB depiction of protein levels of various RA-target genes in F9 cells treated with RA (G–J) or in F9 RARα−/− cells transduced by RARα or S77A (K).
DISCUSSION
Loss of RARα phosphorylation by CAK mediates transition from G1 exit to differentiation in cancer cells
Differentiation is coupled with cell cycle exit, which often occurs in cells arrested in G1 phase (47, 48). CAK regulates cell cycle G1 exit (26), a stage where cells commonly commit to proliferation or to differentiation (47, 48). The mechanisms by which RA induces arrested G1 cells to differentiation remain unknown. RARα is a substrate for CAK (14), and decreased CAK phosphorylation of RARα is associated with a transition from G1 arrest to leukemia cell differentiation (10, 17, 23). Here we demonstrate that in RA-sensitive, RA-resistant, and RARα−/− cells, either RA-induced or RARαS77A-mimicked loss of CAK phosphorylation of RARα induces differentiation in G1 arrested cells or in RA-resistant cells (Figs. 1 and 6 and Supplemental Fig. 1). During this process, hypophosphorylated RARα mediates a proliferation/differentiation (P/D) transition in arrested G1 cells by remodeling repressive RARα-chromatin interaction to induce transactivation of RA-target gene (Figs. 345). Hence, our studies demonstrate that RA couples post-translational modification to transcriptional control of the cancer cell P/D transition by suppressing CAK phosphorylation of RARα.
Hypophosphorylated RARα induces transactivation by decompacting repressive RARα-chromatin interactions
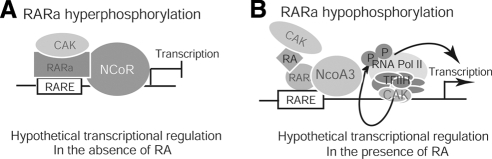
Why does decreased DNA binding result in transcription induction? A current model of transcriptional regulation by retinoids (12) suggests that unliganded and DNA-bound retinoid receptors repress gene transcription by interacting with corepressors in chromatin. To activate transcription, liganded retinoid receptors must remodel repressive chromatin by dissociating corepressors and recruiting coactivators to the promoter of target gene. Previous studies showed that RA treatment diminishes binding of PML/RARα to the RARE region of target genes in human leukemia NB4 cells, and this decreased binding intensity is associated with increased transactivation and cell differentiation (49). Furthermore, studies from Kizaki’s group (50) showed that in the presence of RA, RARα reduces binding to the RARE region of the thrombopoietin (TPO) promoter while up-regulating TPO transactivation. They raised an important question: It is not clear why ATRA stimulation diminished the binding of RARα to TPO-RARE while it stimulated transactivation of TPO. Recent studies show that decreased CAK phosphorylation of RARα by RA lead to inhibition of proliferation and induction of differentiation in several cancer cell lines (10, 15,16,17). RARαS77A mimics the effects of RA on inhibition of proliferation and stimulation of differentiation in both malignant and normal hematopoietic cells (15, 18). These studies suggest that RA-induced loss of RARαS77 phosphorylation by CAK remodels the repressive chromatin environment to induce transactivation of RA-target genes. By testing this novel idea, our studies demonstrate that in contrast to RARα hyperphosphorylation, RA-induced RARα hypophosphorylation or RARαS77A-mimicked RARα hypophosphorylation decreases RARα binding to the RARE of RA-target genes (Figs. 2345). Such decreased interaction is accompanied by hypophosphorylated RARα disassociation from N-CoR and association with NCoA-3, leading to gene transcription (Figs. 345). In association with these changes in protein-chromatin interactions in F9 cells treated with RA or in F9 RARα−/− cells expressing RARαS77A, we find that up-regulated protein levels of the differentiation regulators RARβ and p21 (Fig. 6I–K) are accompanied by proliferation inhibition, G1 arrest, morphological differentiation, decreased SSEA-1 levels, and expression of differentiation marker-genes (Figs. 1 and 6). These data suggest a hypothetical model in RA-induced cancer cell differentiation, by which hypophosphorylated RARα might transactivate differentiation-target genes by decreasing binding to repressive chromatin in order to dissociate from N-CoR and to interact with NCoA-3 (Fig. 7).
Figure 7.
Hypothetical prediction of decreased RARα phosphorylation in transactivation of RA-target genes. A) Hyperphosphorylation in absence of RA. B) Hypophosphorylation in presence of RA.
How does decreased DNA binding result in transcription induction? It is known that in addition to the role of CAK in cell cycle G1 regulation, CAK also serves as a kinase subunit in TFIIH complex (24) to mediate transcription initiation (27,28,29) by phosphorylating the largest subunit of RNA Pol II (27, 29). Thus, it could be that the mobilization of NCoA-3 by hypophosphorylated RARα may facilitate the recruitment of TFIIH to the promoter of RA-target genes to initiate transcription through phosphorylation of RNA Pol II by TFIIH-containing CAK. On the other hand, RA can also induce proteasome-dependent degradation of either RARα or RARα fusion protein (51,52,53). Such decreased RARα levels could be one of the key factors that decreases RARα-RARE binding, leading to RARα dissociation from corepressor and association with coactivator to induce transcription. Moreover, RA isomer formation or 9cRA conversion in cells may also be involved in mediating RA-induced and RARα/RXRα-mediated transcriptional control of cancer cell differentiation. It is therefore important, in future studies, to investigate those possible mechanistic correlations with decreased CAK phosphorylation of RARα in coordinating CAK-dependent G1 arrest with TFIIH-mediated transcriptional control of cancer cell differentiation.
RA-induced earlier transient phosphorylation of RARα
In addition to the studies focusing on RA-induced loss of RARα phosphorylation in human cancer cell differentiation, other groups have reported some interesting findings about the effect of RA-induced earlier transient phosphorylation of RARα (14, 54). They find that by using a short period of RA stimuli (within 24 h) in either Cos-1 cells or HD2 cells (human fibroblasts hybridized with HeLa cells), RARα phosphorylation and transactivation increased, although the biological significance of these earlier transient events remains unknown. Moreover, some studies also show that in the presence of RA stimuli up to 60 min, both RARα and RARαS77A not only interact with TFIIH but also are phosphorylated by TFIIH (S77 and S369 at RARα while S369 at RARαS77A) for up to 50 min and then disappeared at 60 min (55). It has been well established that cancer cell differentiation requires cell cycle exit, by which at least two waves of signaling events are involved: induction of cell cycle arrest as well as transition into transactivation of target genes that regulates differentiation (47, 48). That is why RA-induced cancer cell differentiation usually takes 3 to 7 d depending on the different cell types (7, 10, 16, 17). Currently, it remains unknown whether RA-induced earlier transient phosphorylation of RARα is related to the effect of decreased RARα phosphorylation on mediating cancer cell differentiation after several days of RA stimuli. Nevertheless, future studies in determining the possible relationship between earlier transient RARα phosphorylation and later stage of decreased RARα phosphorylation on mediating cancer cell differentiation would advance the field of retinoid.
In summary, our studies reveal a novel mechanism of RA-mediated transactivation, by which decreased CAK phosphorylation of RARα diminishes RARα-chromatin interaction, leading to gene transcription via hypophosphorylated RARα dissociation from corepressor and association with coactivator. This enhanced knowledge of RA-mediated transcriptional regulation would in turn suggest ways to design improved therapies that direct cancer cell terminal differentiation.
Supplementary Material
Acknowledgments
The authors thank Dr. Lorraine J. Gudas (Cornell University, Ithaca, NY, USA) for providing F9 RARα−/− cells; and Dr. J. Song (University of California, Los Angeles, CA, USA) for providing βRARE-TK-Luc plasmids. The authors acknowledge the Vector Core at Childrens Hospital Los Angeles (CHLA) and Roger Hollis for technical expertise in lentiviral-vector production. The authors thank Dr. Srinivas Somanchi for technical expertise in constructing lentiviral vector. This work was supported by grants from the National Institutes of Health (R21 CA111440 and R01 CA120512 to L.W.).
References
- Chambon P. The retinoid signaling pathway: molecular and genetic analyses. Semin Cell Biol. 1994;5:115–125. doi: 10.1006/scel.1994.1015. [DOI] [PubMed] [Google Scholar]
- Zhang X K, Lehmann J, Hoffmann B, Dawson M I, Cameron J, Graupner G, Hermann T, Tran P, Pfahl M. Homodimer formation of retinoid X receptor induced by 9-cis retinoic acid. Nature. 1992;358:587–591. doi: 10.1038/358587a0. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D J, Evans R M. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- Losel R M, Falkenstein E, Feuring M, Schultz A, Tillmann H C, Rossol-Haseroth K, Wehling M. Nongenomic steroid action: controversies, questions, and answers. Physiol Rev. 2003;83:965–1016. doi: 10.1152/physrev.00003.2003. [DOI] [PubMed] [Google Scholar]
- Liao Y P, Ho S Y, Liou J C. Non-genomic regulation of transmitter release by retinoic acid at developing motoneurons in Xenopus cell culture. J Cell Sci. 2004;117:2917–2924. doi: 10.1242/jcs.01153. [DOI] [PubMed] [Google Scholar]
- Zanotto-Filho A, Cammarota M, Gelain D P, Oliveira R B, Delgado-Canedo A, Dalmolin R J, Pasquali M A, Moreira J C. Retinoic acid induces apoptosis by a non-classical mechanism of ERK1/2 activation. Toxicol In Vitro. 2008;22:1205–1212. doi: 10.1016/j.tiv.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Taneja R, Rochette-Egly C, Plassat J L, Penna L, Gaub M P, Chambon P. Phosphorylation of activation functions AF-1 and AF-2 of RAR alpha and RAR gamma is indispensable for differentiation of F9 cells upon retinoic acid and cAMP treatment. EMBO J. 1997;16:6452–6465. doi: 10.1093/emboj/16.21.6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochette-Egly C, Plassat J L, Taneja R, Chambon P. The AF-1 and AF-2 activating domains of retinoic acid receptor-alpha (RARalpha) and their phosphorylation are differentially involved in parietal endodermal differentiation of F9 cells and retinoid-induced expression of target genes. Mol Endocrinol. 2000;14:1398–1410. doi: 10.1210/mend.14.9.0527. [DOI] [PubMed] [Google Scholar]
- Lamkin T J, Chin V, Yen A. All-trans retinoic acid induces p62DOK1 and p56DOK2 expression which enhances induced differentiation and G0 arrest of HL-60 leukemia cells. Am J Hematol. 2006;81:603–615. doi: 10.1002/ajh.20667. [DOI] [PubMed] [Google Scholar]
- Wang J, Barsky L W, Shum C H, Jong A, Weinberg K I, Collins S J, Triche T J, Wu L. Retinoid-induced G1 arrest and differentiation activation are associated with a switch to cyclin-dependent kinase-activating kinase hypophosphorylation of retinoic acid receptor alpha. J Biol Chem. 2002;277:43369–43376. doi: 10.1074/jbc.M206792200. [DOI] [PubMed] [Google Scholar]
- Soprano D R, Qin P, Soprano K J. Retinoic acid receptors and cancers. Annu Rev Nutr. 2004;24:201–221. doi: 10.1146/annurev.nutr.24.012003.132407. [DOI] [PubMed] [Google Scholar]
- Bastien J, Rochette-Egly C. Nuclear retinoid receptors and the transcription of retinoid-target genes. Gene. 2004;328:1–16. doi: 10.1016/j.gene.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–954. [PubMed] [Google Scholar]
- Rochette-Egly C, Adam S, Rossignol M, Egly J M, Chambon P. Stimulation of RAR alpha activation function AF-1 through binding to the general transcription factor TFIIH and phosphorylation by CDK7. Cell. 1997;90:97–107. doi: 10.1016/s0092-8674(00)80317-7. [DOI] [PubMed] [Google Scholar]
- Crowe D L, Kim R. A phosphorylation defective retinoic acid receptor mutant mimics the effects of retinoic acid on EGFR mediated AP-1 expression and cancer cell proliferation. Cancer Cell Int. 2002;2:15. doi: 10.1186/1475-2867-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, He Q, Peng H, Tedeschi-Blok N, Triche T J, Wu L. MAT1-modulated cyclin-dependent kinase-activating kinase activity cross-regulates neuroblastoma cell G(1) arrest and neurite outgrowth. Cancer Res. 2004;64:2977–2983. doi: 10.1158/0008-5472.can-03-4018. [DOI] [PubMed] [Google Scholar]
- Wang J G, Barsky L W, Davicioni E, Weinberg K I, Triche T J, Zhang X K, Wu L. Retinoic acid induces leukemia cell G1 arrest and transition into differentiation by inhibiting cyclin-dependent kinase-activating kinase binding and phosphorylation of PML/RARalpha. FASEB J. 2006;20:2142–2144. doi: 10.1096/fj.06-5900fje. [DOI] [PubMed] [Google Scholar]
- Luo P, Wang A, Payne K J, Peng H, Wang J G, Parrish Y K, Rogerio J W, Triche T J, He Q, Wu L. Intrinsic retinoic acid receptor alpha-cyclin-dependent kinase-activating kinase signaling involves coordination of the restricted proliferation and granulocytic differentiation of human hematopoietic stem cells. Stem Cells. 2007;25:2628–2637. doi: 10.1634/stemcells.2007-0264. [DOI] [PubMed] [Google Scholar]
- Wu L, Yee A, Liu L, Carbonaro-Hall D, Venkatesan N, Tolo V T, Hall F L. Molecular cloning of the human CAK1 gene encoding a cyclin-dependent kinase-activating kinase. Oncogene. 1994;9:2089–2096. [PubMed] [Google Scholar]
- Fisher R P, Morgan D O. A novel cyclin associates with MO15/CDK7 to form the CDK-activating kinase. Cell. 1994;78:713–724. doi: 10.1016/0092-8674(94)90535-5. [DOI] [PubMed] [Google Scholar]
- Tassan J P, Jaquenoud M, Fry A M, Frutiger S, Hughes G J, Nigg E A. In vitro assembly of a functional human CDK7-cyclin H complex requires MAT1, a novel 36 kDa RING finger protein. EMBO J. 1995;14:5608–5617. doi: 10.1002/j.1460-2075.1995.tb00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankulov K Y, Bentley D L. Regulation of CDK7 substrate specificity by MAT1 and TFIIH. EMBO J. 1997;16:1638–1646. doi: 10.1093/emboj/16.7.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Peng H, Collins S J, Triche T J, Wu L. Retinoid-modulated MAT1 ubiquitination and CAK activity. FASEB J. 2004;18:1734–1736. doi: 10.1096/fj.04-2182fje. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J H, Egly J M, Vermeulen W. TFIIH: a key component in multiple DNA transactions. Curr Opin Genet Dev. 1996;6:26–33. doi: 10.1016/s0959-437x(96)90006-4. [DOI] [PubMed] [Google Scholar]
- Nigg E A. Cyclin-dependent kinase 7: at the cross-roads of transcription, DNA repair and cell cycle control? Curr Opin Cell Biol. 1996;8:312–317. doi: 10.1016/s0955-0674(96)80003-2. [DOI] [PubMed] [Google Scholar]
- Wu L, Chen P, Shum C H, Chen C, Barsky L W, Weinberg K I, Jong A, Triche T J. MAT1-modulated CAK activity regulates cell cycle G(1) exit. Mol Cell Biol. 2001;21:260–270. doi: 10.1128/MCB.21.1.260-270.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feaver W J, Svejstrup J Q, Henry N L, Kornberg R D. Relationship of CDK-activating kinase and RNA polymerase II CTD kinase TFIIH/TFIIK. Cell. 1994;79:1103–1109. doi: 10.1016/0092-8674(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Rossignol M, Kolb-Cheynel I, Egly J M. Substrate specificity of the cdk-activating kinase (CAK) is altered upon association with TFIIH. EMBO J. 1997;16:1628–1637. doi: 10.1093/emboj/16.7.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larochelle S, Chen J, Knights R, Pandur J, Morcillo P, Erdjument-Bromage H, Tempst P, Suter B, Fisher R P. T-loop phosphorylation stabilizes the CDK7–cyclin H–MAT1 complex in vivo and regulates its CTD kinase activity. EMBO J. 2001;20:3749–3759. doi: 10.1093/emboj/20.14.3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S J, Robertson K A, Mueller L. Retinoic acid-induced granulocytic differentiation of HL-60 myeloid leukemia cells is mediated directly through the retinoic acid receptor (RAR-alpha) Mol Cell Biol. 1990;10:2154–2163. doi: 10.1128/mcb.10.5.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochette-Egly C, Chambon P. F9 embryocarcinoma cells: a cell autonomous model to study the functional selectivity of RARs and RXRs in retinoid signaling. Histol Histopathol. 2001;16:909–922. doi: 10.14670/HH-16.909. [DOI] [PubMed] [Google Scholar]
- Boylan J F, Gudas L J. Overexpression of the cellular retinoic acid binding protein-I (CRABP-I) results in a reduction in differentiation-specific gene expression in F9 teratocarcinoma cells. J Cell Biol. 1991;112:965–979. doi: 10.1083/jcb.112.5.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Lu Y C, Yokoyama K, Rossi J, Chiu R. Cyclophilin A is required for retinoic acid-induced neuronal differentiation in p19 cells. J Biol Chem. 2004;279:24414–24419. doi: 10.1074/jbc.M311406200. [DOI] [PubMed] [Google Scholar]
- Sucov H M, Murakami K K, Evans R M. Characterization of an autoregulated response element in the mouse retinoic acid receptor type beta gene. Proc Natl Acad Sci U S A. 1990;87:5392–5396. doi: 10.1073/pnas.87.14.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leid M, Kastner P, Chambon P. Multiplicity generates diversity in the retinoic acid signalling pathways. Trends Biochem Sci. 1992;17:427–433. doi: 10.1016/0968-0004(92)90014-z. [DOI] [PubMed] [Google Scholar]
- Montagne M, Naud J F, Lavigne P. Elucidation of the structural determinants responsible for the specific formation of heterodimeric Mxd1/Max b-HLH-LZ and its binding to E-box sequences. J Mol Biol. 2008;376:141–152. doi: 10.1016/j.jmb.2007.11.062. [DOI] [PubMed] [Google Scholar]
- Robertson K A, Emami B, Collins S J. Retinoic acid-resistant HL-60R cells harbor a point mutation in the retinoic acid receptor ligand-binding domain that confers dominant negative activity. Blood. 1992;80:1885–1889. [PubMed] [Google Scholar]
- Strickland S, Mahdavi V. The induction of differentiation in teratocarcinoma stem cells by retinoic acid. Cell. 1978;15:393–403. doi: 10.1016/0092-8674(78)90008-9. [DOI] [PubMed] [Google Scholar]
- De The H, Vivanco-Ruiz M M, Tiollais P, Stunnenberg H, Dejean A. Identification of a retinoic acid responsive element in the retinoic acid receptor beta gene. Nature. 1990;343:177–180. doi: 10.1038/343177a0. [DOI] [PubMed] [Google Scholar]
- Wang J, Hoshino T, Redner R L, Kajigaya S, Liu J M. ETO, fusion partner in t(8;21) acute myeloid leukemia, represses transcription by interaction with the human N-CoR/mSin3/HDAC1 complex. Proc Natl Acad Sci U S A. 1998;95:10860–10865. doi: 10.1073/pnas.95.18.10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura A, vn Peer M, Schroder A J, Rothman P B. The transcriptional co-activator p/CIP (NCoA-3) is up-regulated by STAT6 and serves as a positive regulator of transcriptional activation by STAT6. J Biol Chem. 2004;279:31105–31112. doi: 10.1074/jbc.M404428200. [DOI] [PubMed] [Google Scholar]
- Park E J, Schroen D J, Yang M, Li H, Li L, Chen J D. SMRTe, a silencing mediator for retinoid and thyroid hormone receptors-extended isoform that is more related to the nuclear receptor corepressor. Proc Natl Acad Sci U S A. 1999;96:3519–3524. doi: 10.1073/pnas.96.7.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler-Guettler H, Yu K, Soff G, Gudas L J, Rosenberg R D. Thrombomodulin gene regulation by cAMP and retinoic acid in F9 embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1992;89:2155–2159. doi: 10.1073/pnas.89.6.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear B T, Ellis A W. Endogenous and transfected mouse alpha-fetoprotein genes in undifferentiated F9 cells are activated in transient heterokaryons. Somat Cell Mol Genet. 1995;21:19–31. doi: 10.1007/BF02255819. [DOI] [PubMed] [Google Scholar]
- Epping M T, Wang L, Edel M J, Carlee L, Hernandez M, Bernards R. The human tumor antigen PRAME is a dominant repressor of retinoic acid receptor signaling. Cell. 2005;122:835–847. doi: 10.1016/j.cell.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Solter D, Knowles B B. Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1) Proc Natl Acad Sci U S A. 1978;75:5565–5569. doi: 10.1073/pnas.75.11.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studzinski G P, Harrison L E. Differentiation-related changes in the cell cycle traverse. Int Rev Cytol. 1999;189:1–58. doi: 10.1016/s0074-7696(08)61384-4. [DOI] [PubMed] [Google Scholar]
- Zhu L, Skoultchi A I. Coordinating cell proliferation and differentiation. Curr Opin Genet Dev. 2001;11:91–97. doi: 10.1016/s0959-437x(00)00162-3. [DOI] [PubMed] [Google Scholar]
- Rosenauer A, Raelson J V, Nervi C, Eydoux P, DeBlasio A, Miller W H., Jr Alterations in expression, binding to ligand and DNA, and transcriptional activity of rearranged and wild-type retinoid receptors in retinoid-resistant acute promyelocytic leukemia cell lines. Blood. 1996;88:2671–2682. [PubMed] [Google Scholar]
- Kinjo K, Miyakawa Y, Uchida H, Kitajima S, Ikeda Y, Kizaki M. All-trans retinoic acid directly up-regulates thrombopoietin transcription in human bone marrow stromal cells. Exp Hematol. 2004;32:45–51. doi: 10.1016/j.exphem.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Scita G, Darwiche N, Greenwald E, Rosenberg M, Politi K, De Luca L M. Retinoic acid down-regulation of fibronectin and retinoic acid receptor alpha proteins in NIH-3T3 cells. Blocks of this response by ras transformation. J Biol Chem. 1996;271:6502–6508. doi: 10.1074/jbc.271.11.6502. [DOI] [PubMed] [Google Scholar]
- Zhu J, Gianni M, Kopf E, Honore N, Chelbi-Alix M, Koken M, Quignon F, Rochette-Egly C, de The H. Retinoic acid induces proteasome-dependent degradation of retinoic acid receptor alpha (RARalpha) and oncogenic RARalpha fusion proteins. Proc Natl Acad Sci U S A. 1999;96:14807–14812. doi: 10.1073/pnas.96.26.14807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf E, Plassat J L, Vivat V, de The H, Chambon P, Rochette-Egly C. Dimerization with retinoid X receptors and phosphorylation modulate the retinoic acid-induced degradation of retinoic acid receptors alpha and gamma through the ubiquitin-proteasome pathway. J Biol Chem. 2000;275:33280–33288. doi: 10.1074/jbc.M002840200. [DOI] [PubMed] [Google Scholar]
- Keriel A, Stary A, Sarasin A, Rochette-Egly C, Egly J M. XPD mutations prevent TFIIH-dependent transactivation by nuclear receptors and phosphorylation of RARalpha. Cell. 2002;109:125–135. doi: 10.1016/s0092-8674(02)00692-x. [DOI] [PubMed] [Google Scholar]
- Bruck N, Vitoux D, Ferry C, Duong V, Bauer A, de The H, Rochette-Egly C. A coordinated phosphorylation cascade initiated by p38MAPK/MSK1 directs RARalpha to target promoters. EMBO J. 2009;28:34–47. doi: 10.1038/emboj.2008.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.