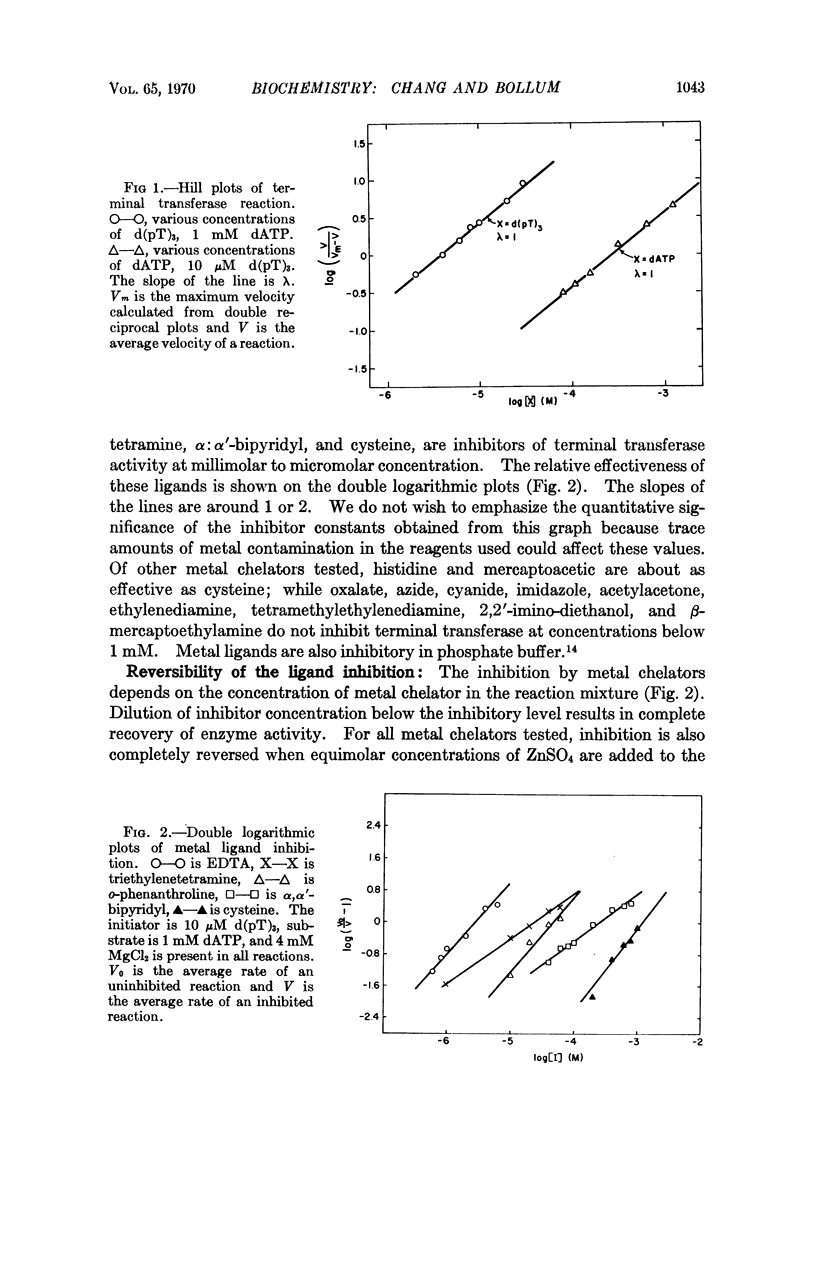
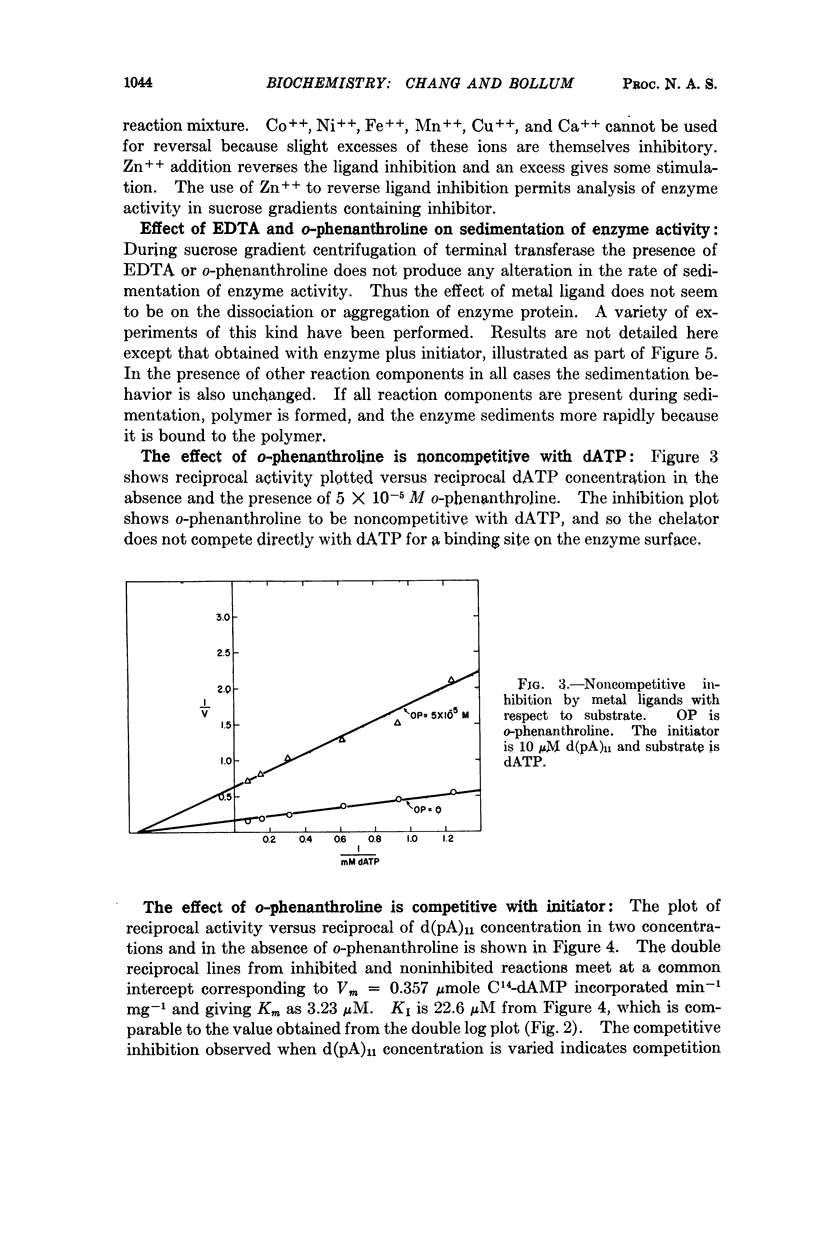
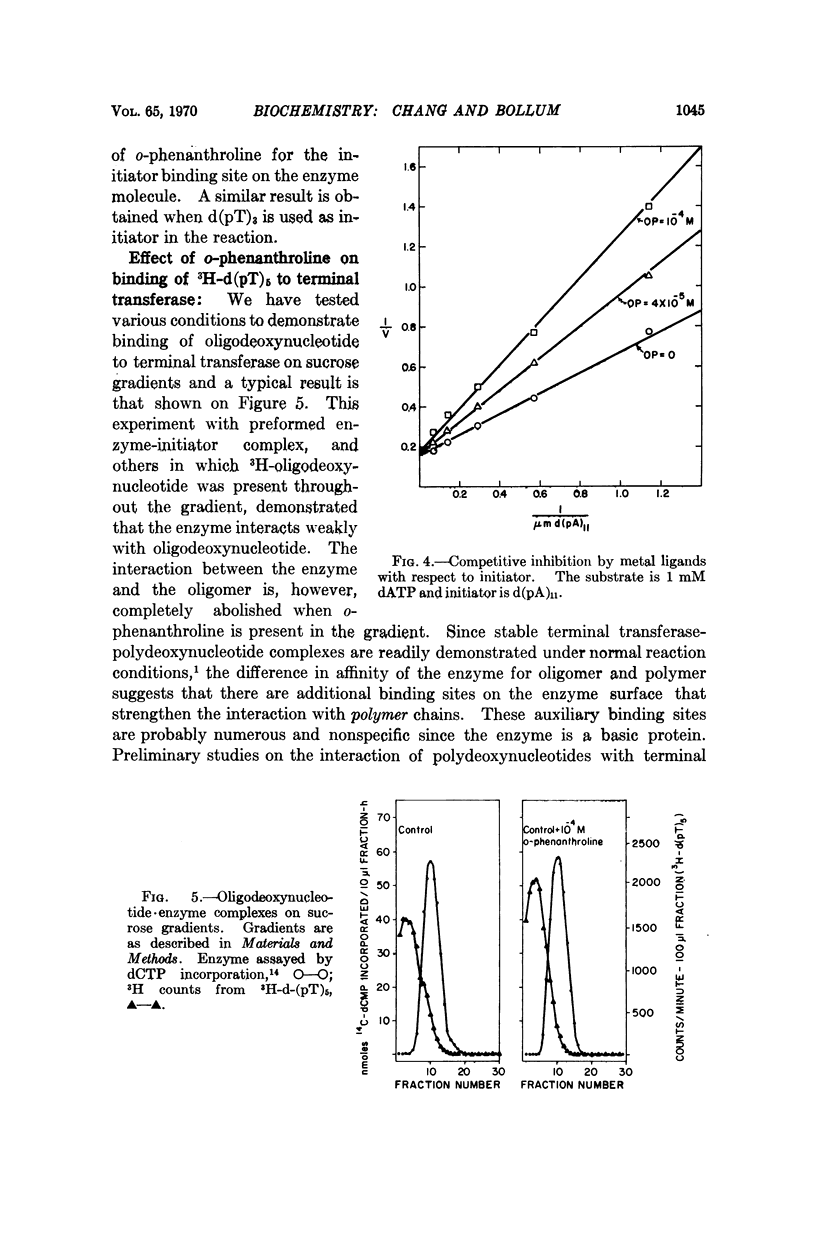
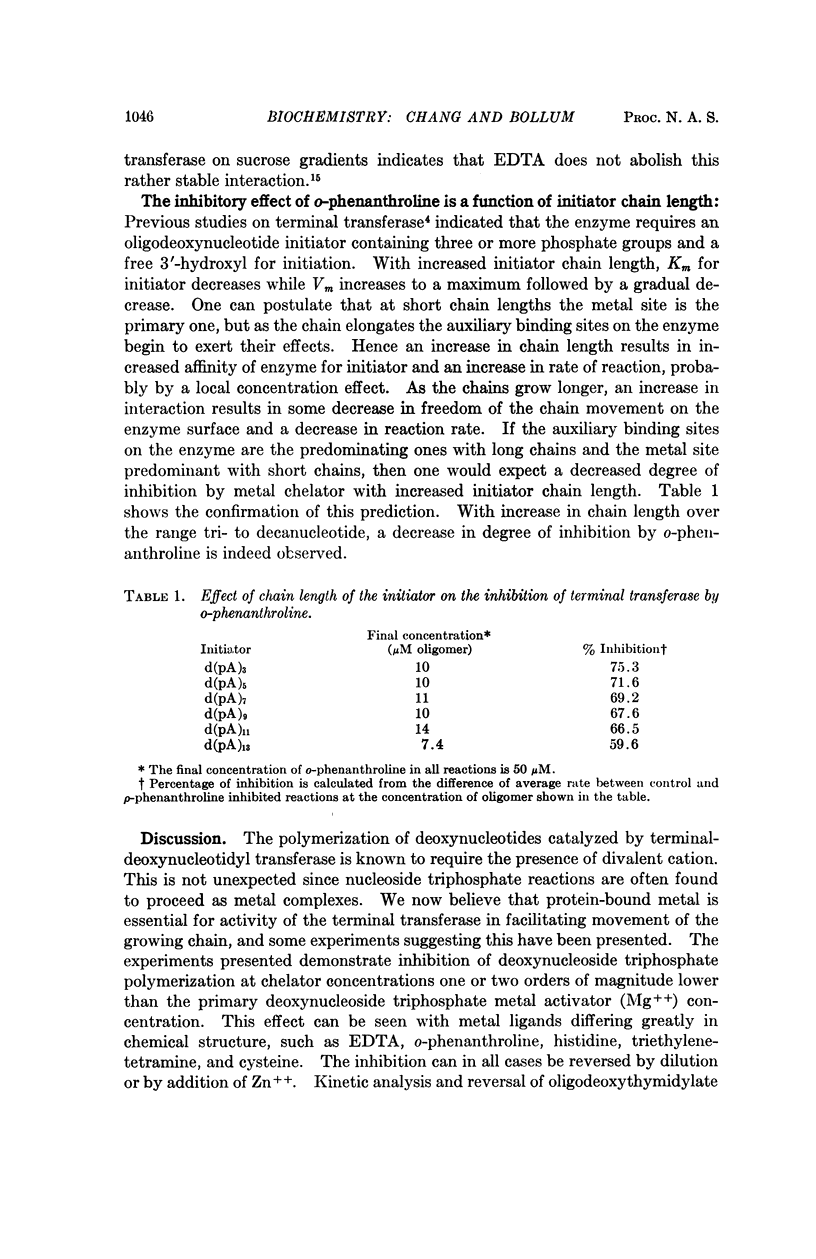
Abstract
The polymerization of deoxynucleoside triphosphates, catalyzed by terminal deoxynucleotidyl transferase from calf thymus gland, is strongly inhibited by various metal chelators. The reaction appears to be first order with respect to enzyme, deoxynucleoside triphosphate, and initiator, indicating that there is only one catalytic center for each enzyme molecule. The presence of metal chelator does not affect the molecular state of the enzyme or the order of reaction. An analysis of the kinetics of o-phenanthroline inhibition demonstrates that the ligand is noncompetitive with nucleoside triphosphate substrate and strictly competitive with oligodeoxynucleotide initiator. These results, obtained in the presence of millimolar Mg++, suggest that the initiator binds to the enzyme through a transition metal atom and ligand inhibition occurs at that site. The precise spatial arrangement of the coordination orbitals around a metal atom provides a model to visualize the specificity of the enzyme for the 3′-OH end of the growing polynucleotide chain.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATKINSON D. E., HATHAWAY J. A., SMITH E. C. KINETICS OF REGULATORY ENZYMES. KINETIC ORDER OF THE YEAST DIPHOSPHOPYRIDINE NUCLEOTIDE ISOCITRATE DEHYDROGENASE REACTION AND A MODEL FOR THE REACTION. J Biol Chem. 1965 Jun;240:2682–2690. [PubMed] [Google Scholar]
- Britten R. J., Roberts R. B. High-Resolution Density Gradient Sedimentation Analysis. Science. 1960 Jan 1;131(3392):32–33. doi: 10.1126/science.131.3392.32. [DOI] [PubMed] [Google Scholar]
- Cassani G. R., Bollum F. J. Oligodeoxythymidylate: polydeoxyadenylate and oligodeoxyadenylate: polydeoxythymidylate interactions. Biochemistry. 1969 Oct;8(10):3928–3936. doi: 10.1021/bi00838a008. [DOI] [PubMed] [Google Scholar]
- FUJIOKA M., LIEBERMAN I. A ZN++ REQUIREMENT FOR SYNTHESIS OF DEOXYRIBONUCLEIC ACID BY RAT LIVER. J Biol Chem. 1964 Apr;239:1164–1167. [PubMed] [Google Scholar]
- Kato K. I., Gonçalves J. M., Houts G. E., Bollum F. J. Deoxynucleotide-polymerizing enzymes of calf thymus gland. II. Properties of the terminal deoxynucleotidyltransferase. J Biol Chem. 1967 Jun 10;242(11):2780–2789. [PubMed] [Google Scholar]
- LIEBERMAN I., ABRAMS R., HUNT N., OVE P. LEVELS OF ENZYME ACTIVITY AND DEOXYRIBONUCLEIC ACID SYNTHESIS IN MAMMALIAN CELLS CULTURED FROM THE ANIMAL. J Biol Chem. 1963 Dec;238:3955–3962. [PubMed] [Google Scholar]
- LIEBERMAN I., OVE P. Deoxyribonucleic acid synthesis and its inhibition in mammalian cells cultured from the animal. J Biol Chem. 1962 May;237:1634–1642. [PubMed] [Google Scholar]
- Loftfield R. B., Eigner E. A. Molecular order of participation of inhibitors (or activators) in biological systems. Science. 1969 Apr 18;164(3877):305–308. doi: 10.1126/science.164.3877.305. [DOI] [PubMed] [Google Scholar]
- YONEDA M., BOLLUM F. J. DEOXYNUCLEOTIDE-POLYMERIZING ENZYMES OF CALF THYMUS GLAND. I. LARGE SCALE PURIFICATION OF TERMINAL AND REPLICATIVE DEOXYNUCLEOTIDYL TRANSFERASES. J Biol Chem. 1965 Aug;240:3385–3391. [PubMed] [Google Scholar]


