Abstract
A well-developed spatial memory is important for many animals, but appears especially important for scatter-hoarding species. Consequently, the scatter-hoarding system provides an excellent paradigm in which to study the integrative aspects of memory use within an ecological and evolutionary framework. One of the main tenets of this paradigm is that selection for enhanced spatial memory for cache locations should specialize the brain areas involved in memory. One such brain area is the hippocampus (Hp). Many studies have examined this adaptive specialization hypothesis, typically relating spatial memory to Hp volume. However, it is unclear how the volume of the Hp is related to its function for spatial memory. Thus, the goal of this article is to evaluate volume as a main measurement of the degree of morphological and physiological adaptation of the Hp as it relates to memory. We will briefly review the evidence for the specialization of memory in food-hoarding animals and discuss the philosophy behind volume as the main currency. We will then examine the problems associated with this approach, attempting to understand the advantages and limitations of using volume and discuss alternatives that might yield more specific hypotheses. Overall, there is strong evidence that the Hp is involved in the specialization of spatial memory in scatter-hoarding animals. However, volume may be only a coarse proxy for more relevant and subtle changes in the structure of the brain underlying changes in behaviour. To better understand the nature of this brain/memory relationship, we suggest focusing on more specific and relevant features of the Hp, such as the number or size of neurons, variation in connectivity depending on dendritic and axonal arborization and the number of synapses. These should generate more specific hypotheses derived from a solid theoretical background and should provide a better understanding of both neural mechanisms of memory and their evolution.
Keywords: birds, comparative analysis, food caching, neuroecology, stereology
1. Introduction
Memory is an important trait for a variety of animals, but it appears to be especially crucial for scatter-hoarding animals (VanderWall 1990). Some scatter-hoarding species can make and retrieve incredible numbers of caches (e.g. as many as 500 000; Haftorn 1956a; Pravosudov 1985; Brodin 1994a) throughout the year, and these caches may be critically important for survival during the winter (Swanberg 1951; Haftorn 1956b; Jansson 1982; Krebs et al. 1989; Sherry et al. 1989; Pravosudov & Grubb 1997; Pravosudov & Lucas 2001). Cache retrieval is often facilitated in part by spatial memory to relocate caches, although other types of memory (e.g. LaDage et al. 2009a) and non-memory-based methods of retrieval can be used as well (see Smulders et al. 2010; e.g. Pravosudov 1985; Brodin 1994b; Lens et al. 1994). Thus, spatial memory is especially important for many scatter-hoarding animals.
The use of scatter-hoarding animals as a paradigm in which to study memory use is inherently integrative (Smulders 2006; Pravosudov 2007). By linking behaviour, physiology, morphology and neurobiology to ecology and evolution (the ‘neuroecological’ approach), recent work in the field has placed memory and memory use into an ecological and evolutionary framework (Pravosudov & Smulders 2010). The leading hypothesis for the evolution of memory used to retrieve caches is the adaptive specialization hypothesis (ASH), which states that selection can modify behaviour and its underlying neural mechanisms if such modifications enhance fitness (Krebs et al. 1989; Sherry et al. 1989, 1992). This hypothesis allows us to address two separate, but related questions: (i) what are the selective pressures and evolutionary processes through which evolution can affect brain structure and function and (ii) how does brain structure relate to brain function?
To address the first question, we need to look for evidence of adaptation in behaviour or cognition. This is often done using comparative studies, in which behavioural and morphological traits from numerous species are correlated with the goal of finding patterns of similarity among the species. In the case of the scatter-hoarding paradigm, the hypothesis is that selection for more efficient memory-based cache retrieval has led to enhanced memory, which in turn has led to changes in brain (hippocampus (Hp)) morphology. Thus, a relationship between caching intensity and Hp morphology has been sought. If such a relationship is indeed identified, then the next step is to examine the specific neural processes underlying these memories that were affected by such selection. Of course, identifying evolutionary patterns across a range of species does not explain exactly how the brain processes memories. Such patterns can, however, provide important directions for future studies directed at identifying specific mechanisms of memory. If a particular feature of the brain follows the same cross-species pattern as the behavioural adaptation, then this feature becomes a candidate for further study of its role in cognitive processing. However, in order to ascertain mechanistic questions and the relationship between structure and function, experimental studies are necessary as the comparative approach is deficient at establishing causal relationships between behaviour and its mechanisms (e.g. Smulders 2006; Healy & Rowe 2007).
The relevance of the neuroecological approach has been criticized by Bolhuis & Macphail (2001) and Macphail & Bolhuis (2001; see also Gahr et al. 1998; Gahr & Daisuke 2007). They rightly argued that the large-scale, comparative approach cannot be used to explain the mechanisms responsible for the differences in behavioural performance. In other words, the specialization of a cognitive trait such as spatial memory capacity cannot be used to explain the specific causal brain mechanisms that underlie this spatial memory capacity. This is the case as problems of (behavioural) function may be solved by evolution in numerous different ways and simply comparing the phenotypic outcomes of the evolutionary process cannot tell us how evolution has solved the problem mechanistically. Bolhuis & Macphail (2001), however, appear to ignore or misunderstand the importance of examining the evolutionary history of the species (see also Hampton et al. 2002; Sherry 2006). It is of course perfectly possible to study in extreme detail how a particular phenomenon (e.g. spatial memory) works in a given organism without any reference to evolution whatsoever. However, as soon as these findings need to be applied to other organisms, it is crucial to understand the evolutionary costs, benefits and trade-offs of the trait in question, as well as the phylogenetic patterns in the evolution of the trait (for a further review of this topic, see Smulders 2009). Likewise, the evolution of a trait depends not only on the selective pressures that adapt a trait to the environment, but also on the constraints placed on evolution by the current mechanisms that underlie the trait in question. Therefore, evolutionary patterns can be better understood when we know the specifics of the mechanisms involved in behaviour, because it allows us to consider the trade-offs and constraints associated with those mechanisms. Therefore, integrating both perspectives will result in a better understanding of the relationship between the brain and memory.
Most of the other criticisms levelled by Bolhuis & Macphail (2001) and Macphail & Bolhuis (2001) are formal and owing to their non-adaptationist approach. This approach has merit (e.g. Gould & Lewontin 1979), but at times remains at odds with the predominating adaptationist approach. As with many arguments, reality is probably somewhere in between the extremes (Pigliucci & Kaplan 2000). Nevertheless, even when the arguments on either side of a debate are based on a different world-view, it should be possible to judge the outcomes from a given approach on their own merits (Brodin & Bolhuis 2008). We will therefore review some of the results from the neuroecological approach in an attempt to better our understanding of the mechanisms that may underlie spatial memory processing.
The validity of some aspects of Bolhuis & Macphail's criticisms, therefore, does not imply that the entire neuroecological paradigm cannot be used to ask questions about brain function, neurological mechanisms, and the evolutionary relevance of such traits. Variation in the brain and in behaviour in many cases has resulted from natural selection, and thus the examination of such variation from an evolutionary perspective may be a powerful and meaningful way to enhance our understanding of the neural mechanisms that underlie such behaviours. In our specific case, this means that investigating variation in food-hoarding behaviour and spatial memory that has been caused by differential selection pressures may give us new insights into the neural mechanisms of memory processing. Not all variation in a trait is necessarily related to the ‘explanatory’ variable we are trying to relate to it. After all, different species have undergone different evolutionary histories, and ancestral states will influence further evolution of a trait (de Kort & Clayton 2006). Still, studying adaptive behaviour in natural contexts will allow us to ask questions that would not be germane in typical laboratory experiments where contexts are less ecologically relevant, e.g. designs with rats in water mazes.
Having established the value of the neuroecological approach to understanding brain evolution and brain mechanisms, we suggest that the application of this approach to date has not lived up to its full potential. We will argue that this is mainly due to the use of a simple-to-use, but potentially problematic measure of brain anatomy: the volume of a brain structure. In the case of food-hoarding animals, the ASH has mainly been tested by relating spatial memory (or a proxy for memory, such as caching intensity) to Hp volume across different species or populations of food hoarders (Krebs et al. 1989; Sherry et al. 1989, 1992; Healy & Krebs 1992, 1996; Garamszegi & Eens 2004; Lucas et al. 2004). The results of this approach have not always been unequivocal, but they have largely provided support for the ASH: the Hp appears to be larger in species that hoard more intensely. Inconsistency and variance that exist in the reported results may be related to the reliability of proxy measures of memory use, such as ‘degree of specialization for hoarding’ (Smulders et al. 2010), or by the use of volume as the measure of Hp specialization. Although it may be assumed that the volume of a brain structure will reflect some aspects of underlying anatomical processes, the nature of these processes is not well understood. Thus, it remains unclear if and how the volume of the Hp itself is related to its function, such as spatial memory.
The main goal of this manuscript is therefore to critically consider the value of volume as the primary measurement of the degree of morphological and physiological adaptation of a brain structure to a cognitive ability. As indicated, we will specifically consider spatial memory in scatter-hoarding animals, and relate it to adaptations of the Hp. We will review the evidence for the adaptive specialization of memory in food-caching animals and discuss the philosophy behind volume as the main currency of brain morphology. We will then examine the problems associated with this approach, focusing on what volume can and cannot actually tell us about spatial memory processing, and suggest alternatives to volume that might yield more specific hypotheses. Finally, we will discuss the implications of better measures of the brain for our understanding of the evolution of memory and food caching in scatter-hoarding animals.
2. The role of the hippocampus in spatial memory
Memory is processed in part in the Hp (for review, see Shettleworth 1998; Bast 2007). For spatial memory in particular, the Hp plays a major role in the acquisition and retrieval of memories. The Hp of birds and mammals is believed to be homologous (for a review, see Colombo & Broadbent 2000; Smulders & DeVoogd 2000) and the importance of the Hp in spatial memory processing is well demonstrated in both birds and mammals. For example, some experiments show that Hp lesions prevent the acquisition of new spatial memories, but not non-spatial memories such as colour (Morris 1983; Sherry & Vaccarino 1989; Hampton & Shettleworth 1996; Shiflett et al. 2003). However, the Hp does not work independently to process memories; other regions of the brain are important as well (Squire et al. 1993; Squire & Zola 1996; Squire 2004). The network of regions involved in memory processing is less well characterized in birds than it is in mammals, but progress is being made at an anatomical level (e.g. Atoji & Wild 2006), and several brain areas have been identified as involved in different types of memory (e.g. Suge & McCabe 2004; Güntürkün 2005).
In addition to being involved in memories, the Hp may play a role in some other functions as well (Bast 2007). For example, the Hp may be involved in motivation, especially as related to appetite (Tracy et al. 2001), hibernation (Horowitz et al. 1987; Spangenberger et al. 1995), emotions and fear response (Bast 2007), as well as non-spatial memory tasks such as reversal learning, extinction, context learning and other flexible forms of declarative-like memory (e.g. Bunsey & Eichenbaum 1996; Eichenbaum 1996). These other functions may covary to some extent with spatial abilities, but until we better understand which brain regions are involved in which behaviours, we will not know. The fact that the Hp may be involved in multiple functions makes the comparative approach more difficult, as unexplained variation in Hp anatomy may be related to functions other than the one under investigation (e.g. spatial memory). For the purposes of this discussion, we will focus only on the role of the Hp in spatial memory, but we will keep the confound of multiple functions in mind.
Within the food-caching animal paradigm, the Hp has been shown to be crucial for the successful retrieval of previously hoarded food: when the Hp is lesioned, birds will still cache food, but they are no better at retrieving their cached food than naive birds (Krushinskaya 1966; Sherry & Vaccarino 1989). This clear involvement of the Hp in processing memories for hidden food items has resulted in the focus on the connection between Hp morphology and spatial memory, with enlargements of the Hp being linked to presumably better memory performance (Krebs et al. 1989; Sherry et al. 1989). Memory can be enhanced by increasing the memory capacity, i.e. the number of things remembered, the duration of the memory, the accuracy of retrieval, the speed of retrieval, or all of the above. Measuring memory capacity and the speed of retrieval is difficult; hence most studies have focused on measuring accuracy and, to a lesser degree, duration (Smulders et al. 2010).
Why would we expect an enlargement of the Hp associated with memory enhancement? Historically, relative brain size (typically when compared with body mass) has been associated with cognitive qualities such as ‘intelligence’ that are associated with plastic behaviour. Numerous comparative studies, even the very recent ones, have maintained this idea (e.g. Iwaniuk & Hurd 2005; Lefebvre & Sol 2008; Sol et al. 2008). However, there is no real biological reason (i.e. no real hypotheses) for this relationship. Why should a relatively larger skull, and thus a relatively larger brain, be a prerequisite for plastic behaviour? Studies that assume a causal relationship between brain size and behavioural plasticity frequently ignore the importance of trade-offs between selection for a larger brain with other selection pressures favouring a reduction in brain size, thereby ignoring important aspects of the ASH. Selection should favour the head volume to body mass ratio that is most adaptive. It is possible that selective pressures that favour a small skull size might counteract selection for behavioural plasticity, cognition and memory, but it is not necessary. Moreover, selection pressures on body size may be independent of those on the head/brain. Such studies can be useful for exploring patterns, but are limited in their interpretation, and certainly cannot be used to causally link structure and function; see Healy & Rowe (2007) for an excellent critique of this issue.
Most studies in the food-caching paradigm have maintained this logic, focusing on a specific section of the brain, the Hp. As the Hp appears to be critical for spatial memory, the logic is that this region might be a reasonable place to expect morphological expressions of memory specialization and the traditional metric has been volume. To what extent the use of volume is really the result of this previous logic or simply the ease of measurement is unclear. Still, the focus on volume is not unique in Hp/memory comparisons, but is also commonly used in other study systems, for example in studies of the relationship between singing behaviour and song nuclei in the brain in passerine birds. Many studies have found that animals that sing more have larger song nuclei (e.g. Nottebohm et al. 1981; Canady et al. 1984), although this relationship does not always hold (e.g. Gahr et al. 1998; Leitner et al. 2001). The approach in such studies has been to treat the brain (and its different regions) as a ‘black box’. There appears to be an association of overall brain volume or the volume of specific regions of the brain, and some interesting behaviours (although the patterns are not completely clear, see below), but why these relationships exist and their relevance for understanding brain function are unclear.
Hence, many questions remain, such as ‘how does the Hp play a role in processing memory?’ and ‘what is the actual physiological expression of memory in the Hp?’ The Hp consists of several well-defined substructures (Atoji & Wild 2006), and it is not known which of these should be important for spatial memory. Thus far, the use of volume has been justified with post hoc explanations. It may well be the case that we should focus our interests on something more specific than gross volume. To date, we just do not know enough about the brains of food-hoarding birds or their function in regards to memory. However, the literature on memory processing in the mammalian brain is large, and too little attention has been paid to this literature in the context of caching memory and the Hp (Roth et al. 2010). In the rest of this paper, we will therefore evaluate the usefulness of comparing volumes across species, populations and individuals (the dominant approach to date), and point out in which directions we believe research on spatial memory and the Hp in scatter hoarders should go next.
3. Evidence for the adaptive specialization of hippocampal volume for spatial memory
If the Hp has been adaptively specialized for spatial memory, a positive association of Hp volume and spatial memory use/capacity has been predicted. Within that framework, species that routinely make and retrieve large quantities of food caches are expected to have a larger Hp region than non-caching species. Indeed, this pattern is generally supported within studies. The seminal works of Krebs et al. (1989) and Sherry et al. (1989) clearly showed that caching birds have considerably larger Hp regions than do non-caching birds. For example, food-storing marsh tits (Poecile palustris) have a 31 per cent larger relative Hp volume than the non-storing great tit (Parus major) (Krebs et al. 1989). Similarly, Hampton et al. (1995) showed that the Hp volume, as well as the caching rates in captivity, of food-storing black-capped chickadees (Poecile atricapillus) was significantly greater than those of two infrequent cachers, Mexican chickadees (Poecile sclateri) and bridled titmouse (Baeolophus wollweberi). Numerous other studies show similar patterns (e.g. Healy & Krebs 1992, 1996). Although most of this work has been done on birds, there is evidence of similar patterns within mammals as well (e.g. microtine rodents (Jacobs et al. 1990); kangaroo rats (Jacobs & Spencer 1994)).
A complicating issue of many of these studies, however, is the common proxy used for memory: caching intensity, how many caches an individual routinely makes during a given time frame. Smulders et al. (2010) provide a full discussion of the relevance of cache intensity versus retrieval for our understanding of spatial memory. Briefly, the relevance of caching intensity for memory function may not be that strong, as it is the amount of caching memorization that is relevant. It has been suggested that caches are not always retrieved by memory, but cache retrieval will still be more relevant as a measure of memory use than a crude measure such as caching intensity (Roth & Pravosudov 2009). A complicating factor here is that memory in some cases could be more important for the caching act than for retrieval (Male & Smulders 2007). Reliable data that are comparable between species on cache retrieval rates and retrieval accuracy are difficult to obtain in natural situations, and the length of time between caching and retrieval itself (i.e. the potential time frame of the memory) is difficult to know. Thus, poorly standardized estimates of caching intensity have become the standard for the comparison of spatial memory capabilities within and among species.
While support for the positive association between caching intensity and Hp volume is clear within studies, support for the pattern across studies is equivocal. The issue has been thoroughly reviewed by Brodin & Bolhuis (2008). Briefly, a relatively recent large-scale comparative study, combining data from previously published studies, found little support for the relationship between caching intensity and Hp volume across species (Brodin & Lundborg 2003). This study focused on the two families that have been best studied in this context: Corvidae (crows, jays, etc.) and Paridae (titmice and chickadees). Pooling a number of studies that each showed a correlation between caching intensity and Hp volume, they found no evidence of an overall link between caching specialization and Hp volume. However, this lack of effect might be the result of some unknown large-scale geographical differences. When Lucas et al. (2004) expanded the Brodin & Lundborg (2003) dataset and controlled for continent, a positive association reappeared. This suggests a major effect of continent where North American species tend to have significantly smaller Hp volumes than European species. This pattern held for caching species as well as non-caching species when phylogeny was considered (Lucas et al. 2004; Garamszegi & Lucas 2005). Consequently, Lucas et al. (2004) suggested that some unknown ecological factors may drive the major differences observed between continents (see also Garamszegi & Lucas 2005). It remains unclear, however, how ecological or evolutionary factors might favour greater Hp size on one continent compared with another. Nevertheless, the discovery by Lucas et al. suggests that the relationship between Hp volume and caching specialization holds.
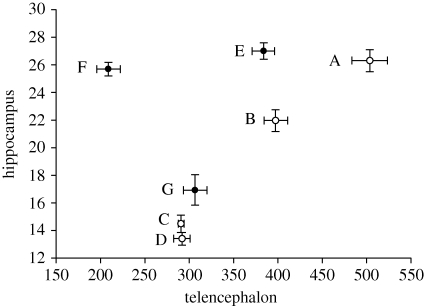
Some additional data, however, seem to complicate the evidence for the entire Hp volume/caching pattern. For example, willow tits (Poecile montanus) and black-capped chickadees are two closely related species (actually considered the same species until recently; e.g. Haftorn 1956a) with similar niches. The black-capped chickadee ranges across much of North America while the willow tit has a similarly impressive range in Europe and Asia. Based on previous studies of brain volume, the willow tit has a larger Hp volume than the black-capped chickadee (Healy & Krebs 1992; Pravosudov & Clayton 2002; Brodin & Lundborg 2003; figure 1), even though both appear to have similar caching rates (Brodin 2005a). However, recent data on black-capped chickadee Hp volumes by Roth & Pravosudov (2009) are not different from those previously reported for willow tits. Likewise, some older willow tit data collected by Cristol (1996) are not different from the original chickadee data (figure 1). Data on black-capped chickadees from Smulders et al. (1995) collected at approximately the same time fall in between these two extremes, although data from Hampton et al. (1995) are more in line with those collected by Pravosudov & Clayton (2002) (figure 1).
Figure 1.
Hippocampal and telencephalic volumes of black-capped chickadees and willow tits. A, Roth & Pravosudov 2009; B, Smulders et al. 1995; C, Hampton et al. 1995; D, Pravosudov & Clayton 2002; E, Healy & Krebs 1992; F, Brodin & Lundborg 2003; G, Cristol 1996. Filled circles, WITI; open circles, BCCH.
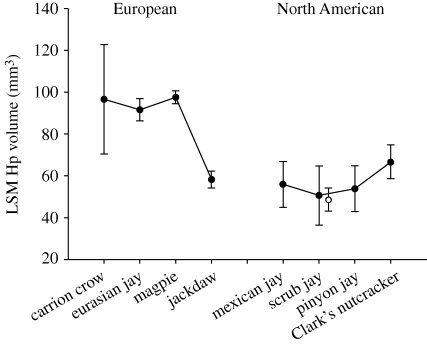
Similar inconsistencies in the supposed continental patterns are created with the inclusion of recent corvid data. North American corvids, even those that cache large amounts and are known to have impressive memories such as the Clark's nutcracker (Nucifraga columbiana), have very small Hp volumes relative to their European counterparts (Basil et al. 1996; Lucas et al. 2004). However, the inclusion of recent data on western scrub-jays (Aphelocoma californica) shows that this picture may not be correct. Pravosudov & de Kort (2006) report Hp volumes (76.19 ± 6.9 mm3) more than two times larger than those previously reported for the species (32.06 ± 3.6 mm3; Basil et al. 1996) and much larger than other North American corvids (figure 2). This is the case even though the scrub-jay is not thought to be a particularly prolific cacher (Vander Wall & Balda 1981). Indeed, the new volumes reported by Pravosudov & de Kort are more in line with those for the European caching corvids (figure 2). If we accept that the continental difference is true, the larger hippocampi of western scrub-jays could only be explained as an adaptive trait if they would possess spatial memory abilities well above those of their more highly specialized food-hoarding relatives in North America. This seems very unlikely. The next section will explore more likely explanations for the inconsistencies pointed out above.
Figure 2.
Least-squared means of Hp volumes of European and North American corvids. European data from Lucas et al. (2004). North American data from Basil et al. (1996; filled circles) and Pravosudov & de Kort (2006; open circle). Error bars represent 1 s.d.
4. Problems with the compatibility of data across studies
The chickadee/tit and corvid examples above are just two illustrations of inconsistencies that make interpretation of comparative Hp volume data difficult. We suspect that additional problems will arise as more data are added. This stems fundamentally from the principal problem of comparability of data across studies. Comparative studies that take their data from a variety of published sources rely on the assumption that data from these different sources are methodologically comparable in that the observed variation is due to the factor of interest and not methodological bias. This assumption, however, appears not to be met in many studies. To address the compatibility issues directly and to decrease the risk of future erroneous and irrelevant interpretations, we will posit some explanations for these inconsistencies and make some recommendations to avoid such problems.
One of the main contributing factors to the incompatibility of data across studies is that there are systematic differences between research groups producing variability among laboratories. The main issues here are that (i) different groups may use slightly different histological techniques, (ii) different studies use different statistical techniques, (iii) different studies may use different measurement decisions, and (iv) regardless of training or technique, studies have been designed to answer different questions and thus treat animals differently, thus creating unforeseen biological variation. Such seemingly minor variation may be very important. In our corvid example above, there is a laboratory and continental confound. The bulk of the data on North American corvid species come from a single study (Basil et al. 1996). Likewise, most of the European corvid data originate from a single study (Healy & Krebs 1992). These differences among groups may have been underestimated (e.g. Garamszegi & Eens 2004; Lucas et al. 2004) for various reasons. It has been suggested that most people who study these aspects of the brain have learned techniques from the same original source (John Krebs’ laboratory in Oxford in the 1980s) and thus should perform their studies in the same way (Lucas et al. 2004). It has also been suggested that there is repeatability in the variables measured (Garamszegi & Eens 2004). Unfortunately, this might not be the case.
(a). Differences in histological techniques
Variation in techniques across research groups and over time may stem from multiple sources. For example, different procedures involved in the collection and preservation of the brain tissue may result in different tissue shrinkage rates. To complicate things, brain shrinkage rates may be different at different times of the year (Smulders 2002; Phillmore et al. 2006). In most studies, the first step in collecting brain tissue is a perfusion, but details of this technique have varied between studies. This method involves pumping fluid through the animal in order to remove the blood from the brain and begin the fixation process from the inside. Perfusion leads to less interference from blood cells during analysis and may reduce tissue damage during processing. The duration of the perfusion and the concentration of the chemicals involved may, however, have strong effects. For example, the concentration of and duration of exposure to formalin (formaldehyde/paraformaldehyde) will affect the degree of tissue shrinkage (Quester & Schroder 1997; Kerns et al. 2008). Formalin fixes tissue by cross-linking proteins (Puchtler & Meloan 1985). In doing so, it denatures and constricts the proteins (Puchtler & Meloan 1985). Thus, all else equal, longer exposure to and/or higher concentrations of formalin in the solutions will produce more tissue shrinkage to the point of full fixation (Quester & Schroder 1997 and references therein). This may lead to systematic biases among laboratories in estimates of Hp volume. More importantly, after the brain tissue is extracted from the animal, it is generally post-fixed in a formalin solution for a period ranging from a day to several weeks (e.g. Pravosudov & Clayton 2002; LaDage et al. 2009b; Roth & Pravosudov 2009). This is yet another very important point where considerable tissue shrinkage may occur (Quester & Schroder 1997 and references therein; T. C. Roth, L. D. LaDage & V. V. Pravosudov, unpublished data).
After the tissue is fixed, it must be sectioned before measurements can occur. Different techniques for sectioning and mounting tissue may cause systematic variation in volume estimates. For example, the two common techniques for preparing tissue for sectioning are embedding the tissue in paraffin wax (e.g. Basil et al. 1996) and freezing the tissue (e.g. Krebs et al. 1989). Systematic comparisons between the two techniques show a clear difference in that tissue shrinks much more when it is embedded in paraffin than when it is frozen (Kretchmann et al. 1982).
Such systematic differences are a major problem when data from different laboratories are pooled since they cannot reliably be corrected post hoc. Different parts of the brain can shrink at different rates with different techniques (Kretchmann et al. 1982). Nevertheless, many studies in the medical field do use post hoc corrections to better estimate the size of the original (i.e. fresh) tissue (Quester & Schroder 1997). These types of studies, however, focus their corrections on specific brain regions (rather than whole brain changes) and use very large sample sizes to compare the effects of different chemicals and techniques, aiming to promote the consistency of the technique in subsequent studies. Such corrections will not be perfect, but may still be a reasonable way, for example, to estimate the original size of a cancerous growth within an individual. The applicability of such techniques to volumetric differences in Hp size across species is less clear, as sample sizes are frequently very small. Thus, we do not recommend such corrections for our field.
(b). Differences in statistical techniques
Another source of systematic bias in estimates of Hp volume can be found in differences and problems with sampling, statistical analysis and interpretation among studies. Many studies suffer from small sample sizes. In the corvid example above, some species (e.g. blue jay Cyanocitta cristata, alpine chough Pyrrhocorax graculus and rook Corvus frugilegus) were represented by single individuals. Yet, other species have been represented by only two individuals (see table 4 in Brodin & Bolhuis 2008). Obviously, the smaller the sample sizes, the less reliable they will be as estimates of a population mean. Besides normal variation between individuals, there is also a possibility that measured individuals are not representative for other reasons (e.g. if they are old, ill or of only one sex). Information on sex, age and health is rarely, if ever, actually included in comparative analyses.
Furthermore, whether and how telencephalon (Te) volume is used in the analysis may also have an effect on the results. Te volume is often used as a control region in studies of the Hp. In effect, using Te as a reference controls for overall brain size, thus making comparisons of Hp volumes between groups more meaningful. However, few studies explicitly describe how the Te was measured. This is a problem as one current method of measuring the Te in the literature is to estimate Te volume only on sections of tissue on which the Hp has also been measured (e.g. Sherry et al. 1993; Cristol et al. 2003; Day et al. 2005, 2008). Since the Te is longer than the Hp, this means that parts of the Te that are anterior and posterior to the Hp may not have been included in the calculation. This will lead to underestimation of the Te volume, thereby inflating the relative size of the Hp. The inflation of relative Hp volume is not only a problem in comparisons of data from different laboratories, but also produces a size bias in that a small (i.e. short) Hp may exclude relatively more of the Te from the analysis than a larger Hp. Using this method can change the estimate of Hp volume as much as 15 per cent (LaDage et al. 2009c).
In the case of the parid example above, there are significant differences in the Te volumes reported both within and between the black-capped chickadee and willow tit (figure 1). Is this variation natural (e.g. owing to differences in populations) or the result of different volume estimates? Overall, depending upon which datasets are used and how the data are analysed, the conclusion could be that black-capped chickadee brain sizes are larger, smaller or not different from those of willow tits. While we do not want to say that we should only use raw Hp measurements for interspecific comparisons, it is clearly important to measure both Hp and Te volumes using comparable methods.
Finally, the definition of the independent variable is problematic. In some studies, a categorical storer/non-storer variable has been used (e.g. Krebs et al. 1989; Sherry et al. 1989). These categories are probably accurate to some degree, but a categorical dichotomous variable gives poor resolution to a relationship. In an attempt to make regression/correlation analyses possible, Healy & Krebs (1992, 1996) introduced three categories on a scale of degree of specialization for caching: non-hoarders, hoarders of intermediate specialization and highly specialized hoarders. This scale has been used in several subsequent studies (e.g. Brodin & Lundborg 2003; Lucas et al. 2004), but how these categories are defined and which species they include are fairly subjective. For example, the willow tit is considered to be a specialized cacher, as it may make more than 100 000 caches in a single season (Haftorn 1956a,b; Pravosudov 1985; Brodin 1994a). However, the black-capped chickadee used to be considered a non-specialized cacher, even though it caches at the same rate (or at a slightly higher rate) as the willow tit (Brodin 2005a). Although the black-capped chickadee has been designated a specialized cacher in later studies (e.g. Lucas et al. 2004), the problem of subjectivity remains. Other species that have been problematic to categorize objectively include the Eurasian magpie Pica pica, the rook C. frugilegus, the coal tit and the marsh tit (Brodin & Lundborg 2003).
Our main point here is that seemingly minor variation in technique may produce large effects in the final estimation of volume if there is a systematic bias. Such a bias may not necessarily be a problem within a study. Assuming that all treatment groups are handled identically, the value of the study by itself should still hold. However, when studies are compared or combined, such biases may make data incompatible. Taken individually, there may be differences in the precise effect that each technique may have on the estimation of brain region volume. Collectively, however, such variation may be compounded in various ways, resulting in large systematic differences across studies (like continental differences). Thus, future studies that wish to examine large-scale patterns between the Hp and caching should consider the use of formal meta-analyses rather than pooling data from various sources.
(c). Differences in measurement decisions
Systematic differences in the designation of different regions of the brain may produce variation between studies. Although the dorsal, ventral and medial boundaries of the avian Hp are unambiguous, the lateral boundary is not. There can be variation between studies in how this boundary is defined and how much of the parahippocampus and Hp proper are included. Similarly, the rostral boundary can be difficult to determine and caudal sections may be lost owing to their small size and shape. Thus, differential treatment of these areas may produce between-study variation, although within-study variation should be minimal. In addition, differences in staining techniques may produce variation in volume estimates by means of labelling different regions or cells in the brain. For example, Gahr (1997) found significant differences in the estimates of brain region volumes using cytoarchitectural, cytochemical and connectional delineation techniques. Moreover, these differences varied depending upon developmental stage. While the majority of studies involving the Hp/memory relationship use Nissl staining (a cytoarchitectural technique), it is important to note this possibility for future studies that may use different techniques.
(d). Unforeseen biological variation
Even if differences in techniques could be controlled for, the problem will still remain that different studies are designed to ask different questions, and thus their results may not be directly comparable. Such differences may make comparisons between and among studies difficult. For example, some studies are designed to ask questions about the behaviour of animals, which is then related to brain morphology. In many such studies, animals are captured and brought into captivity for experiments (e.g. Hampton et al. 1995; Pravosudov & Clayton 2002). While mentioned as a possible source of variation, the effect of captivity on the brain has not been thoroughly investigated (Smulders et al. 2000a). The amount of stimulation and movement will be very small in captivity compared with an animal's natural environment. Recent work by LaDage et al. (2009b) and Tarr et al. (2009) suggests that captivity alone has a large effect on Hp volume, but no effect on Hp neuron number or the rest of the Te volume. Hoshooley & Sherry (2004) found an effect of captivity (one to two weeks) on neurogenesis rates, but not neuron number or volume. It has been suggested that spending time in captivity will lead to reduced connectivity within the Hp, which itself may result in changes in volume without affecting total neuron numbers. Such changes may be in part due to stress (McEwen 1999; Sousa & Almeida 2002), although there are probably multiple factors that could produce such a captivity effect. Whether such volumetric changes have consequences for behaviour (i.e. a reduction in memory) is also unknown.
In addition to a captivity effect on wild-caught animals, other animals have been obtained from zoos or the pet trade (e.g. Healy & Krebs 1992). In such cases, animals may have spent most of their lives in captivity or may have belonged to a strain that has been held in captivity for generations. It is not known whether animals that have spent their entire lives in captivity are comparable to animals that have been brought into captivity as adults, although domestication is known to have effects on brain anatomy (e.g. Rehkamper et al. 2008). For example, the alpine chough and red-billed blue magpie in Healy & Krebs (1992) were taken from long-term captive sources. If these individuals had reduced Hp volumes relative to their wild counterparts (as did captive juncos in Smulders et al. (2000a), mountain chickadees in LaDage et al. (2009b) and black-capped chickadees in Tarr et al. (2009)), their Hp volumes may not be representative and thus the relationship between the degree of hoarding and Hp volume may not hold.
Another potential bias is when and where animals are collected. We know from previous work that there may be an effect of season on volume (Smulders et al. 1995; Smulders 2002; but see Hoshooley & Sherry 2004). Thus, animals collected at different times of the year may not be directly comparable. In addition, there is also clear evidence of variation in relative Hp volume among populations. Pravosudov & Clayton (2002) and Roth & Pravosudov (2009) showed that black-capped chickadees from northern populations have larger Hp volumes and more neurons than those from more southern populations. Thus, even within the same season, birds from different areas should not be assumed to be directly comparable without first considering the effects of region. Differences in caching rates and the possible associated Hp morphology may occur in neighbouring populations for various reasons.
Furthermore, there could be other confounding variables that may be more important than date and location for capture. Large-scale comparative studies traditionally focus on species-level analyses. The premise of such studies is that if we find a relationship between a variable of interest, such as caching intensity, and some other trait, such as brain morphology, we can predict the latter from the former. However, species-level analyses are prone to unexplained variation owing to other ecological variations between species. Variation in factors such as diet, evolutionary history, physiology, etc., can be highly influential and difficult to explain (e.g. Volman et al. 1997). To complicate matters, spatial memory may respond to selection for reasons other than food caching and the Hp is involved in processes other than spatial memory (as mentioned earlier). When comparing species, it is therefore important firstly to know if there is variation in spatial demands between these groups that depend on other factors such as territory size, movement patterns, habitat structure, etc. For example, the migratory subspecies of white-crowned sparrows (Zonotrichia leucophrys) has a relatively larger Hp volume and more Hp neurons than their sedentary conspecifics (Pravosudov et al. 2006). Also, spatial memory demands from activities such as territory maintenance and movements during the pursuit of mates may be high during the breeding season but low at other times of the year (e.g. Jacobs et al. 1990; Lavenex et al. 2000). Likewise, individuals that maintain territories might need to memorize the area of their territories. This could create higher demands for spatial memory in territorial individuals than in non-territorial ones, potentially confusing species-level comparisons of the Hp. Such differences in spatial memory requirements have been demonstrated to affect Hp morphology (e.g. LaDage et al. 2009d). While speculative, we feel that it is important to note that the selection pressure for high spatial memory capacity created by food hoarding may be confounded by selection for high spatial memory capacity for other behaviours, and indeed by any other processes that require the Hp. Thus, it is important to know and consider the ecology of the species before attempting to compare them based on their food-hoarding status alone, and even then, additional variation owing to un-measured variables is likely to remain.
One way to reduce these problems is to compare separate populations within a species. Just as in comparisons between species, variation in morphology and behaviour can occur within a species and is just as reasonably the result of natural selection. Theoretically, the variation in hoarding specialization and Hp volume that has been compared between species could also occur between populations within one species. If we can find intraspecific variation in these traits, we can reduce variation from unrelated factors. For example, Brodin & Bolhuis (2008) suggest that the strongest evidence for the adaptive specialization of the Hp comes from two intraspecific comparisons of black-capped chickadees. This species has a wide range from Alaska to New Mexico. As a result, they occupy a range with a great deal of climatic variation. Chickadees in more northern populations experience a more severe climate (lower temperatures, more snow, shorter day lengths) than their southern conspecifics. This makes it logical to assume that northern birds need more cached food during the winter. If caching locations are memorized this should select for better spatial memory in northern populations. Indeed, both laboratory experiments and fieldwork suggest that northern chickadees have better spatial memory (Pravosudov & Clayton 2002) and relatively larger Hp volumes with more neurons (Roth & Pravosudov 2009) than their southern counterparts. We suggest that future questions should take better advantage of intraspecific variation in these traits both across (e.g. Pravosudov & Clayton 2002; Roth & Pravosudov 2009) and within populations (e.g. LaDage et al. 2009d).
5. Is hippocampal volume a relevant morphological measure of spatial memory adaptation?
Despite the problems with comparisons across studies, the consistent within-study patterns show that there does seem to be good evidence for a positive association of Hp volume and spatial memory ability (see also the review by Smulders 2006). While the unexplained variation in interspecific studies produces confusion, it can be addressed somewhat by selecting appropriate species for comparison and by careful treatment of data. Moreover, intraspecific studies strongly support such an association. At this point, however, we as a field now need to ask, ‘What do volumetric differences in Hp volume really mean?’ As discussed earlier, volume may traditionally have been used as a convention rather than a factor of theoretical importance, with the vague assumption of ‘the bigger, the better’. The biological relevance of variation in volume is not clear. Variation in Hp volume may reflect several different processes at a cellular level: a larger Hp could be due to more/larger blood vessels, more glia, larger glia, more neurons and/or larger neurons (either in the cell body, in dendritic arborization, axonal arborization or any combination of these). Knowing which of these cellular mechanisms underlie the observed differences in Hp volume will have a major implication for how we interpret the observed patterns. It might therefore be more appropriate and meaningful to examine the Hp at these finer scales in order to identify morphological expressions of variation in memory. If we can identify fine anatomical Hp features that correlate strongly with variation in cache memory use, while others do not, then the former will be stronger candidates to be involved in the mechanisms underlying the processing of these memories. In the rest of this review, we will focus on the properties of neurons, as these have been studied to some degree in the Hp of food-hoarding birds.
(a). Variation in neurons
Neurons and glia are the basic cell types within the Hp. As such, they represent one aspect of the brain's capacity to process information. The total number of Hp neurons, in particular, may be an important factor underlying differences in Hp volume. If neurons represent the integrators in the brain's neural network, then more neurons may be associated with more processing power. As such, the number of neurons may be a more relevant factor in predicting spatial memory ability than gross Hp volume per se (e.g. Smulders et al. 2000b; Pravosudov & Clayton 2002; Pravosudov & de Kort 2006; Roth & Pravosudov 2009). Healy et al. (1994), for example, suggested that the differences in Hp volume and neuron density between caching and non-caching species may arise as a result of an interaction between genetics and memory-based food-caching experience. Increased numbers of neurons may be distributed unevenly throughout different substructures of the Hp. Since the subsections of the avian Hp are not easily delineated, very few studies to date have investigated whether the relative size of subsections of the Hp differs between species that vary in Hp volume. Gould et al. (2001), for example, showed that the medial substance P-field is larger in hoarding black-capped chickadees than in non-hoarding great tits, blue tits and dark-eyed juncos. This increase in size was proportional to the larger Hp in the chickadee compared with the other three species. Another issue that remains to be explored is the exact neuron types that represent the increase in neuron numbers. Different neuron types perform different functions, and knowing which types are responsible for the difference in Hp volume would provide important new insights. Indeed, independent of whether or not they contribute to differences in volume, the relative proportions of different neuron types are important to investigate in the context of the ASH.
Similarly, neuron size might be informative. Neurons consist of relatively small cell bodies connected to a relatively large arborization of dendrites and axons. Neurons with larger dendritic arbors may make more connections with adjacent neurons. A larger cell body may also affect the integration properties of the neurons. Montagnese et al. (1993) showed that food-hoarding species have larger calbindin-positive neuronal cell bodies in the dorsal Hp than closely related non-hoarders. The functional significance of these differences, however, is not yet clear.
(b). Neural connectivity
While the number of neurons might be important, the degree of connectivity between neurons may be equally crucial for memory (Lamprecht & LeDoux 2004; Brodin 2005b). One measure of that connectivity is the amount of branching and the length of the dendritic branches. If the differences in dendritic arborization are large enough, and apply to enough neurons, this may have a significant effect on the overall volume of the brain structure. All else being equal, more branching will produce more connectivity between neurons, which produces more efficient transfer of information between and among neurons with an ensuing increase in potential memory capacity (McEwen 1999). Moreover, the level of dendritic branching and connectivity is a factor that can easily and quickly be modified within an individual. For example, several mammalian species show a reduction in dendritic branching under chronic levels of stress as well as a result of changes in hormone levels (reviewed in McEwen 1999), and these effects may be reversible (Radley et al. 2005). Interestingly, the changes in dendrite morphology can occur very rapidly. For example, changes in dendritic shape can occur within seconds (Fisher et al. 1998), and the formation of new dendrites can occur within minutes (Maletic-Savatic et al. 1999). Thus, the dendritic response may be an adaptive way for an animal to rapidly create or eliminate the hardware needed for memory and, alternatively, to rapidly increase neuronal connections when the demands for memory become high. If differences in volume are due to differences in dendritic tree size, then this may also be reflected in the number of synapses. Although difficult, quantifying synapse numbers and synapse size at an electron microscopic level would allow the examination of the degree of connectivity between neurons.
Of course, the fine-scale changes at the levels of synapses that may underlie changes in cognitive performance may not necessarily be reflected in changes in Hp volume. In such cases, studies of synaptic function such as long-term potentiation (LTP) could be useful to show how quickly and efficiently signals can actually be transmitted. There is strong evidence that modification in synaptic strength is part of the mechanism that underlies memory storage (Bliss & Collingridge 1993; Malenka & Bear 2004). The higher the degree of potentiation, the stronger the connection will be, and this will produce a higher degree of chemical stimulation between neurons. Independent of neuron number and size, the level of potentiation may reflect levels of communication between neurons and hence may be an important physiological expression of variation in memory capacity. Thus, large differences in memory capabilities may be due to small-scale synaptic differences that would not necessarily produce large-scale differences in volume.
While LTP is one of the most frequently studied aspects of neural plasticity in biomedical studies, its applicability to behavioural ecology has not been fully appreciated but may be of great importance (Brodin 2005b; Roth et al. 2010). In the absence of existing studies that focus on LTP in food-hoarding birds, Stewart et al. (1999) compared the expression of N-methyl-d-aspartate (NMDA) glutamate receptors in the Hp of hoarding and non-hoarding tits, and found these to be different. NMDA receptors are a crucial part of most forms of LTP and are important in memory formation in the Hp of food-hoarding birds (Shiflett et al. 2003). Therefore, studies such as this one might give us much better insights into the kinds of changes that may have occurred as the Hp was adapted to the food-hoarding lifestyle.
(c). Neurogenesis
Another important aspect of studying neurons is neurogenesis, or the creation of new neurons. For the purposes of this discussion, we will only consider the role of neurogenesis in adult animals, although the role of neurogenesis in developing animals may be important as well (e.g. Patel et al. 1997). Hoshooley & Sherry (2007) reported that food-caching black-capped chickadees had more new neurons following injection of a cell proliferation marker (bromodeoxyuridine; BrdU) compared with non-caching house sparrows (Passer domesticus), which suggests that adult Hp neurogenesis is especially important in food-caching species (see also Sherry & Hoshooley 2010). In addition, Barnea & Nottebohm (1994) found an increase in the percentage of incorporated neurons clearly following the fall caching peak in wild black-capped chickadees (Pravosudov 2006). This reported pattern of seasonal variation in neurogenesis rates is consistent with some other studies showing seasonal changes in other aspects of brain morphology such as Hp volume (Smulders et al. 1995) and Hp neuron counts (Smulders et al. 2000b; see below).
While there appears to be a correlation between caching and adult neurogenesis in the Hp, there may be some ambiguity surrounding which aspects of neurogenesis are being measured. Neurogenesis consists of two stages, the production of new neurons and their survival. Both of these processes may be affected by various ecological factors, but survival of new neurons may respond especially quickly to exogenous factors affecting memory and hence experimental manipulation (Gould et al. 1999). So, while Barnea & Nottebohm (1994) found an increase in neuron incorporation in fall chickadees six weeks after injection, they did not investigate the neuron production rates; thus, it remains possible that either new neuron production, neuron survival, or both could have contributed to the reported pattern. Hoshooley & Sherry (2004) found no seasonal effect of neurogenesis rates, while Hoshooley et al. (2007) reported seasonal effects, with a peak in neuron production occurring in January (when caching rates are significantly lower than during the autumn peak; Pravosudov 2006). They analysed the tissue shortly (one to two weeks) after BrdU injections, suggesting that they may have been measuring neuron production only. So while it is possible that neuron survival, but not neuron production, may vary seasonally, it is impossible to conclude unambiguously because no study to date has investigated both neuron production and neuron survival rates with regard to seasonal variation in food-caching behaviour.
It may be logical to expect better memory with more neurons, more connections or stronger synapses, and much literature supports these ideas. It is also important to point out that the morphological basis for variation in spatial memory capacity could be expressed as more Hp neurons with the same level of connections, more connections with the same number of neurons, or stronger connections with the same number of neurons and dendrites, or any combinations of these. It is very likely that these factors are more relevant for memory capacity than is gross Hp volume. The investigation of such factors on a finer scale will make it possible to test specific mechanisms of variation, to create specific predictions and, perhaps most importantly, to identify factors that will make consistent comparisons possible. The exact mechanisms of memory and its storage are still debated and remain an important topic of current research. Thus, they represent exciting possible directions and new insights for our field. Number, size and distribution of neurons may be particularly easy to address as the methodology used to study them (e.g. Nissl stains, stereology) is not greatly different from that used to estimate volume. The study of neurogenesis may be complex using traditional techniques (e.g. BrdU), although new techniques such as doublecortin labelling (e.g. Gleeson et al. 1998; LaDage et al. 2010) are more tractable and may produce similarly interpretable results (Brown et al. 2003; Rao & Shetty 2004).
Variation in some or all of these aspects of Hp anatomy may be the main causes of some of the extraneous variation in volume that we currently see. For example, the loss of neurons or dendrites might allow the brain to shrink more during the fixation process as there is less internal structure in the tissue. This phenomenon is well known in the medical field where, for example, the more fibrous dermal tissue of adults will shrink less than that of juveniles when fixed in formalin (Kerns et al. 2008). Thus, if neuron numbers or dendritic branching change (e.g. Smulders et al. 2000b), this may result in different perceived volumes in the processed tissue, even though the actual volume (volume of the brain in the animal's skull) does not change. Measuring such factors as neurons, dendrites and synapses may provide us with more accurate measures that can be used to explain the variation in memory capacity. The reason for this is that these measurements are much less prone to variation owing to tissue shrinkage or laboratory-based variation in technique. Consequently, these variables may prove to be more relevant currencies of memory capacity, especially for comparative analyses. Similarly, interesting patterns may emerge when some of these factors change while others do not. For example, LaDage et al. (2009b) observed a change in volume with no change in neuron numbers in captive mountain chickadees. The authors suggested that this pattern may reflect a change in dendritic connections without a change in neuron number, which may reflect different costs at different levels of brain morphology.
We have outlined a number of possible mechanisms that can underlie volumetric differences in the Hp. In general, we would expect observed differences to be a function of the costs of manufacturing and maintaining different brain structures, the benefits of having those structures and current selection pressures working on the system. Thus, the importance of these mechanisms to variation in brain volume may be apparent at different taxonomic scales. For example, since neurons are probably expensive to create and maintain relative to changes in neural connectivity and synaptic strength, the higher the taxonomic level and the larger the differences in selection pressures, the more likely we would see larger, more profound differences in neural anatomy. Among species, then, differences in neuron numbers would probably be most apparent, with the differences in Hp volume between species most probably reflecting differences in neuron numbers. Among populations within the same species, neuron numbers might also be different at selection extremes (e.g. Roth & Pravosudov 2009), but connectivity differences among existing neurons might be important as well. Relatively short-term variation in Hp volumes among individuals, on the other hand, more likely reflects variation in connectivity rather than large-scale variation in the number of neurons (e.g. LaDage et al. 2009b). However, if there was a high cost to maintain neurons and a low cost to generate them, or a great benefit to have additional neurons at a particular time, then we would expect neuronal density changes could occur within individuals, which might occur for example during the peak of seasonal caching (e.g. Smulders et al. 2000b). Finally, within individuals, changes in connectivity should be the most prevalent and rapid anatomical change, but still changes in the number of neurons via adult neurogenesis might also be important. These predictions are currently quite speculative as we have no real understanding of the costs associated with whole brain maintenance or function, let alone the costs of specific parts. Once we have a grasp of the cost of forming and maintaining neural structures, we should be better poised to understand how morphological and taxonomic variation may be related.
6. Environment, memory and the hippocampus
Across taxa, we see a high degree of variation in specialization for scatter hoarding, spatial memory and Hp volume. In this section, we will address the role of plasticity in the relationship between the brain and scatter-hoarding behaviour. A precise memory for cache locations will facilitate retrieval of caches. In cases when the costs of memory are high, caches might also be retrieved by other mechanisms such as idiosyncratic preferences (Haftorn 1956a; Pravosudov 1985; Lens et al. 1994; Brodin 2005b; Brodin & Bolhuis 2008), although this could also lead to increased rates of pilferage. Still, the importance of accurate spatial memory to facilitate cache retrieval should remain. A more specific understanding of the morphological features in the brain that are associated with spatial memory capacity may provide us with a better understanding of the evolution of scatter-hoarding behaviour.
Selection for increased memory capacity for cache retrieval can occur in two main ways. First, improvements in spatial memory capabilities may be completely genetically fixed. In this scenario, natural selection would favour a genetic component producing more efficient memory processing, storage and recall, for example with more neurons or a higher degree of connectivity. This might be expected in highly specialized hoarders such as Clark's nutcracker (N. columbiana) that always rely on caches for winter survival. Alternatively, selection could favour plasticity in memory abilities and behaviour. In this situation, the brain could respond to experienced spatial memory demands. If no caching experiences occurred, and thus spatial memory was not in great demand, then there would be no effect on Hp morphology. The advantage of plasticity would be that brain resources, both energetically and morphologically, could be allocated to other important needs.
Such plasticity could be facilitated either ontogenetically or post-developmentally and may be most common in facultative cachers. An example of facultative development of caching specialization could be the marsh tit. In central Scandinavia, these are large-scale hoarders of the same magnitude as willow tits (Haftorn 1956a), whereas they are hoarders of intermediate specialization in England (Lucas et al. 2004). Clayton & Krebs (1994) showed a developmental effect on Hp growth in naive marsh tits in captivity. The tits required some level of experience in caching early in their development to develop an Hp of similar volume as wild birds. This study also showed a threshold effect as volume development was not proportional to the number of experiences (Clayton & Krebs 1994). Clayton (2001) showed a similar effect in mountain chickadees, a more specialized cacher, in that the development of the Hp can be triggered by as few as three caching experiences. Interestingly, caching alone was not sufficient to produce Hp enlargement, which specifically required caching in combination with cache retrieval experience (Clayton 2001). Furthermore, this study suggested that ontogenetic plasticity might be combined with elements of post-developmental plasticity since continued experience seems to be required to maintain the Hp. The results of two other studies suggest that this may only be true for developing cachers since deprivation of caching and retrieval experience in caching-experienced birds had no effect on Hp volume or the total number of Hp neurons (Cristol 1996; LaDage et al. 2009b). Since such experiments are rare, we cannot know for sure if this pattern represents the standard development of caching behaviour or if it is specific to facultative cachers.
The post-developmental Hp plasticity observed in adult cachers has been investigated in a number of studies. For example, Smulders et al. (1995, 2000b) reported seasonal variation in Hp volume and neuron numbers in field-caught black-capped chickadees. Hippocampal volume and neuron number peaked in October when hoarding rates in the field were assumed to peak. However, several laboratory experiments and field studies have failed to find any evidence of an effect that is large enough to change gross Hp volume. In the laboratory, seasonal changes have been simulated by photoperiod manipulations. This technique created high motivation for storing with ensuing high caching rates, but no detectable effects on Hp morphology in black-capped chickadees (Krebs et al. 1995; MacDougall-Shackleton et al. 2003). In the field, Barnea & Nottebohm (1994), Hoshooley & Sherry (2004) and Hoshooley et al. (2007) failed to find a change in Hp morphology over the season in the black-capped chickadee.
The difference between Smulders et al. (1995) and the other studies could be a function of methodology. It is now known that short times spent in captivity may affect Hp volume (Smulders et al. 2000a; LaDage et al. 2009b; Tarr et al. 2009). In that case, the difference might be explained in part by confounding effects mediated by differences in time spent in captivity. For example Hoshooley et al. (2007) kept the birds in cages for 7 days before they sacrificed the birds, whereas Smulders et al. (1995) sacrificed their birds the same day that they were captured. Barnea & Nottebohm (1994) sacrificed their birds shortly after capture and found significant seasonal effects on neurogenesis. They also found that birds kept in captivity had much lower neuronal incorporation rates than their wild-caught birds. Therefore, captivity may have interfered with the detection of seasonal variation in other studies, although we cannot be certain. Experimental studies with food-caching mountain chickadees (LaDage et al. 2009b, 2010) suggest that spending time in captivity results in significant (approx. 25%) reductions of Hp volume and neurogenesis rates. This reduction in volume and neurogenesis rates occurred without changes in the total number of neurons. It remains unclear how and whether such captivity effects would affect seasonal patterns of variation in Hp morphology in captive birds. It could be argued that even with the possible effects of captivity, Hoshooley & Sherry (2004) should have detected a volumetric difference if it existed since all of the birds were held for the same time period. However, this assumes that the captivity effect is consistent across season, which is unknown. It is possible that the captivity effect reduced the (presumably) larger Hp volumes during October more than at other times during the year to a constant level. If the captivity effect is an adaptive response to expensive brain machinery in combination with a reduced ‘need’ for memory, then we might expect similar effects on Hp volume in different individuals in similar laboratory environments. Furthermore, while the motivation to cache might have been higher in the laboratory birds during October, this will be manifested in only modest increases in caching rates under laboratory conditions. Such small changes in caching rates might be insufficient to induce detectable increases in Hp volume, but they do seem to be sufficient to induce changes in neurogenesis (LaDage et al. 2009b, 2010).
Also, it remains unclear what specific morphological changes might lead to seasonal changes in the HP volume. Smulders et al. (2000a,b) reported seasonal variation in Hp neuron numbers, but their estimates were determined by volume extrapolation rather than unbiased stereological techniques. Others (Barnea & Nottebohm 1994; Hoshooley & Sherry 2004), on the other hand, did not find any seasonal variation in the neuron numbers. To further explain this discrepancy and to better understand the effect of plasticity and seasonality, we need to better match the collecting of animals and tissue to the actual caching peaks and treat them the same across studies.
Which factor is then most relevant for spatial memory adaptations: genetics, developmental states or behavioural experience? Probably all three are important to some extent. To some degree, genetics must play a role in the absolute level of memory by ensuring that the machinery is in place for change. For example, the same spatial memory experience that triggered Hp growth in food-hoarding marsh tits failed to do so in non-hoarding blue tits (Clayton 1995). Brain plasticity is likely to be important too, as it may be very expensive to maintain parts of the brain also under conditions when they are not needed. If maintaining connections is energetically expensive but new connections can be created relatively easily, then they should be reduced when not needed (e.g. captivity work; see above). In addition, experience may be needed to activate absolute and/or plastic memory. Overall, we know very little about the influence of selection on the brain and the development of behaviour.
7. Conclusion
So what do we hope to learn in the future from the study of spatial memory and caching behaviour? What can the variation in Hp morphology tell us? The evidence that there is a relationship between the spatial memory of scatter-hoarding animals and the Hp is strong. To better understand the nature of this relationship, we now need to turn our attention to some more complex questions relating to specific mechanisms underlying memory. Using the paradigm of scatter-hoarding animals, we can specifically target memory and address the adaptive specialization of specific regions of the Hp beyond a simple correlation of Hp volume and caching intensity. In addition, we can address factors that might be important for selection and investigate how selection for increased memory capacity is actually realized in the brain. This may help us to better understand how selection influences the brain at different scales. The goal here is a better understanding of the process of natural selection itself and its impact on memory and the brain.
At a smaller scale, we can attempt to address how variation in morphology influences behaviour in more detail and how behavioural experiences and environmental variation affect morphology. If we have variation in a behaviour such as caching propensity, we could then get a better understanding of how we should relate this to variation in Hp morphology. By answering these questions, we can more specifically explore the mechanisms of memory from an ecological and evolutionary perspective. For example, what are the relative contributions of neuron number, dendritic branching and synaptic strength for memory across taxa? How might different strategies trade-off the costs and benefits of these? How and where is memory stored, modified and achieved in the brain? The consideration of more specific variables, such as the number of neurons, to test more specific hypotheses should enhance our current understanding of the relationship between the brain and behaviour.
To summarize, our current understanding of the evolution of spatial memory and its association with the brain in food-hoarding animals is largely based on volumetric analyses of the Hp. We have argued that the biological relevance and reliability of volumetric measures are low. Estimations of volume are prone to variation from a variety of different sources, making comparisons between studies difficult. Moreover, the significance of volumetric analyses to the brain/memory relationship remains unclear, as such measures only represent a coarse proxy of other changes occurring within the brain. Consequently, conclusions drawn from studies attempting to relate brain volume and memory or caching behaviour have sometimes produced weak or conflicting results. We have also pointed out that the comparative approach can only provide a correlation between two variables (e.g. Hp volume and the degree of caching specialization) and can only inform our understanding of large-scale evolutionary patterns. It cannot test directly the causal mechanisms of variation in memory processing or morphological changes within the brain. The critics of the neuroecological approach have accused the entire field of attempting to explain too much (causal mechanisms of memory and the relevance of morphological changes in the Hp) with too little evidence (comparative analyses based on data collected using inconsistent methodology) (sensu Bolhuis & Macphail 2001). However, we should not be so quick to conclude that the larger neuroecological approach is flawed. Instead, as a field, we need to expand the paradigm beyond the questionable significance of volume and the valuable, yet limited in scope, correlative approach to more biologically relevant measures, specific hypotheses and manipulative experiments. Comparative work is an important step in the long way to a full understanding of the relationship between two variables, but should not be considered the final word. We must employ both the large-scale evolutionary approach as well as the small-scale mechanistic approach to inform our understanding of the brain. Mechanism may be better understood when placed in an evolutionary context, yet evolutionary patterns cannot be used to explain how the brain processes memories; each can be used to provide clues to the other. Testing specific mechanistic hypotheses derived from a solid evolutionary theoretical background with more relevant and reliable measurements under consistent situations will be crucial for our further understanding of the evolution of the brain and behaviour.
Acknowledgements
We thank two anonymous reviewers for their comments on earlier drafts of this manuscript. T.C.R., L.D.L. and V.V.P. were supported by grants from the National Science Foundation (IOB-0615021) and the National Institutes of Health (MH079892, MH076797) to V.V.P. A.B. was supported by the Swedish Research Council, VR. T.V.S. was supported by a travel grant from the Royal Society of London.
Footnotes
One contribution of 10 to a Theme Issue ‘Integrating ecology, psychology and neurobiology within a food-hoarding paradigm’.
References
- Atoji Y., Wild J. M.2006Anatomy of the avian hippocampal formation. Rev. Neurosci. 17, 3–15 [DOI] [PubMed] [Google Scholar]
- Barnea A., Nottebohm F.1994Seasonal recruitment of hippocampal neurons in adult free-ranging black-capped chickadees. Proc. Natl Acad. Sci. USA 91, 11 217–11 221 (doi:10.1073/pnas.91.23.11217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basil J. A., Kamil A. C., Balda R. P., Fite K. V.1996Differences in hippocampal volume among food storing corvids. Brain Behav. Evol. 47, 156–164 (doi:10.1159/000113235) [DOI] [PubMed] [Google Scholar]
- Bast T.2007Toward an integrative perspective on hippocampal function: from the rapid encoding of experience to adaptive behavior. Rev. Neurosci. 18, 253–281 [DOI] [PubMed] [Google Scholar]
- Bliss T. V. P., Collingridge G. L.1993A synaptic model of memory—long-term potentiation in the hippocampus. Nature 361, 31–39 (doi:10.1038/361031a0) [DOI] [PubMed] [Google Scholar]
- Bolhuis J. J., Macphail E. M.2001A critique of the neuroecology of learning and memory. Trends Cogn. Sci. 4, 426–433 [DOI] [PubMed] [Google Scholar]
- Brodin A.1994aTime aspects on food hoarding in the willow tit. PhD dissertation, Stockholm University, Stockholm, Sweden [Google Scholar]
- Brodin A.1994bSeparation of caches between individual willow tits hoarding under natural conditions. Anim. Behav. 47, 1031–1035 (doi:10.1006/anbe.1994.1141) [Google Scholar]
- Brodin A.2005aHippocampal volume does not correlate with food-hoarding rates in the black-capped chickadee and willow tit. Auk 122, 819–828 (doi:10.1642/0004-8038(2005)122[0819:HVDNCW]2.0.CO;2) [Google Scholar]
- Brodin A.2005bMechanisms of cache retrieval in long-term hoarding birds. J. Ethol. 23, 77–83 (doi:10.1007/s10164-005-0147-5) [Google Scholar]
- Brodin A., Bolhuis J. J.2008Memory and brain in food storing birds: space oddities or adaptive specialisations? Ethology 114, 633–645 (doi:10.1111/j.1439-0310.2008.01508.x) [Google Scholar]
- Brodin A., Lundborg K.2003Is hippocampal volume affected by specialisation for food hoarding in birds? Proc. R. Soc. Lond. B 270, 1555–1563 (doi:10.1098/rspb.2003.2413) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. P., Couillard-Despres S., Cooper-Kuhn C. M., Winkler J., Aigner L., Kuhn H. G.2003Transient expression of doublecortin during adult neurogenesis. J. Comp. Neurol. 467, 1–10 (doi:10.1002/cne.10874) [DOI] [PubMed] [Google Scholar]
- Bunsey M., Eichenbaum H.1996Conservation of hippocampal memory function in rats and humans. Nature 379, 255–257 (doi:10.1038/379255a0) [DOI] [PubMed] [Google Scholar]
- Canady R. A., Kroodsma D. E., Nottebohm F.1984Population differences in complexity of a learned skill are correlated with the brain space involved. Proc. Natl Acad. Sci. USA 81, 6232–6234 (doi:10.1073/pnas.81.19.6232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton N. S.1995Development of memory and the hippocampus: comparison of food-storing and nonstoring birds on a one-trial associative memory task. J. Neurosci. 15, 2796–2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton N. S.2001Hippocampal growth and maintenance depend on food-caching experience in juvenile mountain chickadees (Poecile gambeli). Behav. Neurosci. 115, 614–625 (doi:10.1037/0735-7044.115.3.614) [DOI] [PubMed] [Google Scholar]
- Clayton N. S., Krebs J. R.1994Hippocampal growth and attrition in birds affected by experience. Proc. Natl Acad. Sci. USA 91, 7410–7414 (doi:10.1073/pnas.91.16.7410) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo M., Broadbent N.2000Is the avian hippocampus a functional homologue of the mammalian hippocampus? Neurosci. Biobehav. Rev. 24, 465–484 (doi:10.1016/S0149-7634(00)00016-6) [DOI] [PubMed] [Google Scholar]
- Cristol D. A.1996Food storing does not affect hippocampal volume in experienced adult willow tits. Behav. Brain Res. 81, 233–236 (doi:10.1016/S0166-4328(96)89083-8) [DOI] [PubMed] [Google Scholar]
- Cristol D. A., Reynolds E. B., LeClerc J. E., Donner A. H., Farabaugh C. S., Ziegenfus C. W. S.2003Migratory dark-eyed juncos, Junco hyemalis, have better spatial memory and denser hippocampal neurons than nonmigratory conspecifics. Anim. Behav. 66, 317–328 (doi:10.1006/anbe.2003.2194) [Google Scholar]
- Day L. B., Westcott D. A., Olster D. H.2005Evolution of bower complexity and cerebellum size in bowerbirds. Brain Behav. Evol. 66, 62–72 (doi:10.1159/000085048) [DOI] [PubMed] [Google Scholar]
- Day L. B., Guerra M., Schlinger B. A., Rothstein S. I.2008Sex differences in the effects of captivity on hippocampus size in brown-headed cowbirds (Molothrus ater obscurus). Behav. Neurosci. 122, 527–534 (doi:10.1037/0735-7044.122.3.527) [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kort S. R., Clayton N. S.2006An evolutionary perspective on caching by corvids. Proc. R. Soc. B 273, 417–423 (doi:10.1098/rspb.2005.3350) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H.1996Is the rodent hippocampus just for ‘place'? Curr. Opin. Neurobiol. 6, 187–195 (doi:10.1016/S0959-4388(96)80072-9) [DOI] [PubMed] [Google Scholar]
- Fischer M., Kaech S., Knutti D., Matus A.1998Rapid actin-based plasticity in dendritic spines. Neuron 20, 847–854 (doi:10.1016/S0896-6273(00)80467-5) [DOI] [PubMed] [Google Scholar]
- Gahr M.1997How should brain nuclei be delineated? Consequences for developmental mechanisms and for correlations of area size, neuron numbers and functions of brain nuclei. Trends Neurosci. 20, 58–62 (doi:10.1016/S0166-2236(96)10076-X) [DOI] [PubMed] [Google Scholar]
- Gahr M., Daisuke Y.2007Sexual differentiation of the vocal control system of birds. In Advances in genetics, pp. 67–105 New York, NY: Academic Press; [DOI] [PubMed] [Google Scholar]
- Gahr M., Sonnenschein E., Wickler W.1998Sex difference in the size of the neural song control regions in a dueting songbird with similar song repertoire size of males and females. J. Neurosci. 18, 1124–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garamszegi L. Z., Eens M.2004The evolution of hippocampus volume and brain size in relation to food hoarding in birds. Ecol. Lett. 7, 1216–1224 (doi:10.1111/j.1461-0248.2004.00685.x) [Google Scholar]
- Garamszegi L. Z., Lucas J. R.2005Continental variation in relative hippocampal volume in birds: the phylogenetic extent of the effect and the potential role of winter temperatures. Biol. Lett. 1, 330–333 (doi:10.1098/rsbl.2005.0328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson J. G., Lin P. T., Flanagan L. A., Walsh C. A.1998Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron 23, 257–271 (doi:10.1016/S0896-6273(00)80778-3) [DOI] [PubMed] [Google Scholar]
- Gould S. J., Lewontin R. C.1979The spandrels of San Marco and the panglossian paradigm: a critique of adaptionist programme. Proc. R. Soc. Lond. B 205, 581–598 (doi:10.1098/rspb.1979.0086) [DOI] [PubMed] [Google Scholar]
- Gould E., Tanapat P., Hastings N. B., Shors T. J.1999Neurogenesis in adulthood: a possible role of learning. Trends Cogn. Sci. 3, 186–192 (doi:10.1016/S1364-6613(99)01310-8) [DOI] [PubMed] [Google Scholar]
- Gould K. L., Newman S. W., Tricomi E. M., DeVoogd T. J.2001The distribution of substance P and neuropeptide Y in four songbird species: a comparison of food-storing and non-storing birds. Brain Res. 918, 80–95 (doi:10.1016/S0006-8993(01)02961-4) [DOI] [PubMed] [Google Scholar]
- Güntürkün O.2005The avian ‘prefrontal cortex' and cognition. Curr. Opin. Neurobiol. 15, 686–693 (doi:10.1016/j.conb.2005.10.003) [DOI] [PubMed] [Google Scholar]
- Haftorn S.1956aContribution to the food biology of tits, especially about storing of surplus food: Part IV. A comparative analysis of Parus atricapillus L., P. cristatus, L., and P. ater L. Kgl. Norske Vidensk. Selsk. Skr. 4, 1–54 [Google Scholar]
- Haftorn S.1956bContribution to the food biology of tits especially about storing of surplus food. Part III: The willow tit (Parus atricapillus). Kgl. Norske Vidensk. Selsk. Skr. 3, 1–79 [Google Scholar]
- Hampton R. R., Shettleworth S. J.1996Hippocampal lesions impair memory for location but not color in passerine birds. Behav. Neurosci. 110, 831–835 (doi:10.1037/0735-7044.110.4.831) [DOI] [PubMed] [Google Scholar]
- Hampton R. R., Sherry D. F., Shettleworth S. J., Khugel M., Ivy G.1995Hippocampal volume and food-storing behavior are related in parids. Brain Behav. Evol. 45, 54–61 (doi:10.1159/000113385) [DOI] [PubMed] [Google Scholar]
- Hampton R. R., Healy S. D., Shettleworth S. J., Kamil A. C.2002‘Neuroecologists’ are not made of straw. Trends Cogn. Sci. 6, 6–7 (doi:10.1016/S1364-6613(00)01821-0) [DOI] [PubMed] [Google Scholar]
- Healy S. D., Krebs J. R.1992Food storing and the hippocampus in corvids—amount and volume are correlated. Proc. R. Soc. Lond. B 248, 241–245 (doi:10.1098/rspb.1992.0068) [Google Scholar]
- Healy S. D., Krebs J. R.1996Food storing and the hippocampus in Paridae. Brain Behav. Evol. 47, 195–199 (doi:10.1159/000113239) [DOI] [PubMed] [Google Scholar]
- Healy S. D., Rowe C.2007A critique of comparative studies of brain size. Proc. R. Soc. B 274, 453–464 (doi:10.1098/rspb.2006.3748) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy S. D., Clayton N. S., Krebs J. R.1994Development of hippocampal specialization in two species of tit (Parus spp.). Behav. Brain. Res. 61, 23–28 (doi:10.1016/0166-4328(94)90004-3) [DOI] [PubMed] [Google Scholar]
- Horowitz J. M., Thomas M. P., Eckerman P.1987Thermal dependence of neural activity in the hamster hippocampal slice preparation. J. Therm. Biol. 12, 97–101 (doi:10.1016/0306-4565(87)90045-3) [DOI] [PubMed] [Google Scholar]
- Hoshooley J. S., Sherry D. F.2004Neuron production, neuron number, and structure size are seasonally stable in the hippocampus of the food-storing black-capped chickadee (Poecile atricapillus). Behav. Neurosci. 118, 345–355 (doi:10.1037/0735-7044.118.2.345) [DOI] [PubMed] [Google Scholar]
- Hoshooley J. S., Sherry D. F.2007Greater hippocampal neuronal recruitment in food-storing than in non-food-storing birds. Dev. Neurobiol. 67, 406–414 (doi:10.1002/dneu.20316) [DOI] [PubMed] [Google Scholar]
- Hoshooley J. S., Phillmore L. S., Sherry D. F., MacDougall-Shackleton S. A.2007Annual cycle of the black-capped chickadee: seasonality of food-storing and the hippocampus. Brain Behav. Evol. 69, 161–168 (doi:10.1159/000096984) [DOI] [PubMed] [Google Scholar]
- Iwaniuk A. N., Hurd P. L.2005The evolution of cerebrotypes in birds. Brain Behav. Evol. 65, 215–230 (doi:10.1159/000084313) [DOI] [PubMed] [Google Scholar]
- Jacobs L. F., Spencer W. D.1994Natural space-use patterns and hippocampal site in kangaroo rats. Brain Behav. Evol. 44, 125–132 (doi:10.1159/000113584) [DOI] [PubMed] [Google Scholar]
- Jacobs L. F., Gaulin S. J. C., Sherry D. F., Hoffman G. E.1990Evolution of spatial cognition—sex-specific patterns of spatial behavior predict hippocampal size. Proc. Natl Acad. Sci. USA 87, 6349–6352 (doi:10.1073/pnas.87.16.6349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson C.1982The year round diets of the willow tit Parus montanus conrad and the crested tit P. cristatus L. PhD thesis, Göteborgs Universitet, Göteborg
- Kerns M. J. J., Darst M. A., Olsen T. G., Fenster M., Hall P., Grevey S.2008Shrinkage of cutaneous specimens: formalin or other factors involved? J. Cutan. Pathol. 35, 1093–1096 (doi:10.1111/j.1600-0560.2007.00943.x) [DOI] [PubMed] [Google Scholar]
- Krebs J. R., Sherry D. F., Healy S. D., Perry V. H., Vaccarino A. L.1989Hippocampal specialisation of food-storing birds. Proc. Natl Acad. Sci. USA 86, 1388–1392 (doi:10.1073/pnas.86.4.1388) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs J. R., Clayton N. S., Hampton R. R., Shettleworth S. J.1995Effects of photoperiod on food-storing and the hippocampus in a food-storing and a non-storing passerine bird. Neuroreport 16, 1701–1704 [DOI] [PubMed] [Google Scholar]
- Kretchmann H. J., Tafesse U., Herrmann A.1982Different volume changes of cerebral cortex and white matter during histological preparation. Microsc. Acta 86, 13–24 [PubMed] [Google Scholar]
- Krushinskaya N.1966Some complex forms of feeding behavior of nutcracker Nucifraga caryocatactes, after removal of old cortex. Z. Evoluz. Biochem. Fisiol II, 563–568 [Google Scholar]
- LaDage L. D., Roth T. C., Fox R. A., Pravosudov V. V.2009aFlexible cue use in food-caching birds. Anim. Cogn. 12, 419–426 (doi:10.1007/s10071-008-0201-0) [DOI] [PubMed] [Google Scholar]
- LaDage L. D., Roth T. C., Fox R. A., Pravosudov V. V.2009bEffects of captivity and memory-based experiences on the hippocampus of mountain chickadees. Behav. Neurosci. 123, 284–291 (doi:10.1037/a0014817) [DOI] [PubMed] [Google Scholar]
- LaDage L. D., Roth T. C., Pravosudov V. V.2009cBiases in measuring the brain: the trouble with the telencephalon. Brain Behav. Evol. 73, 253–258 (doi:10.1159/000225623) [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaDage L. D., Riggs B. J., Sinervo B., Pravosudov V. V.2009dDorsal cortex volume in male side-blotched lizards (Uta stansburiana) is associated with different space use strategies. Anim. Behav. 78, 91–96 (doi:10.1016/j.anbehav.2009.03.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaDage L. D., Roth T. C., Fox R. A., Pravosudov V. V.2010Ecologically-relevant spatial memory use modulates hippocampal neurogenesis. Proc. R. Soc. B (doi:10.1098/rspb.2009.1769) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht R., LeDoux J.2004Structural plasticity and memory. Nat. Rev. Neurosci. 5, 45–54 (doi:10.1038/nrn1301) [DOI] [PubMed] [Google Scholar]
- Lavenex P., Steele M. A., Jacobs L. F.2000Sex differences, but no season variation in the hippocampus of food-caching squirrels: a stereological study. J. Comp. Neurol. 425, 152–166 (doi:10.1002/1096-9861(20000911)425:1<152::AID-CNE13>3.0.CO;2-Y) [PubMed] [Google Scholar]
- Lefebvre L., Sol D.2008Brains, lifestyles and cognition: are there general trends? Brain Behav. Evol. 72, 135–144 (doi:10.1159/000151473) [DOI] [PubMed] [Google Scholar]
- Leitner S., Voight C., Garcia-Segura L. M., Van't Hof T., Gahr M.2001Seasonal activation and inactivation of song motor memories in wild canaries is not reflected in neuroanatomical changes of forebrain song areas. Hormon. Behav. 40, 160–168 (doi:10.1006/hbeh.2001.1700) [DOI] [PubMed] [Google Scholar]
- Lens L., Adriaensen F., Dhondt A. A.1994Age-related hoarding strategies in the crested tit—should the cost of subordination be reassessed. J. Anim. Ecol. 63, 749–755 [Google Scholar]
- Lucas J. R., Brodin A., de Kort S. R., Clayton N. S.2004Does hippocampal size correlate with the degree of caching specialisation? Proc. R. Soc. Lond. B 271, 2423–2429 (doi:10.1098/rspb.2004.2912) [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDougall-Shackleton S. A., Sherry D. F., Clark A. P., Pinkus R., Hernandez A. M.2003Photoperiodic regulation of food storing and hippocampus volume in black-capped chickadees, Poecile atricapillus. Anim. Behav. 65, 805–812 (doi:10.1006/anbe.2003.2113) [Google Scholar]
- Macphail E. M., Bolhuis J. J.2001The evolution of intelligence: adaptive specialisations versus general process. Biol. Rev. 76, 341–364 [DOI] [PubMed] [Google Scholar]
- Male L. H., Smulders T. V.2007Memory for food caches: not just for retrieval. Behav. Ecol. 18, 456–459 (doi:10.1093/beheco/arl107) [Google Scholar]
- Malenka R. C., Bear M. F.2004LTP and LTD: an embarrassment of riches. Neuron 44, 5–21 (doi:10.1016/j.neuron.2004.09.012) [DOI] [PubMed] [Google Scholar]
- Maletic-Savatic M., Malinow R., Svoboda K.1999Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science 283, 1923–1927 (doi:10.1126/science.283.5409.1923) [DOI] [PubMed] [Google Scholar]
- McEwen B. S.1999Stress and hippocampal plasticity. Annu. Rev. Neurosci. 22, 105–122 (doi:10.1146/annurev.neuro.22.1.105) [DOI] [PubMed] [Google Scholar]
- Montagnese C. M., Krebs J. R., Szekely A. D., Csillag A.1993A subpopulation of large calbindin-like immunopositive neurones is present in the hippocampal formation in food-storing but not in non-storing species of bird. Brain Res. 614, 291–300 (doi:10.1016/0006-8993(93)91047-V) [DOI] [PubMed] [Google Scholar]
- Morris R. G. M.1983An attempt to dissociate ‘spatial-mapping’ and ‘working-memory’ theories of hippocampal function. In Neurobiology of the hippocampus. (ed. Seifert W.), pp. 405–432 London, UK: Academic Press [Google Scholar]
- Nottebohm F., Nasparian S., Pansazis C.1981Brain space for a learned task. Brain Res. 213, 99–109 (doi:10.1016/0006-8993(81)91250-6) [DOI] [PubMed] [Google Scholar]
- Patel S. N., Clayton N. S., Krebs J. R.1997Spatial learning induces neurogenesis in the avian brain. Behav. Brain Res. 89, 115–128 (doi:10.1016/S0166-4328(97)00051-X) [DOI] [PubMed] [Google Scholar]
- Phillmore L. S., Hoshooley J. S., Sherry D. F., MacDougall-Shackleton S. A.2006Annual cycle of the black-capped chickadee: seasonality of singing rates and vocal-control brain regions. J. Neurobiol. 66, 1002–1010 (doi:10.1002/neu.20282) [DOI] [PubMed] [Google Scholar]
- Pigliucci M., Kaplan J.2000The fall and rise of Dr Pangloss: adaptationism and the Spandrels paper 20 years later. Trends Ecol. Evol. 15, 66–70 (doi:10.1016/S0169-5347(99)01762-0) [DOI] [PubMed] [Google Scholar]
- Pravosudov V. V.1985Search for and storage of food by Parus cinctus lapponicus and P. montanus borealis (Paridae). Zool. Z. 64, 1036–1043 [Google Scholar]
- Pravosudov V. V.2006On seasonality in food-storing behavior in parids: do we know the whole story? Anim. Behav. 71, 1455–1460 (doi:10.1016/j.anbehav.2006.01.006) [Google Scholar]
- Pravosudov V. V.2007The relationship between environment, food caching, spatial memory, and the hippocampus in chickadees. In Ecology and behavior of chickadees and titmice: an integrated approach (ed. Otter K.), pp. 25–41 Oxford, UK: Oxford University Press [Google Scholar]
- Pravosudov V. V., Clayton N. S.2002A test of the adaptive specialisation hypothesis: population differences in caching, memory, and the hippocampus in black-capped chickadees (Poecile atricapilla). Behav. Neurosci. 116, 515–522 (doi:10.1037/0735-7044.116.4.515) [PubMed] [Google Scholar]
- Pravosudov V. V., de Kort S.2006Is the western scrub-jay (Aphelocoma californica) really an underdog among food-caching corvids when it comes to hippocampal volume and food caching propensity? Brain Behav. Evol. 67, 1–9 (doi:10.1159/000088855) [DOI] [PubMed] [Google Scholar]
- Pravosudov V. V., Grubb T. C.1997Management of fat reserves and food caches in tufted titmice (Parus bicolor) in relation to unpredictable food supply. Behav. Ecol. 8, 332–339 (doi:10.1093/beheco/8.3.332) [Google Scholar]
- Pravosudov V. V., Lucas J. R.2001A dynamic model of short-term energy management in small food-caching and non-caching birds. Behav. Ecol. 12, 207–218 (doi:10.1093/beheco/12.2.207) [Google Scholar]
- Pravosudov V. V., Smulders T. V.2010Integrating ecology, psychology and neurobiology within a food-hoarding paradigm. Phil. Trans. R. Soc. B 365, 859–867 (doi:10.1098/rstb.2009.0216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravosudov V. V., Kitaysky A. S., Omanska A.2006The relationship between migratory behavior, memory and the hippocampus—an intraspecific comparison. Proc. R. Soc. B 273, 2641–2649 (doi:10.1098/rspb.2006.3624) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchtler H., Meloan S. N.1985On the chemistry of formaldehyde fixation and its effects on immunohistochemical reactions. Histochem 82, 201–204 (doi:10.1007/BF00501395) [DOI] [PubMed] [Google Scholar]
- Quester R., Schroder R.1997The shrinkage of the human brain stem during formalin fixation and embedding in paraffin. J. Neurosci. Methods 75, 81–89 (doi:10.1016/S0165-0270(97)00050-2) [DOI] [PubMed] [Google Scholar]
- Radley J. J., Rocher A. B., Janssen W. G. M., Hof P. R., McEwen B. S., Morrison J. H.2005Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exp. Neurol. 196, 199–203 (doi:10.1016/j.expneurol.2005.07.008) [DOI] [PubMed] [Google Scholar]
- Rao M. S., Shetty A. K.2004Efficacy of doublecortin as a marker to analyse the absolute number of and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur. J. Neurosci. 19, 234–246 (doi:10.1111/j.0953-816X.2003.03123.x) [DOI] [PubMed] [Google Scholar]
- Rehkamper G., Frahm H. D., Cnotka J.2008Mosaic evolution and adaptive brain component alteration under domestication seen on the background of evolutionary theory. Brain Behav. Evol. 71, 115–126 [DOI] [PubMed] [Google Scholar]
- Roth T. C., Pravosudov V. V.2009Hippocampal volume and neuron numbers increase along a gradient of environmental harshness: a large-scale comparison. Proc. R. Soc. B 276, 401–405 (doi:10.1098/rspb.2008.1184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth T. C., Rattenborg N. C., Pravosudov V. V.2010The ecological relevance of sleep: the trade-off between sleep, memory, and energy conservation. Phil. Trans. R. Soc. B 365, 945–959 (doi:10.1098/rstb.2009.0209) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry D. F.2006Neuroecology. Ann. Rev. Psychol. 57, 167–197 (doi:10.1146/annurev.psych.56.091103.070324) [DOI] [PubMed] [Google Scholar]
- Sherry D. F., Hoshooley J. S.2010Seasonal hippocampal plasticity in food-storing birds. Phil. Trans. R. Soc. B 365, 933–943 (doi:10.1098/rstb.2009.0220) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry D. F., Vaccarino A. L.1989Hippocampus and memory for food caches in black-capped chickadees. Behav. Neurosci. 103, 308–318 (doi:10.1037/0735-7044.103.2.308) [Google Scholar]
- Sherry D. F., Vaccarino A. L., Buckenham K., Herz R. S.1989The hippocampal complex of food-storing birds. Brain Behav. Evol. 34, 308–317 (doi:10.1159/000116516) [DOI] [PubMed] [Google Scholar]
- Sherry D. F., Jacobs L. F., Gaulin S. J. C.1992Spatial memory and adaptive specialisation of the hippocampus. Trends Neurosci. 15, 298–303 (doi:10.1016/0166-2236(92)90080-R) [DOI] [PubMed] [Google Scholar]
- Sherry D. F., Forbes M. R. L., Khurgel M., Ivy G. O.1993Females have a larger hippocampus than males in the brood-parasitic brown-headed cowbird. Proc. Natl Acad. Sci. USA 90, 7839–7843 (doi:10.1073/pnas.90.16.7839) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shettleworth S. J.1998Cognition, evolution, and behavior Oxford, UK: Oxford University Press [Google Scholar]
- Shiflett M. W., Smulders T. V., Benedict L., DeVoogd T. J.2003Reversible inactivation of the hippocampal formation in food-storing black-capped chickadees (Poecile atricapillus). Hippocampus 13, 437–444 (doi:10.1002/hipo.10065) [DOI] [PubMed] [Google Scholar]
- Smulders T. V.2002Natural breeding conditions and artificial increases in testosterone have opposite effects on the brains of adult male songbirds. Horm. Behav. 41, 156–169 (doi:10.1006/hbeh.2001.1748) [DOI] [PubMed] [Google Scholar]
- Smulders T. V.2006A multi-disciplinary approach to understanding hippocampal function in food-hoarding birds. Rev. Neurosci. 17, 53–69 [DOI] [PubMed] [Google Scholar]
- Smulders T. V.2009The relevance of brain evolution for the biomedical sciences. Biol. Lett. 5, 138–140 (doi:10.1098/rsbl.2008.0521) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smulders T. V., DeVoogd T. J.2000The avian hippocampal formation and the memory for hoarded food: spatial learning out in the real world. In Brain, perception, memory. Advances in cognitive neuroscience (ed. Bolhuis J. J.), pp. 127–148 Oxford, UK: Oxford University Press [Google Scholar]
- Smulders T. V., Sasson A. D., DeVoogd T. J.1995Seasonal variation in hippocampal volume in a food-storing bird, the black-capped chickadee. J. Neurobiol. 27, 15–25 (doi:10.1002/neu.480270103) [DOI] [PubMed] [Google Scholar]
- Smulders T. V., Casto J. M., Nolan V., Jr, Ketterson E. D., DeVoogd T. J.2000aEffects of captivity and testosterone on four brain regions in the dark-eyed junco (Junco hyemalis). J. Neurobiol. 43, 244–253 (doi:10.1002/(SICI)1097-4695(20000605)43:3<244::AID-NEU3>3.0.CO;2-#) [PubMed] [Google Scholar]
- Smulders T. V., Shiflett M. W., Sperling A. J., DeVoogd T. J.2000bSeasonal changes in neuron numbers in the hippocampal formation of a food-hoarding bird: the black-capped chickadee. J. Neurobiol. 44, 414–422 (doi:10.1002/1097-4695(20000915)44:4<414::AID-NEU4>3.0.CO;2-I) [DOI] [PubMed] [Google Scholar]
- Smulders T. V., Gould K. L., Leaver L. A.2010Using ecology to guide the study of cognitive and neural mechanisms of different aspects of spatial memory in food-hoarding animals. Phil. Trans. R. Soc. B 365, 883–900 (doi:10.1098/rstb.2009.0211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sol D., Bacher S., Reader S. M., Lefebvre L.2008Brain size predicts the success of mammal species introduced into novel environments. Am. Nat. 172, S63–S71 (doi:10.1086/588304) [DOI] [PubMed] [Google Scholar]
- Sousa N., Almeida O. F. X.2002Corticosteroids: sculptors of the hippocampal formation. Rev. Neurosci. 13, 59–84 [DOI] [PubMed] [Google Scholar]
- Spangenberger H., Nikmanesh F. G., Igelmund P.1995Long-term potentiation at low temperature is stronger in hippocampal slices from hibernating Turkish hamsters compared to warm acclimated hamsters and rats. Neurosci. Lett. 194, 127–129 (doi:10.1016/0304-3940(95)11723-A) [DOI] [PubMed] [Google Scholar]
- Squire L. R.2004Memory systems of the brain: a brief history and current perspective. Neurobiol. Learn. Mem. 82, 171–177 (doi:10.1016/j.nlm.2004.06.005) [DOI] [PubMed] [Google Scholar]
- Squire L. R., Zola S. M.1996Structure and function of declarative and nondeclarative memory systems. Proc. Natl Acad. Sci. USA 93, 13 515–13 522 (doi:10.1073/pnas.93.24.13515) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire L. R., Knowlton B., Musen G.1993The structure and organization of memory. Annu. Rev. Psychol. 44, 453–495 (doi:10.1146/annurev.ps.44.020193.002321) [DOI] [PubMed] [Google Scholar]
- Stewart M. G., Cristol D., Philips R., Steele R. J., Stamatakis A., Harrison E., Clayton N.1999A quantitative autoradiographic comparison of binding to glutamate receptor sub-types in hippocampus and forebrain regions of a food-storing and a non-food-storing bird. Behav. Brain Res. 98, 89–94 (doi:10.1016/S0166-4328(98)00055-2) [DOI] [PubMed] [Google Scholar]
- Suge R., McCabe B. J.2004Early stages of memory formation in filial imprinting: Fos-like immunoreactivity and behavior in the domestic chick. Neuroscience 123, 847–856 (doi:10.1016/j.neuroscience.2003.11.002) [DOI] [PubMed] [Google Scholar]
- Swanberg P. O.1951Food storage, territory and song in the thick-billed nutcracker. Proc. 10th Int. Ornithol. Congr. 10, 545–554 [Google Scholar]
- Tarr B., Rabinowitz J., Ali Imtiaz M., DeVoogd T. J.2009Captivity reduces hippocampal volume but not survival of new cells in a food-storing bird. Dev. Neurobiol. 69, 972–981 (doi:10.1002/dneu.20736) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy A. L., Jarrard L. E., Davidson T. L.2001The hippocampus and motivation revisited: appetite and activity. Behav. Brain Res. 127, SI13–SI23 [DOI] [PubMed] [Google Scholar]
- VanderWall S. B.1990Food hoarding in animals. Chicago, IL: University of Chicago Press [Google Scholar]
- Vander Wall S. B., Balda R. P.1981Ecology and evolution of food-storage behavior in conifer-seed-caching Corvids. J. Comp. Ethol 56, 217–242 [Google Scholar]
- Volman S. F., Grubb T. C., Schuett K. C.1997Relative hippocampal volume in relation to food-storing behaviour in four species of woodpeckers. Brain Behav. Evol. 49, 110–1120 (doi:10.1159/000112985) [DOI] [PubMed] [Google Scholar]